 Open Access Article
Open Access ArticleNuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits
Joe A.
Vinson
* and
Yuxing
Cai
Department of Chemistry, University of Scranton, Scranton, PA 18510, USA. E-mail: vinson@scranton.edu; Fax: +1-570-941-7510; Tel: +1-570-941-7551
First published on 21st December 2011
Abstract
Free and total (after basic hydrolysis) polyphenols in nine types of raw and roasted nuts and two types of peanut butter (54 commercial samples) were analyzed after methanol extraction by a single step Folin-Ciocalteu reagent using catechin as standard. Walnuts had the highest free and total polyphenols in both the combined raw and roasted samples. Total polyphenols in the nuts were significantly higher than free polyphenols. Roasting had little effect on either free or total polyphenols in nuts. Raw and roasted walnuts had the highest total polyphenols. The efficacy of raw and roasted nut antioxidants was assessed by measuring the ability of the free polyphenol nut extracts to inhibit the oxidation of lower density lipoproteins (LDL + VLDL). A nut polyphenol, catechin, was measured after binding of three nut extracts to lower density lipoproteins. Walnut polyphenols had the best efficacy among the nuts and also the highest lipoprotein-bound antioxidant activity. Based on USDA availability data, the per capita total polyphenols was 162 mg from nuts per day in 2008. This corresponds to 19% of the total polyphenols from fruits and vegetables, nuts, grains, oils and spices in the US diet. Nuts provided 158 mg of polyphenols per day to the European Union diet. Nuts are high in polyphenol antioxidants which by binding to lipoproteins would inhibit oxidative processes that lead to atherosclerosis in vivo. In human supplementation studies nuts have been shown to improve the lipid profile, increase endothelial function and reduce inflammation, all without causing weight gain. These qualities make nuts a nutritious healthy snack and food additive.
Introduction
Since nuts have favorable fatty acid and nutrient profiles, there is growing interest in evaluating their role in a healthy diet. While fat accounts for 50–75% of the weight of the nuts, the amount of saturated fatty acids is quite low ranging from 4–15%. Among the nuts, walnuts have the highest weight % of PUFA and also the highest % 18:2 (n–6), and the highest % 18:3 (n–3), 47, 38, and 9%, respectively.1 When consuming the high-energy dense nuts, there is a satiety effect and low metabolizable energy. In addition, nut consumption increases elevated resting energy expenditure and elicits a thermogenic effect of feeding.2 These mechanisms provide the rationale for the fact that clinical studies show that nuts are not associated with weight gain.3,4 In fact in a Spanish study those who ate nuts twice a week were 31% less likely to gain weight than those who did not eat any nuts.5Nut consumption also has a favorable effect on the lipid profile and cardiovascular disease risk factors as demonstrated by numerous supplementation studies involving almonds, hazelnuts, Macademia, peanuts, pistachios, walnuts, and mixed nuts.6–20 The lipid effect was examined in subjects with normal and with high cholesterol. Interestingly the cholesterol-lowering effect of the nuts as shown by supplementation is greater than that predicted based on the amount and nature of fat consumed.21 These results suggest that there are other bioactive constituents present in the nuts besides the fats. We hypothesize that these compounds are polyphenols. This hypothesis is strengthened by the human supplementation studies. Almonds, cashews, peanuts and walnuts have been shown to increase plasma antioxidant capacity and/or plasma oxidized LDL and oxidative stress.12,22–27 Nuts and oxidation has been recently reviewed.28
Plant polyphenols include simple phenolic acids, flavonoids, stilbenes and a variety of other polyphenolic plasma antioxidant capacity and/or decrease plasma oxidized compounds which possess hydroxy groups bound to an aromatic group. Flavonoid content of nuts has been measured by HPLC from four regions of the US by the Nutrient Data Laboratory of the USDA.29 Twenty compounds were analyzed and the highest total flavonoids were found in pecans (9.5 mg per 28 g serving size) with almonds second at 4.2 mg. No flavonoids were detected in Brazil or Macademia nuts. Proanthocyanidins, large molecules not bioavailable to humans, are present in very high amounts in nuts, ranging from 140 mg in hazelnuts and pecans to 3 mg in cashews. Total polyphenols as measured by ox-redox reactions are best done after hydrolysis to liberate any polyphenols bound as esters or ethers primarily to the polysaccharide matrix (fiber) which is high in nuts ranging from 5.9% in cashews to 10.4% in hazelnuts.1 We measured free and total polyphenols in both fresh and roasted nuts. In addition we measured the efficacy of the antioxidants in the nuts and determined whether the polyphenols in the nuts bind to lower density lipoproteins and protected the lipoproteins from oxidation. Using per capita consumption data we also calculated the contribution of nuts to the daily antioxidants in the US diet.
Materials and methods
Nine types of nuts (n = 50) and peanut butter (n = 4) were purchased from local supermarkets and from the internet. The samples were refrigerated before analysis. Nut samples were ground in a mortar and pestle under liquid nitrogen and stored at 4 °C prior to assay. Fat and the fat-soluble vitamins in one gram samples were removed by two extractions with 5 ml of hexane. After pouring out the liquid the residual hexane in the nuts was evaporated with nitrogen. A 100 mg sample of hexane-treated ground nuts (in duplicate) was put into a 10 ml screw-capped plastic tubes and shaken with 10 ml of methanol for 48 h at room temperature. The extracts were quantitatively transferred to a 10 ml volumetric flask and then diluted to volume with methanol. This is the sample extract for measurement of free polyphenols. Hydrolysis was carried out by adding 100 mg of defatted ground nuts (in duplicate) to 5 ml of 1.2 M NaOH in methanol in a screw-capped tube and shaken for 3 h at 37 °C. The extract was diluted to 10 ml in a volumetric flask. This is the extract for the assay of total polyphenols. Extracts were stored at −80 °C until assayed.Polyphenol assay
Kinetic studies were done in order to determine the optimum time for extraction and also for hydrolysis. For extraction, cashews were shaken with methanol at room temperature and aliquots removed at 24, 48 and 72 h prior to Folin assay. Maximum extraction occurred at 48 h. For hydrolysis, almonds were shaken with NaOH in methanol at 37 °C and aliquots removed every 30 min for 5 h. Three hours were needed for maximal extraction and hydrolysis. We measured the amount of interferences in the water eluates from the Polyclar columns from the free and total polyphenol extracts. The amount of interferences ranged from 0 to 3% of the Folin values among all the nut samples.
Polyphenols were measured in the extracts using the Folin-Ciocalteu reagent (Sigma Chemical Co.) diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 with nanopure water (Millipore Q). Catechin hydrate (Sigma) was used at 5 to 50 μM in the cuvette and measurements were made after 30 min at 750 nm vs. a reagent blank with 100 μL of methanol to generate the standard curve. Due to possible interferences present in the nut extracts which may give a positive response with the Folin reagent, we used a new solid phase method to adsorb polyphenols, and the eluate contained water-soluble interferences. Polyvinylpyrrolidone (Polyclar VT, GAF Chemicals, Wayne, NJ) was used as the solid phase and 0.38 g of the powder was added to a 10 ml syringe with 0.1 g of cotton used as a bed. The Polyclar column was prepared by washing with 5ml of methanol, followed with 5 ml of 0.012M HCl. One ml of nut extracts (free and total) were each diluted to 10 ml after 4M HCl addition to acidify the solution to pH 2–4. 4 ml of diluted nut extract was pipetted on the column and 4 ml of water used to wash the column. The eluants were combined and diluted to 10 ml with water and the solid phase discarded. The Folin assay was then done using a larger volume of extract due to the dilution. For the Folin assay sugars are often listed as interferences with the standard two step method of Singleton.30 Our single step Folin method showed no positive interferences with 1 mM glucose and fructose.
9 with nanopure water (Millipore Q). Catechin hydrate (Sigma) was used at 5 to 50 μM in the cuvette and measurements were made after 30 min at 750 nm vs. a reagent blank with 100 μL of methanol to generate the standard curve. Due to possible interferences present in the nut extracts which may give a positive response with the Folin reagent, we used a new solid phase method to adsorb polyphenols, and the eluate contained water-soluble interferences. Polyvinylpyrrolidone (Polyclar VT, GAF Chemicals, Wayne, NJ) was used as the solid phase and 0.38 g of the powder was added to a 10 ml syringe with 0.1 g of cotton used as a bed. The Polyclar column was prepared by washing with 5ml of methanol, followed with 5 ml of 0.012M HCl. One ml of nut extracts (free and total) were each diluted to 10 ml after 4M HCl addition to acidify the solution to pH 2–4. 4 ml of diluted nut extract was pipetted on the column and 4 ml of water used to wash the column. The eluants were combined and diluted to 10 ml with water and the solid phase discarded. The Folin assay was then done using a larger volume of extract due to the dilution. For the Folin assay sugars are often listed as interferences with the standard two step method of Singleton.30 Our single step Folin method showed no positive interferences with 1 mM glucose and fructose.
Antioxidant efficacy
The efficacy of antioxidants in the hydrolyzed and neutralized nut extracts was examined using our standard published in vitro “heart disease in a test tube” model in which lower density lipoprotein (LDL + VLDL) is isolated from the plasma of a normocholesterolemic subject after approval was obtained from the Institutional Review Board. Lipoproteins were subjected to oxidation with or without added antioxidants added at different concentrations.31Oxidation is at physiological pH (7.4) and temperature (37 °C) and is initiated by addition of cupric ion (final concentration 25 μM) and oxidation is continued for 6 h. Lipid oxidation is measured by fluorometry in a control with no added antioxidants and in the samples with thiobarbituric acid reagent from Sigma. The concentration to inhibit the oxidation by 50% (IC50) is determined as the measure of the efficacy of the antioxidants. One raw and one roasted nut of each type was measured in duplicate. Phenol antioxidant index (PAOXI) is a combined measure of both quantity and efficacy of the antioxidants present. It is calculated by dividing the quantity of polyphenols in raw nuts and peanut butter in μm kg−1 units by the IC50 in uM units.32Lipoprotein binding and antioxidant activity
A standard method was used31 for determination of binding and lipoprotein-bound antioxidant activity. LDL + VLDL isolated in 2.7% NaCl was diluted to 0.7% NaCl to mimic physiological conditions. 4 ml was used for the spiking experiments. Methanol, pure catechin, gallic acid and α-tocopherol (Sigma) in methanol and three unhydrolyzed and neutralized nut extracts were added separately at 1 and 5 μM polyphenol concentrations (Folin) to the LDL + VLDL and allowed to equilibrate at 37 °C for 2 h, before 2.5 ml was then put through a heparin-agarose (Sigma) lipoprotein affinity column and free polyphenol eluted with 0.7% NaCl. Polyphenol-bound LDL + VLDL was isolated by elution with 2.5% NaCl. After analysis of protein this latter eluate was then subjected to oxidation with cupric ion at 37 °C after dilution with PBS to a protein concentration of 70 μg ml−1, and the formation of conjugated dienes measured at 234 nm. Lag time was determined for the blank and samples. The concentration of pure polyphenols and catechin in the bound polyphenol sample was measured by HPLC using a C18 column and methanol/phosphoric acid 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 adjusted to pH 2.5 with potassium dihydrogen phosphate. Electrochemical detection at the optimum voltage was done by the ESA Coularray detector.
10 adjusted to pH 2.5 with potassium dihydrogen phosphate. Electrochemical detection at the optimum voltage was done by the ESA Coularray detector.
Per capita calculations
Per capita nut and other food consumption data was obtained from the USDA database using the most recent data from 2008.33 It was combined with our total polyphenol assay (average of raw and roasted) to estimate the per capita consumption of nuts in comparison to our fruits and vegetables estimates updated to 2008.34,35 For the European Union (EU), a 2007 market information database was used.36Results
Quantity of antioxidants
Results of free and total polyphenols for all the nut samples are displayed in Table 1. Walnuts were significantly higher in free and total polyphenols with raw and roasted data combined than all the other nuts (p < 0.002). Total polyphenols in nuts were significantly higher than free polyphenols for all nuts combined (p < 0.00001). The order for total polyphenols in raw nuts is the following: walnut > Brazil ≫ pistachio ∼ pecan ∼ peanut ∼ almond ≫ Macademia ≫ cashew ∼ hazelnut. The order of total polyphenols in roasted nuts is the following: walnut ≫ Brazil ≫ hazelnut > peanut ∼ pecan ∼ cashew > Macademia ∼ almond > pistachio. Total polyphenols in peanut butter were similar in the creamy and crunchy varieties and considerably lower than roasted Peanuts but the difference was not significant. Statistically total polyphenols for roasted walnut were higher than almond (p < 0.01) and Brazil was higher than almond (p < 0.05). Except for Brazil, walnut total polyphenols were significantly higher than all the other nuts (p < 0.02). There was no significant difference between free polyphenols in raw and roasted nuts. Total polyphenols were slightly higher in roasted than raw nuts. The % free polyphenols in the nuts and peanut butters ranged from 18.9% in roasted cashews to 90.0% in roasted hazelnuts. There was no significant difference between % free in raw and roasted nuts.| Nut | Raw | Roasted | ||
|---|---|---|---|---|
| Free | Total | Free | Total | |
| Almond (n = 11) | 34.8 ± 1.1 (34.0 to 36.0) | 48.0 ± 28.9 (27.5 to 68.4) | 36.8 ± 7.4 (25.7 to 47.8) | 44.8 ± 2.0 (29.2 to 74.8) |
| Brazil (n = 4) | 33.7 ± 0.0 (33.7) | 66.1 ± 8.8 (59.8 to 72.3) | 17.0 ± 0.8 (16.4 to 17.6) | 73.2 ± 8.5 (67.2 to 79.2) |
| Cashew (n = 7) | 21.0 ± 1.6 (17.9 to 22.8) | 28.6 ± 2.3 (25.9 to 30.2) | 19.3 ± 2.5 (17.9 to 22.1) | 49.3 ± 24.0 (26.4 to 71.6) |
| Hazelnut (n = 4) | 21.6 ± 0.6 (21.2 to 22.0) | 26.7 ± 2.0 (25.3 to 28.1) | 22.6 ± 0.6 (20.4 to 23.1) | 61.4 ± 43.6 (38.5 to 100) |
| Macademia (n = 4) | 15.0 ± 2.9 (12.9 to 17.0) | 39.1 ± 10.1 (31.9 to 46.2) | 11.1 ± 4.2 (8.1 to 14.0) | 45.1 ± 23.2 (28.6 to 61.5) |
| Peanut (n = 6) | 24.0 ± 4.1 (21.1 to 26.9) | 48.5 ± 9.4 (41.8 to 55.1) | 8.7 ± 5. (13.7 to 22.8) | 50.3 ± 24.0 (27.5 to 73.5) |
| Peanut Butter (Creamy) (n = 2) | N/A | N/A | 16.2 ± 0.4 (15.9 to 16.5) | 33.5 ± 7.6 (28.1 to 38.9) |
| Peanut Butter (Crunchy (n = 2) | N/A | N/A | 16.0 ± 0.8 (14.5 to 15.4) | 32.5 ± 1.8 (31.2 to 33.8) |
| Pecan (n = 4) | 22.3 ± 3.4 (19.9 to 24.7) | 49.9 ± 23.1 (33.5 to 66.2) | 16.6 ± 0.6 (16.2 to 17.0) | 50.0 ± 17.9 (37.4 to 62.7) |
| Pistachio (n = 6) | 15.8 ± 1.7 (14.6 to 17.0) | 51.9 ± 0.4 (51.6 to 52.1) | 19.9 ± 3.7 (15.4 to 20.8) | 39.7 ± 24.5 (25.3 to 76.1) |
| Walnut (n = 4) | 31.9 ± 6.9 (27.0 to 36.8) | 69.3 ± 16.5 (57.6 to 81.0) | 65.5 ± 9.1 (59.1 to 71.9) | 107 ± 12.1 (97.9 to 115) |
Antioxidant efficacy
The antioxidant efficacy of the different nuts is shown by the IC50 value in Fig. 1. The order of decreasing efficacy (increasing IC50) for raw nuts is walnut > cashew > hazelnut ∼ pecan ∼ almond ∼ Macademia > pistachio > Brazil > peanut. For roasted nuts and peanut butters the order is almond ∼ Macademia ∼ pecan ∼ peanut butter crunchy > peanut butter creamy > peanut > pistachio > hazelnut ∼ walnut > Brazil > cashew. Roasting causes a decline in efficacy; the average IC50 for raw nuts is 4.3 ± 2.1 μM and for roasted nuts (not including Peanut Butters) is 6.9 ± 3.9 μM. This is a 37% decrease but it is not quite significant, p = 0.14. In addition to the nuts, tocopherol and gallic acid which are both present in nuts, were tested and their IC50 values were 25.8 and 3.5 μM, respectively. Thus nuts on average had over 3 times better antioxidant efficacy than α-tocopherol, ranging from 15 times for raw walnut to 1.9 times for roasted cashew, the poorest antioxidant among the nuts. Raw walnut had the highest antioxidant efficacy among all the nuts. The phenol antioxidant index (PAOXI), a combined measure of quantity and efficacy, was highest for pecan and closely followed by walnut. Nuts average 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 which was higher than 23 vegetables, 9170,35 and lower than 20 fruits, 27
100 which was higher than 23 vegetables, 9170,35 and lower than 20 fruits, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400.34
400.34
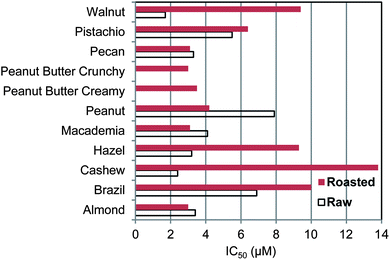 | ||
| Fig. 1 Antioxidant efficacy of nut and peanut butter polyphenols as measured by the concentration to inhibit the oxidation of LDL + VLDL by 50% compared to control with no added antioxidants. | ||
Lipoprotein binding
Results for the binding experiment are shown in Table 2. Catechin was found in all three of the unhydrolyzed nut samples and it was analyzed in the bound LDL + VLDL fraction. There is a dose-response effect for binding of nut polyphenols indicating that saturation of protein and nut binding sites has not occurred. This situation allows for valid binding comparisons to be made. With similar amounts of catechin bound for almond, walnut and peanut (∼2.5 nmol), the antioxidant effect as measured by the % increase in lag time vs. the control was much greater for walnut than the other nuts. Since binding of catechin was similar for the 3 nuts this is an indication that catechin did not interfere with the binding of other nut polyphenols (unidentified). The most accurate method to compare the lipoprotein-bound antioxidant activity is CLT50 which is the concentration to increase the lag time by 50% vs. the control. For almond it is 2.1 μM, peanut 5.6 μM and walnut 1.0 μM. Thus walnut-bound polyphenol antioxidants are twice as effective as almond and 5 times those of peanut.| Sample | Phenol concentration (μM) | LDL-bound polyphenol concentration (nmol/70 μg protein) | % Increase in lag time vs. control |
|---|---|---|---|
| Almond | 1 | 0.57 | 5.2 |
| Almond | 5 | 2.64 | 64.8 |
| Peanut | 1 | 0.60 | −13.6 |
| Peanut | 5 | 2.50 | 8.2 |
| Peanut | 10 | 5.40 | 48.3 |
| Walnut | 1 | 0.55 | 26.4 |
| Walnut | 5 | 2.37 | 118 |
Per capita consumption
Per capita consumption of nuts was calculated from nut consumption data and the total polyphenol concentration in roasted nuts except for Brazil and Walnut which are eaten raw. Brazil and Cashew consumption were assumed to be equal for our estimate of per capita polyphenol consumption in the USA in 2008/09. Total nut consumption (tree nuts and peanuts) in the USA was estimated at 12.9 g day−1 which is similar to the 2007 EU consumption of 11.1 g day−1. Peanuts comprised 65% of the nut consumption in the US and 45% in the EU. In the USA the total tree nut polyphenol consumption is an estimated 73.3 mg day−1 and peanuts including peanut butter 88.9 mg day−1 for a total nut contribution in the USA of 162 mg day−1. In the US, almonds were the largest tree nut source of polyphenols very closely followed by walnuts. The greater contribution of polyphenols from peanuts was due to the higher peanut consumption; a total of 7.5 g day−1 of peanuts vs. 4.4 g day−1 of tree nuts. Almonds were the most highly consumed tree nut at 1.6 g day−1 followed by walnuts and pecans at 0.6 g day−1. In the EU 5.0 g of peanuts per day were consumed, contributing 71.5 mg of polyphenols. Tree nuts totalled 86.9 mg of polyphenols and thus nut contribution of polyphenols to the EU diet amounted to 158 mg day−1, which was very similar to the US diet.Discussion
Quantity of antioxidants
In our assay, which includes raw and roasted nuts, walnuts were superior in antioxidant content to the other nuts. Six other published articles using several different assays and standards measured free polyphenols in raw nuts and four used Folin assays.37–42 Walnuts ranked first in 3/4 studies that used Folin and first in the two studies that used a FRAP antioxidant assay. Walnuts were first in both free and total polyphenols in the study that did a hydrolysis.39 Raw walnuts, on a serving size basis and using our assay provide the 7th largest amount of total polyphenols among common foods and beverages.Roasted nuts had 14% more total polyphenols than raw nuts and the % free polyphenols decreased. These two results indicate that hydrolysis took place during roasting. This conclusion agrees with studies on almonds and hazelnuts which showed an increase in antioxidant activity following roasting.43,44
Antioxidant efficacy
Our dose-response efficacy assay is equivalent to a comparison of nut polyphenols on a molecule to molecule basis and to other single molecule antioxidants with respect to their ability to react with free radicals. Roasting caused an average decline in efficacy, but the effect was not consistent as seen in Fig. 1. Some nuts decreased in efficacy and others increased during roasting. This is hypothesized to be due to chemical reactions which decrease the concentrations of some polyphenols and increase the concentration of others. This was seen in the aforementioned Hazelnut study in which gallic acid increased and procatechuic acid decreased during roasting.44 Walnuts had the highest antioxidant efficacy in our assay indicating their polyphenolic composition was of higher quality than the other nuts. Walnut extract at 1 μM (gallic acid equivalents) was previously found to inhibit the oxidation of LDL 84%.45 All nuts had more efficacious antioxidants than α-tocopherol. The normal non-supplemented human plasma levels of tocopherol range from 8 to 28 μM, and average 21μM.46 Thus at a possible and reasonable polyphenol plasma concentration of 0.1 to 1 μM walnuts may have appreciable plasma antioxidant activity in the presence of tocopherol since walnut's antioxidants have 15 times the efficacy of vitamin E. An in vivo post-prandial LDL antioxidant effect occurred after human consumption of pecans.25 Additionally another study showed that post-prandial oxidized LDL significantly decreased with walnut consumption with a high fat meal and endothelial function was also improved.47 This antioxidant effect is not due to the change in LDL fat composition since walnuts have the highest PUFA content of all the nuts and PUFA are the most oxidizable fats. Walnuts have the highest content of beneficial linoleic acid and α-linolenic acids among the nuts.48Linoleic acid is 3 times higher in walnuts than almonds and almonds have only trace levels of linolenic acid.49 These post-prandial results are evidence implicating nut polyphenols or their metabolites as in vivoantioxidants.There has been few published investigations of absorption and metabolism of nut polyphenols in humans. In a single dose human pecan study unmetabolized epigallocatechin gallate was found in the plasma and reached a maximum of 116 nM at 2 h post-consumption.25 In a comprehensive human study with almond skins monomeric polyphenols were poorly absorbed and appeared as phase II metabolites (glucuronides and sulfates).50 In this study it was found that almond skin polyphenols were 24% absorbed as measured by Folin polyphenols in the almond and Folin levels in urine pre- and post-consumption. Microbial-derived conjugated phenolic acid metabolites were the predominant compounds found in plasma and urine and were derived from degradation of monomers and polymeric flavonoids. Over 20 compounds were identified and all of them were conjugated. Our efficacy data in the present study was for aglycones. We have found ferulic acid glucuronide to have better antioxidant efficacy for LDL + VLDL oxidation than the parent compound ferulic acid (Vinson, unpublished results) and this was also the case for LDL oxidation.51 We have also found that ferulic acid glucuronide bound more strongly to LDL + VLDL than ferulic acid (Vinson, unpublished results). Binding to proteins represents one of the non-antioxidant mechanisms for polylphenols in vivo. The health benefits of nuts are not necessarily specifically related to their antioxidant activity. However one study showed a significant positive correlation between nut polyphenol content and cellular antiproliferative activity.39
Most recently there has been a supplementation study with mixed nuts (50% walnuts plus almonds and hazelnuts) to subjects with metabolic syndrome.52 Metabolomics using LC-MS found that urolithin A (glucuronides and sulfates) and serotonin metabolites were significant biomarkers of nut consumption. Urolithin A is a characteristic microbial-derived metabolite of walnut ellagitannins53 and was the most discriminative of the phenolics found in the urine. A thorough search of the literature revealed that only walnuts of the mixed nuts contained ellagitannins.54 Walnuts are also considered by the authors to be the source of the serotonin in the urine since walnuts are very high in serotonin.55 The same mixed nuts after supplementation to metabolic syndrome subjects produced an improvement in fasting insulin, insulin resistance and inflammatory markers.56
Lipoprotein binding
In our study, walnuts were superior lipoprotein-bound antioxidants to both peanuts and almonds. At similar catechin-bound concentrations for the nuts, walnuts had a 5-fold longer lag time than almonds, and peanuts had a negative effect of lag time vs. the control, i.e. pro-oxidant. At 2.6 nmol/70 μg protein walnut increased the lag time 2-fold compared to almond and 8-fold compared to peanut. Thus at presumably low physiological concentrations (nM to μM) walnuts would be better in vivoantioxidants than peanut or almond. Catechins have been found in plasma after pecan (human) and almond (hamster) consumption.25,57 We have shown previously that a wide variety of pure polyphenols and extracts from foods and beverages bind to LDL + VLDL.58Polyphenols from olive oil and red wine have been found bound to LDL after human consumption59,60 and thus nut polyphenols could be potent in vivoantioxidants. The binding of nut polyphenols to lipoproteins provides a mechanism for the heart-beneficial decrease in oxidized LDL after nut consumption, specifically for walnuts and almonds.6,47Per capita consumption
The US per capita consumption of vegetables and fruits has declined 13% and 6% since our estimation of polyphenol consumption during the 90's. In 2008 we estimate that 204 mg of polyphenols/day are provided by vegetables and 223 mg day−1 by fruits. Nuts provide a significant amount of polyphenols, 162 mg day−1. We have also analyzed total polyphenols in other foods such as grains, spices and oils and the total polyphenols from all food sources is conservatively estimated as 954 mg day−1 in 2008. In the USA, nuts provide 19% of total food polyphenols per day, see Fig. 2. In Spain nuts, including peanuts, provided 12% of free food polyphenols on a daily basis.61,62 A single serving (28.4 g) of raw walnuts would provide 575 mg of polyphenols which is greater than the daily sum of these antioxidants provided by fruits and vegetables combined.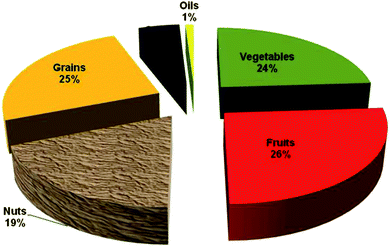 | ||
| Fig. 2 Daily per capita contribution of polyphenols from food sources to the US diet in 2008. | ||
Conclusions
Thus nuts are a major source of antioxidants in the US diet. Nuts are high in fiber, low in saturated fats, high in beneficial unsaturated fats and very high in antioxidants. Nuts are a nutritious snack and food additive providing both nutrients and bioactive antioxidants which provide significant health benefits to the consumer.References
- U.S. Department of Agriculture Nutrient Data Base at http://www.nal.usda.gov/fnic/cgi-bin/nut_search.pl, Accessed January 11, 2011.
- R. D. Mattes and M. L. Dreher, Nuts and healthy body weight maintenance mechanisms, Asia Pac. J. Clin. Nutr., 2010, 19, 137–141 Search PubMed.
- D. Zambón, J. Sabaté, S. Muñoz, B. Campero, E. Casals, M. Merlos, J. C. Laguna and E. Ros, Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial, Ann. Intern. Med, 2000, 132, 538–546 Search PubMed.
- J. Salas-Salvadó, J. Fernández-Ballart, E. Ros, M. A. Martínez-González, M. Fitó, R. Estruch, D. Corella, M. Fiol, E. Gómez-Gracia, F. Arós, G. Flores, J. Lapetra, R. Lamuela-Raventós, V. Ruiz-Gutiérrez, M. Bulló, J. Basora and M. I. Covas, Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. PREDIMED Study Investigators, Arch. Intern. Med., 2008, 168, 2449–2458 CrossRef.
- M. Bes-Rastrollo, N. M. Wedick, M. A. Martinez-Gonzalez, T. Y. Li, L. Sampson and F. B. Hu, Prospective study of nut consumption, long-term weight change, and obesity risk in women, Am. J. Clin. Nutr., 2009, 89, 1913–1919 CrossRef CAS.
- D. J. A. Jenkins, C. W. Kendall, A. Marchie, T. L. Parker, P. W. Connelly, W. Qian, J. S. Haight, D. Faulkner, E. Vidgen, K. G. Lapsley and G. A. Spiller, Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial, Circulation, 2002, 106, 1327–1332 CrossRef CAS.
- D. J. Jenkins, C. W. Kendall, D. A. Faulkner, T. Nguyen, T. Kemp, A. Marchie, J. M. Wong, R. de Souza, A. Emam, E. Vidgen, E. A. Trautwein, K. G. Lapsley, C. Holmes, R. G. Josse, L. A. Leiter, P. W. Connelly and W. Singer, Assessment of the longer-term effects of a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia, Am. J. Clin. Nutr, 2006, 83, 582–591 CAS.
- S. M. Mercanligil, P. Arslan, C. Alasalvar, E. Okut, E. Akgül, A. Pinar, P. O. Geyik, L. Tokgözoğlu and F. Shahidi, Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men, Eur. J. Clin. Nutr, 2007, 61212–61220 Search PubMed . Epub 2006.
- S. L. Tey, R. C. Brown, A. W. Chisholm, C. M. Delahunty, A. R. Gray and S. M. Williams, Effects of different forms of hazelnuts on blood lipids and α-tocopherol concentrations in mildly hypercholesterolemic individuals, Eur. J. Clin. Nutr., 2011, 65, 117–124 CrossRef CAS.
- A. E. Griel, D. D. CaoBagshaw, A. M. Cifelli, B. Holub and P. M. Kris-Etherton, A Macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women, J. Nutr., 2008, 138, 761–767 CAS.
- S. M. Mercanligil, P. Arslan, C. Alasalvar, E. Okut, E. Akgül, A. Pinar, P. O. Geyik, L. Tokgözoğlu and F. Shahidi, Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men, Eur. J. Clin. Nutr., 2007, 61, 212–220 CrossRef CAS.
- N. M. Ghadimi, A. Kimiagar, A. Abadi, M. Mirzazadeh and G. Harrison, Peanut consumption and cardiovascular risk, Public Health Nutr., 2010, 13, 1581–1586 CrossRef . Epub 2009.
- A. Kocyigit, A. A. Koylu and H. Keles, Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers, Nutr., Metab. Cardiovasc. Dis., 2006, 16, 202–209 CrossRef CAS.
- S. K. Gebauer, S. G. West, C. D. Kay, P. Alaupovic, D. Bagshaw and P. M. Kris-Etherton, Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study, Am. J. Clin. Nutr, 2008, 88, 651–659 CAS.
- Z. Li., R. Song, C. Nguyen, A. Zerlin, H. Karp, K. Naowamondhol, G. Thames, K. Gao, L. Li, C. H. Tseng, S. M. Henning and D. Heber, Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program, J. Am. Coll. Nutr, 2010, 29, 198–203 CAS.
- M. Iwamoto, K. Imaizumi, M. Sato, Y. Hirooka, K. Sakai, A. Takeshita and M. Kono, Serum lipid profiles in Japanese women and men during consumption of walnuts, Eur. J. Clin. Nutr., 2002, 56, 629–637 CrossRef CAS.
- D. Zambón, J. Sabaté, S. Muñoz, B. Campero, E. Casals, M. Merlos, J. C. Laguna and E. Ros, Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial, Ann. Intern. Med, 2000, 132, 538–546 Search PubMed . Erratum in: Ann. Intern. Med. 2000.
- M. Iwamoto, K. Imaizumi, M. Sato, Y. Hirooka, K. Sakai, A. Takeshita and M. Kono, Serum lipid profiles in Japanese women and men during consumption of walnuts, Eur. J. Clin. Nutr., 2002, 56, 629–637 CrossRef CAS.
- B. Olmedilla-Alonso, F. Granado-Lorencio, C. Herrero-Barbudo, I. Blanco-Navarro, S. Blázquez-García and B. Pérez-Sacristán, Consumption of restructured meat products with added walnuts has a cholesterol-lowering effect in subjects at high cardiovascular risk: a randomised, crossover, placebo-controlled study, J. Am. Coll. Nutr, 2008, 27, 342–348 CAS.
- R. Estruch, M. A. Martínez-González, D. Corella, J. Salas-Salvadó, V. Ruiz-Gutiérrez, M. I. Covas, M. Fiol, E. Gómez-Gracia, M. C. López-Sabater, E. Vinyoles, F. Arós, M. Conde, C. Lahoz, J. Lapetra, G. Sáez and E. Ros, PREDIMED Study Investigators Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial, Ann. Intern. Med, 2006, 4145, 1–11 Search PubMed.
- P. M. Kris-Etherton, S. Yu-Poth, J. Sabaté, H. E. Ratcliffe, G. Zhao and T. D. Etherton, Nuts and their bioactive constituents: effects on serum lipids and other factors that affect disease risk, Am. J. Clin. Nutr, 1999, 70, 504S–511S CAS.
- D. J. Jenkins, C. W. Kendall, A. R. Josse, S. Salvatore, F. Brighenti, L. S. Augustin, P. R. Ellis, E. Vidgen and A. V. Rao, Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals, J. Nutr, 2006, 136, 2987–2992 CAS.
- J. Mukuddem-Petersen, W. S. Oosthuizen, J. C. Jerling, S. M. Hanekom and Z. White, Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial, Br. J. Nutr., 2007, 97, 1144–1153 CrossRef CAS.
- I. Sari, Y. Baltaci, C. Bagci, V. Davutoglu, O. Erel, H. Celik, O. Ozer, N. Aksoy and M. Aksoy, Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study, Nutrition, 2010, 26, 399–404 CrossRef CAS , Epub 2009.
- C. Hudthagosol, E. H. Haddad, K. McCarthy, P. Wang, K. Oda and J. Sabaté, Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans, J. Nutr., 2011, 141, 56–62 CrossRef CAS.
- M. Fitó, M. Guxens, D. Corella, G. Sáez, R. Estruch, R. de la Torre, F. Francés, C. Cabezas, M. C. López-Sabater, J. Marrugat, A. García-Arellano, F. Arós, V. Ruiz-Gutierrez, E. Ros, J. Salas-Salvadó, M. Fiol, R. Solá and M. I. Covas, for the PREDIMED Study Investigators, Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial, Arch. Intern. Med., 2007, 167, 1195–1203 CrossRef.
- D. L. McKay, C. Y. Chen, K. J. Yeum, N. R. Matthan, A. H. Lichtenstein and J. B. Blumberg, Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study, Nutr. J., 2010, 9, 21 CrossRef.
- P. López-Uriarte, M. Bulló, P. Casas-Agustench, N. Babio and J. Salas-Salvadó, Nuts and oxidation: a systematic review, Nutr. Rev., 2009, 67, 497–508 CrossRef.
- J. M. Harnly, R. F. Doherty, G. R. Beecher, J. M. Holden, D. B. Haytowitz, S. Bhagwat and S. Gebhardt, Flavonoid content of U.S. fruits, vegetables, and nuts, J. Agric. Food Chem., 2006, 54, 9966–9977 CrossRef CAS.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, Analysis of total phenols and other oxidation substrates by means of Folin-Ciocalteu reagent, Methods Enzymol., 1999, 299, 152–178 CAS.
- J. A. Vinson, J. Proch and P. Bose, Determination of quantity and quality of polyphenol antioxidants in foods and beverages, Methods Enzymol., 2001, 335, 103–114 CAS.
- J. A. Vinson and B. A. Hontz, Antioxidant Effectiveness of Red and White Wines, J. Agric. Food Chem., 1995, 43, 401–403 CrossRef CAS.
- Economic Research Service (ERS), U.S. Department of Agriculture (USDA). Food Availability (Per Capita) Data System. http://www.ers.usda.gov/Data/FoodConsumption, Accessed January 11, 2011.
- J. A. Vinson, X. Su, L. Zubik and P. Bose, Phenol antioxidant quantity and quality in foods: fruits, J. Agric. Food Chem., 2001, 49, 5315–5321 CrossRef CAS.
- J. A. Vinson, Y. Hao, X. Su and L. S. Zubik, Phenol antioxidant quantity and quality in foods: vegetables, J. Agric. Food Chem., 1998, 46, 3630–3634 CrossRef CAS.
- CBI Market Survey: The Edible Nuts and Dried Fruit and Vegetables Market in the EU, Centre for the Promotion of imports from developing countries, Rotterdam, The Netherlands, 2009 Search PubMed.
- M. Kornsteiner, K.-H. Wagner and I. Elmadfa, Tocopherols and total phenolics in 10 different nut types, Food Chem., 2006, 98, 381–387 CrossRef CAS.
- N. Pellegrini, M. Serafini, S. Salvatore, D. Del Rio, M. Bianchi and F. Brighenti, Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays, Mol. Nutr. Food Res., 2006, 50, 1030–1038 CAS.
- J. Yang, R. H. Liu and L. Halim, Antioxidant and antiproliferative activities of common edible nut seeds, LWT–Food Sci. Technol., 2009, 42, 1–8 CrossRef CAS.
- X. Wu, G. R. Beecher, J. M. Holden, D. B. Haytowitz, S. E. Gebhardt and R. L. Prior, Lipophilic and hydrophilic antioxidant capacities of common foods in the United States, J. Agric. Food Chem., 2004, 52, 4026–4037 CrossRef CAS.
- R. Blomhoff, M. H. Carlsen, L. F. Andersen and D. R. Jacobs Jr, Health benefits of nuts: potential role of antioxidants, Br. J. Nutr., 2006, 96(Suppl. 2), S52–60 CrossRef CAS.
- B. J. Macfarlane, W. R. Bezwoda, T. H. Bothwell, R. D. Baynes, J. E. Bothwell, A. P. MacPhail, R. D. Lamparelli and F. Mayet, Inhibitory effect of nuts on iron absorption, Am. J. Clin. Nutr, 1988, 47, 270–274 CAS.
- I. Garrido, M. Monagas, C. Gómez-Cordovés and B. J. Bartolomé, Polyphenols and antioxidant properties of almond skins: influence of industrial processing, J. Food Sci., 2008, 73, C106–115 CrossRef CAS.
- V. Schmitzer, A. Slatnar, R. Veberic, F. Stampar and A. Solar, Roasting Affects Phenolic Composition and Antioxidative Activity of Hazelnuts (Corylus avellana L.), J. Food Sci., 2011, 76, S14–19 CrossRef CAS.
- K. J. Anderson, S. S. Teuber, A. Gobeille, P. Cremin, A. L. Waterhouse and F. M. Steinberg, Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation, J. Nutr, 2001, 131, 2837–2842 CAS.
- A. C. Chang, Normal plasma vitamin E concentration, Biochem. J, 1989, 260, 623 Search PubMed.
- B. Cortés, I. Núñez, M. Cofán, R. Gilabert, A. Pérez-Heras, E. Casals, R. Deulofeu and E. Ros, Acute effects of high-fat meals enriched with Walnuts or olive oil on postprandial endothelial function, J. Am. Coll. Cardiol., 2006, 48, 1666–1671 CrossRef.
- E. Ros and J. Mataix, Fatty acid composition of nuts–implications for cardiovascular health, Br. J. Nutr., 2006, 96(Suppl. 2), S29–35 CrossRef CAS.
- S. K. Sathe, N. P. Seeram, H. H. Kshirsagar, D. Heber and K. A. Lapsley, Fatty acid composition of California grown almonds, J. Food Sci., 2008, 773, C607–614 CrossRef.
- M. Urpi-Sarda, I. Garrido, M. Monagas, C. Gómez-Cordovés, A. Medina-Remón, C. Andres-Lacueva and B. Bartolomé, Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans, J. Agric. Food Chem., 2009, 57, 10134–42 CrossRef CAS.
- T. Ohta, T. Nakano, Y. Egashira and H. Sanada, Antioxidant activity of ferulic acid beta-glucuronide in the LDL oxidation system, Biosci., Biotechnol., Biochem., 1997, 61, 1942–3 CrossRef CAS.
- S. Tulipani, R. Llorach, O. Jáuregui, P. López-Uriarte, M. Garcia-Aloy, M. Bullo, J. Salas-Salvadó and C. Andrés-Lacuaeva, Metabolomics unveils urinary change in subjects with metabolic Syndrome, J. Proteome Res., 2011, 10, 5047–8 CrossRef CAS.
- B. Cerdá, F. A. Tomás-Barberán and J. C. Espín, Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability, J. Agric. Food Chem., 2005, 53, 227–35 CrossRef.
- L. Tieme, F. M. Lajola and M. I. Genovese, Comparison of polyphenol content and antioxidant capacity of nuts, Cienc. Tecnol. Aliment., 2010, 30, 254–9 CrossRef.
- L. Kema, A. Schellings, G. Meiborg, C. Hoppenbrouwers and F. Muskiet, Influence of a serotonin- and dopamine-rich diet on platelet serotonin content and urinary excretion of biogenic amines and their metabolites, Clin. Chem, 1992, 38, 1730–6 Search PubMed.
- F. Casas-Agustench, F. López-Uriarte, M. Bulló, E. Ros, J. J. Cabré-Villa and J. Salas-Salvadó, Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with metabolic syndrome, Nutr., Metab. Cardiovasc. Dis., 2011, 21, 126–35 CrossRef.
- C. Y. Chen, P. E. Milbury, K. Lapsley and J. B. Blumberg, Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation, J. Nutr, 2005, 135, 1366–1373 CAS.
- J. A. Vinson, J. Jang, Y. A. Dabbagh, M. S. Serry and S. Cai, Plant polyphenols exhibit lipoprotein-bound antioxidant activity using an in vitro oxidation model for heart disease, J. Agric. Food Chem., 1995, 43, 2798–2799 CrossRef CAS.
- M. I. Covas, K. de la Torre, M. Farré-Albaladejo, J. Kaikkonen, M. Fitó, C. López-Sabater, M. A. Pujadas-Bastardes, J. Joglar, T. Weinbrenner, R. M. Lamuela-Raventós and R. de la Torre, Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans, Free Radical Biol. Med., 2006, 40, 608–616 CrossRef CAS.
- M. Urpí-Sardà, O. Jáuregui, R. M. Lamuela-Raventós, W. Jaeger, M. Miksits, M. I. Covas and C. Andres-Lacueva, Uptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry, Anal. Chem., 2005, 77, 3149–3155 CrossRef.
- M. I. Covas, K. de la Torre, M. Farré-Albaladejo, J. Kaikkonen, M. Fitó, C. López-Sabater, M. A. Pujadas-Bastardes, J. Joglar, T. Weinbrenner, R. M. Lamuela-Raventós and R. de la Torre, Nut consumption in Spain and other countries, Br. J. Nutr, 2006, 96(Suppl. 2), S-3–11 Search PubMed.
- F. Saura-Calixto and I. Goni, Antioxidant capacity of the Spanish Mediterranean diet, Food Chem., 2006, 94, 442–47 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
