Understanding the competition between hydrodechlorination and Friedel–Crafts alkylation in PVC dechlorination with silylium ions†
Nicholas J.
Roubineau‡
a,
Eunice C.
Castro‡
 a,
Kaustubh
Rane
a,
Kaustubh
Rane
 b,
Zachary A.
Wood
b,
Zachary A.
Wood
 a,
Angelyn N.
Nguyen
a,
Nancy G.
Bush
a,
Angelyn N.
Nguyen
a,
Nancy G.
Bush
 a,
Ayon
Das
a,
Ayon
Das
 a,
Shaama
Mallikarjun Sharada
a,
Shaama
Mallikarjun Sharada
 *ab and
Megan E.
Fieser
*ab and
Megan E.
Fieser
 *ac
*ac
aDepartment of Chemistry, University of Southern California, Los Angeles, CA 90089, USA. E-mail: fieser@usc.edu
bMork Family Department of Chemical Engineering and Materials Science, University of Southern California, Los Angeles, CA 90089, USA
cWrigley Institute for Environment and Sustainability, University of Southern California, Los Angeles, CA 90089, USA
First published on 6th May 2025
Abstract
The combination of hydrodechlorination and Friedel–Crafts alkylation using silylium ions is a rapid route to fully dechlorinate poly(vinyl chloride) (PVC), while producing organic and chlorine-based products that have value for a second life. This silylium reaction has been optimized for full dechlorination under ambient conditions for a range of temperatures. Additionally, tetramethyldisiloxane is introduced as a cheaper and more moisture-stable silane, which shows similar activity as triethylsilane. While high selectivity for Friedel–Crafts alkylation has been achieved for molecular silylium chemistry, analogous attempts with PVC as a substrate have revealed a maximum arene incorporation below 30%. However, reaction conditions and choice of aromatic solvent show dramatic changes in thermal properties of the polyethylene-co-poly(vinyl arene) polymer product. These differences in thermal properties align with a variable side reaction of secondary Friedel–Crafts alkylation, either intramolecularly to form polyindene repeating units or intermolecularly to form crosslinks.
Introduction
Poly(vinyl chloride) (PVC) is a versatile polymer for countless applications, making it the third-most produced plastic worldwide.1 However, this material has one of the most harmful ends-of-life, leading to low recycling rates and initiatives to ban the use of PVC. The consequential end-of-life of PVC often lies in the leaching of toxic additives, including heavy metals and plasticizers, and the generation of hydrochloric acid and dioxins upon thermal or UV treatment.2 The diverse additives prevent practical mechanical recycling of PVC items. Although PVC is not accepted at most recycling facilities, its presence, even as small impurities, can harm the polyolefin streams (polyethylene and polypropylene), where the hydrochloric acid (HCl) can disrupt both pyrolysis and mechanical recycling of these polymers.3 With the large quantities already produced and the difficulty to replace PVC for many applications, environmentally friendly management of PVC waste is a critical need.There have been several emerging strategies to repurpose PVC waste.4,5 Most strategies have aimed to improve efforts for pyrolysis or mechanical recycling of polyolefin waste by minimizing the impact of HCl produced and/or converting the polymer chain to better represent the general polyolefin waste stream. One route has been to perform a pre-dechlorination step via dehydrochlorination, at a lower temperature in the presence of a base prior to pyrolysis.6–15 This lowers the amount of HCl in a pyrolysis reactor and prolongs the lifetime of a pyrolysis catalyst. However, when pyrolyzed, the polyacetylene-like product still disrupts product distributions through the formation of polyaromatics. One way to improve upon this strategy is to introduce a sequential hydrogenation reaction to convert the polyacetylene-like product to polyethylene.16–18 Hydrogenation reactions to high conversion without disrupting the polymer backbone are still challenging with this strategy, as the conjugation in polyacetylene resists functionalization. Direct hydrodechlorination of PVC can eliminate HCl production entirely, while producing polyethylene-like materials for pyrolysis or mechanical recycling.19–21 Although metal-catalyzed routes and silylium-catalyzed routes have been realized, cross-linking and branching formation have altered materials from linear polyethylene. While these strategies decrease the toxicity of the waste PVC and could serve an important role in the processing of mixed plastics waste, they do not add value to the organic product and may not produce valuable chlorinated products.
There are even fewer examples focused on the conversion of PVC to value-added organic and chlorinated products, without the production of harmful byproducts (Fig. 1). These strategies would be much better suited to create value through the repurposing of direct PVC waste streams. Groups have been interested in the dehydrochlorination of PVC, followed by olefin metathesis to generate small molecule olefins (Fig. 1a).22,23 While the PVC materials have been broken down into smaller molecules, full dechlorination and selectivity for a molecular product have not yet been achieved. McNeil and coworkers have taken a unique strategy to electrochemically repurpose the chlorine through the chlorination of arenes (Fig. 1b).24 Excitingly, plasticizer additives that are often considered challenges for catalyst survival are crucial for this route, making this method well-suited for commercial PVC items. However, the partially chlorinated polymer product is not prioritized, which maintains a degree of waste. Stache and coworkers have reported an alternative photothermal route to generate and use the HCl from PVC to hydrochlorinate styrene and other alkenes (Fig. 1c).25 In most of these cases, either the chlorine or the organic material is prioritized, leaving the remaining material as waste.
Our group has recently identified a method to use silylium cation catalysis to perform a tandem Friedel–Crafts alkylation and hydrodechlorination (or reduction) to form polyethylene (PE)-co-polystyrene (PS) random copolymers (Fig. 1d).26 In this method, [Ph3C][B(C6F5)4] serves as the initiator, triethylsilane (Et3SiH) serves as a hydride and silylium cation source, and the arenes used were benzene, toluene and xylenes. When using methylated arenes, at 0.25 mol% catalyst loading and 1.1 equiv. Et3SiH, full dechlorination of PVC could be realized in less than 5 minutes at 110 °C. In all cases, arene incorporation into the polymer chain was always close to 20%, with high selectivity for PE at approximately 80% incorporation. These copolymers are of interest for battery membrane applications and as compatibilizers,27–34 but they can be difficult to make from ethylene and styrene directly,35–38 which has often led to creative synthetic strategies with metathesis polymerization.34,39–42 The chlorine byproduct, Et3SiCl, has a higher cost than the original Et3SiH and has numerous commercial uses, leaving minimal waste and adding value to all products in the overall process.
Silylium catalysis has been used in molecular chemistry to prioritize reduction or Friedel–Crafts alkylation selectivity, but not prioritizing a combination of both methods in the same pot.43,44 The result of this type of catalysis in the case of repurposing PVC would vary the amount of PE and PS-like repeat units in the polymer product, presumably altering the properties.45 As an extreme, our group has recently reported the direct selectivity to PE, where we achieved polymer products that match the properties of polyethylene with tunable degrees of branching under mild conditions and on the benchtop with the same silylium chemistry.20
Herein, the efforts made to better understand the method for dual hydrodechlorination and Friedel–Crafts alkylation of PVC to form polyethylene-co-poly(vinyl arene) copolymers are reported. Efforts to increase arene incorporation into the polymer product have led to the discovery of intramolecular Friedel–Crafts alkylation to form polyindene-like repeating units in the product. Polyindene formation can be tuned based on the arene reactant and the reaction temperature, leading to a wide variety of polymer products with tunable thermal properties.
Results and discussion
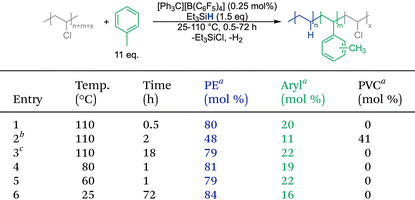
Prior studies showed that dechlorination can be achieved with benzene, toluene and xylene, with toluene and all three xylenes showing more rapid dechlorination than benzene.26 In addition to the influence of the electron-donating methyl substituents, this difference in reactivity is likely due to the formation of more cross-linked materials with benzene, through secondary Friedel–Crafts alkylation reactions on the same aromatic ring, generating less soluble products. Evidence of this cross-linking was previously seen in Friedel–Crafts alkylation of PVC with benzene and other catalyst systems.46–49 Toluene was selected as the primary aromatic solvent for these studies, as the polymer products are more soluble and toluene is a common, inexpensive solvent. Chlorine loss from the product is confirmed by attenuated total reflectance (ATR) Fourier-transform infrared (FT-IR) spectroscopy and 1H nuclear magnetic resonance (1H NMR) spectroscopy.Air stability of the method
Repetitions of the prior reported air-free toluene studies for 5 minutes did not lead to full dechlorination of PVC, leaving 12 mol% PVC remaining in the polymer. This inconsistency could be due to several factors, including the humidity, water content in the aromatic solvent, and the purity of the batch of Et3SiH and [Ph3C][B(C6F5)4] purchased. In our prior report, this tandem process could be achieved under ambient conditions, with benzene, Et3SiH and PVC all stored outside the glovebox and [Ph3C][B(C6F5)4] taken out of the glovebox right before the reaction.26 Using the prior optimized air-free conditions for dechlorination with toluene as the solvent did not support full dechlorination of PVC under ambient conditions either.Further optimization of this method with toluene, as described in the ESI,† was able to achieve reproducible, full dechlorination of PVC under ambient conditions (Table 1, entry 1). In this case, the low 0.25 mol% initiator loading could be maintained, while the Et3SiH loading needed to be slightly higher (1.5 equiv.), toluene was dried in a solvent system, and 30 minutes of reaction time was needed. This presents a trade-off between reactions conducted air-free, which require more preparation, and ambient conditions, which require more reagent and longer time. Additionally, control reactions showed that the [Ph3C][B(C6F5)4] initiator can be stored outside of the glovebox as long as 71 days without losing the ability to fully dechlorinate PVC. Nevertheless, reproducible full dechlorination of PVC under ambient conditions in less than 2 hours is still quite rapid and eliminating the need to dry and store all reagents air-free allows for faster reaction set-up.
Selectivity for organic product
Previously, the toluene incorporation into the product was consistently near 20 mol%, with approximately 80 mol% ethylene units formed on the backbone, based on 1H NMR spectroscopy. It is important to note that the 1H NMR spectra of these samples are extremely broad, making it difficult to observe subtle changes in the arene incorporation. The broad 1H NMR spectra also speak to a key difference in these materials from polyethylene with precise phenyl groups added, which have sharp and easily resolved peaks.34,40,41 The ability to tune the arene incorporation to a wider array of products would improve the practicality of this method for commercial PVC repurposing.For the optimized ambient reaction conditions with toluene, variations were made to alter the arene incorporation into the polymer product. As previously demonstrated, removal of the arene solvent can lead to full hydrodechlorination of PVC and formation of PE products.20 In this case, the silane loading impacted how much carbocation rearrangement could occur, leading to tailored branching in the product. Therefore, adjustments to the reaction conditions were conducted to increase the arene incorporation, to get closer to a PS-like polymer. Molecular literature indicates the rate-determining step to likely be the dehalogenation with a silylium cation.50 Therefore, product selectivity likely relies on the relative reactivity of the carbocation with the silane and the aromatic solvent.
Silane structure
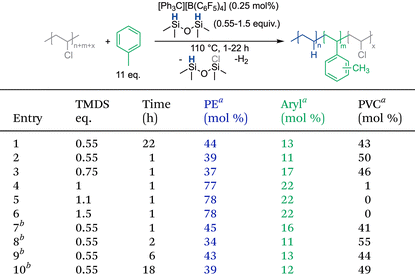
While Et3SiH has been the most common silane source for silylium chemistry, it is a stoichiometric reagent, therefore cost is important. However, the Et3SiCl byproduct is often sold at a higher price than its precursor and can be used in applications such as drying metal salts and in polymerizations.51 Nonetheless, having a less expensive silane source could provide additional options for this strategy. One such option that is more moisture-stable is 1,1,3,3-tetramethyldisiloxane (TMDS); however, there are no reports using it for dehalogenation of organic molecules. It was questioned whether both hydrides could be used, in which the method would only need 0.5 equivalents of a cheaper silane source. As shown in Table 2, TMDS is an active silane source for the dechlorination of PVC. Yet only one hydride is consumed, even when less than 1 equivalent of TMDS is provided and the reaction is run for 22 hours (Table 2, entry 1). Due to its higher moisture stability, consistent full dechlorination can be achieved with just 1.1 equivalents of TMDS, making it more efficient under ambient conditions than Et3SiH (Table 2, entry 5). Even with this dramatically different silane structure, arene incorporation remains near 20 mol%. It was hypothesized that TMDS might degrade in the reaction conditions at 110 °C, which could explain why the second hydride cannot be used. Unfortunately, reactions performed at 60 °C were also not able to use the second hydride. It is therefore likely that once one hydride is replaced with a chloride, the second hydride is much harder to abstract. Calculations indicate that the use of the second hydride in TMDS should be thermodynamically favorable (as described in the ESI†), which could indicate a high kinetic barrier for this second hydride abstraction.Arene substitutions
As shown in prior work, addition of methyl groups to the arene solvent enhanced the rate of dechlorination, but did not greatly impact the selectivity preference for hydrodechlorination over Friedel–Crafts alkylation. Reactions with benzene, and xylene (ortho, meta and para) at different temperatures show the same behavior as toluene, in which full dechlorination is achieved with approximately 20 mol% incorporation of the arene solvent (see Tables S5–S8†).Thermal properties
In our prior report, the glass transition temperature (Tg) of the PE/PS copolymer products were much higher in temperature (>51 °C) than expected for a PE/PS copolymer.26 Further, when comparing these products to past reported copolymers with approximately the same amount of arene content, the poly(ethylene-co-styrene) products from past literature had a lower Tg of around 17–23 °C.34,40,41 In these polymers, the arenes were evenly displaced across the polymer, and the 1H NMR spectra contains reasonably sharp signals. However, 1H NMR spectra of the polymer products in this study are extremely broad. One explanation for these observations could be due to a second, intramolecular Friedel–Crafts alkylation, leading to polyindene formation (Fig. 2). The proposed polyindene formed would be slightly different in structure than the product of indene polymerization, although the thermal properties are expected to be similar. Polyindene is shown to have broad 1H NMR spectra and Tgs above 200 °C. Polyindene formation has been previously hypothesized as a side reaction in the Friedel–Crafts alkylation of PVC with AlCl3.46,47,52 Therefore, it was hypothesized that increased presence of polyindene would be a likely cause of the increase in the observed Tg for these materials. | ||
| Fig. 2 Proposed intramolecular Friedel–Crafts alkylation leading to formation of polyindene segments. | ||
DSC studies of the polymers produced with different arenes at different temperatures were measured (Fig. 3). While the ratios of hydrodechlorination to Friedel–Crafts alkylation did not seem to be changing, the difference between intramolecular and intermolecular Friedel–Crafts alkylation would be expected to be thermally dependent. In the case of toluene, the Tg of the material increases from 74 to 109 °C as the reaction temperature decreases from 110 to 25 °C, suggesting more polyindene formation at lower temperatures (Fig. 3a). This would be expected, as intramolecular reactions should be favored more at low temperature than the intermolecular hydrodechlorination or Friedel–Crafts alkylation. When shifting to benzene, the change in Tg is much more pronounced across the different reaction temperatures (74 to 124 °C) with most Tgs being higher than the relative Tgs for toluene reactions. This is likely due to both polyindene formation and cross-link formation due to additional Friedel–Crafts alkylation to phenyl groups on a second polymer. Use of para-xylene shows a similar behavior to toluene, albeit with a lower Tg for the product of the reaction at 110 °C. Shifting to ortho- and meta-xylene show a significant drop in Tg of the products run at 25 and 60 °C, with a much lower change in Tg across the system (Fig. 3b). We hypothesize that the placement of the electron-donating methyl groups can negatively influence the intramolecular Friedel–Crafts alkylation. To further confirm that polyindene formation is responsible for the increased Tg, mesitylene was used as the aromatic solvent, in which methyl groups would block the intramolecular Friedel–Crafts alkylation (Fig. 3a). As expected, the resulting polymer for mesitylene reactions has a much sharper 1H NMR spectrum. The Tgs are the lowest of all arenes studied, but still influenced by the reaction temperature, with a change from 51–75 °C. These results suggest that all arene solvents likely do allow some cross-link formation, though not enough to severely influence the solubility of the polymers. Importantly, all copolymers reported herein have higher Tgs than previously reported poly(ethylene-co-styrene) materials (17–23 °C), along with similar degradation temperatures (Td,5% > 350 °C). Due to their higher thermal rigidity, they may show improved applicability in battery membranes that require high operating temperatures. Finally, reactions done in toluene with the TMDS silane show very similar behavior to that of Et3SiH, suggesting that the silane structure is not relevant for this selectivity difference (Fig. 3c). These results indicate that polymers with tunable, high thermal stability could be formed through silylium dechlorination of PVC.
Computational analysis
The starting point for the computations is the carbocation formed upon cleavage of one of the two secondary C–Cl bonds in the model PVC molecule by a silylium cation. The energy of this structure is added to that of two molecules of benzene and two molecules of triethylsilane to constitute the reference state, labelled ‘REF’. The REF state is either attacked by a hydride from silane or undergoes Friedel–Crafts alkylation via benzene addition. Thermodynamic calculations comparing the free energies of formation of the hydrogenated (H) and alkylated (Ph) products are reported in Fig. 4. The data used to generate the figure and the structures constituting each state are reported in Table S12 and Fig. S123 of the ESI,† respectively.The primary finding from the computational study is that hydrodechlorination is energetically more favorable than Friedel–Crafts alkylation, which agrees with experiments. If one C–Cl is replaced with C–H (or C–Ph), hydride addition is once again thermodynamically more favorable than Friedel–Crafts alkylation. Intramolecular Friedel–Crafts alkylation to form an indene product following the first Friedel–Crafts alkylation is energetically slightly more favorable than the addition of another phenyl group via Friedel–Crafts alkylation (POLY).
Conclusion
The combined hydrodechlorination and Friedel–Crafts alkylation of PVC with silylium ions and aromatic solvents to form polyethylene and poly(vinyl arene) copolymers have been optimized under ambient conditions, with full dechlorination being achieved with reaction temperatures between 25 and 110 °C. Extension to less expensive and less moisture-sensitive TMDS allows for full dechlorination of PVC, although only one of the two hydrides is delivered for every molecule of TMDS. Attempts to increase the poly(vinyl arene) repeating units in the polymer product could not go beyond 30 mol%, with most samples being near 20 mol% poly(vinyl arene) repeating units. With the same poly(vinyl arene) content, reactions run at different temperatures and/or different arene solvents revealed products with dramatically different Tgs, ranging from 51–124 °C, all of which are much higher than discrete polyethylene-co-polystyrene copolymers synthesized from ethylene and styrene monomers directly. These variations are attributed to secondary intramolecular Friedel–Crafts alkylations to form polyindene repeating units and intermolecular Friedel–Crafts alkylations to form cross-links. These results show that while the poly(vinyl arene) content cannot be varied widely, the thermal properties of the polymer products produced from PVC can be altered by impacting the presence and quantity of secondary Friedel–Crafts alkylations.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding for this project was provided by the National Science Foundation (CHE-2203756). Instrumentation in the USC Chemistry Instrument Facility was acquired with support from USC Research and Innovation Awards. Additionally, funds were provided by the National Science Foundation (DBI-0821671, CHE-0840366) and National Institute of Health (S10 RR25432) to support the acquisition of the NMR spectrometers used in our work. Polymer analysis instrumentation (DSC) was supported by the National Institute of Standards and Technology (NIST Award 60NANB22D134).References
- J. Martín, C. Mundell, S. D. Jaydev and J. Pérez-Ramírez, Chem, 2021, 7, 1487–1533 Search PubMed.
- S. Xu, Z. Han, K. Yuan, P. Qin, W. Zhao, T. Lin, T. Zhou and F. Huang, Nat. Rev. Methods Primers, 2023, 3, 44 CrossRef CAS.
- M. Roosen, N. Mys, M. Kusenberg, P. Billen, A. Dumoulin, J. Dewulf, K. M. Van Geem, K. Ragaert and S. De Meester, Environ. Sci. Technol., 2020, 54, 13282–13293 CrossRef CAS PubMed.
- R. K. Jha, B. J. Neyhouse, M. S. Young, D. E. Fagnani and A. J. McNeil, Chem. Sci., 2024, 15, 5802–5813 RSC.
- J. Sun, J. Dong, L. Gao, Y.-Q. Zhao, H. Moon and S. L. Scott, Chem. Rev., 2024, 124, 9457–9579 CrossRef CAS PubMed.
- P. A. Kots, B. C. Vance, C. M. Quinn, C. Wang and D. G. Vlachos, Nat. Sustain., 2023, 6, 1258–1267 CrossRef.
- Q. Zhou, C. Tang, Y.-Z. Wang and L. Zheng, Fuel, 2004, 83, 1727–1732 CrossRef CAS.
- T. Bhaskar, R. Negoro, A. Muto and Y. Sakata, Green Chem., 2006, 8, 697–700 RSC.
- K.-B. Park, S.-J. Oh, G. Begum and J.-S. Kim, Energy, 2018, 157, 402–411 CrossRef CAS.
- T. Okada, S. Sutoh, K. Sejima, H. Tomohara and S. Mishima, Polym. Degrad. Stab., 2020, 171, 109040 CrossRef CAS.
- A. Marino, A. Aloise, H. Hernando, J. Fermoso, D. Cozza, E. Giglio, M. Migliori, P. Pizarro, G. Giordano and D. P. Serrano, Catal. Today, 2022, 390–391, 210–220 CrossRef CAS.
- L. Guo, G. Shi and Y. Liang, Polymer, 2001, 42, 5581–5587 CrossRef CAS.
- Y. Chen, S. Zhang, X. Han, X. Zhang, M. Yi, S. Yang, D. Yu and W. Liu, Energy Fuels, 2018, 32, 2407–2413 CrossRef CAS.
- P. Zhao, J. Cleaner Prod., 2017, 15, 38–46 CrossRef.
- P. Zhao, T. Li, W. Yan and L. Yuan, Environ. Technol., 2018, 39, 977–985 CrossRef PubMed.
- G. O'Rourke, T. Hennebel, M. Stalpaert, A. Skorynina, A. Bugaev, K. Janssens, L. Van Emelen, V. Lemmens, R. De Oliveira Silva, C. Colemonts, P. Gabriels, D. Sakellariou and D. E. De Vos, Chem. Sci., 2023, 14, 4401–4412 RSC.
- G. O'Rourke, A. Skorynina, I. Beckers, S. V. Minnebruggen, C. Colemonts, P. Gabriels, P. Van der Veken and D. E. De Vos, EES Catal., 2024, 2, 1006–1018 RSC.
- A. Alshaikh, S. Ezendu, D. Ryoo, P. S. Shinde, J. L. Anderson, T. Szilvási, P. A. Rupar and J. E. Bara, ACS Appl. Polym. Mater., 2024, 16, 9656–9662 CrossRef.
- N. G. Bush, M. K. Assefa, S. Bac, S. Mallikarjun Sharada and M. E. Fieser, Mater. Horiz., 2023, 10, 2047–2052 RSC.
- Z. A. Wood, E. C. Castro, A. N. Nguyen and M. E. Fieser, Chem. Sci., 2024, 15, 8766–8774 RSC.
- N. G. Bush, A. Das, J. A. Bowen and M. E. Fieser, ChemSusChem, 2025, e202402689 CrossRef PubMed.
- D. Glas, J. Hulsbosch, P. Dubois, K. Binnemans and D. E. De Vos, ChemSusChem, 2014, 7, 610–617 CrossRef CAS PubMed.
- P. W. Skelly, C. F. Chang and R. Braslau, ChemPlusChem, 2023, 88, e20230018 CrossRef PubMed.
- D. E. Fagnani, D. Kim, S. I. Camarero, J. F. Alfaro and A. J. McNeil, Nat. Chem., 2023, 15, 222–229 CrossRef CAS PubMed.
- H. Jiang, E. A. Medina and E. E. Stache, J. Am. Chem. Soc., 2025, 147, 2822–2828 CrossRef CAS PubMed.
- M. K. Assefa and M. E. Fieser, J. Mater. Chem. A, 2023, 11, 2128–2132 RSC.
- X.-L. Wang, I.-K. Oh and T.-H. Cheng, Polym. Int., 2010, 59, 305–312 CrossRef CAS.
- H. F. Guo, S. Packirisamy, R. S. Mani, C. L. Aronson, N. V. Gvozdic and D. J. Meier, Polymer, 1998, 39, 2495–2505 CrossRef CAS.
- T. H. Nguyen, C. Wang and X. Wang, J. Membr. Sci., 2009, 342, 208–214 CrossRef CAS.
- M. Annala, S. Lipponen, T. Kallio and J. Seppälä, J. Appl. Polym. Sci., 2012, 124, 1511–1519 CrossRef CAS.
- M. A. Elseifi, G. W. Flintsch and I. L. Al-Qadi, J. Mater. Civ. Eng., 2003, 15, 32–40 CrossRef CAS.
- S.-Y. Shim and R. Weiss, Polym. Int., 2005, 54, 1220–1223 CrossRef CAS.
- J. M. Serpico, S. G. Ehrenberg, J. J. Fontanella, X. Jiao, D. Perahia, K. A. McGrady, E. H. Sanders, G. E. Kellogg and G. E. Wnek, Macromolecules, 2002, 35, 5916–5921 CrossRef CAS.
- A. Kendrick IV, W. J. Neary, J. D. Delgado, M. Bohlmann and J. G. Kennemur, Macromol. Rapid Commun., 2018, 39, 1800145 CrossRef PubMed.
- G. Xu and S. Lin, Macromolecules, 1997, 30, 685–693 CrossRef CAS.
- K.-S. Son and R. M. Waymouth, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1579–1585 CrossRef CAS.
- D. J. Arriola, M. Bokota, R. E. Campbell, J. Klosin, R. E. LaPointe, O. D. Redwine, R. B. Shankar, F. J. Timmers and K. A. Abboud, J. Am. Chem. Soc., 2007, 129, 7065–7076 CrossRef CAS PubMed.
- H. Zhang and K. Nomura, Macromolecules, 2006, 39, 5266–5274 CrossRef CAS.
- E. Elacqua, Nat. Synth., 2024, 3, 1322–1324 CrossRef CAS.
- W. J. Neary and J. G. Kennemur, Macromol. Rapid Commun., 2016, 37, 975–979 CrossRef CAS PubMed.
- R. J. Kieber III, W. J. Neary and J. G. Kennemur, Ind. Eng. Chem. Res., 2018, 57, 4916–4922 CrossRef.
- M. D. Watson and K. B. Wagener, Macromolecules, 2000, 33, 8963–8970 CrossRef CAS.
- T. He, H. F. T. Klare and M. Oestreich, ACS Catal., 2021, 11, 12186–12193 CrossRef CAS.
- C. Douvris, C. M. Nagaraja, C.-H. Chen, B. M. Foxman and O. V. Ozerov, J. Am. Chem. Soc., 2010, 132, 4946–4953 CrossRef CAS PubMed.
- H. Y. Chen, E. V. Stepanov, S. P. Chum, A. Hiltner and E. Baer, Macromolecules, 2000, 33, 8870–8877 CrossRef CAS.
- N. Cascaval, I. A. Schneider and I. C. Poinescu, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 2259–2268 CrossRef.
- D. Hace and V. Vurdelja, J. Polym. Sci., Polym. Symp., 1974, 47, 225–240 CrossRef CAS.
- J. C. Bevington and R. G. W. Norrish, J. Chem. Soc., 1948, 771–774 RSC.
- J. F. Rabek, J. Lucki, H. Kereszti, T. Hjertber and Q. B. Jun, J. Appl. Polym. Sci., 1990, 39, 1569–1586 CrossRef CAS.
- H. F. T. Klare, L. Albers, L. Süsse, S. Keess, T. Müller and M. Oestreich, Chem. Rev., 2021, 121, 5889–5985 CrossRef CAS PubMed.
- A. D. Beck, S. Haufe and S. R. Waldvogel, ChemElectroChem, 2023, 10, e202201149 CrossRef CAS.
- Ph. Teyssié and G. Smets, J. Polym. Sci., 1956, 20, 351–369 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5py00242g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |