Open Access Article
Open Access ArticleGrape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats†
Àngela
Casanova-Martí‡
a,
Joan
Serrano‡
a,
Kevin J.
Portune
 b,
Yolanda
Sanz
b,
M. Teresa
Blay
b,
Yolanda
Sanz
b,
M. Teresa
Blay
 a,
Ximena
Terra
a,
Ximena
Terra
 a,
Anna
Ardévol
a,
Anna
Ardévol
 *a and
Montserrat
Pinent
*a and
Montserrat
Pinent
 a
a
aMoBioFood Research Group. Departament de Bioquímica i Biotecnologia, Universitat Rovira i Virgili, c/Marcel·lí Domingo no 1, 43007 Tarragona, Spain. E-mail: anna.ardevol@urv.cat; Fax: +34 977 558232; Tel: +34 977 55 9566
bMicrobial Ecology, Nutrition & Health Research Unit. Institute of Agrochemistry and Food Technology, National Research Council (IATA-CSIC), Av. Agustín Escardino, 7, 46980, Valencia, Spain
First published on 20th February 2018
Abstract
Grape seed proanthocyanidin extract (GSPE) modulates several parameters involved in metabolic syndrome. GSPE is a mixture of compounds, some which are rapidly absorbed, while others remain in the lumen where they might have effects that are translated to the whole organism. Our aim was to decipher if the 8-day treatment of GSPE, previously shown to reduce food intake, induces changes in the microbiota and enterohormone secretion. The ratio of Firmicutes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bacteroidetes was lower in the microbiota of GSPE-treated rats compared to controls, and differences in several taxonomic families and genera were observed. Such modulation led to a reduction in cecal butyrate content. GSPE also increased plasma glucagon-like-peptide-1 (GLP-1). Gallic acid did not induce major changes in the microbiota profile nor in GLP-1 secretion. Correlations between several microbiota taxa and plasma triacylglycerol, adiposity, and enterohormones were observed. Modulation of microbiota may be one of the mechanism by which GSPE impacts metabolic health.
Bacteroidetes was lower in the microbiota of GSPE-treated rats compared to controls, and differences in several taxonomic families and genera were observed. Such modulation led to a reduction in cecal butyrate content. GSPE also increased plasma glucagon-like-peptide-1 (GLP-1). Gallic acid did not induce major changes in the microbiota profile nor in GLP-1 secretion. Correlations between several microbiota taxa and plasma triacylglycerol, adiposity, and enterohormones were observed. Modulation of microbiota may be one of the mechanism by which GSPE impacts metabolic health.
1. Introduction
Grape seed proanthocyanidins (GSPE) have been shown to possess several beneficial health properties in animals and human studies.1–4 Some of the effects could directly be mediated by the phenolic compounds of the extract or their metabolites, since derived structural components have been found in several tissues.5 However, other effects might be mediated through interactions with the gastrointestinal tract where they act by inhibiting enzymes,6 modulating inflammation and/or gut barrier properties,7 or modulating enteroendocrine secretion.8,9 The effects of flavonoids on gut microbiota have been recently reviewed;7 but there are few studies analyzing the role of proanthocyanidins on gut microbiota which could also mediate their physiological effects.Satiety-related enterohormones such as glucagon-like-peptide-1 (GLP-1), peptide YY (PYY), cholecystokinin (CCK) and ghrelin are signaling molecules that could act through the vagus nerve to signal to the brain. GSPE acutely increases plasma GLP-1,10 and this has been linked to an amelioration of glucose tolerance10 and an induction of satiety.11 Satiating effects have been shown to be maintained during 8 days of treatment with GSPE 0.5 g per kg BW in rats. Interestingly this treatment also increased energy expenditure, which together with reduced food intake led to a lower body weight gain.8 The increased satiety has been linked to ghrelin secretion modulation, but whether other enterohomones are regulated in this specific treatment has not been shown. These results point out GSPE as an interesting candidate for ameliorating obesity and diabetes. Some authors postulate a key role of microbiota-derived short chain fatty acids (SCFA)12 on satiety-related mechanisms. In this sense, an emerging role for microbiota and therefore of food that modulate its composition in the control of metabolic homeostasis is being acknowledged. In this context polyphenols, and more specifically flavonoids, have been suggested to act as prebiotic components. In a small human study moderate red wine consumption for 4 weeks increased the phylum Bacteroidetes in humans, and this was correlated with a decrease in plasma triacylglycerol (TAG) and high density lipoprotein (HDL)-cholesterol.13 In mice, cranberry extract, which is rich in proanthocyanidins and flavanols, modulated gut microbiota by increasing the proportion of the mucin-degrading bacterium Akkermansia when administered to mice for 8 weeks undergoing a high-fat sucrose diet.14
Therefore, in the present study we explore the mechanisms that could help to explain GSPE effects on at the level of the gastrointestinal tract. We hypothesized that modulation of gut microbiota could also be a mechanism for grape seed proanthocyanidins to exert their effects on host metabolic health.
2. Materials and methods
2.1. Materials
GSPE was obtained from Les Dérivés Résiniques et Terpéniques (Dax, France). The same batch (#124029) was used in all studies. According to the manufacturer, the extract contains monomeric (21.3%), dimeric (17.4%), trimeric (16.3%), tetrameric (13.3%) and oligomeric (5–13 U; 31.7%) proanthocyanidins. The small molecules were previously characterized by liquid chromatography-tandem mass spectrometry.15 Gallic acid (GA) was obtained from Sigma (St Louis, USA).2.2. Animals and experimental design
Female Wistar rats weighing 180–200 g were obtained from Harlan (Barcelona, Spain). The subjects were single housed at 22 °C under a 12 h light/dark cycle (lights on at 8 am) with access to standard chow pellets (Teklad Global Diets #2014, Harlan, Barcelona) and tap water ad libitum during a 1 week adaptation period. The study complied with all institutional and national guidelines, according to the Catalan regulation about animal experimentation: Llei5/1995, to protect animals to be used for research and other scientific purposes; Decret 214/1997 that defines the use of animals for research and other scientific purposes, and Decret 286/1997 that modifies Decret 214/1997. The protocol were approved by the Experimental Animal Ethics Committee of the Universitat Rovira i Virgili (code: 0152S/4655/2015).Rats were introduced (1 week) to a daily 4 h fasting and chow was replaced in the dark-onset. After the adaptation, rats were daily treated during 8 days with GSPE or gallic acid 1 h prior to chow replacement by gavage, using tap water as vehicle. A control group (vehicle treated) was performed in parallel. Rats (n = 9 for each group) were sacrificed 80 min after the last dose. Animals were sacrificed by aortal exsanguination under pentobarbital anesthesia. The blood was collected and aliquoted. Samples to measure active GLP-1 were treated with a commercial DPP4 inhibitor (Millipore, Madrid, Spain) and a serine protease inhibitor, Pefabloc SC (Roche, Barcelona, Spain). The samples to analyze active ghrelin were treated with serine protease inhibitor, Pefabloc SC (Roche, Barcelona, Spain) and 0.1 M HCl. All the samples were stored at −80 °C. Cecal content together with intestinal segments from the duodenum, jejunum, ileum and proximal colon were immediately frozen in liquid nitrogen and then stored at −80° C for further enzyme activity and gene expression analysis.
2.3. Microbiota composition analysis
gDNA from cecal content of rats was extracted using a Fast DNA Stool Mini Kit (Qiagen) according to the manufacturer's instructions with minor variations. 180–220 mg aliquots of cecal content were placed in sterile tubes filled with glass beads and one mL of Inhibitex buffer (Qiagen). Samples were homogenized in a beadbeater for 2 successive rounds for 1 min with intermittent cooling on ice. Samples were then heated to 95 °C for 10 min and DNA extraction was carried out according to the manufacturer's standard protocol. Samples were amplified in triplicate via PCR using primers (S-D-Bact-0563-a-S-15/S-D-Bact-0907-b-A-20) that target the V4–V5 variable regions of the 16S rDNA.16 Triplicate reactions consisted of final concentrations of Buffer HF (1×), dNTPs (0.11 μM), primers (0.29 μM each) and Taq Phusion High Fidelity (0.007 U μL−1) in final volumes of 35 μL. Cycling conditions consisted of 98 °C for 3 min, followed by 25 cycles of 95 °C for 20 s, 55 °C for 20 s, and 72 °C for 20 s, followed by a final extension step of 72 °C for 5 min. Each sample was tagged with a barcode to allow multiplexing during the sequencing process. Triplicate sample amplicons were combined and purified using the Illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare) according to the manufacturer's instructions and combined in equimolar concentrations before carrying out sequencing on a MiSeq instrument (Illumina). All raw sequence data has been submitted to ENA-EMBL (accession number: PRJEB21445).Bioinformatic processing of data was carried out using the software QIIME.17 Using QIIME, paired-end forward and reverse Illumina reads were joined into contigs, barcodes were extracted, reads were demultiplexed, and then primers were removed using Mothur.18 Using the UPARSE pipeline for MiSeq amplicon data,19 reads were clustered into OTUs, chimeras were removed and an OTU abundance table was created by remapping reads to representative sequences from OTUs. QIIME was then used for all downstream processing. All samples from the OTU abundance table were rarefied to the sample with the lowest number of reads and singletons were removed. Taxonomy was assigned to representative OTUs using UCLUST20 against the Greengenes database (version 13.8).21
In order to carry out alpha and beta diversity analysis, representative OTU sequences were aligned using PYNAST22 against the Greengenes’ core set alignment. The alignment was filtered using QIIME's default settings and a phylogenetic tree was constructed. Alpha diversity metrics and richness estimators (Shannon's, Simpson's reciprocal, Chao1, Simpson's evenness, Observed OTUs) were then calculated. In order to examine β diversity, distance matrices (using weighted and unweighted UniFrac distances) and Principal Coordinates Analyses (PCoA) were performed using QIIME and PCoAs were visualized using the R package PhyloSeq.23 A heatplot of correlation analysis was constructed using heatmap2() function in the R package gplots using default parameters.
2.4. Short chain fatty acid quantification
Cecal content (∼500 mg) was added to 2 ml tubes containing 900 μl QH2O and H3PO4 (0.5% final concentration) and homogenized vigorously followed by centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min at 4 °C). 350 μl of supernatant was used for the derivatization of SCFAs according to the methods used in Kristensen et al.24 Derivatized SCFAs were analyzed using a gas chromatograph-mass spectrometer (Agilent Technologies 5977A MSD) with a low-resolution quadrupole analyzer and gas chromatograph (Agilent 7890B) equipped with an apolar capillary column (30 m, 0.25 mm ID; 0.25 μm film). The carrier gas used was helium. A constant flow mode was used (split 30
000g, 10 min at 4 °C). 350 μl of supernatant was used for the derivatization of SCFAs according to the methods used in Kristensen et al.24 Derivatized SCFAs were analyzed using a gas chromatograph-mass spectrometer (Agilent Technologies 5977A MSD) with a low-resolution quadrupole analyzer and gas chromatograph (Agilent 7890B) equipped with an apolar capillary column (30 m, 0.25 mm ID; 0.25 μm film). The carrier gas used was helium. A constant flow mode was used (split 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 30 ml min−1 split flow), with an initial flow of 1 ml min−1, initial temperature of 90 °C for 2 min, 5 °C min−1 to 222 °C for 5 min, followed by 20 °C min−1 to 280 °C for 2 minutes, and an interphase temperature of 280 °C. Three ethyl acetate washings were performed prior to injection of samples.
1; 30 ml min−1 split flow), with an initial flow of 1 ml min−1, initial temperature of 90 °C for 2 min, 5 °C min−1 to 222 °C for 5 min, followed by 20 °C min−1 to 280 °C for 2 minutes, and an interphase temperature of 280 °C. Three ethyl acetate washings were performed prior to injection of samples.
2.5. Enterohormone and plasmatic parameters quantification
Enterohormones were analysed using commercial ELISA kits for insulin (Mercodia, Uppsala, Sweden), GLP-1 7–37 amide (Millipore, Billerica, MA, USA), total CCK (Peninsula Laboratories, San Carlos, CA, USA), PYY (Phoenix Pharmaceuticals, Burlingame, CA, USA) and specific octanoyl ghrelin (Phoenix Pharmaceuticals, Burlingame, CA, USA). Glucose and TAG plasma levels were analysed with an enzymatic colorimetric kit (Glucose Oxidase-Peroxidase method from QCA, Tarragona, Spain).2.6. Measurement of DPP-IV activity
DPP-IV was extracted from rat intestine segments as previously described.6 Briefly, intestine segments were homogenized using lysis buffer (PBS containing 100 KIU mL−1 aprotinin and 1% Triton X-100). Then, the obtained samples were centrifuged at 1000g at 4 °C for 10 min to eliminate the cellular debris, then centrifuged twice at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 10 min. Supernatants were stored at −80 °C until analysis.
000g at 4 °C for 10 min. Supernatants were stored at −80 °C until analysis.
To determine DPP-IV activity, intestinal lysates were incubated with 0.2 mM – chromogenic substrate Gly-Pro-pNA (Bachem, Bubendorf, Switzerland) in Tris−HCl buffer at 37 °C. The mixture was measured at 405 nm at 37 °C for 30 min in a microplate reader.
2.7. Quantitative real-time RT-PCR analysis
Total RNA was extracted using Trizol (Ambion, USA) and trichloromethane-ethanol (Panreac, Barcelona, Spain), and purified using a Qiagen RNAeasy kit (Qiagen, Hilden, Germany). The cDNA was generated using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, USA). Quantitative PCR amplification was performed using specific TaqMan probes (Applied Biosystems, Waltham, USA): Rn00563215_m1 for CCK, and Rn00562293_m1 for proglucagon. The relative expression of each gene was calculated against the control group using the 2−ΔΔCt method, with cyclophilin A, PPIA (Rn00690933_m1), as reference.2.8. Statistical analysis
Morphometric, plasmatic, food intake parameters, together with enterhormone parameters and SCFAs are presented as mean ± SEM and were analysed with SPSS (IBM, Chicago, USA). These data were analysed by Student t-tests. Differences between means were considered significant when P < 0.05.All statistics and data visualization for microbiota data were carried out using the R statistical software and related R packages or QIIME. Comparison of relative abundances of each phylogenetic group between treatments was carried out for each phylotype (dependent variable) by Kruskal–Wallis tests followed by Wilcoxon rank sum tests to identify significant differences between treatments. Comparison of alpha diversity among dietary treatments was also performed by Kruskal–Wallis and Wilcoxon rank sum tests. To compare bacterial community structure, PERMANOVAs were carried out using the QIIME script compare_categories.py and the adonis method using weighted and unweighted UniFrac distance matrices. Correlation analysis was conducted in order to compare physiological/metabolic parameters with relative abundances of taxonomic groups using cor.test in R. Significance values were corrected for multiple comparisons using false discovery rate (FDR) and a q-value <0.05 was selected as significant.
3. Results
3.1. Enterohormone profile is differentially modulated by GSPE and gallic acid
A GSPE treatment (500 mg GSPE per kg bw for 8 days), previously shown to be effective at inhibiting food intake in male rats,8 was reproduced in female rats in this study. To discriminate between the effect of GSPE versus one of its monomeric components, for which satiating properties have been described,25 we included a group treated with gallic acid at an equivalent amount to that present in the GSPE. Since we aimed to analyze whether there was a modulation of enterohormone secretion, we sacrificed the animals in a condition where anorexigenic hormone secretion is stimulated, by allowing the animals to eat for 20 minutes after a fasting period. At sacrifice, plasma levels of enterohormones were measured. GSPE-treated rats had higher active GLP-1 and active ghrelin levels, while treatment with gallic acid did not significantly change such parameters (Table 1). Instead, gallic acid tended to increase CCK levels (P = 0.064).| Control | Gallic acid | GSPE | ||
|---|---|---|---|---|
| Enterohormones | Active GLP-1 (pM) | 0.63 ± 0.03a | 0.72 ± 0.1a | 1.17 ± 0.24b |
| PYY (pg ml−1) | 105.48 ± 22.9a | 149.4 ± 28.2a | 162.75 ± 46.9a | |
| CCK (ng ml−1) | 1.31 ± 0.2a | 7.1 ± 1.69a | 6.45 ± 2.89a | |
| Active Ghrelin (pg ml−1) | 303.92 ± 29.8a | 236.1 ± 29.41a | 465.61 ± 47.5b | |
| Plasma parameters | Glucose (mM) | 9.48 ± 0.53a | 9.81 ± 0.12a | 9.70 ± 0.62a |
| TAG (mM) | 0.21 ± 0.08a | 0.31 ± 0.08a | 0.07 ± 0.03a | |
| Insulin (μg L−1) | 2.31 ± 0.43a | 2.59 ± 0.36a | 2.57 ± 0.34a | |
| Morphometric parameters | Final weight (g) | 263.11 ± 3.40a | 266.56 ± 5.80a | 253.44 ± 4.30a |
| MWAT (g) | 2.82 ± 0.23a | 3.65± 0.39a | 3.33 ± 0.28a | |
| OWAT (g) | 3.34 ± 0.35a | 3.77 ± 0.42a | 2.67 ± 0.18a | |
| RWAT (g) | 6.39 ± 0.59a | 8.76 ± 0.89a | 6.56 ± 0.89a | |
| BAT (g) | 0.41 ± 0.03a | 0.51 ± 0.05a | 0.36 ± 0.04a | |
| Sum WAT (g) | 12.55 ± 0.81a | 15.77 ± 1.25a | 12.55 ± 1.18a | |
| Food intake | kcal (sum total intake) | 375.28 ± 5.72a | 384.95 ± 8.27a | 342.28 ± 10.29b |
| Short chain fatty acids (μmol g−1 wet intestine content) | Acetic acid | 17.64 ± 1.87 a | 17.49 ± 0.97a | 15.57 ± 1.88a |
| Propionic | 2.82 ± 0.24a | 2.46 ± 0.08a | 3.35 ± 0.3a | |
| Butyric acid | 9.87 ± 1.02a | 10.02 ± 0.94a | 5.59 ± 0.98b | |
| Valeric acid | 0.15 ± 0.04a | 0.20 ± 0.01a | 0.14 ± 0.03a | |
GLP-1 and CCK gene expression was assessed in the ileum and colon of the animals. GSPE caused a significant up-regulation of CCK gene expression in the ileum, while the monomer gallic acid did not induce any change in gene expression compared to the control animals (Table 2). In the colon there was no modification either by GSPE or the GA treatment (Table 2).
| Ileum | Colon | ||||||
|---|---|---|---|---|---|---|---|
| Control | Gallic acid | GSPE | Control | Gallic acid | GSPE | ||
| mRNA | GLP-1 | 1.12 ± 0.2a | 0.96 ± 0.2a | 1.24 ± 0.1a | 1.11 ± 0.2a | 0.65 ± 0.2a | 0.96 ± 0.2a |
| CCK | 0.87 ± 0.1a | 1.15 ± 0.3a | 2.25 ± 0.2b | 1.63 ± 0.5a | 3.03 ± 1.1a | 0.61 ± 0.1a | |
| Protein | GLP-1 (pmol g−1 tissue) | 355.36 ± 18.8 | NA | 365.95 ± 47.6 | 186.08 ± 59.1 | NA | 234.64 ± 17.4 |
The amount of GLP-1 protein was also measured in intestinal tissue of GSPE-treated and control animals. GSPE did not significantly modify GLP-1 protein either in the ileum or in the colon (Table 2).
We previously showed that an increase in GLP-1 secretion by an acute GSPE treatment was in part due to inhibition of DPP-IV activity. Therefore we measured this activity in different intestinal segments. Our results showed no difference in any of the segments due to GSPE treatment. Curiously, gallic acid increased DPP-IV activity in the duodenum (Table S1†).
In the conditions in which the animals were sacrificed, neither GSPE nor gallic acid significantly changed other biochemical plasmatic parameters, i.e. glucose, insulin and triglycerides (Table 1). It was also found that in this model, GSPE but not gallic acid reduced food intake. The weight of different adipose depots was also not significantly modified by GSPE or gallic acid.
3.2. GSPE reduces butyrate in cecal content
Short chain fatty acids were measured in the cecal content of treated animals. GSPE led to a reduction in butyrate concentration (Table 1), while no differences were observed for acetic, propionic nor valeric acid. This led to significant differences (p ≤ 0.05, Student T-test) in the ratio of acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propionate
propionate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyrate, which was 58.0 ± 2
butyrate, which was 58.0 ± 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.4 ± 0
9.4 ± 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.6 ± 2 for controls and 63.7 ± 1
32.6 ± 2 for controls and 63.7 ± 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.4 ± 1
14.4 ± 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21.9 ± 2 for GSPE. Gallic acid did not induce any change compared to controls in SCFA cecal content (Table 1) nor in the ratio acetate
21.9 ± 2 for GSPE. Gallic acid did not induce any change compared to controls in SCFA cecal content (Table 1) nor in the ratio acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propionate
propionate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyrate (58.6 ± 2
butyrate (58.6 ± 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.4 ± 1
8.4 ± 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.9 ± 2).
32.9 ± 2).
3.3. GSPE changes microbiota profile, while gallic acid does not
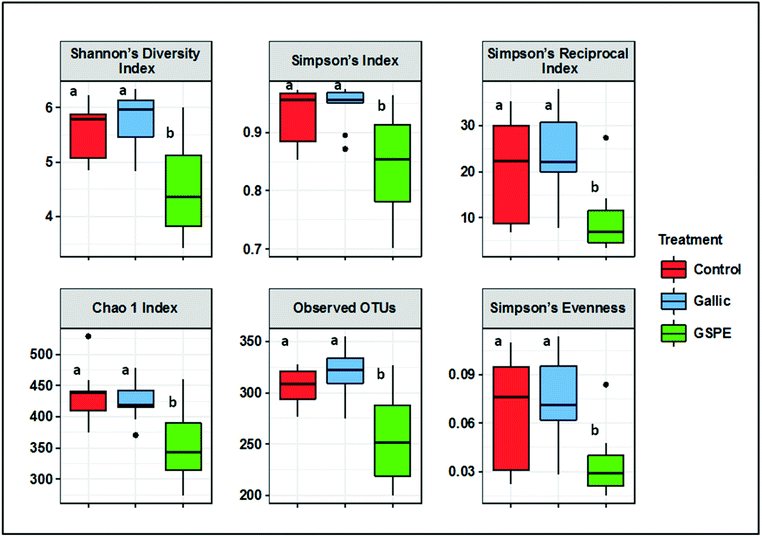
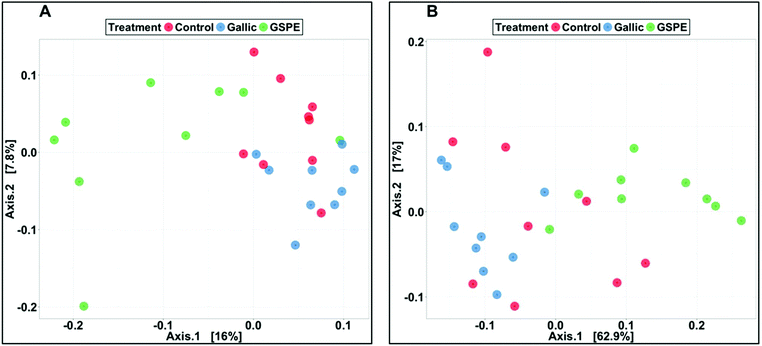
Several diversity indices (Shannon's, Simpson's, Simpson's Reciprocal, Simpson's evenness) and richness estimators (Chao1, observed OTUs) were significantly lower in the GSPE group compared to both Control and Gallic groups, whereas no differences were detected between the control and Gallic groups (Fig. 1).Bacterial composition in cecal content as revealed by PCoA was significantly different in the GSPE group compared to both Control and Gallic groups using both unweighted (P < 0.001) and weighted (P < 0.001) UniFrac distances (Fig. 2A and B).
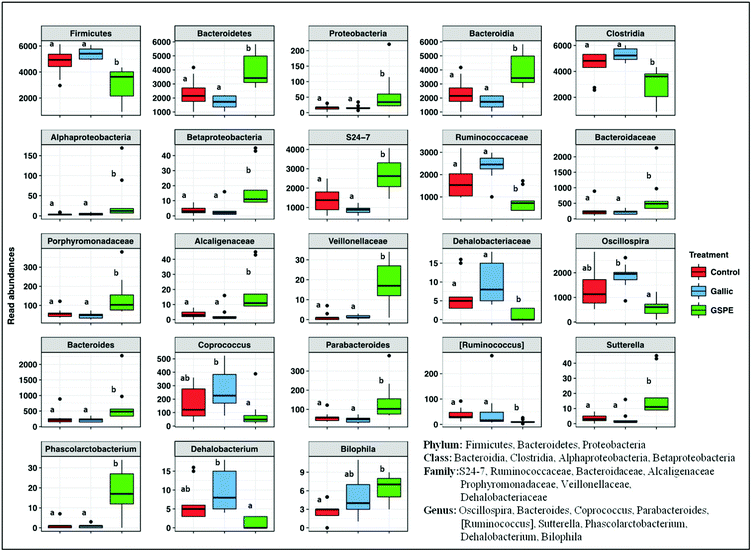
Concerning taxonomic phyla, Firmicutes was significantly higher in the control and Gallic groups compared to the GSPE group, while Bacteroidetes and Proteobacteria were higher in the GSPE group (Fig. 3). Primarily, bacteria from the classes Clostridia, Bacteroidia and Alphaproteobacteria and Betaproteobacteria were responsible for these observed patterns (Fig. 3). The families S24-7, Bacteroidaceae and Porphyromonadaceae were the notable groups from the class Bacteroidia that were larger in the GSPE treatment compared to the other two treatment groups, while Alcaligenaceae (Betaproteobacteria) and Veillonellaceae (Clostridia) had similar trends (Fig. 3). In contrast, Ruminococcacea and Dehalobacteriaceae were larger in the Control and Gallic group compared to the GSPE (Fig. 3). Prominent bacterial genera that were significantly higher in the GSPE treatment compared to the Control and Gallic groups include Bacteroides, Parabacteroides, Sutterella and Phascolarctobacterium, while Bilophila was significantly higher in the GSPE group compared to only the control. [Ruminococcus] was significantly lower in the GSPE compared to the other 2 groups and Oscillospira, Coprococcus and Dehalobacterium were significantly lower in the GSPE treatment compared to the Gallic treatment (Fig. 3).
