Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multibranched aliphatic side chains for π-conjugated polymers with a high density of ‘unshielded’ aromatics†
Simeng
Wang
a,
Jessica
Shaw
a,
Yang
Han
b,
Zhuping
Fei
c,
Florian
Glöcklhofer
 *a and
Martin
Heeney
*a and
Martin
Heeney
 *a
*a
aDepartment of Chemistry and Centre for Processable Electronics, Imperial College London, Molecular Sciences Research Hub, 80 Wood Lane, London W12 0BZ, UK. E-mail: f.glocklhofer@imperial.ac.uk; m.heeney@imperial.ac.uk
bSchool of Materials Science and Engineering, Tianjin University, Tianjin 300072, P. R. China
cInstitute of Molecular Plus, Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin 300072, P. R. China
First published on 7th September 2020
Abstract
The synthesis of strongly solubilising multibranched aliphatic side chains for π-conjugated polymers is reported. The solubilising capability of the side chains and their effect on the polymer properties are studied on the example of copolymers composed of up to six unsubstituted, ‘unshielded’ thiophene units per side chain-substituted naphthalene diimide unit.
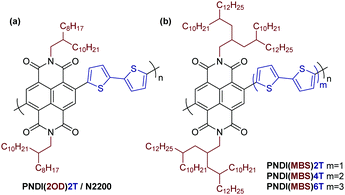
π-Conjugated polymers have been widely studied as organic semiconductors for applications such as solar cells, thin-film transistors and light-emitting diodes.1,2 A key advantage of π-conjugated polymers and other organic semiconductors over their inorganic counterparts is their processability from solution. For conjugated polymers, this property is usually achieved by attaching aliphatic side chains to the π-conjugated backbone, modifying the interchain packing and enabling solubility in organic solvents.3–5 The attachment of linear side chains such as hexyl or octyl chains can often sufficiently solubilize the polymers, but for polymers with more rigid, planar conjugated backbones or donor–acceptor copolymers with strong intermolecular interactions, branched instead of linear aliphatic side chains are usually required to achieve good solubility.6 2-Ethylhexyl or 2-octyldodecyl (2OD) chains are among the most popular branched aliphatic side chains.3
While many well-investigated π-conjugated polymers such as poly(3-hexylthiophene) (P3HT) or poly(9,9-dioctylfluorene-alt-benzothiadiazole) (F8BT) feature aliphatic side chains on every or every other unit of the conjugated backbone, some polymers are composed of two or even three unsubstituted aromatic units per side chain-substituted unit. Poly(9-(1-octylnonyl)carbazole-alt-4,7-dithienylbenzothiadiazole) (PCDTBT) and copolymers of naphthalene diimide (NDI) and bithiophene (2T), such as PNDI(2OD)2T (Scheme 1a), are two examples of such polymers that show good performance in organic solar cells or transistors.7,8
Interestingly, although attaching simple branched aliphatic side chains is well investigated, the attachment of side chains with multiple branching point has, to the best of our knowledge, not yet been reported. Such multibranched aliphatic side chains (MBS) potentially offer further increased solubilizing capability, allowing for the synthesis of soluble polymers with a higher density of unsubstituted aromatic units in the conjugated backbone than with previously reported side chains. A high density of unsubstituted, ‘unshielded’ aromatics in the backbone may have a beneficial effect on various applications: it may allow for closer interaction with other semiconductors, dopant molecules, and analytes (if used in sensors) and may even improve the photocatalytic performance of the polymers.9
Here we report a simple synthetic route to an MBS with three branching points, 4-decyl-2-(2-decyltetradecyl)hexadecyl, and study its solubilizing capability and effect on the properties of a series of π-conjugated polymers as an important first step towards an investigation in above-mentioned applications. We aimed to introduce the side chain in donor–acceptor copolymers composed of NDI and unsubstituted thiophene units and to gradually increase the number of thiophene units per NDI from two, as in PNDI(2OD)2T, to four and six (Scheme 1b). As mentioned, such donor–acceptor copolymers are often difficult to dissolve as a result of strong intermolecular interactions and NDI-thiophene copolymers are no exception in this regard.10 Furthermore, the solubility of unsubstituted oligothiophenes decreases rapidly when increasing the number of repeat units.
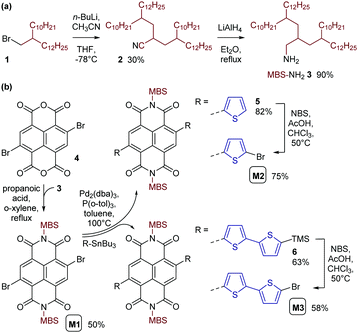
For introducing the MBS in the NDI unit, we synthesized the respective amine MBS-NH23 in just two steps (Scheme 2a). In the first step, acetonitrile (CH3CN) was dialkylated with 1-bromo-2-decyltetradecane 1 to give the multibranched aliphatic nitrile 2 in 30% yield following column chromatography. This reaction was inspired by previous reports of (mono)alkylation of acetonitrile11–13 and, in our case, is considered to proceed in two consecutive deprotonation and alkylation steps. n-Butyllithium (n-BuLi) served as the base for the initial deprotonation of CH3CN in our reaction. An excess (2.5 eq.) of the deprotonated CH3CN was then reacted with alkyl bromide 1, with the deprotonated CH3CN also serving as a base for a subsequent second deprotonation and alkylation. Nitrile 2 was then reduced to the amine 3 in the second step of the synthesis. Using LiAlH4 for the reduction, crude amine 3 was obtained in a yield of 90% and used for the synthesis of monomer M1 without further purification.
 | ||
| Scheme 2 (a) Synthesis of multibranched aliphatic amine 3. (b) Synthesis of monomers M1-3 using amine 3 for the introduction of the multibranched aliphatic side chains (MBS). | ||
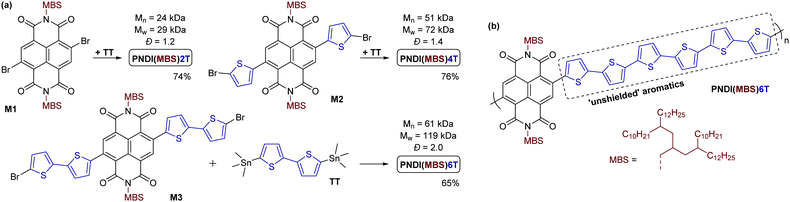
Monomer M1 was obtained by reacting 2,6-dibromonaphthalene-1,4,5,8-tetracarboxylic dianhydride 4 with amine 3 under conditions previously used for the introduction of monobranched aliphatic side chains (Scheme 2b).14 Purification by column chromatography afforded pure monomer M1 as an orange viscous oil in 50% yield. M1 was then used for Stille polymerization with distannylated bithiophene TT, giving the dark blue polymer PNDI(MBS)2T in good yields (Scheme 3a). However, M1 was also used for the synthesis of monomers M2 and M3 by Stille coupling with monostannylated (bi)thiophenes, yielding compounds 5 and 6, and subsequent bromination (Scheme 2b). Stille polymerization of these monomers with TT afforded the dark green polymers PNDI(MBS)4T and PNDI(MBS)6T (Scheme 3a). In this synthetic approach, which was previously used for the synthesis of PNDI(2OD)4T,15,16 the additional thiophene units of the polymers are introduced in the NDI comonomer, as opposed to extending the distannylated bithiophene comonomer TT. Direct arylation polycondensation of M1 and the respective oligothiophenes may be a suitable alternative approach, considering previous reports.17
All polymerizations were performed in a microwave oven in a reaction time of approx. 50 min, gradually increasing the temperature from 100 °C to 200 °C. As for the Stille reactions towards compounds 5 and 6, Pd2(dba)3 and tri(o-tolyl)phosphine was used as the catalytic system for the polymerizations. The yields given in Scheme 3a represent the soluble polymer fractions obtained by Soxhlet extraction with hexane (for PNDI(MBS)2T, PNDI(MBS)4T) or chloroform (for PNDI(MBS)6T). Medium to high molecular weight polymers were obtained (gel permeation chromatography (GPC) data provided in Scheme 3a). The higher molecular weight and dispersity (Đ) observed for PNDI(MBS)6T can be attributed to the use of the better solvent chloroform instead hexane for the final Soxhlet extraction step of this polymer.
