Copper-catalyzed [3 + 2]/[3 + 2] carboannulation of dienynes and arylsulfonyl chlorides enabled by Smiles rearrangement: access to cyclopenta[a]indene-fused quinolinones†
Liwei
Zhou‡
a,
Xiaodong
Liu‡
a,
Haiyan
Lu
a,
Guobo
Deng
 a,
Yun
Liang
a,
Yun
Liang
 *a,
Yuan
Yang
*a,
Yuan
Yang
 *a and
Jin-Heng
Li
*a and
Jin-Heng
Li
 *abc
*abc
aNational & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources, Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education), and Key Laboratory of the Assembly and Application of Organic Functional Molecules of Hunan Province, Hunan Normal University, Changsha 410081, China. E-mail: yliang@hunnu.edu.cn; yuanyang@hunnu.edu.cn; jhli@hnu.edu.cn
bKey Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, Nanchang Hangkong University, Nanchang 330063, China
cState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, China
First published on 13th July 2021
Abstract
On one hand, the construction of two fused five-membered carbocyclic rings remains an extremely challenging topic in organic synthesis. On the other hand, transition-metal-catalyzed dienyne cycloaddition reactions have become one of the most powerful methods for the construction of diverse cyclic frameworks. Nevertheless, these methods are limited to noble transition-meal catalysis and non-radical cycloaddition modes. Here, a radical Smiles rearrangement strategy for allowing an unprecedented [3 + 2]/[3 + 2] carboannulation of dienynes with arylsulfonyl chlorides using cheap copper catalysis is described. This cascade method represents a novel cycloaddition mode for dienyne chemistry, which occurs by the sequence of addition of arylsulfonyl radical to a dienyne, a Smiles rearrangement and radical annulation to access cyclopenta[a]indene-fused quinolinones with excellent diastereoselectivity.
Introduction
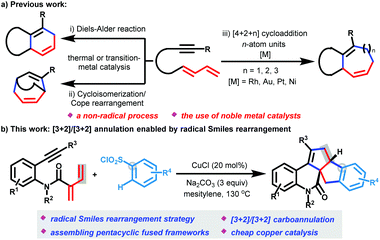
Until now, the construction of two fused five-membered carbocyclic rings, especially with two vicinal chiral carbon atoms, has remained an extremely challenging topic in organic synthesis. Conventional methods often require multiple steps with low overall yields or the use of cyclopentane-based starting materials.1 Therefore, the development of one-pot strategies for directly accessing two fused five-membered carbocyclic frameworks is in great demand.The cycloaddition reaction is well-known and recognized as one of the most powerful and straightforward methodologies for efficient access to cyclic compounds in a single step, often along with the generation of multiple diastereo-centers.2 In the past several decades, cycloaddition of dienynes (generally alkyne-tethered 1,3-dienes) has received considerable attention because this methodology is particularly appealing for rapid construction of diverse complex carbo- and hetero-cyclic structures, including the challenging seven- to nine-membered ring systems.3–6 Typical methodologies can be divided into three types of non-radical annulation modes (Scheme 1a), including (i) Diels–Alder reaction via intramolecular [4 + 2] cycloaddition/isomerization,4 (ii) intramolecular cycloisomerization/Cope rearrangement,5 and (iii) intermolecular [4 + 2 + n] annulation.6 However, these methods require expensive transition metal catalysts (such as Rh, Au, Pt, and Ni) and/or harsh thermal conditions. Although the last [4 + 2 + n] cycloaddition of dienynes with external n-atom units can readily incorporate functional groups as well as controllably tune the ring scaffolds and has been well investigated, the scope of external n-atom units (such as carbene units, alkyne, ethyl cyclopropylideneacetate, and boryl(isopropoxy)silane) is limited. To the best of our knowledge, approaches that employ dienynes to undergo cycloaddition with external x-atom units for the construction of five-membered carbocyclic frameworks, especially those including two carbocyclic rings in a single step, have never been reported. Thus, the development of novel dienyne cycloaddition modes, especially including mechanistically distinguishable strategies using inexpensive transition metal catalysis, to widely expand the dienyne applications is desirable.
The Smiles rearrangement has emerged as one of the most powerful methods for the formation of chemical bonds in synthesis, and has been applied to difunctionalization of unsaturated hydrocarbons (such as alkenes and alkynes) by releasing SO2 in recent years.7–11 In a pioneering study, the group of Nevado has reported a new Smiles rearrangement tactic for enabling the addition of various radicals across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of N-(arylsulfonyl)acrylamides and their derivatives through radical addition/aryl migration/desulfonylation cascades to produce diverse N-heterocycles.8 However, these methods largely rely on tailored acryl sulfonamide motifs.8,9 Recently, Stephenson11a and Zhu11b–e made a significant breakthrough in the radical Smiles rearrangement to realize two-component difunctionalization of alkenes, in which external sulfonyl-based starting materials are converted to nitrogen- or sp3 hybridized carbon-center radicals, and then they undergo addition across the C
C bonds of N-(arylsulfonyl)acrylamides and their derivatives through radical addition/aryl migration/desulfonylation cascades to produce diverse N-heterocycles.8 However, these methods largely rely on tailored acryl sulfonamide motifs.8,9 Recently, Stephenson11a and Zhu11b–e made a significant breakthrough in the radical Smiles rearrangement to realize two-component difunctionalization of alkenes, in which external sulfonyl-based starting materials are converted to nitrogen- or sp3 hybridized carbon-center radicals, and then they undergo addition across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and Smiles rearrangement cascades. On this basis, we envisioned that by the addition of the external sulfonyl radicals across the C
C bonds and Smiles rearrangement cascades. On this basis, we envisioned that by the addition of the external sulfonyl radicals across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of the dienynes would arise an unprecedented cycloaddition reaction for the construction of new cyclic systems.
C bonds of the dienynes would arise an unprecedented cycloaddition reaction for the construction of new cyclic systems.
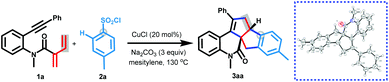
Here, we report an unprecedented copper-catalyzed [3 + 2]/[3 + 2] carboannulation of dienynes with arylsulfonyl chlorides for diastereoselectively producing cyclopenta[a]indene-fused quinolinones (Scheme 1b). This method enables one-pot formation of four new C–C bonds in a single step via radical addition cyclization, Smiles rearrangement and annulation cascades, and represents a new dienyne annulation mode to form two fused five-membered carbocyclic rings in a single step.
Results and discussion
We commenced our studies with the reaction of N-methyl-2-methylene-N-(2-(phenylethynyl)phenyl)but-3-enamide 1a with 4-methylbenzenesulfonyl chloride 2a in the presence of copper catalysts (Table 1). After investigating various reaction parameters, we were pleased to find that the [3 + 2]/[3 + 2] carboannulation could be successfully achieved by a simple catalytic system composed of CuCl (10 mol%), Na2CO3 (3 equiv.), and mesitylene at 130 °C, leading to the formation of 3aa in 58% yield (entry 1). The structure of 3aa was clearly confirmed by X-ray crystallography. Other alternative copper catalysts, including monovalent (CuBr, Cu2O, and CuOAc) and bivalent copper salts (CuCl2), did not give better results (entries 2–5). Further examination of other bases, including K2CO3, K3PO4, NaOAc, and Cs2CO3, revealed that all of them were less effective than Na2CO3 (entries 6–9). Solvents were proved to have a vital effect on this reaction: using toluene derivatives such as o-xylene, m-xylene, and p-xylene as media could smoothly afford the target products, albeit with greatly reduced yields (entries 10–12). However, no desired product was observed when mesitylene was replaced by DMF as medium (entry 13). Finally, this reaction showed lower conversion efficiency on changing the reaction temperature to 120 °C or 140 °C (entries 14 and 15). Notably, we attempted to perform the reaction scaling up to 1 mmol of 1a, delivering the product 3aa in a 46% yield (entry 1).| Entry | Variation from the optimal conditions | Yield (%)b |
|---|---|---|
a Standard conditions: 1a (0.15 mmol), 2a (0.3 mmol), CuCl (20 mol%), Na2CO3 (3 equiv.), and mesitylene (1.5 mL) at 130 °C under a N2 atmosphere for 12 h. Only the cis-diastereoisomer (dr >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained, which is determined by 1H NMR and GC-MS analyses of the crude product.
b Yield of the isolated product.
c
1a (1 mmol) and mesitylene (8 mL). 1) was obtained, which is determined by 1H NMR and GC-MS analyses of the crude product.
b Yield of the isolated product.
c
1a (1 mmol) and mesitylene (8 mL).
|
||
| 1 | None | 58 (46)c |
| 2 | CuBr instead of CuCl | 13 |
| 3 | Cu2O instead of CuCl | 36 |
| 4 | CuOAc instead of CuCl | 21 |
| 5 | CuCl2 instead of CuCl | 33 |
| 6 | K2CO3 instead of Na2CO3 | 15 |
| 7 | K3PO4 instead of Na2CO3 | 16 |
| 8 | NaOAc instead of Na2CO3 | 26 |
| 9 | Cs2CO3 instead of Na2CO3 | Trace |
| 10 | o-Xylene instead of mesitylene | 40 |
| 11 | m-Xylene instead of mesitylene | 33 |
| 12 | p-Xylene instead of mesitylene | 35 |
| 13 | DMF instead of mesitylene | 0 |
| 14 | At 120 °C | 38 |
| 15 | At 140 °C | 49 |
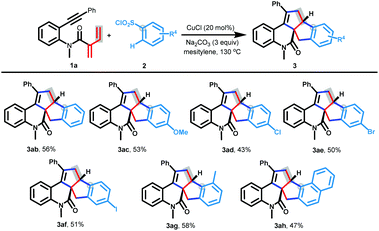
With the optimal reaction conditions identified, we set out to examine the substrate scope of this protocol with respect to arylsulfonyl chlorides. As shown in Scheme 2, a range of arylsulfonyl chlorides 2b–h were subjected to [3 + 2]/[3 + 2] carboannulation with aniline-linked conjugated dienynes 1a to furnish the desired products 3ab–ah in moderate yields. For example, benzenesulfonyl chloride could afford benzo[4,5]pentaleno[6a,1-c]quinolin-12-one 3ab in a 56% yield. Electron-donating methoxy and methyl substituted sulfonyl chlorides were also competent substrates, delivering the target products in 53% and 58% yields (3ac and 3ag). Importantly, halogen atom substituted sulfonyl chlorides at the para-position of the benzene ring were smoothly converted into products 3ad–af, which could provide a promising opportunity for further manipulation. Gratifyingly, the reaction of naphthalene-1-sulfonyl chloride 2h with 1a proceeded favorably, resulting in a 47% yield of 3ah.
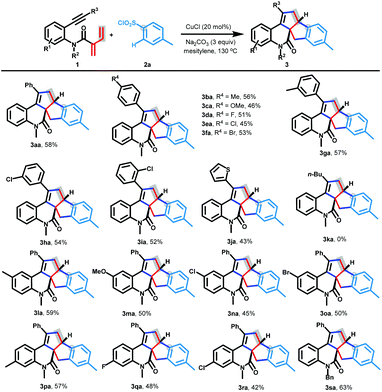
We next turned our attention to the investigation of the scope of aniline-linked conjugated dienynes 1. To our delight, the [3 + 2]/[3 + 2] carboannulation was applicable to a wide range of conjugated dienynes 1, thus furnishing the target products 3ba–ta in moderate yields (Scheme 3). Initially, the alkyne moiety of conjugated dienynes 1 was studied in detail. The reaction tolerated a variety of substrates 1b–i with different aromatic substituents (4-MeC6H4, 4-OMeC6H4, 4-FC6H4, 4-ClC6H4, 4-BrC6H4, 3-MeC6H4, 3-ClC6H4, and 2-ClC6H4) at the alkyne terminus, furnishing pentacyclic-fused quinolin-12-one 3ba–ia in moderate yields. The electronic and steric effects of substituents on the benzene ring had no significant influence on the cascade reaction. The heterocyclic thienyl group could also survive, delivering quinolin-12-one 3ja in 43% yield. Unfortunately, chain alkynes did not give the desired product 3ka. Afterward, we tested the variations in the aniline moiety. To our delight, various substituents such as electron-donating groups (Me and OMe) and electron-withdrawing groups (F, Cl, and Br) on the benzene ring were all well tolerated (3la–ra). When the methyl group on the nitrogen atom was replaced by a benzyl group, the anticipated product 3sa could be obtained in 63% yield.
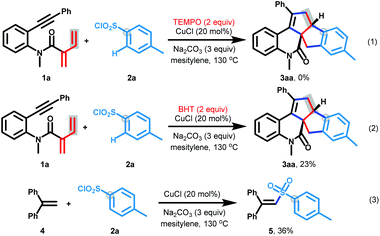
Several control experiments were carried out to explore if a radical process was involved in this reaction (Scheme 4). First, the formation of product 3aa was greatly suppressed when a stoichiometric amount of radical inhibitors such as TEMPO and BHT was added to the model reaction of 1a with 2a under the standard conditions (eqn (1) and (2)). 1,1-Diphenyl ethylene 4 and 4-methylbenzenesulfonyl chloride 2a were next subjected to the above optimal reaction conditions, leading to the generation of (2-tosylethene-1,1-diyl)dibenzene 5 in 36% yield (eqn (3)). These results indicated that the [3 + 2]/[3 + 2] carboannulation possibly involved a radical pathway, which was initiated by arylsulfonyl radicals generated from arylsulfonyl chlorides 2.
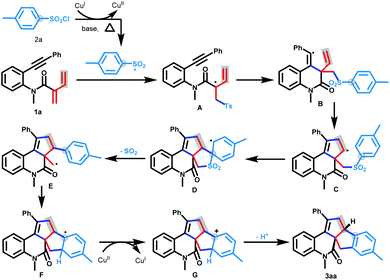
On the basis of the above results and previous reports,10 a possible mechanism for the [3 + 2]/[3 + 2] carboannulation protocol is presented in Scheme 5. Initially, the reaction is triggered by a single electron transfer process of 4-methylbenzenesulfonyl chloride and CuI species under heating and basic conditions, thus producing 4-methylbenzenesulfonyl radicals and CuII species. Subsequently, the addition of the 4-methylbenzenesulfonyl radical to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of conjugated dienynes generates the allyl radical intermediate A, which continues to undergo a radical addition/annulation tandem reaction to form intermediate C. Then, the Smiles rearrangement of intermediate C, including ipso-cyclization, 1,4-arylmigration, and SO2-release, occurs to afford the alkyl radical intermediate E. The intramolecular radical addition to the vicinal benzene ring results in a cyclization of intermediate E to form intermediate F. Eventually, intermediate F undergoes a sequence of single electron oxidation with the CuII species and deprotonation to afford the desired fused pentacyclic product 3aa.
C double bond of conjugated dienynes generates the allyl radical intermediate A, which continues to undergo a radical addition/annulation tandem reaction to form intermediate C. Then, the Smiles rearrangement of intermediate C, including ipso-cyclization, 1,4-arylmigration, and SO2-release, occurs to afford the alkyl radical intermediate E. The intramolecular radical addition to the vicinal benzene ring results in a cyclization of intermediate E to form intermediate F. Eventually, intermediate F undergoes a sequence of single electron oxidation with the CuII species and deprotonation to afford the desired fused pentacyclic product 3aa.
Conclusions
In summary, we have developed an unprecedented copper-catalyzed [3 + 2]/[3 + 2] carboannulation of dienynes with arylsulfonyl chlorides via a radical Smiles rearrangement strategy. In this protocol, arylsulfonyl radicals generated from readily available arylsulfonyl chlorides undergo a radical addition cyclization/Smiles rearrangement/ring closure sequence to achieve [3 + 2]/[3 + 2] carbocyclization of dienynes, thus providing cyclopenta[a]indene-fused quinolinone pentacyclic frameworks with excellent diastereoselectivity. Note that four C–C bonds can be formed by this complex radical cascade process. Further applications of the dienynes are still in progress in our laboratory.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 21625203, 21871126, 21901071 and 21971061) and the Science and Technology Planning Project of Hunan Province (2018TP1017) for financial support.Notes and references
- (a) C. Varela, K. Nilsson, M. Torneiro and A. Mourin, Synthesis of Tetracyclic Analogues of Calcitriol (1α,25-Dihydroxyvitamin D3) with Side-Chain-Locked Spatial Orientations at C(20), Helv. Chim. Acta, 2002, 85, 3251–3261 CrossRef CAS; (b) J. Holtsclaw and M. Koreeda, A Ring-Rearrangement Metathesis Approach toward the Synthesis of Cyclopenta- and Cyclohexa[c]indene Systems, Org. Lett., 2004, 6, 3719–3722 CrossRef CAS; (c) E. Moman, D. Nicoletti and A. Mouriño, Strained Polycycles by H5C5x Free-Radical Cascades, Org. Lett., 2006, 8, 1249–1251 CrossRef CAS PubMed; (d) S. A. Snyder, in Biomimetic Organic Synthesis, ed. E. Poupon and B. Nay, Wiley-VCH, Weinheim, 2011 Search PubMed; (e) B. S. Matsuura, M. H. Keylor, B. Li, Y. Lin, S. Allison, D. A. Pratt and C. R. J. Stephenson, A Scalable Biomimetic Synthesis of Resveratrol Dimers and Systematic Evaluation of their Antioxidant Activities, Angew. Chem., Int. Ed., 2015, 54, 3754–3757 CrossRef CAS.
- (a) Cycloaddition Reactions in Organic Synthesis, ed. S. Kobayashi and K. A. Jorgensen, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2002 Search PubMed; (b) Methods and Applications of Cycloaddition Reactions in Organic Syntheses, ed. N. Nishiwaki, Wiley-VCH, Hoboken, 2014 Search PubMed; (c) G. Dyker, Transition Metal Catalyzed Synthesis of Seven–Membered Carbocyclic Rings, Angew. Chem., Int. Ed. Engl., 1995, 34, 2223–2224 CrossRef CAS; (d) M. Lautens, W. Klute and W. Tam, Transition Metal-Mediated Cycloaddition Reactions, Chem. Rev., 1996, 96, 49–92 CrossRef CAS PubMed; (e) H.-W. Frühauf, Metal-Assisted Cycloaddition Reactions in Organotransition Metal Chemistry, Chem. Rev., 1997, 97, 523–596 CrossRef PubMed; (f) J.-J. Feng and J. Zhang, Synthesis of Unsaturated N-Heterocycles by Cycloadditions of Aziridines and Alkynes, ACS Catal., 2016, 6, 6651–6661 CrossRef CAS; (g) H. Pellissier, Recent Developments in the [5 + 2] Cycloaddition, Adv. Synth. Catal., 2018, 360, 1551–1583 CrossRef CAS; (h) P. Gandeepan, T. Mueller, D. Zell, G. Cera, S. Warratz and L. Ackermann, 3d Transition Metals for C–H Activation, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed; (i) S. Rej, Y. Ano and N. Chatani, Bidentate Directing Groups: An Efficient Tool in C–H Bond Functionalization Chemistry for the Expedient Construction of C–C Bonds, Chem. Rev., 2020, 120, 1788–1887 CrossRef CAS PubMed; (j) J.-H. Qin, J.-H. Li and D.-L. An, Recent Advances in Cycloaddition Reactions with Alkynes to Construct Heterocycles, Synthesis, 2020, 52, 3818–3836 CrossRef CAS.
- For reviews, see: (a) V. Michelet, P. Y. Toullec and J.-P. Genêt, Cycloisomerization of 1,n-Enynes: Challenging Metal-Catalyzed Rearrangements and Mechanistic Insights, Angew. Chem., Int. Ed., 2008, 47, 4268–4315 CrossRef CAS; (b) L.-N. Wang and Z.-X. Yu, Transition-Metal-Catalyzed Cycloadditions for the Synthesis of Eight-Membered Carbocycles: an Update from 2010 to 2020, Chin. J. Org. Chem., 2020, 40, 3536–3558 CrossRef.
- For papers on Diels–Alder cycloaddition of dienynes, see: (a) P. A. Wender and T. E. Jenkins, Nickel-catalyzed intramolecular [4 + 2] dienyne cycloadditions: an efficient new method for the synthesis of polycycles containing cyclohexa-1,4-dienes, J. Am. Chem. Soc., 1989, 111, 6432–6434 CrossRef CAS; (b) R. S. Jolly, G. Luedtke, D. Sheehan and T. Livinghouse, Novel cyclization reactions on transition metal templates. The catalysis of intramolecular [4 + 2] cycloadditions by low-valent rhodium complexes, J. Am. Chem. Soc., 1990, 112, 4965–4966 CrossRef CAS; (c) S. R. Gilbertson, G. S. Hoge and D. G. Genov, Rhodium-Catalyzed Asymmetric [4 + 2] Cycloisomerization Reactions, J. Org. Chem., 1998, 63, 10077–10080 CrossRef CAS; (d) S.-J. Paik, S. U. Son and Y. K. Chung, Highly Efficient Intra- and Intermolecular [4 + 2] Cycloaddition Reaction Catalyzed by Rhodium Complex, Org. Lett., 1999, 1, 2045–2047 CrossRef CAS; (e) B. Wang, P. Cao and X. Zhang, An efficient Rh-catalyst system for the intramolecular [4 + 2] and [5 + 2] cycloaddition reactions, Tetrahedron Lett., 2000, 41, 8041–8044 CrossRef CAS; (f) D. Motoda, H. Kinoshita, H. Shinokubo and K. Oshima, Phosphane–Free Rhodium Catalyst in an Anionic Micellar System for [4 + 2] Annulation of Dienynes, Angew. Chem., Int. Ed., 2004, 43, 1860–1862 CrossRef CAS; (g) S. I. Lee, S. Y. Park, J. H. Park, I. G. Jung, S. Y. Choi, Y. K. Chung and B. Y. Lee, Rhodium N-Heterocyclic Carbene-Catalyzed [4 + 2] and [5 + 2] Cycloaddition Reactions, J. Org. Chem., 2006, 71, 91–96 CrossRef CAS PubMed; (h) S. M. Kim, J. H. Park and Y. K. Chung, Au(PPh3)OPOF2-catalyzed intramolecular [4 + 2] cycloaddition reaction of dienynes, Chem. Commun., 2011, 47, 6719–6722 RSC; (i) N. J. Kramer, T. T. Hoang and G. B. Dudley, Reaction Discovery Using Neopentylene-Tethered Coupling Partners: Cycloisomerization /Oxidation of Electron-Deficient Dienynes, Org. Lett., 2017, 19, 4636–4639 CrossRef CAS PubMed.
- For papers on intramolecular cycloisomerization/Cope rearrangement of dienynes, see: (a) H. Kusama, Y. Onizawa and N. Iwasawa, W(CO)5(L)-Catalyzed Tandem Intramolecular Cyclopropanation/Cope Rearrangement for the Stereoselective Construction of Bicyclo[5.3.0]decane Framework, J. Am. Chem. Soc., 2006, 128, 16500–16501 CrossRef CAS PubMed; (b) A. Furstner and C. C. Stimson, Two Manifolds for Metal-Catalyzed Intramolecular Diels-Alder Reactions of Unactivated Alkynes, Angew. Chem., Int. Ed., 2007, 46, 8845–8849 CrossRef; (c) Y. Onizawa, M. Hara, T. Hashimoto, H. Kusama and N. Iwasawa, Synthetic Studies on and Mechanistic Insight into [W(CO)5(L)]-Catalyzed Stereoselective Construction of Functionalized Bicyclo[5.3.0]decane Frameworks, Chem. – Eur. J., 2010, 16, 10785–10796 CrossRef CAS PubMed; (d) S. Y. Kim, Y. Park and Y. K. Chung, Sequential Platinum-Catalyzed Cycloisomerization and Cope Rearrangement of Dienynes, Angew. Chem., Int. Ed., 2010, 49, 415–418 CrossRef CAS; (e) Z. Cao and F. Gagosz, Gold-Catalyzed Tandem Cycloisomerization/Cope Rearrangement: An Efficient Access to the Hydroazulenic Motif, Angew. Chem., Int. Ed., 2013, 52, 9014–9018 CrossRef CAS PubMed; (f) P.-J. Cai, Y. Wang, C.-H. Liu and Z.-X. Yu, Gold(I)-Catalyzed Polycyclization of Linear Dienediynes to Seven-Membered Ring-Containing Polycycles via Tandem Cyclopropanation/Cope Rearrangement/C-H Activation, Org. Lett., 2014, 16, 5898–5901 CrossRef CAS PubMed; (g) Y. Wang, P.-J. Cai and Z.-X. Yu, Mechanistic Study on Gold-Catalyzed Cycloisomerization of Dienediynes Involving Aliphatic C-H Functionalization and Inspiration for Developing a New Strategy to Access Polycarbocycles, J. Am. Chem. Soc., 2020, 142, 2777–2786 CrossRef CAS PubMed.
- For papers on intermolecular [4 + 2 + x] carboannulation of dienynes, see: (a) A. Padwa, K. E. Krumpe, Y. Gareau and U. Chiacchio, Rhodium(II)-Catalyzed Cyclization Reactions of Alkynyl-Substituted α-DiazoKetones, J. Org. Chem., 1991, 56, 2523–2530 CrossRef CAS; (b) D. F. Harvey and K. P. Lund, Cyclization Reactions of a Molybdenium Carbene Complex with 1,3-Nonadien-8-ynes, J. Am. Chem. Soc., 1991, 113, 5066–5068 CrossRef CAS; (c) D. F. Harvey, E. M. Grenzer and P. K. Gantzel, Effect of Alkene Substituents on Molybdenum and Chromium Carbene Complex Mediated Cyclization Reactions, J. Am. Chem. Soc., 1994, 116, 6719–6732 CrossRef CAS; (d) S. R. Gilbertson and B. DeBoef, Rhodium Catalyzed [4 + 2 + 2] Cycloaddition and Alkyne Insertion: A New Route to Eight-Membered Rings, J. Am. Chem. Soc., 2002, 124, 8784–8785 CrossRef CAS; (e) Y. Ni and J. Montgomery, An Efficient [4 + 2 + 1] Entry to Seven-Membered Rings, J. Am. Chem. Soc., 2004, 126, 11162–11163 CrossRef CAS PubMed; (f) Y. Ni and J. Montgomery, Synthetic Studies and Mechanistic Insight in Nickel-Catalyzed [4 + 2 + 1] Cycloadditions, J. Am. Chem. Soc., 2006, 128, 2609–2614 CrossRef CAS PubMed; (g) B. DeBoef, W. R. Counts and S. R. Gilbertson, Rhodium-Catalyzed Synthesis of Eight-Membered Rings, J. Org. Chem., 2007, 72, 799–804 CrossRef CAS PubMed; (h) S. Saito, K. Maeda, R. Yamasaki, T. Kitamura, M. Nakagawa, K. Kato, I. Azumaya and H. Masu, Synthesis of Nine–Membered Carbocycles by the [4 + 3 + 2] Cycloaddition Reaction of Ethyl Cyclopropylideneacetate and Dienynes, Angew. Chem., Int. Ed., 2010, 49, 1830–1833 CrossRef CAS PubMed; (i) R. Yamasaki, M. Ohashi, K. Maeda, T. Kitamura, M. Nakagawa, K. Kato, T. Fujita, R. Kamura, K. Kinoshita, H. Masu, I. Azumaya, S. Ogoshi and S. Saito, Ni-Catalyzed [4 + 3 + 2] Cycloaddition of Ethyl Cyclopropylideneacetate and Dienynes: Scope and Mechanistic Insights, Chem. – Eur. J., 2013, 19, 3415–3425 CrossRef CAS PubMed; (j) I. Sasaki, T. Ohmura and M. Suginome, Construction of Silicon-Containing Seven-Membered Rings by Catalytic [4 + 2 + 1] Cycloaddition through Rhodium Silylenoid, Org. Lett., 2020, 22, 2961–2966 CrossRef PubMed.
- For selected reviews, see: (a) A. Studer and M. Bossart, Radical aryl migration reactions, Tetrahedron, 2001, 57, 9649–9667 CrossRef CAS; (b) Z.-M. Chen, X.-M. Zhang and Y.-Q. Tu, Radical aryl migration reactions and synthetic applications, Chem. Soc. Rev., 2015, 44, 5220–5245 RSC; (c) C. M. Holden and M. F. Greaney, Modern Aspects of the Smiles Rearrangement, Chem. – Eur. J., 2017, 23, 8992–9008 CrossRef CAS PubMed.
- (a) W. Kong, M. Casimiro, E. Merino and C. Nevado, Copper-Catalyzed One-Pot Trifluoromethylation/Aryl Migration/Desulfonylation and C(sp2)-N Bond Formation of Conjugated Tosyl Amides, J. Am. Chem. Soc., 2013, 135, 14480–14483 CrossRef CAS PubMed; (b) W. Kong, M. Casimiro, N. Fuentes, E. Merino and C. Nevado, Metal-Free Aryltrifluoromethylation of Activated Alkenes, Angew. Chem., Int. Ed., 2013, 52, 13086–13090 CrossRef CAS PubMed; (c) W. Kong, E. Merino and C. Nevado, Arylphosphonylation and Arylazidation of Activated Alkenes, Angew. Chem., Int. Ed., 2014, 53, 5078–5082 CAS; (d) N. Fuentes, W. Kong, L. Fernandez-Sanchez, E. Merino and C. Nevado, Cyclization Cascades via N-Amidyl Radicals toward Highly Functionalized Heterocyclic Scaffolds, J. Am. Chem. Soc., 2015, 137, 964–973 CrossRef CAS PubMed; (e) W. Kong, N. Fuentes, A. García-Domínguez, E. Merino and C. Nevado, Stereoselective Synthesis of Highly Functionalized Indanes and Dibenzocycloheptadienes through Complex Radical Cascade Reactions, Angew. Chem., Int. Ed., 2015, 54, 2487–2491 CrossRef CAS PubMed.
- For selected examples, see: (a) M. Pudlo, I. Allart-Simon, B. Tinant, S. Gèrard and J. Sapi, First domino radical cyclisation/Smiles rearrangement combination, Chem. Commun., 2012, 48, 2442–2445 RSC; For other selected examples, see: (b) Z. He, P. Tan, C. Ni and J. Hu, Fluoroalkylative Aryl Migration of Conjugated N-Arylsulfonylated Amides Using Easily Accessible Sodium Di- and Monofluoroalkanesulfinates, Org. Lett., 2015, 17, 1838–1841 CrossRef CAS PubMed; (c) H. Zhang, C. Pan, N. Jin, Z. Gu, H. Hu and C. Zhu, Metal-free cascade construction of C-C bonds by activation of inert C(sp3)-H bonds, Chem. Commun., 2015, 51, 1320–1323 RSC; (d) Y.-L. Zhu, B. Jiang, W.-J. Hao, J.-K. Qiu, J. Sun, D.-C. Wang, P. Wei, A.-F. Wang, G. Li and S.-J. Tu, Catalytic Arylsulfonyl Radical Triggered 1,7-Enyne Bicyclizations, Org. Lett., 2015, 17, 6078–6081 CrossRef CAS PubMed; (e) Z. Ni, X. Huang and Y. Pan, Metal-Free Mediated Meerwein-Type Reaction: A Radical Cascade Arylation/Aryl Migration/Desulfonylation of Conjugated Alkenes, Org. Lett., 2016, 18, 2612–2615 CrossRef CAS PubMed; (f) F.-L. Tan, R.-J. Song, M. Hu and J.-H. Li, Metal-Free Oxidative 1,2-Arylmethylation Cascades of N-(Arylsulfonyl)acrylamides Using Peroxides as the Methyl Resource, Org. Lett., 2016, 18, 3198–3201 CrossRef CAS PubMed; (g) X.-F. Xia, S.-L. Zhu, C. Chen, H. Wang and Y.-M. Liang, Silver-Catalyzed Decarboxylative Addition/Cyclization of Activated Alkenes with Aliphatic Carboxylic Acids, J. Org. Chem., 2016, 81, 1277–1284 CrossRef CAS PubMed; (h) K. Liu, L.-C. Sui, Q. Jin, D.-Y. Li and P.-N. Liu, CuBr-mediated radical cascade difluoroacetamidation of acrylamides using α,α-difluoro-α-(TMS)-acetamides, Org. Chem. Front., 2017, 4, 1606–1611 RSC; (i) H. Huang and Y. Li, Sustainable Difluoroalkylation Cyclization Cascades of 1,8-Enynes, J. Org. Chem., 2017, 82, 4449–4457 CrossRef CAS PubMed; (j) D. M. Whalley, H. A. Duong and M. F. Greaney, Alkene Carboarylation through Catalyst-Free, Visible Light-Mediated Smiles Rearrangement, Chem. – Eur. J., 2019, 25, 1927–1930 CrossRef CAS PubMed; (k) M. Li, C.-T. Wang, Q.-F. Bao, Y.-F. Qiu, W.-X. Wei, X.-S. Li, Y.-Z. Wang, Z. Zhang, J.-L. Wang and Y.-M. Liang, Copper-Catalyzed Radical Aryl Migration Approach for the Preparation of Cyanoalkylsulfonylated Oxindoles/Cyanoalkyl Amides, Org. Lett., 2021, 23, 751–756 CrossRef CAS PubMed.
- (a) J. Zheng, Y. Li, J. Han, T. Xiong and Q. Zhang, Radical cascade reaction of alkynes with N-fluoroarylsulfonimides and alcohols, Nat. Commun., 2015, 6, 7011–7019 CrossRef PubMed; (b) E. Brachet, L. Marzo, M. Selkti, B. König and P. Belmont, Visible light amination/Smiles cascade: access to phthalazine derivatives, Chem. Sci., 2016, 7, 5002–5006 RSC; (c) Z.-S. Wang, Y.-B. Chen, H.-W. Zhang, Z. Sun, C. Zhu and L.-W. Ye, Ynamide Smiles Rearrangement Triggered by Visible-Light-Mediated Regioselective Ketyl-Ynamide Coupling: Rapid Access to Functionalized Indoles and Isoquinolines, J. Am. Chem. Soc., 2020, 142, 3636–3644 CrossRef CAS PubMed; (d) J. Yan, H. W. Cheo, W. K. Teo, X. Shi, H. Wu, S. B. Idres, L.-W. Deng and J. Wu, A Radical Smiles Rearrangement Promoted by Neutral Eosin Y as a Direct Hydrogen Atom Transfer Photocatalyst, J. Am. Chem. Soc., 2020, 142, 11357–11362 CrossRef CAS PubMed; (e) J. J. Douglas, H. Albright, M. J. Sevrin, K. P. Cole and C. R. J. Stephenson, A Visible-Light-Mediated Radical Smiles Rearrangement and its Application to the Synthesis of a Difluoro-Substituted Spirocyclic ORL-1 Antagonist, Angew. Chem., Int. Ed., 2015, 54, 14898–14902 CrossRef CAS PubMed; (f) D. Alpers, K. P. Cole and C. R. J. Stephenson, Visible Light Mediated Aryl Migration by Homolytic C-N Cleavage of Aryl Amines, Angew. Chem., Int. Ed., 2018, 57, 12167–12170 CrossRef CAS PubMed.
- (a) T. M. Monos, R. C. McAtee and C. R. J. Stephenson, Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation, Science, 2018, 361, 1369–1373 CrossRef CAS PubMed; (b) J. Yu, Z. Wu and C. Zhu, Efficient Docking-Migration Strategy for Selective Radical Difluoromethylation of Alkenes, Angew. Chem., Int. Ed., 2018, 57, 17156–17160 CrossRef CAS PubMed; (c) M. Wang, H. Zhang, J. Liu, X. Wu and C. Zhu, Radical Monofluoroalkylative Alkynylation of Olefins by a Docking-Migration Strategy, Angew. Chem., Int. Ed., 2019, 58, 17646–17650 CrossRef CAS PubMed; (d) J. Liu, S. Wu, J. Yu, C. Lu, Z. Wu, X. Wu, X.-S. Xue and C. Zhu, Polarity Umpolung Strategy for the Radical Alkylation of Alkenes, Angew. Chem., Int. Ed., 2020, 59, 8195–8202 CrossRef CAS PubMed; (e) H. Zhang, M. Wang, X. Wu and C. Zhu, Heterocyclization Reagents for Rapid Assembly of N-Fused Heteroarenes from Alkenes, Angew. Chem., Int. Ed., 2021, 60, 3714–3719 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2059099. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1qo00703c |
| ‡ The authors contributed equally. |
| This journal is © the Partner Organisations 2021 |