A soft shape memory reactor with controllable catalysis characteristics†
Xinzhen Wu,
Jie Hu*,
Songjun Li,
Rong Zhao and
Di Wang
School of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, P.R. China. E-mail: hujiechem@hotmail.com; Fax: +86-511-88790191; Tel: +86-511-88790191
First published on 17th July 2014
Abstract
A shape memory reactor comprised of a temperature responsive control layer and non-responsive substrate layer has been developed following a facile procedure. This unique structure with Ag nanoparticles contained in the control layer results in a catalytic activity controlled by its shape change when the temperature is varied.
It is estimated that catalysts are responsible for the production of over 60% of all chemicals and are used in some 90% of all chemical processes worldwide.1 Interest in the use of metal nanoparticles in catalysis has dramatically increased in recent years, due to the many different advantages they can offer.2,3 Owing to their large surface-to-volume ratio, metal nanoparticles bring together the benefits of heterogeneous (recovery and recyclability) and homogeneous catalysts (relatively high activity and good selectivity).4 Nevertheless, due to their high surface area, metal clusters tend to aggregate into larger particles in order to minimize their surface energy, which leads to a loss of the catalytic activity. To solve this key problem, restricting noble metal nanoparticles on supporting materials is an ideal solution since it prevents particle migration and the corresponding decay in the efficiency of the catalyst.
Different sorts of materials, e.g. organic micelles,5–8 polymers9–13 and inorganic materials,14–17 have been utilized to prevent metal nanoparticles from sintering and leaching. Among these supporting materials, smart polymeric hydrogels that can be switched “on” and “off” by an external stimulus have attracted more and more attention in recent years. Besides isolating and protecting the metal nanoparticles, those polymer gels can effectively regulate the reaction process and behavior under stimuli. For example, Zhang and coworkers18 reported the use of a thermoresponsive hydrogel based on crosslinked poly(glycidyl methacrylate-co-N-isopropylacrylamide) as a thermotunable and recyclable nanoreactor of gold nanoparticles. It was found that the reduction of 4-nitrophenol catalyzed by the thermoresponsive hybrid could be tuned by temperature change, in which the reduction rate at T < LCST was much lower than that at T > LCST. Results from Zhang's group19,20 indicated that hydrogels was indeed a suitable reaction medium where organic synthesis could be accelerated in comparison to the processes in water, since the hydrophobic reactants and metal catalyst could indeed become highly enriched within the hydrogel. Recently, Lu et al. reported an Au/PNIPAm yolk–shell catalytic system. They found that the polymer based nanoreactor showed a high catalytic activity for 4-nitrophenol. When the matrix became hydrophobic with increased external temperature, the shrinkage of the nanoreactor thereby caused a sharp decrease in the reaction rate.21 Investigations by Mei et al.22 and Perez-Juste et al.23,24 demonstrated that the volume transition within a thermosensitive network could be used as a switch and systems having a thermoresponsive shell with limited crosslinking allowed for particularly efficient control of the catalysis.
Although many important achievements have been obtained in this field, there is none successful example, to our best knowledge, reported on a smart reactor that can regulate its catalytic behavior by changing its shape under an external stimulus, and can be judged its “on” or “off” state through observing its shape simply with naked eyes. Here, we consider a conceptually novel design to intelligent polymer based reactor in which catalytic activity is modulated by the shape changing of a shape memory hydrogel under temperature variation. It is known that shape memory polymers (SMPs) are one kind of the most potential advanced materials which have the capability of changing their shapes upon application of an external thermal stimulus.25,26 These SMPs can perform even more complex shape changes when prepared in a two- or multi-layer form composed of control layer and substrate layer, in which the shape change is automatic and reversible without needing any external mechanical manipulation.27–29 Comparing with the traditional shape memory alloys, SMPs are much softer and therefore possess many advantages including excellent processability, lightweight, and great flexibility, which seems to provide a promising prospect for the stranded catalytic reactors.30,31 Furthermore, some special shape changes of the reactor responding to the external stimulus, such as an extended shape denoting the “on” state and the high catalytic activity of the reactor, and a coiled roll denoting the “off” state and the low catalytic activity, would have many benefits in practical process. It can not only facilitate the operating process of catalytic reaction, utilizing and protecting the metal nanoparticles contained in SMPs in a better way, but people can judge the real catalytic state of reactors very simply with eyes.
Inspired by this principle, we herein report a novel soft shape memory reactor with controllable catalytic characteristics. As outlined in Scheme 1, a two-layer shape memory reactor is prepared, in which Poly(N-isopropylacrylamide) (PNIPAm) crosslinked with methylenebisacrylamide form the thermosensitive control layer, and polyacrylamide (PAAm) crosslinked with methylenebisacrylamide form the substrate. Silver nanoparticles contained in the control layer play the catalyst role. When temperature is above the LCST of PNIPAm, the temperature responsive control layer shrinks drastically while the substrate layer does not undergo any noticeable changes in volume. The two-layer reactor curls to form a coiled roll, which results in the difficult access of reactants to silver particles contained in the control layer. On the contrary, when temperature is below the LCST, the swelling of the control layer induces the two-layer reactor to expand to form an extended strip, which results in the easy access of reactants to silver particles. So, upon the temperature variation, the catalytic activity can be regulated effectively with the change in shape of the smart reactor.
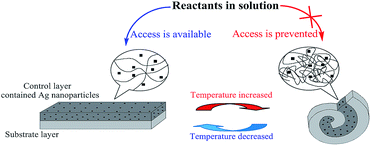 | ||
| Scheme 1 Schematic presentation of the soft shape memory reactor with controllable catalytic characteristics. | ||
Just as shown in Scheme S1,† the preparation route of the two-layer shape memory reactor, this novel shape memory reactor is fabricated in a two-layer form composed of a PNIPAm control layer containing Ag nanoparticles and a PAAM substrate layer. There should be no doubt concerning the main polymer compositions of the two layers, because N-isopropyl acrylamide and acrylamide are the only monomers used for the polymerization to form the control layer and substrate layer, respectively. As shown in Fig. S1,† the substrate layer is clear and colorless. The control layer, however, showed the black color because of Ag nanoparticles contained. Both layers are estimated to be about 2.5 mm. The Ag nanoparticles contained in the control layer are tested with XRD. As shown in Fig. S2,† the peaks indexed to diffractions from the {1 1 1}, {2 0 0}, {2 2 0}, and {3 1 1} planes indicate existence of the face-centered cubic silver. For a comparative study, the XRD patterns of the both controls, AgSR-D and SR-A, are also provided. Just as expected, the diffraction pattern of AgSR-D also shows peaks at the same 2theta values with AgSR-A, demonstrating the silver metal in it. In the diffraction pattern of SR-A, however, no any peaks is observed, which agrees with the absence of Ag. Fig. S3† shows the TEM images of the shape memory reactor and two controls. Similar with the results discussed in XRD analysis, there is no Ag nanoparticles observed in SR-A. The average sizes of silver particles contained in AgSR-A and AgSR-D are estimated to be 25 nm and 20 nm, respectively. Themogravimetric analysis is shown in Fig. S4.† It is found that the three hydrogels are stable below 250 °C. Beyond about 600 °C, SR-A decomposes completely. However, there are 15.3% and 10.9% mass left respectively for AgSR-A and AgSR-D, which is thought to be the residual silver nanoparticles. More content Ag in AgSR-A than in AgSR-D might result from the greater electronic density in N atom induced by the electron denoting effect of neighboring isopropyl group. As a result, the coordinating ability with Ag+ of N atom in NIPAm is strong than that in AAm.
The shape change of the AgSR-A and SR-A with temperature variation are shown in Fig. 1. It is found that both AgSR-A and SR-A exhibit extended strips at 25 °C. Increasing the temperature, both two-layer hydrogels bend to form the similar circle shape at 35 °C, and curl to become coiled rolls at 45 °C. It seems that Ag nanoparticles barely affect the thermo-responsive shape change of the two-layer hydrogel. Most interesting, the change in shape is automatical and reversible upon the external temperature change, which might mainly be resulted from the switchable expanding/shrinkage of PNIPAm control layer in the two-layer reactors while the substrate layer does not undergo any noticeable changes. The defined shape at a given temperature demonstrates the shape memory effect of AgSR-A and SR-A. No shape change, however, is observed for AgSR-D, although not being represented here.
Fig. 2 shows the thermally induced shape changes of AgSR-A over time when immersed into a 45 °C water bath. It is found that the straight strip bends quickly when heated, and curls to a roll within 200 s. Its relatively fast thermo-responsive shape changing ability is believed to facilitate its “on” and “off” states switched sensitively by temperature in controllable catalytic reaction.
To further discuss the shape change characteristics of these two-layer hydrogels, DLS is utilized to evaluate the relative volume change of the thermosensitive control layer compared with the substrate layer. Through determining the dependence of Rc upon temperature (Rc, the difference of size changing at a given temperature between grinded small particles from separated control layer and substrate layer, whose detailed calculation method was described in the Experimental part), the shape changing temperature or temperature range of AgSR-A and SR-A can be estimated. As can be seen from Fig. 3, Rc values of AgSR-A and SR-A show a sharp change in the range of 30–38 °C, which is compatible with the fact that the volume of PNIPAm gel shrinks drastically at temperatures higher than its LCST, about 32 °C,32 whereas the volume of PAAm gel does not. As a result, when the bilayer strips of AgSR-A and SR-A are heated uniformly, they gradually bend and finally curl into rolls. Because most shape changes are developed in the range of 30–38 °C, 25 °C and 45 °C, which are lower and higher than the shape changing temperature range, respectively, are selected in the following switchable “on” and “off” studies.
 | ||
| Fig. 3 Plots of size change difference between control layer and substrate layer upon temperature variation. | ||
Electrochemical experiments are performed to get information on the switchable “on” and “off” states by determining the dependence of the availability of external ions to the smart reactor prepared upon the shape change with temperature variation. In an electrochemical workstation, a Pt wire inserted in the control layer of the two-layer hydrogel is used as the working electrode in a conventional three-electrode configuration. The redox process of HPO42−/H2PO4− is adopted as a probe to achieve information on the controllable access of external mass into the control layer to contact with Pt wire by detecting the current change upon temperature variation. Two typical temperatures, 25 °C and 45 °C, are selected for the comparative study, at which the shape memory reactor exhibited shapes of the extended strip and the coiled roll, respectively. As shown in Fig. S5(a),† a reduction peak as high as 113.80 μA for AgSR-A at 25 °C is observed, which indicates the easy availability of the external ions for the Pt wire. When temperature increases to 45 °C, however, the current peak decreases significantly to 44.27 μA, which reveals the difficult access of the external ions into the reactor. It is believed that hydrophobic phase transition and shrinkage of the control layer, in which the Pt wire is inserted, result in the sharp decreasing of the current peak. It makes the external mass contact with the Pt wire much more difficultly. These phenomena observed demonstrate controllable access of external mass into AgSR-A by its shape change with temperature variation, which provides excellent mechanism for a reactor with switchable “on” and “off” characteristics. As can be seen from Fig. S5(b),† SR-A also shows the similar controllable ability companied with the change in shape. As shown in Fig. S5(c),† however, no significant current change is observed in AgSR-D with the temperature variation, which could be well understood if take the fact that the both layers in AgSR-D comprised only of PAAM for consideration.
The catalytic property of the smart reactor prepared is evaluated utilizing the reducing reaction of SF with NaBH4 catalyzed by Ag nanoparticles. Two typical temperatures, 25 °C and 45 °C, are also selected for the comparative study. As indicated in Fig. 4(a), SR-A is not found obvious catalytic ability due to the absence of Ag nanoparticles. Although exhibiting catalytic ability, no controlled catalytic behavior had been observed on AgSR-D, in which both layers are built up by PAAm and no PNIPAm layer included. The little difference of conversion when changing temperature is only because of the faster reaction rate induced by higher temperature. Most interesting, as shown in Fig. 4(b),† AgSR-A shows tunable catalysis as expected. After 10 min, the reaction conversion at 25 °C, being 51%, is much higher than that at 45 °C, only 10%. It is believed that hydrophobic phase transition and shrinkage of the control layer containing Ag nanoparticles, along with the embedding of PNIPAm layer in the coiled roll combine to respond for the sharp decreasing of the reaction rate when temperature increases. Fig. 4(c) illustrates the catalytic reaction progress of AgSR-A undergone an alternate temperature change between 45 °C and 25 °C. It is found that the catalytic reaction in the present of AgSR-A is significantly accelerated if temperature changes from 45 °C to 25 °C, and reaction rate falls dramatically when temperature increased back to 45 °C. The marked and quick change in slope of the conversion-time curve with temperature variation indicates the fast responsibility of AgSR-A to temperature. It is consistent with the fast shape changing described above, in which the two-layer hydrogel completes its shape changing within 200 s. So, it is clear that the soft smart reactor switches quickly between the “on” and the “off” states, when it changes its shape between the extended strip and the coiled roll with the temperature varies between 25 °C and 45 °C.
In summary, a novel thermosensitive SMR with controllable catalysis characteristics was reported. Comprised of a PNIPAm control layer and a PAAM substrate layer, this soft two-layer hydrogel revealed temperature responsive shape memory features. The reversible expanding and shrinkage of the PNIPAm layer with the temperature variation between 25 °C and 45 °C resulted in the corresponding switchable extended bar and coiled roll shape change of the two-layer hydrogel. Electrochemical experiments demonstrated controllable access of external mass by the shape change of SMR with temperature variation. Most interesting, this SMR indicated a tunable catalytic ability companied with its shape change, which displayed weak reactivity when curled to a roll at high temperature and high catalytic activity if changed to an extended strip at low temperature.
Although PNIPAm and PAAm have been chosen as the control layer and substrate layer, respectively, the design principles and synthetic methods are applicable to a wide variety of soft materials that can change their shape under external stimuli. Therefore, the advanced properties of this self-switchable shape memory system have considerable potential to open a new avenue for tailoring the catalytic activity of metal nanoparticles toward a given reaction.
Acknowledgements
Financial support of this study by China Postdoctoral Science Foundation (no. 2012M521007 and no. 2013T60563) is gratefully acknowledged.Notes and references
- C. H. Bartholomew, and R. J. Farrauto, Fundamentals of Industrial Catalytic Processes, Wiley–AIChE, 2nd edn, 2005 Search PubMed.
- D. Astruc, Nanoparticles and Catalysis, Wiley-VCH, Weinheim, 2008 Search PubMed.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed.
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS PubMed.
- Y. Wei, S. Han, J. Kim, S. Soh and B. A. Grzybowski, J. Am. Chem. Soc., 2010, 132, 11018 CrossRef CAS PubMed.
- M. Boutonnet, J. Kizling and P. Stenius, Colloids Surf., A, 1982, 5, 209 CAS.
- P. Bagwerahul and C. Rhilarkartic, Langmuir, 2000, 16, 905 CrossRef.
- Y. F. Shi, C. L. Tu and Q. Zhu, Nanotechnology, 2008, 19, 445609 CrossRef PubMed.
- S. Li, Y. Ge, A. Tiwari and S. Cao, small, 2010, 6, 2453 CrossRef CAS PubMed.
- Y. Lu and M. Ballauff, Prog. Polym. Sci., 2011, 36, 767 CrossRef CAS PubMed.
- A. Biffis, N. Orlandi and B. Corain, Adv. Mater., 2003, 15, 1551 CrossRef CAS.
- L. Cen, K. G. Neoh and E. T. Kang, Adv. Mater., 2005, 17, 1656 CrossRef CAS.
- D. Diaz, D. Kuhbeck and R. J. Koopmans, Chem. Soc. Rev., 2011, 40, 427 RSC.
- M. Sanes-Sobrido, M. Perez-Lorenzo, B. Rodiguez-Gonzalez, V. Salgueirino and M. A. Correa-Duarte, Angew. Chem., Int. Ed., 2012, 51, 3877 CrossRef PubMed.
- Y. J. Wong, L. Zhu, W. S. Teo, Y. W. Tan, Y. Yang, C. Wang and H. Chen, J. Am. Chem. Soc., 2011, 133, 11422 CrossRef CAS PubMed.
- M. Perez-Lorenzo, B. Vaz, V. Salgueirino and M. A. Correa-Duartz, Chem.–Eur. J., 2013, 19, 12196 CrossRef CAS PubMed.
- H. Wang, L. Chen, Y. Feng and H. Chen, Acc. Chem. Res., 2013, 46, 1636 CrossRef CAS PubMed.
- X. Jiang, D. Xiong, Y. An, P. Zheng, W. Zhang and L. Shi, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2812 CrossRef CAS.
- Y. Wang, G. Wei, F. Wen, X. Zhang, W. Zhang and L. Shi, J. Mol. Catal. A: Chem., 2008, 280, 1 CrossRef CAS PubMed.
- Y. Wang, J. Zhang, W. Zhang and M. Zhang, J. Org. Chem., 2009, 74, 1923 CrossRef CAS PubMed.
- S. Wu, J. Dzubiella, J. Kaiser, M. Drechsler, X. Guo, M. Ballsuff and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 2229 CrossRef CAS PubMed.
- Y. Mei, Y. Lu, F. Polzer, M. Ballauff and M. Drechsler, Chem. Mater., 2007, 19, 1062 CrossRef CAS.
- S. Carregal-Romero, N. J. Buurma, J. Perez-Juste, L. M. Liz-Marzan and P. Herves, Chem. Mater., 2010, 22, 3051 CrossRef CAS.
- S. Carregal-Romero, N. J. Buurma, J. Perez-Juste, L. M. Liz-Marzan and P. Mulvaney, Langmuir, 2010, 26, 1271 CrossRef CAS PubMed.
- J. R. Kumpfer and S. J. Rowan, J. Am. Chem. Soc., 2011, 133, 12866 Search PubMed.
- A. Lendlein and S. Kelch, Angew. Chem., Int. Ed., 2002, 41, 2034 CrossRef CAS.
- J. Hu, Y. Zhu, H. Huang and J. Lu, Prog. Polym. Sci., 2012, 37, 1720 CrossRef CAS PubMed.
- Z. Hu, X. Zhang and Y. Li, Science, 1995, 269, 525 CAS.
- T. Xie, X. Xiao and Y. Cheng, Macromol. Rapid Commun., 2009, 30, 1823 CrossRef CAS PubMed.
- M. C. Serrano, L. Carbajal and G. A. Ameer, Adv. Mater., 2011, 23, 2211 CrossRef CAS PubMed.
- S. A. Madbouly and A. Lendlein, Adv. Polym. Sci., 2010, 226, 41 CrossRef CAS.
- K. N. Plunkett, X. Zhu, J. S. Moore and D. E. Leckband, Langmuir, 2006, 22, 4259 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis details, characterization and spectral data. See DOI: 10.1039/c4ra04051a |
| This journal is © The Royal Society of Chemistry 2014 |









