Artemisinin as a therapeutic vs. its more complex Artemisia source material
Pamela J.
Weathers

Department of Biology and Biotechnology, 100 Institute Rd, Worcester Polytechnic Institute, Worcester, MA 01609, USA. E-mail: weathers@wpi.edu
First published on 21st December 2022
Abstract
Covering: up to 2017–2022
Many small molecule drugs are first discovered in nature, commonly the result of long ethnopharmacological use by people, and then characterized and purified from their biological sources. Traditional medicines are often more sustainable, but issues related to source consistency and efficacy present challenges. Modern medicine has focused solely on purified molecules, but evidence is mounting to support some of the more traditional uses of medicinal biologics. When is a more traditional delivery of a therapeutic appropriate and warranted? What studies are required to establish validity of a traditional medicine approach? Artemisia annua and A. afra are two related but unique medicinal plant species with long histories of ethnopharmacological use. A. annua produces the sesquiterpene lactone antimalarial drug, artemisinin, while A. afra produces at most, trace amounts of the compound. Both species also have an increasing repertoire of modern scientific and pharmacological data that make them ideal candidates for a case study. Here accumulated recent data on A. annua and A. afra are reviewed as a basis for establishing a decision tree for querying their therapeutic use, as well as that of other medicinal plant species.
1. Introduction: artemisinin, its derivatives, and Artemisia sp. vs. many diseases
Artemisinin (ARTi; Fig. 1), produced by the medicinal plant Artemisia annua L., was identified and isolated in the 1960's by the Chinese Project 523 and in 2015 Tu Youyou was awarded the Nobel Prize in Medicine for the discovery of artemisinin as the primary active molecule in A. annua.1 Use of decoctions of the plant were documented, however, in the Chinese Materia Medica and used for >2000 years1,2 to treat fever, thought to be malaria. Although many people in low- and middle-income countries (LIMC) still use decoctions of the plant to treat disease, artemisinin has been isolated and derivatized into more bioavailable forms that are now combined with another antimalarial and commercialized as artemisinin combination therapies (ACTs) mainly to thwart the emergence of artemisinin drug resistance.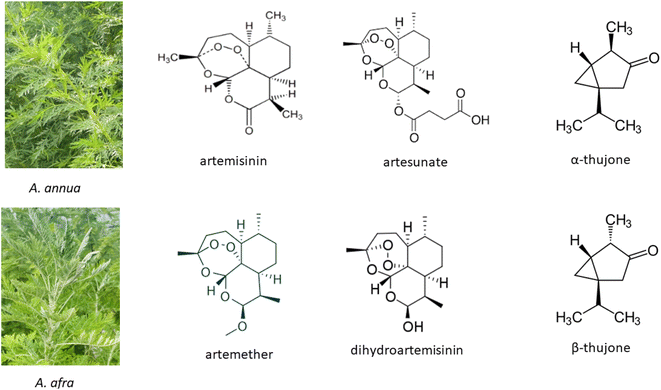 | ||
| Fig. 1 Artemisia annua and A. afra and key phytochemicals including artemisinin and thujones (found in some A. afra cultivars) and artemisinin semisynthetic derivatives. | ||
Artemisinin and A. annua have broad antimicrobial potential and also are showing promise in alleviating chronic conditions including diabetes, inflammation, and fibrosis (Table 1). With an increasing number of studies, it is becoming apparent that A. annua is at least equal in therapeutic efficacy to artemisinin at the same concentration and is often more potent. Together those results beg the question: why not instead use the plant? Here we examine the evidence and considerations related to a more traditional use of A. annua and A. afra and whether such use translates readily for other medicinal species to develop qualifying plants as Botanical Drugs as defined by the US Food and Drug Administration (FDA).
| Disease/condition | Model | Reference |
|---|---|---|
| Malaria | In vitro/rodent | 3–6 |
| Schistosomiasis | In vitro/rodent | 7–9 |
| Lyme (Borrelia) | In vitro | 10–12 |
| Tuberculosis | In vitro | 13–15 |
| Leishmaniasis | In vitro/rodent | 16–18 |
| Trypanosomiasis | In vitro/rodent | 19 and 20 |
| Acanthamoebiasis | In vitro/rodent | 21 |
| Periodontitis | In vitro | 22 |
| HIV | In vitro | 23 and 24 |
| SARS-CoV-2 | In vitro | 25–30 |
| Hepatitis B | In vitro | 31 |
| Human papillomavirus | In vitro | 32 and 33 |
| Fibrosis | In vitro | 34 |
| Diabetes | In vitro/rodent | 35–37 |
| Inflammation | In vitro/rodent | 38–40 |
This review aims to summarize recent research comparing efficacy and safety of Artemisia annua, A. afra and artemisinin in treatment of five exemplar infectious diseases. These terms were combined with Boolean operators to select 69 articles from a total of 504 articles: Artemisia annua, Artemisia afra, COVID-19, tuberculosis, schistosomiasis, malaria, Borrelia, synergism, toxicity, and artemisinin. The search was undertaken December 7, 2022 on PubMed and SCOPUS, limited to 2017–2022 publications excluding most reviews and book chapters excepting some context-setting older articles.
1.1. A. afra an Artemisia alternative
Compared to the annual species A. annua, perennial A. afra Jacq. ex Willd has not been as well studied. For two well-documented comprehensive reviews on this species, see those by the van der Kooy lab.41,42 However, like A. annua, this medicinal plant has been used for centuries with the earliest reported documentation in 1932 mainly by indigenous southern Africans.42 Although A. afra has many phytochemicals in common with A. annua, there are two major differences. It produces no or scarcely detectable artemisinin, but unlike A. annua, it may contain thujone (Fig. 1).42 Thujone has had a mixed reputation regarding toxicity as later noted. Because A. afra is also increasing in global traditional use, it has been included in some of the topics discussed herein.2. Safety: artemisinin vs. Artemisia
Similar to pure drugs, a Botanical Drug used for therapeutic or prophylactic purposes must comply with rigorous regulatory oversight to meet two key requirements: efficacy and safety.43 For approval, a Botanical Drug also must be available as a consistent product that does not vary in quality or dosing efficacy. Compared to pure molecules that are either extracted from plants, e.g. artemisinin, or synthesized into a final form, e.g., artesunate (AS), plants respond to environmental changes that can result in considerable variation in phytochemical content. Genetic variations can also arise from cross-pollination, favored by A. annua, so seed planting can yield wide variations in artemisinin and other phytochemicals. Long-term consistency of phytochemicals including artemisinin can be achieved by clonal propagation through rooted cuttings44–46 or controlled self-pollination to provide homozygous seed.47,48 Having target molecules to track, e.g., artemisinin in A. annua, enables validation of stable production of plant material. A botanical drug should have at least one identified molecular constituent that can be tracked through its growth, harvest, and processing. Once stable production is achieved in a specific geographical location, other botanical product goals are achievable.A. annua and A. afra have been used for millennia by indigenous peoples to treat various ailments and are accepted as safe traditional medicines. Such use, while important, belies scientific evidence of safety, so more recently both species have been studied using standard in vitro and in vivo methods to establish overall safety. A. annua is a generally regarded as safe (GRAS) herb,49,50 yet some countries, e.g., in the EU and New Zealand, have banned or limited its sale or use. Such occurrences are the exception and the result of not being listed in their pharmacopeias; concerns about misuse as a malaria prophylactic by travelers (EU);51 an isolated case study;52 or unusual formulations, e.g., as a supercritical CO2 extract into grapeseed oil.53 Until recent rodent studies,54–56 far less was known about the safety of A. afra. Concerns had focused on the presence of thujones, generally present in A. afra but not in A. annua. Thujones are nervous system antagonists affecting 5-hydroxytryptamine (serotonin)57 and γ-aminobutyric acid-A (GABAA).58 However, given that the LD50 of thujone is 192, 230, 396 mg kg−1 body weight in rats, mice, and guinea pigs, respectively,59 the small amounts (0–0.86 mg g−1 dry plant mass) in aqueous A. afra extracts,60 and thujone is only partially extractable into water,61 toxicity of orally consumed A. afra from a traditional infusion is not a concern.
Rodent studies measured acute toxicity in rats of p.o. delivered A. afra reconstituted tea infusions resuspended in 0.9% saline from the freeze-dried extract of 100 g dried leaves, boiled 30 min, cooled, and filtered.54 The single p.o. doses were 0–24 g kg−1 body weight (BW) and monitored for 14 days. There were no symptoms of toxicity to 2 g kg−1 BW, after which symptoms began to appear. Chronic p.o. administration of A. afra at 100 mg kg−1 BW delivered daily for three months showed there were no adverse events including 10 hematological and biochemical blood parameters. Using the same A. afra extraction protocol, Sunmonu and Afolayan55 measured >20 hematological and biochemical blood parameters in streptozotocin-induced diabetic rats treated with 50–200 mg kg−1 BW of extract daily for 15 days. Compared to diabetic-induced rats, the A. afra extract restored all liver function and hematological indices to normal except for platelets and neutrophils that although greatly improved, were not equal to untreated controls. More recently Kane et al.56 compared extracts of A. afra dried leaves in dichloromethane, hexane, and ethanol at acute p.o. doses of 1000–2500 mg kg−1 BW in mice. Similar to prior studies,54,55 there was no apparent toxicity. Both AST (aspartate aminotransferase) and ALT (alanine aminotransferase), important indicators of liver function, improved or remained equivalent to untreated controls in all studies. Together these rodent studies suggested that p.o.-delivered A. afra is safe.