3.4. Correlations between microbiota and morphometric and metabolic variables
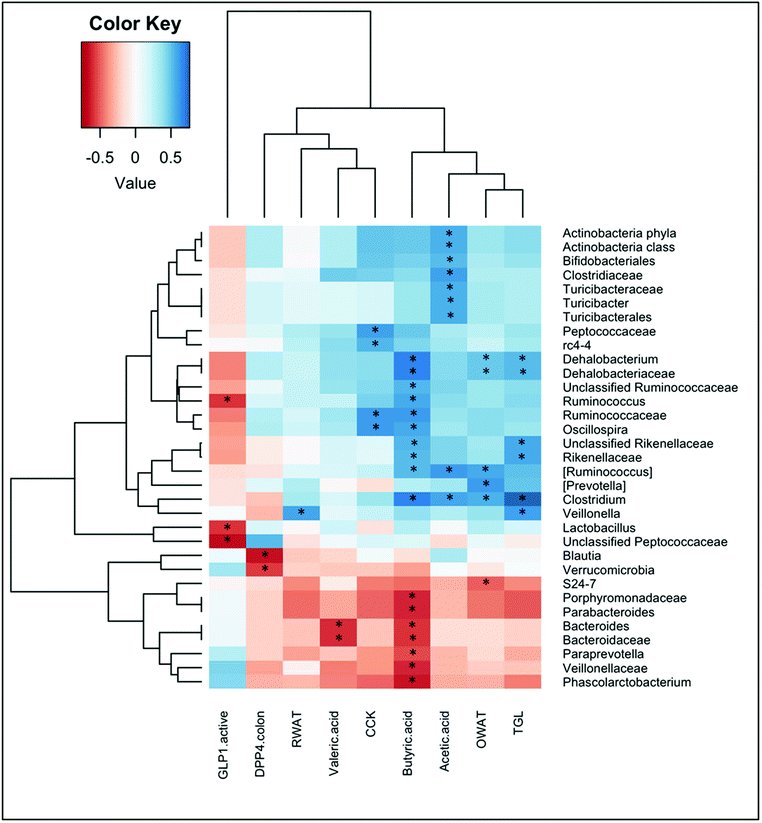
To identify whether bacterial taxa are associated with metabolic and morphometric variables, or with the different parameters that were measured in the intestine, we calculated the Spearman's rank correlation coefficient or Spearman's rho for these parameters. Significant correlations are shown in Fig. 4.We found significant correlations between the levels of short chain fatty acids and multiple bacterial taxa. Acetic acid positively correlated with some families that belong to the Firmicutes phyla, that is the Clostridiaceae (and more specifically with the Clostridium genera) and the Turicibacteraceae (and more specifically with the Turicibacter genera). Positive correlations for acetic acid were also found for the phyla Actinobacteria, and within it for the Bifidobacteriales. Butyric acid correlated positively with several genera of the families Dehalobacteriaceae and Ruminococcaaceae within the Firmicutes phylum, as well as with the Rikenellaceae family of the Bacteroidetes phylum. In contrast, butyrate levels negatively correlated with the families Veillonellaceae (Firmicutes) and Porphyromonadaceae and Bacteroidaceae (Bacteroidetes). Valeric acid also negatively correlated with this last family, and specifically with the Bacteroides genera.
Fig. 4 also shows significant correlations between some enterohormones and bacterial taxa. Plasma levels of CCK were positively associated with the genera Oscillospira (family Ruminococcaceae) and the genera rc4-4 (family Peptococcaceae) within the Firmicutes phylum. Instead, negative correlations were found between active GLP-1 levels in plasma and the genera Lactobacillus, Ruminococcus, and the unclassified Peptococcaceae, all of them belonging to the Firmicutes phylum.
We also found negative correlations between DPP-IV activity in the colon and the phylum Verrucomicrobia, as well as with the genera Blautia (phylum Firmicutes).
Ovarian WAT also positively correlated with the genera Clostridium, [Ruminococcus] and Dehalobacterium (Firmicutes phylum), and negatively correlated with the S24-7 family (within Bacteroidetes phyla). In contrast, retroperitoneal WAT positively correlated with the Veillonella genera (Firmicutes).
Finally, the only metabolic parameter that significantly correlated with bacterial taxa was the plasma triglycerides, which showed a strong positive association with the genus Clostridium, and also correlated with Veillonella, and Dehalobacterium (from the Firmicutes phylum), and the family Rikenellaceae (from the Bacteroidetes phylum).
4. Discussion
Grape seed proanthocyanidins have several demonstrated beneficial effects including a potential to alleviate metabolic syndrome parameters. They modulate body weight gain and lipidemia through several different mechanisms.26 In fact, an 8-day treatment with 500 mg per kg bw of GSPE was demonstrated to reduce body weight gain through inhibition of food intake and activation of energy expenditure.27 Provided the emerging acknowledged role of microbiota in obesity and the few studies showing that phenolic compounds might have some effect on its composition,28–30 in the present paper we analysed whether such GSPE treatment (500 mg per kg bw) during 8 days could modulate microbiota. Concomitantly we investigated other effects at the intestinal level, i.e. regulation of enterohormones, which could help to explain the effects of the GSPE treatment on food intake. We also compared the effects of the whole extract with that of one of its compounds, gallic acid, which is rapidly absorbed in upper intestine to identify the active components in the gut ecosystem.We found that GSPE changes the microbiota profile, and our results are in agreement with the general view that polyphenols increase Bacteroidetes and decrease Firmicutes phyla.7 Although there is controversy in regard to whether the Firmicutes/Bacteroidetes ratio is a microbiome-marker of obesity, this ratio has been shown to have some health implications, in particular, higher values of this fraction have been associated with obesity and type 2 diabetes in different human and animal studies.31–33 The few studies concerning flavanols and microbiota modulation suggest that acting on intestinal microbiota could be a mechanism for flavonoids to exert beneficial effects, as suggested for red wine34,35 or by cocoa flavanols.36 Conclusions of these reviews agree that more evidence is required to define the relationship between flavanols, microbiota and health effects. Our results clearly support the hypothesis that flavanols act at least partly by modulating gut microbiota composition, and such modulation might contribute to explain the previously shown beneficial effects that exert a similar GSPE treatment.27
In mice fed a high-fat-sucrose diet, highly polymeric procyanidins (PP) from apple increased the Firmicutes/Bacteroidetes ratio to the level observed in the standard-fed mice after 20 weeks of treatment.37 In that study, PP increased proportions of sequences assigned to the Adlerceitzia, Roseburia, S24-7, Bacteroides, Anaerovorax, rc4-4, and Akkermansia taxa. In contrast, the proportion of reads assigned to Clostridium, Lachnospiraceae, and Bifidobacterium were reduced by PP administration.37 Another study showed that grape polyphenols dramatically increased the relative abundance of A. muciniphila within the Verrucomicrobia phylum, in mice under a high fat diet after 13-week treatment.38 Accordingly, in mice under a high-fat-sucrose diet, cranberry extract, rich in the flavanols group including procyanidins, increased the proportion of the mucin-degrading bacterium Akkermansia when administered to mice for 8 weeks.14 Recently, it has been shown that a grape seed proanthocyanidin extract administered for 7 weeks together with a high fat diet increased Clostridium XIVa, Roseburia and Prevotella.39 All of these studies analyzed the effects of treatments that were several-weeks long together with a high fat or high-fat-sucrose diet. Instead, we describe that GSPE changed the microbiota composition after only one week of treatment and in animals fed a standard diet. Indeed the study of Ahnê et al. showed that pretreatment with cranberry extract 1 week before high-fat-sucrose feeding was not associated with specific changes in the baseline metagenome.14 Differences with our results might be attributed to the different compounds of the extract (i.e. A-type procyanidin in cranberry versus B-type in grapes), dose (200 mg cranberry extract per kg bw versus 500 mg GSPE per kg bw) and animal model (mice versus rat). Furthermore, the specific taxonomic families and genera that we find significantly modified by GSPE are mostly different from the above mentioned, with the exception of the S24-7 family and the Bacteroides genera, that were previously described as a targets for polyphenols.37 Thus, we define several new target taxonomic groups, described at the genera level, influenced by proanthocyanidins, including Sutterella, Pharscolarctobacterium, Parabacteroides, Bilophila, and Ruminococcus.
