Open Access Article
Open Access ArticleEnantioseparation and chiral induction in Ag29 nanoclusters with intrinsic chirality†
Hiroto
Yoshida
a,
Masahiro
Ehara
 *b,
U. Deva
Priyakumar
*b,
U. Deva
Priyakumar
 c,
Tsuyoshi
Kawai
c,
Tsuyoshi
Kawai
 a and
Takuya
Nakashima
a and
Takuya
Nakashima
 *a
*a
aDivision of Materials Science, Graduate School of Science and Technology, Nara Institute of Science and Technology (NAIST), Ikoma, Nara 630-01921, Japan. E-mail: ntaku@ms.naist.jp
bInstitute for Molecular Science, Research Center for Computational Science, Myodai-ji, Okazaki 444-8585, Japan. E-mail: ehara@ims.ac.jp
cCentre for Computational Natural Sciences and Bioinformatics, International Institute of Information Technology, Hyderabad 500032, India
First published on 20th January 2020
Abstract
The optical activity of a metal nanocluster (NC) is induced either by an asymmetric arrangement of constituents or by a dissymmetric field of a chiral ligand layer. Herein, we unveil the origin of chirality in Ag29 NCs, which is attributed to the intrinsically chiral atomic arrangement. The X-ray crystal structure of a Ag29(BDT)12(TPP)4 NC (BDT: 1,3-benzenedithiol; TPP: triphenylphosphine) manifested the presence of intrinsic chirality in the outer shell capping the icosahedral achiral Ag13 core. The enantiomers of the Ag29(BDT)12(TPP)4 NC are separated by high-performance liquid chromatography (HPLC) using a chiral column for the first time, showing mirror-image circular dichroism (CD) spectra. The CD spectra are reproduced by time-dependent density functional theory (TDDFT) calculations based on enantiomeric Ag29 models with achiral 1,3-propanedithiolate ligands. The mechanism of chiral induction in the synthesis of Ag29(DHLA)12 (DHLA: α-dihydrolipoic acid) NCs with a chiral ligand system is further discussed with the aid of DFT calculations. The use of the enantiomeric DHLA ligand preferentially leads to a one-handed atomic arrangement which is more stable than the opposite one, inducing the enantiomeric excess in the population of intrinsically chiral Ag29 NCs with CD activity.
Introduction
Chiral metal nanoclusters (NCs) with atomically precise structures have garnered great interest over the recent two decades because of their fascinating structural, electronic and optical properties.1 Chirality can be introduced into the electronic properties of NCs either by intrinsically chiral atomic arrangements2–18 or by employing chiral organic ligands such as chiral thiols.19–25 While intrinsically chiral NCs possess a chiral atomic arrangement in the NC-core including the surface metal-thiolate networks as a stable structure, the use of achiral ligands leads to the formation of a racemic mixture.2–17 A few studies successfully separated one enantiomer or both enantiomers from the racemic mixture through chiral HPLC columns,6–8,16 phase transfer with a chiral surfactant,10 and interaction with chiral reagents and ions.3b,13 However, there has been no report on the enantioseparation of atomically precise chiral silver NCs, possibly because of reactive and less stable properties of silver NCs compared to gold NCs.1,26Ag29 NCs serve as one of the representative examples of atomically precise silver NCs with remarkable photoluminescence properties.27–30 The X-ray single crystal structure of Ag29 NCs was first revealed for Ag29(BDT)12(TPP)4 NCs.27a A similar structure and composition were proposed for water soluble silver NCs prepared with bidentate DHLA ligands29 by mass spectrometry data corresponding to an m/z value corresponding to the [Ag29(DHLA)12]3− composition, exhibiting absorption and photoluminescence spectra similar to those of Ag29(BDT)12(TPP)4 NCs.29 We have recently imparted optical activity to the NCs by using enantiomeric DHLAs and demonstrated mirror image CD and circular polarized luminescence (CPL) spectra.30 While the origin of optical activity remained unclear, the chirality analysis of the single crystal structure of the Ag29(BDT)12(TPP)4 NC27a by Whetten and co-workers29d suggested intrinsic chirality in the shell structure surrounding the icosahedral Ag13 core. Their finding motivated us to separate enantiomers of Ag29(BDT)12(TPP)4 NCs which are prepared with achiral ligands and demonstrate chiroptical properties. In this work, the enantiomers of Ag29(BDT)12(TPP)4 NCs are successfully separated with a chiral HPLC column, exhibiting absorption and luminescence spectra identical to those of the racemic mixture but affording mirror image CD spectra. The CD spectra were reproduced by TDDFT calculations based on model NCs employing the 1,3-propanedithiolate ligand in place of BDT. We further discuss the chirality induction mechanism in the synthesis of water soluble analogous Ag29(DHLA)12 NCs with the aid of DFT calculations.
Experimental
Synthesis of Ag29(BDT)12(TPP)4 NCs
Ag29(BDT)12(TPP)4 NCs were prepared according to a method reported in the literature.27a For the enantioseparation, the purified NCs were dispersed in ethanol.Synthesis of Ag29(R-DHLA)12 NCs
Ag29(R-DHLA)12 NCs were also prepared according to the reported procedure.30 The reaction temperature was controlled with a temperature-controlled bath until the formation of Ag29 NCs was confirmed with spectroscopic measurements.Enantioseparation of Ag29(BDT)12(TPP)4 NCs
Enantioseparation of Ag29(BDT)12(TPP)4 NCs was carried out using a JASCO EXTREMA HPLC system equipped with a PU-4180 pump, a UV-4075 detector and a CD-4095 detector. For the enantioseparation, a DAICEL CHILALPAK IA column (5 μm, 4.6 × 250 mm) was used in a JASCO CO-4060 column oven at 20 °C. As a typical condition, 50 μl of ethanol solution (0.02 mg ml−1) was injected and then the separation was performed using ethanol as the eluent at a flow rate of 0.5 ml min−1. The eluting solutions were monitored by optical absorption and a CD signal at 450 nm simultaneously.Characterization
Electrospray ionization mass (ESI-MS) spectrometry was conducted with a JEOL JMS-T100LC AccuTOF. UV-vis absorption and photoluminescence spectra were recorded with a JASCO V-760 spectrometer and a JASCO FP-8500 spectrofluorometer, respectively. CD spectra were measured on a JASCO J-725 spectropolarimeter.DFT calculations
DFT and TDDFT calculations were performed for the model Ag29 NCs using the B3LYP functional.31 Relativistic effective core potential LANL2DZ32 was used for silver atoms and the basis sets of other atoms were 3-21G.33 For simulating ECD spectra, 200 excited states were solved to cover the spectrum in the energy range up to about 300 nm and the rotatory strength was calculated in the velocity form. All calculations were conducted using the Gaussian 09 suite of programs version E.01.34Results and discussion
Ag29(BDT)12(TPP)4 NCs were prepared according to the method reported by Bakr and co-workers.27a The purified NCs were subjected to the HPLC separation study. The HPLC technique was applied to the size-dependent separation of monolayer protected gold NCs,35 and this technique has been further advanced to achieve high-resolution separation of metal NCs depending on the size, core- and ligand-compositions and coordination isomers.36,37 While reverse phase HPLC has been mostly used for the polarity-based partitioning of NCs with hydrophobic surface ligands,37 the separation of enantiomeric NCs with the same composition requires chirality-dependent interactions. Bürgi et al. employed a chiral column filled with silica gel tethering aryl-functionalized cellulose as a stationary phase for the enantioseparation of intrinsically chiral gold NCs with the 2-phenylethanethiolate ligand.6,7 We chose the DAICEL CHILALPAL IA column having aryl-substituted amylose (amylose tris(3,5-dimethylphenyl-carbamate)) in the stationary phase for the separation. The chiral π–π interactions between 3,5-dimethyl units in the stationary phase and phenyl groups on the surface of Ag29(BDT)12(TPP)4 should work as a primary force for the enantioseparation.The purified NCs were found to be soluble in DMF, ethanol and acetone with moderate polarity but less soluble in acetonitrile, methanol and 1-propanol. When we first tested DMF as a solvent for injection and as a mobile phase, both the enantiomers gave an identical retention time. Since the separation stems from the difference in partition equilibrium between the mobile and stationary phases, the NCs may be too soluble in DMF. We then employed ethanol showing moderate dissolving capability for the NCs. Although the complete separation was not accomplished, two separate peaks at 16.9 and 20 min were confirmed with the UV-vis detector (Fig. 1). The presence of negative and positive signals corresponding to the former and latter peaks in the CD-detected chromatogram clearly demonstrated the successful separation of enantiomers. The separation conditions particularly related to the eluting solvent were further investigated by mixing an additional solvent with ethanol such as DMF, methanol, acetonitrile and hexane. However, all the mixed solvent systems could not much improve the peak separation, resulting in peak broadening or clogging of the column (Fig. S1, ESI†). Therefore, fractions were collected over several separation runs using ethanol and optical properties were measured for the collected fractions.
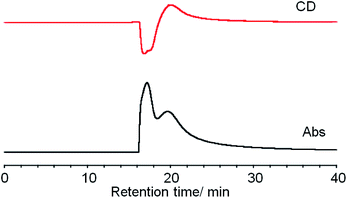 | ||
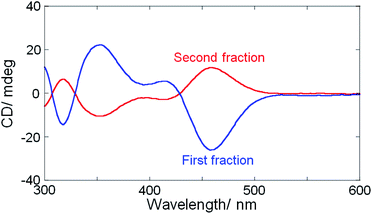
| Fig. 1 Simultaneous CD (top) and absorbance (bottom) detected (at 450 nm) chiral HPLC chromatograms of Ag29(BDT)12(TPP)4 NCs. | ||
Each collected ethanol solution was subjected to ESI-MS measurements, providing an m/z value corresponding to the [Ag29(BDT)12]3− composition with loss of TPP molecules (Fig. S2, ESI†).27a The strong π–π interactions between TPP ligands and the aryl groups in the stationary phase may remove them from the surface of NCs since phosphines were reported to weakly bind to the NCs compared to dithiolates.27a Meanwhile, UV-vis and photoluminescence spectra of the isolated fraction were identical to those of the racemic mixture before the HPLC separation, also confirming the preservation of the Ag29(BDT)12 NC composition after the separation (Fig. S3, ESI†).
The separated fractions afforded mirror image CD spectra, clearly demonstrating the intrinsic chirality of Ag29(BDT)12 NCs having organic ligands with no chiral group (Fig. 2). Very weak first Cotton effect signals were observed around 550 nm with negative and positive signs for the first and second fractions, respectively. Then, each fraction afforded a more prominent CD signal at 460 nm with the same sign as the first Cotton effect. Although the presence of TPP was reported to have little effect on the UV-vis absorption and photoluminescence spectral profiles,27a CD spectra were slightly modified by the addition of TPP. Re-dispersion of the separated NCs in DMF resulted in the CD profiles unaltered from those in as-separated ethanol solutions. The prominent changes include a blue-shift by 5–10 nm and the sign-reversal of first Cotton effect signals above 500 nm after the addition of 10 mM TPP (Fig. S4, ESI†). This result further confirmed the loss of TPP molecules and suggested the non-negligible effect of TPP on the electronic structure of NCs contributing to the CD activity. Because the first fraction was less contaminated by the opposite enantiomer,38 a larger CD amplitude was obtained compared to the second one. We attempted further analysis of each fraction with the chiral HPLC to estimate the enantiomeric excess (ee),7 whereas it failed because of the low stability of as-separated samples without TPP.27a The anisotropy factors (|gabs| = |Δε/ε|) were therefore estimated tentatively to be no less than 1.1 × 10−3 at 460 nm which is in a similar range to those obtained for Ag29(R- or S-DHLA)12 NCs30 (1.5 × 10−3 at 500 nm, Fig. S5, ESI†).
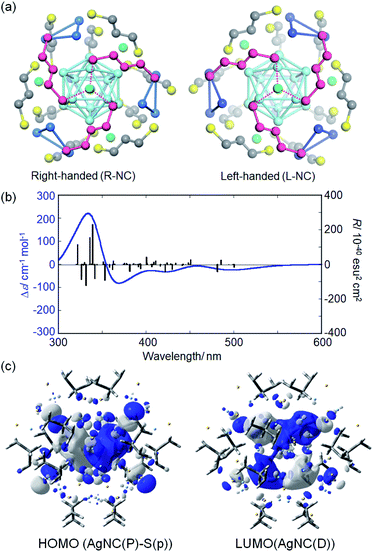
To predict the NC structure responsible for each HPLC fraction, TDDFT calculations were performed based on model NCs with 1,3-propanedithiolate ligands. A careful analysis of the single crystal data of Ag29(BDT)12(TPP)4 NCs27a suggested that the cubic lattice is composed of both enantiomeric NCs (Fig. S6, ESI†). Both enantiomeric NCs were derived from the crystallographic lattice structure27a to construct the model NC enantiomers. The BDT ligands were replaced by 1,3-propanedithiolate and TPP molecules were removed to reduce the computational cost. The loss of TPP ligands was also suggested by the HPLC separation study. The model structures of Ag29(1,3-propanedithiolate)12(3−) were first optimized by the DFT method. Absorption and CD spectra were then simulated based on the ground-state optimized structure. Each optimized enantiomer is composed of a highly symmetric Ag13 icosahedral core (Fig. 3a, light blue) and an exterior shell with a chiral atomic arrangement. In the exterior shell, 12 silver atoms capping all 12 silver atoms of the icosahedral Ag13 core form four triangles (blue) and the remaining 4 silver atoms (green) face-cap the icosahedral core. These two respective types of silver atoms, i.e. triangles and face-capping atoms, in the shell were found to be arranged in a chirally distorted tetrahedral manner as reported by Whetten and co-workers.29d Along with the exterior silver atoms, the bidentate ligands are also oriented in a chiral manner. A view with a focus on one of the face-capping silver atoms clearly demonstrates that the bidentate ligands (purple) are arranged in a C3-symmetry with a right- or left-handed orientation in the Ag(1,3-dithiolate)3 unit (Fig. 3a). It is apparent that, therefore, the chirality of Ag29 NCs stems from the asymmetric atomic arrangement in the exterior shell. Meanwhile, the TPP ligands orthogonally cap the center of the Ag(1,3-dithiolate)3 unit in the Ag29(BDT)12(TPP)4 NC in a monodentate manner, having a limited effect on the intrinsically chiral geometry in the exterior shell.
The TDDFT calculation based on the ground-state optimized structures simulated the chiroptical properties. Unlike most of the ordinary small molecules, the electronic transitions in NC systems involve a large number of excited states. The model NC with a right-handed ligand orientation (R-NC, Fig. 3a) afforded a CD spectrum similar to that of the first fraction with a small negative first Cotton effect (Fig. 3b and S7† for the simulated UV-vis absorption data), while the one with left-handed orientation (L-NC) resembled that of the second fraction (Fig. S8, ESI†). The deviations of transition energy and rotatory strength (R) between the experimental and simulation data may be attributed to the used functional and basis set in DFT calculations (Fig. S9, ESI†). The triply degenerate lowest state (T symmetry) has a considerable rotatory strength and is characterized by the HOMO–LUMO transitions (Fig. S10 and Table S1, ESI†). The HOMOs (no. 611–613, Fig. S10, ESI†) correspond to the P orbitals of the superatom of the Ag13 core and to the p orbitals of helically arranged S atoms, while the LUMOs (LUMO and LUMO+1, no. 614–618) correspond to the D orbitals of the Ag13 core. The electronic transitions simulated above 430 nm correspond to the transitions to the LUMOs from the HOMO, HOMO−1 (no. 608–610) and HOMO−3 (no. 603–605), with the considerable contributions of orbitals localized in the chiral shell, suggesting the involvement of charge transfer between the exterior shell and the Ag13 core (Fig. S10 and Table S1, ESI†).
The intrinsic chirality of the Ag29 NC system was unveiled by the chiral HPLC separation of Ag29(BDT)12(TPP)4 NCs and the DFT calculations based on the Ag29(1,3-propanedithiolate)12 NC models. We further discuss the chirality induction mechanism in the synthesis of Ag29(DHLA)12 NCs30 with the aid of DFT calculations. The detailed investigation by de Groot and co-workers29e into the synthesis of silver NCs in the presence of racemic DHLA suggested that the composition of Ag29(DHLA)12 is formed in an exclusive manner as the most stable structure. Furthermore, the Ag29(DHLA)12 NC is expected to have an intrinsic chirality in the structure considering the analogous composition and optical properties to those of Ag29(BDT)12(TPP)4 NCs, whereas its crystal structure has not been obtained yet. It is reasonably assumed that the use of enantiomeric DHLA gives an energy difference between the L- and R-NCs with left- and right-handed ligand orientations, respectively. To gain an insight into this assumption, the DFT calculations were performed.
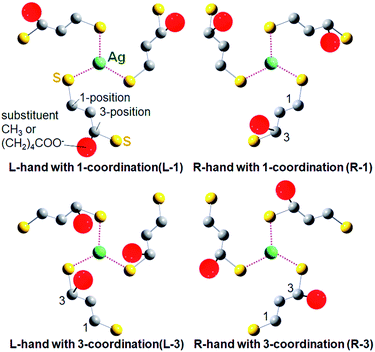
For the DFT simulation, NC models with right- and left-handed ligand orientations were constructed with employing a mixed ligand system including four (R)-DHLA and eight (R)-butane-1,3-dithiol ligands to reduce the calculation cost (total charge; −7). These ligands were distributed homogeneously so that each Ag(1,3-dithiolate)3 unit focusing on one of the face-capping silver atoms possesses one (R)-DHLA and two (R)-butane-1,3-dithiolate ligands. There are two typical possible orientation patterns in the coordination of 3-position-substituted 1,3-propanedithiolate derivatives for each one-handed NC as shown in Fig. 4. Given the Ag(1,3-dithiolate)3 unit, the dithiolate ligands can coordinate to the central silver atom with a thiolate group either at the 1- or 3-position (Fig. 4). Based on the combinations of the handedness of the NC (L- or R-NC) and ligand coordination orientation, the four possible model NCs were labelled L-1, R-1, L-3 and R-3 NCs (Fig. 4). Among these four patterns, L-1 and R-3 NC models are less stable than others, in which bulky substituents at the 3-position are located outward from the silver center, resulting in higher steric repulsion with an adjacent ligand in the neighboring Ag(1,3-dithiolate)3 unit (see Fig. S11 and Table S2 in the ESI† for the result of Ag29(R-butane-1,3-dithiolate)12 models). Therefore, R-1 and L-3 NC models were compared (see Fig. S12 in the ESI† for the entire optimized NC structures). Interestingly, the carboxylate group (COO−) in the (R)-DHLA ligand was found to coordinate to the central silver atom in each Ag(1,3-dithiolate)3 unit for both models, contributing to the stabilization of the whole NC structure in a similar manner to the role of the TPP ligand in Ag29(BDT)12(TPP)4 NCs.27 Compared with the R-1 NC, the L-3 NC model was estimated to be more stable by 0.48 kcal mol−1. Thus the left-handed NC was suggested to preferentially form in the presence of (R)-DHLA.
To support the calculation reliability, the TDDFT method was applied to the L-3 NC model, simulating absorption and CD spectra. Fig. 5 compares the simulated CD with the experimental data of Ag29(R-DHLA)12 NCs.30 The TDDFT simulation well reproduced the positive first Cotton effect at around 500 nm. The difference between the simulated and experimental spectra can be partly attributed to the simplified ligand structure in the model and the used functional in the DFT calculation. However, it should be noted that the R-1 NC model with the opposite handedness gave the negative first Cotton effect in the same region and a very different CD spectrum from the experimental one (Fig. S13, ESI†). The preferential formation of L-NCs in the presence of (R)-DHLA was thus supported by the CD simulation study with the TDDFT method. A calculation based on the full structure model of Ag29(R-DHLA)12 might increase the energy difference between the left- and right-handed NCs, whereas it had difficulty with converging by our calculation method.
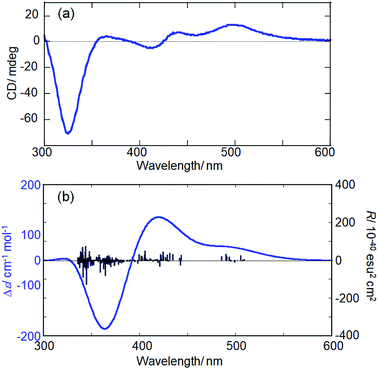 | ||
| Fig. 5 Experimental (a) and simulated (b) CD spectra of Ag29(R-DHLA)12 NCs and the L-3 NC model, respectively. | ||
To gain further insight into the chirality induction mechanism in the Ag29(DHLA)12 NCs, the effect of preparation temperature on the enantiomeric excess (ee) of NCs (between the left- and right-handed NCs) was investigated using the enantiopure (R)-DHLA ligand. Interestingly, the CD amplitude of Ag29(R-DHLA)12 NCs increased with increasing preparation temperature (Fig. S14, ESI†). Given that the maximum gabs value of 1.93 × 10−3 (at 500 nm) for Ag29(R-DHLA)12 NCs prepared at 60 °C corresponds to an ee of 1 (100% selectivity of L-NCs), an enantioselectivity of 80% was estimated for NCs synthesized at 20 °C (90% L-NCs and 10% R-NCs). The ee value further drops to 0.64 at 10 °C (82% L-NCs and 18% R-NCs). A small energy difference between the most stable L-NCs and the second most stable R-NCs resulted in the formation of NCs with both handedness even with the use of a homochiral (R)-ligand, which is in accordance with the DFT calculation result. The formation of Ag29 NCs is considered to involve the nucleation of larger size nanoparticles and the subsequent size-focusing processes.29e The rapid nucleation process should proceed via a kinetically controlled reduction reaction while the size-focusing one occurs with oxidation-assisted etching of larger size nanoparticles.29e Temperature should have a more prominent effect on the latter process than the former one, introducing thermodynamic controllability in the formation of NCs. Therefore, the increase of ee with increasing preparation temperature suggested the presence of a considerable activation barrier in the chirality inversion39 between the L- and R-NCs. That is, the increase in the preparation temperature facilitates the introduction of thermodynamic control in the formation of NCs, resulting in the distribution between the R- and L-NCs on the basis of energy difference (ΔΔH, Fig. S15, ESI†). We further investigated the possibility of chirality inversion after the completion of NC formation. The Ag29(R-DHLA)12 NCs prepared at 10 °C with relatively low purity of NC-handedness (gabs at 500 nm = 1.2 × 10−3) were treated at 60 °C for 90 min, resulting in the increase of the gabs value up to 1.7 × 10−3 (Fig. S14, ESI†). This result indicated the inversion of R-NCs to L-NCs with a more thermodynamically stable structure upon heating. The chirality inversion requires the rearrangement of bidentate ligand orientation in the exterior shell, which should serve as one of the origins of the activation barrier.39 Meanwhile, further increase in the preparation temperature (>70 °C) impaired the stability of NCs, decreasing the net gabs value.
Conclusions
In summary, we have succeeded in the enantioseparation of intrinsically chiral Ag29(BDT)12(TPP)4 NCs with a chiral HPLC method and the separated fractions afforded mirror image CD spectra. The DFT-optimized models clearly demonstrated the emergence of the chiral structure in the atomic arrangement in the exterior shell surrounding the icosahedral Ag13 core. The TDDFT calculation based on the model structure revealed that the optical activity stems from the electronic transition between the molecular orbitals delocalized in the whole NCs including the ligand-shell layer. The use of an enantiomeric ligand such as DHLA successfully led to the preferential formation of one-handed intrinsically chiral NCs, which was supported by the DFT calculations giving an energy difference between the right- and left-handed NCs with employing a homochiral ligand system. The chiral NCs with a precise atomic structure are expected to serve as a platform to work on with functional ligand assemblies with a precise chiral orientation on the NC.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI grant numbers JP16H06522 (T. N.) and 16H06511 (M. E.) in Scientific Research on Innovative Area, “Coordination Asymmetry”. We are grateful to Ms Yoshiko Nishikawa for her support in the ESI-MS measurement.Notes and references
- (a) C. Noguez and I. L. Garzón, Chem. Soc. Rev., 2009, 38, 757–771 RSC; (b) Y. Wang, J. Xu, Y. Wang and H. Chen, Chem. Soc. Rev., 2013, 42, 2930–2962 RSC; (c) S. Knoppe and T. Bürgi, Acc. Chem. Res., 2014, 47, 1318–1326 CrossRef CAS PubMed; (d) T. Bürgi, Nanoscale, 2015, 7, 15553–15567 RSC; (e) J. Kumar, K. G. Thomas and L. M. Liz-Marzán, Chem. Commun., 2016, 52, 12555–12569 RSC; (f) W. Ma, L. Xu, A. F. de Moura, X. Wu, H. Kuang, C. Xu and N. A. Kotov, Chem. Rev., 2017, 117, 8041–8093 CrossRef CAS PubMed; (g) I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed; (h) C. Zeng and R. Jin, Chem.–Asian J., 2017, 12, 1839–1850 CrossRef CAS PubMed; (i) N. V. Karimova and C. M. Aikens, Part. Part. Syst. Charact., 2019, 36, 1900043 CrossRef.
- C. Zeng, C. Liu, Y. Chen, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2014, 136, 11922–11925 CrossRef CAS PubMed.
- (a) X.-K. Wan, S.-F. Yuan, Z.-W. Lin and Q.-M. Wang, Angew. Chem., Int. Ed., 2014, 53, 2923–2926 CrossRef CAS PubMed; (b) Y. Zhu, H. Wang, K. Wan, J. Guo, C. He, Y. Yu, L. Zhao, Y. Zhang, J. Lv, L. Shi, R. Jin, X. Zhang, X. Shi and Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 9059–9063 CrossRef CAS PubMed.
- C. Zeng, T. Li, A. Das, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2013, 135, 10011–10013 CrossRef CAS PubMed.
- (a) O. Lopez-Acevedo, H. Tsunoyama, T. Tsukuda, H. Häkkinen and C. M. Aikens, J. Am. Chem. Soc., 2010, 132, 8210–8218 CrossRef CAS PubMed; (b) H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS PubMed.
- I. Dolamic, S. Knoppe, A. Dass and T. Bürgi, Nat. Commun., 2012, 3, 798 CrossRef PubMed.
- S. Knoppe, I. Dolamic, A. Dass and T. Bürgi, Angew. Chem., Int. Ed., 2012, 51, 7589–7591 CrossRef CAS PubMed.
- C. Zeng, T. Li, A. Das, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2013, 135, 10011–10013 CrossRef CAS PubMed.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS PubMed.
- S. Knoppe, O. A. Wong, S. Malola, H. Hakkinen, T. Bürgi, T. Verbiest and C. J. Ackerson, J. Am. Chem. Soc., 2014, 136, 4129–4132 CrossRef CAS PubMed.
- (a) C. Zeng, Y. Chen, K. Kirschbaum, K. J. Lambright and R. Jin, Science, 2016, 354, 1580–1584 CrossRef CAS PubMed; (b) C. Zeng, Y. Chen, K. Kirschbaum, K. Appavoo, M. Y. Sfeir and R. Jin, Sci. Adv., 2015, 1, e1500045 CrossRef PubMed.
- F. Tian and R. Chen, J. Am. Chem. Soc., 2019, 141, 7107–7114 CrossRef CAS PubMed.
- J. Yan, H. Su, H. Yang, C. Hu, S. Malola, S. Lin, B. K. Teo, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2016, 138, 12751–12754 CrossRef CAS PubMed.
- H. Yang, J. Yan, Y. Wang, G. Deng, H. Su, X. Zhao, C. Xu, B. K. Teo and N. Zheng, J. Am. Chem. Soc., 2017, 139, 16113–16116 CrossRef CAS PubMed.
- C. Liu, T. Li, H. Abroshan, Z. Li, C. Zhang, H. J. Kim, G. Li and R. Jin, Nat. Commun., 2018, 9, 744 CrossRef PubMed.
- S. Jin, F. Xu, W. Du, X. Kang, S. Chen, J. Zhang, X. Li, D. Hu, S. Wang and M. Zhu, Inorg. Chem., 2018, 57, 5114–5119 CrossRef CAS PubMed.
- R. S. Dhayal, Y. R. Lin, J. H. Liao, Y. J. Chen, Y. C. Liu, M. H. Chiang, S. Kahlal, J. Y. Saillard and C. W. Liu, Chem.–Eur. J., 2016, 22, 9943–9947 CrossRef CAS PubMed.
- B. Li, Y. J. Kong, S. Li, X. F. Liu, P. Luo and S. Q. Zang, Part. Part. Syst. Charact., 2019, 36, 1900069 CrossRef.
- (a) T. G. Schaaff, G. Knight, M. N. Shafigullin, R. F. Borkman and R. L. Whetten, J. Phys. Chem. B, 1998, 102, 10643–10646 CrossRef CAS; (b) T. G. Schaaff and R. L. Whetten, J. Phys. Chem. B, 2000, 104, 2630–2641 CrossRef CAS.
- H. Yao, K. Miki, N. Nishida, A. Sasaki and K. Kimura, J. Am. Chem. Soc., 2005, 127, 15536–15543 CrossRef CAS PubMed.
- M. Zhu, H. Qian, X. Meng, S. Jin, Z. Wu and R. Jin, Nano Lett., 2011, 11, 3963–3969 CrossRef CAS PubMed.
- (a) N. Nishida, H. Yao, T. Ueda, A. Sasaki and K. Kimura, Chem. Mater., 2007, 19, 2831–2841 CrossRef CAS; (b) N. Cathcart, P. Mistry, C. Makra, B. Pietrobon, N. Coombs, M. Jelokhani-Niaraki and V. Kitaev, Langmuir, 2009, 25, 5840–5846 CrossRef CAS PubMed; (c) M. Farrag, M. Tschurl and U. Heiz, Chem. Mater., 2013, 25, 862–870 CrossRef CAS.
- H. Yao, M. Saeki and K. Kimura, J. Phys. Chem. C, 2010, 114, 15909–15915 CrossRef CAS.
- A. Sánchez-Castillo, C. Noguez and I. L. Garzón, J. Am. Chem. Soc., 2010, 132, 1504–1505 CrossRef PubMed.
- H. Yao, Prog. Nat. Sci.: Mater. Int., 2016, 26, 428–439 CrossRef CAS.
- R. Jin, S. Zhao, Y. Xing and R. Jin, CrystEngComm, 2016, 18, 3996–4005 RSC.
- (a) L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. Del Gobbo, R. G. AbdulHalim, M. Eddaoudi, D. E. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970–11975 CrossRef CAS PubMed; (b) X. Kang, S. Wang and M. Zhu, Chem. Sci., 2018, 9, 3062–3068 RSC; (c) A. Nag, P. Chakraborty, M. Bodiuzzaman, T. Ahuja, S. Antharjanam and T. Pradeep, Nanoscale, 2018, 10, 9851–9855 RSC; (d) X. Wei, X. Kang, Q. Yuan, C. Qin, S. Jin, S. Wang and M. Zhu, Chem. Mater., 2019, 31, 4945–4952 CrossRef CAS.
- (a) B. Adhikari and A. Banerjee, Chem. Mater., 2010, 22, 4364–4371 CrossRef CAS; (b) W. T. Chen, Y. J. Hsu and P. V. Kamat, J. Phys. Chem. Lett., 2012, 3, 2493–2499 CrossRef CAS PubMed; (c) P. T. Chin, M. van der Linden, E. J. van Harten, A. Barendregt, M. T. Rood, A. J. Koster, F. W. van Leeuwen, C. de Mello Donega, A. J. Heck and A. Meijerink, Nanotechnology, 2013, 24, 075703 CrossRef PubMed.
- (a) I. Russier-Antoine, F. Bertorelle, R. Hamouda, D. Rayane, P. Dugourd, Z. Sanader, V. Bonacic-Koutecky, P. F. Brevet and R. Antoine, Nanoscale, 2016, 8, 2892–2898 RSC; (b) M. van der Linden, A. Barendregt, A. J. van Bunningen, P. T. Chin, D. Thies-Weesie, F. M. de Groot and A. Meijerink, Nanoscale, 2016, 8, 19901–19909 RSC; (c) D. M. Black, G. Robles, P. Lopez, S. B. H. Bach, M. Alvarez and R. L. Whetten, Anal. Chem., 2018, 90, 2010–2017 CrossRef CAS PubMed; (d) P. Lopez, H. H. Lara, S. M. Mullins, D. M. Black, H. M. Ramsower, M. M. Alvarez, T. L. Williams, X. Lopez-Lozano, H.-C. Weissker, A. P. García, I. L. Garzón, B. Demeler, J. L. Lopez-Ribot, M. J. Yacamán and R. L. Whetten, ACS Appl. Nano Mater., 2018, 1, 1595–1602 CrossRef CAS; (e) M. van der Linden, A. J. van Bunningen, M. U. Delgado-Jaime, B. Detlefs, P. Glatzel, A. Longo and F. M. F. de Groot, J. Phys. Chem. C, 2018, 122, 28351–28361 CrossRef CAS PubMed.
- J. Kumar, T. Kawai and T. Nakashima, Chem. Commun., 2017, 53, 1269–1272 RSC.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270–283 CrossRef CAS.
- J. S. Binkley, J. A. Pople and W. J. Hehre, J. Am. Chem. Soc., 1980, 102, 939–947 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. A. Petersson, et al., Gaussian 09, Wallingford, CT, 2009 Search PubMed.
- V. L. Jimenez, M. C. Leopold, C. Mazzitelli, J. W. Jorgenson and R. W. Murray, Anal. Chem., 2003, 75, 199–206 CrossRef CAS PubMed.
- (a) Y. Negishi, C. Sakamoto, T. Ohyama and T. Tsukuda, J. Phys. Chem. Lett., 2012, 3, 1624–1628 CrossRef CAS PubMed; (b) Y. Niihori, M. Matsuzaki, T. Pradeep and Y. Negishi, J. Am. Chem. Soc., 2013, 135, 4946–4949 CrossRef CAS PubMed; (c) Y. Niihori, Y. Kikuchi, A. Kato, M. Matsuzaki and Y. Negishi, ACS Nano, 2015, 9, 9347–9356 CrossRef CAS PubMed; (d) Y. Niihori, Y. Koyama, S. Watanabe, S. Hashimoto, S. Hossain, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, J. Phys. Chem. Lett., 2018, 9, 4930–4934 CrossRef CAS PubMed.
- (a) Y. Niihori, C. Uchida, W. Kurashige and Y. Negishi, Phys. Chem. Chem. Phys., 2016, 18, 4251–4265 RSC; (b) Y. Niihori, K. Yoshida, S. Hossain, W. Kurashige and Y. Negishi, Bull. Chem. Soc. Jpn., 2019, 92, 664–695 CrossRef CAS.
- K. Shimomura, T. Ikai, S. Kanoh, E. Yashima and K. Maeda, Nat. Chem., 2014, 6, 429–434 CrossRef CAS PubMed.
- (a) S. Knoppe, I. Dolamic and T. Bürgi, J. Am. Chem. Soc., 2012, 134, 13114–13120 CrossRef CAS PubMed; (b) B. Varnholt, I. Dolamic, S. Knoppe and T. Bürgi, Nanoscale, 2013, 5, 9568–9571 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc05299b |
| This journal is © The Royal Society of Chemistry 2020 |