Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transition metal ion induced hydrogelation by amino-terpyridine ligands†
Sandip
Bhowmik
a,
Biswa Nath
Ghosh
ab and
Kari
Rissanen
*a
aDepartment of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland. E-mail: kari.t.rissanen@jyu.fi; Fax: +358 14 2602501; Tel: +358-50-5623721
bDepartment of Chemistry, Veer Surendra Sai University of Technology, Burla, Sambalpur 768018, Odisha, India
First published on 29th September 2014
Abstract
Hydrogelation behavior of two amino-terpyridine ligands in the presence of divalent metal ions in water was studied in detail. The effect of ligand structure and different counter anions on the gel morphologies was also explored.
In the past few decades, there has been great interest in elucidating mechanisms responsible for self-assembly of small molecules into hierarchical nanostructures. Supramolecular gelators have attracted particular attention because of the expanding usage of gels in fields such as optoelectronics,1 medicines,2 organic–inorganic hybrid materials3etc. Although, most commercial gels are polymeric in nature,4 one great advantage of supramolecular gels lies in the dynamic nature of the assemblies which allow structural modulations at the molecular level. Several such functional materials have been reported in recent literature where the physical properties of the material could be modified by applying external stimuli5 such as pH,6 light,7 chemical agents8etc., thus greatly expanding the scope of such systems for target oriented applications. Among various chemical stimuli applied to modify gel behavior, cations and anions have been the most common regulators.9–12 In cases of hydrogelators with metal binding motifs, introduction of metal ions in water was shown to have profound effects on the self-assembly process. In many of the metallogels, the formation of the 3D gel network was found to be solely dependent on the presence of a particular metal ion.13–15 Also, in some cases, gelators have been deliberately designed to have metal-binding motifs to enhance selectivity.16
Terpyridines have been extensively used to complex transition metal ions and have often served as building blocks for larger macromolecular structures.17 However, due to low solubility in water, very few studies exist about their self-assembling properties in aqueous media.9,18,19
Recently, we discovered selective gelation behavior of an amino-terpyridine ligand upon binding Hg(II) ion. The results were particularly intriguing considering the lack of any specific Hg(II) binding motif on the gelator.20 When analogues terpyridines were studied in details for clues regarding metal induced gelation, identity of metal ions as well as subtle changes in the ligand structure was found to have critical impact on the overall gelation process. In certain cases these gels have interesting properties such as pyrophosphate detection at nanomolar concentrations.21
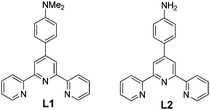
Herein we report interesting gelation behaviors of 4′-(4-N,N′-dimethylaminophenyl)-2,2′:6′,2′′-terpyridine (L1) and 4′-(4-aminophenyl)-2,2′:6′,2′′-terpyridine (L2) (Scheme 1) in presence of divalent transition metal ions and how small changes in the gelator molecule led to significant changes in the self-assembly process.
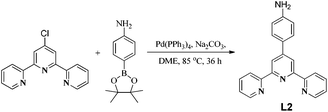
Ligand L1 was synthesized following a literature method.22 Ligand L2 was synthesized by the Suzuki cross-coupling reaction between 4′-chloro-2,2′:6′,2′′-terpyridine and 4-aminophenylboronic acid pinacol ester in dimethoxyethane solvent in the presence of Na2CO3 using Pd(PPh3)4 as catalyst (Scheme 2).
Uniform conditions were used for gelation experiments with both the ligands. L1 and L2 were solubilized in an aqueous solution of 0.15 N HCl. At this pH, electrostatic interactions between partially protonated ligands and water were too strong to allow self-assembly and resulted in clear solutions. However, as observed previously,20,21 metal coordination significantly altered solubility and charge polarization in the complexes, leading to self-assembly and eventual gelation. Several divalent metal ions were screened for the ability to induce gelation (Table 1).
| Ligand | Mg(II) | Ca(II) | Mn(II) | Fe(II) | Ni(II) | Co(II) | Cu(II) | Zn(II) | Cd(II) | Hg(II) |
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | S | S | P | P | P | P | G | G | G | P |
| L2 | S | S | P | P | P | P | P | G | G | G |
Although, most of the metal ions caused precipitation of the resulting complexes, it was found that addition of Zn(II)21 and Cd(II) to both ligands led to gelation. More interesting results were observed for Cu(II) and Hg(II) cations. Cu(II) was found to induce gelation upon complexation with L1 but not with L2, whereas Hg(II) showed the exact reverse behavior (Fig. 1). This was quite intriguing considering only minor structural differences exist between the two ligands.
 | ||
| Fig. 1 Hydrogels of (a) L1 with Cu(II), Zn(II), Cd(II) and (precipitate with) Hg(II) (left to right); (b) L2 with Cu(II) (precipitate), Zn(II), Cd(II) and Hg(II) (left to right). | ||
The metal to ligand ratio was optimized to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for all the gelation experiments. It was observed that the acidic environment and hence the protonation of the amino terminal was crucial for gel formation. It can also be assumed that π–π stacking interactions as well as the salt bridge interactions would play a major role in the self-assembly of the complexes. However the properties of the metal ion that differentiate between precipitation and gelation are not absolutely clear at this point. Although the trends for both the ligands suggested that gelation is favored in case of larger cations where hydration is weak enough to allow other interactions. During the course of our investigation, all the gels formed were found to be thermo-irreversible and thixotropic.
1 for all the gelation experiments. It was observed that the acidic environment and hence the protonation of the amino terminal was crucial for gel formation. It can also be assumed that π–π stacking interactions as well as the salt bridge interactions would play a major role in the self-assembly of the complexes. However the properties of the metal ion that differentiate between precipitation and gelation are not absolutely clear at this point. Although the trends for both the ligands suggested that gelation is favored in case of larger cations where hydration is weak enough to allow other interactions. During the course of our investigation, all the gels formed were found to be thermo-irreversible and thixotropic.
To better understand the nucleation and propagation of the self-assembly in different cases, the gel morphology was elucidated by SEM (scanning electron microscopy) and TEM (transmission electron microscopy) techniques. Although all gel networks mostly comprised of thin fibers, distinct structural differences were observed in case of each metal ion. Significant morphological variations were also observed between the two ligands L1 and L2 for the same metal ion (Fig. 2).
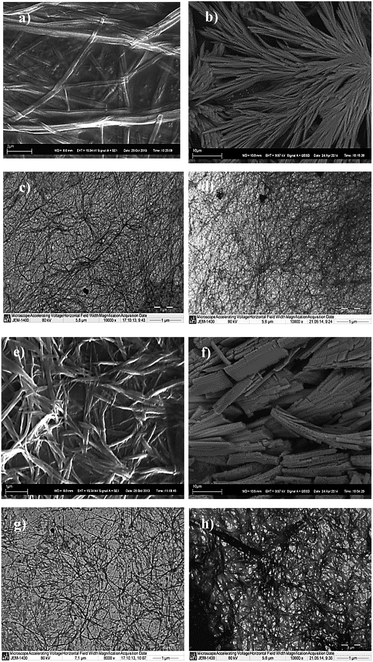 | ||
| Fig. 2 SEM images of xerogels of (a) Zn(II):L1, (b) Zn(II):L2, (e) Cd(II):L1 and (f) Cd(II):L2; TEM images of xerogels of (c) Zn(II):L1, (d) Zn(II):L2, (g) Cd(II):L1 and (h) Cd(II):L2. | ||
The dried Zn(II) hydrogels (xerogels) with both L1 and L2 showed uniform fibrous morphology in both SEM and TEM. While TEM images of the gels in both cases revealed dense network of thin fibers (∼50 nm width), presence of larger aggregates (formed by higher order assemblies of smaller fibers) were discovered through SEM analysis. However, distinct structural differences were obtained in case of Cd(II) gels. While Cd(II) gel of L1 showed typical fibrous structure (∼80–100 nm width), both SEM and TEM analysis showed Cd(II):L2 xerogel to be consisting of unusual flake like aggregates (small flat fibers of 120–150 nm width).
Both Cu(II):L1 and Hg(II):L2 gels also have fibrous morphology. However the fibers were found to be much thinner in case of Cu(II):L1 gel than Hg(II):L2 gel (see ESI† for SEM and TEM images).
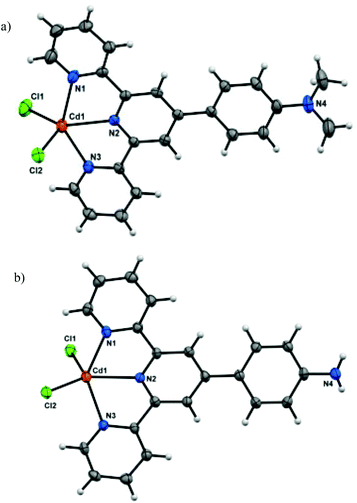
To better understand the structural anomalies and varying nature of interactions responsible for such behavior, crystal structures of the parent complexes were analyzed in detail. Due to extreme difficulty in obtaining the single crystals under exactly the same conditions which led to the gelation, the corresponding hydrogels were dissolved in DMF and slowly evaporated to afford single crystals of the coordination complexes CuCl2(L1), ZnCl2(L1),21 CdCl2(L1), ZnCl2(L2), CdCl2(L2) and HgCl2(L2). The crystal structures confirmed the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes in which the metal centers adopt distorted trigonal bipyramidal N3Cl2 coordination. Fig. 3a shows the crystal structure of CdCl2(L1), while structure of CdCl2(L2) is depicted in Fig. 3b.
1 complexes in which the metal centers adopt distorted trigonal bipyramidal N3Cl2 coordination. Fig. 3a shows the crystal structure of CdCl2(L1), while structure of CdCl2(L2) is depicted in Fig. 3b.
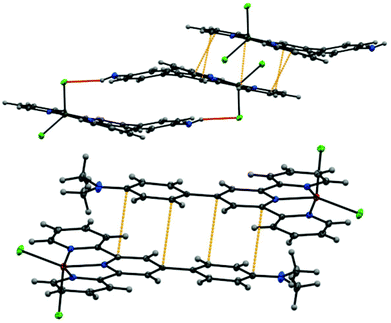
Although the coordination geometry around the metal centers in all the complexes is identical, some minor differences were observed when comparing the structures of the complexes. While the ligand L1 was found to be planar in all the three complexes, the aniline moiety of ligand L2 in each of the three complexes was found to be distorted from the planarity. Analysis of crystal packing of metal complexes of L2 revealed the presence of π⋯π interactions between the terpyridine domain of the neighboring complexes as well as N–H⋯Cl interactions (between the terminal Cl atom and adjacent anilinic H-atoms) (Fig. 4). However, the crystal packing of metal complexes of L1 was mainly governed by π⋯π interactions (Fig. 4).
The evidence from the crystal packing of the complexes suggested that the weak interactions such as π⋯π interactions between neighboring complexes might play an important role in the self-assembly. In addition, the N–H⋯Cl interactions have also influenced the self-assembly of L2 complexes. Anions were found to have a profound effect on gelation process, especially in cases where metal coordination is involved.23 Dong et al. recently demonstrated that the fiber dimensions can be regulated by Cl− ions controlling the charge distribution on π-conjugated molecules.24 This prompted us to investigate the effect of anions on the gelation process. For this purpose, the gelation experiments were repeated by dissolving ligands L1 and L2 in 0.15 N of different acids (only strong acids were selected to ensure complete dissociation and to maintain constant counter anion concentrations25). Gelation occurred uninterrupted with Zn(II), Cd(II) and Hg(II) complexes irrespective of the counter anion present.25 However, in case of Cu(II), gelation (with ligand L1) was found to be critically influenced by the presence of anions and only happened in the presence of HCl and HBr (Fig. 5).
 | ||
| Fig. 5 Cu(II):L1 (8 mM/8 mM) system after dissolving L1 in HCl, HBr, H2SO4, HNO3, HClO4, p-toluenesulfonic acid, CF3SO3H and CH3SO3H (left to right). | ||
To investigate the role of anions further, the following study was conducted. Addition of 1 equivalent of Cu(ClO4)2 to L1 (dissolved individually in 0.15 N of HNO3, H2SO4, HBr, HClO4 and p-toluenesulfonic acid) in presence of 1 equivalent of Cl− (NaCl), always led to gelation irrespective of the large excess of other counter anions in the system (Fig. 6).
 | ||
| Fig. 6 Cu(II):L1 (8 mM/8 mM) Gels in presence of 8 mM NaCl after dissolving L1 in HNO3, H2SO4, HBr, HClO4 and p-toluenesulfonic acid (left to right). | ||
The results confirmed the importance of Cl− ion in the gelation process. Although the reason for this interesting gelation behavior is not well understood, the smaller size of Cu(II) ion and hence stronger polarisability might have a critical impact on the self-assembly.
In conclusion, the self-assembly behavior of divalent metal complexes of two simple amino-terpyridine ligands L1 and L2 in water was explored in detail. It was observed that hydrogelation depended critically on the metal ions and also on minor changes in the ligand structure. The crystal packing of both the ligand-complexes showed definite differences that might have led to the morphological changes in the corresponding gel structures. The observation of the role of the metal ion in controlling the dimensions of such aggregated structures was intriguing. Given that the terpyridine based gelators were not explored until recently, this study could provide valuable insights into the dynamics of such system and would help creating new class of metal ion responsive materials.
Mr Petri Papponen, Mr Hannu Salo and Dr Rakesh Puttreddy are kindly acknowledged for their help in TEM, SEM and X-ray measurements, respectively. Academy of Finland (KR. grant no. 256259, 26337, 265328 and 263256) is kindly acknowledged for financial support.
Notes and references
- L. Korala, Z. Wang, Y. Liu, S. Maldonado and S. L. Brock, ACS Nano, 2013, 7, 1215–1223 CrossRef CAS PubMed.
- P. K. Vemula, J. Li and G. John, J. Am. Chem. Soc., 2006, 128, 8932–8938 CrossRef CAS PubMed.
- S. Bhowmik, T. Gorai and U. Maitra, J. Mater. Chem. C, 2014, 2, 1597–1600 RSC.
- H. A. Aliyar, P. D. Hamilton and N. Ravi, Biomacromolecules, 2004, 6, 204–211 CrossRef PubMed.
- E. Carretti, M. Bonini, L. Dei, B. H. Berrie, L. V. Angelova, P. Baglioni and R. G. Weiss, Acc. Chem. Res., 2010, 43, 751–760 CrossRef CAS PubMed.
- Y. Hisamatsu, S. Banerjee, M. B. Avinash, T. Govindaraju and C. Schmuck, Angew. Chem., Int. Ed., 2013, 52, 12550–12554 CrossRef CAS PubMed.
- J. Eastoe, M. Sánchez-Dominguez, P. Wyatt and R. K. Heenan, Chem. Commun., 2004, 2608–2609 RSC.
- S. Banerjee, R. K. Das and U. Maitra, J. Mater. Chem., 2009, 19, 6649–6687 RSC.
- O. Kotova, R. Daly, C. M. G. dos Santos, M. Boese, P. E. Kruger, J. J. Boland and T. Gunnlaugsson, Angew. Chem., Int. Ed., 2012, 51, 7208–7212 CrossRef CAS PubMed.
- T. Tu, W. Fang, X. Bao, X. Li and K. H. Dötz, Angew. Chem., Int. Ed., 2011, 50, 6601–6605 CrossRef CAS PubMed.
- J. Park, J. H. Lee, J. Jaworski, S. Shinkai and J. H. Jung, Inorg. Chem., 2014, 53, 7181–7187 CrossRef CAS PubMed.
- M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489–497 CrossRef CAS PubMed.
- A. Kumar, M. Dubey, A. Kumar and D. S. Pandey, Chem. Commun., 2014, 50, 10086–10089 RSC.
- A. Y.-Y. Tam and V. W.-W. Yam, Chem. Soc. Rev., 2013, 42, 1540–1567 RSC.
- M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960–2004 CrossRef CAS PubMed.
- T. Sato, M. Ebara, S. Tanaka, T.-A. Asoh, A. Kikuchi and T. Aoyagi, Phys. Chem. Chem. Phys., 2013, 15, 10628–10635 RSC.
- A. Wild, A. Winter, F. Schlütter and U. S. Schubert, Chem. Soc. Rev., 2011, 40, 1459–1511 RSC.
- L. Sambri, F. Cucinotta, G. D. Paoli, S. Stagni and L. D. Cola, New J. Chem., 2010, 34, 2093–2096 RSC.
- A. Griffith, T. J. Bandy, M. Light and E. Stulz, Chem. Commun., 2013, 49, 731–733 RSC.
- B. N. Ghosh, S. Bhowmik, P. Mal and K. Rissanen, Chem. Commun., 2014, 50, 734–736 RSC.
- S. Bhowmik, B. N. Ghosh, V. Marjomäki and K. Rissanen, J. Am. Chem. Soc., 2014, 136, 5543–5546 CrossRef CAS PubMed.
- J. Wang and G. S. Hanan, Synlett, 2005, 1251–1254 CAS.
- J. W. Steed, Chem. Soc. Rev., 2010, 39, 3686–3699 RSC.
- B. Dong, T. Sakurai, Y. Bando, S. Seki, K. Takaishi, M. Uchiyama, A. Muranaka and H. Maeda, J. Am. Chem. Soc., 2013, 135, 14797–14805 CrossRef CAS PubMed.
- The gelation experiment with H2SO4 solution of L1 was done in presence of 0.075 M Na2SO4 to balance the SO42− concentration.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, gelation procedure, X-ray single crystal structure analysis. CCDC 1021539–1021543. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ob01867b |
| This journal is © The Royal Society of Chemistry 2014 |