Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluoride binding in water with the use of micellar nanodevices based on salophen complexes†
Flore
Keymeulen
a,
Paolo
De Bernardin
ab,
Ilaria
Giannicchi
b,
Luciano
Galantini
b,
Kristin
Bartik
*a and
Antonella
Dalla Cort
*b
aEngineering of Molecular NanoSystems, Université libre de Bruxelles, 50 avenue F.D. Roosevelt, B-1050 Brussels, Belgium. E-mail: kbartik@ulb.ac.be; Fax: (+32) 2 650 3606; Tel: (+32) 2 650 2063
bDepartment of Chemistry and IMC-CNR, Università La Sapienza, Piazzale Aldo Moro 5, 00185 Roma, Italy. E-mail: antonella.dallacort@uniroma1.it; Fax: (+39) 06 4904 21; Tel: (+39) 06 4991 3087
First published on 11th December 2014
Abstract
The use of micelles to transpose lipophilic receptors, such as uranyl-salophen complexes, into an aqueous environment is a valuable and versatile tool. Receptor 1 incorporated into CTABr micelles forms a supramolecular system that exhibits excellent binding properties towards fluoride in water, despite the competition of the aqueous medium. To fully evaluate the potential of micellar nanodevices, we extended our previous study to other types of surfactants and to a uranyl-salophen receptor with a more extended aromatic surface. Paramagnetic relaxation enhancement experiments were used to obtain information on the location of the two receptors within the micelles and complementary information was obtained from dynamic light scattering experiments. With these data it is possible to account for the key factors necessary to obtain an efficient supramolecular device for anion binding in water.
Introduction
Anion recognition by artificial receptors that operate in aqueous solution is a field that has been growing exponentially in the last few decades due to the biological and environmental significance of these species. Fluoride can be considered as a biologically important anion but its excessive intake has been linked to health issues, such as dental and skeletal fluorosis.1,2 The detection of fluoride in water is consequently a valuable target to pursue despite the intrinsic difficulty in doing so due to the fact that fluoride is a small but a highly hydrated anion.Several neutral receptors which are able to recognize fluoride have been designed and studied in organic solvents.3,4 Many of them rely on hydrogen bonding5,6 or on a Lewis acid–base type interaction.7–10 Important colorimetric changes, enabling easy sensing, are obtained when deprotonation by fluoride11 or intramolecular charge transfer occurs.12 Uranyl-salophen complexes which rely on a Lewis acid–base interaction (Fig. 1, Receptors 1 and 2) are known to selectively and efficiently bind fluoride in organic solvents8–10,13–15 and furthermore, if properly functionalized with hydrophilic substituents, can partly maintain this affinity in water.16,17 The use and advantages of these metal complexes have been discussed and reviewed in the literature.9
We have recently reported that Receptor 1, which is easily synthesized through the one-pot reaction of 1,2-phenylenediamine with two moles of salicylaldehyde in the presence of uranyl acetate,18 can be transposed into water with the help of CTABr (cetyltrimethylammonium bromide) micelles.19,20 Remarkably the supramolecular receptor/micelle assembly exhibits an affinity for fluoride of the order of 104 M−1 which is one of the highest values ever reported for fluoride binding by a neutral receptor in water and, very interestingly, higher than the one observed with the hydro-soluble version of Receptor 1.16 The selectivity observed in organic media10 is furthermore maintained versus sulphate, acetate, nitrate, phosphate and other halides.19 Using micelles avoids the time-consuming synthetic procedures needed to obtain water soluble receptors and the potential of this strategy has already been exploited by a few groups interested in the binding or sensing of different analytes in water using a variety of receptors.19,21–32
With the aim to better understand these supramolecular systems, we investigated a number of factors that can influence their binding efficiency. We report here the study carried out with Receptor 1 and with Receptor 2, which is characterized by a more extended aromatic surface, in the presence of four different surfactants which are suitable for anion recognition (CTABr; cetyltrimethylammonium chloride, CTACl; dodecylphosphocholine, DPC; p-tert-octylphenol-polyoxyethylene ether, Triton X-100; Fig. 1). Both of these receptors are not soluble in water. The affinity of the systems for fluoride was investigated by UV-vis or NMR titrations and their structural characteristics were probed via NMR Paramagnetic Relaxation Enhancement (PRE) experiments, with the aim of correlating binding efficiency to the location of the receptors in the micelles. DLS experiments were also undertaken.