Although most studies do not adequately provide enough detail describing extraction of the plant material, there are rodent studies showing that A. annua also is not toxic. For example, in an acute toxicity test in mice Siddiqui et al.62 provided 60% ethanolic extracts of A. annua p.o at 50–5000 mg kg−1 BW, then observed animals for 14 days and observed no toxic effects at any level. In another acute study in mice, Park et al.63 compared healthy mice to those treated with LPS/D-galactosamine (LPS/D-gaIN) to induce liver failure. The LPS/D-gaIN mice were treated p.o. with either an ethanolic or water extract of A. annua at 100 mg kg−1 BW daily for 14 days. The water extract was significantly better than the ethanolic extract at improving the AST and ALT levels in animals. In a 13 week chronic toxicity study Park et al.63 fed a water extract of A. annua to rats and also observed no adverse effects in 14 hematological and 18 biochemical blood parameters at the 1000 mg kg−1 BW dose. As noted for A. afra, AST and ALT levels in all A. annua treated animals either improved or remained equivalent to untreated healthy controls regardless of length of treatment, indicating a positive effect on the liver. More recently, an ethanolic (∼70%) extract of A. annua stems and leaves were tested in male and female rats for acute oral toxicity according to the OECD Test Guideline 425 (2008) limit test and also showed no toxicity at the delivered dose.
Recently evidence of safety in humans was documented in a small randomized, double-blind controlled clinical trial by Han et al.64 who measured the safety of an undefined A. annua hot water extract powder (SPB-201). They compared the extract powder to a crystalline cellulose placebo p.o in patients with non-alcoholic mild liver dysfunction. For 8 weeks about 48 patients per group were provided with 2 tablets TID of SPB-201 for a daily dose of 480 mg per day of A. annua dried extract or placebo tablets. There were no significant differences in 23/24 hematological parameters between placebo and A. annua treatments; only hematocrit declined a bit more in SPB-201 treated patients than those given placebo (p = 0.0462). Multidimensional fatigue scale (MFS) assessments were also significantly better in the SPB-201 group. Both AST and ALT were significantly reduced at 4 and 8 weeks in the SPB-201 patients compared to placebo indicating improved liver function, a result similar to a human trial using tea infusions of A. annua and A. afra in several hundred malaria patients.65 When compared to the standard malaria treatment recommended by WHO for that region of Africa, neither ALT nor AST were significantly altered post-treatment with A. afra or A. annua.65 Together these human trials demonstrated the safety of both A. afra and A. annua.
Compared to A. annua and A. afra, artemisinin and its derivatives seem to have more adverse effects, but direct comparison is challenging and even LD50s where available cannot be directly compared (Table 2). Of 11 possible and monitored adverse effects, Munyangi et al.65 observed that only 5% of malaria patients treated with tea infusions of A. annua or A. afra reported nausea or vomiting, while 42.8% of patients treated with ASAQ (the recommended ACT for the study region) reported adverse effects, especially asthenia, abdominal pain, hypoglycemia, pruritis, nausea, and vomiting. Based on that small study it seemed the Artemisia infusions were better tolerated by patients.
| Species or drug | LD50 mg kg−1 BW | Solvent | Animal | Ref. |
|---|---|---|---|---|
| a Specific alcohol not stated, but likely ethanol; NA, not available. | ||||
| A. annua | 2750 | Hexane | Mouse | 66 |
| 7425 | Ether | 67 | ||
| 4162 | Dilute alcohola | 67 | ||
| A. afra | >5000 | Water | Mouse | 68 |
| >5000 | Water | Rats | 69 and 70 | |
| ART | 5105 | NA | Mouse | 67 |
| 4228 | 71 | |||
| AS | 1000–1300 | NA | Mouse | 71 |
| AM | >800 | NA | Rat | 71 |
| 1000 | Dog | |||
| DHA (artenimol) | 700–1500 | NA | Mouse | 71 |
3. Bioavailability: artemisinin vs. Artemisia-delivered artemisinin
There are concerns that A. annua does not provide enough artemisinin into the bloodstream to combat the malaria parasite, and hence, fosters resistance.72In vivo rodent studies measuring serum levels of artemisinin showed that artemisinin is >40-fold more bioavailable when delivered per os via A. annua,73 and later studies supported those results.74,75 This is consistent with earlier pharmacokinetic studies76 showing A. annua tea infusions containing 94.5 mg artemisinin/delivered dose/subject delivered high levels of artemisinin into the serum of healthy human subjects, and with a Cmax of 240 μg L−1 plasma, that was well above the 9 μg L−1 minimum antimalarial threshold required to kill the parasite.77Artemisinin is four times more soluble in digestive fluid when delivered as plant material,78 and in a Caco-2 cell model of intestinal permeability, plant-delivered artemisinin subsequently crossed the intestinal epithelial barrier more efficiently than pure artemisinin.79 Furthermore, phytochemicals in the Artemisia extracts inhibit cytochrome P450 2B6 and 3A4, crucial in Phase I metabolism in the intestine and liver, thereby making artemisinin highly bioavailable when delivered via the plant.38 In rats there was significantly greater serum artemisinin delivered from orally ingested A. annua than from similarly administered pure artemisinin, results consistent with the P450 data. Likewise, there was significantly more artemisinin in rat organs and tissues, and a greater decline in inflammation when delivered via A. annua than from pure artemisinin. Tea infusions of both A. annua and A. afra inhibited enzymatic activity of both CYP2B6 and CYP3A4 hepatic P450s and provided mechanistic data showing the effect was not only at the level of enzymatic activity, but also at the transcription and translation steps of these two P450s.80
4. Therapeutic examples
4.1. Malaria
Malaria is the most studied artemisinin-susceptible disease and artemisinin combination therapy (ACT) is the currently approved treatment for malaria patients. Despite widespread use, ACTs are still not available to all because of rural logistic challenges and cost. Although WHO states that a full ACT treatment costs <US $2, that is still too expensive for a family of four as each could easily be infected twice a year. Assuming $16 for a family of four infected twice a year, that is about 4–5% of an annual subsistence income of $350–400 where, for example, in Ghana ACT costs are likely much higher and people live on $3.2–5.5 per day. ACTs actually cost ∼$10 for a treatment course of artemether–lumefantrine,81 so a family of four would need ∼$40 for one bout of malaria (as of July 30, 2021, https://www.statista.com/statistics/1221864/middle-income-poverty-rate-in-ghana-by-level/). The goal is to produce a malaria treatment for <US $0.50,82,83 but highly subsidized ACTs are well beyond that price point. Consequently, many with financial limitations, especially in LMI countries, are moving towards using a more traditional use of A. annua or A. afra as a tea infusion, a growing practice that unfortunately is discouraged by the WHO.72 A series of recent studies are providing a base of scientific knowledge to better guide the traditional use of Artemisia for treating malaria.Malaria is caused by numerous Plasmodium species with P. falciparum being the most lethal, and it has numerous stages requiring therapeutic treatment: liver stages (asexual), blood stages (asexual and sexual), and dormant stages (hypnozoites in the liver). The sexual gametocyte stage transfers the parasite back to the mosquito vector with the next bite helping to complete the parasite's life cycle. Asexual parasites include trophozoite, schizont, and ring stages. The sexual stage includes male and female gametocytes that can reside in the blood for some time acting as a parasite reservoir and are responsible for asymptomatic malaria. A. annua and A. afra tea infusions kill both blood asexual5,6,84 and gametocyte stages5,6in vitro, results that corroborate clinical observations.65,85,86 Recently, Ashraf et al.5 showed that tea infusions of the artemisinin-deficient species, A. afra, were more effective than artemisinin-containing A. annua against both rings and schizonts, and even more effective than dihydroartemisinin (DHA). Using bioassay-guided fractionation of A. afra, Moyo et al. identified two guaianolide sesquiterpene lactones, yomogiartemin and 1α, 4α-dihydroxybishopsolicepolide, that were effective against both gametocytes and asexual blood stages of P. falciparum, with IC50s vs. late-stage gametocytes of 15.8 and 6.3 μM, respectively.87 Neither was cytotoxic in vitro and the latter was especially potent against late vs. early stage gametocytes.
