Synthesis of heterocyclic-fused benzopyrans via the Pd(ii)-catalyzed C–H alkenylation/C–O cyclization of flavones and coumarins†
Abstract
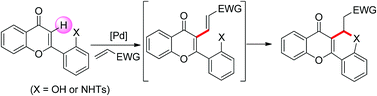
An efficient and practical method for effecting a tandem C–H alkenylation/C–O cyclization has been achieved via the C–H functionalization of flavone derivatives. The synthetic utility of the one-pot sequence was demonstrated by obtaining convenient access to coumarin-annelated benzopyrans. The reaction scope for the transformation was found to be fairly broad, affording good yields of a wide range of flavone- or coumarin-fused benzopyran motifs, which are privileged structures in many biologically active compounds.

 Please wait while we load your content...
Please wait while we load your content...