Thermogravimetric analysis (TGA) revealed the high thermal stability of the polymers, with the decomposition starting above 400 °C in all three cases and a 5% mass loss between 447 °C and 450 °C (Fig. S7, ESI†). For PNDI(MBS)4T and PNDI(MBS)6T, differential scanning calorimetry (DSC) (Fig. S8, ESI†) showed endothermic transitions on heating with peaks at 177 °C and 176 °C, respectively, and exothermic transitions with peaks at 157 °C and 160 °C on cooling. These transitions can be attributed to melting and crystallization of the polymers. Broad, lower temperature transitions are also observed for these polymers (midpoints at 91 °C/61 °C on heating/cooling) possibly corresponding to glass transitions. No transitions were observed for PNDI(MBS)2T. Comparison to PNDI(2OD)2T, for which a 5% mass loss at approx. 450 °C,18 a glass transition at 74 °C,19 and a molecular weight dependent melting point between 280 and 310 °C were reported,17 confirmed that the MBS does not affect the stability of the polymers but has a pronounced effect on the other thermal transitions.
To evaluate the solubilizing capability of the MBS, we tested the solubility of the polymers in chlorobenzene (CB), a solvent often used for processing conjugated polymers from solution, and in n-hexane. For PNDI(MBS)2T, we found the solubility to be >200 g L−1 in CB and >20 g L−1 in n-hexane, a surprising observation considering that the latter is known to be a very poor solvent for conjugated polymers. For PNDI(MBS)4T, the solubility was found to be approx. 100 g L−1 in CB and 8 g L−1 in n-hexane. PNDI(MBS)6T could not be dissolved in n-hexane, but the solubility in CB was approx. 70 g L−1, despite the high density of unsubstituted thiophene units. For PNDI(2OD)2T, our tests showed a lower solubility of 25 g L−1 in CB. Previously, “solubilities […] in conventional organic solvents such as xylene and dichlorobenzene (DCB) as high as 60 g L−1” were reported, possibly reflecting molecular weight differences.8
UV-vis absorption spectra (Fig. 1) in CB showed an absorption maximum at 368 nm and a charge-transfer (CT) absorption band with a peak at 617 nm for PNDI(MBS)2T. The peaks are clearly blueshifted compared to PNDI(2OD)2T, which (despite having the same π-conjugated backbone) showed peaks at 394 and 706 nm in the same solvent (Fig. S9, ESI†). This blueshift indicates considerably reduced aggregation of PNDI(MBS)2T in CB as a result of the MBS, a conclusion further supported by the fact that the absorption peaks of PNDI(2OD)2T shifted to similar values (approx. 370 and 620 nm) when measured in chloronaphthalene (CN), a solvent known to suppress aggregation.20 For PNDI(MBS)2T no further blueshift was observed when measured in CN and at lower concentrations in CB (Fig. S11, ESI†).
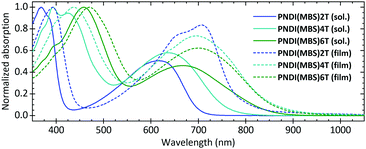 | ||
| Fig. 1 Normalized UV-vis absorption spectra of PNDI(MBS)2T-6T in CB (0.1 g L−1) and as annealed films. | ||
Measuring PNDI(MBS)4T and PNDI(MBS)6T in CB, we observed a redshift of both peaks with an increasing density of thiophene units. Compared to PNDI(MBS)2T, the peaks in the spectrum of PNDI(MBS)4T redshifted by 26 and 19 nm, followed by a redshift of another 63 and 31 nm in PNDI(MBS)6T. Similarly, the absorption onset shifted from 700 nm (1.77 eV) to 760 nm (1.63 eV) and 846 nm (1.47 eV). In annealed thin films, a similar trend was observed for the onsets of the CT absorption bands, which shifted from 783 nm (1.58 eV) to 855 nm (1.45 eV) and 864 nm (1.44 eV). However, while the absorption maximum was following the same trend, the CT absorption peak did not; similar behaviour has been previously noted in donor–acceptor copolymers as the length of the donor was increased.21
In thin films, PNDI(MBS)2T showed the most redshifted peak at 706 nm, redshifted by 89 nm compared to solution and very close to the peak of PNDI(2OD)2T at 705 nm (Fig. S9, ESI†). For PNDI(MBS)4T and PNDI(MBS)6T, the smaller redshifts of 61 and 32 nm resulted in peaks at 697 and 699 nm, respectively. Comparison of annealed and pristine films revealed little influence of the annealing on the spectra (Fig. S12, ESI†).
Photoluminescence spectra (Fig. 2) in CB showed a pronounced shift of the emission maximum from 828 nm for PNDI(2OD)2T to 716 nm for PNDI(MBS)2T and a significant increase in intensity. Measuring PNDI(2OD)2T in CN for reduced aggregation shifted its emission maximum to a similar wavelength of 721 nm and increased the intensity, confirming that the differences in CB can be attributed to reduced aggregation as a result of the MBS. In contrast to PNDI(MBS)2T, PNDI(MBS)4T and PNDI(MBS)6T were found to be very weak emitters, possibly due to the increased oligothiophene length leading to enhanced relaxation of the excited state via interannular rotations.22 Similar observations were made at lower concentration (Fig. S13, ESI†).
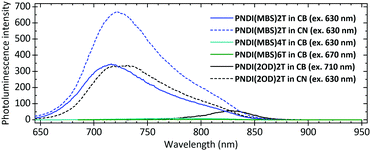 | ||
| Fig. 2 Photoluminescence spectra of PNDI(MBS)2T-6T and PNDI(2OD)2T in chlorobenzene (CB) and chloronaphthalene (CN). Conc.: 0.1 g L−1. | ||
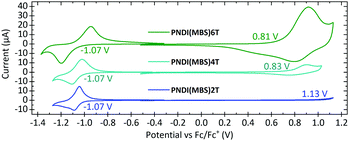
Cyclic voltammetry (CV) measurements of drop-cast films (Fig. 3) revealed that the half-wave potential in the reduction measurements of PNDI(MBS)2T-6T is unaffected by the increased density of thiophene units. The redox potential was determined to be −1.07 V vs. ferrocene/ferrocene+ (Fc/Fc+) for all three polymers, which corresponds to an estimated lowest unoccupied molecular orbital (LUMO) energy level of −3.73 eV (Table 1) and indicates that the LUMO is dominated by the NDI units of the polymers. For estimating the highest occupied molecular orbital (HOMO) energy levels, we determined the redox potentials from the inflection points in the oxidation measurements. In contrast to the half-wave potentials in the reduction measurements, the redox potentials in the oxidation measurements shifted from 1.13 V to 0.83 V and 0.81 V vs. Fc/Fc+, corresponding to estimated HOMO energy levels of −5.93 eV, −5.63 eV and −5.61 eV, respectively. As for the optical bandgap, the redox potentials of PNDI(MBS)4T and PNDI(MBS)6T are very similar. However, while the peak currents in the reduction measurements are similar as well, the anodic peak current in the oxidation measurements increased drastically when increasing the density of thiophene units.
| Polymer | λ max,sol. (nm) | λ max,film (nm) | E g,opt (eV) | E Homo (eV) | E Lumo (eV) |
|---|---|---|---|---|---|
| a From measurements in chlorobenzene. b From film measurements. c Estimated from CV measurements using ferrocene as the reference. | |||||
| PNDI(MBS)2T | 368/617 | 393/706 | 1.58 | −5.93 | −3.73 |
| PNDI(MBS)4T | 394/636 | 437/697 | 1.45 | −5.63 | −3.73 |
| PNDI(MBS)6T | 457/667 | 470/699 | 1.44 | −5.61 | −3.73 |
In conclusion, we can report that the attachment of the multibranched aliphatic side chain (MBS) 4-decyl-2-(2-decyltetradecyl)hexadecyl to the backbone of conjugated polymers can have a drastic effect on the polymer properties. We attached the MBS to donor–acceptor copolymers composed of naphthalene diimide (NDI) and thiophene units and found it to drastically reduce the aggregation and blueshift the absorption and photoluminescence of PNDI(MBS)2T compared to the well-investigated polymer PNDI(2OD)2T, while retaining the excellent thermal stability. The MBS has an impressively high solubilizing capability, as we could demonstrate by increasing the number of unsubstituted thiophene units per side chain-substituted NDI unit in our set of polymers from two (in PNDI(MBS)2T) to four and six (in PNDI(MBS)4T and PNDI(MBS)6T). The high density of unsubstituted, ‘unshielded’ thiophene units in the latter polymers has a pronounced effect on the oxidation in cyclic voltammetry (CV) measurements, significantly increasing the current and shifting the redox potential from 1.13 V to 0.81 V vs. Fc/Fc+. The redox potential of the reduction remained unaffected.
Considering the properties and facile synthesis of the MBS presented in this work, we believe it will find broad use in polymers for applications that benefit from reduced aggregation, increased solubility, and/or from a high density of unsubstituted, ‘unshielded’ aromatics in the polymer backbone.
We thank the EPSRC and Merck Chemicals for an iCase award and the Royal Society and the Wolfson Foundation (Royal Society Wolfson Fellowship) for funding. We further acknowledge funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 796024.
Conflicts of interest
There are no conflicts to declare.References
- A. Facchetti, Chem. Mater., 2011, 23, 733–758 CrossRef CAS.
- A. C. Grimsdale, K. Leok Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897–1091 CrossRef CAS.
- J. Mei and Z. Bao, Chem. Mater., 2014, 26, 604–615 CrossRef CAS.
- Z. Liu, G. Zhang and D. Zhang, Acc. Chem. Res., 2018, 51, 1422–1432 CrossRef CAS.
- Y. Yang, Z. Liu, G. Zhang, X. Zhang and D. Zhang, Adv. Mater., 2019, 31, 1903104 CrossRef CAS.
- H. Bronstein, C. B. Nielsen, B. C. Schroeder and I. McCulloch, Nat. Rev. Chem., 2020, 4, 66–77 CrossRef CAS.
- N. Blouin, A. Michaud and M. Leclerc, Adv. Mater., 2007, 19, 2295–2300 CrossRef CAS.
- H. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dötz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679–686 CrossRef CAS.
- C. Dai and B. Liu, Energy Environ. Sci., 2020, 13, 24–52 RSC.
- X. Guo, F. S. Kim, M. J. Seger, S. A. Jenekhe and M. D. Watson, Chem. Mater., 2012, 24, 1434–1442 CrossRef CAS.
- J. Berlan, H. Delmas, I. Duée, J. L. Luche and L. Vuiglio, Synth. Commun., 1994, 24, 1253–1260 CrossRef.
- S. Arseniyadis, K. S. Kyler and D. S. Watt, Addition and Substitution Reactions of Nitrile-Stabilized Carbanions, in Organic Reactions, ed. W. G. Dauben, John Wiley & Sons, Inc., 2005, vol. 31 Search PubMed.
- D. F. Taber and S. Kong, J. Org. Chem., 1997, 62, 8575–8576 CrossRef CAS.
- Z. Chen, Y. Zheng, H. Yan and A. Facchetti, J. Am. Chem. Soc., 2009, 131, 8–9 CrossRef.
- A. Luzio, D. Fazzi, D. Natali, E. Giussani, K.-J. Baeg, Z. Chen, Y.-Y. Noh, A. Facchetti and M. Caironi, Adv. Funct. Mater., 2014, 24, 1151–1162 CrossRef.
- M. M. Szumilo, E. H. Gann, C. R. McNeill, V. Lemaur, Y. Oliver, L. Thomsen, Y. Vaynzof, M. Sommer and H. Sirringhaus, Chem. Mater., 2014, 26, 6796–6804 CrossRef CAS.
- R. Matsidik, H. Komber, A. Luzio, M. Caironi and M. Sommer, J. Am. Chem. Soc., 2015, 137, 6705–6711 CrossRef CAS.
- L. Hu, J. Han, W. Qiao, X. Zhou, C. Wang, D. Ma, Y. Li and Z. Y. Wang, Polym. Chem., 2018, 9, 327–334 RSC.
- R. Xie, A. R. Weisen, Y. Lee, M. A. Aplan, A. M. Fenton, A. E. Masucci, F. Kempe, M. Sommer, C. W. Pester, R. H. Colby and E. D. Gomez, Nat. Commun., 2020, 11, 893 CrossRef CAS.
- R. Steyrleuthner, M. Schubert, I. Howard, B. Klaumünzer, K. Schilling, Z. Chen, P. Saalfrank, F. Laquai, A. Facchetti and D. Neher, J. Am. Chem. Soc., 2012, 134, 18303–18317 CrossRef CAS.
- P. M. Beaujuge, C. M. Amb and J. R. Reynolds, Acc. Chem. Res., 2010, 43, 1396–1407 CrossRef CAS.
- S. C. Rasmussen, S. J. Evenson and C. B. McCausland, Chem. Commun., 2015, 51, 4528–4543 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, thermal analysis, solubility tests, UV-vis absorption, photoluminescence, cyclic voltammetry, 1H and 13C NMR spectra. See DOI: 10.1039/d0cc04967k |
| This journal is © The Royal Society of Chemistry 2020 |