Open Access Article
Open Access ArticleRuthenium or osmium? On the role of the metal in carbonylchlorido complexes for photodynamic therapy
Gina Elena Giacomazzoa,
Fortuna Ponteb,
Valentina Ceccherinia,
Lorenzo Gianassia,
Gloria Mulasc,
Emilia Sicilia b,
Francesca Cencetti
b,
Francesca Cencetti c,
Barbara Valtancolia,
Luca Conti
c,
Barbara Valtancolia,
Luca Conti *a and
Claudia Giorgi
*a and
Claudia Giorgi *a
*a
aDepartment of Chemistry “Ugo Schiff”, University of Florence, Via della Lastruccia 3, 50019, Sesto Fiorentino (FI), Italy. E-mail: luca.conti@unifi.it; claudia.giorgi@unifi.it
bDepartment of Chemistry and Chemical Technologies University of Calabria, via Pietro Bucci, 87036 Arcavacata Rende, Cs, Italy
cDepartment of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Viale Morgagni 50, Florence 50134, Italy
First published on 13th January 2026
Abstract
Two novel carbonylchlorido complexes for photodynamic therapy (PDT), RuCOCl and OsCOCl, were synthesized via a unified synthetic strategy. Comparative photophysical, computational and preliminary biological studies reveal the crucial influence of the metal in governing their PDT performance, highlighting the potential of this largely underestimated class of complexes for PDT applications.
Ruthenium(II) polypyridyl complexes (RPCs) have long stood as the photosensitizers (PSs) of choice as inorganic compounds for photodynamic therapy (PDT),1–5 mainly thanks to the rich chemical-physical repertoire, encompassing tunable photophysical properties, efficient singlet oxygen (1O2) generation and interaction with key biological targets.6,7 However, in recent years osmium(II)-based complexes have emerged as a promising alternative,8–11 offering superior properties in terms of red-shifted absorptions,10,12 spin–orbit coupling and chemical stability.11,13 Yet, despite these advantages, their actual potential over Ru(II) analogues still remains largely unexplored, owing to the limited number of direct comparative investigations on the role of the metal in translating these features into tangible benefits for PDT.
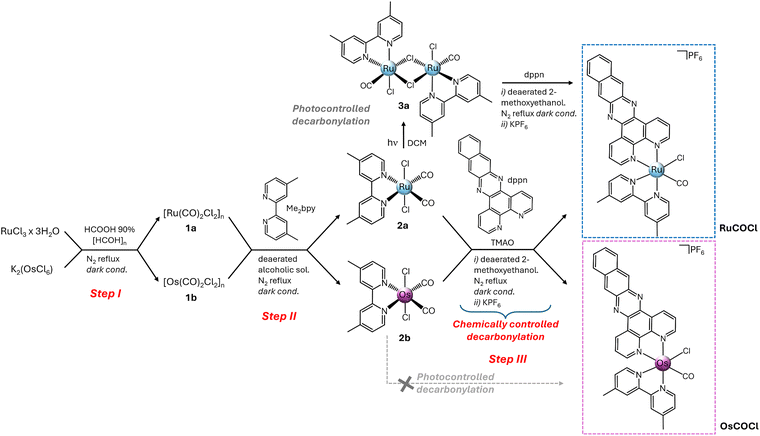
Prompted by this scenario, herein we present a comparative study of two novel Ru(II) and Os(II) carbonylchlorido complexes, namely RuCOCl and OsCOCl, featuring the polypyridyl ligands 4,4′-dimethyl-2,2′-bipyridine (Me2bpy) and benzo[i]dipyrido[3,2-a:2′,3′-c]phenazine (dppn) (Scheme 1). Although carbonylchlorido complexes are well known for different applications, spanning from carbon dioxide reduction14–16 to hydrogen production,17,18 their use in PDT remains, to the best of our knowledge, unexplored, leaving an important gap to be fulfilled. Incorporation of the dppn ligand in the two complexes is intended to enhance their PDT potential by enabling long-lived 3IL (3ππ*) states for 1O2 sensitization, red-shifting the absorption and facilitating membrane permeation.19–21 From a synthetic point of view, among the various strategies for the preparation of heteroleptic Ru(II) carbonylchlorido complexes [Ru(NN)(N′N′)COCl]+ (NN and N′N′ = different polypyridyl chelates), one of the most straightforward was described by Spiccia et al.,22 involving a key photodecarbonylation step of a dicarbonyl [Ru(NN)(CO)2Cl2] intermediate (Scheme S1, SI). However, this route is not straightforward for Os(II), owing to higher-energy d-d states and stronger CO back-bonding that hinder photodecarbonylation.23 Alternative methods replacing this step with chloride-triflate exchange have indeed been explored, although they lead to the dicarbonyl products rather than the desired carbonylchlorido analogues (Scheme S2, SI).24 The different nature of the two metals, therefore, makes a unified synthetic method a non-trivial task yet essential to compare their reactivity. To this end, we developed a new strategy, applicable to both metals, for the preparation of analogous carbonylchlorido complexes (Scheme 1).
As shown, and described in detail in the SI (paragraph 1), the first step afforded the polymeric precursors [Ru(CO)2Cl2]n (1a) and [Os(CO)2Cl2]n (1b) by refluxing RuCl3·3H2O with formic acid and paraformaldehyde, in line with the literature for 1a22 and with slight modifications from Keene et al. for 1b.24 These showed similar reactivity in step II, coordinating Me2bpy in alcoholic solvent to give [Ru(Me2bpy)(CO)2Cl2] (2a) and [Os(Me2bpy)(CO)2Cl2] (2b), both of them showing cis-dicarbonyl trans-dichloro configuration, according to the literature and in line with the C2v molecular symmetries of NMR analysis (Fig. S1 and S2, SI).22,24 RuCOCl and OsCOCl were then obtained by chemical decarbonylation of 2a and 2b, which were reacted with dppn in 2-methoxyethanol in the presence of trimethylamine N-oxide (TMAO) as a decarbonylating agent, leading to the final complexes as hexafluorophosphate salts, following KPF6 precipitation (step III). The overall yield over three synthetic steps was 29% for RuCOCl and 18% for OsCOCl. Ru(II) and Os(II) complexes were obtained as nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of trans-CO and trans-Cl isomers, as shown by NMR data and consistent with the literature (Fig. S3, S5, S6, S8, SI).24,25 HR-(ESI) MS analysis further confirmed the identity of the compounds, as denoted by the isotopic patterns of the mono positively charged [RuCOCl-PF6]+ and [OsCOCl-PF6]+, centered at 681.07566 and 771.13319 (m/z = 1) (Fig. S4, S7, SI). Isomer separation was not achievable chromatographically; however, a trans-CO enriched mixture (67
1 mixtures of trans-CO and trans-Cl isomers, as shown by NMR data and consistent with the literature (Fig. S3, S5, S6, S8, SI).24,25 HR-(ESI) MS analysis further confirmed the identity of the compounds, as denoted by the isotopic patterns of the mono positively charged [RuCOCl-PF6]+ and [OsCOCl-PF6]+, centered at 681.07566 and 771.13319 (m/z = 1) (Fig. S4, S7, SI). Isomer separation was not achievable chromatographically; however, a trans-CO enriched mixture (67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33), referred to as OsCOCl-67, was obtained by exploiting their different solubilities in acetone and dichloromethane (Fig. S8, SI); this sample was also included in the chemical-physical analysis, shown in Fig. 1.
33), referred to as OsCOCl-67, was obtained by exploiting their different solubilities in acetone and dichloromethane (Fig. S8, SI); this sample was also included in the chemical-physical analysis, shown in Fig. 1.
The introduction of a controlled chemical decarbonylation in step III was crucial to give OsCOCl, since 2b does not undergo photodecarbonylation unlike its Ru(II) analogue, and therefore, to provide a common synthetic pathway for both the final complexes. The difference in reactivity of dicarbonyl intermediates, clearly imparted by the nature of the metal, was further investigated by density functional theory (DFT) and time-dependent density functional theory (TDDFT) calculations, performed on 2a and 2b in both the ground and excited state. All calculations, including geometry optimizations, relaxed scan analyses, UV-visible spectral simulations, and natural bond orbital (NBO) analysis, were performed at the B3PW91 level of theory and are described in detail in paragraph 5.1, SI. Our data showed that, when irradiated, the CO release from the Ru(II)-based 2a occurs with a low activation barrier (2.1 kcal mol−1) following an exergonic reaction, whereas for 2b the energy barrier is higher (17 kcal mol−1) and the process is endergonic. Thus, CO dissociation is both kinetically and thermodynamically favoured for the Ru(II) intermediate but disfavoured for its Os(II) analogue (Fig. S13, SI). This, along with the NBO analysis which revealed a weaker LP(Ru) → π(CO)* back-donation compared to Os(II), corroborated the observed higher inertness of the Os(II)-CO bond in 2b and the consequent need for a more energetic decarbonylation reaction.
Absorption and emission spectra of the complexes in acetonitrile and water are shown in Fig. 1; their molar extinction coefficients (ε), fluorescence maxima (λem) and fluorescence emission quantum yields (φL) are listed in Table 1. Of particular relevance in the UV-vis spectra is the panchromatic 1MLCT absorption of OsCOCl, with a tail extending beyond 550 nm, better matching the therapeutic window. This effect, which represents a key reason for the strong appeal of Os(II) complexes in PDT,26,27 is even more evident in water (Fig. 1b). Fluorescence emissions are overall moderate, consistent with the population of low-lying triplet states of mixed ligand centered/MLCT character (Fig. 1d), with a significant contribution from poorly emissive, dppn-centered 3LC (3ππ*) states (see also paragraph 5, SI). Pronounced solvent-dependent differences were found between the two complexes. In acetonitrile, OsCOCl exhibits the strongest emission (λem 550 nm), while RuCOCl is ∼7-fold less emissive, as also witnessed by ϕL values of 15.6(6) × 10−3 and 2.24(9) × 10−3, respectively (Table 1 and Table S1, SI). Water markedly affects the luminescence signals, with OsCOCl being almost completely quenched and RuCOCl retaining a residual signal. Interestingly enough, the trans-CO enriched OsCOCl-67 is almost non-emissive compared to OsCOCl (Fig. 1a), hinting at a possible influence of the CO ligand geometry in the balance between radiative and non-radiative decay pathways.28,29
| Complex | λmax/nm (ε × 103/M−1 cm−1) in CH3CN | λmax/nm (ε × 103/M−1 cm−1) in H2O | λem/nm in CH3CN | λem/nm in H2O | ΦL CH3CN (× 10−3) | ΦL H2O (× 10−3) | ΦΔ 1O2 CH3CN |
|---|---|---|---|---|---|---|---|
| RuCOCl | 260 (52.6), 312 (53.5), 396 (8.09), 408 (8.06) | 262 (45.6), 312 (45.0), 396 (6.64), 408 (5.82) | 571 | 614 | 2.24 ± 0.09 | 4.3 ± 0.2 | 0.60 ± 0.06 |
| OsCOCl | 240 (36.1), 262 (37.1), 321 (55.6), 395 (9.15), 412 (8.94) | 265 (31.8), 321 (36.4), 395 (11.5), 412 (10.5) | 550 | — | 15.6 ± 0.6 | 0.87 ± 0.06 | 0.88 ± 0.06 |
As a fundamental prerequisite for their PDT application, the singlet oxygen quantum yields (ΦΔ) of the metal complexes were determined by direct measurement of the 1O2 phosphorescence at 1270 nm upon irradiation of air-saturated acetonitrile solutions, a more reliable approach compared to the use of indirect probes, often sensitive to other ROS species.30 Results for OsCOCl are reported in Fig. 1c, with those for the other compounds in paragraph 2 (SI); ΦΔ values were determined as previously described31,32 using Ru(phen)3Cl2 as a standard (ΦΔ = 0.38 ± 0.06)33 and are listed in Table 1. As shown, all complexes display strong sensitizing properties, with OsCOCl being almost 1.5-fold more efficient than RuCOCl (ΦΔ = 0.88 ± 0.06 vs. ΦΔ = 0.60 ± 0.06). The different ability to sensitize 1O2 conferred by the two metals was further investigated by DFT and TDDFT calculations. Detailed information is reported in paragraph 5.2, SI. As shown in the Jablonski-like diagram of Fig. 1d, our results indicated significantly higher spin–orbit coupling (SOC) for OsCOCl, ∼ 10 times higher relative to Ru(II), in line with the stronger spin–orbit coupling/heavy-atom effect of the former and therefore well corroborating the experimental findings.
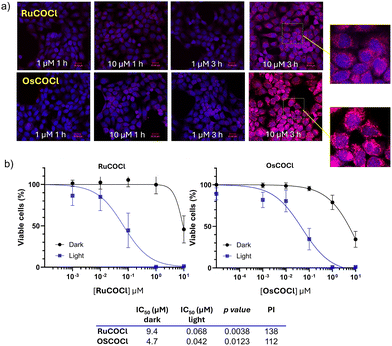
Preliminary to the biological tests, the stability of the complexes in PBS solutions was assessed by UV-Vis spectroscopy. The absence of appreciable spectral variations in the dark over a total period of 24 hours demonstrated a remarkable stability under these conditions (Fig. S10, SI). Their photoreactivity was also evaluated spectrophotometrically in PBS and acetonitrile to monitor their structural integrity under prolonged LED irradiation (λmax 462 nm, 160 mW, time up to 2 h). These experiments, complemented by HR ESI-MS analysis (Fig. S11 and S12, paragraph 4, SI), indicated a tendency, particularly pronounced for RuCOCl, for CO release/partial release upon extended photoactivation, also resulting in a detrimental effect on singlet oxygen sensitization. However, this effect remains negligible within the reduced irradiation duration of the PDT biological assays (vide infra). Yet, the investigation of these complexes as suitable photoCORMs represents an interesting aspect that will be explored in future studies. Focusing on the remarkable PDT potential of RuCOCl and OsCOCl, the biological behaviour of the complexes was preliminarily evaluated in A431 vulvar carcinoma cells, chosen as a human cancer model. The internalization of the complexes was first evaluated by confocal laser scanning fluorescence microscopy (CLSM), quantified by inductively coupled plasma mass spectrometry (ICP-MS) and then followed by evaluation of their photodynamic effect by MTT analysis (see SI for the experimental details). As shown by CLSM imaging (Fig. 2a), both RuCOCl and OsCOCl were promptly and efficiently internalized in tumoral cells, showing a marked time- and concentration-dependent uptake (1 to 3 h, 1–10 µM).
ICP-MS quantification confirmed intracellular metal contents of approximately 200 ng of Ru and 450 ng of Os per 106 cells after 3 h incubation at 10 µM (Fig. S14, SI). Notably, both complexes tended to accumulate within the cytoplasmic region surrounding the DAPI-stained nuclei. In the dark, MTT assays (Fig. 2b) showed that RuCOCl was essentially non-toxic up to 1 µM, whereas OsCOCl caused a moderate decrease in cell viability of 20% at the same concentration. Upon 15 min photoactivation (λmax = 462 nm, 160 mW), both compounds exhibited pronounced cytotoxic activity, consistent with their strong photosensitizing properties, resulting in a marked shift of the IC50 values from the micromolar to the sub-micromolar range (Fig. 2b). Interestingly, despite OsCOCl's superior photophysical properties, RuCOCl exhibited the highest photoreactivity index (PI = 138), while the lower PI value of OsCOCl (ca. 112) likely reflects its higher dark cytotoxicity.
In summary, the aim of this comparative study is to highlight the potential of the two novel carbonylchlorido complexes RuCOCl and OsCOCl for PDT, beyond shedding light on the role of the metal in shaping their properties as effective photosensitizers. Following the synthesis, accomplished by introducing a central chemically controlled decarbonylation of the dicarbonylic precursors, our data evidenced the favourable features of RuCOCl and OsCOCl for their PDT application, in particular those inferred by Os(II), namely red-shifted absorption profiles and augmented emissive and sensitization properties compared to its lighter congener. DFT and TDDFT calculations were also undertaken to corroborate the experimental findings. Lastly, preliminary in vivo investigations confirmed the perspectives of these systems in the PDT of vulvar carcinoma, although the higher in the dark cytotoxicity of OsCOCl partially counteracts its superior photophysical properties.
Which metal therefore performs better? Clearly, there is no simple answer; the choice of metal has to be rather considered alongside the overall complex/drug design, taking into account the careful balance between reactivity, photophysical and biological properties of this largely underestimated class of potential PDT agents.
Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: detailed experimental procedures, additional tables and figures. See DOI: https://doi.org/10.1039/d5cc06225j.Acknowledgements
G. E. G. and V. C. respectively thank Fondazione Umberto Veronesi and the Next Generation EU Project B12B23000300006, for the financial support.References
- J. Karges, Angew. Chem., Int. Ed., 2022, 61, e202112236 CrossRef CAS PubMed.
- L. Conti, E. Macedi, C. Giorgi, B. Valtancoli and V. Fusi, Coord. Chem. Rev., 2022, 469, 214656 CrossRef CAS.
- X. Y. Ng, K. W. Fong, L. V. Kiew, P. Y. Chung, Y. K. Liew, N. Delsuc, M. Zulkefeli and M. L. Low, J. Inorg. Biochem., 2024, 250, 112425 CrossRef CAS PubMed.
- Y. Xu, C. Li, S. Lu, Z. Wang, S. Liu, X. Yu, X. Li and Y. Sun, Nat. Commun., 2022, 13, 2009 CrossRef CAS PubMed.
- M. Li, J. Xiong, Y. Zhang, L. Yu, L. Yue, C. Yoon, Y. Kim, Y. Zhou, X. Chen, Y. Xu, X. Peng and J. S. Kim, Chem. Soc. Rev., 2025, 54, 7025 RSC.
- Y.-A. Deng, S.-J. Tang, M.-F. Wang, X. Ren, X.-L. Li, L.-Z. Zeng, D.-N. Ren, M.-R. Wang, W.-L. Xiao, Z.-Y. Cai, D. Zhang, H. Zhang and F. Gao, Inorg. Chem. Front., 2023, 10, 4552–4561 RSC.
- L. Conti, G. E. Giacomazzo, B. Valtancoli, M. Perfetti, A. Privitera, C. Giorgi, P. S. Sfragano, I. Palchetti, S. Pecchioli, P. Bruni and F. Cencetti, Int. J. Mol. Sci., 2022, 23, 13302 CrossRef CAS PubMed.
- K. M. Kuznetsov, K. Cariou and G. Gasser, Chem. Sci., 2024, 15, 17760–17780 RSC.
- S. M. Meier-Menches, C. Gerner, W. Berger, C. G. Hartinger and B. K. Keppler, Chem. Soc. Rev., 2018, 47, 909–928 RSC.
- A. Mani, T. Feng, A. Gandioso, R. Vinck, A. Notaro, L. Gourdon, P. Burckel, B. Saubaméa, O. Blacque, K. Cariou, J.-E. Belgaied, H. Chao and G. Gasser, Angew. Chem., Int. Ed., 2023, 135, e202218347 CrossRef.
- M. Stitch, R. Z. Boota, A. S. Chalkley, T. D. Keene, J. C. Simpson, P. A. Scattergood, P. I. P. Elliott and S. J. Quinn, Inorg. Chem., 2022, 61, 14947–14961 CrossRef CAS PubMed.
- B. Carlson, G. D. Phelan, W. Kaminsky, L. Dalton, X. Jiang, S. Liu and A. K. Y. Jen, J. Am. Chem. Soc., 2002, 124, 14162–14172 CrossRef CAS PubMed.
- A. Gul, M. Ahmad, A. Ali, D. Boateng, L. Yang, Y. Kang and W. Liao, Colloids Surf., B, 2025, 255, 114935 CrossRef CAS PubMed.
- H. Ishida, K. Fujiki, T. Ohba, K. Ohkubo, K. Tanaka, T. Terada and T. Tanaka, J. Chem. Soc., Dalton Trans., 1990, 2155–2160 RSC.
- J.-M. Lehn and R. Ziessel, J. Organomet. Chem., 1990, 382, 157–173 CrossRef CAS.
- M. N. Collomb-Dunand-Sauthier, A. Deronzier and R. Ziessel, J. Phys. Chem., 1993, 97, 5973–5979 CrossRef CAS.
- H. Ishida, H. Tanaka, K. Tanaka and T. Tanaka, J. Chem. Soc., Chem. Commun., 1987, 131–132 RSC.
- K. Tanaka, M. Morimoto and T. Tanaka, Chem. Lett., 1983, 901–904 CrossRef CAS.
- S. P. Foxon, C. Metcalfe, H. Adams, M. Webb and J. A. Thomas, Inorg. Chem., 2007, 46(2), 409–416 CrossRef CAS PubMed.
- G. E. Giacomazzo, M. Schlich, L. Casula, L. Galantini, A. Del Giudice, G. Pietraperzia, C. Sinico, F. Cencetti, S. Pecchioli, B. Valtancoli, L. Conti, S. Murgia and C. Giorgi, Inorg. Chem. Front., 2023, 10, 3025–3036 RSC.
- G. E. Giacomazzo, G. Mulas, I. Palchetti, L. Conti, F. Tadini-Buoninsegni, B. Valtancoli, F. Cencetti and C. Giorgi, Bioinorg. Chem. Appl., 2025, 8899727 CrossRef CAS.
- L. Spiccia, G. B. Deacon and C. M. Kepert, Coord. Chem. Rev., 2004, 248, 1329–1341 CrossRef CAS.
- B. P. Sullivan, J. V. Caspar, T. J. Meyer and S. Johnson, Organometallics, 1984, 3, 1241–1251 CrossRef CAS.
- E. Z. Jandrasics and F. R. Keene, J. Chem. Soc., Dalton Trans., 1997, 153–160 RSC.
- C. M. Kepert, G. B. Deacon, N. Sahely, L. Spiccia, G. D. Fallon, B. W. Skelton and A. H. White, Inorg. Chem., 2004, 43, 2818–2827 CrossRef CAS PubMed.
- E. C. Glazer, Photochem. Photobiol., 2017, 93, 1326–1328 CrossRef CAS PubMed.
- A. Hernández-García, L. Marková, M. D. Santana, J. Prachařová, D. Bautista, H. Kostrhunová, V. Novohradský, V. Brabec, J. Ruiz and J. Kašpárková, Inorg. Chem., 2023, 62, 6474–6487 CrossRef PubMed.
- C. Daniel and A. Veillard, Inorg. Chem., 1989, 28, 1170–1173 CrossRef CAS.
- S. J. Carrington, I. Chakraborty, J. R. Alvarado and P. K. Mascharak, Inorg. Chim. Acta, 2013, 407, 121–125 CrossRef CAS PubMed.
- S. Mondal, R. B. Jethwa, B. Pant, R. Hauschild and S. A. Freunberger, Faraday Discuss., 2024, 248, 175–189 RSC.
- G. E. Giacomazzo, S. Doria, A. Revilla-Cuesta, N. De Monte, M. Pagliai, G. Pietraperzia, B. Valtancoli, T. Torroba, L. Conti, M. Di Donato and C. Giorgi, Inorg. Chem., 2024, 63, 6248–6259 CrossRef CAS PubMed.
- G. Sambucari, C. Coutant, A. Di Michele, G. E. Giacomazzo, P. Nun, V. Sol, N. Renard, T. Ouk, V. Coeffard and M. D. Donato, Adv. Opt. Mater., 2025, 13, 1–11 Search PubMed.
- L. Conti, A. Bencini, C. Ferrante, C. Gellini, P. Paoli, M. Parri, G. Pietraperzia, B. Valtancoli and C. Giorgi, Chem. – Eur. J., 2019, 25, 10606–10615 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |