Luminescent tetraphenylethene-cored, carbazole- and thiophene-based microporous polymer films for the chemosensing of nitroaromatic analytes†
A.
Palma-Cando
a,
D.
Woitassek
a,
G.
Brunklaus
b and
U.
Scherf
 *a
*a
aMacromolecular Chemistry Group, Bergische Universität Wuppertal, Gaußstraße 20, D-42119 Wuppertal, Germany. E-mail: scherf@uni-wuppertal.de
bWestfälische Wilhelms-Universität, Institut für Physikalische Chemie, Corrensstr. 46, D-48149 Münster, Germany
First published on 16th December 2016
Abstract
A series of novel microporous polymer networks (MPNs) have been prepared from tetraphenylethene (TPE)-cored, multifunctional carbazole- or thiophene-based monomers by using chemical and electrochemical oxidative coupling methods. Octafunctional monomers lead to MPNs with a high degree of cross-linking and optimized specific surface areas (SBET) of up to 2200 m2 g−1. Aggregation-induced emission (AIE) activity of the monomers is demonstrated. The MPN films are tested for application in the chemical sensing of nitroaromatic analytes by fluorescence quenching.
Introduction
Microporous polymer networks (MPNs) are covalently bonded highly crosslinked materials which show permanent microporosity and high surface areas. Carbazole-based MPNs have been discussed for potential applications in the field of gas storage and separation.1 Light-emitting MPNs containing extended π-conjugated segments have been used in chemosensing experiments towards different chemicals since they provide a high interfacial area for analyte interaction.2,3 The chemical structure of the core as well as the number and size of the linkers in the monomeric building blocks (tectons) play a significant role in the resulting properties of the MPNs, e.g. the specific surface area (SBET).4,5 However, the generation of MPN films is still challenging. Thin MPN films can be achieved by electrochemical, oxidative coupling of electroactive, multifunctional monomers.6 Usually carbazolyl or thienyl groups are used due to the low oxidative potentials required for oxidation and consecutive coupling/dimerization.7,8 In a previous report, we showed that the number of carbazolyl linkers in the monomers (two, three or four) strongly influence the porosity of the resulting MPN films.9 Increasing the number of reactive groups leads to a higher cross-linking density and rigidity, and therefore to an enhanced surface area. Recently, we studied how a further increase of the number of carbazolyl units (up to eight carbazolyls around a tetraphenylmethane core) influenced the microporosity of the resulting MPNs.10 Thereby, almost similar SBET values resulted for either tetra- or octacarbazole-based MPNs, probably related to incomplete cross-linking caused by steric hindrance. This drawback may be overcome by the introduction of additional spacers between core and linker units.The study of aggregation-induced emission (AIE) phenomena has received enormous response from the scientific community since the first report by Tang and co-workers in 2001.11 The main cause for the AIE effect is the restriction of intramolecular rotations and vibrations.12 Based on this mechanism, many different propeller-shaped small molecules13 and polymers14 have been synthesized, with the tetraphenylethene (TPE) core as the most prominent structural motif. AIE luminogens are connected to numerous potential applications as biological probes, for cell imaging, in organic light-emitting diodes and chemical sensors, among others.15 By combining MPN films and AIE approaches, Jiang and co-workers studied electrogenerated MPN films made from carbazolyl-substituted TPE-cored monomers for use as chemical sensors for nitroaromatic explosives.16
In this work, we have synthesized a series of six TPE-cored monomers containing four or eight electroactive carbazolyl or thienyl substituents. The AIE activity of the monomers and the porosity of the corresponding MPNs are studied in relation to the structure and number of electroactive groups and the presence of additional 1,4-phenylene linkers. Bulk polymers as well as MPN films (obtained by chemical or electrochemical oxidative coupling, respectively) show high specific BET surface areas of up to 2200 m2 g−1. Whereas the AIE-active monomers show high photoluminescence quantum yields of up to 83% in the aggregated state, the corresponding electrogenerated MPN films are less emissive but show a high sensitivity of up to the ppm level in chemosensing experiments towards nitroaromatic analytes.
Results and discussion
The synthesis of a series of six TPE-cored monomers followed literature reports (see the ESI† for details). Scheme 1 presents the chemical structures of the monomers. The first pair of monomers has four functionalities (carbazolyl or thienyl) around the TPE core (TPETCz and TPETTh); the second pair of monomers with four groups contains additional 1,4-phenylene linkers (TPETPTCz and TPETPTTh); and the third pair of monomers with eight functionalities holds additional 1,3,5-phenylene linkers (TPETPOcCz and TPETPOcTh). 1,1,2,2-Tetra(4-(carbazol-9-yl)phenyl)ethene (TPETCz) was synthesized from bis(4-(carbazol-9-yl)phenyl)methanone (produced by the reaction of bis(4-fluorophenyl)methanone and carbazole) in a McMurry-type reductive coupling in 80% yield. The intermediate 1,1,2,2-tetra(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)ethene that is used for the generation of five other monomers was synthesized by tetrabromination of tetraphenylethene followed by Miyaura borylation. 1,1,2,2-Tetra(4-(thiophen-2-yl)phenyl)ethene (TPETTh), 1,1,2,2-tetra(4′-(carbazol-9-yl)-[1,1′-biphenyl]-4-yl)ethene (TPEPTCz), 1,1,2,2-tetra(4′-(thiophen-2-yl)-[1,1′-biphenyl]-4-yl)ethene (TPEPTTh), 1,1,2,2-tetra(3′,5′-di(carbazol-9-yl)-[1,1′-biphenyl]-4-yl)ethene (TPEPOcCz), and 1,1,2,2-tetra(3′,5′-di(thiophen-2-yl)-[1,1′-biphenyl]-4-yl)ethene (TPEPOcTh) were generated by Suzuki-type cross-couplings of the TPE intermediate with 2-bromothiophene, 9-(4-bromophenyl)-carbazole, 2-(4-bromophenyl)thiophene, 9,9′-(5-bromo-1,3-phenylene)bis-(carbazole), or 2,2′-(5-bromo-1,3-phenylene)dithiophene, respectively, with good to moderate yields.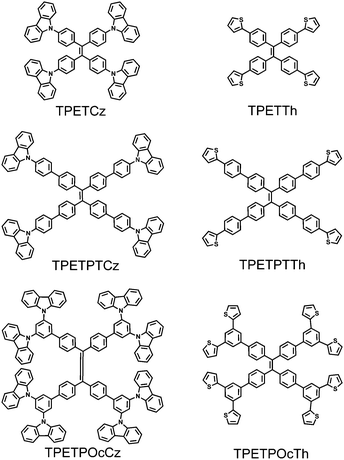 | ||
| Scheme 1 Chemical structures of the six investigated carbazolyl- (left) and thienyl-substituted (right) monomers. | ||
The bulk polymer networks were generated as powdery materials by chemical oxidative coupling of the monomers with iron(III) chloride in chloroform. After washing and drying, the fine powders were obtained in good to excellent yields (73–99%). These MPNs exhibit solid state luminescence. Thermogravimetric analysis of the polymer networks showed good thermal stability under argon up to 300 °C (see Fig. S1 and S2, ESI†). Solid state 13C{1H} cross-polarization magic-angle spinning (CPMAS) spectra of the MPNs agree well with the proposed chemical structures (see Fig. S3, ESI†). Signals from 110 nm to 130 nm are mainly related to protonated aromatic carbons, while signals at lower fields from 130 nm to 150 nm are ascribed to substituted vinyl and phenyl carbons. The FT-IR spectra of monomers and bulk polymers are shown in Fig. S4 (ESI†). For carbazole-based polymers, the appearance of a peak at ca. 800 cm−1 is mainly attributed to the formation of trisubstituted carbazole rings.10,17 In the case of thiophene-based monomers, a strong peak at ca. 690 cm−1 is attributed to out-of-plane CH bending vibrations of mono-substituted thiophene rings.18 Disappearance of this signal and the emergence of two bands in the range from 799 cm−1 to 840 cm−1 document the formation of α,α′-disubstituted bithiophene moieties in the corresponding bulk polymers.19 Nitrogen gas adsorption isotherms recorded at 77 K revealed the microporosity of all bulk polymers, as documented in a rapid gas uptake at low relative pressures of <0.1 (see Fig. S5, ESI†).20 The average pore diameters were calculated based on the nonlocal density functional theory (NLDFT) model to be 0.40–1.43 nm. On the other hand, significant gas adsorption at high relative pressures (>0.9) and the apparent hysteresis between the adsorption and desorption for all materials reflect the presence of additional meso- and macropores.21Table 1 lists the calculated SBET values of the bulk polymers by applying the Brunauer–Emmett–Teller equation to the corresponding N2 adsorption isotherms. The following points should be mentioned: (i) PTPETCz and PTPETTh show very similar SBET values of 1097 m2 g−1 and 1085 m2 g−1, respectively; both structures contain tectons with four carbazolyl or thienyl linkers around TPE cores. The chemical syntheses of PTPETCz and PTPETTh have already been reported under similar conditions by Han and co-workers5,22 resulting in SBET values of 1430 m2 g−1 and 681 m2 g−1, respectively. When an additional 1,4-phenylene spacer was introduced into the tectons, similarly reduced SBET values are observed for PTPETPTCz (1039 m2 g−1) and PTPETPTTh (956 m2 g−1). This phenomenon might be explained by the formation of intercalated networks due to an increase of the pore diameter.17 (ii) Remarkably high SBET values were found for PTPETPOcCz (2203 m2 g−1) and PTPETPOcTh (1767 m2 g−1) made from octafunctionalized monomers. Both tectons contain 1,3,5-trisubtituted phenylene linkers for attachment of the functional groups. The increased porosity might be related to the higher number of reactive sites leading to higher cross-linking densities and increased rigidity.
It is possible that the 1,3,5-trisubtituted spacer may also decrease the probability of chain intercalation. Please note that in a recent publication,10 we found quite similar SBET values for tetra- or octa-carbazolyl substituents around a tetraphenylmethane core (1322 m2 g−1 and 1331 m2 g−1, respectively), thus illustrating the role of the structure of the tectons. (iii) As a tendency, higher SBET values were calculated for carbazole-based MPNs in relation to the corresponding, structurally similar thiophene-based MPNs. Thereby, the increased size of the carbazolyl group may possibly play a role. Due to the high microporosity and the polar character of the MPNs, they may find applications in gas storage and separation.23,24 CO2 uptake capacities in the range of 5.33–8.95% at 25 °C and 1 bar were calculated for our chemically synthesized powdery networks (see Fig. S6 and Table S1, ESI†). By applying Henry's law, the maximum values of CO2/N2 and CO2/CH4 gas adsorption selectivities of 13.0 and 2.8, respectively, have been observed for PTPETPOcCz.
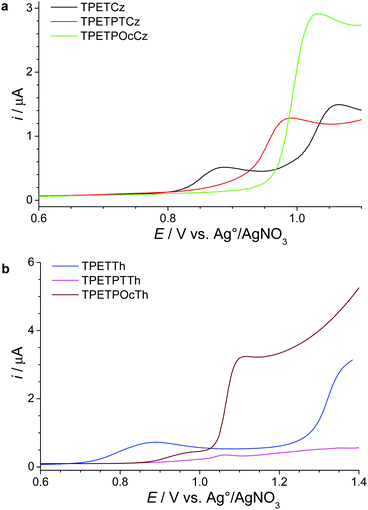
Electrochemical oxidative polymerization is a powerful technique that allows for a well-controlled deposition of polymer films on suitable electrodes from a monomer solution.25 Thereby, for the carbazole-based monomers diluted 0.1 mM solutions in dichloromethane were used together with 0.1 M tetrabutylammonium tetrafluoroborate (TBABF4) as a supporting electrolyte. For the thiophene-based monomers, addition of 20% (V/V) boron trifluoride diethyl etherate (BFEE) allows for an optimum polymerization into MPN films. As shown in a previous study,26 BFEE mainly decreases the required oxidation potential for electrochemical coupling resulting in boosted porosity of the MPN films. Fig. 1 shows the first anodic scan voltammograms for polymerization at Pt working electrodes for the carbazole-based (Fig. 1a) and thiophene-based (Fig. 1b) monomers. TPETCz and TPETTh show the presence of two oxidation peaks (see also Table 1). For both monomers, the reversible peaks at around 0.89 V (see insets of Fig. S7a and b, ESI†) might be related to the formation of stable, conjugated radical cations as reported for 1,4-dicarbazolyl-substituted phenylene monomers.27 At higher potentials of 1.06 V and 1.36 V, respectively, the second irreversible oxidation peaks are observed for these carbazole- or thiophene-based monomers. These peaks should be related to the dimerization/cross-linking and deposition of the MPN films as reflected by the gradual current increase during multiple cycling (see Fig. S7a and b, ESI†). TPETPTCz and TPETPTTh exhibit single oxidation peaks at around 0.99 V and 1.06 V, respectively, for the electrochemical coupling reaction (see Fig. S7c and d, ESI†). The observed lower current for TPETPTTh stems from the low solubility of this monomer (monomer concentration <0.1 mM). Finally, for the coupling of TPETPOcCz and TPETPOcTh, slightly higher oxidation potentials of 1.03 V and 1.12 V, respectively, are needed if compared to the corresponding tetrasubstituted monomers (see Fig. S7e and f, ESI†). Moreover, the observed higher oxidation current peaks for the octasubstituted monomers reflect the doubled amount of electroactive groups in the tectons. Cyclic voltammograms of the electrogenerated films in monomer-free solutions at different scan rates from 0.005 to 0.20 V s−1 exhibited two reversible peaks for carbazole-based MPNs and one reversible peak for thiophene-based MPNs (potential range: 0.5–1.1 V, see Fig. S8, ESI†). These peaks are related to charging/discharging processes in conducting polymer films via reversible formation of radical cations and/or dications.28 Additionally, the observed linear relation between peak currents and scan rate documents the formation of well-adhered deposits where the electron transfer is not a diffusion-controlled process (see insets of Fig. S8, ESI†).
In particular, potentiostatic oxidative polymerization allows for the formation of thicker, free-standing MPNs upon increasing the monomer concentration to 0.5 mM. After repeated washing and drying of the films, they were used to extract the corresponding krypton gas sorption data (77 K, relative pressure of 0–0.6) as shown in the representative sorption isotherms of Fig. 2a. A fast krypton uptake at low relative pressures of <0.1 again documents microporosity (see Fig. 2b for nitrogen uptake in the corresponding bulk polymers). Slightly reduced SBET values are observed for most of the electrogenerated MPN films compared to the corresponding bulk polymers (see Table 1). Strikingly, electrogenerated PTPETTh films display a dramatically lowered SBET value of 433 m2 g−1 (1085 m2 g−1 for the corresponding bulk polymer). This finding can be related to the relatively high oxidation potential of 1.4 V necessary for film deposition, probably resulting in overoxidation and occurrence of side reactions. The SBET value of PTPETPTTh films was not determined by krypton adsorption measurements due to the poor monomer solubility leading to the formation of only thin deposits.
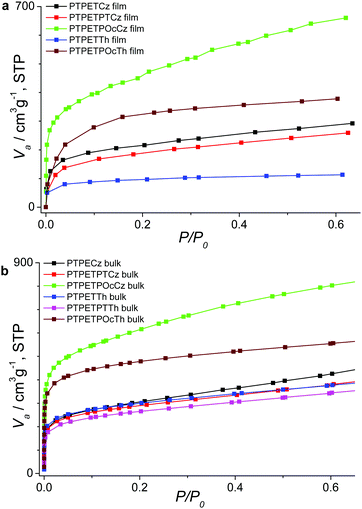 | ||
| Fig. 2 Adsorption isotherms of electropolymerized MPN films (a) and the corresponding bulk polymers synthesized by oxidative coupling with iron(III) chloride (b), using Kr or N2 gas, respectively. | ||
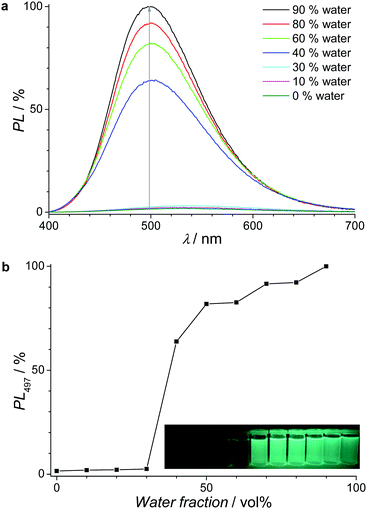
Organic materials with high solid state photoluminescence (PL) are of increasing importance for many potential applications, e.g. in electronic devices or sensors. One concept towards this goal, the so-called aggregation-induced emission (AIE) of small molecules and polymers, has been intensively studied during the last 15 years. In particular, materials based on the tetraphenylethylene motif have been extensively investigated.29 Herein, the AIE properties of our TPE-cored monomers were studied due to their good synthetic accessibility and favorable optical properties. Subsequently, the use of MPN films made from these monomers was explored for the chemical detection of nitroaromatic analytes. The optical spectra of the monomers are depicted in Fig. S9 (ESI†). Red-shifted absorption spectra are observed for the monomer films in relation to their corresponding solutions in chloroform. Photoluminescence spectra show solid state PL maxima between 497 nm for TPETCz and 553 nm for TPETTh (see Table 2 and Fig. S10, ESI†). The corresponding photoluminescence quantum yields (PL-QYs) in solution and in the solid state are summarized in Table 2. Please notice: (i) for all monomers, the transition from solution to the solid state leads to up to 63 times increased PL-QYs (for TPETCz) due to the AIE effect. (ii) The carbazole-based monomers generally display higher PL-QYs compared to their corresponding thiophene-based counterparts. For completion, the AIE effect was also investigated in solvent/non-solvent mixtures. As an example, Fig. 3a shows the PL spectra of 0.01 mM TPETPOcCz in THF/water mixtures with the increasing non-solvent fraction (0% to 90% water). The low PL intensity for water contents <30% rapidly increases for the water contents between 30% and 40% (see Fig. 3b) due to aggregation of AIE-active TPE-cored molecules. The PL intensity reaches a maximum for 90% water, with a 67-fold increase compared to the THF solution. The aggregated state is expected to restrict intramolecular rotations and block non-radioactive deactivation channels thus leading to a strong PL increase.30 To finish up the monomer section, we explored the PL quenching properties of aggregated TPETPOcCz dispersions in THF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) caused by a prototypical nitroaromatic analyte, 1,3,5-trinitrobenzene (TNB). A progressive decrease of the PL intensity was obtained by adding TNB at the ppm level (0–25 ppm, see Fig. S11a, ESI†). A possible mechanism of PL quenching would be energy transfer between the excited, electron-rich host and the electron-deficient nitroaromatic quencher. Thereby, the driving force for the electron transfer stems from the offset between the LUMO levels of the host and the quencher.31 Stern–Volmer analysis displays an upward bended plot at high TNB concentrations thus pointing to amplified PL quenching (see Fig. S11b, ESI†).32 Three quenching constants (KSV) were calculated depending on the TNB concentration: 6.78 × 104 M−1 ± 7.69 × 102 M−1 below 30 μM, 1.52 × 105 M−1 ± 1.78 × 103 M−1 between 30 μM and 105 μM, and 2.53 × 105 M−1 ± 5.66 × 104 M−1 between 105 μM and 120 μM. This phenomenon of amplified quenching has been attributed to the formation of small voids in the three dimensional structures of the aggregates thus creating channels for the diffusion of analyte molecules and leading to an amplification of the quenching process.33
9) caused by a prototypical nitroaromatic analyte, 1,3,5-trinitrobenzene (TNB). A progressive decrease of the PL intensity was obtained by adding TNB at the ppm level (0–25 ppm, see Fig. S11a, ESI†). A possible mechanism of PL quenching would be energy transfer between the excited, electron-rich host and the electron-deficient nitroaromatic quencher. Thereby, the driving force for the electron transfer stems from the offset between the LUMO levels of the host and the quencher.31 Stern–Volmer analysis displays an upward bended plot at high TNB concentrations thus pointing to amplified PL quenching (see Fig. S11b, ESI†).32 Three quenching constants (KSV) were calculated depending on the TNB concentration: 6.78 × 104 M−1 ± 7.69 × 102 M−1 below 30 μM, 1.52 × 105 M−1 ± 1.78 × 103 M−1 between 30 μM and 105 μM, and 2.53 × 105 M−1 ± 5.66 × 104 M−1 between 105 μM and 120 μM. This phenomenon of amplified quenching has been attributed to the formation of small voids in the three dimensional structures of the aggregates thus creating channels for the diffusion of analyte molecules and leading to an amplification of the quenching process.33
| Monomer | PL λmax film [nm] | PL-QY sol. [%] | PL-QY film [%] |
|---|---|---|---|
| TPETCz | 497 | 1.3 | 83 |
| TPETPTCz | 512 | 5.8 | 56 |
| TPETPOcCz | 520 | 2.9 | 73 |
| TPETTh | 553 | 0.8 | 7 |
| TPETPTTh | 503 | 2.5 | 4 |
| TPETPOcTh | 514 | 1.7 | 38 |
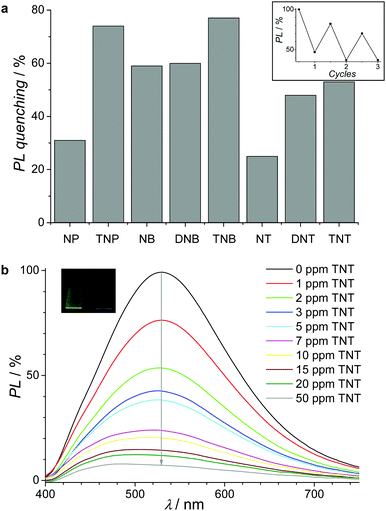
As previously mentioned, the electrogenerated MPN films are also characterized by an intense PL, especially the carbazole-based materials. Therefore, we next studied the optical properties of the carbazolyl MPN films. The presence of high permanent microporosity and intense PL should provide a unique combination of properties, especially, for PL quenching applications. Towards this end, MPN films were potentiodynamically generated on ITO electrodes from 0.1 mM monomer solutions by applying ten cycles in a potential range of −1 V to 1.1 V with a scan rate of 0.1 V s−1. Tapping mode AFM images of the films show a smooth topology (see Fig. S12, ESI†) with an average roughness (Rq) of 4–13 nm at thicknesses of 25–75 nm (see Table S2, ESI†). The absorption spectra of the MPN films display long wavelength absorption maxima at ca. 350 nm (see Fig. S13a, ESI†).16 Upon excitation at 340 nm, these MPN thin films emit green-yellowish light with PL maxima centered at 527 nm for PTPETCz, 542 nm for PTPETPTCz, and 529 nm for PTPETPOcCz (see Fig. S13b, ESI†). Moderately high PL-QY values of 3.9% (PTPETCz), 11.5% (PTPETPTCz), and 3.1% (PTPETPOcCz) were found, with the values still higher than the ones of the corresponding monomers in chloroform solutions. As shown in a previous study,34 MPN films are better suited than the corresponding monomer films for the detection of low concentrations of nitroaromatic analytes thus demonstrating the added value of the microporosity in the sensing potential. Therefore, we have explored the potential of electrogenerated PTPETPOcCz films for the chemical sensing of nitroaromatic compounds. Fig. 4a illustrates the PL quenching upon contacting these MPN films with 100 ppm solutions of 4-nitrophenol (NP), 2,4,6-trinitrophenol (TNP), nitrobenzene (NB), 1,3-dinitrobenzene (DNB), 1,3,5-trinitrobenzene (TNB), 4-nitrotoluene (NT), 2,6-dinitrotoluene (DNT), and 2,4,6-trinitrotoluene (TNT) in acetonitrile for 1 min. The PL quenching correlates well with the number of nitro groups in the analyte, with the maximum PL quenching of 77% for TNB. Assuming excited state energy transfer between the electron-rich MPN film and the electron-deficient quencher, this trend may be easily rationalized. Finally, PTPETPOcCz thin films were tested for the detection limit towards TNT, due to its importance to public health and security as well as for the environment.35Fig. 4b displays a progressively decreased PL intensity for ppm concentrations with a clearly detectable quenching already for 1 ppm TNT. Examples of explosive detection techniques36 based on various sensory materials37,38 have been reported with detection limits of up to the ppt level (see Table S3, ESI†). However, our fluorometric technique based on electrogenerated, microporous films may be optimized towards enhanced detection sensitivities especially by switching to gas-phase detection schemes. PTPETPOcCz films also show good reusability after removal of the analyte by washing with acetonitrile and drying under reduced pressure at 100 °C (see the inset of Fig. 4a).
Conclusions
To sum up, a series of six tetraphenylethene-cored, carbazole- or thiophene-based monomers were synthesized and used in the generation of the corresponding microporous polymer networks by chemical or electrochemical oxidative coupling. All polymers showed high microporosity with BET surface areas of up to 2203 m2 g−1. The monomers displayed the expected AIE activity especially for the carbazole-based monomers. Thin films produced by electrochemical coupling were used for PL quenching-based sensing of nitroaromatic explosives reacting at a low level of ca. 1 ppm for TNT.Experimental
Details of the syntheses of all monomers and the corresponding bulk microporous polymer networks are presented in the ESI.†For electrochemical polymerization and characterization, a potentiostat/galvanostat PAR VersaSTAT 4 was used in combination with a three-electrode cell. For polymerization, 10 mL of 0.1 mM solutions of carbazole- or thiophene-based monomers in dichloromethane or BFEE/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), respectively, together with 0.1 M TBABF4 as a supporting electrolyte were placed in a three-electrode cell under an argon atmosphere at 25 °C. A platinum disc electrode (Pt, 1 mm diameter) or ITO on glass (∼1.5 × 1.2 cm deposit area) was used as a working electrode (WE); platinum as a counter-electrode (CE); and Ag0/AgNO3 (0.01 M AgNO3/0.1 M TBAP in acetonitrile, 0.56 V vs. NHE) as a reference electrode (RE). Thick films on ITO were produced by applying a static oxidative potential of 1.2 V (TPETCz, TPETPTCz, TPETPOcCz, TPETPTTh, and TPETPOcTh) or 1.4 V (TPETTh) for 20 min. A potential of −1.0 V was applied for 120 s in order to dedope the deposits. After removal from the electrodes, rinsing and careful drying, these films were used for krypton gas sorption measurements. Carbazole-based MPN thin films on ITO for the sensing experiments were obtained in a potentiodynamic regime by applying multiple cycles from 0 V to 1.1 V with a scan rate of 0.10 V s−1.
4), respectively, together with 0.1 M TBABF4 as a supporting electrolyte were placed in a three-electrode cell under an argon atmosphere at 25 °C. A platinum disc electrode (Pt, 1 mm diameter) or ITO on glass (∼1.5 × 1.2 cm deposit area) was used as a working electrode (WE); platinum as a counter-electrode (CE); and Ag0/AgNO3 (0.01 M AgNO3/0.1 M TBAP in acetonitrile, 0.56 V vs. NHE) as a reference electrode (RE). Thick films on ITO were produced by applying a static oxidative potential of 1.2 V (TPETCz, TPETPTCz, TPETPOcCz, TPETPTTh, and TPETPOcTh) or 1.4 V (TPETTh) for 20 min. A potential of −1.0 V was applied for 120 s in order to dedope the deposits. After removal from the electrodes, rinsing and careful drying, these films were used for krypton gas sorption measurements. Carbazole-based MPN thin films on ITO for the sensing experiments were obtained in a potentiodynamic regime by applying multiple cycles from 0 V to 1.1 V with a scan rate of 0.10 V s−1.
Acknowledgements
We thank Silvia Adamczyk for the AFM measurements. A. Palma-Cando wishes to thank the German Academic Exchange Service (DAAD) for granting him a PhD scholarship.Notes and references
- Q. Chen, M. Luo, P. Hammershøj, D. Zhou, Y. Han, B. W. Laursen, C.-G. Yan and B.-H. Han, J. Am. Chem. Soc., 2012, 134, 6084–6087 CrossRef CAS PubMed.
- X. Liu, Y. Xu and D. Jiang, J. Am. Chem. Soc., 2012, 134, 8738–8741 CrossRef CAS PubMed.
- Y. Zhang, S. A, Y. Zou, X. Luo, Z. Li, H. Xia, X. Liu and Y. Mu, J. Mater. Chem. A, 2014, 2, 13422–13430 CAS.
- Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012–8031 RSC.
- Q. Chen, D.-P. Liu, M. Luo, L.-J. Feng, Y.-C. Zhao and B.-H. Han, Small, 2014, 10, 308–315 CrossRef CAS PubMed.
- A. Palma-Cando and U. Scherf, Macromol. Chem. Phys., 2016, 217, 827–841 CrossRef CAS.
- A. Ringk, A. Lignie, Y. Hou, H. N. Alshareef and P. M. Beaujuge, ACS Appl. Mater. Interfaces, 2016, 8, 12091–12100 CAS.
- X.-M. Hu, Z. Salmi, M. Lillethorup, E. B. Pedersen, M. Robert, S. U. Pedersen, T. Skrydstrup and K. Daasbjerg, Chem. Commun., 2016, 52, 5864–5867 RSC.
- A. Palma-Cando and U. Scherf, ACS Appl. Mater. Interfaces, 2015, 7, 11127–11133 CAS.
- A. Palma-Cando, E. Preis and U. Scherf, Macromolecules, 2016, 49, 8041–8047 CrossRef CAS.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- A. Qin, J. W. Y. Lam and B. Z. Tang, Prog. Polym. Sci., 2012, 37, 182–209 CrossRef CAS.
- R. Hu, Y. Kang and B. Z. Tang, Polym. J., 2016, 48, 359–370 CrossRef CAS.
- C. Gu, N. Huang, Y. Wu, H. Xu and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 11540–11544 CrossRef CAS PubMed.
- X. Zhang, J. Lu and J. Zhang, Chem. Mater., 2014, 26, 4023–4029 CrossRef CAS.
- B. Wang, J. Zhao, C. Cui, J. Liu and Q. He, Sol. Energy Mater. Sol. Cells, 2012, 98, 161–167 CrossRef CAS.
- S. Yuan, S. Kirklin, B. Dorney, D.-J. Liu and L. Yu, Macromolecules, 2009, 42, 1554–1559 CrossRef CAS.
- S. Qiao, Z. Du and R. Yang, J. Mater. Chem. A, 2014, 2, 1877–1885 CAS.
- E. Preis, W. Dong, G. Brunklaus and U. Scherf, J. Mater. Chem. C, 2015, 3, 1582–1587 RSC.
- Q. Chen, J.-X. Wang, F. Yang, D. Zhou, N. Bian, X.-J. Zhang, C.-G. Yan and B.-H. Han, J. Mater. Chem., 2011, 21, 13554–13560 RSC.
- X. Zhang, Y.-Z. Lv, X.-L. Liu, G.-J. Du, S.-H. Yan, J. Liu and Z. Zhao, RSC Adv., 2016, 6, 76957–76963 RSC.
- F. Jiang, J. Sun, R. Yang, S. Qiao, Z. An, J. Huang, H. Mao, G. Chen and Y. Ren, New J. Chem., 2016, 40, 4969–4973 RSC.
- G. Inzelt, M. Pineri, J. W. Schultze and M. A. Vorotyntsev, Electrochim. Acta, 2000, 45, 2403–2421 CrossRef CAS.
- A. Palma-Cando, G. Brunklaus and U. Scherf, Macromolecules, 2015, 48, 6816–6824 CrossRef CAS.
- B. R. Kaafarani, A. a. O. El-Ballouli, R. Trattnig, A. Fonari, S. Sax, B. Wex, C. Risko, R. S. Khnayzer, S. Barlow, D. Patra, T. V. Timofeeva, E. J. W. List, J.-L. Bredas and S. R. Marder, J. Mater. Chem. C, 2013, 1, 1638–1650 RSC.
- J. Heinze, B. A. Frontana-Uribe and S. Ludwigs, Chem. Rev., 2010, 110, 4724–4771 CrossRef CAS PubMed.
- Z. Zhao, J. W. Y. Lam and B. Z. Tang, Curr. Org. Chem., 2010, 14, 2109–2132 CrossRef CAS.
- S. Baysec, E. Preis, S. Allard and U. Scherf, Macromol. Rapid Commun., 2016, 37, 1802–1806 CrossRef CAS PubMed.
- S. Content, W. C. Trogler and M. J. Sailor, Chem. – Eur. J., 2000, 6, 2205–2213 CrossRef CAS.
- W. Dong, T. Fei, A. Palma-Cando and U. Scherf, Polym. Chem., 2014, 5, 4048–4053 RSC.
- J. Wang, J. Mei, W. Yuan, P. Lu, A. Qin, J. Sun, Y. Ma and B. Z. Tang, J. Mater. Chem., 2011, 21, 4056–4059 RSC.
- A. Räupke, A. Palma-Cando, E. Shkura, P. Teckhausen, A. Polywka, P. Görrn, U. Scherf and T. Riedl, Sci. Rep., 2016, 6, 29118 CrossRef PubMed.
- T. Fei, K. Jiang and T. Zhang, Sens. Actuators, B, 2014, 199, 148–153 CrossRef CAS.
- J. S. Caygill, F. Davis and S. P. J. Higson, Talanta, 2012, 88, 14–29 CrossRef CAS PubMed.
- X. Sun, Y. Wang and Y. Lei, Chem. Soc. Rev., 2015, 44, 8019–8061 RSC.
- S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871–2883 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods; TGA plots and gas sorption isotherms of bulk polymers; cyclic voltammograms of the electropolymerization of monomers on Pt disc electrodes; AFM images of MPN films, including surface roughness and thickness data; and absorption and photoluminescence spectra of monomers and MPN films. See DOI: 10.1039/c6qm00281a |
| This journal is © the Partner Organisations 2017 |