 Open Access Article
Open Access ArticleA triosmium carbonyl cluster that inhibits α-synuclein aggregation and disassembles preformed aggregates†
Xin
Liang
a,
Balasz
Gulyas
b,
Mathangi
Palanivel
 b and
Weng Kee
Leong
b and
Weng Kee
Leong
 *a
*a
aDivision of Chemistry and Biological Chemistry, School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore 637371, Singapore. E-mail: chmlwk@ntu.edu.sg
bLee Kong Chian School of Medicine, Nanyang Technological University, 59 Nanyang Drive, Singapore 636921, Singapore
First published on 17th April 2025
Abstract
Two triosmium carbonyl clusters, viz., Os3(μ-H)(μ-SC6H4-p-NO2)(CO)10 (1) and Os3(μ-H)(kO,μ-O′-2-flavone)(CO)9 (2), effectively inhibited α-synuclein aggregation, a key signature of Parkinson's disease (PD), in both wild-type and A53T-mutant α-synuclein models. Cluster 2 showed superior efficacy and a significantly better safety profile, and could also disassemble preformed aggregates.
Parkinson's disease (PD) is a progressive neurodegenerative disorder that can affect motor functions, leading to life-threatening complications such as pneumonia in its advanced stages.1–3 Diagnosis is typically based on motor dysfunction and tremors, which may not be prominent in the early stages, making it challenging for individuals with non-motor symptoms. There is currently no treatment available to reverse progression; hence, early intervention strategies for halting or reversing advanced PD are highly desirable.4–11 One biochemical hallmark of PD is the abnormal aggregation of α-synuclein to form toxic inclusions known as Lewy bodies.12–18 Targeting the aggregation of α-synuclein or promoting the disassembly of its aggregates are among the promising therapeutic approaches.14–16 One strategy to modulate α-synuclein aggregation is through small molecule inhibitors and some, such as Anle138b and NPT200-11, have reached clinical trials (Fig. 1).17–23 Their ring systems appear to be essential as studies have indicated that even compounds with a single ring can effectively inhibit α-synuclein aggregation;24 for example, ZPDm has been shown to both inhibit α-synuclein aggregation and disassemble preformed fibrils.25
Metal-based compounds have found potential in biomedicine, including the ruthenium complex NAMI-A, which has also been shown to exhibit potential as a therapeutic agent for PD.26–46 The larger ligand sphere in di- and multi-nuclear complexes may be expected to offer greater potential for incorporating multi-target or -drug capabilities; their larger molecular size may also help reduce protein–protein interactions and aggregation. In this paper, we report on our investigations into the potential of two triosmium carbonyl clusters, viz., Os3(μ-H)(μ-SC6H4-p-NO2)(CO)10 (1) and Os3(μ-H)(kO,μ-O′-2-flavone)(CO)9 (2), for PD treatment (Scheme 1).
In our previous SAR studies, we have found that the cytotoxicity of triosmium carbonyl clusters such as 1 is associated with the formation of a vacant site on the triosmium core for binding of a biomolecule; factors that affect this would impact cytotoxicity.47 We conjectured that replacement of the bridging moiety (O for S) and of the aromatic ring with a larger ring system may further reduce cytotoxicity; the latter should also strengthen interaction with α-synuclein. Both clusters have been fully characterized spectroscopically (IR, NMR, HRMS) and by single-crystal X-ray crystallography. The structure of 1 has already been reported;47 an ORTEP plot for 2 is shown in Fig. 2. The pattern for the CO vibrations in the IR spectrum and the metal hydride resonance at −9.27 ppm in the 1H NMR spectrum of cluster 2 are consistent with those reported for triosmium carbonyl clusters containing hydroxypyrone.48 Its UV absorption spectrum exhibits bands typical of flavonoids, with absorption maxima in the 240–270 nm and 320–380 nm ranges, along with an additional band at 520 nm (Fig. S3, ESI†),49–52 while the emission spectrum shows a single emission peak at ∼540 nm under 350 nm excitation, which is attributable to a charge transfer (CT) band (Fig. S4, ESI†); the color of the solution is solvent-dependent, changing from yellow-orange in DCM to dark red in DMSO.
MTT assays carried out on clusters 1 and 2 against the SH-SY5Y cell line (human-derived neuroblastoma) commonly used in Parkinson's disease (PD) studies showed that 2 was significantly less cytotoxic than 1; no signs of cytotoxicity were observed up to at least 50 μM for the former, while an IC50 of 28 ± 6 μM was determined for the latter (Fig. S5, ESI†). The latter IC50 value is also worth comparing with a value of 72 ± 8 μM against triple negative breast carcinoma (MDA-MB-231) under the same treatment conditions.42
The inhibitory efficacy of 1 and 2 on wild-type α-synuclein aggregation (the form most commonly associated with PD) was assessed through a thioflavin T (ThT) fluorescence assay, with curcumin, a well-established compound for preventing aggregation in α-synuclein, as a positive control.53–57 The results showed that the inhibitory effect of both clusters was immediate and sustained, and superior to that of curcumin (Fig. 3 and Fig. S8, ESI†). Inhibition of aggregation was also visible in the transmission electron microscopy (TEM) images, which showed that the treated samples exhibited remarkably fewer aggregates, with smaller and shorter structures, compared to the untreated sample (negative control) which showed amyloid fibrils as long, twisted filamentous structures approximately 7–10 nm in diameter (Fig. S6, ESI†). The inhibitory effects were also concentration-dependent, with 2 exhibiting slightly higher efficacy; an optimal inhibitory concentration of 10–20 μM reaching ∼92% inhibition at 20 μM, was obtained, as compared to 20–40 μM for 1 (Fig. S7, ESI†). This optimal inhibitory concentration of 2 was well below its cytotoxicity range. The fact that the inhibitory efficacy of 2 was significantly greater than that for free 3HF also suggested that the cluster core played a role in the inhibition of aggregation.
The inhibitory effect of 2 on A53T mutant α-synuclein aggregation was also examined; this mutation is linked to genetically inherited PD and is characterized by the formation of toxic fibrils at a much faster rate than wild-type α-synuclein.58–60 The results were surprisingly promising; while the optimal inhibitory concentration remained the same as for the wild type (10–20 μM), it demonstrated greater efficacy at lower concentrations (Fig. 4). Notably, at 5 μM concentration, there was a significant drop in inhibition compared to the wild type (∼50% and 26%, respectively). Thus, 2 is effective in inhibiting aggregation of both wild-type and A53T mutant α-synuclein.
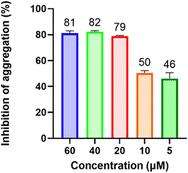 | ||
| Fig. 4 Inhibition of A53T mutant α-synuclein aggregation after treatment with varying concentrations of 2. | ||
The in vivo inhibition of α-synuclein aggregation was also evaluated using an immunofluorescence assay (IFA) on SH-SY5Y cells overexpressing A53T-mutated α-synuclein, with aggregation induced by rotenone, a widely used environmental toxicant in Parkinson's disease research to mimic neurodegenerative conditions.61–64 The results show that cells treated with 1 or 2 exhibited negligible aggregate formation compared to the negative control (Fig. 5).
The disassembly of preformed fibrils is a desirable outcome for reversing the effects of Parkinson's disease (PD) and is particularly crucial in late-stage PD. We have found that 2 could effectively disassemble preformed fibrils, including those formed by A53T mutant α-synuclein. This may be attributable to the presence of the aromatic ring system, which facilitates π–π interactions with aromatic residues in α-synuclein fibrils, such as tyrosine, phenylalanine, and tryptophan.65 Additionally, the bulky nature of the triosmium core introduces steric strain and hydrophobic interactions, leading to local structural perturbations and increasing the susceptibility of aggregates to disassemble.66 This is supported by the observation that while 20 μM of 3HF resulted in only 10% disassembly, it was 33% disassembly with 2 even at 5 μM concentration (Fig. 6). Our findings thus suggest that the triosmium cluster core plays a critical role in disrupting the stabilizing forces within the fibrils, effectively promoting their breakdown.
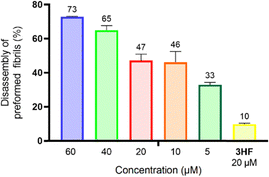 | ||
| Fig. 6 Disassembly of preformed A53T mutant α-synuclein fibrils following treatment with varying concentrations of 2, with 3HF (20 μM) as a positive control. | ||
In this study, we have shown that the triosmium carbonyl clusters 1 and 2 could inhibit the aggregation of α-synuclein, with 2 also capable of disassembling preformed aggregates. This effect was observed in both wild-type and A53T mutant α-synucleins. Notably, 2 exhibited optimal activity at concentrations well below that at which any antiproliferative effects could be observed. We propose that the metal cluster core plays an important role in its activity and while the precise mode of action remains unclear, these findings suggest that compounds of this type hold great potential for further development.
Funding support from the Ministry of Education, Singapore, through the university research grants RG12/20 and RG1/21 is gratefully acknowledged.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 2 has been deposited with the Cambridge Crystallographic Data Centre as CCDC 2426212.†Conflicts of interest
We declare that there are no conflicts of interest.References
- J. Jankovic and E. K. Tan, J. Neurol., Neurosurg. Psychiatry, 2020, 91, 795 CrossRef.
- D. Aarsland, L. Batzu, G. M. Halliday, G. J. Geurtsen, C. Ballard, K. Ray Chaudhuri and D. Weintraub, Nat. Rev. Dis. Primers, 2021, 7, 47 CrossRef PubMed.
- G. DeMaagd and A. Philip, Pharm. Ther., 2015, 40, 504 Search PubMed.
- C. Kobylecki, Clin. Med., 2020, 20, 393 CrossRef.
- J. Fujikawa, R. Morigaki, N. Yamamoto, H. Nakanishi, T. Oda, Y. Izumi and Y. Takagi, Life, 2023, 13, 78 CrossRef.
- X. Dong-Chen, C. Yong, X. Yang, S. Chen-Yu and P. Li-Hua, Signal Transduction Targeted Ther., 2023, 8, 73 CrossRef PubMed.
- R. N. Rees, A. P. Acharya, A. Schrag and A. J. Noyce, F1000Res, 2018, 7, 1106 Search PubMed.
- V. Carroll, R. Rossiter and D. Blanchard, Aust. J. Gen. Pract., 2021, 50, 812 CrossRef PubMed.
- R. Armañanzas, C. Bielza, K. R. Chaudhuri, P. Martinez-Martin and P. Larrañaga, Artif. Intell. Med., 2013, 58, 195 CrossRef PubMed.
- T. Pardo-Moreno, V. García-Morales, S. Suleiman-Martos, A. Rivas-Domínguez, H. Mohamed-Mohamed, J. J. Ramos-Rodríguez, L. Melguizo-Rodríguez and A. González-Acedo, Pharmaceutics, 2023, 15, 770 CrossRef CAS PubMed.
- Y. Wu, X. Meng, W.-Y. Cheng, Z. Yan, K. Li, J. Wang, T. Jiang, F. Zhou, K.-H. Wong and C. Zhong, et al. , Front. Neurosci., 2024, 18, 1210447 CrossRef PubMed.
- M. Sharma and J. Burré, Trends Neurosci., 2023, 46, 153 CrossRef CAS PubMed.
- J. Burré, J. Parkinsons Dis., 2015, 5, 699 Search PubMed.
- D. Baggett, A. Olson and M. S. Parmar, Brain Disord., 2024, 16, 100163 CrossRef CAS.
- C. R. Fields, N. Bengoa-Vergniory and R. Wade-Martins, Front. Mol. Neurosci., 2019, 12, 299 CrossRef CAS.
- M. Vidović and M. G. Rikalovic, Cells, 2022, 11, 1732 CrossRef.
- G. Henriquez and M. Narayan, Explor. Neuroprotect. Ther., 2023, 3, 207 Search PubMed.
- I. M. Sandoval, D. J. Marmion, K. T. Meyers and F. P. Manfredsson, J. Parkinsons Dis., 2021, 11, S189–S197 CAS.
- M. Izco, J. Blesa, M. Schleef, M. Schmeer, R. Porcari, R. Al-Shawi, S. Ellmerich, M. de Toro, C. Gardiner and Y. Seow, et al. , Mol. Ther., 2019, 27, 2111 Search PubMed.
- J. S. Lee and S. J. Lee, J. Mov. Disord., 2016, 9, 14 CrossRef PubMed.
- B. Xiao and E.-K. Tan, J. Transl. Med., 2023, 21, 178 Search PubMed.
- J. Levin, N. Sing, S. Melbourne, A. Morgan, C. Mariner, M. G. Spillantini, M. Wegrzynowicz, J. W. Dalley, S. Langer and S. Ryazanov, et al. , eBioMedicine, 2022, 80, 104021 Search PubMed.
- D. L. Price, A. Khan, R. Angers, A. Cardenas, M. K. Prato, M. Bani, D. W. Bonhaus, M. Citron and A.-L. Biere, npj Parkinson's Dis., 2024, 10, 60 CrossRef CAS PubMed.
- D. S. Pena and S. Ventura, Neural Regen. Res., 2022, 17, 508 Search PubMed.
- S. Peña-Díaz, J. Pujols, F. Pinheiro, J. Santos, I. Pallarés, S. Navarro, M. Conde-Gimenez, J. García, X. Salvatella and E. Dalfó, et al. , Front. Bioeng. Biotechnol., 2020, 8, 588947 Search PubMed.
- T. A. Sales, I. G. Prandi, A. A. de Castro, D. H. S. Leal, E. F. F. da Cunha, K. Kuca and T. C. Ramalho, Int. J. Mol. Sci., 2019, 20, 1829 Search PubMed.
- E. Carboni and P. Lingor, Metallomics, 2015, 7, 395 Search PubMed.
- R. Moons, A. Konijnenberg, C. Mensch, R. Van Elzen, C. Johannessen, S. Maudsley, A.-M. Lambeir and F. Sobott, Sci. Rep., 2020, 10, 16293 Search PubMed.
- F. Schifano, S. Dell'Acqua, S. Nicolis, L. Casella and E. Monzani, Antioxidants, 2023, 12, 791 Search PubMed.
- A. Binolfi, L. Quintanar, C. W. Bertoncini, C. Griesinger and C. O. Fernández, Coord. Chem. Rev., 2012, 256, 2188 Search PubMed.
- V. N. Uversky, J. Li and A. L. Fink, J. Biol. Chem., 2001, 276, 44284 CrossRef CAS PubMed.
- Z. Jansen van Rensburg, S. Abrahams, S. Bardien and C. Kenyon, Mol. Neurobiol., 2021, 58, 5920 CrossRef CAS PubMed.
- A. Wypijewska, J. Galazka-Friedman, E. R. Bauminger, Z. K. Wszolek, K. J. Schweitzer, D. W. Dickson, A. Jaklewicz, D. Elbaum and A. Friedman, Parkinsonism Relat. Disord., 2010, 16, 329 CrossRef PubMed.
- B. Chen, X. Wen, H. Jiang, J. Wang, N. Song and J. Xie, Free Radic. Biol. Med., 2019, 141, 253 Search PubMed.
- Q. Zhao, Y. Tao, K. Zhao, Y. Ma, Q. Xu, C. Liu, S. Zhang and D. Li, J. Mol. Biol., 2023, 435, 167680 Search PubMed.
- R. J. Ward, D. T. Dexter, A. Martin-Bastida and R. R. Crichton, Int. J. Mol. Sci., 2021, 22, 3338 Search PubMed.
- R. B. Mounsey and P. Teismann, Int. J. Cell Biol., 2012, 2012, 983245 Search PubMed.
- K. Cao, Y. Zhu, Z. Hou, M. Liu, Y. Yang, H. Hu, Y. Dai, Y. Wang, S. Yuan, G. Huang, J. Mei, P. J. Sadler and Y. Liu, Angew. Chem., 2023, 135, e202215360 Search PubMed.
- I. Tolbatov, E. Barresi, S. Taliani, D. La Mendola, T. Marzo and A. Marrone, Inorg. Chem. Front., 2023, 10, 2226 RSC.
- S. La Manna, C. Di Natale, V. Panzetta, M. Leone, F. A. Mercurio, I. Cipollone, M. Monti, P. A. Netti, G. Ferraro and A. Terán, et al. , Inorg. Chem., 2024, 63, 564 Search PubMed.
- E. Meggers, Chem. Commun., 2009, 1001 RSC.
- A. Temesgen, H. C. Ananda Murthy, A. Z. Enyew, R. Revathi and R. Venkatesha Perumal, Chem. Select, 2023, 8, e202302113 Search PubMed.
- Z. Pei, L. Li, N. Yang, S. Sun, N. Jiang and L. Cheng, Coord. Chem. Rev., 2024, 517, 215969 Search PubMed.
- H. Jiang, L. Li, Z. Li and X. Chu, Biomed. Microdevices, 2024, 26, 12 CrossRef PubMed.
- S. Abdolmaleki, A. Aliabadi and S. Khaksar, Coord. Chem. Rev., 2024, 501, 215579 Search PubMed.
- S. Bhattacharya, T. Adon, K. Dsouza and H. Y. Kumar, Chem. Select, 2025, 10, e202404147 CAS.
- X. Liang and W. K. Leong, J. Med. Chem., 2024, 67(23), 20980 Search PubMed.
- H. Z. Lee, W. K. Leong, S. Top and A. Vessières, ChemMedChem, 2014, 9, 1453 Search PubMed.
- X. Zhao, X. Li, S. Liang, X. Dong and Z. Zhang, RSC Adv., 2021, 11, 28851 RSC.
- H. Gao and X. Wu, Chem. Heterocycl. Compd., 2018, 54, 125 CrossRef CAS.
- K. W. Fan, H. L. Luk and D. L. Phillips, Molecules, 2023, 28, 3966 CrossRef CAS PubMed.
- V. I. Tomin and D. V. Ushakou, Polym. Test., 2017, 64, 77 CrossRef CAS.
- N. Ahsan, S. Mishra, M. K. Jain, A. Surolia and S. Gupta, Sci. Rep., 2015, 5, 9862 CrossRef CAS PubMed.
- B. Xu, J. Chen and Y. Liu, ACS Omega, 2022, 7, 30281 CrossRef CAS PubMed.
- P. K. Singh, V. Kotia, D. Ghosh, G. M. Mohite, A. Kumar and S. K. Maji, ACS Chem. Neurosci., 2013, 4, 393 CrossRef CAS PubMed.
- B. Ahmad and L. J. Lapidus, J. Biol. Chem., 2012, 287, 9193 CrossRef CAS.
- Tinku, S. A. Shaikh, I. K. Priyadarsini and S. Choudhary, J. Mol. Liq., 2024, 405, 125063 CrossRef CAS.
- L. Narhi, S. J. Wood, S. Steavenson, Y. Jiang, G. M. Wu, D. Anafi, S. A. Kaufman, F. Martin, K. Sitney and P. Denis, et al. , J. Biol. Chem., 1999, 274, 9843 CrossRef CAS PubMed.
- M. Perni, A. van der Goot, R. Limbocker, T. J. van Ham, F. A. Aprile, C. K. Xu, P. Flagmeier, K. Thijssen, P. Sormanni and G. Fusco, et al. , Front. Cell. Dev. Biol., 2021, 9, 552549 CrossRef PubMed.
- Y. Guan, X. Zhao, F. Liu, S. Yan, Y. Wang, C. Du, X. Cui, R. Li and C. X. Zhang, Front. Cell. Neurosci., 2020, 14, DOI:10.3389/fncel.2020.00159.
- T. Ranasinghe, Y. Seo, H.-C. Park, S.-K. Choe and S.-H. Cha, J. Hazard. Mater., 2024, 480, 136215 Search PubMed.
- L. Zou, Z. Che, K. Ding, C. Zhang, X. Liu, L. Wang, A. Li and J. Zhou, Antioxidants, 2023, 12(5), 1134 Search PubMed.
- N. Xiong, J. Xiong, M. Jia, L. Liu, X. Zhang, Z. Chen, J. Huang, Z. Zhang, L. Hou and Z. Luo, et al. , Behav. Brain Funct., 2013, 9(1), 13 CrossRef CAS PubMed.
- I. O. Ishola, I. O. Awogbindin, T. G. Olubodun-Obadun, A. E. Olajiga and O. O. Adeyemi, NeuroToxicol., 2023, 96, 37 CrossRef CAS PubMed.
- Y. Zhou, Y. Yao, Z. Yang, Y. Tang and G. Wei, Phys. Chem. Chem. Phys., 2023, 25, 14471–14483 RSC.
- G. A. De Oliveira, M. d A. Marques, C. Cruzeiro-Silva, Y. Cordeiro, C. Schuabb, A. H. Moraes, R. Winter, H. Oschkinat, D. Foguel and M. S. Freitas, Sci. Rep., 2016, 6, 37990 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2426212. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc01141h |
| This journal is © The Royal Society of Chemistry 2025 |