The study of Anhe et al. on cranberry extract, suggested that the related increase in Akkermansia population might be sufficient to prevent the negative metabolic phenotype associated with obesity-driven dysbiosis without major modifications in the proportions of Firmicutes and Bacteroidetes.14 To try to discern whether changes in microbiota were related to modulation of morphometric and metabolic parameters, we performed correlation tests. Our results showed positive correlations between the genera Clostridium, and Dehalobacterium (both Firmicutes), and negative correlation for S24-7 with ovarian WAT. Changes in these specific taxa by GSPE could be linked to an impairment of adipose weight increase, despite that in the present conditions (standard diet, 8–day treatment) we do not find significant modulation of total visceral WAT as expected from this experimental model. Actually, the same GSPE treatment (500 mg per kg bw, 8 days) has previously been shown to increase energy expenditure through increased lipid oxidation in adipose tissue.27 Furthermore, we found a positive correlation of circulating plasma TAG with Clostridium, Dehalobacterium and Veillonella. GSPE has previously been shown to reduce plasma triglycerides (reviewed in ref. 26) despite that in the present study we do not find statistically significant differences, again probably due to the short-term treatment and standard diet used in the study. Administration of a putative probiotic Lactobacillus rhamnosus for 28 days in diet-induced hyperlipidemic rats led to a positive correlation between Clostridium leptum and serum triglycerides, which were reduced by the treatment.40 For other types of putative prebiotics, reduction in Clostridium has been observed together with reduction in blood lipids.41 In our experiment, reduction by GSPE of several taxa that belong to Firmicutes is accompanied with a reduction in butyrate, an energy source for colonocytes. Our results in fact show a positive correlation between butyric acid and several genera, that are reduced by GSPE (e.g. Dehalobacterium, Ruminococcus), but also negative correlation with other genera within the Bacteroidetes phylum, that are increased by GSPE. In this sense, proanthocyanidins’ modulation of the microbiota profile is associated with a reduction of butyrate, that is, of energy harvest from the diet. These results led us to hypothesize that it could be a mechanism to protect from increased TAG accumulation in cases of diet induced-obesity, at least complementary to other well described mechanisms.26 Further studies on GSPE treatments in high fat diet-fed animals are required to discern whether the genera that we describe as new targets for being modulated by GSPE, are also involved in the beneficial metabolic effects of GSPE. In humans, consumption of red wine polyphenols for 4 weeks significantly increased the number of Enterococcus, Prevotella, Bacteroides, Bifidobacterium, Bacteroides uniformis, Eggerthella lenta, and Blautia coccoides–Eubacterium rectale groups,13 suggesting that the possible prebiotic effect of proanthocyanidins could be extended to humans.
In the present study, we found increased active GLP-1 in plasma after an 8-day GSPE treatment. Our results of intestinal GLP-1 gene and protein expression suggest that the effect on circulating GLP-1 levels is not due to a modification of GLP-1 production, but of its secretion. Since the last GSPE dose was administered 80 minutes before sacrifice, it could be that the previously shown acute GLP-1-stimulatory GSPE effects8,10 are maintained after 8 days of treatment, as has been described for ghrelin.27 In humans, the intake of a specific strain of Lactobacillus (i.e. L. reuteri) has been shown to increase GLP-1 secretion.42 However, we found that the levels of active GLP-1 negatively correlated with three genera (Lactobacillus, Ruminococcus and Unclassified Peptococcaceaea) within the Firmicutes phylum. Although the Lactobacillus genus identified in our study was not further classified to the species level, it appears that not all strains of Lactobacillus demonstrate the same GLP-1 secretion patterns in the host. It has been shown that the gut microbiota fermentation of specific prebiotics or other non-digestible carbohydrates is associated with the secretion of enteroendocrine peptides produced by L-cells.43 One possible mechanism is that SCFAs produced by the fermentation of dietary fibers bind to the G-protein-coupled receptors (GPCRs) GPR41 and GPR43, thereby triggering GLP-1 secretion by the L-cells.43 Our results show that GSPE significantly reduced cecal butyrate content, and modulated the ratio of acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propionate
propionate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyrate, although whether these changes could signal to enhance GLP-1 secretion should be further assessed. Alternatively, a direct link between gut microbiota and intestinal bioactive lipids related to the endocannabinoids and involved in enteroendocrine peptide secretion has been suggested as another mechanism for enhanced GLP-1 secretion observed with prebiotics.43 To our knowledge, there is no information concerning the possible relationship between these three genera and endocannabinoids, thus future studies will reveal whether this may be a mechanism for GSPE.
butyrate, although whether these changes could signal to enhance GLP-1 secretion should be further assessed. Alternatively, a direct link between gut microbiota and intestinal bioactive lipids related to the endocannabinoids and involved in enteroendocrine peptide secretion has been suggested as another mechanism for enhanced GLP-1 secretion observed with prebiotics.43 To our knowledge, there is no information concerning the possible relationship between these three genera and endocannabinoids, thus future studies will reveal whether this may be a mechanism for GSPE.
GSPE is a mixture of different compounds. Masumoto et al. demonstrated that modification in the proportion of Akkermansia in the gut microbiota is due to non-absorbable PPs and that the degree of PP polymerization is an important factor.37 In the present paper we show that gallic acid, one of the main acids found in the extract does not induce significant modification of microflora or short chain fatty acids, at least in the concentration assayed, which is equivalent to that found in 500 mg GSPE per kg. Moreover we show that gallic acid does not mimic the changes in enterohormone profile induced by GSPE. Furthermore, in this model it does not reduce food intake or adiposity.
