From unprecedented 2,2′-bisimidazole-bridged rare earth organometallics to magnetic hysteresis in the dysprosium congener†
Florian
Benner
 and
Selvan
Demir
and
Selvan
Demir
 *
*
Department of Chemistry, Michigan State University, 578 South Shaw Lane, East Lansing, Michigan 48824, USA. E-mail: sdemir@chemistry.msu.edu
First published on 31st May 2023
Abstract
The first use of the bare 2,2′-bisimidazole (H2bim) ligand in rare earth metal chemistry is presented. A series of symmetric dinuclear complexes [(Cp*2RE)2(μ-bim)] were synthesized from the salt metathesis reaction of the lithium salt Li2(bim) with Cp*2RE(BPh4) (RE = Y (1), Gd (2), Dy (3); Cp* = 1,2,3,4,5-pentamethylcyclopentadienyl). The isostructural complexes 1–3 were unambiguously characterized through elemental analysis, NMR, IR and UV/Vis spectroscopy, single-crystal X-ray diffraction, SQUID magnetometry and density functional theory (DFT) calculations. Intriguingly, the compounds are redox-inactive both on the timescale of chemical and electrochemical experiments. Herein, a rationale for the redox innocence of the bim2− ligand is provided by calculations of the electron affinity and ionization potential, both correlating well with topologically similar structures of comparable complexes. Remarkably, the Dy complex 3 shows open magnetic hysteresis loops up to 5 K which is rare for lanthanide SMMs with bridging diamagnetic entities. AC magnetic susceptibility measurements at zero field revealed slow magnetic relaxation up to 26 K leading to an effective energy barrier to spin reversal of Ueff = 154(2) cm−1 and τ0 = 5(1) × 10−8 s. The lanthanides are weakly antiferromagnetically coupled, where the J value for the Gd-congener 2 was determined to be −0.074(2) cm−1.
Introduction
The rational design of polynuclear molecular lanthanide (Ln) complexes has sparked tremendous interest among chemists to uncover novel catalytic reactivity,1 luminescence properties2–4 and single-molecule magnet (SMM) behavior. The latter requires the molecule's magnetic moment to exhibit a preferred orientation, giving rise to a bistable ground state, where spin “up” and “down” are separated by an energy barrier to spin reversal, Ueff. Since the first observation of slow magnetic relaxation in a molecule in the [Mn12O12(CH3COO)16(H2O)4] complex in 1993,5,6 milestones in terms of SMM characteristics have been achieved by the use of highly anisotropic LnIII ions such as DyIII or TbIII to form mononuclear7 and multinuclear complexes. The latter advance benefited from the implementation of radical bridges that are able to couple two lanthanide centers strongly together.8 Among those, owing to the short, highly charged bridge, dinitrogen radical-bridged systems are particularly impressive, where the organometallic terbium complex [K(crypt-222)][(Cptet2Tb)2(μ-N23−˙)] (where crypt-222 = 2.2.2-cryptand; Cptet = 1,2,3,4-tetramethylcyclopentadienyl) exhibits open hysteresis loops up to 30 K and maximum coercivity of 8 T, both the highest for a dinuclear compound containing a radical moeity.9 This is only surpassed by the mixed-valent dinuclear compound (CpiPr5)2Dy2I3 which features record metrics for SMMs in terms of spin-reversal barrier, blocking temperature, and coercivity.10 These advances are a testament of the enormous potential of coupling highly anisotropic lanthanide ions strongly together, and the number of such examples is small, thus, research towards this direction is desirable.11–13An underexplored ligand system is the tetraaza-analogue of fulvalene, 2,2′-bisimidazole (bim), which is composed of two bridged five-membered rings, each featuring two N atoms. Both the protonated (H2bim) and deprotonated (bim2−) have been used in coordination chemistry. Transition metal (TM) complexes feature the ligand in its protonated (H2bim),14–22 ligands singly deprotonated (Hbim−)19,24,25 or doubly deprotonated (bim2−)14,17,19,21,25–28 form. The degree of protonation affects the denticity of the ligand and provided access to mono-,14,16–21,25 di-,14,15,17,21,22,24–26,29–31 tri-,27,32 or tetranuclear complexes19,27,28Chart 1. This structural richness gave rise to a wide range of applications where the TM complexes were luminescent,22 catalytically active,15 or intriguing for their spin-crossover properties.14,16 The various binding modes also allowed the construction of metal–organic frameworks28,31,32 or polyoxometallates.15,30 Furthermore, many complexes of H2bim show strong ion pairing effects through hydrogen bonding of the N–H groups with anionic species,16,20 yielding exotic N–H-bridged dimeric complexes19 or potential applications in anion sensing.14
By comparison, H2bim/bim2− ligands are entirely unknown in molecular rare earth metal chemistry. In multidimensional systems, only four examples are known all of which are lanthanide (Ln) MOFs, where three of them contain N-carboxylate-substituted bim ligands33–35 and one features direct Ln–N-bonds to the bim.29 This scarcity is surprising as its six-membered counterpart 2,2′-bipyrimidine (bpym) has found many promising applications in the fields of small-molecule activation36,37 and SMMs.38
Here, we present the synthesis and characterization of the first bim-bridged rare earth (RE) complexes (Cp*2RE)2bim (where Cp* = pentamethylcyclopentadienyl) with the RE metals yttrium (1), gadolinium (2), and dysprosium (3). The Cp*2 ligand field was judiciously chosen due to its ability to reinforce the preferred axial orientation of the DyIII magnetic moment while providing sufficient steric hindrance to avoid equatorial coordination of solvent molecules.
Results and discussion
The dinuclear bisimidazolate-bridged complexes were pursued via a salt metathesis strategy aiming at the synthesis of a pro-ligand and a metal source first which can readily exchange their counterions to produce a thermodynamically more stable side product. We employed the proligand [(Li(PMDTA))2bim] (PMDTA: 1,1,4,7,7-pentamethyldiethylenetriamine), where the Li cation is encapsuled in the tetradentate PMDTA chelate. This known lithium compound was prepared through deprotonation of commercially available H2bim with nBuLi in the presence of the chelating PMDTA.26 As a metal source, we used Cp*2RE(BPh4) (RE = Y, Gd, Dy),38 each featuring a weakly coordinated BPh4− anionic ligand, which can easily be displaced via anionic ligands in salt elimination reactions.The neutral complexes [(Cp*2RE)2(μ-bim)] (RE = Y (1), Gd (2), Dy (3)) were generated through salt metathesis reactions of Cp*2RE(BPh4) (RE = Y, Gd, Dy) with [(Li(PMDTA))2bim] in THF at room temperature in 31% (1), 52% (2) and 52% (3) crystalline yields (Fig. 1). Crystals suitable for X-ray diffraction analysis were obtained from cooling concentrated toluene solutions to −30 °C over the course of three days (Fig. 1, Table 1 and Tables S1–S3†). Compounds 1–3 crystallize in the monoclinic space group P21/c where each metal center is formally eight-coordinated by two Cp* and two N atoms of a slightly asymmetrically coordinated bridging bim ligand with average RE–N distances (2.405(3) (1), 2.430 (2), 2.414(4) Å (3)) comparable to the average 2.393(3) Å of the structurally related dysprosocenium complex [K(cryp-222)][(Cp*2Ln)(N,N′-bpyB)] bearing a dianionic 2,2′-bipyridine ligand (N,N′-bpyB = 5,5′-bis(dimesitylboranyl)-2,2′-bipyridine).39 Recently, we isolated the structurally closely related yttrium complex [(Cp*2Y)2(μ-Bbim)] in which the bridge constitutes annulated bim, namely bisbenzimidazole (Bbim).40 The observed Cnt–RE–Cnt angles in 1–3 are slightly smaller (difference (Δ) = −1.5°) and the RE⋯RE-distances significantly shorter (Δ = −0.109 Å) than in the respective Bbim complex, indicative of a smaller steric repulsion between the two Cp*2RE scaffolds owing to the smaller bridging ligand. Moreover, the metal centers are symmetry-related through an inversion center residing on the bim C2–C′2 bond. In certain cases, the tetraphenylborate moiety within Cp*2RE(BPh4) is able to reduce redox-active ligands innate to accessible redox potentials such as phenazine (−1.83 V vs. Fc/Fc+),41 and thus, could potentially undergo a redox reaction with bim2− to produce a radical bridge with a 3−˙ oxidation state, where the an unpaired electron is localized in the ligands π*-orbitals.42 However, the central C2–C′2 distance of 1.438(15) Å (1), 1.45(2) Å (2), and 1.448(4) Å (3), relative to 1.433(15) Å of the bim2−-bridged diosmium complex [(OsII(bpy)2)2(bim)](ClO4)2,14 as the structurally closest example, alluded to an unambiguous assignment of the bim oxidation state as −2 in 1–3. Importantly, the radical oxidation state of 3−˙ for bim is hitherto unknown.
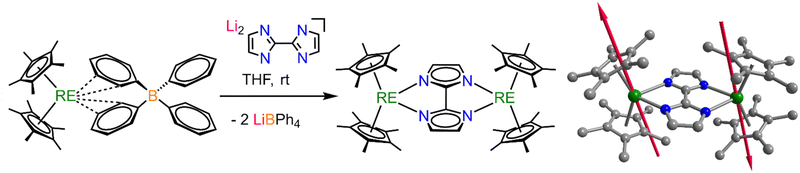 | ||
| Fig. 1 Synthesis of [(Cp*2RE)2(μ-bim)] RE = Y (1), Gd (2), Dy (3), Li = [Li(PMDTA)]+ (left) via salt metathesis reaction of Cp*2RE(BPh4) and Li2bim. Structure of 3 (right). Green, blue, and grey spheres represent dysprosium, nitrogen, and carbon atoms, respectively. All hydrogen atoms and lattice solvent molecules for 3 are omitted for clarity. Red arrows represent the orientation of the magnetic anisotropy of the MJ = ± 15/2 state of DyIII as calculated by Magellan.23 | ||
| 1 | 2 | 3 | |
|---|---|---|---|
| a Cnt = centroid of the pentamethylcyclopentadienyl ring. | |||
| RE–N | 2.412(3), 2.397(3) | 2.424(3), 2.435(3) | 2.417(2), 2.403(2) |
| Cnta–RE | 2.362, 2.369 | 2.405, 2.398 | 2.363, 2.374 |
| C2–C′2 | 1.438(8) | 1.452(7) | 1.452(5) |
| M⋯M | 6.105(1) | 6.169(1) | 6.119(1) |
| Cnt–RE–Cnt | 137.1 | 137.8 | 137.6 |
| RE–N–N–RE | 13.5(5) | 15.5(3) | 15.2(3) |
Owing to the very similar coordination sphere of around each metal center, a comparison of [(Cp*2Y)2(μ-Bbim)] with 1 can aid to further understand structural trends arising from modifications of the bridging ligand. In spite of the presence of differing metal ions, in the first approximation the bonding situation is transferable to the dysprosium conger due to the similar ionic radii (Y: 1.019 Å, DyIII: 1.027 Å)43 and electronic configuration 4d05s0 (YIII) and 4f95d06s0 (DyIII), which leaves the DyIII 4f valence shell strongly contracted and hindered for orbital overlap with ligand orbitals.
Despite the annulation of the bim moiety the ligand's electronic structure and bonding remain largely unaffected as proven by essentially identical C2–C′2 bond distances of 1.438(8) Å (1), 1.452(7) Å (2), 1.448(4) Å (3) relative to 1.445(6) Å in [(Cp*2Y)2(μ-Bbim)].
The successful complexation of the bim2− ligand can easily be traced by 1H NMR spectroscopy on complex 1, since the two diamagnetic YIII ion facilitate execution and interpretation of nuclear magnetic resonance measurements, Fig. S1–S3.† In the 1H-NMR spectrum of 1, the presence of one aromatic singlet at 6.75 ppm and one aliphatic singlet at 1.87 ppm with an integral ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 can be associated with the bim2− protons and Cp* methyl protons, respectively. In addition, paramagnetic 1H NMR spectra of 2 and 3 were recorded at room temperature in C6D6 solutions. For 3, a set of two broad signals at δ = −1.99 and −15.29 ppm occurs where the broadness is attributed to the presence of two paramagnetic DyIII ions. The integration of these signals hints at a more pronounced shielding of the Cp* protons relative to the bim2− proton resonances. For 2, only one clearly paramagnetic signal at −25.1 ppm is observed, whereas the expected second signal might be obstructed by residual nhexane. As observed for the other paramagnetic Ln ions before,44–46 the paramagnetic nature of GdIII and DyIII ions render 13C NMR experiments challenging. In fact, no 13C NMR signals were observed for complex 2 (compare Fig. S4 to S5†).
60 can be associated with the bim2− protons and Cp* methyl protons, respectively. In addition, paramagnetic 1H NMR spectra of 2 and 3 were recorded at room temperature in C6D6 solutions. For 3, a set of two broad signals at δ = −1.99 and −15.29 ppm occurs where the broadness is attributed to the presence of two paramagnetic DyIII ions. The integration of these signals hints at a more pronounced shielding of the Cp* protons relative to the bim2− proton resonances. For 2, only one clearly paramagnetic signal at −25.1 ppm is observed, whereas the expected second signal might be obstructed by residual nhexane. As observed for the other paramagnetic Ln ions before,44–46 the paramagnetic nature of GdIII and DyIII ions render 13C NMR experiments challenging. In fact, no 13C NMR signals were observed for complex 2 (compare Fig. S4 to S5†).
The complexation of the bim2− ligand engenders also pronounced changes of the UV/Vis absorption spectra of 1–3 compared to reported spectra of the starting material Cp*2Dy(BPh4), Fig. 2A and S9.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 Most prominently, a broad band at ∼300 nm emerged after complexation. This band stretches from 275 to 350 nm and is split into six discernible transitions at 281, 286, 294, 302, 311 and 320 nm for 1 and as such differs from the UV/Vis spectrum collected for [(Cp*2Y)2(μ-Bbim)], which features a broad transition between 275 and 395 nm with additional sharp transitions at 343 and 364 nm.40 Hence, in both cases, the UV/Vis spectra must be strongly affected by transitions involving orbitals located on the bridging ligand, where the hypsochromic shift likely stems from a larger HOMO–LUMO gap in 1–3 owing to the absence of electron donating phenyl rings (Table S7†).
47 Most prominently, a broad band at ∼300 nm emerged after complexation. This band stretches from 275 to 350 nm and is split into six discernible transitions at 281, 286, 294, 302, 311 and 320 nm for 1 and as such differs from the UV/Vis spectrum collected for [(Cp*2Y)2(μ-Bbim)], which features a broad transition between 275 and 395 nm with additional sharp transitions at 343 and 364 nm.40 Hence, in both cases, the UV/Vis spectra must be strongly affected by transitions involving orbitals located on the bridging ligand, where the hypsochromic shift likely stems from a larger HOMO–LUMO gap in 1–3 owing to the absence of electron donating phenyl rings (Table S7†).
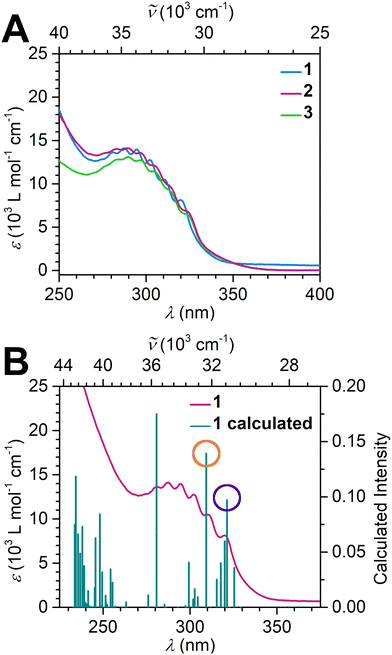 | ||
| Fig. 2 Magnified UV/Vis spectra of [(Cp*2RE)2(μ-bim)] (blue line: Y (1); purple line: Gd (2); green line: Dy (3)) between 250 and 400 nm, taken in THF (A). Full spectra are shown in Fig. S9.† Overlay of experimental UV/Vis spectrum of 1 (purple line) with TDDFT-calculated transitions (green bars). (B) Orange and purple circles highlight the transitions at 309 nm and 321 nm described in the text, which primarily involve bim-based transitions. Concentrations: 22.38 μmol L−1 (1), 28.65 μmol L−1 (2), 24.60 μmol L−1 (3). | ||
TDDFT calculations on 1 were carried out to gain insight into the electronic excitation energies (Fig. 2B and Table S4†) using ORCA 5.0.3 (see ESI† for computational details).48 The calculated, most intense transitions between 280 and 375 nm are excitations into the LUMO, which comprises a vacant Y centered d-orbital with negligible bim contributions (Fig. 3). By contrast, the HOMO exhibits bim-based π-character with smaller Cp*–π contributions. The calculated most intense excitation into the LUMO at 281 nm constitutes to 83% of a low-lying Cp*–Y bonding orbital. The second most intense transition at 309 nm is composed of 39% of HOMO and 32% HOMO−1 excitations into the LUMO, representing π → (Y d)* transitions. Similarly, the weaker 321 nm band constitutes a combination of 33% bonding (Cp*–Y) → π* and 37% π → (Y d)* contributions. Direct HOMO → LUMO transitions also substantially contribute to the bands at 325 nm and 309 nm (35% and 39%, respectively), inducing ligand to metal charge transfer (LMCT) processes. Multiple weak excitations are predicted from the bim-centered HOMO and HOMO−1 into the HOMO+2 between 329 and 321 nm, corroborating with π → π* transitions.
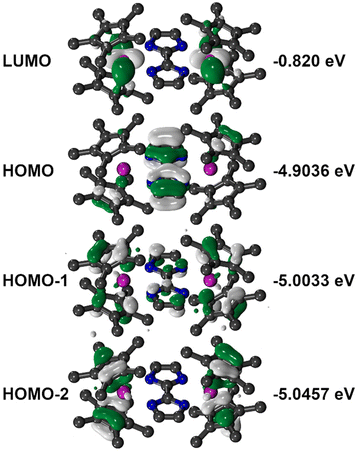 | ||
| Fig. 3 Graphical representation of calculated frontier orbitals partaking in the highlighted transitions. Orbital energies are given on the right in eV. | ||
For the sake of comparison, we also performed TDDFT calculations on [(Cp*2Y)2(μ-Bbim)] complex and contrasted the reported UV/Vis spectrum with the results in hand (Fig. S10†).40 Similarly to 1, the most intense transitions between 416 and 288 nm are the excitations into the LUMO, which is majorly a Bbim-based π*-orbital. At 416 nm, a transition from the Cp*-based HOMO into the LUMO is anticipated. A transition involving mostly Bbim-based orbitals is expected at 252 nm from HOMO−4 → HOMO+5, in accordance with a π → π* transition. This shows that the annulation of the bim moiety with two benzene rings in fact induces a significant blue shift of the π → π* transition by ∼22%. By contrast, the HOMO → LUMO transition of 1 is blue-shifted by ∼31% relative to the Bbim-bridged complex, which corresponds to an inhibited electron uptake in the smaller arene system.
The potential for ligand-based redox activity of 1 can be theoretically assessed by calculating the approximate adiabatic electron affinity EEA and the ionization potential EIvia EEA = E(1) − E(1−) and EI = E(1+) − E(1), Table 2 and Table S7.† The energies E(1−) and E(1+) represent the final energies of the hypothetical one-electron reduced and oxidized form of 1, respectively.49 These energies were obtained by performing unrestricted DFT geometry optimizations of the crystal coordinates of 1 with the respective charges and spin states set to +1/2 for 1+, 0/1 for 1 and −1/2 for 1−. The calculations provided values of EEA = −0.2042 eV and EI = 6.0225 eV, which imply that the chemical oxidation of 1 to a monoanionic bim−˙ radical is exceedingly harder than the chemical reduction to a trianionic bim3−˙ radical. Intriguingly, 1 was robust to all conducted chemical reduction experiments, even with the extremely strong reductant KC8. Applying the same methodology, EEA and EI were also calculated for [(Cp*2Y)2(μ-Bbim)] innate to a diamagnetic Bbim2− unit which, however, can be transformed to the trianionic bim3−˙ radical when exposed to strong chemical reductants. This gives the unique opportunity to evaluate the effect of the additional fused phenyl rings on the oxidizability/reducibility of the central bisimidazole moiety. The shift of the energy level following the annulation of bisimidazole may be the key factor to enable chemical reduction of Bbim, namely through the narrower HOMO–LUMO gap and a positive EEA value.
| E I (eV) | E EA (eV) | |
|---|---|---|
| E EA: electron affinity; EI: ionization potential. | ||
| [(Cp*2Y)2(μ-bim)] (1) | 6.0225 | −0.2042 |
| [(Cp*2Y)2(μ-Bbim)] | 5.9663 | 0.6372 |
The calculations on [(Cp*2Y)2(μ-Bbim)] yielded EEA of 0.6372 eV and EI of 5.9663 eV and point to a similar trend to 1, which is that the chemical oxidation of Bbim2− to a monoanionic Bbim−˙ radical is much harder than the chemical reduction to a trianionic bim3−˙ radical. This interpretation is in good agreement with our successful chemical reduction experiment to a bim3−˙ radical-bridged compound, while chemical oxidation attempts precluded access to a monoanionic radical-bridged complex. Notably, the calculated values obtained for 1 and [(Cp*2Y)2(μ-Bbim)] are especially different with respect to the sign of EEA: 1 is negative EEA and (Cp*2Y)2Bbim is positive. A positive EEA value signifies an electronically stable anionic state since energy is required to remove an electron. Whereas a negative EEA value indicates an electronically metastable anion that can spontaneously eject an electron to regenerate the neutral species.50,51 Therefore, 1 is anticipated to be unstable upon chemical reduction, whereas the singly reduced [(Cp*2Y)2(μ-Bbim˙)]− complex anion is expected to be stable. In fact, this computational finding is consistent with the isolation of the Bbim radical-bridged complex [K(crypt-222)][(Cp*2Y)2(μ-Bbim˙)] containing a reduced bim3−˙ anion.40
Lastly, the electrochemical properties of 1 and 3 were investigated via cyclic voltammetry to elucidate the redox activity of the bim2− ligand captured between two metal ions (Fig. S11†). No redox activity was monitored through scanning towards negative potential, whereas scanning towards positive potentials revealed broad, irreversible oxidation events at applied potentials >0.47 V (vs. Fc+/Fc0). These features substantially decreased in intensity upon repeated scanning cycles which may originate from irreversible chemical reactions of the analytes. The lack of any reversible features in all scans hints at the absence of an accessible, stable radical oxidation state for the bim ligand. Thus, the electrochemical results are in accord with the foregoing theoretical analysis and confirm the redox inactivity of the coordinated bim2− ligand since the reduction is projected to be easier than the oxidation.
Static magnetic susceptibility behaviour
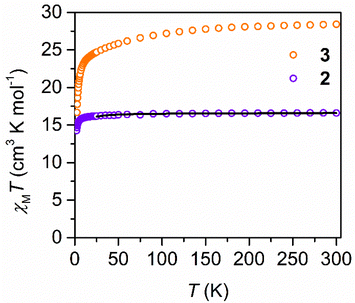
Direct current (dc) magnetic susceptibility data were collected on polycrystalline samples of 2 and 3 in applied magnetic fields of 0.1 T, 0.5 T and 1.0 T from 2 to 300 K (Fig. 4 and S12, S13†). The room temperature χMT value of 16.60 cm3 K mol−1 for 2 at 0.1 T (15.81 cm3 K mol−1 at 1.0 T) is in accordance with the expected value of 15.75 cm3 K mol−1 for two uncoupled GdIII ions. Between 300 and 50 K, the χMT value remain almost unchanged and drop significantly at temperatures below 10 K, which is attributed to the thermal depopulation of low-lying excited states and/or antiferromagnetic coupling. GdIII ions offer the unique opportunity to quantify the strength of magnetic exchange coupling because the orbital singlet is free of complications arising from spin–orbit coupling which typically occurs for ions such as DyIII. Thus, the χMT vs. T data at 0.1 T for 2 was fit to a Heisenberg exchange Hamiltonian Ĥ = −2JGd–GdŜGd(1)ŜGd(2) where JGd–Gd accounts for the exchange constant ascribed to the intramolecular GdIII–GdIII coupling and ŜGd(n) is the spin operator for each GdIII ion using Phi.52 A small antiferromagnetic intramolecular exchange coupling constant of −0.074(2) cm−1 with a small intermolecular coupling (zJ′) of −0.0075 cm−1 was found for 2. Notably, the inclusion of a zJ′ contribution improved substantially the quality of the fit (see ESI† for details) alluding to the presence of a small antiferromagnetic intermolecular coupling with additional weak intramolecular ferromagnetic coupling. Such a small J value indicates weak coupling between the two metal ions which is typical for polynuclear LnIII where the metal ions are bridged by diamagnetic ligands,53,54 since the lanthanides’ valence 4f orbitals are extremely contracted preventing effective spin density transfer onto the bridging ligands. However, the exchange coupling can be greatly strengthened by implementing heavy p-block or radical ligands as bridges where their diffuse orbitals allow efficient suppression of undesirable quantum tunneling processes (QTM).55–59 An qualitative indication for strong magnetic coupling between the LnIII ions would be rise in χMT at lower temperatures which is lacking in 2 and 3 further corroborating the weak coupling.Unlike 2, the χMT vs. T plot for 3 showed first a steady decline with decreasing temperature from 300 to 12 K, followed by a steep drop culminating at a value of 18.09 cm3 K mol−1 at 2 K. The room temperature χMT value of 28.62 cm3 K mol−1 at 0.1 T is in excellent agreement with the expected value of 28.33 cm3 K mol−1 for two non-interacting DyIII ions. The overall similar trend of the shape of the χMT vs. T plot of 3 relative to 2 points at similar weak coupling between the DyIII ions.
Although the closest intermolecular Ln⋯Ln distances of 8.558(1) Å (2) and 8.538(1) Å (3) are rather long, close in-plane interactions are observed between the outer carbon atoms of the bim ligand and carbon atoms of the neighboring Cp* ring (3.478(5) Å (2)), hinting at a potential antiferromagnetic dipolar coupling pathway.
Dynamic magnetic susceptibility properties
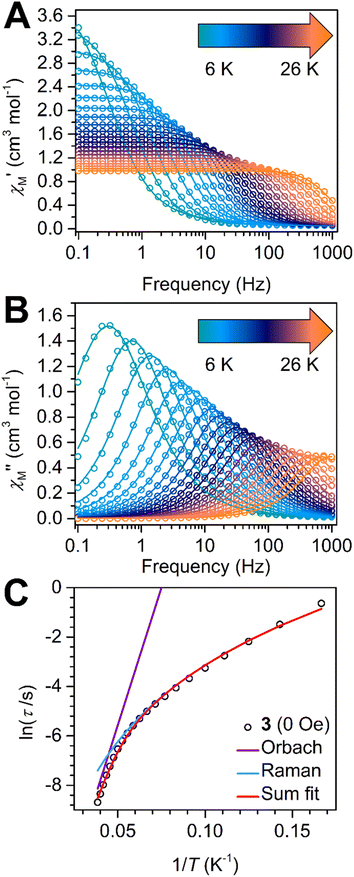
Bisimidazolate ligands have never been employed in Ln-based single-molecule magnets (SMMs). Therefore, we were interested in exploring the interplay of exchange coupling between the anisotropic LnIII ions and the local crystal field around each metal center. Although the intramolecular magnetic coupling is rather small, the magnetic moments of 3 may still relax slowly mostly owing to the local crystal field of the DyIII ions as the single-ion magnetic anisotropy of each oblate-shaped DyIII ion is generally exemplified when sandwiched in an axial coordination environment as in this case imposed by the two Cp* ligands. If additionally equatorial ligands around the lanthanide ion are avoided, then the anisotropy can be maximized as impressively demonstrated in mononuclear complexes.7,60,61 The most recent such example of the impact of a strongly axial ligand sphere is the retention of magnetic memory for 100 s at 66 K in a bis(amino)borolide DyIII complex.62,63To investigate the relaxation dynamics, variable-frequency variable-temperature in-phase (χM′) and out-of-phase (χM′′) ac magnetic susceptibility data were collected for a polycrystalline sample of 3 under a 3 Oe ac field at zero applied dc field, Fig. 5. Here, the observation of χM′′ signals imply the presence of an energy barrier to spin-reversal. Indeed, the Dy complex 3 exhibits temperature-dependent χM′′ peak maxima between 2 and 26 K in the 0.1 and 1000 Hz frequency range which shift towards higher frequencies with rising temperatures. The collected ac magnetic susceptibility data were used to generate χM′ vs. χM′′ plots (Cole–Cole plots) at each temperature (Fig. S14†) and were then fitted by a generalized Debye model (program CCfit)64 to extract the relaxation times, τ. The ln(τ) vs. 1/T plots (Arrhenius plots) can be used to deconvolute the operating relaxation mechanisms by considering different temperature profiles (Fig. 5C and S15, S16†). The temperature dependence of relaxation times provides insight into the operative relaxation processes at the given temperatures. An energy exchange of the system with the lattice via phonons to ascend to the cusp of the barrier corresponds to an activation barrier of spin relaxation which is referred to as Orbach process and is reflected by the exponential function τ−1 = τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/kBT), where Ueff is the energy barrier to spin reversal, τ0 is the preexponential factor (or attempt time) and kB is the Boltzmann constant. Temperature-independent relaxation times are commonly associated with quantum tunnelling of the magnetization and typically expressed as τQTM−1. In addition, a relaxation process obeying a power law (τ−1 = CTn) is associated with the simultaneous absorption and emission of lattice phonons.
exp(−Ueff/kBT), where Ueff is the energy barrier to spin reversal, τ0 is the preexponential factor (or attempt time) and kB is the Boltzmann constant. Temperature-independent relaxation times are commonly associated with quantum tunnelling of the magnetization and typically expressed as τQTM−1. In addition, a relaxation process obeying a power law (τ−1 = CTn) is associated with the simultaneous absorption and emission of lattice phonons.
The best fit to the extracted τ values for 3 considered an Orbach and a Raman process giving rise to Ueff = 154(2) cm−1 and τ0 = 5(1) × 10−8 s, C = 7.9(2) × 10−4 s−1 K−n, n = 4.47(2). The inclusion of a QTM contribution yielding τQTM = 103.00(1) s, had no impact on the Orbach and Raman parameters, hinting at a negligible through-barrier tunnelling process within the investigated temperature regime (Fig. S16†). By comparison, a linear fit to the relaxation times observed at the highest temperatures between 24 and 26 K gave an effective barrier to spin relaxation of Ueff = 153.9(5) cm−1 and a pre-exponential factor of τ0 = 3.31(8) × 10−8 s (Fig. S15†). This attained barrier height from considering a pure Orbach process at high temperatures matches perfectly with the Ueff value obtained for the full temperature range. Since the extraction of the τ data is limited by the frequency range measurable through a conventional ac magnetometer, the relaxation times below 6 K were not accessible through this method.
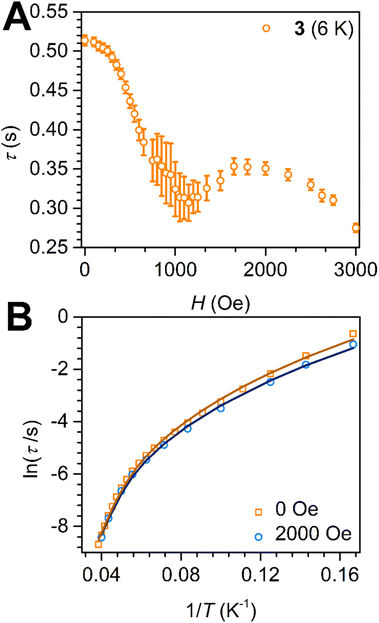
The application of a dc field during ac magnetic measurements can attenuate QTM by moving the corresponding ±MJ states out of resonance. The effect of dc fields of up to 3000 Oe on the relaxation behaviour of 3 was probed at 6 K, Fig. 6 and S17.† If the application of dc fields engenders a suppression of QTM, oftentimes a shift of the χM′′ maxima towards lower frequencies occurs giving rise to longer τ times.65 By contrast, subjecting 3 to increasing magnetic fields amplified the magnetic relaxation as indicated by the high-frequency shifts of the χM′′ maxima, Fig. S17.† The plot of the extracted relaxation times versus dc fields exhibits first a gradual decline of τ with stronger dc fields passing through a minimum at 1150 Oe, followed by an ascent of τ to a local maximum at ∼2000 Oe, and finally transitioning into a second descend, Fig. 6A. At 3000 Oe, the value for τ was diminished by 47% which suggests the onset of additional relaxation processes at higher fields, which is described as τ−1 = AH4T. A direct process only occurs when the Zeeman splitting of a given state matches exactly a lattice phonon's energy and is therefore only observed under applied magnetic fields.66 Variable-frequency, variable-temperature in-phase (χM′) and out-of-phase (χM′′) ac magnetic susceptibility data were collected for 3 at the optimum dc field of 2000 Oe, Fig. 6B and S18.† However, the obtained τ values were again best fit to a sum of Orbach and Raman processes, giving rise to Ueff = 156(1) cm−1 and τ0 = 4.8(1) × 10−8 s, C = 1.5(2) × 10−3 s−1 K−n, n = 4.30(1), where Ueff is only marginally affected by the applied magnetic field, Fig. S19.† These findings emphasize the absence of a direct relaxation process within the field-range investigated here and rather a magnitude amplification of the Raman relaxation process through the dc field.
To explore the relaxation times below 6 K, we conducted dc magnetic relaxation experiments between 1.8 K and 4.0 K. In this technique a high magnetic field is applied to the sample to saturate the magnetization. The field is then quickly removed, which will cause the onset of magnetic relaxation that will follow an exponential decay. Hence, fitting these curves to an exponential function allows the extraction of τ at these low temperatures unattainable through ac techniques, Fig. S20–S26.† Fitting the combined Arrhenius plot of τ derived from ac and dc measurements versus inverse temperature was possible considering QTM, Raman and Orbach processes, Table S9 and Fig. S27.† The inclusion of all three relaxation processes allows for the phenomenological deconvolution of the relaxation processes as a function of temperature. At the lowest temperatures the relaxation times are temperature-dependent which is associated with quantum tunnelling of the magnetization. This is commonly observed for polynuclear Dy complexes featuring diamagnetic bridging ligands. A change between the temperature-independent QTM and power-law dependent Raman regime occurs around 3.2 K, where the relaxation times begin to be temperature-dependent. A second transition appears at around 16 K, where the Orbach process becomes dominant corresponding to a linear temperature dependence of the relaxation times.
Magnetization under variable fields
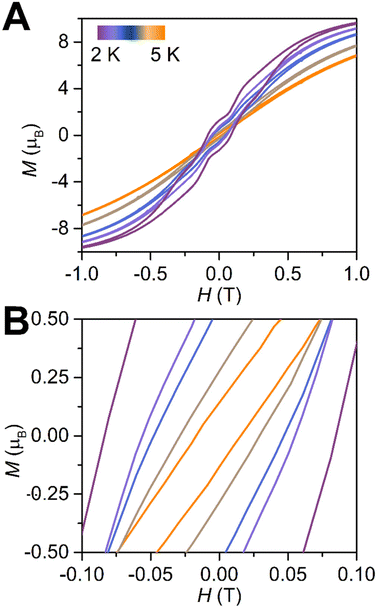
The final inspection of the efficacy of a single-molecule magnet behaviour represents the evaluation of the magnetic hysteresis behaviour, which is a key feature for information storage materials. This property is probed via field-dependent magnetization (M vs. H) experiments, which most prominently show either open hysteresis loops corresponding to a lack of QTM or waist-restricted curves, possibly with step-like features, hinting at the presence of QTM (Fig. 7). Noteworthy, this magnetic blocking which is essentially the retention of a magnetic moment after removal of the external field, is unlikely to occur for a dinuclear DyIII complex containing a diamagnetic bridging ligand with nitrogen donor atoms.To this end, a M vs. H curve was collected for 3 at 2 K between −7 and +7 T at a 100 Oe s−1 sweep rate. Excitingly, this measurement revealed the retention of magnetization upon removal of the external magnetic field giving rise to a coercive field HC of 825 Oe at 2 K. With rising temperatures, the coercive fields decrease in value. The hysteresis loops remain open until 5 K, where HC is smaller than the sweep rate. Noteworthy, the observation that hysteresis loops are open at such high temperatures is extremely rare for polymetallic lanthanide compounds where the metal ions are bridged by closed-shell ligands of the first row p-block elements.67–69 For example, comparable dinuclear benzotriazole/aminobipyrimidine or indigo (ind) bridged dysprosocenium complexes exhibit waist-constricted hysteresis loops at 1.8 K with no significant coercivity.67,68 Oftentimes, this is attributed to the presence of strong equatorial interaction of these ligands with the DyIII f-electron density, which instils mixing of the MJ states and gives rise to effective QTM. Furthermore, the hysteresis curves for 3 exhibit steps, indicative of the presence of QTM at these temperatures. To probe whether the steps in the hysteresis loops and the relaxation observed in the ac magnetic susceptibility data collected at 6 K under varying field can be potentially correlated, the first derivative of the magnetization (dM/dH vs. H) was investigated, where accelerations of the magnetic relaxation should become visible as signals (Fig. S31†). At 2 K, three asymmetric, yet clearly discernible peaks at 1168, −1082 and −3513 Oe (scanning from +7 T towards −7 T) became apparent, alongside of two minima at 0 and −2407 Oe. The positions of the maxima and minima remain invariant traversing from negative to positive fields indicating that the first derivative is symmetric. As the temperatures were increased, the peaks gradually vanished, where the signal at ∼−3000 Oe decreases faster than the ones at ∼±1000 Oe, until at 6 K these features are superimposed by noise.
Interestingly, although the variable-field magnetization data were collected at a different timescale relative to the ac data, the first signals coincide very well with the drop around 1000 Oe in the field-dependent AC measurements at 6 K (Fig. 6A). These peaks coincide with turning points of the hysteresis loops displayed in Fig. 7 and could be an indication for potential level crossings due to intramolecular coupling or hyperfine coupling. Given a small intramolecular zJ′ was taken into account to fit the χMT vs. T data for the Gd complex 2, it can be hypothesised that these intermolecular effects are equally operative in the DyIII congener and contribute to an increase of relaxation times. The effects of hyperfine coupling in various Dy isotopolgues can be detrimental for the relaxation behaviour,70 although the fields at which the steps in the hysteresis loops are largely invariant with different DyIII isotopes. However, diamagnetic dilution of 162Dy versus163Dy samples result in open magnetic hysteresis loops which without dilution show a butterfly-shaped hysteresis loop. Future studies will focus on deconvolution of these type of mechanisms.
In addition, isothermal magnetization curves were collected for 2 and 3 between 0 and 7 T at temperatures between 2 and 10 K (Fig. S28†). The curves for both complexes show gradual growth without inflection points signifying the lack of strong magnetic coupling and/or magnetic blocking. The saturation magnetization is reached for both samples at 2 K, plateauing at moments of 14.90 μB (2) and 11.11 μB (3), respectively. These moments are substantially lower than the expected values of 2 × 7.94 μB and 2 × 10.65μB for two noninteracting GdIII and DyIII ions, respectively. However, the experimental values are close to the saturation magnetization values observed for other bridged didysprosocenium complexes44,71e.g. 14.03 μB (Gd) and 10.39 μB (Dy) in [(Cp*2Ln)2(μ-ind)].71 In fact, crystal field effects may engender a reduced magnetization since they remove the degeneracy of the spin ground states and induce splitting of the MJ states. Notably, these crystal field effects are reflected in the reduced magnetization plots (M vs. H/T), Fig. S28.† For 2, these curves are essentially superimposed in accordance with weak crystal field effects on the MJ manifold, and the curves for 3 deviate significantly from ideal superposition, reflecting a considerable splitting among the MJ states. This also highlights the presence of substantial magnetic anisotropy in 3. Similar to the studies of the magnetic hysteresis loops, the first derivative of the magnetization data was scrutinized. At 2 K, the derivative exhibits a feature with two maxima at 676 and 2624 Oe, which disappears at 6 K (Fig. S29 and S30†). This is in accord with the peaks observed in the derivative obtained from the hysteresis data (starting at 0 Oe applied field), but occur at much lower fields, possibly owing to differing measurement modes employed for both data sets and not of molecular origin. Such features in the derivative magnetization of other didysprosocene complexes containing diamagnetic bridges have been ascribed to metamagnetization-like behavior.72
The strong magnetic anisotropy in 3 is also reflected in the orientation of the MJ = ± 15/2 state of DyIII as calculated by Magellan,23 where the anisotropy is determined through the Cp* framework despite the presence of the equatorial dianionic bim ligand (Fig. 1, assuming −0.5 charges residing on each bridging N atom and −0.2 on each central Cp* C atom).
Conclusions
The foregoing findings demonstrate that 2,2′-bisimidazole could be successfully employed in rare earth metal chemistry to generate a series of new organometallic compounds in good yields. In fact, the results on hand constitute the first utilization of the bisimidazole bridging ligand in the realm of lanthanide-containing single-molecule magnets. The Dy congener demonstrates zero-field dynamic magnetic properties with an effective energy barrier to spin reversal of Ueff = 154(2) cm−1 and remarkably, and open magnetic hysteresis loops below 5 K. Noteworthy, such magnetic memory is unusual for polynuclear dysprosium complexes with bridging, diamagnetic organic ligands. The strength of magnetic exchange coupling was quantified for the Gd congener, revealing weak antiferromagnetic coupling with J = −0.074(2) cm−1 (at 0.1 T). The similar trend for the χMT vs. T plot of 3 alludes to weak DyIII–DyIII coupling which is indicative of the SMM properties to be likely originating from single-ion anisotropy, rather than from a coupled state. The electronic structure of 1 was studied through DFT calculations and an in-depth comparison of the dianions bim2− and the larger analogue Bbim2− were carried out. This provided a profound insight into the redox inactivity of bim2− relative to the redox activity of Bbim2−. Importantly, the calculated electron affinities confirm the experimentally observed redox inactivity of bim and the redox activity of Bbim. Hence, the annellation of the central bim moiety in 1–3 tunes the bridging ligand's electronic structure such that the population of a low-lying π* becomes possible and therefore enables the isolation of the stable trianionic Bbim3−˙ radical. Thus, the electron affinity could serve as a theoretical tool to predict the redox activity of bridging entities. This discovery has huge ramifications moving forward in exploring new bridging ligands and assessing their reducibility. The foregoing results also pave the way for the construction of both higher nuclearity arrays innate to more than two paramagnetic lanthanide ions by utilizing the bim2− ligand, and molecular qubits.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. D. is grateful to the Department of Chemistry at Michigan State University (MSU) for generous start-up funds. We are grateful to Dr. Rui Huang (MSU) for assistance with CHN analyses. This work was supported in part through computational resources and services provided by the Institute for Cyber-Enabled Research, as well as the Max T. Rodgers NMR facility at MSU. Funding for the single-crystal X-ray diffractometer was provided through the MRI program of the National Science Foundation under Grant No. CHE-1919565.Notes and references
- P. L. Arnold, M. W. McMullon, J. Rieb and F. E. Kühn, C-H bond Activation by f-block Complexes, Angew. Chem., Int. Ed., 2015, 54, 82–100 CrossRef CAS PubMed.
- J. C. G. Bünzli and C. Piguet, Taking Advantage of Luminescent Lanthanide Ions, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC.
- H. F. Li, P. F. Yan, P. Chen, Y. Wang, H. Xu and G. M. Li, Highly Luminescent Bis-Diketone Lanthanide Complexes with Triple-Stranded Dinuclear Structure, Dalton Trans., 2012, 41, 900–907 RSC.
- A. P. Bassett, S. W. Magennis, P. B. Glover, D. J. Lewis, N. Spencer, S. Parsons, R. M. Williams, L. De Cola and Z. Pikramenou, Highly Luminescent, Triple- and Quadruple-Stranded, Dinuclear Eu, Nd, and Sm(III) Lanthanide Complexes Based on Bis-Diketonate Ligands, J. Am. Chem. Soc., 2004, 126, 9413–9424 CrossRef CAS PubMed.
- R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Magnetic Bistability in a Metal-Ion Cluster, Nature, 1993, 365, 141–143 CrossRef CAS.
- A. Caneschi, D. Gatteschi, R. Sessoli, A. L. Barra, L. C. Brunei and M. Guillot, Alternating Current Susceptibility, High Field Magnetization, and Millimeter Band EPR evidence for a ground S = 10 state in [Mn12O12(CH3COO)16(H2O)4]·2CH3COOH·4H2O, J. Am. Chem. Soc., 1991, 113, 5873–5874 CrossRef CAS.
- F. Guo, B. M. Day, Y. Chen, M. Tong, A. Mansikkamäki and R. A. Layfield, Magnetic Hysteresis up to 80 Kelvin in a Dysprosium Metallocene Single-Molecule Magnet, Science, 2018, 362, 1400–1403 CrossRef CAS PubMed.
- S. Demir, M. I. Gonzalez, L. E. Darago, W. J. Evans and J. R. Long, Giant Coercivity and High Magnetic Blocking Temperatures for N23− Radical-Bridged Dilanthanide Complexes upon Ligand dissociation, Nat. Commun., 2017, 8, 2144 CrossRef PubMed.
- S. Demir, I. R. Jeon, J. R. Long and T. D. Harris, Radical ligand-containing single-molecule magnets, Coord. Chem. Rev., 2015, 289–290, 149–176 CrossRef CAS.
- C. A. Gould, K. R. Mcclain, D. Reta, J. G. C. Kragskow, D. A. Marchiori, E. Lachman, E. Choi, J. G. Analytis, R. D. Britt, N. F. Chilton, B. G. Harvey and J. R. Long, Ultrahard Magnetism from Mixed-Valence Dilanthanide Complexes with Metal–Metal Bonding, Science, 2021, 202, 198–202 Search PubMed.
- Z. Zhu and J. Tang, Metal-Metal Bond in Lanthanide Single-Molecule Magnets, Chem. Soc. Rev., 2022, 51, 9469–9481 RSC.
- Z. Zhu and J. Tang, Lanthanide Single-Molecule Magnets with High Anisotropy Barrier: Where to From Here?, Natl. Sci. Rev., 2022, 9, 5–7 Search PubMed.
- H. Li, S. Wu and M. Tong, Lanthanide–Radical Single-Molecule Magnets: Current Status and Future Challenges, Chem. Commun., 2023, 59, 6159–6170 RSC.
- A. Das, S. M. Mobin and G. K. Lahiri, Recognition of Fractional Non-Innocent Feature of Osmium Coordinated 2,2′-Biimidazole or 2,2′-Bis(4,5-dimethylimidazole) and Their Interactions with Anions., Dalton Trans., 2015, 44, 13204–13219 RSC.
- Y. Li, X. R. Chang, X. J. Sang, J. S. Li, Y. H. Luo, Z. M. Zhu and W. S. You, Keggin-Type Polyoxometalate Modified Ag/Graphene Composite Materials for Electrocatalytic Water Oxidation, Eur. J. Inorg. Chem., 2019, 3597–3604 CrossRef CAS.
- S. Fortin and A. L. Beauchamp, Preparations, Characterizations, and Structures of (Biimidazole)dihalobis(triphenylphosphine)Rhenium(III) Salts: Strong Ion-Pairing and Acid–Base Properties, Inorg. Chem., 2001, 40, 105–112 CrossRef CAS PubMed.
- M. J. Calhorda and A. R. Dias, Di-η5-Cyclopentadienylmetal Complexes with Nitrogen Donor Atom Ligands: New Imidazole, N-Methylimidazole, Pyrazole and 2,2′-Bisimidazole Complexes of Molybdenum and Tungsten, J. Organomet. Chem., 1980, 197, 291–302 CrossRef CAS.
- G. S. Matouzenko, J. F. Létard, S. Lecocq, A. Bousseksou, L. Capes, L. Salmon, M. Perrin, O. Kahn and A. Collet, Two-step spin crossover in a mononuclear compound [Fe(DPEA)(bim)](ClO4)2·0.5H2O [DPEA = (2-aminoethyl)-bis(2-pyridylmethyl)amine, bim = 2,2-bisimidazole] – Crystal Structure, Magnetic Properties, Mössbauer Spectroscopy, and Photomagnetic Effects, Eur. J. Inorg. Chem., 2001, 2935–2945 CrossRef CAS.
- M. Tadokoro, K. Isogai, S. Harada, T. Kouchi, T. Yamane, T. Sugaya and H. Kamebuchi, Evidence of Poton-Coupled Mixed-Valency by Electrochemical Behavior on Transition Metal Complex Dimers Bridged by two Ag+ Ions, Dalton Trans., 2019, 48, 535–546 RSC.
- Y. R. Zhong, M. L. Cao, H. J. Mo and B. H. Ye, Syntheses and Crystal Structures of Metal Complexes with 2,2′-biimidazole-like Ligand and Chloride: Investigation of X-H⋯Cl (X = N, O, and C) Hydrogen Bonding and Cl-π (imidazolyl) interactions, Cryst. Growth Des., 2008, 8, 2282–2290 CrossRef CAS.
- D. P. Rillema, R. Sahai, P. Matthews, A. K. Edwards, R. J. Shaver and L. Morgan, Multimetallic Ruthenium(II) Complexes Based on Biimidazole and Bibenzimidazole: Effect of Dianionic Bridging Ligands on Redox and Spectral Properties, Inorg. Chem., 1990, 29, 167–175 CrossRef CAS.
- P. Maślewski, K. Kazimierczuk, Z. Hnatejko and A. Dołęga, Isostructural Zinc and Cadmium Silanethiolates with Bridging Biimidazole Co-Ligands – Enhanced Luminescence of Zinc Complex, Inorg. Chim. Acta, 2017, 459, 22–28 CrossRef.
- N. F. Chilton, D. Collison, E. J. L. McInnes, R. E. P. Winpenny and A. Soncini, An Electrostatic Model for the Determination of Magnetic Anisotropy in Dysprosium Complexes, Nat. Commun., 2013, 4, 2551 CrossRef PubMed.
- R. P. Sharma, A. Singh, P. Brandão, V. Félix and P. Venugopalan, Syntheses, Characterization, Thermal Properties and Single Crystal Structure Determination of Cobalt(III) Complexes with 2,2′-Biimidazole and 1,10-Phenanthroline Ligands, Polyhedron, 2011, 30, 2759–2767 CrossRef CAS.
- S. W. Kaiser, R. B. Saillant, W. M. Butler and P. G. Rasmussen, Rhodium and Iridium Complexes of Biimidazole. 1. Mononuclear and Dinuclear Species, Inorg. Chem., 1976, 15, 2681–2688 CrossRef CAS.
- B. F. Fieselmann, D. N. Hendrickson and G. D. Stucky, Synthesis, Electron Paramagnetic Resonance, and Magnetic Studies of Binuclear Bis(η5-Cyclopentadienyl)Titanium(III) Compounds with Bridging Pyrazolate, Biimidazolate, and Bibenzimidazolate Anions, Inorg. Chem., 1978, 17, 2078–2084 CrossRef.
- K. K. Kamar, L. R. Falvello, P. E. Fanwick, J. Kim and S. Goswami, Designed Synthesis of a Multimetallic System Having Ru4Cu2 Core using Trimetallic Coordination of 2,2′-Biimidazolate Ion, Dalton Trans., 2004, 1827–1831 RSC.
- Y. H. Tan, J. S. Wu, C. S. Yang, Q. R. Liu, Y. Z. Tang, Y. R. Zhong and B. H. Ye, Spontaneous Resolution of Novel Zn Complexes in the Formation of 3d Metal-Organic Frameworks based on 2,2′-Biimidazole Ligand, Inorg. Chim. Acta, 2013, 399, 45–49 CrossRef CAS.
- S. Y. Shi, L. Y. Chen, T. H. Zhu, J. Zhang and X. B. Cui, Two new Compounds of Polyoxoanions, Transition Metal Complexes and Organic Amines, Inorg. Chim. Acta, 2018, 477, 292–299 CrossRef CAS.
- Z. Shi, J. Peng, X. Yu, Y. Shen, Z. Zhang, K. Alimaje and X. Wang, A new 3d Keggin-POM based Metal-Organic Framework of Cu and H2Biim: Assembly and Properties of Fluorescence and Electrochemistry, Inorg. Chem. Commun., 2013, 28, 85–89 CrossRef CAS.
- J. C. Zhong, S. Z. Ge, F. Wan, Y. Q. Sun and Y. P. Chen, A Series of Luminescent Lanthanide-Organic Complexes Constructed from in situ generated Dithiobenzoicacid, J. Inorg. Organomet. Polym. Mater., 2014, 24, 633–643 CrossRef CAS.
- S. S. Pedro, P. Brandão, F. N. Shi, J. C. G. Tedesco and M. S. Reis, A new Metal Organic Framework constructed of Co(II) Ions six and seven-coordinated: Synthesis, Structure and Magnetism, Polyhedron, 2014, 81, 210–215 CrossRef CAS.
- R. L. Sang and L. Xu, Unprecedented Infinite Lanthanide Hydroxide Ribbons [Ln3(μ3-OH)3]n6n+ in a 3-D Metal-Organic Framework, Chem. Commun., 2013, 49, 8344–8346 RSC.
- H. J. Zhang, R. Q. Fan, X. M. Wang, P. Wang, Y. L. Wang and Y. L. Yang, Preparation, Characterization, and Properties of PMMA-doped Polymer Film Materials: A Study on the Effect of Terbium Ions on Luminescence and Lifetime Enhancement, Dalton Trans., 2015, 44, 2871–2879 RSC.
- L. X. You, L. X. Cui, B. B. Zhao, G. Xiong, F. Ding, B. Y. Ren, Z. L. Shi, I. Dragutan, V. Dragutan and Y. G. Sun, Tailoring the Structure, Ph Sensitivity and Catalytic Performance in Suzuki-Miyaura Cross-Couplings of Ln/Pd MOFs based on the 1,1′-di(p-Carboxybenzyl)-2,2′-diimidazole Linker, Dalton Trans., 2018, 47, 8755–8763 RSC.
- V. Goudy, A. Jaoul, M. Cordier, C. Clavaguéra and G. Nocton, Tuning the Stability of Pd(IV) Intermediates using a Redox Non-Innocent Ligand Combined with an Organolanthanide Fragment, J. Am. Chem. Soc., 2017, 139, 10633–10636 CrossRef CAS PubMed.
- D. Wang, J. Moutet, M. Tricoire, M. Cordier and G. Nocton, Reactive Heterobimetallic Complex Combining Divalent Ytterbium and Dimethyl Nickel Fragments, Inorganics, 2019, 7, 58 CrossRef CAS PubMed.
- S. Demir, J. M. Zadrozny, M. Nippe and J. R. Long, Exchange Coupling and Magnetic Blocking in Bipyrimidyl Radical-Bridged Dilanthanide Complexes, J. Am. Chem. Soc., 2012, 134, 18546–18549 CrossRef CAS PubMed.
- C. Chen, Z. B. Hu, H. Ruan, Y. Zhao, Y. Q. Zhang, G. Tan, Y. Song and X. Wang, Tuning the Single-Molecule Magnetism of Dysprosium Complexes by a Redox-Noninnocent Diborane Ligand, Organometallics, 2020, 39, 4143–4148 CrossRef CAS.
- F. Benner and S. Demir, Isolation of the Elusive Bisbenzimidazole Bbim3−˙ Radical Anion and its Employment in a Metal Complex, Chem. Sci., 2022, 13, 5818–5829 RSC.
- K. Kotwica, I. Wielgus and A. Proń, Azaacenes Based Electroactive Materials: Preparation, Structure, Electrochemistry, Spectroscopy and Applications – A Critical Review, Materials, 2021, 14, 5155 CrossRef CAS PubMed.
- M. R. MacDonald, J. W. Ziller and W. J. Evans, Coordination and Reductive Chemistry of Tetraphenylborate Complexes of Trivalent Rare-Earth Metallocene Cations, [(C5Me5)2Ln][(μ-Ph)2BPh2], Inorg. Chem., 2011, 50, 4092–4106 CrossRef CAS PubMed.
- P. artman and H. K. Chan, Application of the Periodic Bond Chain (PBC) Theory and Attachment Energy Consideration to derive the Crystal Morphology of Hexamethylmelamine, Pharm. Res., 1993, 10, 1052–1058 CrossRef PubMed.
- E. Castellanos, F. Benner and S. Demir, Taming salophen in Rare Earth Metallocene Chemistry, Inorg. Chem. Front., 2022, 9, 1325–1336 RSC.
- W. J. Evans, J. R. Walensky, F. Furche, A. G. Dipasquale and A. L. Rheingold, Trigonal-Planar versus Pyramidal Geometries in the Tris(Ring) Heteroleptic Divalent Lanthanide Complexes (C5Me5)Ln(μ-η6:η1-Ph)2BPh2: Crystallographic and Density Functional Theory Analysis, Organometallics, 2009, 28, 6073–6078 CrossRef CAS.
- W. J. Evans, The Importance of Questioning Scientific Assumptions: Some Lessons from f-Element Chemistry, Inorg. Chem., 2007, 46, 3435–3449 CrossRef CAS PubMed.
- S. Demir, J. M. Zadrozny and J. R. Long, Large Spin-Relaxation Barriers for the Low-Symmetry Organolanthanide Complexes [Cp*2Ln(BPh4)] (Cp* = Pentamethylcyclopentadienyl; Ln = Tb, Dy), Chem. – Eur. J., 2014, 20, 9524–9529 CrossRef CAS PubMed.
- F. Neese, Software Update: The Orca Program System—Version 5.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, 1–15 Search PubMed.
- C. G. Zhan, J. A. Nichols and D. A. Dixon, Ionization Potential, Electron Affinity, Electronegativity, Hardness, and Electron Excitation Energy: Molecular Properties from Density Functional Theory Orbital Energies, J. Phys. Chem. A, 2003, 107, 4184–4195 CrossRef CAS.
- J. Simons, Theoretical Study of Negative Molecular Ions, Annu. Rev. Phys. Chem., 2011, 62, 107–128 CrossRef CAS PubMed.
- C. P. Vibert and D. J. Tozer, Simple DFT Scheme for Estimating Negative Electron Affinities, J. Chem. Theory Comput., 2019, 15, 241–248 CrossRef CAS PubMed.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynuclear d- and f-Block Complexes, J. Comput. Chem., 2013, 34, 1164–1175 CrossRef CAS PubMed.
- P. Evans, D. Reta, C. A. P. Goodwin, F. Ortu, N. F. Chilton and D. P. Mills, A Double-Dysprosocenium Single-Molecule Magnet Bound together with Neutral Ligands, Chem. Commun., 2020, 56, 5677–5680 RSC.
- D. Errulat, B. Gabidullin, A. Mansikkamäki and M. Murugesu, Two Heads are better than one: Improving Magnetic Relaxation in the Dysprosium Metallocene DyCp*2BPh4 upon Dimerization by Use of an Exceptionally Weakly-Coordinating Anion, Chem. Commun., 2020, 56, 5937–5940 RSC.
- P. Zhang, F. Benner, N. F. Chilton and S. Demir, Organometallic Lanthanide Bismuth Cluster Single-Molecule Magnets, Chem, 2022, 8, 717–730 CAS.
- F. Tuna, C. A. Smith, M. Bodensteiner, L. Ungur, L. F. Chibotaru, E. J. L. McInnes, R. E. P. Winpenny, D. Collison and R. A. Layfield, A High Anisotropy Barrier in a Sulfur-Bridged Organodysprosium Single-Molecule Magnet, Angew. Chem., Int. Ed., 2012, 51, 6976–6980 CrossRef CAS PubMed.
- T. Pugh, A. Kerridge and R. A. Layfield, Yttrium Complexes of Arsine, Arsenide, and Arsinidene Ligands, Angew. Chem., Int. Ed., 2015, 54, 4255–4258 CrossRef CAS PubMed.
- T. Pugh, V. Vieru, L. F. Chibotaru and R. A. Layfield, Magneto-Structural Correlations in Arsenic- and Selenium-Ligated Dysprosium Single-Molecule Magnets, Chem. Sci., 2016, 7, 2128–2137 RSC.
- T. Pugh, F. Tuna, L. Ungur, D. Collison, E. J. L. McInnes, L. F. Chibotaru and R. A. Layfield, Influencing the Properties of Dysprosium Single-Molecule Magnets with Phosphorus Donor Ligands, Nat. Commun., 2015, 6, 7492 CrossRef PubMed.
- F. S. Guo, B. M. Day, Y. C. Chen, M. L. Tong, A. Mansikkamäki and R. A. Layfield, A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit, Angew. Chem., Int. Ed., 2017, 56, 11445–11449 CrossRef CAS PubMed.
- C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Molecular Magnetic Hysteresis at 60 Kelvin in Dysprosocenium, Nature, 2017, 548, 439–442 CrossRef CAS PubMed.
- J. C. Vanjak, B. O. Wilkins, V. Vieru, N. S. Bhuvanesh, J. H. Reibenspies, C. D. Martin, L. F. Chibotaru and M. Nippe, A High-Performance Single-Molecule Magnet utilizing Dianionic Aminoborolide Ligands, J. Am. Chem. Soc., 2022, 144, 17743–17747 CrossRef CAS PubMed.
- A. H. Vincent, Y. L. Whyatt, N. F. Chilton and J. R. Long, Strong Axiality in a Dysprosium(III) Bis(borolide) Complex leads to Magnetic Blocking at 65 K, J. Am. Chem. Soc., 2023, 145, 1572–1579 CrossRef CAS PubMed.
- D. Reta and N. F. Chilton, Uncertainty Estimates for Magnetic Relaxation Times and Magnetic Relaxation Parameters, Phys. Chem. Chem. Phys., 2019, 21, 23567–23575 RSC.
- H. L. C. Feltham and S. Brooker, Review of Purely 4f and Mixed-Metal nd-4f Single-Molecule Magnets containing only one Lanthanide Ion, Coord. Chem. Rev., 2014, 276, 1–33 CrossRef CAS.
- D. Aravena and E. Ruiz, Spin Dynamics in Single-Molecule Magnets and Molecular Qubits, Dalton Trans., 2020, 49, 9916–9928 RSC.
- R. A. Layfield, J. J. W. McDouall, S. A. Sulway, F. Tuna, D. Collison and R. E. P. Winpenny, Influence of the N-Bridging Ligand on Magnetic Relaxation in an Organometallic Dysprosium Single-Molecule Magnet, Chem. – Eur. J., 2010, 16, 4442–4446 CrossRef CAS PubMed.
- J. O. Moilanen, A. Mansikkamäki, M. Lahtinen, F. S. Guo, E. Kalenius, R. A. Layfield and L. F. Chibotaru, Thermal Expansion and Magnetic Properties of Benzoquinone-Bridged Dinuclear Rare-Earth Complexes, Dalton Trans., 2017, 46, 13582–13589 RSC.
- P. Zhang, M. Perfetti, M. Kern, P. P. Hallmen, L. Ungur, S. Lenz, M. R. Ringenberg, W. Frey, H. Stoll, G. Rauhut and J. van Slageren, Exchange Coupling and Single Molecule Magnetism in Redox-Active Tetraoxolene-Bridged Dilanthanide Complexes, Chem. Sci., 2018, 9, 1221–1230 RSC.
- J. Flores Gonzalez, F. Pointillart and O. Cador, Hyperfine coupling and Slow Magnetic Relaxation in Isotopically Enriched DyIII Mononuclear Single-Molecule Magnets, Inorg. Chem. Front., 2019, 6, 1081–1086 RSC.
- F.-S. Guo and R. A. Layfield, Strong Direct Exchange Coupling and Single-Molecule Magnetism in Indigo-Bridged Lanthanide Dimers, Chem. Commun., 2017, 53, 3130–3133 RSC.
- Y. S. Meng, J. Xiong, M. W. Yang, Y. Sen Qiao, Z. Q. Zhong, H. L. Sun, J. B. Han, T. Liu, B. W. Wang and S. Gao, Experimental Determination of Magnetic Anisotropy in Exchange-Bias Dysprosium Metallocene Single-Molecule Magnets, Angew. Chem., Int. Ed., 2020, 59, 13037–13043 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2251266 (1), 2251267 (2) and 2251271 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00546a |
| This journal is © the Partner Organisations 2023 |