Results and discussion
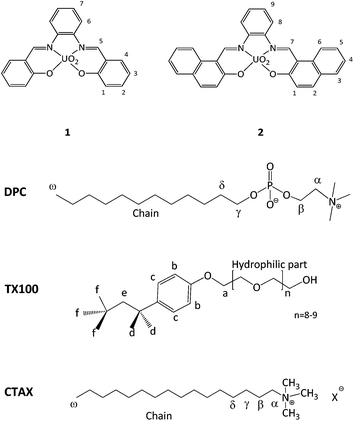
Receptors 1 and 2 were first studied in CH2Cl2 and it was observed that they bind fluoride with affinity constants of the order of 106 M−1 (see ESI†). In order to evaluate their binding capacities in water, they were solubilized in CTABr, CTACl and Triton X-100 micelles (50 mM surfactant concentration) and in DPC micelles (20 mM surfactant concentration). These concentrations are all well above the cmc of the surfactants and are comparable to those reported in previous studies.19,20,33Fluoride binding was monitored by UV-vis titrations for the receptors incorporated into the CTABr and CTACl micelles and Fig. 2 reports the data for the Receptor 2/CTABr system. The changes in the spectra triggered by the addition of increasing amounts of KF were sufficient to allow a precise assessment of the binding affinity. This was not the case for the receptors incorporated into Triton X-100 and DPC micelles and, for these systems, 1H NMR was used to monitor the recognition process. Fig. 3 shows the spectra recorded for Receptor 2 in Triton X-100 (see ESI† for Receptor 1 in Triton X-100). Fluoride binding data for the two receptors in the four environments are reported in Table 1.
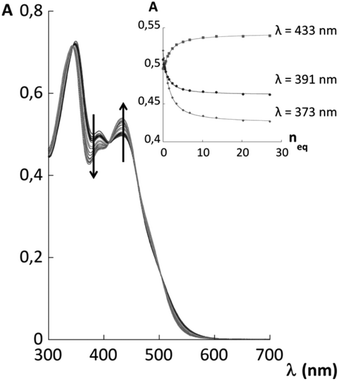 | ||
Fig. 2 UV-vis titration of a 5.05 × 10−5 M solution of Receptor 2 in 50 mM CTABr with KF and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherms with parametric adjustment. 1 binding isotherms with parametric adjustment. | ||
 | ||
| Fig. 3 1H NMR spectrum of a 1.5 mM solution of Receptor 2 in 50 mM Triton X-100. Receptor signals are amplified and shown for different additions of KF. (●) Surfactant signals. The assignment of the receptor signals was made by comparison with the spectrum of the receptor in CTAX micelles. For proton labelling see Fig. 1. | ||
As previously observed, Receptor 1 in CTABr micelles shows a high affinity for fluoride despite the predictable strong competition of the surfactant counterion, Br−, and of the medium.19 Receptor 2 shows the same behaviour and the binding constant is even slightly higher. Similar results were obtained when the two receptors were incorporated into CTACl micelles. The affinity for fluoride however drops drastically when the receptors are incorporated into Triton X-100 or DPC micelles.
The data reported in Table 1 were not easy to rationalize. For example we would have expected, since chloride is more competitive than bromide, to obtain much lower apparent binding constants with CTACl micelles than the ones determined.
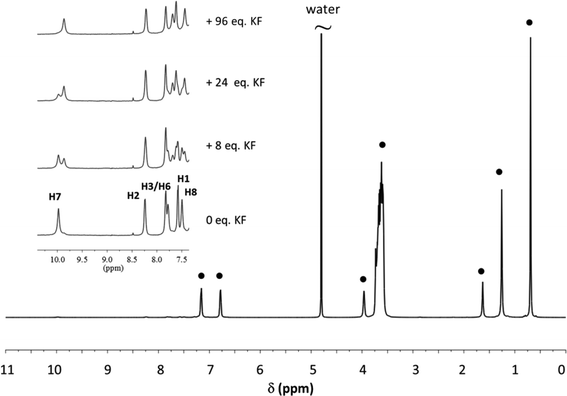
To evaluate how the location of the receptor within the micelle influences the affinity of the systems for fluoride, the positioning of the receptors was monitored by NMR Paramagnetic Relaxation Enhancement (PRE) experiments. Several studies reported in the literature have shown that these experiments are particularly well suited to obtain information on the localisation and orientation of (bio)molecules inside micelles.19,20,34–36 Precise distance information can be obtained if a calibration procedure is first undertaken.34,37 As we recently showed it is however possible to obtain reliable information on the localisation and orientation of receptors in micelles by simply comparing the PRE data obtained for the receptor protons with those of the surfactant protons of the micelles.19,20 This protocol was applied in this study and for each receptor/micelle system, the longitudinal relaxation rate of the different receptor and surfactant protons were measured in the presence of increasing amounts of the paramagnetic species (K3[Cr(CN)6]). When plotting the relaxation rate enhancement (Δ1/T1) as a function of paramagnetic species concentration, a linear relationship was obtained and the slope, known as the relaxivity (ϕ), was extracted. The normalized relaxivities (value relative to the one obtained for the head protons of the surfactant) of the receptor and surfactant protons of all the systems studied are reported in the ESI.† A careful comparison of these normalized relaxivity values has enabled us to localise the receptors in the micelles and Fig. 4 gives a schematic representation of the results obtained for receptors in the different micelles. The PRE data for superimposed signals must be interpreted with care as the relaxivity of such signals is dominated by the relaxivity of the proton which is least affected by the paramagnetic species (see ESI†).
 | ||
| Fig. 4 Schematic representation of the localisation of the receptor within the different types of micelles. | ||
In DPC micelles, the PRE measurements clearly show that Receptor 1 is located next to the surfactant's phosphate group and this most certainly explains the extremely small apparent affinity constant of the system for fluoride. It is indeed known that phosphate binds with a good affinity to uranyl-salophens.10 In Triton-X-100 micelles, the PRE experiments show that the receptors are located in the highly hydrated palisade layer. They are consequently not protected from the aqueous environment and the higher competition with water will contribute to the poor binding capacity of the receptors.
As far as the CTAX micelles are concerned, the PRE data show that the receptors are essentially protected from the aqueous environment. Receptor 1 is located close to the surface of the micelles with its binding site oriented towards the outside. In the CTABr micelles, the proton next to the guest coordination site of the receptor (proton 1) has a relaxivity value which is even larger than the relaxivity of the surfactant head protons (normalized relaxivity > 1), suggesting that it is protruding into the Stern layer. Receptor 2 is more deeply buried in the hydrophobic core than Receptor 1, in both types of micelles, and this can be easily explained by its more extended lipophilic structure. This difference has a small beneficial impact on fluoride recognition which could be explained by the fact that Receptor 2 is better protected from the aqueous environment.
In order to verify if fluoride binding influences the localisation of the receptor within the micelle, PRE measurements were also undertaken with Receptors 1 and 2 in CTACl and CTABr micelles in the presence of 4 equivalents of KF (the receptors are saturated under these conditions). These PRE values were compared to those obtained in the absence of fluoride (Fig. 5). In the case of Receptor 2, signals of protons 1 and 8 are superimposed in the 1H NMR spectrum and the normalized relaxivity of proton 1 is without doubt higher than the values obtained for this signal (see ESI†).
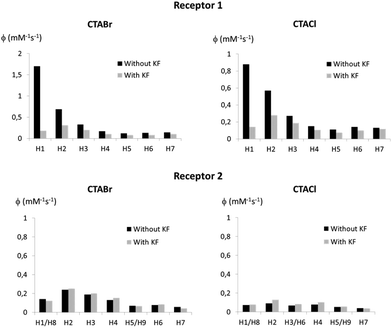 | ||
| Fig. 5 Normalized relaxivity values for the protons of Receptors 1 (top) and 2 (bottom) solubilized in 50 mM CTABr micelles (left) and 50 mM CTACl micelles (right) before and after addition of 4 equivalents of KF. All normalized PRE are given in the ESI.† | ||
The results shown in Fig. 5 indicate that Receptor 1, which is located at the very interface of the micelles in the absence of bound fluoride, buries itself into the micelle upon fluoride binding, both in the case of CTABr and CTACl micelles. Indeed, the relaxivities of the H1 and H2 protons of the receptor drop significantly and have values which are comparable to those of the surfactant chain protons. The receptor becomes negatively charged upon anion binding and thus appears to position itself so as to put its charge next to the positively charged polar head of the surfactant molecules. The burying of a charged species in CTABr micelles has already been described in the literature.38,39 It has been reported that phenol, which is located at the micelle surface when protonated, buries itself into CTABr micelles when it is deprotonated, driven by the favourable electrostatic interactions between the negatively charged phenolate ions and the positive charge of the cationic surfactant head group. As far as Receptor 2 is concerned, the PRE experiments show that its location is not markedly affected by complexation with fluoride and this can be explained by the fact that its lipophilicity keeps it in the internal core where it was already hosted before the addition of fluoride.
To gain more insight into the structure of the particularly efficient CTAX/receptor systems, dynamic light scattering (DLS) measurements were undertaken on 50 mM CTABr and CTACl solutions in the presence of increasing concentrations of Receptor 1 or 2. KCl or KBr was added to the solutions so as to minimize the electrostatic interaction effects on the measured apparent diffusion coefficients.40,41 The derived hydrodynamic radii are plotted as a function of receptor concentration in Fig. 6 (see tables in ESI†). The results clearly show that CTABr micelles swell when the receptors are incorporated whereas no significant size variation is observed in the case of CTACl. The observed different behaviours of these two surfactants is not astonishing since it is known that the nature of the counter ions plays a crucial role in the structural features of cationic micelles and controls micellar growth when hydrophobic compounds, such as benzene, are incorporated.42–47 Chloride counterions, which are more hydrated and consequently bulkier, are described to place themselves in front of the head groups while bromide counterions are reported to interact specifically with the positively charged head groups of the CTA surfactant molecules and to locate themselves between these head groups.42,46,47 In the case of CTABr micelles, the higher affinity observed could therefore be due not only to the fact that the counterion is less competitive than in the case of the CTACl micelles, but also because the swelling of the micelles makes the receptors more accessible while still protecting the binding site from the aqueous environment.
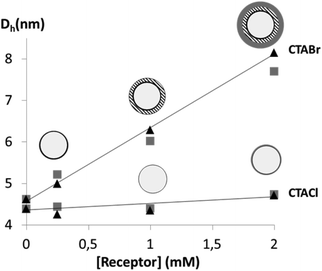 | ||
Fig. 6 Hydrodynamic diameters of CTABr and CTACl micelles upon incorporation of different concentrations of Receptor 1 ( ) or 2 (▲). ) or 2 (▲). | ||
Conclusions
In summary, the results obtained in this study clearly show that the use of micelles to transpose lipophilic receptors, such as uranyl-salophen complexes, into an aqueous environment is a valuable and versatile tool. The surfactant must however be chosen with care.In relation to the receptor/micelle nanodevices based on uranyl-salophen complexes and their ability to bind fluoride in water, the best systems are those obtained with CTABr and CTACl. The assemblies that are formed behave as supramolecular systems increasing the receptor affinity towards fluoride despite the competitiveness of the medium. The two receptors exhibit a very similar affinity for fluoride, despite the different lipophilicity that affects their location inside the micelle, as shown via PRE experiments. Receptor 2 is more deeply buried inside the micelle compared to Receptor 1 which is at the very interface. Recognition of fluoride by the metal centre transforms the complexes from neutral to negatively charged. Receptor 1 after fluoride binding buries itself closer to the cationic head, driven perhaps by the possibility of establishing a favourable electrostatic interaction. No significant difference in the location of the more lipophilic Receptor 2, before and after fluoride binding, is observed. DLS measurements established that the inclusion of the receptors leads to swelling of the CTABr, but not the CTACl micelles, making the receptors more accessible to the external environment. This could perhaps explain, in conjunction with the fact that bromide is a less competitive counterion, that the fluoride binding is slightly more efficient within the CTABr nanodevices.
The work reported can be seen as a contribution to the development of simple and efficient supramolecular devices to bind analytes in water using lipophilic receptors.
Experimental section
Chemicals
Triton X-100, KF, TBAF·3H2O, KBr, KCl, and K3[Cr(CN)6] were purchased from Aldrich and used without further purification. CTABr and CTACl were purchased from Aldrich and were purified following a protocol detailed in the literature.48 DPC was purchased from Avanti Lipids and used without further purification. D2O was purchased from Aldrich. Receptors 1 and 2 were available from previous investigations.49,50Solutions of surfactants were prepared by dissolving the surfactant in H2O, for UV-vis spectroscopy, or D2O, for NMR experiments. For the DLS experiments, surfactant solutions were prepared by dissolving the surfactant in 100 mM KCl solutions in H2O for CTACl and in 50 mM KBr solutions for CTABr. The micelle–receptor solutions were prepared by adding a weighed amount (to obtain mM concentrations) of the receptor to a 50 mM of CTABr or CTACl or Triton X-100 solution or to a 20 mM DPC solution. The solution was then stirred for 30 min. For the UV-experiments the solutions were diluted with surfactant solutions.
With mM receptor concentrations, considering the aggregation number of the different surfactants, it can be expected that 2–3 receptors are incorporated per micelle.
UV-Vis spectroscopy
UV-vis spectra were recorded on a Perkin Elmer UV-vis spectrophotometer Lambda 40 using a quartz cell with an optical path length of 1 cm. Spectra were recorded at 30 °C with the micellar solutions and at room temperature for the organic solutions. Solutions with a receptor concentration of around 10−5 M were titrated with aliquots of a TBAF solution for the titration in CH2Cl2 or with KF in the case of aqueous solutions. The reported spectra were corrected for dilution. To determine the binding constants the experimental points were fitted using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm (Benesi–Hildebrand equation).
1 binding isotherm (Benesi–Hildebrand equation).
NMR spectroscopy
All the NMR experiments were performed on a Varian spectrometer operating at 14.1 T (599.9 MHz for 1H) at 30 °C for CTAX solutions and at 25 °C for DPC and Triton X-100 solution. 500 μl of different micelle solutions were placed in the NMR tube.For the titration experiments, aliquots of freshly prepared concentrated solutions of potassium fluoride in water in the presence of 50 mM CTAX/Triton X-100 or 20 mM DPC were added directly into the NMR tube. The change in receptor concentration was <10%. Binding constants were obtained via integration (following deconvolution if needed) of the H7 (Receptor 2) or H5 (Receptor 1) protons.
For the PRE experiments, aliquots of 5 μl of a concentrated D2O solution of K3[Cr(CN)6] (2.7 mM for cationic micelles and 210 mM for DPC and Triton X-100) were added. T1 measurements were undertaken using the classical inversion recovery (180-τ-90-acquisition) sequence, with 16 points and the delay varying between 0 and 6 s. The delays were adapted according to the amount of added chromium salt. Data treatment was performed using the Varian VNMRJ software. To obtain the T1 values, eqn (1) was adjusted to the values of the signal integrals:
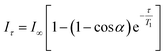 | (1) |
For each signal monitored, the increase in the longitudinal relaxation rate induced by the paramagnetic species (Δ1/T1; the difference between the longitudinal relaxation rate measured in the presence and in the absence of the paramagnetic species) was plotted as a function of the concentration of paramagnetic species. A linear regression was undertaken with the experimental data points from which the relaxivity value was derived (slope of the line).
DLS
DLS experiments were undertaken at 30 °C on a Malvern NanoZetasizer apparatus, equipped with a 4 mW HeNe laser source (632.8 nm). In this apparatus, the light scattered by the sample, placed in a thermostated cell-holder, is collected at an angle of 173°. Unimodal intensity weighted distributions of the hydrodynamic diameters were obtained by analysing the measured autocorrelation functions using the CONTIN algorithm. The values corresponding to the peaks were considered as apparent hydrodynamic diameters Dh. Averaged values of five measurements were reported, and the repeated measurements were reproducible within ±5%.Acknowledgements
The authors acknowledge the European Cooperation in Science and Technology (COST Action “Supramolecular Chemistry in Water”, CM1005). A.D.C. and L.G. acknowledge “Ricerca scientifica di Ateneo 2013” and MIUR “PRIN 2010CX2TLM”. F.K. thanks the “Fonds de la Recherche Scientifique” (Belgium) for her FRIA doctoral grant. P.D.B. thanks the Fonds Van Buuren and the Fonds de Meurs-François for their contribution to his PhD financing.References
- Guidelines for Drinking-water, Fourth Edition Quality,World Health Organization, http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/, 2011 Search PubMed.
- J. Fawell, K. Bailey, J. Chilton, E. Dahi, L. Fewtrell and Y. Magara, Fluoride in drinking water, World Health Organisation, http://www.who.int/water_sanitation_health/publications/fluoride_drinking_water/en/, 2006 Search PubMed.
- M. Cametti and K. Rissanen, Chem. Commun., 2009, 2809–2829 RSC.
- M. Cametti and K. Rissanen, Chem. Soc. Rev., 2013, 42, 2016–2038 RSC.
- V. Amendola, L. Fabbrizzi, L. Mosca and F.-P. Schmidtchen, Chem. – Eur. J., 2011, 17, 5972–5981 CrossRef CAS PubMed.
- R. Pérez-Ruiz, Y. Díaz, B. Goldfuss, D. Hertel, K. Meerholz and A. G. Griesbeck, Org. Biomol. Chem., 2009, 7, 3499–3504 Search PubMed.
- C. R. Wade, A. E. J. Broomsgrove, S. Aldridge and F. P. Gabbaï, Chem. Rev., 2010, 110, 3958–3984 CrossRef CAS PubMed.
- M. Cametti, M. Nissinen, A. Dalla Cort, L. Mandolini and K. Rissanen, J. Am. Chem. Soc., 2005, 127, 3831–3837 CrossRef CAS PubMed.
- A. Dalla Cort, P. De Bernardin, G. Forte and F. Y. Mihan, Chem. Soc. Rev., 2010, 39, 3863–3874 RSC.
- M. Cametti, A. Dalla Cort, L. Mandolini, M. Nissinen and K. Rissanen, New J. Chem., 2008, 32, 1113–1116 RSC.
- M. Boiocchi, L. Del Boca, D. E. Gómez, L. Fabbrizzi, M. Licchelli and E. Monzani, J. Am. Chem. Soc., 2004, 126, 16507–16514 CrossRef CAS PubMed.
- N. Kumari, S. Jha and S. Bhattacharya, J. Org. Chem., 2011, 76, 8215–8222 CrossRef CAS PubMed.
- M. Cametti, M. Nissinen, A. Dalla Cort, L. Mandolini and K. Rissanen, Chem. Commun., 2003, 2420–2421 RSC.
- S. Le Gac, M. Luhmer, O. Reinaud and I. Jabin, Tetrahedron, 2007, 63, 10721–10730 CrossRef CAS PubMed.
- D. M. Rudkevich, W. P. R. V. Stauthamer, W. Verboom, J. F. J. Engbersen, S. Harkema and D. N. Reinhoudt, J. Am. Chem. Soc., 1992, 114, 9671–9673 CrossRef CAS.
- A. Dalla Cort, G. Forte and L. Schiaffino, J. Org. Chem., 2011, 76, 7569–7572 CrossRef CAS PubMed.
- E. Bedini, G. Forte, C. De Castro, M. Parrilli and A. Dalla Cort, J. Org. Chem., 2013, 78, 7962–7969 CrossRef CAS PubMed.
- M. M. G. Antonisse, B. H. M. Snellink-Ruël, I. Yigit, J. F. J. Engbersen and D. N. Reinhoudt, J. Org. Chem., 1997, 62, 9034–9038 CrossRef CAS.
- M. Cametti, A. Dalla Cort and K. Bartik, ChemPhysChem, 2008, 9, 2168–2171 CrossRef CAS PubMed.
- F. Keymeulen, P. De Bernardin, A. Dalla Cort and K. Bartik, J. Phys. Chem. B, 2013, 117, 11654–11659 CrossRef CAS PubMed.
- A. Dorazco-Gonzalez, Organometallics, 2014, 33, 868–875 CrossRef CAS.
- S. Elsayed, A. Agostini, L. E. Santos-Figueroa, R. Martínez-Máñez and F. Sancenón, ChemistryOpen Commun., 2013, 2, 58–62 CrossRef CAS PubMed.
- R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 122, 5035–5038 CrossRef.
- A. Agostini, I. Campos, M. Milani, S. Elsayed, L. Pascual, R. Martínez-Máñez, M. Licchelli and F. Sancenón, Org. Biomol. Chem., 2014, 12, 1871–1874 CAS.
- T. Riis-Johannessen and K. Severin, Chem. – Eur. J., 2010, 16, 8291–8295 CrossRef CAS PubMed.
- T. Riis-Johannessen, K. Schenk and K. Severin, Inorg. Chem., 2010, 49, 9546–9553 CrossRef CAS PubMed.
- M. P. Schramm, R. J. Hooley and J. J. Rebek, J. Am. Chem. Soc., 2007, 129, 9773–9779 CrossRef CAS PubMed.
- O. Jurček, M. Cametti, M. Pontini, E. Kolehmainen and K. Rissanen, Org. Biomol. Chem., 2013, 11, 4585–4590 Search PubMed.
- N. Kumari, S. Jha and S. Bhattacharya, Chem. – Asian J., 2014, 9, 830–837 CrossRef CAS PubMed.
- S. Bhattacharya and A. Gulyani, Chem. Commun., 2003, 1158–1159 RSC.
- N. Dey, S. K. Samanta and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 8394–8400 CAS.
- N. Kumari, N. Dey, S. Jha and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 2438–2445 CAS.
- S. Javor and J. J. Rebek, J. Am. Chem. Soc., 2011, 133, 17473–17478 CrossRef CAS PubMed.
- K. Zangger, M. Respondek, C. Göbl, W. Hohlweg, K. Rasmussen, G. Grampp and T. Madl, J. Phys. Chem. B, 2009, 113, 4400–4406 CrossRef CAS PubMed.
- S. Kosol, E. Schrank, M. Bukvić-Krajačić, G. E. Wagner, N. H. Meyer, C. Göbl, G. N. Rechberger, K. Zangger and P. Novak, J. Med. Chem., 2012, 55, 5632–5636 CrossRef CAS PubMed.
- M. Lindberg and A. Gräslund, FEBS Lett., 2001, 497, 39–44 CrossRef CAS.
- M. Franzmann, D. Otzen and R. Wimmer, ChemBioChem, 2009, 10, 2339–2347 CrossRef CAS PubMed.
- C. A. Bunton and C. P. Cowelli, J. Colloid Interface Sci., 1988, 122, 154–162 CrossRef CAS.
- P. Sabatino, A. Szczygiel, D. Sinnaeve, M. Hakimhashemi, H. Saveyn, J. C. Martins and P. Van der Meeren, Colloids Surf., A, 2010, 370, 42–48 CrossRef CAS PubMed.
- R. B. Dorshow, J. Briggs, C. A. Bunton and D. F. Nicoli, J. Phys. Chem., 1982, 86, 2388–2395 CrossRef CAS.
- L. Galantini, S. M. Giampaolo, L. Mannina, N. V. Pavel and S. Viel, J. Phys. Chem. B, 2004, 108, 4799–4805 CrossRef CAS.
- G. Cerichelli and G. Mancini, Langmuir, 2000, 16, 182–187 CrossRef CAS.
- J. V. Joshi, V. K. Aswal and P. S. Goyal, J. Macromol. Sci. Phys., 2008, 47, 338–347 CrossRef CAS.
- V. K. Aswal and P. S. Goyal, Chem. Phys. Lett., 2002, 364, 44–50 CrossRef CAS.
- V. K. Aswal and P. S. Goyal, Chem. Phys. Lett., 2003, 368, 59–65 CrossRef CAS.
- N. Hedin, R. Sitnikov, I. Furó, U. Henriksson and O. Regev, J. Phys. Chem. B, 1999, 103, 9631–9639 CrossRef CAS.
- U. Henriksson, L. Odberg, J. C. Eriksson and L. Westman, J. Phys. Chem., 1977, 87, 76–82 CrossRef.
- E. F. J. Duynstee and E. Grunwald, J. Am. Chem. Soc., 1959, 81, 4540–4542 CrossRef CAS.
- M. Antonisse and D. Reinhoudt, Chem. Commun., 1998, 443–448 RSC.
- M. Goursaud, P. De Bernardin, A. Dalla Cort, K. Bartik and G. Bruylants, Eur. J. Org. Chem., 2012, 3570–3574 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ob02298j |
| This journal is © The Royal Society of Chemistry 2015 |