We had previously shown that gallic acid reduced CCK secretion after an acute treatment in a rat duodenum ex vivo model.9 Now we found that CCK plasma levels tended to increase after an 8-day treatment with gallic acid. These differences might be due to the different length of time of each treatment (acute vs. 8 days). Despite our results showing no significant modulation of CCK gene expression in ileal and colonic tissue of gallic acid-treated rats, suggesting that there is not an increased production, we also observed a correlation between plasma CCK levels and some microbiota taxa. Plasma CCK positively correlated with the Oscillospira genus and the corresponding Ruminococcae family. The mechanism and whether there is a relation with CCK secretion remain unresolved, but altogether results confirm that gallic acid is not the main active molecule in the GSPE extract to explain its effects at the gastrointestinal tract.
5. Conclusion
In conclusion, our results show for the first time a clear short-term effectiveness of GSPE at modifying microbiota, increasing the amount of Bacteroidetes and reducing that of Firmicutes, and altering specific genera within these phyla. Modifications in the microbiota led to changes in the short chain fatty acid profile from the cecal content. Our correlation analysis suggests that these changes in the microbiota may be linked to the modulation of plasma TAG, adiposity, and enterohormone secretion induced by GSPE.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by a grant (AGL2014-55347-R) and AGL2014-52101-P from the Spanish government (MINECO). Kevin J. Portune was supported by the European Union's Seventh Framework Program under the grant agreement no 613979 (MyNewGut). Àngela Casanova received a grant for PhD students from the Universitat Rovira i Virgili. Joan Serrano received a pre-doctoral fellowship from the Government of Catalonia. Montserrat Pinent is a Serra Húnter fellow. We would like to thank Niurka Llopiz, Rosa Pastor, and the graduate students Berta Cava and Ingrid Pino for their technical support.References
- K. Gil-Cardoso, I. Ginés, M. Pinent, A. Ardévol, X. Terra and M. Blay, A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats, Br. J. Nutr., 2017, 117, 218–229 CrossRef CAS PubMed.
- L. Guerrero, M. Margalef, Z. Pons, M. Quiñones, L. Arola, A. Arola-Arnal and B. Muguerza, Serum metabolites of proanthocyanidin-administered rats decrease lipid synthesis in HepG2 cells, J. Nutr. Biochem., 2013, 24, 2092–2099 CrossRef CAS PubMed.
- M. Quiñones, L. Guerrero, M. Suarez, Z. Pons, A. Aleixandre, L. Arola and B. Muguerza, Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats, Food Res. Int., 2013, 51, 587–595 CrossRef.
- I.-J. Chen, C.-Y. Liu, J.-P. Chiu and C.-H. Hsu, Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial, Clin. Nutr., 2016, 35, 592–599 CrossRef CAS PubMed.
- M. Margalef, Z. Pons, F. I. Bravo, B. Muguerza and A. Arola-Arnal, Tissue distribution of rat flavanol metabolites at different doses, J. Nutr. Biochem., 2015, 26, 987–995 CrossRef CAS PubMed.
- N. González-Abuín, N. Martínez-Micaelo, M. Blay, G. Pujadas, S. Garcia-Vallvé, M. Pinent and A. Ardévol, Grape seed-derived procyanidins decrease dipeptidyl-peptidase 4 activity and expression, J. Agric. Food Chem., 2012, 60, 9055–9061 CrossRef PubMed.
- K. Gil-Cardoso, I. Ginés, M. Pinent, A. Ardévol, M. Blay and X. Terra, Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity, Nutr. Res. Rev., 2016, 29, 234–248 CrossRef CAS PubMed.
- J. Serrano, À. Casanova-Martí, I. Depoortere, M. T. Blay, X. Terra, M. Pinent and A. Ardévol, Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols, Mol. Nutr. Food Res., 2016, 60, 2554–2564 CAS.
- À. Casanova-Martí, J. Serrano, M. T. Blay, X. Terra, A. Ardévol and M. Pinent, Acute selective bioactivity of grape seed proanthocyanidins on enteroendocrine secretions in the gastrointestinal tract, Food Nutr. Res., 2017, 61, 1321347 CrossRef PubMed.
- N. González-Abuín, N. Martínez-Micaelo, M. Margalef, M. Blay, A. Arola-Arnal, B. Muguerza, A. Ardévol and M. Pinent, A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats, Food Funct., 2014, 5, 2357–2364 Search PubMed.
- J. Serrano, À. Casanova-Martí, K. Gil-Cardoso, M. T. Blay, X. Terra, M. Pinent and A. Ardévol, Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent, Food Funct., 2016, 7, 483–490 CAS.
- E. S. Chambers, D. J. Morrison and G. Frost, Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms?, Proc. Nutr. Soc., 2015, 74, 328–336 CrossRef CAS PubMed.
- M. I. Queipo-Ortuño, Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers, Am. J. Clin. Nutr., 2012, 95, 1323–1334 CrossRef PubMed.
- F. F. Anhê, D. Roy, G. Pilon, S. Dudonné, S. Matamoros, T. V. Varin, C. Garofalo, Q. Moine, Y. Desjardins, E. Levy and A. Marette, A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice, Gut, 2015, 64, 872–883 CrossRef PubMed.
- M. Margalef, L. Iglesias-Carres, Z. Pons, F. I. Bravo, B. Muguerza and A. Arola-Arnal, Age related differences in the plasma kinetics of flavanols in rats, J. Nutr. Biochem., 2016, 29, 90–96 CrossRef CAS PubMed.
- M. J. Claesson, Q. Wang, O. O'Sullivan, R. Greene-Diniz, J. R. Cole, R. P. Ross and P. W. O'Toole, Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions, Nucleic Acids Res., 2010, 38(22), e200 CrossRef PubMed.
- J. G. Caporaso, J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, A. G. Peña, J. K. Goodrich, J. I. Gordon, G. A. Huttley, S. T. Kelley, D. Knights, J. E. Koenig, R. E. Ley, C. A. Lozupone, D. McDonald, B. D. Muegge, M. Pirrung, J. Reeder, J. R. Sevinsky, P. J. Turnbaugh, W. A. Walters, J. Widmann, T. Yatsunenko, J. Zaneveld and R. Knight, QIIME allows analysis of high-throughput community sequencing data, Nat. Methods, 2010, 7, 335–336 CrossRef CAS PubMed.
- P. D. Schloss, S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. a. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. Van Horn and C. F. Weber, Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microbiol., 2009, 75, 7537–7541 CrossRef CAS PubMed.
- R. C. Edgar, UPARSE: highly accurate OTU sequences from microbial amplicon reads, Nat. Methods, 2013, 10, 996–998 CrossRef CAS PubMed.
- R. C. Edgar, Search and clustering orders of magnitude faster than BLAST, Bioinformatics, 2010, 26, 2460–2461 CrossRef CAS PubMed.
- D. McDonald, M. N. Price, J. Goodrich, E. P. Nawrocki, T. Z. Desantis, A. Probst, G. L. Andersen, R. Knight and P. Hugenholtz, An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea, ISME J., 2012, 6, 610–618 CrossRef CAS PubMed.
- J. G. Caporaso, K. Bittinger, F. D. Bushman, T. Z. Desantis, G. L. Andersen and R. Knight, PyNAST: a flexible tool for aligning sequences to a template alignment, Bioinformatics, 2010, 26, 266–267 CrossRef CAS PubMed.
- P. J. McMurdie and S. Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data, PLoS One, 2013, 8, e61217 CAS.
- N. B. Kristensen, S. G. Pierzynowski and A. Danfær, Net portal appearance of volatile fatty acids in sheep intraruminally infused with mixtures of acetate, propionate, isobutyrate, butyrate, and valerate, J. Anim. Sci., 2000, 78, 1372–1379 CrossRef CAS PubMed.
- Z. Glick, Modes of action of gallic acid in suppressing food intake of rats, J. Nutr., 1981, 111, 1910–1916 CrossRef CAS PubMed.
- M. J. Salvadó, E. Casanova, A. Fernández-Iglesias, L. Arola and C. Bladé, Roles of proanthocyanidin rich extracts in obesity, Food Funct., 2015, 6, 1053–1071 Search PubMed.
- J. Serrano, À. Casanova-Martí, A. Gual, A. M. Pérez-Vendrell, M. T. Blay, X. Terra, A. Ardévol and M. Pinent, A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats, Eur. J. Nutr., 2016, 1–8 Search PubMed.
- J. Baldwin, B. Collins, P. G. Wolf, K. Martinez, W. Shen, C. C. Chuang, W. Zhong, P. Cooney, C. Cockrell, E. Chang, H. R. Gaskins and M. K. McIntosh, Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice, J. Nutr. Biochem., 2016, 27, 123–135 CrossRef CAS PubMed.
- B. Collins, J. Hoffman, K. Martinez, M. Grace, M. A. Lila, C. Cockrell, A. Nadimpalli, E. Chang, C. C. Chuang, W. Zhong, J. Mackert, W. Shen, P. Cooney, R. Hopkins and M. McIntosh, A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice, J. Nutr. Biochem., 2016, 31, 150–165 CrossRef CAS PubMed.
- M. Van Hul, L. Geurts, H. Plovier, C. Druart, A. Everard, M. Ståhlman, M. Rhimi, K. Chira, P.-L. Teissedre, N. M. Delzenne, E. Maguin, A. Guilbot, A. Brochot, P. Gerard, F. Bäckhed and P. D. Cani, Reduced obesity, diabetes and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier, Am. J. Physiol.: Endocrinol. Metab., 2017 DOI:10.1152/ajpendo.00107.2017.
- R. E. Ley, F. Backhed, P. Turnbaugh, C. a. Lozupone, R. D. Knight and J. I. Gordon, Obesity alters gut microbial ecology, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11070–11075 CrossRef CAS PubMed.
- R. Ley, P. Turnbaugh, S. Klein and J. Gordon, Microbial ecology: human gut microbes associated with obesity, Nature, 2006, 444, 1022–1023 CrossRef CAS PubMed.
- S. H. Duncan, G. E. Lobley, G. Holtrop, J. Ince, A. M. Johnstone, P. Louis and H. J. Flint, Human colonic microbiota associated with diet, obesity and weight loss, Int. J. Obes., 2008, 32, 1720–1724 CrossRef CAS PubMed.
- C. Cueva, I. Gil-Sánchez, B. Ayuda-Durán, S. González-Manzano, A. M. González-Paramás, C. Santos-Buelga, B. Bartolomé and M. Victoria Moreno-Arribas, An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health, Molecules, 2017, 22, 99 CrossRef PubMed.
- I. Fernandes, R. Pérez-Gregorio, S. Soares, N. Mateus, V. De Freitas, C. Santos-Buelga and A. S. Feliciano, Wine Flavonoids in Health and Disease Prevention, Molecules, 2017, 22, 292 CrossRef PubMed.
- K. M. Strat, T. J. Rowley, A. T. Smithson, J. S. Tessem, M. W. Hulver, D. Liu, B. M. Davy, K. P. Davy and A. P. Neilson, Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders, J. Nutr. Biochem., 2016, 35, 1–21 CrossRef CAS PubMed.
- S. Masumoto, A. Terao, Y. Yamamoto, T. Mukai, T. Miura and T. Shoji, Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes, Sci. Rep., 2016, 6, 31208 CrossRef CAS PubMed.
- D. E. Roopchand, R. N. Carmody, P. Kuhn, K. Moskal, P. Rojas-Silva, P. J. Turnbaugh and I. Raskin, Dietary polyphenols promote growth of the gut bacterium akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome, Diabetes, 2015, 64, 2847–2858 CrossRef CAS PubMed.
- W. Liu, S. Zhao, J. Wang, J. Shi, Y. Sun, W. Wang, G. Ning, J. Hong and R. Liu, Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice, Mol. Nutr. Food Res., 2017, 61, 1601082 Search PubMed.
- D. Chen, Z. Yang, X. Chen, Y. Huang, B. Yin, F. Guo, H. Zhao, J. Huang, Y. Wu and R. Gu, Effect of Lactobacillus rhamnosus hsryfm 1301 on the gut microbiota and lipid metabolism in rats fed a high-fat diet, J. Microbiol. Biotechnol., 2015, 25, 687–695 CrossRef CAS PubMed.
- S. O. Park and B. S. Park, Bifidogenic effect of grain larvae extract on serum lipid, glucose and intestinal microflora in rats, J. Biosci., 2015, 40, 513–520 CrossRef CAS PubMed.
- M. C. Simon, K. Strassburger, B. Nowotny, H. Kolb, P. Nowotny, V. Burkart, F. Zivehe, J. H. Hwang, P. Stehle, G. Pacini, B. Hartmann, J. J. Holst, C. Mackenzie, L. B. Bindels, I. Martinez, J. Walter, B. Henrich, N. C. Schloot and M. Roden, Intake of Lactobacillus reuteri Improves Incretin and Insulin Secretion in Glucose-Tolerant Humans : A Proof of Concept, Diabetes Care, 2015, 38, 1827–1834 CrossRef CAS PubMed.
- A. Everard and P. D. Cani, Gut microbiota and GLP-1, Rev. Endocr. Metab. Disord., 2014, 15, 189–196 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7fo02028g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |