Bay-linked perylene bisimides as promising non-fullerene acceptors for organic solar cells†
Wei
Jiang
a,
Long
Ye
b,
Xiangguang
Li
a,
Chengyi
Xiao
a,
Fang
Tan
a,
Wenchao
Zhao
b,
Jianhui
Hou
*b and
Zhaohui
Wang
*a
aKey Laboratory of Organic Solids, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: wangzhaohui@iccas.ac.cn; Fax: +86-10-62653617; Tel: +86-10-62653617
bState Key Laboratory of Polymer Physics and Chemistry, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: hjhzlz@iccas.ac.cn
First published on 30th October 2013
Abstract
A series of bay-linked perylene bisimides as non-fullerene acceptors for organic solar cells are designed. The best power conversion efficiency up to 3.63% based on s-diPBI (1b) is demonstrated by fine-tuning optoelectronic properties resulting from different degrees of twisting and flexibility by bay-linkages.
Organic solar cells (OSCs), featuring a bulk heterojunction (BHJ) architecture with an electron donor and an electron acceptor, have been spurring increasing attention due to low-cost, mechanical flexibility, and chemical versatility.1 Significant progress has been made by combining rational molecular design and device engineering to afford power conversion efficiencies (PCEs) close to or exceeding 10% for typical polymer/fullerene based BHJ OSCs.2 Complementary absorption, aligned energy levels, effective charge separation and transporting are pivotal for achieving higher efficiencies. Despite the diversity of donor materials,3 alternatives to fullerenes as electron acceptors would bring about more potential,4 since the fullerene family suffer less tunable electronic structure and weak absorption in the visible region.5
Perylene bisimides (PBIs) are promising alternatives as electron acceptors because of their comparable electron affinities to fullerenes,6 excellent photochemical stability, tunable electronic structure and properties.7 However, PBI-based small molecules and polymers serving as electron acceptors in OSCs yield relatively modest efficiencies even with optimization of donor and device structure.8 The inferior PCE of the present PBI systems primarily lies on the relatively low fill factor (FF) and reduced short-circuit current density (JSC), which are probably traceable to the π-stacked aggregation and crystallinity of PBIs.9
We are particularly interested in π-expanded PBI dyes with different bay-linkages between the PBI subunits, for instance well-defined singly-linked, chiral doubly-linked, and graphene-like triply-linked PBI oligomers.10 They have either a flexibly twisted structure with about 70° angle between two PBI units (singly), or a locked twisted structure with nearly perpendicular PBI systems (doubly), or rigidly and almost planar superstructure by the presence of triple linkage (triply).11 The resulting differences of twisting and flexible structure allow us to efficiently screen a structural series of bay-linked PBIs with defined properties in OSC applications. Keeping this in mind, three bay-linked perylene bisimides (diPBIs) characterized by branched alkyl chains as electron acceptors were chosen for integration into solution-processed BHJ OSC devices (Fig. 1). By combining a 2D conjugated polymer based on alkylthiophene-2-yl-substituted benzo[1,2-b:4,5-b′]dithiophene (PBDTTT-C-T) with broadened light absorption and downshifted HOMO levels as electron donor,12 the 6-undecyl substituted singly-linked diPBI (1b, C5,5-s-diPBI) exhibited the best PCEs up to 3.63% for their better matching energy levels resulting from their flexibility and twisted structure.
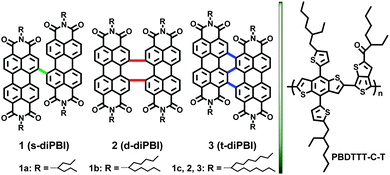 | ||
| Fig. 1 The chemical structures of bay-linked PBIs (diPBIs, 1–3) and donor polymer (PBDTTT-C-T) used in this study. | ||
The synthetic route to diPBIs by one-step homo-coupling of halogenated PBIs is shown in Scheme S2 (ESI†), using minor modifications of previously reported procedures.11 A PBI dimer, previously synthesized by some of us from isomeric dibromoPBI gave a promising PCE of 4.03%,13 and here we explore further structurally defined PBI dimers. Our synthetic route to bay-linked dimers is particularly based on monobrominated PBI, which render more precise control of molecular conformation and fine tuning of energy levels. Moreover, branched alkyl chains were introduced to balance the molecular aggregation as a result of strong π–π interactions.
As expected, the flexibly twisted s-diPBIs (1) showed much broadened absorption bands in the range of 400 to 600 nm, while d-diPBI (2) showed a narrowed spectrum similar to that of the parent PBI. In contrast, new and pronounced red-shifted absorption peaks throughout from 300 to 800 nm in t-diPBI (3) were observed (Fig. 2). The donor polymer absorbed in the wavelength range from 500 to 750 nm, which well matched with these diPBIs that will enhance light harvesting in the range of the solar spectrum. The optical energy gaps were determined from the absorption onsets in film with 2.03–2.08 eV for s-diPBIs, and 2.22 eV for d-diPBI, and 1.69 eV for t-diPBI, respectively (Table S2, ESI†). Calculated by their onset of reduction potentials in film, the LUMO levels were −3.91, −3.87, −3.82, −3.79 and −4.09 eV for s-diPBI to t-diPBI, which are slightly upward shifted of about 0.2–0.5 eV to that of PCBM (Fig. S4, ESI†). The differences can be ascribed to the different degrees of twisting and flexibility by bay-linkages that results in different conjugation over the whole π-system.11
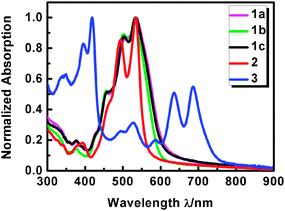 | ||
| Fig. 2 Normalized UV-vis absorption spectra of thin films of bay-linked PBIs (diPBIs, 1–3) on quartz. | ||
Electron transporting properties of five diPBIs were also measured by field-effect transistors (FETs) in air at room temperature and listed in Table S3 (ESI†). It is observed that the doubly- and triply-linked diPBIs with the same alkyl substituents (8-pentadecyl) showed higher mobilities by two orders of magnitude than that of the singly-linked one. However, the electron transporting properties can be largely enhanced by reducing the branched alkyl chain length to achieve a moderate mobility of ∼10−3 cm2 V−1 s−1 for 6-undecyl substituted s-diPBI (1b), suggesting that structural modification by appropriate substituents indeed exert influences on crystallinity and self-assembly morphology of s-diPBI in the solid state. These electron mobilities without intense optimization, comparable to that of PCBM usually measured in nitrogen, are necessary for functioning as electron acceptors in OSCs.14
The photovoltaic properties of the PBDTTT-C-T/diPBI blends have been assessed in typical solar cell devices with the structure of ITO|PEDOT:PSS (∼35 nm)|PBDTTT-C-T:diPBI|Ca (∼20 nm)|Al (∼80 nm). The ratio of donor to acceptor (D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) was initially optimized by using dichlorobenzene (DCB) as the processing solvent. The best D
A) was initially optimized by using dichlorobenzene (DCB) as the processing solvent. The best D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio is 1
A ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for all the studied diPBIs with the exception of t-diPBI (3). Then, we carefully examined their performances as electron acceptors by gradually adding 1,8-diiodooctane (DIO) additive (1, 3 or 5 vol%). The detailed photovoltaic parameters of the OSC devices with different D
1 for all the studied diPBIs with the exception of t-diPBI (3). Then, we carefully examined their performances as electron acceptors by gradually adding 1,8-diiodooctane (DIO) additive (1, 3 or 5 vol%). The detailed photovoltaic parameters of the OSC devices with different D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
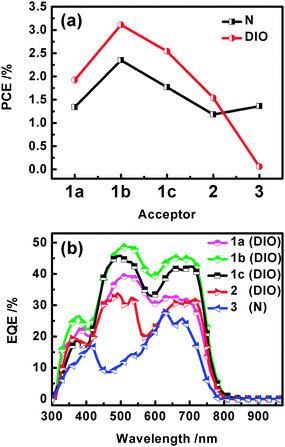
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios and additive amounts are listed in Tables S4–S8 (ESI†). As presented in Fig. 3a, distinct photovoltaic characteristics were observed from the different bay-linked diPBIs with or without DIO processing. The promoted PCEs can be obtained accompanied with increased JSC
A ratios and additive amounts are listed in Tables S4–S8 (ESI†). As presented in Fig. 3a, distinct photovoltaic characteristics were observed from the different bay-linked diPBIs with or without DIO processing. The promoted PCEs can be obtained accompanied with increased JSC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 when using 3% DIO processing with the exception of t-diPBI (3). Comparing the three diPBIs with same alkyl substituents, s-diPBI showed better PCEs than those of other two analogues. As summarized in Table 1, the open circuit voltage (VOC) values of devices based on s-diPBI (1c) are similar to that of d-diPBI (2) and much higher than that of t-diPBI (3), which is mainly ascribed to their different arrangement of LUMO energy levels, while the obtained JSC values of s-diPBI are significantly increased in comparison with those of d-diPBI and t-diPBI. The primary reason is traceable to the flexibility and twisting of the singly-linked derivatives with disrupting the crystallinity and appropriate charge-transporting properties.16 In contrast, the device based on t-diPBI (3) exhibited a PCE of 1.36% at the ratio of 1
15 when using 3% DIO processing with the exception of t-diPBI (3). Comparing the three diPBIs with same alkyl substituents, s-diPBI showed better PCEs than those of other two analogues. As summarized in Table 1, the open circuit voltage (VOC) values of devices based on s-diPBI (1c) are similar to that of d-diPBI (2) and much higher than that of t-diPBI (3), which is mainly ascribed to their different arrangement of LUMO energy levels, while the obtained JSC values of s-diPBI are significantly increased in comparison with those of d-diPBI and t-diPBI. The primary reason is traceable to the flexibility and twisting of the singly-linked derivatives with disrupting the crystallinity and appropriate charge-transporting properties.16 In contrast, the device based on t-diPBI (3) exhibited a PCE of 1.36% at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 when pure DCB was used, but almost vanished when using 3% DIO as the additive, resulting from the sharply decreased JSC and the reduced FF by a half due to π-stacked aggregation that is induced by its almost-planar structure.
3 when pure DCB was used, but almost vanished when using 3% DIO as the additive, resulting from the sharply decreased JSC and the reduced FF by a half due to π-stacked aggregation that is induced by its almost-planar structure.
| Acceptor | D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio A ratio |
Additives | V OC/V | J SC/mA cm−2 | FF (%) | PCEa (%) |
|---|---|---|---|---|---|---|
| a Tested under illumination of AM 1.5G 100 mW cm−2. | ||||||
| 1a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N | 0.62 | 6.09 | 35.39 | 1.34 |
| 3% DIO | 0.67 | 6.68 | 42.94 | 1.92 | ||
| 1b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N | 0.68 | 8.47 | 40.80 | 2.35 |
| 3% DIO | 0.72 | 10.36 | 42.08 | 3.11 | ||
| 1c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N | 0.69 | 7.51 | 33.87 | 1.77 |
| 3% DIO | 0.72 | 8.86 | 39.75 | 2.54 | ||
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N | 0.66 | 4.76 | 37.43 | 1.18 |
| 3% DIO | 0.74 | 5.76 | 36.41 | 1.54 | ||
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
N | 0.46 | 5.77 | 50.58 | 1.36 |
| 3% DIO | 0.50 | 0.50 | 23.45 | 0.06 | ||
We speculated that further alkyl modification might play an important role on crystallinity and interpenetrating nanostructure formed by the two components, which also influence corresponding JSC and FF, and thus PCE. As a result, much better performance of 3.11% for C5,5-s-diPBI (1b) by binary co-solvent (DCB/3% DIO) processing was obtained when shortened 6-undecyl was adopted. Further optimization by selecting another binary solvent mixture of DCB/3% CN (1-chloronaphthalene), and a ternary solvent mixture of DCB/1.5% DIO/1.5% CN, we found that a PCE of 3.63% with a VOC of 0.73 V, a JSC of 10.58 mA cm−2 and a FF of 46.80% was recorded by the use of the ternary solvent with finely tuning the morphology of the composites (Table 2).17 It can be seen that the EQE curves of all these devices cover the wavelength range from 300 to 800 nm (Fig. 3b). Their quantum yields become much higher when a 3% DIO additive was added, with the exception of a sharp decrease for t-diPBI. It is also noteworthy that the maximum EQE peak approaches 48.62% at 530 nm for devices utilizing 1b as electron acceptor.
In summary, a series of bay-linked (singly, doubly and triply) perylene bisimides featuring branched alkyl chains as non-fullerene acceptors have been designed and demonstrated their electron accepting properties in solution-processed BHJ OSC devices by utilizing PBDTTT-C-T as the polymer donor. By screening the bay-linkage and further alkyl modification, the singly-linked diPBIs have proved their superior performances up to 3.63% owing to their better matching energy levels resulting from the flexibility and twisted structure. In addition, the triply-linked analogue also revealed its promising nature owing to broad absorptions and preferable charge transporting. Further improvements in non-fullerene solar cells could be achieved by molecular design of bay-linkage and alkyl modification in PBIs towards suitable energy levels as well as enhanced mobility.
For financial support of this research, we thank 973 Program (Grant 2014CB643500 and 2012CB932903), the National Natural Science Foundation of China (21225209, 91027043, 21190032, and 51203164), NSFC-DFG Joint Project TRR61, and the Chinese Academy of Sciences.
Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS.
- (a) J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C.-C. Chen, J. Gao and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed; (b) Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 Search PubMed.
- (a) Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2011, 11, 44 CrossRef PubMed; (b) Y.-X. Xu, C.-C. Chueh, H.-L. Yip, F.-Z. Ding, Y.-X. Li, C.-Z. Li, X. Li, W.-C. Chen and A. K.-Y. Jen, Adv. Mater., 2012, 24, 6356 CrossRef CAS PubMed; (c) J. Zhou, Y. Zuo, X. Wan, G. Long, Q. Zhang, W. Ni, Y. Liu, Z. Li, G. He, C. Li, B. Kan, M. Li and Y. Chen, J. Am. Chem. Soc., 2013, 135, 8484 CrossRef CAS PubMed; (d) U. Mayerhöffer, K. Deing, K. Gruß, H. Braunschweig, K. Meerholz and F. Würthner, Angew. Chem., Int. Ed., 2009, 48, 8776 CrossRef PubMed.
- For examples: (a) Y. Lin, Y. Li and X. Zhan, Adv. Energy Mater., 2013, 3, 724 CrossRef CAS; (b) Y. Zhou, L. Ding, K. Shi, Y.-Z. Dai, N. Ai, J. Wang and J. Pei, Adv. Mater., 2012, 24, 957 CrossRef CAS PubMed; (c) G. Ren, E. Ahmed and S. A. Jenekhe, Adv. Energy Mater., 2011, 1, 946 CrossRef CAS; (d) Y. Zhou, Y.-Z. Dai, Y.-Q. Zheng, X.-Y. Wang, J.-Y. Wang and J. Pei, Chem. Commun., 2013, 49, 5802 RSC; (e) Y. Zhou, Q. Yan, Y.-Q. Zheng, J.-Y. Wang, D. Zhao and J. Pei, J. Mater. Chem. A, 2013, 1, 6609 RSC.
- (a) R. B. Ross, C. M. Cardona, D. M. Guldi, S. G. Sankaranarayananan, M. O. Reese, N. Kopidakis, J. Peet, B. Walker, G. C. Bazan, E. V. Keuren, B. C. Holloway and M. Grees, Nat. Mater., 2009, 8, 208 CrossRef CAS PubMed; (b) Y. He, H.-Y. Chen, J. Hou and Y. Li, J. Am. Chem. Soc., 2010, 132, 1377 CrossRef CAS PubMed.
- (a) C. Li and H. Wonneberger, Adv. Mater., 2012, 24, 613 CrossRef CAS PubMed; (b) B. Liu, W. Zhu, W. Wu, K. Mu Ri and H. Tian, J. Photochem. Photobiol., A, 2008, 194, 268 CrossRef CAS PubMed.
- (a) B. A. Jones, M. J. Ahrens, M. H. Yoon, A. Facchetti, T. J. Marks and M. R. Wasielewski, Angew. Chem., Int. Ed., 2004, 43, 6363 CrossRef CAS PubMed; (b) M. Gsänger, J. H. Oh, M. Könemann, H. W. Höffken, A.-M. Krause, Z. Bao and F. Würthner, Angew. Chem., Int. Ed., 2010, 49, 740 CrossRef PubMed.
- For recent examples: (a) E. Zhou, J. Cong, Q. Wei, K. Tajima, C. Yang and K. Hashimoto, Angew. Chem., Int. Ed., 2011, 50, 2799 CrossRef CAS PubMed; (b) A. Sharenko, C. M. Proctor, T. S. van der Poll, Z. B. Henson, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2013, 25, 4403 CrossRef CAS PubMed; (c) Q. Yan, Y. Zhou, Y.-Q. Zheng, J. Pei and D. Zhao, Chem. Sci., 2013, 4, 4389 RSC.
- (a) J. J. Dittmer, E. A. Marseglia and R. H. Friend, Adv. Mater., 2000, 12, 1270 CrossRef CAS; (b) T. W. Holcombe, J. E. Norton, J. Rivnay, C. H. Woo, L. Goris, C. Piliego, G. Griffini, A. Sellinger, J.-L. Brédas, A. Salleo and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 12106 CrossRef CAS PubMed.
- (a) H. Qian, Z. Wang, W. Yue and D. Zhu, J. Am. Chem. Soc., 2007, 129, 10664 CrossRef CAS PubMed; (b) H. Qian, F. Negri, C. Wang and Z. Wang, J. Am. Chem. Soc., 2008, 130, 17970 CrossRef CAS PubMed; (c) Y. Zhen, W. Yue, Y. Li, W. Jiang, S. Di Motta, E. Di Donato, F. Negri, S. Ye and Z. Wang, Chem. Commun., 2010, 46, 6078 RSC; (d) J. Zhang, L. Tan, W. Hu and Z. Wang, J. Mater. Chem. C, 2013, 1, 3200 RSC.
- W. Jiang, C. Xiao, L. Hao, Z. Wang, H. Ceymann, C. Lambert, S. Di Motta and F. Negri, Chem.–Eur. J., 2012, 18, 6764 CrossRef CAS PubMed.
- L. Huo, S. Zhang, X. Guo, F. Xu, Y. Li and J. Hou, Angew. Chem., Int. Ed., 2011, 50, 9697 CrossRef CAS PubMed.
- X. Zhang, Z. Lu, L. Ye, C. Zhan, J. Hou, S. Zhang, B. Jiang, Y. Zhao, J. Huang, S. Zhang, Y. Liu, Q. Shi, Y. Liu and J. Yao, Adv. Mater., 2013, 25, 5791 CrossRef CAS PubMed.
- C.-Z. Li, C.-C. Chueh, H.-L. Yip, J. Zou, W.-C. Chen and A. K.-Y. Jen, J. Mater. Chem., 2012, 22, 14976 RSC.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619 CrossRef CAS PubMed.
- S. Rajaram, R. Shivanna, S. K. Kandappa and K. S. Narayan, J. Phys. Chem. Lett., 2012, 3, 2405 CrossRef CAS.
- L. Ye, S. Zhang, W. Ma, B. Fan, X. Guo, Y. Huang, H. Ade and J. Hou, Adv. Mater., 2012, 24, 6335 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of the synthesis and characterization of starting materials and as-synthesized diPBIs. See DOI: 10.1039/c3cc47204c |
| This journal is © The Royal Society of Chemistry 2014 |