Open Access Article
Open Access ArticleProtected amino acids as a nonbonding source of chirality in induction of single-handed screw-sense to helical macromolecular catalysts†
Shoma
Ikeda
,
Ryohei
Takeda
,
Takaya
Fujie
,
Naoto
Ariki
,
Yuuya
Nagata‡
* and
Michinori
Suginome
 *
*
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: suginome@sbchem.kyoto-u.ac.jp; nagata@icredd.hokudai.ac.jp
First published on 26th May 2021
Abstract
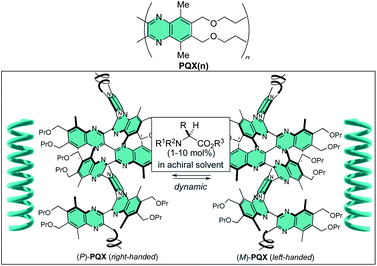
Chiral nonbonding interaction with N-protected amino acid methyl esters used as chiral additives in achiral solvents allows dynamic induction of single-handed helical conformation in poly(quinoxaline-2,3-diyl)s (PQX) bearing only achiral substituents. Ac-L-Pro-OMe, for instance, allows induction of energy preference of 0.16 kJ mol−1 per monomer unit for the M-helical structure over the P-helix in t-butyl methyl ether (MTBE). With this new mode of screw-sense induction, homochiral screw-sense has been induced in virtually achiral poly(quinoxaline-2,3-diyl)s 1000-mer containing phosphine pendants (PQXphos). Use of PQXphos as a helically dynamic ligand along with Ac-Pro-OMe (L or D) as a chiral additive in MTBE allowed a highly enantioselective Suzuki–Miyaura coupling reaction with up to 95% enantiomeric excess.
Introduction
Induction of nonracemic screw-sense in dynamic helical macromolecular structures has gained ever-increasing interest1 because unique chiral functions of dynamic nonracemic helical macromolecules have rapidly been developed in chiral separation,2 chiral detection,3 selective emission/reflection of circularly polarized light,4,5 and asymmetric catalysis.6 Recent efforts have enabled the use of external chiral sources for the induction of helical macromolecules that have no covalently bonded chiral groups.7–10 Although the use of covalently bonded chiral side chains has been a quite robust strategy to form nonracemic helical main chain conformations,11 the utilization of external chiral additives as sources of chirality is quite advantageous because it allows escaping from the tedious and costly synthesis of monomers containing chiral groups.Chiral additives that interact with polymer chains through dynamic covalent bonds,7 ionic interactions,8 hydrogen bonding,9 and host–guest interactions10 have been used to shift the equilibrium between right- and left-handed helical conformations. Particular interest is currently focused on the utilization of weak nonbonding interactions such as dipole–dipole and dispersion interactions for the induction of single-handed screw-sense.12 Even though the polymer has no specific receptor sites to interact with chiral additives, unfunctionalized chiral molecules including chiral hydrocarbons and haloalkanes allow inducing biased screw-sense to the polymer main chain. This induction mode is remarkable in that significant screw-sense induction has been achieved despite the weak nondirectional molecular interactions. Macromolecular scaffolds allow amplifying such small energy differences per monomer units in large macromolecular scaffolds.13 Through this mode of chirality induction, detection of “hidden” chirality of saturated hydrocarbons with quaternary stereocenters has been enabled.3
Utilization of a chiral nonbonding interaction allowed us to induce single-handed screw-sense to virtually achiral poly(quinoxaline-2,3-diyl)s (PQX hereafter) using chiral solvents including limonene.14,15 Application of these macromolecules as a chiral ligand in highly enantioselective asymmetric catalysis has been demonstrated as the first example for the use of chiral solvent as a source of chirality in asymmetric catalysis.16 In the system, the nonbonding interactions, including dispersion forces, between chiral solvent and the backbone of PQX may play a crucial role in determining the position of equilibrium between right- and left-handed helical conformations. Although the details of their molecular interaction await further clarification, the scope of chiral guests is important practically to find more applications of this unique phenomenon. Particularly important is the utilization of naturally occurring chiral feedstocks as chiral additives and reduction of their loading amounts. In this paper, we screened natural amino acid derivatives as new chiral additives for induction of single-handed screw-sense, which leads to an asymmetric Suzuki–Miyaura coupling reaction in the presence of a virtually achiral macromolecular phosphine ligand along with a small amount of fully protected amino acids such as Ac-L-Pro-OMe.
Results and discussion
To test the ability of protected amino acids in screw-sense induction, circular dichroism (CD) spectra of PQX n-mer (PQX(n)) bearing n-propoxymethyl side chains in various achiral solvents with the protected amino acids were measured (Scheme 1). Firstly, CD spectra of PQX(100) in THF containing 16 enantiopure Boc-protected amino acid methyl esters were compared (amino acid/THF = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (mol/mol)). All spectra showed the same CD signals with varied intensities and signs (see the ESI†). The signs and intensities of the CD signal (dissymmetry factor; g value) at 366–371 nm at 293 K are summarized in Table 1. For the sake of comparison, when used as a chiral additive in THF (11 mol%), (R)-limonene afforded gabs of 0.53 × 10−3, which corresponds roughly to 20–25% screw-sense excess (se) (entry 0).
90 (mol/mol)). All spectra showed the same CD signals with varied intensities and signs (see the ESI†). The signs and intensities of the CD signal (dissymmetry factor; g value) at 366–371 nm at 293 K are summarized in Table 1. For the sake of comparison, when used as a chiral additive in THF (11 mol%), (R)-limonene afforded gabs of 0.53 × 10−3, which corresponds roughly to 20–25% screw-sense excess (se) (entry 0).
 | ||
| Scheme 1 Two enantiomeric (P- and M-) conformations of achiral PQX, of which equilibrium is shifted by N-protected amino acid esters used as additives in an achiral solvent. | ||
| Entry | Chiral additives | CD intensities (gabs/10−3)b |
|---|---|---|
a In THF containing the amino acid (AA) derivatives (molar ratio of AA derivatives and THF = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) with PQX(100) (ca. 7 × 10−4 M) at 293 K.
b Δε/ε at 367–371 nm (293 K).
c 11 90) with PQX(100) (ca. 7 × 10−4 M) at 293 K.
b Δε/ε at 367–371 nm (293 K).
c 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89 molar ratio of limonene and THF. 89 molar ratio of limonene and THF.
|
||
| 0 | (R)-limonenec | +0.53 (P) |
| 1 | Boc-L-Pro-OMe | −1.16 (M) |
| 2 | Boc-D-Pip-OMe | +0.80 (P) |
| 3 | Boc-L-Ala-OMe | −0.80 (M) |
| 4 | Boc-L-Thr-OMe | −0.63 (M) |
| 5 | Boc-L-t-Leu-OMe | −0.35 (M) |
| 6 | Boc-L-Glu(OMe)–OMe | −0.34 (M) |
| 7 | Boc-L-Ile-OMe | −0.28 (M) |
| 8 | Boc-L-Asn-OMe | −0.22 (M) |
| 9 | Boc-L-Tyr-OMe | −0.15 (M) |
| 10 | Boc-L-Ser-OMe | −0.28 (M) |
| 11 | Boc-L-Gln-OMe | −0.19 (M) |
| 12 | Boc-L-Val-OMe | −0.07 (M) |
| 13 | Boc-L-Phe-OMe | +0.08 (P) |
| 14 | Boc-L-Cys-OMe | +0.10 (P) |
| 15 | Boc-L-Asp(OMe)–OMe | +0.29 (P) |
| 16 | Boc-L-Leu-OMe | +0.18 (P) |
The proline derivative showed the most efficient induction, leading to the formation of left-handed (M) helix with se higher than 50% (entry 1). A six-membered ring derivative Boc-Pip-OMe (D-isomer) and L-Ala also showed efficient screw-sense induction to the same direction as L-Pro in terms of the relationship between the absolute configuration of the additives and the induced screw sense. Indeed, the observed screw-sense induction was significantly higher than the induction by (R)-limonene (entry 0). The majority of the L- and D-amino acid derivatives induced M- and P-helical conformation, respectively, with varied degrees of screw-sense induction (entries 1–12). However, four of the tested L-amino acids including the leucine and asparagine derivatives (entries 15 and 16) induced right-handed (P) helical conformation albeit with low screw sense excesses (entries 13–16). No clear relationship was found between the sense/degree of screw-sense induction and the structure of the amino acid derivatives.
We then evaluated the effect of protective groups of proline on the screw-sense induction (Table 2). In terms of the groups at the C-termini, protection with ester was found to be more effective than amide or acid functionality (entries 1–5). Among a series of esters, methyl esters showed the highest induction. In terms of N-protection, trifluoroacetamide and acetamide showed a much more efficient induction of M-helical sense (entries 8 and 9, 80–85% se) than did the others.
| Entry | Amino acid derivatives | CD intensities (gabs/10−3)b |
|---|---|---|
a In THF containing the amino acid (AA) derivatives (molar ratio: 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) with PQX(100) (ca. 7 × 10−4 M) at 293 K.
b Δε/ε at 367–371 nm (293 K). 10) with PQX(100) (ca. 7 × 10−4 M) at 293 K.
b Δε/ε at 367–371 nm (293 K).
|
||
| 1 | Boc-L-Pro-OMe | −1.16 (M) |
| 2 | Boc-L-Pro-NH2 | −0.29 (M) |
| 3 | Boc-L-Pro-OH | −0.52 (M) |
| 4 | Boc-L-Pro-OEt | −0.79 (M) |
| 5 | Boc-L-Pro-O-n-C6H13 | −1.01 (M) |
| 6 | Cbz-L-Pro-OMe | −1.08 (M) |
| 7 | Piv-L-Pro-OMe | −1.31 (M) |
| 8 | Ac-L-Pro-OMe | −1.75 (M) |
| 9 | TFAc-L-Pro-OMe | −1.64 (M) |
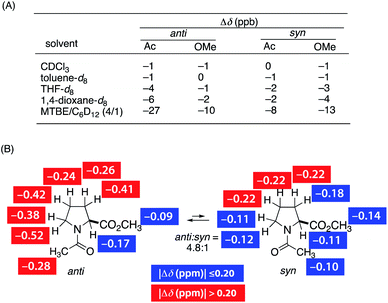
We found a strong effect of solvent on the screw-sense induction to PQX(30) with TFAc-L-Pro-OMe (Fig. 1, red bars). In comparison to THF used in the above measurements, significantly weaker induction was obtained in chloroform. By contrast, t-butyl methyl ether (MTBE) showed much more effective induction than did THF. This trend was maintained in the induction to PQX(30) with Ac-L-Pro-OMe (Fig. 1, blue bars). This result suggests that chloroform has a strong nonbonding interaction with PQX, thereby preventing interaction of the chiral guests with PQX, while MTBE has a weak interaction with PQX, thus maximizing the screw-sense induction. Although we cannot exclude the other possibility where the chiral additive anyhow interacts with polymer preferentially over the solvent to form “supramolecular complex”, of which screw-sense is steered by solvent effect. However, our NMR measurements of Ac-L-Pro-OMe in the presence of PQX(100) in different solvents revealed that the chemical shifts of Ac-L-Pro-OMe sharply depends on solvent (Fig. 2(A)). Whereas no apparent change of the chemical shift was observed in CHCl3 and toluene, appreciable change was observed in THF, dioxane, and MTBE. In particular, remarkable change of chemical shift was observed in MTBE. This result may support the former assumption that there is competitive interaction by chiral additives and achiral solvent. In the 1H NMR measurements of Ac-L-Pro-OMe, all the signals of Ac-L-Pro-OMe were up-field shifted in the presence of PQX(100). The Δδ observed in cyclohexane-d12, in which the up-field shift was even more pronounced, are shown in Fig. 2(B). It should be noted that the hydrophobic region, i.e., the Ac methyl group and ring methylenes, of the major anti-conformer of Ac-L-Pro-OMe showed larger change of chemical shifts than did its other part, which contains polar carbonyl oxygens. Although this observation still gives no clear information on the nature of the nonbonding interaction, it is likely that the hydrophobic region of Ac-L-Pro-OMe is more favorably incorporated into the backbone of PQX, of which quinoxaline rings may bring about the observed up-field shift in the NMR measurements.
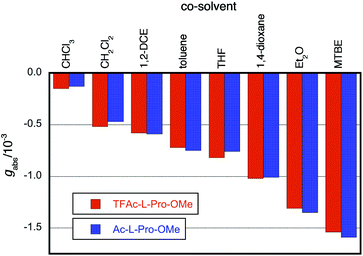 | ||
| Fig. 1 CD intensities (gabs at 367–371 nm at 293 K) of PQX(30) in various solvents in the presence of TFAc-L-Pro-OMe and Ac-L-Pro-OMe (10 mol% in the solvents). | ||
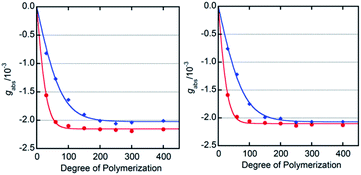
We determined the energy profile of the P/M equilibria in THF and MTBE in the presence of Ac- and TFAc-L-Pro-OMe (10 mol%) by measuring the CD spectra of PQX(n) with different polymerization degrees (n = 30–400), which were selectively synthesized using living polymerization (Fig. 3). The plot of CD intensities against polymerization degrees showed that higher screw-sense induction was achieved with increase in polymerization degree. The curve fitting according to Green's theory13 brings about the energy difference between M- and P-helices per unit (ΔGh) of 0.16 kJ mol−1 for Ac-L-Pro-OMe in MTBE (Table 3, entry 5). The ΔGh with Ac-L-Pro-OMe in MTBE is significantly higher than that in pure limonene, even though the amino acid additives are used in a small quantity. Even the use of 0.25 mol% Ac-L-Pro-OMe in MTBE allows the induction of M-helix with 88% se to PQX(1000) (Fig. 4).
| Entry | Chiral additive (mol%) | Solvent | ΔGh (kJ mol−1) | g max/10−3 |
|---|---|---|---|---|
| 1 | (R)-Limonene (100) | None | 0.104 | +2.37 (P) |
| 2 | TFAc-L-Pro-OMe (10) | THF | 0.060 | −2.02 (M) |
| 3 | TFAc-L-Pro-OMe (10) | MTBE | 0.148 | −2.15 (M) |
| 4 | Ac-L-Pro-OMe (10) | THF | 0.059 | −2.07 (M) |
| 5 | Ac-L-Pro-OMe (10) | MTBE | 0.157 | −2.10 (M) |
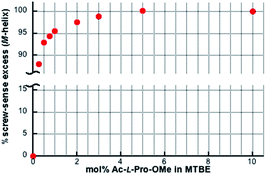 | ||
| Fig. 4 Screw-sense excesses of PQX(1000) (ca. 7 × 10−4 M based on monomer units) in MTBE in the presence of Ac-L-Pro-OMe with varied concentrations. | ||
We then sought the possibility of application of the particular helix induction in asymmetric catalysis, by taking Suzuki–Miyaura coupling of naphthyl bromide 1 with naphthylboronic acid 2 as a model reaction (Scheme 2).6b,14,17,18 Achiral PQXphos (1000-mer) containing diphenylphosphino groups was used as a ligand. In advance, we confirmed that no product was obtained in the absence of PQXphos and that racemic coupling product was obtained in the absence of a chiral additive (Table 4, entries 1 and 2). Use of 10 mol% (R)-limonene as a chiral additive resulted in the formation of the coupling product 3 with 43% enantiomeric excess (ee) (entry 3). TFAc-L-Pro-OMe was then used as a chiral additive in THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 molar ratio) in asymmetric Suzuki–Miyaura coupling. We observed the formation of 3 with 92% ee, although the chemical yield was disappointingly low (entry 4). The use of achiral low-molecular weight phosphines such as BPDPP and QXphos in the presence of TFAc-L-Pro-OMe resulted in the formation of racemates with low chemical yields (entries 5 and 6). These results suggested that the chiral reaction space is not formed directly by the chiral additive through its coordination to palladium metal, but rather, created by screw-sense induction to the polymer backbone. The results also suggested that the added TFAc-L-Pro-OMe significantly inhibited the coupling reactions. When we reduced the loading of the chiral additive in the reactions with PQXphos, the reaction yields were improved, but enantioselectivity decreased significantly (entries 7–10). In MTBE, we observed even stronger inhibition of the reaction, even though the higher enantioselectivity was obtained (entry 11). We found that the degree of reaction inhibition was improved significantly with use of Ac-L-Pro-OMe in MTBE, which afforded 3 in much better yield with 95% enantioselectivity (entry 12). By reducing the loading of the chiral additive to 1 mol% in MTBE, we obtained higher chemical yields without affecting the enantioselectivity significantly (entries 13–15). The use of enantiomeric Ac-D-Pro-OMe as a chiral guest led to the formation of an enantiomeric (S)-coupling product under the same reaction conditions (entry 16).
9 molar ratio) in asymmetric Suzuki–Miyaura coupling. We observed the formation of 3 with 92% ee, although the chemical yield was disappointingly low (entry 4). The use of achiral low-molecular weight phosphines such as BPDPP and QXphos in the presence of TFAc-L-Pro-OMe resulted in the formation of racemates with low chemical yields (entries 5 and 6). These results suggested that the chiral reaction space is not formed directly by the chiral additive through its coordination to palladium metal, but rather, created by screw-sense induction to the polymer backbone. The results also suggested that the added TFAc-L-Pro-OMe significantly inhibited the coupling reactions. When we reduced the loading of the chiral additive in the reactions with PQXphos, the reaction yields were improved, but enantioselectivity decreased significantly (entries 7–10). In MTBE, we observed even stronger inhibition of the reaction, even though the higher enantioselectivity was obtained (entry 11). We found that the degree of reaction inhibition was improved significantly with use of Ac-L-Pro-OMe in MTBE, which afforded 3 in much better yield with 95% enantioselectivity (entry 12). By reducing the loading of the chiral additive to 1 mol% in MTBE, we obtained higher chemical yields without affecting the enantioselectivity significantly (entries 13–15). The use of enantiomeric Ac-D-Pro-OMe as a chiral guest led to the formation of an enantiomeric (S)-coupling product under the same reaction conditions (entry 16).
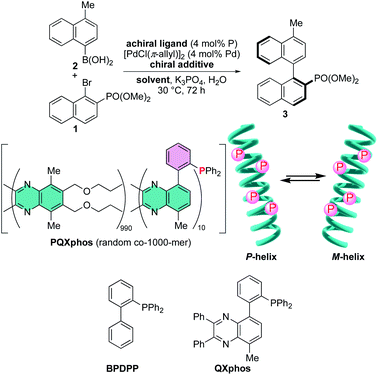 | ||
| Scheme 2 Asymmetric Suzuki–Miyaura coupling in the presence of achiral phosphine ligands including PQXphos with chiral additives. | ||
| Entry | Chiral additive (mol% in solvent) | Ligand | Solvent | Yield/% | ee/% |
|---|---|---|---|---|---|
| a Standard reaction conditions: PQXphos (30 mg, 1.0 μmol P) and [PdCl(π-allyl)]2 (1.0 μmol Pd) were stirred in a solvent (0.50 mL) at 30 °C for 1 h. Chiral additive was added to the mixture, which was stirred at 30 °C for 24 h. The bromide 1 (0.025 mmol), the boronic acid 2 (0.050 mmol), K3PO4 (0.075 mmol), and H2O (25 mL) were added, and the resultant mixture was stirred at 30 °C for 72 h. | |||||
| 1 | None | PQXphos | THF | 63 | 0 |
| 2 | TFAc-L-Pro-OMe (10) | None | THF | 0 | — |
| 3 | (R)-Limonene (10) | PQXphos | THF | 57 | 43 |
| 4 | TFAc-L-Pro-OMe (10) | PQXphos | THF | 16 | 92 |
| 5 | TFAc-L-Pro-OMe (10) | BPDPP | THF | 12 | 0 |
| 6 | TFAc-L-Pro-OMe (10) | QXphos | THF | 17 | 0 |
| 7 | TFAc-L-Pro-OMe (7) | PQXphos | THF | 27 | 87 |
| 8 | TFAc-L-Pro-OMe (5) | PQXphos | THF | 49 | 84 |
| 9 | TFAc-L-Pro-OMe (3) | PQXphos | THF | 49 | 54 |
| 10 | TFAc-L-Pro-OMe (1) | PQXphos | THF | 55 | 36 |
| 11 | TFAc-L-Pro-OMe (5) | PQXphos | MTBE | 10 | 95 |
| 12 | Ac-L-Pro-OMe (10) | PQXphos | MTBE | 48 | 95 |
| 13 | Ac-L-Pro-OMe (5) | PQXphos | MTBE | 60 | 91 |
| 14 | Ac-L-Pro-OMe (3) | PQXphos | MTBE | 64 | 91 |
| 15 | Ac-L-Pro-OMe (1) | PQXphos | MTBE | 71 | 87 |
| 16 | Ac-D-Pro-OMe (3) | PQXphos | MTBE | 62 | 92 (S) |
We tried to reduce the amount of chiral additives, keeping the concentration of chiral additive at 3 mol% in MTBE, but increasing the concentration of PQXphos in the helix induction step. In a 10-fold reaction scale, Ac-L-Pro-OMe (20 mg) and MTBE (0.50 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97 molar ratio) were used in the equilibration step (Scheme 3). The amount of Ac-L-Pro-OMe corresponds to 0.5 equiv. of 1 and 0.13 equiv. of monomer units of PQXphos. After the removal of MTBE used in the equilibration, 1-propanol (5 mL) was added as a reaction solvent to the solid catalyst before starting the reaction. The heterogeneous reaction, in which the catalyst was hardly dissolved, afforded 3 with 90% ee in 67% yield. By contrast, when the reaction was carried out in MTBE without switching the solvent to 1-propanol, 3 was obtained with 68% ee (73% yield). These results suggested that homochiral M-helix sense was induced in the equilibration step at high concentration with a substoichiometric amount of Ac-L-Pro-OMe, and that the induced helix was maintained during the progress of the reaction in the solid state using 1-propanol as a reaction solvent.
97 molar ratio) were used in the equilibration step (Scheme 3). The amount of Ac-L-Pro-OMe corresponds to 0.5 equiv. of 1 and 0.13 equiv. of monomer units of PQXphos. After the removal of MTBE used in the equilibration, 1-propanol (5 mL) was added as a reaction solvent to the solid catalyst before starting the reaction. The heterogeneous reaction, in which the catalyst was hardly dissolved, afforded 3 with 90% ee in 67% yield. By contrast, when the reaction was carried out in MTBE without switching the solvent to 1-propanol, 3 was obtained with 68% ee (73% yield). These results suggested that homochiral M-helix sense was induced in the equilibration step at high concentration with a substoichiometric amount of Ac-L-Pro-OMe, and that the induced helix was maintained during the progress of the reaction in the solid state using 1-propanol as a reaction solvent.
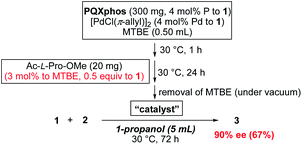 | ||
| Scheme 3 Asymmetric Suzuki–Miyaura coupling with the use of a substoichiometric amount of chiral nonbonding guest. | ||
Conclusions
We found that protected amino acids enable the screw-sense induction to virtually achiral poly(quinoxaline-2,3-diyl)s (PQX) that have no chiral group or receptor site. The induction power of proline derivatives such as Ac-Pro-OMe and TFAc-Pro-OMe were significantly stronger than limonene, which previously showed the most powerful screw-sense induction among the chiral solvents. A significant effect of achiral solvent was noted: ether solvents, particularly MTBE, resulted in better screw-sense induction than did halogenated solvents such as chloroform, probably because of weaker interaction with PQX. Upon using achiral PQXphos containing diphenylphosphino coordinating groups, asymmetric Suzuki–Miyaura coupling proceeded with high enantioselectivities with up to 95% ee in the presence of protected proline derivatives as a sole chiral source. For use in catalysis, Ac-Pro-OMe was found to be most effective, while TFAc-Pro-OMe significantly retarded the catalysis. These results clearly demonstrate that nonbonding interaction between the chiral additives and dynamic helical polymer can serve as an effective driving force to shift the equilibrium of helical conformations, leading to highly enantioselective asymmetric catalysis.Data availability
The datasets supporting this article have been uploaded as part of the supplementary material.Author contributions
The study is conceptualized and supervised by M. S. and Y. N. Experiments are conducted by S. I., T. F., and N. A. with initial support by R. T. The manuscript is written by M. S. and checked by all the co-authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI on Innovative Areas (Grant Number JP15H05811 in Precisely Designed Catalysts with Customized Scaffolding), KAKENHI (S) (Grant Number 20H05674), KAKENHI (A) (Grant Number 20H00377), and JST CREST (Grant Number JPMJCR14L1 in Establishment of Molecular Technology toward the Creation of New Function).Notes and references
- (a) E. Yashima, K. Maeda, H. Iida, Y. Furusho and K. Nagai, Chem. Rev., 2009, 109, 6102–6211 CrossRef CAS PubMed; (b) E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed.
- (a) K. Shimomura, T. Ikai, S. Kanoh, E. Yashima and K. Maeda, Nat. Chem., 2014, 6, 429–434 CrossRef CAS PubMed; (b) D. Hirose, A. Isobe, E. Quiñoá, F. Freire and K. Maeda, J. Am. Chem. Soc., 2019, 141, 8592–8598 CrossRef CAS PubMed.
- K. Maeda, D. Hirose, N. Okoshi, K. Shimomura, Y. Wada, T. Ikai, S. Kanoh and E. Yashima, J. Am. Chem. Soc., 2018, 140, 3270–3276 CrossRef CAS PubMed.
- Chirality-switchable emission of circularly polarized luminescence: T. Nishikawa, Y. Nagata and M. Suginome, ACS Macro Lett., 2017, 6(4), 431–435 CrossRef CAS.
- Chirality-switchable reflection of circularly polarized light: (a) Y. Nagata, K. Takagi and M. Suginome, J. Am. Chem. Soc., 2014, 136, 9858–9861 CrossRef CAS PubMed; (b) Y. Nagata, M. Uno and M. Suginome, Angew. Chem., Int. Ed., 2016, 55, 7126–7130 CrossRef CAS PubMed . For the seminal works on the use of helical polymers for CPL reflection, see: ; (c) G. Maxein, H. Keller, B. M. Novak and R. Zentel, Adv. Mater., 1998, 10, 341–345 CrossRef CAS; (d) J. Watanabe, H. Kamee and M. Fujiki, Polym. J., 2001, 33, 495–497 CrossRef CAS; (e) K. E. Shopsowitz, H. Qi, W. Y. Hamad and M. J. MacLachlan, Nature, 2010, 468, 422–425 CrossRef CAS PubMed; (f) M. K. Khan, M. Giese, M. Yu, J. A. Kelly, W. Y. Hamad and M. J. MacLachlan, Angew. Chem., Int. Ed., 2013, 52, 8921–8924 CrossRef CAS PubMed.
- (a) T. Yamamoto, T. Yamada, Y. Nagata and M. Suginome, J. Am. Chem. Soc., 2010, 132, 7899–7901 CrossRef CAS PubMed; (b) T. Yamamoto, Y. Akai, Y. Nagata and M. Suginome, Angew. Chem., Int. Ed., 2011, 50, 8844–8847 CrossRef CAS PubMed; (c) Y. Akai, T. Yamamoto, Y. Nagata, T. Ohmura and M. Suginome, J. Am. Chem. Soc., 2012, 134, 11092–11095 CrossRef CAS PubMed; (d) T. Yamamoto, R. Murakami and M. Suginome, J. Am. Chem. Soc., 2017, 139, 2557–2560 CrossRef CAS PubMed; (e) Y. Yoshinaga, T. Yamamoto and M. Suginome, J. Am. Chem. Soc., 2020, 142, 18317–18323 CrossRef CAS PubMed; (f) T. Yamamoto, T. Takahashi, R. Murakami, N. Ariki and M. Suginome, Bull. Chem. Soc. Jpn., 2021, 94, 943–949 CrossRef . For the seminal work on the use of static helical polymers as chiral catalysts, see: ; (g) M. Reggelin, M. Schultz and M. Holbach, Angew. Chem., Int. Ed., 2002, 41, 1614–1617 CrossRef CAS; (h) G. Roelfes and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 3230–3232 CrossRef CAS PubMed; (i) A. J. Boersma, R. P. Megens, B. L. Feringa and G. Roelfes, Chem. Soc. Rev., 2010, 39, 2083–2092 RSC.
- (a) E. Yashima and K. Maeda, Macromolecules, 2008, 41, 3–12 CrossRef CAS; (b) E. Yashima, T. Nimura, T. Matsushima and Y. Okamoto, J. Am. Chem. Soc., 1996, 118, 9800–9801 CrossRef CAS; (c) T. Yamamoto, R. Murakami, S. Komatsu and M. Suginome, J. Am. Chem. Soc., 2018, 140, 3867–3870 CrossRef CAS PubMed; (d) Y. Nagata, S. Ohashi and M. Suginome, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1564–1571 CrossRef CAS.
- (a) E. Yashima, T. Matsushima and Y. Okamoto, J. Am. Chem. Soc., 1995, 117, 11596–11597 CrossRef CAS; (b) E. Yashima, T. Nimura, T. Matsushima and Y. Okamoto, J. Am. Chem. Soc., 1996, 118, 9800–9801 CrossRef CAS; (c) E. Yashima, T. Matsushima and Y. Okamoto, J. Am. Chem. Soc., 1997, 119, 6345–6359 CrossRef CAS; (d) K. Maeda, K. Morino, Y. Okamoto, T. Sato and E. Yashima, J. Am. Chem. Soc., 2004, 126, 4329–4342 CrossRef CAS PubMed; (e) Y. Hase, K. Nagai, H. Iida, K. Maeda, N. Ochi, K. Sawabe, K. Sakajiri, K. Okoshi and E. Yashima, J. Am. Chem. Soc., 2009, 131, 10719–10732 CrossRef CAS PubMed.
- (a) J. Tabei, R. Nomura, F. Sanda and T. Masuda, Macromolecules, 2003, 36, 8603–8608 CrossRef CAS; (b) M. Inouye, M. Waki and H. Abe, J. Am. Chem. Soc., 2004, 126, 2022–2027 CrossRef CAS PubMed; (c) K. Maeda, H. Tsukui, Y. Matsushita and E. Yashima, Macromolecules, 2007, 40, 7721–7726 CrossRef CAS.
- R. Nonokawa and E. Yashima, J. Am. Chem. Soc., 2003, 125, 1278–1283 CrossRef CAS PubMed.
- (a) R. Ishidate, A. J. Markvoort, K. Maeda and E. Yashima, J. Am. Chem. Soc., 2019, 141, 7605–7614 CrossRef CAS PubMed; (b) Y. Nagata, T. Nishikawa, M. Suginome, S. Sato, M. Sugiyama, L. Porcar, A. Martel, R. Inoue and N. Sato, J. Am. Chem. Soc., 2015, 137, 4070–4073 CrossRef CAS PubMed; (c) Y. Nagata, T. Nishikawa and M. Suginome, J. Am. Chem. Soc., 2015, 137, 4070–4073 CrossRef CAS PubMed; (d) Y. Nagata, T. Nishikawa and M. Suginome, J. Am. Chem. Soc., 2014, 136, 15901–15904 CrossRef CAS PubMed; (e) Y. Nagata, T. Yamada, T. Adachi, Y. Akai, T. Yamamoto and M. Suginome, J. Am. Chem. Soc., 2013, 135, 10104–10113 CrossRef CAS PubMed; (f) T. Yamada, Y. Nagata and M. Suginome, Chem. Commun., 2010, 46, 4914–4916 RSC.
- M. M. Green, C. Khatri and N. C. Peterson, J. Am. Chem. Soc., 1993, 115, 4941–4942 CrossRef CAS.
- S. Lifson, C. Andreola, N. C. Peterson and M. M. Green, J. Am. Chem. Soc., 1989, 111, 8850–8858 CrossRef CAS.
- Y. Nagata, R. Takeda and M. Suginome, ACS Cent. Sci., 2019, 5, 1235–1240 CrossRef CAS PubMed.
- For the previous works on the induction of asymmetry of helical polymers using limonene, see: (a) Y. Kawagoe, M. Fujiki and Y. Nakano, New J. Chem., 2010, 34, 637–647 RSC; (b) M. Fujiki, A. Jalilah, N. Suzuki, M. Taguchi, W. Zhang, M. M. Abdellatif and K. Nomura, RSC Adv., 2012, 2, 13038 RSC.
- (a) D. Seebach and H. A. Oei, Angew. Chem., Int. Ed. Engl., 1975, 14, 634–636 CrossRef; (b) D. Seebach, G. Crass, E.-M. Wilka, D. Hilvert and E. Brunner, Helv. Chim. Acta, 1979, 62, 2695–2698 CrossRef CAS; (c) W. H. Laarhoven and T. J. H. M. Cuppen, J. Chem. Soc., Chem. Commun., 1977, 47 RSC.
- Selected examples for asymmetric biaryl synthesis via Suzuki-Miyaura coupling: (a) J. Yin and S. L. Buchwald, J. Am. Chem. Soc., 2000, 122, 12051–12052 CrossRef CAS; (b) A. N. Cammidge and K. V. L. Crépy, Chem. Commun., 2000, 1723–1724 RSC; (c) X. Shen, G. O. Jones, D. A. Watson, B. Bhayana and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 11278–11287 CrossRef CAS PubMed; (d) M. Genov, A. Almorín and P. Espinet, Chem. - Eur. J., 2006, 12, 9346–9352 CrossRef CAS PubMed; (e) A. Bermejo, A. Ros, R. Fernández and J. M. Lassaletta, J. Am. Chem. Soc., 2008, 130, 15798–15799 CrossRef CAS; (f) Y. Uozumi, Y. Matsuura, T. Arakawa and Y. M. A. Yamada, Angew. Chem., Int. Ed., 2009, 48, 2708–2710 CrossRef CAS PubMed; (g) G. Xu, W. Fu, G. Liu, C. H. Senanayake and W. Tang, J. Am. Chem. Soc., 2014, 136, 570–573 CrossRef CAS PubMed; (h) D. Shen, Y. Xu and S.-L. Shi, J. Am. Chem. Soc., 2019, 141, 14938–14945 CrossRef CAS PubMed; (i) H. Yang, J. Sun, W. Gu and W. Tang, J. Am. Chem. Soc., 2020, 142, 8036–8043 CrossRef CAS PubMed.
- Y. Akai, L. Konnert, T. Yamamoto and M. Suginome, Chem. Commun., 2015, 51, 7211–7214 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc01764k |
| ‡ Current address: Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Kita 21, Nishi 10, Kita-ku, Sapporo, Hokkaido 001-0021, Japan. |
| This journal is © The Royal Society of Chemistry 2021 |