A contrasting study used A. annua mutants that were impaired either in artemisinin or total flavonoid production.88 The mutant CHI1-1, deficient in chalcone isomerase, shut down major flavonoid production. Two other lines produced little to no artemisinin: AMS-silenced had insufficient amorphadiene synthase, and CYP71AV1-1 was deficient in a key P450; both enzymes are required for artemisinin biosynthesis. Compared to WT the EC50s (ng mL−1) of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform
1 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol extracts against the Dd2 strain of P. falciparum were: 15.6, 25.9, 350, and 4220, for WT, CHI1-1, AMS-silenced, and CYP71AV1-1, respectively. Authors concluded that despite numerous reports of individual antimalarial activity of many flavonoids as summarized in Gruessner and Weathers,46 flavonoids played no role in the antimalarial activity of A. annua. How can one explain this discrepancy with those other antimalarial flavonoid studies and more recent ones involving the traditional infusion5,6? It may be in the methodology. For example, Snider and Weathers6 initiated experiments using dichloromethane (DCM) extracts and did not see much activity and had control vehicle (DMSO) issues (data not included in the published report). Testing hot water infusions, however, provided greater antiparasitic activity in vitro and water-based infusions are also more biosimilar. In personal communications with Dominique Mazur (Sorbonne University/Inserm/CNRS), similar solvent problems were observed and more informative results were obtained with biosimilar hot water infusions.5 In testing A. annua extracts against SARS-CoV-2 variants, initial DCM extracts were at best challenging to interpret. Switching to the water-based infusion also yielded more reproducible and consistent results with no DMSO vehicle issues and far less cytotoxicity than with DCM (see Section 4.2 for summary data).
ethanol extracts against the Dd2 strain of P. falciparum were: 15.6, 25.9, 350, and 4220, for WT, CHI1-1, AMS-silenced, and CYP71AV1-1, respectively. Authors concluded that despite numerous reports of individual antimalarial activity of many flavonoids as summarized in Gruessner and Weathers,46 flavonoids played no role in the antimalarial activity of A. annua. How can one explain this discrepancy with those other antimalarial flavonoid studies and more recent ones involving the traditional infusion5,6? It may be in the methodology. For example, Snider and Weathers6 initiated experiments using dichloromethane (DCM) extracts and did not see much activity and had control vehicle (DMSO) issues (data not included in the published report). Testing hot water infusions, however, provided greater antiparasitic activity in vitro and water-based infusions are also more biosimilar. In personal communications with Dominique Mazur (Sorbonne University/Inserm/CNRS), similar solvent problems were observed and more informative results were obtained with biosimilar hot water infusions.5 In testing A. annua extracts against SARS-CoV-2 variants, initial DCM extracts were at best challenging to interpret. Switching to the water-based infusion also yielded more reproducible and consistent results with no DMSO vehicle issues and far less cytotoxicity than with DCM (see Section 4.2 for summary data).
Using P. yoelii in mice, Li et al.89 compared high (100 mg kg−1) and low (25 mg kg−1) oral doses of artemisinin to a combination treatment of artemisinin + AB + AA + scopoletin (each at 25 mg kg−1) and observed the 4-drug combination was equal in efficacy to the 100 mg kg−1 dose of artemisinin in eliminating the parasite. AUC, Cmax and Tmax for the 4-drug combination were about double that of the high artemisinin dose. Efficacy of low dose artemisinin (25 mg kg−1) was about a third that of the high dose artemisinin (100 mg kg−1). Results indicated that nonartemisinin compounds present in A. annua synergistically improved the activity of artemisinin.
A recent report90 reexamined the original studies by the Project 523 team and discovered notes indicating that a NaOH-treated ether extract also had antimalarial activity in malaria patients. Based on that rediscovered information, ether extracts ± a subsequent alkali extraction were tested in P. yoelii-infected mice to search for alternative and synergistic antimalarial compounds. Of the 113 identified compounds that included sesquiterpenoids, flavonoids, and other phytochemicals, those with antimalarial activity fell within two activity groups: (1), artemisinin and non-artemisinin antimalarial compounds, and (2), synergistic compounds. The non-artemisinin antimalarial active phytochemicals included 5α-hydroperoxy-eudesma-4(15),11-diene while the synergistic phytochemicals included arteannuin B, arteannuin B analogues, and polymethoxy flavonoids.
In some malaria species, e.g., P. vivax, hypnozoites, a dormant parasite stage, form in the liver and can later relapse into a clinical infection. Because hypnozoite metabolism is so slow, they are not particularly susceptible to antimalarial drugs that require active growth to be therapeutically effective. Recently, Ashraf et al.5 showed that tea infusions of both A. afra and A. annua disrupted hypnozoites. Considering that long term consumption of A. annua is safe with no significant adverse effects, hypnozoites could be eliminated using the plant. Together these studies5,89 show that both A. annua and A. afra tea infusions are effective, and since A. afra lacks artemisinin, efficacy is not solely dependent upon artemisinin which was further substantiated by Shi et al.90
There is an effort to discourage the use of A. annua and A. afra to treat malaria. This is unfortunate, but not surprising considering the low price point of the plant as it would outcompete ACTs thereby obviating the profits of modern pharmaceuticals. While widespread use of a tea infusion is challenging, this traditional preparation offers a cost effective and accessible first line treatment of malaria in regions where ACTs are not readily accessible.
4.2. SARS-CoV-2 (COVID-19)
COVID-19 (SARS-CoV-2) emerged in 2019 and now is a rapidly evolving pandemic virus with estimates of >650 million cases and >6.5 million deaths all of which are deemed underestimates due to inadequate global tracking (https://coronavirus.jhu.edu/map.html). Several groups have reported that extracts of A. annua and A. afra have potent anti-SARS-CoV-2 activity.25,26,28–30 All testing thus far has been in vitro using hot water and/or solvent extracts of A. annua.In 2021,25 seven different dried leaf cultivars from four continents were prepared as hot water extracts (DLAe) and tested against an original strain, US WA1. All were effective with IC50s (based on artemisinin content) of 0.1–8.7 μM artemisinin representing 23.4–57.4 μg leaf dry mass. One dry leaf sample was from leaves stored for >12 years that was equally as potent as one-year old material, indicating long term stability of anti-SARS-CoV-2 activity in dried leaves. A. annua DLAe also minimally affected Vero E6 or Calu-3 human lung cells infected with a VSV-spike pseudovirus containing the SARS-CoV-2 spike protein. Those results suggested that A. annua inhibits SARS-CoV-2 primarily by targeting the virus post-entry step. Although artemisinin and several other antimalarials including amodiaquine (AQ), artemether (AM), deoxyartemisinin (dART), and DHA were tested, most were not as potent as DLAe, yet a Spearman correlation analysis of all the artemisinin-based IC50s indicated that potency increased with decreasing artemisinin content, suggesting compounds other than artemisinin in the plant were responsible for the observed antiviral efficacy and that in complex combinations, artemisinin may be antagonistic. Nie et al.29 separately confirmed non-artemisinin activity of Artemisia when they showed that A. afra lacking artemisinin also had an IC50 of 0.65 μg mL−1, comparable to that from four different cultivars of A. annua (ranging from 0.88 to 3.42 μg mL−1). Although the IC50s for all the Artemisia cultivars tested were similar to that for artemisinin, the selectivity index (SI) varied substantially from 3.52–26.89 for the Artemisia cultivars and was 4.3 for artemisinin. The higher the SI the less toxic the extract; two A. annua extracts had an SI < 7. It is possible that those two cultivars had artemisinin contents equivalent to the pure artemisinin that was tested. Unfortunately, no artemisinin contents were reported.
Nair et al. has continued to test the original (frozen) DLAe samples for efficacy against emerging SARS-CoV-2 variants including delta,26 omicron, and BA.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and to date the A. annua hot water extracts have remained potent. Although thus far there is no identification of the non-artemisinin active phytochemicals, there are several likely candidates including isorhamnetin, luteolin, and rosmarinic acid, which are present in A. annua with IC50 values of 8.42, 11.81, and 9.43 μM, respectively, against 3CLpro, the main chymotrypsin-like protease involved in SARS-CoV-2 viral replication.91 Arteannuin B, present in A. annua, also seems to have post-viral entry activity with an IC50 of 12.03 μM.92 Other A. annua phytochemicals, e.g., quercetin and myricetin, showed activity against SARS-CoV-2 NTPase/helicase.93,94 Many of these small molecules, especially flavonoids, are showing antiviral potential and likely work synergistically as antiviral agents. Future studies are needed to identify and validate the activity of these anti-SARS-CoV-2 therapeutic compounds, and to also validate efficacy of DLAe and putatively active phytochemicals in rodent models.
30 and to date the A. annua hot water extracts have remained potent. Although thus far there is no identification of the non-artemisinin active phytochemicals, there are several likely candidates including isorhamnetin, luteolin, and rosmarinic acid, which are present in A. annua with IC50 values of 8.42, 11.81, and 9.43 μM, respectively, against 3CLpro, the main chymotrypsin-like protease involved in SARS-CoV-2 viral replication.91 Arteannuin B, present in A. annua, also seems to have post-viral entry activity with an IC50 of 12.03 μM.92 Other A. annua phytochemicals, e.g., quercetin and myricetin, showed activity against SARS-CoV-2 NTPase/helicase.93,94 Many of these small molecules, especially flavonoids, are showing antiviral potential and likely work synergistically as antiviral agents. Future studies are needed to identify and validate the activity of these anti-SARS-CoV-2 therapeutic compounds, and to also validate efficacy of DLAe and putatively active phytochemicals in rodent models.
Other variations of artemisinin and A. annua extracts have been tested mainly in vitro against SARS-CoV-2. Although we and others showed artemisinin is not the sole anti-SARS-CoV-2 compound in Artemisia25,29 it does have some anti-SARS-CoV-2 activity.25,27–29,92,95,96 There have been some small nonrandomized clinical studies in patients treated with artemisinin-piperaquine (ART-PQ) or placebo.97 Compared to placebo at 19.3 days, the mean time for recovery for ART-PQ treated patients (no PCR-detectable virus) was 10.6 days. All ART-PQ treated patients were virus-free after 21 days compared to 36 days for placebo. In another small trial patients who used ArtemiC, an oral spray containing artemisinin, curcumin, frankincense, and vitamin C98 had faster recovery vs. placebo. To my knowledge, however, there are no reports of well controlled clinical trials solely using A. annua or its extracts.
4.3. Tuberculosis
Tuberculosis (TB), a communicable disease caused by respiratory transfer of Mycobacterium tuberculosis (Mtb), accounted for ∼1.2 million deaths of an estimated 10 million cases in 2020 (https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021). TB is currently treated with a series of drugs, mainly rifampicin and isoniazid, taken for about 6 mon and although deaths recently rose for the first time in years, likely attributed to the SARS-CoV-2 pandemic, there were fewer cases of drug resistant TB (https://www.who.int/news/item/14-10-2021-tuberculosis-deaths-rise-for-the-first-time-in-more-than-a-decade-due-to-the-covid-19-pandemic). Nevertheless, because of emerging drug resistance there is an ongoing search for new therapeutics.A. afra and A. annua showed some promise as TB therapeutics. Using M. aurum and Mtb H37Rv, Ntutela et al.14 compared efficacy of A. afra aqueous (AQ), DCM, and MeOH extracts in vitro and only the DCM extract yielded significant growth inhibition, albeit less than the isoniazid control. Upon fractionation of the DCM extract, the C8 fraction had a minimum inhibitory concentration (MIC) equivalent to the isoniazid control. However, when mice infected with the Mtb were treated with a daily 1 g dose of the C8 fraction, and DCM and AQ extracts and then compared to isoniazid, there was no significant decrease in either spleen or pulmonary bacilli even after 26 weeks treatment.
These results were in contrast to results from a small human trial in North Kivu (Democratic Republic of Congo) where a small number of TB patients were treated with A. annua or A. afra tea infusions (10 g dry mass per L), 330 mL every 8 h each day for 10 days.99 Using the Ziehl–Neelsen sputum assay, samples were negative for bacilli 10 and 15 days after treatment with A. afra and A. annua, respectively, suggesting that both species were effective in vivo; however, more study is needed. Taken together these studies suggest there may be merit to using A. afra and A. annua in treating TB; however, considerably more work is needed with well-controlled randomized clinical trials (RCTs) and to discern the mechanism of action of artemisinin and the different plant extracts.
Artemisinin was reported to block DosRST, a key stress response system in Mtb,15 and appeared to reduce viability in hypoxia, the granulosis stage of TB infection where the bacilli are less susceptible to drugs. Even in a DosR deletion mutant, artemisinin-induced gene expression changes suggesting other possible targets. Other work suggests that artemisinin may also affect lipid peroxidation in Mtb.100,101 Subsequent MIC analysis of artemisinin, A. annua, and A. afra DCM extracts against Mtb (H37Rv ΔRD1 ΔpanCD) in vitro showed that the A. annua extract was strongly bactericidal.13 In contrast, artemisinin at the same concentration, although also bactericidal, had slower killing kinetics. Results suggested that A. annua extracts killed Mtb through a combination of artemisinin and other phytochemicals found in the plant. Indeed, the A. afra extract with only negligible amounts of artemisinin also killed Mtb, but much slower than the A. annua extract. Similar effects were seen when the more virulent Mtb Erdman strain was tested.13 While the non-artemisinin active components in A. annua have not yet been identified, Bhowmick et al. suggested that artemisinic acid (AA) and deoxyartemisinin (dART) may have antimycobacterial roles.102 They reported a four-fold decrease in MIC when the AA + dART combination was tested against M. smegmatis, a mycobacterium species often used as a model to screen for anti-Mtb compounds. Others reported synergistic effects of some terpenes with rifampicin, isoniazid, and ethambutol on Mtb.103 Unfortunately, efficacy in M. smegmatis was not particularly predictive, as Martini et al.13 reported that despite significantly affecting growth, neither artemisinin nor the Artemisia extracts fully inhibited growth or killed M. smegmatis.
4.4. Lyme disease
Lyme disease affects millions of people and is mainly caused by the spirochetes, Borrelia burgdorferi, B. afzelii, and B. garinii (https://emedicine.medscape.com/article/330178-overview), but may also be attributed to or co-infected with other tick-borne parasites including human Babesia (https://www.bayarealyme.org/blog/lyme-with-a-side-or-two-of-babesia-the-most-common-co-infection-that-is-frequently-missed/). Although artemisinin12,104,105 and A. annua106 have shown in vitro efficacy against B. burgdorferi Lyme, in a mouse model they were ineffective against Babesia microti.107B. burgdorferi afflicts humans first by an acute infection that if caught early is readily treatable with a short course of doxycycline. However, infection is not always obvious and can be missed, leading to no treatment for several weeks often resulting in “post-treatment Lyme disease syndrome” (PTLDS) with clinical symptoms that can last 6 months or longer. Although not well characterized, one posited explanation for PTLDS is the formation of a slow-growing form of the spirochete termed a persister that is especially drug tolerant. Persisters can develop in vitro and display a round body morphology in response to exposure to Lyme antibiotics, e.g. amoxicillin (Amx), doxycycline (Dox), and cefuroxime (Cef).105 In a screen of an FDA approved drug library against stationary phase cultures of B. burgdorferi, one of the top hits was artemisinin showing about 50% greater potency than Dox and Amx, and equivalent to Cef, but less potent than daptomycin (DAP).12 In a follow-up study using Amx-induced persisters as well as stationary-phase cultures, artemisinin was compared with a number of drugs including Dox, DAP, and cephoperazone (CefP) and in combinations at 10 μg mL−1 ART + Dox was not substantially different from artemisinin in its effect on persisters or stationary phase cells. However, two 3-drug combinations, ART + Dox + CefP and ART + Dox + DAP, substantially reduced both persisters and stationary phase live cell numbers. Others observed similar effects from artemisinin104 and measured MIC for artemisinin, Dox, and DAP at 5.0, 0.08, and >10 μg mL−1, respectively, against stationary phase B. burgdorferi. Using the Cmax drug concentrations determined from the MIC analyses, cell viability was 53.6% and 65.8% for artemisinin and Dox, respectively. Artemisinin and Dox combinations were not reported, and other drug combinations showed greater potency than artemisinin or Dox. Feng et al.10 recently compared a variety of natural product ethanolic extracts including A. annua in 60% ethanol and measured stationary phase cell viability at 44% at MICs of 0.5 and 1%. At 1.0 and 0.5%, however, residual cells in treated cultures regrew after subculture. Dox-treated cells had 74% viability after initial treatment and also regrew upon subculture. Overall, both artemisinin and A. annua showed activity against B. burgdorferi, but considerably more studies are needed including, e.g., A. annua water extracts, multiple dosing, mechanistic studies, and testing in an animal model, to determine therapeutic potential.
4.5. Schistosomiasis
Schistosomiasis (bilharzia) is the result of trematode (blood fluke) infections caused by the genus Schistosoma transmitted by freshwater snails. WHO reports that in 2019 >105 million people were treated out of >236 million who needed a preventative treatment (July 15, 2022 accessed, https://www.who.int/news-room/fact-sheets/detail/schistosomiasis). Currently, the preferred therapy is the cost-effective praziquantel (PZQ), but there are concerns regarding a need for prophylaxis and possible emerging drug resistance.108Earlier studies suggested that when PZQ was combined with an artemisinin, e.g., AM or AS, clinical outcomes improved,109 but AS alone was not as effective as either PZQ or PZQ + AS. AM and AS also were prophylactic against schistosomiasis compared to placebo.109 Due to the above concerns and anecdotal evidence suggesting Artemisia had anti-schistosomal efficacy, a large clinical trial compared PZQ to A. annua and A. afra tea infusions at 1 L infusion/d for 7 days and showed faster elimination of the parasite than PZQ with fewer side effects.110 Although the study was later retracted, it provided clinical safety data on Artemisia-treated patients and stimulated subsequent in vitro and rodent studies8,9,111,112 that confirmed the efficacy of Artemisia vs. schistosomiasis.
In a recent in vitro study, S. mansoni worms were treated with A. annua (ANAMED A3) and A. afra tea infusions or extracts at 10 and 100 μg mL−1.9 After 24 h, only the 10 μg mL−1 dichloromethane (DCM) extract of A. afra killed 100% of worms, while at 72 h the hexane extract was also 100% effective. A. afra tea infusions were 100% effective at 24 and 72 h, but only at 100 μg mL−1. Hexane and DCM extracts of A. annua were 100% effective, but only at 100 μg mL−1 and at 72 h incubation. Although both Artemisia species were effective, artemisinin-free A. afra was more potent and authors posited this was due to as yet unidentified novel molecules. Yang et al.8 compared artemisinin and A. annua in per os treated mice to untreated infected and uninfected controls 7 days post-infection with the free-swimming larval stage (cercariae) of S. japonicum. Treatments were at 300 mg kg−1 of artemisinin dosing given once a week for 4 weeks. There was no significant difference between artemisinin and A. annua treatments in reduction of both worm (95.7 vs. 98.3%, respectively) and egg levels (98.4 and 100%, respectively), indicating the effect of the commercial plant extract (Henan Yuzhou Heima Pharmaceutical Co., Ltd Batch No. 20210715) was equivalent to artemisinin, albeit less costly to produce. In another study, Ado et al.111 used methanolic and aqueous extracts of A. annua and showed efficacy even against shedding of S. mansoni cercariae by its snail vector, Biomphlaria pfeifferi. More recently, Fadladdin et al.112 confirmed the in vitro efficacy of a cold-water extract of A. annua against adult male and female S. mansoni worms with subsequent validation in Golden hamsters. Histochemical analysis of the hamster tissues showed improved liver parenchyma morphology and the absence of eggs compared to untreated infected controls. Together these studies indicate that A. afra and A. annua have anti-schistosomal activity, and while artemisinin may play some part in that response, nonartemisinin phytochemicals mainly appear to be responsible.
5. Artemisinin drug resistance
Despite concerns113 about A. annua inducing artemisinin resistance, there is no evidence that use of the plant induces artemisinin drug resistance. Not only has the plant been used for millennia to treat variousfever-related diseases2 with no evidence of artemisinin resistance emerging from its use, there is also in vivo evidence4 that in a rodent malaria model the plant delivered at the same artemisinin level as pure artemisinin controls was at least three times more resilient against the evolution of artemisinin drug resistance than an equal amount of pure artemisinin. Furthermore, A. annua proved effective at eliminating artemisinin-resistant parasites in another rodent model, in contrast to pure artemisinin.4 Indeed, there are many phytochemicals in the plant that have antimalarial activity, albeit weaker than artemisinin as summarized in Gruessner and Weathers84 and that may explain the observed in vitro antimalarial effects of artemisinin-free Artemisia sp.5,6 Although the WHO argues that use of A. annua causes artemisinin resistance, they have not offered any direct evidence to support that stance. The WHO claims that A. annua does not provide enough artemisinin into the bloodstream to combat the parasite, and hence, fosters resistance. As noted previously, in vivo rodent studies measuring serum levels of artemisinin showed that artemisinin is >40-fold more bioavailable when delivered per os via A. annua,73 and later studies supported those results.74,75 This is consistent with earlier pharmacokinetic studies76 showing A. annua tea infusions containing 94.5 mg artemisinin/delivered dose/subject delivered high levels of artemisinin into the plasma of healthy human subjects, well above the 9 μg L−1 minimum antimalarial threshold required to kill the parasite.776. Is artemisinin the sole Artemisia therapeutic
Although artemisinin is a potent antimicrobial molecule, it may not always be the sole active therapeutic in A. annua. Monotherapies for treating infectious diseases are to be avoided to prevent the emergence of drug resistance and the World Health Organization (WHO) has continued to argue that the use of A. annua is an artemisinin monotherapy.72 Indeed, in their 2019 whitepaper, WHO ignored at least one significant prior report that countered the claim that use of A. annua would induce artemisinin drug resistance. The 2015 study4 showed that when A. annua dried leaves were given per os to mice infected with Plasmodium chabaudi, artemisinin drug resistance developed three-fold slower in the mouse model than for pure artemisinin delivery at the same artemisinin dose. Furthermore, in artemisinin-resistant strains of P. yoelii, the plant proved more effective than artemisinin at eliminating the artemisinin resistant parasite. Together those results indicated that compared to pure artemisinin, whole plant delivery of artemisinin was significantly more resilient to development of artemisinin drug resistance, a problem now emerging in Africa.114The WHO also focused only on artemisinin as the active principle in the plant. While artemisinin is well-established as a potent bioactive molecule with significant antimalarial activity, a growing number of studies have shown that there is considerable antimalarial activity from the plant that extends beyond artemisinin. For example, the studies already described in Section 4.1 on malaria5,6,65,84–86 clearly demonstrate that artemisinin is not the sole antimalarial molecule in A. annua. In fact, Ashraf et al., 2022 showed that artemisinin-free A. afra has potent antimalarial activity.5 Although the active phytochemical(s) are not yet identified, many with in vitro antimalarial activity found in A. annua and A. afra are summarized in a recent review46 and in other recent studies89,90 previously discussed in Section 4.1. These non-artemisinin antimalarial responses are often synergistic with artemisinin, and there was some evidence of that effect in early in vitro studies where the flavonoids chrysoplenol-D, cirsilineol, and eupatorin reduced the IC50 against P. falciparum by >50%.115 To clearly elucidate such a complicated therapeutic response requires further extensive investigation using biochemometric methods and such studies are in progress for malaria, TB, and COVID-19.
Another example of artemisinin not functioning as a sole therapeutic in A. annua therapy is against SARS-CoV-2. In that case, artemisinin-free A. afra had a potent anti-SARS-CoV-2 response29 and the results of Nair et al.25 suggested that artemisinin might be antagonistic to the antiviral effect of A. annua.
7. Conclusions
This presentation aimed to serve as an example of the types of questions that require empirical answers while also demonstrating the challenges that are present in trying to establish a medicinal plant that could be widely and cost-effectively implemented as a Botanical Drug. For approval as a drug, a therapeutic must be efficacious and safe. A. annua and A. afra are also edible plants, and while not necessarily palatable, they are safe for per os consumption so that makes this plant somewhat unusual. Nevertheless, any edible medicinal plant should first be considered as a holistic therapeutic or prophylactic entity with different potential delivery modes and their relevant caveats (Table 3).| Delivery mode | Pros | Cons |
|---|---|---|
| Traditional tea infusion at 5 g dried leaves infused in 1 L boiled water for 10 min, cool, strain, drink 1 L per day in 3 equal aliquots | Meshes well with traditional cultures prevalent in low- and middle-income countries | Not all compounds fully extracted |
| Artemisinin fully extracted if properly prepared | No storage beyond 12 h | |
| Artemisinin very bioavailable | ||
| Uses boiled water so provides modicum of safety during ingestion | ||
| Most cost-effective delivery mode | ||
| Encapsulated dried leaves: ∼0.33 g powdered dried leaves/capsule (size 00) | Acceptable delivery mode for people in more developed countries | Not a traditional delivery mode, so may be less acceptable to people in low- and middle-income countries |
| No losses due to extraction | Requires potable water to consume | |
| Artemisinin is very bioavailable | A bit more costly than tea infusion | |
| Can be used as a rectal suppository in neonates and children too young to swallow a capsule or drink tea | Must ingest large number of capsules to equate with tea infusion delivery/day | |
| Offers long term storage |
If there are active phytochemicals in the plant, those molecules would provide a molecular tracking system. In A. annua a key tracker molecule is artemisinin with others emerging as extracts are fractionated and analyzed for therapeutic activity. For A. afra there is no specific identified molecule to track. Nevertheless, one can establish phytochemical fingerprints or chemical profiles that link to therapeutic activity. Phytochemical fingerprints also help establish consistency of plant material. Examples of tracking methods include ultraviolet, infrared, Fourier transformed infrared, mass spectroscopy, high performance liquid chromatography (HPLC), gas chromatography (GC), thin layer chromatography (TLC), capillary zone electrophoresis, liquid chromatography-mass spectrometry (LC-MS), and GC-mass spectroscopy. Although not fully addressed here, consistency of plant crops is essential for establishing a medicinal plant as an approved Botanical Drug. Cultivation, processing, and use of a crude extract or a whole plant material, e.g., dried plant material used for infusion, powdered leaves encapsulated or compressed into a tablet, also are more sustainable options. They require fewer resources than pure molecules to process as a drug, they can be more locally sourced with less likelihood of counterfeiting, and they are considerably more cost-effective. Nevertheless, key requirements must be met regarding product consistency, safety, and of course efficacy. In the US meeting those requirements enables a product to be classified as a Botanical Drug.116
For approval as a Botanical Drug that has specific therapeutic claims, the same rules generally apply as for a pure molecule drug and these have been recently summarized for Botanical Drugs.43,117–120 Although as for any drug off-label use is possible, a botanical drug's approval is specific to its use as verified through RCTs. For approval of either A. annua or A. afra as Botanical Drugs, two key tasks are lacking: demonstration of a long-term supply of plant material with a consistent phytochemical content, and successful RCTs specific to at least one disease. While Botanical Drug approval is possible in the US for specific diseases like COVID-19, tuberculosis, Lyme disease etc., it is likely not a practical goal for LMI countries mainly because of the stringent quality control requirements. Once quality control criteria are met, however, Botanical Drug approval would be possible and could lead to off-label use for other artemisinin/Artemisia-susceptible diseases. Obtaining such approval, e.g. by the US FDA, would also provide validated support for more global use of these important medicinal plants as cost-effective therapeutics for many of the Artemisia-susceptible diseases that plague not only people in developed countries but also those in the developing world.
8. Abbreviations
| AA | Artemisinic acid |
| AB | Arteannuin B |
| ACT | Artemisinin combination therapy |
| ALT | Alanine aminotransferase |
| AM | Artemether |
| AMX | Amoxicillin |
| AQ | Aqueous extract |
| ART | Artemisinin |
| AS | Artesunate |
| AST | Aspartate aminotransferase |
| Cef | Cefuroximine |
| CefP | Cephoperazone |
| Dap | Daptomycin |
| dART | Deoxyartemisinin |
| DCM | Dichloromethane |
| DHA | Dihydroartemisinin |
| DLAe | Dried leaf hot water extract |
| Dox | Doxycycline |
| GRAS | Generally recognized as safe |
| LMIC | Low- and middle-income countries |
| MIC | Minimum inhibitory concentration |
| Mtb | Mycobacterium tuberculosis |
| PZQ | Praziquantel |
| RCT | Randomized clinical trial |
| SI | Selectivity index |
9. Conflicts of interest
There are no conflicts to declare.10. Acknowledgements
Thanks to Melissa J. Towler (PhD), Worcester Polytechnic Institute, for critical review of the manuscript. Award Number NIH-2R15AT008277-02 to PJW from the National Center for Complementary and Integrative Health funded some of the studies reported herein. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Center for Complementary and Integrative Health or the National Institutes of Health.11. References
- Y. Tu, Angew. Chem., Int. Ed., 2016, 55, 10210–10226 CrossRef CAS PubMed.
- E. Hsu, Br. J. Clin. Pharmacol., 2006, 61, 666–670 CrossRef PubMed.
- M. A. Elfawal, M. J. Towler, N. G. Reich, D. Golenbock, P. J. Weathers and S. M. Rich, PLoS One, 2012, 7, e52746 CrossRef CAS PubMed.
- M. A. Elfawal, M. J. Towler, N. G. Reich, P. J. Weathers and S. M. Rich, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 821–826 CrossRef CAS PubMed.
- K. Ashraf, S. Tajeri, C.-S. Arnold, N. Amanzougaghene, J.-F. Franetich, A. Vantaux, V. Soulard, M. Bordessoulles, G. Cazals, T. Bousema, G.-J. van Gemert, R. L. Grand, N. Dereuddre-Bosquet, J.-C. Barale, B. Witkowski, G. Snounou, R. Duval, C. Y. Botté and D. Mazier, Life Sci. Alliance, 2021, 5(3), e202101237 CrossRef PubMed.
- D. Snider and P. J. Weathers, J. Ethnopharmacol., 2021, 268, 113638 CrossRef CAS PubMed.
- J. F. Ferreira, P. Peaden and J. Keiser, Parasitol. Res., 2011, 109, 1585–1592 CrossRef PubMed.
- M. Yang, Y. Xu, J. Sun, Y. Guo, P. Jia, R. Mi, H. Yan and C. Zhu, 2022.
- L. Taljaard, A. Probst, R. Tornow, J. Keiser, R. K. Haynes and F. van der Kooy, Phytomedicine, 2022, 2, 100279 CrossRef.
- J. Feng, J. Leone, S. Schweig and Y. Zhang, Front. Med., 2020, 7, 6 CrossRef PubMed.
- J. Feng, T. Li, R. Yee, Y. Yuan, C. Bai, M. Cai, W. Shi, M. Embers, C. Brayton and H. Saeki, Discov. Med., 2019, 27, 125–138 Search PubMed.
- J. Feng, W. Shi, S. Zhang and Y. Zhang, Emerging Microbes Infect., 2015, 4, 1–15 Search PubMed.
- M. Martini, T. Zhang, J. Williams, R. Abramovitch, P. Weathers and S. Shell, J. Ethnopharmacol., 2020, 262, 113191 CrossRef CAS PubMed.
- S. Ntutela, P. Smith, L. Matika, J. Mukinda, H. Arendse, N. Allie, D. M. Estes, W. Mabusela, P. Folb and L. Steyn, Tuberculosis, 2009, 89, S33–S40 CrossRef PubMed.
- H. Zheng, C. J. Colvin, B. K. Johnson, P. D. Kirchhoff, M. Wilson, K. Jorgensen-Muga, S. D. Larsen and R. B. Abramovitch, Nat. Chem. Biol., 2017, 13, 218–225 CrossRef CAS PubMed.
- F. Ghaffarifar, F. E. Heydari, A. Dalimi, Z. M. Hassan, M. Delavari and H. Mikaeiloo, Iran. J. Parasitol., 2015, 10, 258 Search PubMed.
- M. Islamuddin, A. Farooque, B. Dwarakanath, D. Sahal and F. Afrin, J. Med. Microbiol., 2012, 61, 1709–1718 CrossRef CAS PubMed.
- L. E. Mesa, D. Vasquez, P. Lutgen, I. D. Vélez, A. M. Restrepo, I. Ortiz and S. M. Robledo, Rev. Soc. Bras. Med. Trop., 2017, 50, 52–60 CrossRef PubMed.
- M. Berrizbeitia de Morgado, Y. Cariaco Sifontes, J. Imery Buiza and P. Lutgen, Enferm. Infecc. Microbiol. Clin., 2017, 390–392 CrossRef PubMed.
- Y. V. Mishina, S. Krishna, R. K. Haynes and J. C. Meade, Antimicrob. Agents Chemother., 2007, 51, 1852–1854 CrossRef CAS PubMed.
- M. Derda, A. Wojtkowiak-Giera, A. Kolasa-Wołosiuk, D. Kosik-Bogacka, E. Hadaś, P. P. Jagodziński and E. Wandurska-Nowak, Exp. Parasitol., 2016, 165, 30–34 CrossRef CAS PubMed.
- D. M. Kim and R. Neiva, J. Periodontol., 2015, 86, S56–S72 CrossRef PubMed.
- A. Lubbe, I. Seibert, T. Klimkait and F. Van der Kooy, J. Ethnopharmacol., 2012, 141, 854–859 CrossRef PubMed.
- A. Devaraj and M. F. Roelofson, World J. Pharm. Res., 2015, 4, 941–946 Search PubMed.
- M. S. Nair, Y. Huang, D. A. Fidock, S. J. Polyak, J. Wagoner, M. J. Towler and P. J. Weathers, J. Ethnopharmacol., 2021, 274, 114016 CrossRef CAS PubMed.
- M. S. Nair, Y. Huang, D. A. Fidock, M. Towler and P. Weathers, J. Ethnopharmacol., 2022, 284, 114797 CrossRef CAS PubMed.
- R. Cao, H. Hu, Y. Li, X. Wang, M. Xu, J. Liu, H. Zhang, Y. Yan, L. Zhao and W. Li, ACS Infect. Dis., 2020, 6, 2524–2531 CrossRef CAS PubMed.
- Y. Zhou, K. Gilmore, S. Ramirez, E. Settels, K. A. Gammeltoft, L. V. Pham, U. Fahnøe, S. Feng, A. Offersgaard, J. Trimpert, J. Bukh, K. Osterrieder, J. M. Gottwein and P. H. Seeberger, Sci. Rep., 2021, 11, 1–14 CrossRef PubMed.
- C. Nie, J. Trimpert, S. Moon, R. Haag, K. Gilmore, B. B. Kaufer and P. H. Seeberger, Virol. J., 2021, 18, 182 CrossRef CAS PubMed.
- M. S. Nair, Y. Huang and P. Weathers, bioRxiv, 2022, preprint.
- M. R. Romero, T. Efferth, M. A. Serrano, B. Castaño, R. I. Macias, O. Briz and J. J. Marin, Antiviral Res., 2005, 68, 75–83 CrossRef CAS PubMed.
- S. K. Goodrich, C. R. Schlegel, G. Wang and J. L. Belinson, Future Oncol., 2014, 10, 647–654 CrossRef CAS PubMed.
- A. Mondal and U. Chatterji, J. Cell. Biochem., 2015, 116, 1968–1981 CrossRef CAS PubMed.
- D. Dolivo, P. Weathers and T. Dominko, Acta Pharm. Sin. B, 2021, 11, 322–339 CrossRef CAS PubMed.
- E. G. Helal, N. Abou-Aouf, A. M. Khattab and M. A. Zoair, Egypt. J. Hosp. Med., 2014, 31, 1–16 Search PubMed.
- M. Ghanbari, M. S. Lamuki, E. Habibi and F. Sadeghimahalli, J. Pharmacopunct., 2022, 25, 130 CrossRef PubMed.
- Y.-y. Jiang, J.-c. Shui, B.-x. Zhang, J.-w. Chin and R.-s. Yue, Front. Pharmacol., 2020, 11, 585487 CrossRef CAS PubMed.
- M. R. Desrosiers, A. Mittelman and P. J. Weathers, Biomolecules, 2020, 10, 254 CrossRef CAS PubMed.
- G. Abate, L. Zhang, M. Pucci, G. Morbini, E. Mac Sweeney, G. Maccarinelli, G. Ribaudo, A. Gianoncelli, D. Uberti and M. Memo, Biomolecules, 2021, 11, 975 CrossRef CAS PubMed.
- C. Shi, H. Li, Y. Yang and L. Hou, Mediators Inflammation, 2015, 2015, 435713 Search PubMed.
- A. Du Toit and F. Van der Kooy, J. Ethnopharmacol., 2019, 244, 112127 CrossRef CAS PubMed.
- N. Liu, F. Van der Kooy and R. Verpoorte, S. Afr. J. Bot., 2009, 75, 185–195 CrossRef CAS.
- J. Dou and P. Weathers, Plant Cell, Tissue Organ Cult., 2022, 149, 105–111 CrossRef CAS PubMed.
- H. Y. Wetzstein, J. A. Porter, J. Janick, J. F. S. Ferreira and T. M. Mutui, Front. Plant Sci., 2018, 9, 358 CrossRef PubMed.
- H. Y. Wetzstein, J. A. Porter, J. Janick, J. F. Ferreira and T. M. Mutui, Front. Plant Sci., 2018, 9, 358 CrossRef PubMed.
- B. M. Gruessner, L. Cornet-Vernet, M. R. Desrosiers, P. Lutgen, M. J. Towler and P. J. Weathers, Phytochem. Rev., 2019, 18, 1509–1527 CrossRef CAS PubMed.
- F. Alejos-Gonzalez, G. Qu, L.-L. Zhou, C. H. Saravitz, J. L. Shurtleff and D.-Y. Xie, Planta, 2011, 234, 685–697 CrossRef CAS PubMed.
- D.-M. Ma, Z. Wang, L. Wang, F. Alejos-Gonzales, M.-A. Sun and D.-Y. Xie, Mol. Plant, 2015, 8, 1580–1598 CrossRef CAS PubMed.
- J. A. Duke, Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants, CRC Press, 2001 Search PubMed.
- U. FDA, CFR – Code of Federal Regulations Title 21, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.510).
- L. Lagarce, N. Lerolle, P. Asfar, Y. Le Govic, P. Lainé-Cessac and L. de Gentile, J. Travel Med., 2016, 23, taw049 CrossRef PubMed.
- F. J. Ruperti-Repilado, S. Haefliger, S. Rehm, M. Zweier, K. M. Rentsch, J. Blum, A. Jetter, M. Heim, A. Leuppi-Taegtmeyer and L. Terracciano, Front. Med., 2019, 6, 221 CrossRef PubMed.
- R. L. Savage, G. R. Hill, J. Barnes, S. H. Kenyon and M. V. Tatley, Front. Pharmacol., 2019, 10, 1448 CrossRef CAS PubMed.
- J. T. Mukinda and J. A. Syce, J. Ethnopharmacol., 2007, 112, 138–144 CrossRef CAS PubMed.
- T. O. Sunmonu and A. J. Afolayan, J. Evidence-Based Complementary Altern. Med., 2013, 2013 Search PubMed.
- N. Kane, M. Kyama, J. Nganga, A. Hassanali, M. Diallo and F. Kimani, S. Afr. J. Bot., 2019, 125, 126–133 CrossRef CAS.
- T. Deiml, R. Haseneder, W. Zieglgänsberger, G. Rammes, B. Eisensamer, R. Rupprecht and G. Hapfelmeier, Neuropharmacology, 2004, 46, 192–201 CrossRef CAS PubMed.
- K. M. Höld, N. S. Sirisoma, T. Ikeda, T. Narahashi and J. E. Casida, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3826–3831 CrossRef PubMed.
- E. M. Agency, Public statement on the use of herbal medicinal products containing thujone, https://www.ema.europa.eu/en/documents/public-statement/draft-public-statement-use-herbal-medicinal-products-containing-thujone_en.pdf Search PubMed.
- M. R. Desrosiers, M. J. Towler and P. J. Weathers, in Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production, ed. S. Malik, Springer International Publishing, Cham, 2019, pp. 197–209, DOI:10.1007/978-3-030-16546-8_6.
- M. Tegtmeier and G. Harnischfeger, Eur. J. Pharm. Biopharm., 1994, 40, 337–340 CAS.
- M. Siddiqui, S. Waghmare, S. Hajare, R. I. S. Deshmukh and S. Ali, J. Pharmacogn. Phytochem., 2018, 7, 1893–1895 CAS.
- C. Y. Park, E. Choi, H. J. Yang, S. H. Ho, S. J. Park, K. M. Park and S. H. Kim, Food Sci. Nutr., 2020, 8, 3738–3749 CrossRef CAS PubMed.
- B. Han, S.-M. Kim, G. E. Nam, S.-H. Kim, S.-J. Park, Y.-K. Park and H. W. Baik, Clin. Nutr. Res., 2020, 9, 258 CrossRef PubMed.
- J. Munyangi, L. Cornet-Vernet, M. Idumbo, C. Lu, P. Lutgen, C. Perronne, N. Ngombe, J. Bianga, B. Mupenda, P. Lalukala, G. Mergeai, D. Mumba, M. Towler and P. Weathers, Phytomedicine, 2019, 57, 49–56 CrossRef CAS PubMed.
- E. Ogbole, Y. Ogbole, J. Peter, M. Builders and J. Aguiyi, 2014.
- M. Willcox, G. Bodeker, G. Bourdy, V. Dhingra, J. Falquet, J. F. Ferreira, B. Graz, H.-M. Hirt, E. Hsu and P. M. de Magalhães, in Traditional medicinal plants and malaria, 2004, vol. 4, ch. 3, pp. 43–59 Search PubMed.
- K. Mekonen, M. Afework, E. Makonnen, A. Debela, W. Ergete and T. Tolessa, Ethiop. J. Health Sci., 2020, 30 Search PubMed.
- C. Muthaura, J. Keriko, C. Mutai, A. Yenesew, J. Gathirwa, B. Irungu, R. Nyangacha, G. Mungai and S. Derese, J. Ethnopharmacol., 2015, 175, 315–323 CrossRef CAS PubMed.
- N. Eshetu, M. Afework, E. Makonnen, A. Debella, W. Ergete and T. Tolesssa, Adv. Biosci. Bioeng., 2016, 4, 1–9 Search PubMed.
- WHO, Artemisinin Derivatives: Summary of Nonclinical Safety Data, https://extranet.who.int/pqweb/sites/default/files/documents/55%20Nonclinical%20overview%20artemisinin%20derivatives_Jan2016_0.pdf, accessed 13 Aug 2022.
- WHO, The use of non-pharmaceutical forms of Artemisia, https://www.who.int/publications/i/item/WHO-CDS-GMP-2019.14, accessed 27 April 2022.
- P. J. Weathers, P. R. Arsenault, P. S. Covello, A. McMickle, K. H. Teoh and D. W. Reed, Phytochem. Rev., 2011, 10, 173–183 CrossRef CAS PubMed.
- M. A. Elfawal, M. J. Towler, N. G. Reich, D. Golenbock, P. J. Weathers and S. M. Rich, PLoS One, 2012, 7(12), e52746 CrossRef CAS PubMed.
- P. J. Weathers, M. A. Elfawal, M. J. Towler, G. K. Acquaah-Mensah and S. M. Rich, J. Ethnopharmacol., 2014, 153, 732–736 CrossRef CAS PubMed.
- K. Räth, K. Taxis, G. Walz, C. H. Gleiter, S.-M. Li and L. Heide, Am. J. Trop. Med. Hyg., 2004, 70, 128–132 CrossRef.
- M. H. Alin and A. Bjorkman, Am. J. Trop. Med. Hyg., 1994, 50, 771–776 CrossRef CAS PubMed.
- M. R. Desrosiers and P. J. Weathers, J. Ethnopharmacol., 2016, 190, 313–318 CrossRef CAS PubMed.
- M. R. Desrosiers and P. J. Weathers, J. Ethnopharmacol., 2018, 210, 254–259 CrossRef CAS PubMed.
- N. F. Kane, B. H. Kiani, M. R. Desrosiers, M. J. Towler and P. J. Weathers, J. Ethnopharmacol., 2022, 15(298), 115587 CrossRef PubMed.
- J. Wang, C. Xu, F. L. Liao, T. Jiang, S. Krishna and Y. Tu, N. Engl. J. Med., 2019, 380, 2087–2089 CrossRef PubMed.
- K. A. O'Connell, H. Gatakaa, S. Poyer, J. Njogu, I. Evance, E. Munroe, T. Solomon, C. Goodman, K. Hanson and C. Zinsou, Malar. J., 2011, 10, 1–14 CrossRef PubMed.
- O. Adeyi and R. Atun, Lancet, 2010, 376, 1869–1871 CrossRef PubMed.
- B. M. Gruessner and P. J. Weathers, Plos One, 2021, 16, e0240874 CrossRef CAS PubMed.
- N. B. Daddy, L. M. Kalisya, P. G. Bagire, R. L. Watt, M. J. Towler and P. J. Weathers, Phytomedicine, 2017, 32, 37–40 CrossRef PubMed.
- M. S. Mueller, N. Runyambo, I. Wagner, S. Borrmann, K. Dietz and L. Heide, Trans. R. Soc. Trop. Med. Hyg., 2004, 98, 318–321 CrossRef PubMed.
- P. Moyo, P. Kunyane, M. A. Selepe, J. N. Eloff, J. Niemand, A. I. Louw, V. J. Maharaj and L.-M. Birkholtz, Malar. J., 2019, 18, 1–11 CrossRef PubMed.
- T. Czechowski, M. A. Rinaldi, M. T. Famodimu, M. Van Veelen, T. R. Larson, T. Winzer, D. A. Rathbone, D. Harvey, P. Horrocks and I. A. Graham, Front. Plant Sci., 2019, 984 CrossRef PubMed.
- J. Li, C. Zhang, M. Gong and M. Wang, Phytother. Res., 2018, 32, 1415–1420 CrossRef CAS PubMed.
- H. Shi, Z. Wang, F. Xu, J. Li, J. Li and M. Wang, Int. J. Mol. Sci., 2022, 23, 14903 CrossRef PubMed.
- N. Shahhamzehei, S. Abdelfatah and T. Efferth, Pharmaceuticals, 2022, 15, 308 CrossRef CAS PubMed.
- Y. Hu, M. Liu, H. Qin, H. Lin, X. An, Z. Shi, L. Song, X. Yang, H. Fan and Y. Tong, Front. Cell. Infect. Microbiol., 2021, 526 Search PubMed.
- M. Russo, S. Moccia, C. Spagnuolo, I. Tedesco and G. L. Russo, Chem.-Biol. Interact., 2020, 328, 109211 CrossRef CAS PubMed.
- J. Solnier and J.-P. Fladerer, Phytochem. Rev., 2021, 20, 773–795 CrossRef CAS PubMed.
- M. Gendrot, J. Andreani, M. Boxberger, P. Jardot, I. Fonta, M. Le Bideau, I. Duflot, J. Mosnier, C. Rolland and H. Bogreau, Travel Med. Infect. Dis., 2020, 37, 101873 CrossRef PubMed.
- M. Gendrot, I. Duflot, M. Boxberger, O. Delandre, P. Jardot, M. Le Bideau, J. Andreani, I. Fonta, J. Mosnier and C. Rolland, Int. J. Infect. Dis., 2020, 99, 437–440 CrossRef CAS PubMed.
- G. Li, M. Yuan, H. Li, C. Deng, Q. Wang, Y. Tang, H. Zhang, W. Yu, Q. Xu and Y. Zou, Int. J. Antimicrob. Agents, 2021, 57, 106216 CrossRef CAS PubMed.
- E. Hellou, J. Mohsin, A. Elemy, F. Hakim, M. Mustafa-Hellou and S. Hamoud, J. Cell. Mol. Med., 2022, 26, 3281–3289 CrossRef CAS PubMed.
- N. B. Daddy, P. Lutgen and P. Gisenya, Pharm. Pharmacol. Int. J., 2021, 9, 58–62 CrossRef.
- M. J. Miller, A. J. Walz, H. Zhu, C. Wu, G. Moraski, U. Mollmann, E. M. Tristani, A. L. Crumbliss, M. T. Ferdig, L. Checkley, R. L. Edwards and H. I. Boshoff, J. Am. Chem. Soc., 2011, 133, 2076–2079 CrossRef CAS PubMed.
- Y. S. Patel, N. Mistry and S. Mehra, Tuberculosis, 2019, 115, 146–153 CrossRef CAS PubMed.
- S. Bhowmick, R. Baptista, D. Fazakerley, K. E. Whatley, K. F. Hoffmann, J. Shen and L. A. Mur, bioRxiv, 2020, preprint.
- E. Sieniawska, M. Swatko-Ossor, R. Sawicki, K. Skalicka-Woźniak and G. Ginalska, Med. Princ. Pract., 2017, 26, 108–112 CrossRef PubMed.
- H. S. Alvarez-Manzo, Y. Zhang, W. Shi and Y. Zhang, Antibiotics, 2020, 9, 542 CrossRef CAS PubMed.
- J. Feng, W. Shi, S. Zhang, D. Sullivan, P. G. Auwaerter and Y. Zhang, Front. Microbiol., 2016, 7, 743 Search PubMed.
- X. Feng, S. Cao, F. Qiu and B. Zhang, Pharmacol. Ther., 2020, 216, 107650 CrossRef CAS PubMed.
- M. A. Elfawal, O. Gray, C. Dickson-Burke, P. J. Weathers and S. M. Rich, Longhua Chin. Med., 2021, 4, 12 CrossRef PubMed.
- N. Vale, M. J. Gouveia, G. Rinaldi, P. J. Brindley, F. Gärtner and J. M. Correia da Costa, Antimicrob. Agents Chemother., 2017, 61, e02582–e02516 CrossRef CAS PubMed.
- L. Perez del Villar, F. J. Burguillo, J. Lopez-Aban and A. Muro, 2012.
- J. Munyangi, L. Cornet-Vernet, M. Idumbo, C. Lu, P. Lutgen, C. Perronne, N. Ngombe, J. Bianga, B. Mupenda and P. Lalukala, Phytomedicine, 2018, 51, 233 CrossRef CAS PubMed.
- I. Ado, Z. Ali, M. Dogara, K. Abdullahi, S. Luka, H. Nock, I. Ndams and A. Madara, Niger. J. Parasitol., 2021, 42, 25–30 CrossRef.
- Y. A. J. Fadladdin, Biomed Res. Int., 2022, 2022, 5172287 Search PubMed.
- E. Ataba, A. M. Dorkenoo, C. T. Nguepou, T. Bakai, T. Tchadjobo, K. D. Kadzahlo, K. Yakpa and T. Atcha-Oubou, Acta Parasitol., 2022, 67, 55–60 CrossRef CAS PubMed.
- A. Uwimana, E. Legrand, B. H. Stokes, J.-L. M. Ndikumana, M. Warsame, N. Umulisa, D. Ngamije, T. Munyaneza, J.-B. Mazarati and K. Munguti, Nat. Med., 2020, 26, 1602–1608 CrossRef CAS PubMed.
- K. Liu, S.-L. Yang, M. Roberts, B. Elford and J. Phillipson, Plant Cell Rep., 1992, 11, 637–640 CrossRef CAS PubMed.
- F. A. Hoffman, Epilepsy Behav., 2015, 52, 338–343 CrossRef PubMed.
- J. McChesney, J. Dou and P. Harrington, Pharmaceut. Reg. Affairs, 2019, 8, 2 Search PubMed.
- B. C. Sorkin, A. J. Kuszak, G. Bloss, N. K. Fukagawa, F. A. Hoffman, M. Jafari, B. Barrett, P. N. Brown, F. D. Bushman and S. J. Casper, FASEB J., 2020, 34, 41–65 CrossRef CAS PubMed.
- J. Dou, J. Beitz and R. Temple, in The Science and Regulations of Naturally Derived Complex Drugs, Springer, 2019, pp. 245–264 Search PubMed.
- C. Wu, S.-L. Lee, C. Taylor, J. Li, Y.-M. Chan, R. Agarwal, R. Temple, D. Throckmorton and K. Tyner, J. Nat. Prod., 2020, 83, 552–562 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |

