A terbium metal–organic framework with stable luminescent emission in a wide pH range that acts as a quantitative detection material for nitroaromatics†
Jinzeng Wanga,
Wei Suna,
Siyuan Changa,
Houting Liua,
Guonan Zhanga,
Yanqin Wanga and
Zhiliang Liu*ab
aCollege of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, 010021, P. R. China. E-mail: cezlliu@imu.edu.cn; Fax: +86-471-4992147; Tel: +86-18686029088
bInner Mongolia Key Lab of Fine Organic Synthesis, Inner Mongolia University, Hohhot, P. R. China
First published on 26th May 2015
Abstract
Nitroaromatics are the principal components of explosives and infamous environmental pollutants in the organic synthesis industry. Although high sensitivity towards the detection of nitroaromatics have been demonstrated, selective and quantitative detection are critical for practical applications. Luminescent MOFs constructed by d10 transition metal ions (Zn/Cd) and π-conjugated ligands for nitro aromatics sensing have been widely reported, but the detection efficiency is insufficient due to the weak and non-characteristic luminescence behaviours. And, luminescent MOFs constructed by lanthanide ions which possess enhanced characteristic and strong luminescence emission for nitroaromatic detection are rare. Herein, we report a fluorescent MOF with stable emissions, [Tb(L1)2/3(L2)1/2(H2O)2]·2H2O (where H3L1 = 2,4,6-tris(4-carboxyphenoxy)-1,3,5-triazine, H2L2 = terephthalic acid), whose characteristic emission intensity can maintain more than 80% in a wide pH range (pH = 4–10). Interestingly, this luminescent MOF can quantitatively detect nitroaromatics.
Introduction
It is known that nitroaromatics are the principal components of explosives and infamous environmental pollutants in the organic synthesis industry. Nitroaromatic compounds such as nitrobenzene (NB), 4-nitrotoluene (4-NT), 2,4-dinitrotoluene (2,4-DNT) and trinitrotoluene (TNT) and so on, are on the black list of anti-terrorist or environment security in the USA, the European Union and China, etc. Therefore, selective, quantitative and easily portable methods for detecting these kinds of compounds are crucial. The instrumental analytical techniques generally used for detecting nitro aromatics include gas chromatography coupled with mass spectrometry, Raman spectroscopy, energy dispersive X-ray diffraction, and ion mobility spectrometry (IMS), but these methods are generally expensive and often not easily portable. Due to the presence of electron withdrawing nitro-group, nitroaromatic compounds can serve as strong oxidants and interact with some conjugated electron donors containing delocalized π electrons. Therefore, chemical sensing methods based upon fluorescence quenching are feasible, and these methods are convenient and in high demand for use in detection or sensing.1The immense interest in designing of metal–organic frameworks (MOFs) materials is of considerable increasing in recent years due to their intriguing structural topologies and potential applications in gas storage,2 drug delivery,3 heterogeneous catalysis,4 separation,5 proton conductively6 and chemical sensing.7 Recently, a large amount of luminescent MOF sensors exhibiting high selectivity and sensitivity for detecting anions,8 cations,9 explosives,10 small molecules,11 and vapor12 have been reported. The ability of luminescent MOFs to propagate the host–guest interaction to detectable changes makes them promising candidates for chemical sensor. As far as the luminescent MOFs for detecting nitroaromatics, most of which were constructed by d10 transition metal ions (Zn/Cd) and π-conjugated ligands.13 The pioneering work of Li et al. and others has demonstrated the potential of luminescent MOFs in nitroaromatics detection.14 The fluorescence of this kinds of MOFs is centered on the organic linking group of the framework, and may be assigned to pure intra-ligand emission. However, the detection efficiency of previous reported luminescent MOFs is insufficient due to the weak and non-characteristic luminescent emission.
It is well known that lanthanide metal ions have characteristic luminescence emission when excited at a particular wavelength. Although, the emission of lanthanide ions suffers from weak light absorption and the spin- or parity-forbidden f–f transition, the organic linker containing π-conjugated in lanthanide based MOFs can act as “antenna”, which greatly enhance the lanthanide characteristic emission of the MOFs. Therefore, the lanthanide based MOFs may be the potential candidates for chemical sensing. Indeed, our research group and some others have reported a few Eu3+/Tb3+ based luminescent MOFs for nitroaromatics detection.15 However, compared to d10 ions based MOFs, a few lanthanide based luminescence MOFs acting as nitroaromatic compounds sensing have been reported.16 Furthermore, lanthanide (e.g. Eu3+/Tb3+) based MOFs have several advantages over d10 ions based MOFs, including strong characteristic emission and readily observed colour by the naked eye under a standard UV lamp.
Herein, we present the MOF [Tb(L1)2/3(L2)1/2(H2O)2]·2H2O, denoted Tb-MOF (where H3L1 = 2,4,6-tris(4-carboxyphenoxy)-1,3,5-triazine, H2L2 = terephthalic acid). It displays a three-dimensional (3D) open framework structure (see Fig. 1) with relatively large channels hosting highly disordered guest (H2O) molecules. Remarkably, we find that Tb-MOF emits high bright characteristic green light readily observed by the naked eye under a standard UV lamp. Tb-MOF possesses relatively stable luminescent properties in wide pH range. The luminescence of Tb-MOF can be selectively and quantitatively quenched by nitroaromatic compounds, indicating its possibility of being a sensing material for nitroaromatics.
Experimental section
Materials and general characterizations
All reagents and solvents were used as received from commercial supplies without further purification. Fourier transform infrared (FTIR) spectra (KBr disk) were measured with a TENSOR 27 FT-IR spectrophotometer. Elemental analyses for C, H, N were obtained from a Perkin-Elmer 2400 elemental analyzer. TGA were measured with NETZSCH TG 209 under heating rate of 10 °C min−1. Powder X-ray diffraction measurements were recorded on a EMPYREAN PANALYTICAL apparatus.Synthesis of [Tb(L1)2/3(L2)1/2(H2O)2]·2H2O
A mixture of Tb(NO3)3·6H2O (0.2 mmol), 2,4,6-tris(4-carboxyphenoxy)-1,3,5-triazine, (0.2 mmol), and terephthalic acid (0.1 mmol) was dissolved in 10 mL of mixture solvent (5 mL distilled water and 5 mL methyl cyanide) and adjusted the pH of solution around 4.5 by NaOH solution. Then it was sealed in a 23 mL Teflon-line stainless-steel autoclave and heated at 120 °C for 72 h followed by cooling to room temperature at the rate of 5 °C h−1. The resulting colorless block crystals were isolated after being filtered, washed with distilled water several times and dried at ambient temperature; yield 75% (0.095 g) based on Tb(NO3)3·6H2O. Anal. calc. for: C, 37.69%; H, 2.85%; N, 4.41%. Found: C, 37.88%; H, 2.73%; N, 4.66%.Single crystal X-ray diffraction
For single crystal XRD measurements, a suitable crystal for the compound was carefully selected under a polarizing microscope and glued to a thin glass fiber. The single crystal diffraction data were collected on a Agilent Xcalibur E X-ray single crystal diffractometer equipped with graphite-monochromatic Cu-Kα radiation at 293 K. Empirical absorption corrections were applied using the SADABS program.17 The structure of Tb-MOF was solved by direct methods and refined anisotropically by full-matrix least squares techniques on F2 values, using the SHELX-97 program.18 Hydrogen atoms were added on appropriate positions in theory and refined with isotropic thermal parameters riding on those parent atoms. All hydrogen atoms in the coordination polymer were generated geometrically and refined isotropically using the riding model. Crystallographic data and structure refinement parameters for Tb-MOF are listed in Table S1.† Selected distances and bond angles for Tb-MOF are listed in Table S2.†Luminescence measurements method
The luminescence spectra for the powdered solid samples were measured at room temperature on a Hitachi F-7000 fluorescence spectrophotometer. The excitation slit and the emission slit were 5.0 nm, PMT voltage = 700 V. The scan speed is 240 nm min−1.The luminescence properties of Tb-MOF were studied in solid states. To investment the sensing properties of Tb-MOF, the fine grinding sample Tb-MOF (3 mg) were ultrasonic agitated for 30 min then dispersed in various nitroaromatics–ethanol solution (5 mL) to form a stable emulsion at room temperature.
The fluorescence titrimetric method was used to measure the concentration dependent luminescence intensity for Tb-MOF dispersed in nitrobenzene (NB) solutions. In this case, 12 mg of Tb-MOF dispersed in 20 mL ethanol and the different amount of NB was added in above suspension system.
Results and discussion
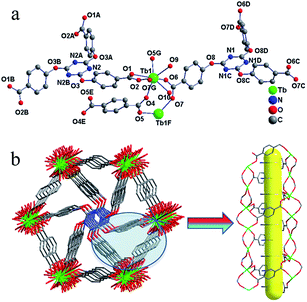
A luminescent three-dimensional Tb-MOF, [Tb(L1)2/3(L2)1/2(H2O)2]·2H2O, was synthesized solvothermally in mixture solvent. Single crystal structure analysis reveals that Tb-MOF crystallized in the triclinic space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) exhibiting a three-dimensional framework. The asymmetric unit of Tb-MOF contains one crystallographically independent Tb3+ ion, two-third of L13− ligand, half a L22− ligand, two coordinated H2O molecules and two isolated solvent H2O molecules.
exhibiting a three-dimensional framework. The asymmetric unit of Tb-MOF contains one crystallographically independent Tb3+ ion, two-third of L13− ligand, half a L22− ligand, two coordinated H2O molecules and two isolated solvent H2O molecules.
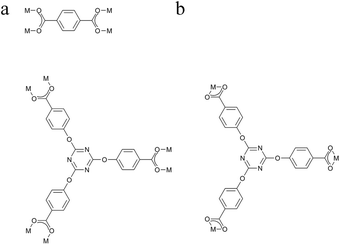
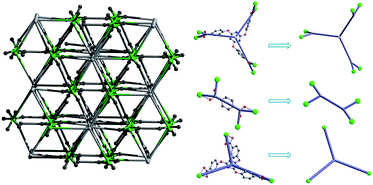
As depicted in Fig. 1, Tb3+ ion is eight-coordinated by two oxygen atoms of one bidentate carboxylate groups from L13− ligand, four monodentate oxygen atoms from L13− and L22− ligands separately and two oxygen atoms from H2O molecules. The Tb–O distances range from 2.271(4) Å to 2.506(4) Å, the O–Tb–O′ angels are between 52.91(12)° and 151.95(15)°. Selected bond lengths and bond angles for Tb-MOF are listed in Table S2 (ESI†). The L13− exhibits types of (a) and (b) coordination mode separately as shown in Fig. 2, acting as sexadentate metal linker and tridentate linker for the latter. The L22− exhibits series a coordination mode and acts as tetradentate metal linker. For the binuclear Tb3+ subunit, the Tb3+ are bridged by two-fold carboxyl groups from L13− and L22− ligands one by one forming a one-dimensional chain along the b direction (Fig. 1). The chains are connected by the carboxylic oxygen atoms of tridentate L13− ligands into a two-dimensional plane then further bridged by the L13− and L22− ligands into a three-dimensional framework. PLATON analysis indicates that the solvent accessible volume and porosity are 442.8 Å3 and 4.2%, respectively. The structure can be simplified as a 3,3,3,3,5-connected framework with the point Schlafli symbol {103}{4 × 102}3{4·82}3{42·83 × 105}3{83}, as shown in Fig. 3.
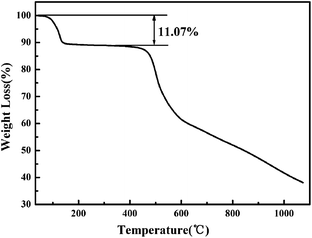
To reveal the thermal stability and further support the molecular formula of the Tb-MOF, the TGA measurement was performed on pure crystal samples of Tb-MOF under an N2 atmosphere with a heating rate of 10 °C min−1 over the range of 30–1100 °C (Fig. 4). The compound shows favourable thermal stability. The TGA curve shows two regions of weight loss. The first weight loss between 100–140 °C is 11.07%, which corresponds to the loss of two isolated water molecules and two coordinated water molecules, calculated to be 11.29%. The second weight loss, above 420 °C, results from the decomposition of the compound. According to the TGA pattern analysis we can conclude that, the isolated water molecules in the pores are easily removed before 140 °C, and in the range of 140–420 °C, the compound remain intact after the removal of the isolated and coordinated water molecules (see Fig. S1 and S2†). Compared to the luminescence emission of as-made sample, the slight red-shift of activated sample is due to the change of Tb3+ energy levels caused by the loss of coordinated water molecules (Fig. S2†).
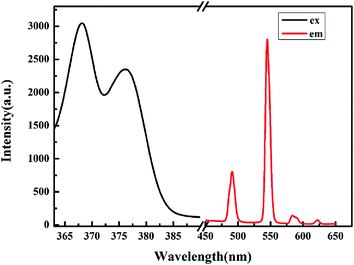
Exposed under the standard UV lamp (254 nm), the Tb-MOF presents very strong green light which can be observed by the naked eye. The solid-state luminescent spectrum of Tb-MOF was recorded under ambient conditions. The high luminescence intensity of Tb-MOF mostly owes to the highly conjugated structure of the organic linkers, which commonly known as “antenna effect” and greatly enhances the optical performance of the Tb3+ ions. The four characteristic emission bands are located at 491 nm, 545 nm, 584 nm, 622 nm which attributed to 5D4–7F6, 5D4–7F5, 5D4–7F4 and 5D4–7F3 transitions of Tb3+, respectively, upon the excitation at 369 nm (Fig. 5).
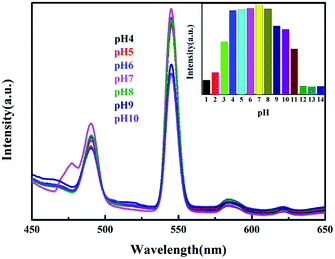
The most prominent characteristic emission peak of Tb-MOF is presented at 545 nm which can be readily observed as bright green light by the naked eye. The strong visible emission provides the possibility of Tb-MOF acting as a luminescence sensor. It is noteworthy that Tb-MOF could keep high characteristic emission intensity in water when the pH value varied from 4 to 10 (as shown the insert in Fig. 6, the characteristic emission intensity can keep more than 80% in pH range of 4–10). From the evidence of PXRD, the framework of Tb-MOF immersed in different pH also can stay stable (see Fig. S3†). Comparing to other chemical sensors,7c this significant luminescent stability in wide pH range makes the Tb-MOF to be an excellent candidate for luminescent sensor.
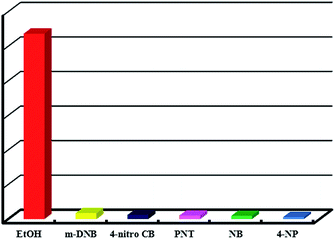
As a matter of fact, luminescent MOFs have several advantages for detecting organic small molecules.19 In this case, MOFs are expected to be highly promising host for guest molecules, and energy transfer between the host-MOFs and guest molecules may produce a synergistic effect for the efficient identification of specific molecules. Thus, we have analyzed the luminescence properties of Tb-MOF after immersed in different selected analytes. To explore the potential luminescence sensing ability of Tb-MOF, the fine grinding samples were dispersed in common organic small molecule solutions (such as the 0.1 mol L−1 ethanol solution of methyl cyanide, trichloromethane, DMF, benzene, toluene, nitrobenzene, m-dinitrobenzene and paranitrotoluene) and the photoluminescent (PL) spectra were recorded to different analytes. As depicted in Fig. 7(a), the significant quenching of fluorescence intensities were observed upon addition of Tb-MOF to nitroaromatic compounds containing solutions such as NB, m-DNB and PNT. Accordingly, Fig. 7(b) presents a megascopic fluorescence quenching effect. Such observations demonstrate the possibility of Tb-MOF for selective sensing of nitroaromatic compounds. Based upon structure analysis, this Tb-MOF shows a rigid, permanently porous structure which contains conjugated aromatic rings within the ordered framework. Thus, the host–guest interaction can propagate between Tb-MOF and aromatic compounds via π–π interactions or dipole–dipole interactions. Thereby, the fluorescence quenching mechanisms between the luminescent MOFs and the nitroaromatics can be explained in previous reports of our research group and some others.14a,d,15d
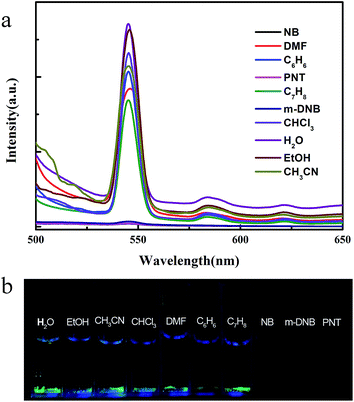 | ||
| Fig. 7 (a) Luminescence spectra of Tb-MOF in different solvents. (b) The picture of Tb-MOF in different solvents under 254 nm UV-lamp. | ||
As shown in Fig. 8, the powder X-ray diffraction (PXRD) patterns of obtained samples are consistent with the corresponding single crystal simulated pattern which indicates the good phase purity of Tb-MOF. To explore the lattice stability of Tb-MOF after immersed in analyte solution, the PXRD patterns of Tb-MOF immersed in NB and m-DNB solution were measured and the result indicates that the nitroaromatics loading only very slightly impacts on the crystalline integrity of the Tb-MOF.
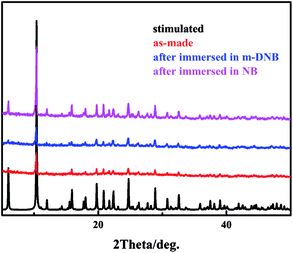 | ||
| Fig. 8 PXRD of Tb-MOF stimulated (black), as-made (red), after immersed in m-DNB (blue), after immersed in NB (pink). | ||
In order to investigate whether the second substituent group of NB influence the detection ability of Tb-MOF, some nitroaromatic compounds with different second substituent group on aromatic ring have been examined with a concentration of 0.1 M analytes in ethanol. According to the experimental results (Fig. 9), no matter what type of second substituent group (e.g. electron donor or acceptor) is introduced on the benzene ring of nitrobenzene, Tb-MOF shows obviously quenching effect towards all selected nitroaromatic derivatives. Namely, Tb-MOF is a potential excellent luminescence detection material for most of the nitroaromatic compounds.
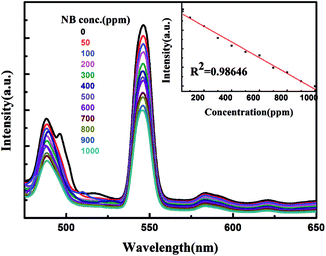
The photoluminescent (PL) spectrum of Tb-MOF dispersed in EtOH exhibits strong emission at ∼545 nm when excited at 369 nm. To explore the ability of Tb-MOF to detect nitroaromatic compounds quantitatively, fluorescence-quenching titrations were performed with incremental addition of nitrobenzene (NB) as representative to Tb-MOF dispersed in EtOH. High fluorescence quenching was observed upon incremental addition of NB solution. The visible bright green emission of Tb-MOF vanished upon the addition of the NB solution, the fluorescence quenching by NB could be quantitatively determined at low concentrations. As depicted in Fig. 10, the quenching of luminescence intensity of Tb-MOF displays an ideal linear correlation with the increasing of the concentration of NB (from 50 ppm to 1000 ppm). Furthermore, m-dinitrobenzene (m-DNB) was investigated, which also presents the similar linear relationship of luminescence-quenching (Fig. S4†). The results provides us a feasible and promising quantitative analytical approach to detect nitroaromatic compounds by using luminescent Tb-MOF material as a chemical sensor.
In addition, detection of NB with Tb-MOF can be fully reversible. After quenching, the photoluminescence of Tb-MOF can be recovered by simply washing the sample with ethanol (3 mg sample, 5 mL ethanol solvent was used under ultrasonic washing condition for two minutes several times). As depicted in Fig. 11, after 5 time recycles, Tb-MOF showed almost identically rapid and evident responses to NB. The slight fluorescence decay of the recovered Tb-MOF may be mainly caused by the sample loss during the recycle experimental process.
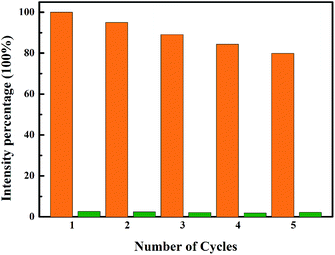 | ||
| Fig. 11 Recycling of Tb-MOF dispersed in NB. The luminescence was recovered by washing with EtOH several times (intensity percentage = I/I0 × 100%). | ||
Conclusions
In conclusion, a highly luminescent Tb-MOF, [Tb(L1)2/3(L2)1/2(H2O)2]·2H2O, was designed and successfully synthesized. It is a novel metal–organic material with stable fluorescence emission in wide pH range. Probably through electric charge transfers luminescent quenching mechanism similar to that in the previous reported systems, Tb-MOF can sense nitroaromatics based on luminescence quenching effect. Interestingly, the visible bright green emission of Tb-MOF could be quantitatively quenched upon the addition of the nitroaromatics at low concentrations. Therefore, this luminescent MOF can quantitatively detect the nitroaromatics. Furthermore, such detection was also proven to be reversible. These remarkable preliminary results point to a new and important application of lanthanide metal–organic framework materials. Comprehensive studies of lanthanide luminescent MOFs are currently underway to further explore their potential in nitroaromatics sensing. It can be envisioned that one can develop a series of lanthanide luminescent MOFs that will have highly sensitive and selective responses towards a particular nitroaromatic compounds.Acknowledgements
We would like to kindly acknowledge the NSFC (21361016) and Inner Mongolia Foundation for Natural Science (2013ZD09) financial support.Notes and references
- (a) S. K. Kim, J. M. Lim, T. Pradhan, H. S. Jung, V. M. Lynch, J. S. Kim, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2014, 136, 495 CrossRef CAS PubMed; (b) Y. H. Lee, H. Liu, J. Y. Lee, S. H. Kim, S. K. Kim, J. L. Sessler, Y. Kim, J. S. Kim, J. S. Kim, D. Kim and J. L. Sessler, Chem.–Eur. J., 2010, 16, 5895 CrossRef CAS PubMed; (c) A. Rose, Z. G. Zhu, C. F. Madigan, T. M. Swager and V. Bulovic, Nature, 2005, 434, 876 CrossRef CAS PubMed; (d) S. W. Thomas, J. P. T. M. Amara, R. E. Bjork and T. M. Swager, Chem. Commun., 2005, 4572 RSC; (e) S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC.
- (a) M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS PubMed; (b) L. Chen, J. P. Mowat, D. Fairen-Jimenez, C. A. Morrison, S. P. Thompson, P. A. Wright and T. Duren, J. Am. Chem. Soc., 2013, 135(42), 15763 CrossRef CAS PubMed.
- (a) M. C. Bernini, D. Fairen-Jimenez, M. Pasinetti, A. J. Ramirez-Pastor and R. Q. Snurr, J. Mater. Chem. B, 2014, 2, 766 RSC; (b) P. Horcajada, C. Serre, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Ferey, Angew. Chem., Int. Ed., 2006, 45(36), 5974 CrossRef CAS PubMed.
- (a) L. Chen, H. Chen, R. Luque and Y. Li, Chem. Sci., 2014, 5, 3708 RSC; (b) K. K. Tanabe, M. S. Ferrandon, N. A. Siladke, S. J. Kraft, G. Zhang, J. Niklas, O. G. Poluektov, S. J. Lopykinski, E. E. Bunel, T. R. Krause, J. T. Miller, A. S. Hock and S. T. Nguyen, Angew. Chem., Int. Ed., 2014, 53(45), 12055 CrossRef CAS PubMed.
- (a) J. L. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44(30), 4670 CrossRef CAS PubMed; (b) S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schröder, Nat. Chem., 2015, 7, 121 CrossRef CAS PubMed.
- (a) A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. El Gabaly, H. P. Yoon, F. Leonard and M. D. Allendorf, Science, 2014, 343(6166), 66 CrossRef CAS PubMed; (b) Q. Tang, Y. Liu, S. Liu, D. He, J. Miao, X. Wang, G. Yang, Z. Shi and Z. Zheng, J. Am. Chem. Soc., 2014, 136(35), 12444 CrossRef CAS PubMed; (c) B. M. Wiers, M. L. Foo, N. P. Balsara and J. R. Long, J. Am. Chem. Soc., 2011, 133(37), 14522 CrossRef CAS PubMed; (d) R. Wang, X.-Y. Dong, H. Xu, R.-B. Pei, M.-L. Ma, S.-Q. Zang, H.-W. Hou and T. C. W. Mak, Chem. Commun., 2014, 50, 9153 RSC.
- (a) X.-Z. Song, S.-Y. Song, S.-N. Zhao, Z.-M. Hao, M. Zhu, X. Meng, L.-L. Wu and H.-J. Zhang, Adv. Funct. Mater., 2014, 24, 4034 CrossRef CAS PubMed; (b) H. L. Jiang, D. Feng, K. Wang, Z. Y. Gu, Z. Wei, Y. P. Chen and H. C. Zhou, J. Am. Chem. Soc., 2013, 135(37), 13934 CrossRef CAS PubMed; (c) M. Zhang, G. Feng, Z. Song, Y. P. Zhou, H. Y. Chao, D. Yuan, T. T. Tan, Z. Guo, Z. Hu, B. Z. Tang, B. Liu and D. Zhao, J. Am. Chem. Soc., 2014, 136(20), 7241–7244 CrossRef CAS PubMed; (d) M. Guo and Z.-M. Sun, J. Mater. Chem., 2012, 22(31), 15939 RSC; (e) H.-Y. Li, Y.-L. Wei, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chem. Mater., 2015, 27, 1327 CrossRef CAS.
- J. Cao, Y. Gao, Y. Wang, C. Du and Z. Liu, Chem. Commun., 2013, 49(61), 6897 RSC.
- (a) Y. Qiu, H. Deng, J. Mou, S. Yang, M. Zeller, S. R. Batten, H. Wu and J. Li, Chem. Commun., 2009, 36, 5415 RSC; (b) P.-F. Shi, B. Zhao, G. Xiong, Y.-L. Hou and P. Cheng, Chem. Commun., 2012, 48(66), 8231 RSC; (c) X.-Y. Dong, R. Wang, J.-Z. Wang, S.-Q. Zang and T. C. W. Mak, J. Mater. Chem. A, 2015, 3, 641 RSC.
- S. Pramanik, C. Zheng, X. Zhang, T. J. Emge and J. Li, J. Am. Chem. Soc., 2011, 133(12), 4153 CrossRef CAS PubMed.
- J. Cui, Z. Lu, Y. Li, Z. Guo and H. Zheng, Chem. Commun., 2012, 48(64), 7967 RSC.
- M. Zhang, G. Feng, Z. Song, Y.-P. Zhou, H.-Y. Chao, D. Yuan, T. T. Y. Tan, Z. Guo, Z. Hu, B. Z. Tang, B. Liu and D. Zhao, J. Am. Chem. Soc., 2014, 136(20), 7241 CrossRef CAS PubMed.
- D. Banerjee, Z. Hu and J. Li, Dalton Trans., 2014, 10668 RSC.
- (a) A. J. Lan, K. H. Li, H. H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. C. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334 CrossRef CAS PubMed; (b) S. Pramanik, C. Zheng, X. Zhang, T. J. Emge and J. Li, J. Am. Chem. Soc., 2011, 133, 4153 CrossRef CAS PubMed; (c) S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Angew. Chem., Int. Ed., 2013, 52, 2881 CrossRef CAS PubMed; (d) K. S. Asha, K. Bhattacharyya and S. Mandal, J. Mater. Chem. C, 2014, 2, 10073 RSC.
- (a) X. H. Zhou, H. H. Li, H. P. Xiao, L. Li, Q. Zhao, T. Yang, J. L. Zuo and W. Huang, Dalton Trans., 2013, 5718 RSC; (b) H. Xu, F. Liu, Y. Cui, B. Chen and G. Qian, Chem. Commun., 2011, 47, 3153 RSC; (c) J. D. Xiao, L. G. Qiu, F. Ke, Y. P. Yuan, G. S. Xu, Y. M. Wang and X. Jiang, J. Mater. Chem. A, 2013, 1, 8745 RSC; (d) W. Sun, J. Wang, H. Liu, S. Chang, X. Qin and Z. Liu, Mater. Lett., 2014, 126, 189 CrossRef CAS PubMed.
- (a) G.-Y. Wang, L.-L. Yang, Y. Li, H. Song, W.-J. Ruan, Z. Chang and X.-H. Bu, Dalton Trans., 2013, 12865 RSC; (b) J.-J. Qian, L.-G. Qiu, Y.-M. Wang, Y.-P. Yuan, A.-J. Xie and Y.-H. Shen, Dalton Trans., 2014, 3978 RSC; (c) D. Banerjee, Z. Hu and J. Li, Dalton Trans., 2014, 10668 RSC; (d) Y.-N. Gong, L. Jiang and T.-B. Lu, Chem. Commun., 2013, 49, 11113 RSC.
- G. M. Sheldrick, Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, SHELXTL, version 5.1, Bruker Analytical X-ray Instruments Inc., Madison, WI, 1998 Search PubMed.
- (a) B. L. Chen, S. C. Xiang and G. D. Qian, Acc. Chem. Res., 2010, 43, 1115–1124 CrossRef CAS PubMed; (b) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallography details and additional figures. X-ray crystallographic data for the structures of Tb-MOF has been deposited with the Cambridge Crystallographic Data Center. CCDC 1056824. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra06308f |
| This journal is © The Royal Society of Chemistry 2015 |