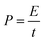2D graphene-like hierarchically porous carbon nanosheets from a nano-MgO template and ZnCl2 activation: morphology, porosity and supercapacitance performance
Binbin Chang*ab,
Shouren Zhangab,
Li Sunab,
Hang Yinab and
Baocheng Yang*ab
aInstitute of Nanostructured Functional Materials, Huanghe Science and Technology College, Zhengzhou, Henan 450006, China. E-mail: binbinchang@hhstu.infmm.cn; baochengyang@yahoo.com
bHenan Provincial Key Laboratory of Nano-composite and Application, Zhengzhou, Henan 450006, China
First published on 20th July 2016
Abstract
Herein, we developed a facile and cost-efficient route to obtain two-dimensional (2D) graphene-like carbon nanosheets with a well-developed hierarchical pore structure (HPCNS) by using nano-MgO spheres as templates and ZnCl2 chemical activation. Importantly, the wrinkled degree of the nanosheet and porosity with various micro/mesopore proportions were controlled by tuning the MgO/ZnCl2 ratio. An optimal sample of the HPCNS-1-2 material displayed an ultrathin sheet-like morphology as well as a well-interconnected hierarchical porous structure, a highly accessible surface area (1415.6 m2 g−1) and a large pore volume (1.57 cm3 g−1). As an electrode material for supercapacitor applications, the HPCNS-1-2 electrode presented a high specific capacitance of 332.8 F g−1 at 1 A g−1 and excellent rate capability of above 66% retention even at 30 A g−1 in a 6 M KOH electrolyte. Meanwhile, the HPCNS-1-2 electrode also exhibited a superior energy density of 45.8 W h kg−1 at a power density of 495.3 W kg−1, and also maintained a 23.1 W h kg−1 energy density at an extremely high power density of 11.3 kW kg−1. In addition, a remarkable long-term cycling stability of about 93.8% capacitance retention was retained after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1 in 6 M KOH.
000 cycles at 5 A g−1 in 6 M KOH.
1. Introduction
Supercapacitors, as a preferred energy storage device, have drawn extensive attention for next generation energy storage applications owing to their ultrahigh power density, fast charge–discharge rate, long cycling stability, high safety, environmental friendliness and widespread range of applications, such as hybrid electrical vehicles, portable electronic equipment, uninterruptible power supplies, pulsing techniques and other devices.1–4 According to energy storage mechanisms, supercapacitors can be categorized into electric double-layer capacitors (EDLCs) and pseudocapacitors.5 Compared with EDLCs, pseudocapacitors usually supply a higher energy density, but suffer a narrow available voltage window, a poor cycling stability and low electrical conductivity, which seriously block the practical application of pseudocapacitors.6 Thus, EDLCs characterized by prominent long-term stability and high power density become the preferred option in industrial application.Carbonaceous porous materials have been considered as the most favored electrode materials for EDLCs, due to their high electrical conductivity, chemical stability, large accessible surface area and the rapid ion transfer rate.7–11 So far, numerous well-developed porous carbons with different morphologies have been extensively investigated, such as porous carbon nanospheres,12 porous carbon nanoflowers,13 carbon nanosheets,14 carbon nanofibers,15 graphene,16 graphite foams17 and so on.18 Among these porous carbon materials, two-dimensional (2D) carbon nanomaterials, especially graphene and 2D carbon nanosheets, are of great potential as electrode materials for highly capacitive performance of EDLCs, owing to some of unique properties, including of high electrical conductivity, large surface area and porous layer structure.19–21 Graphene, as a typical 2D planar structure material with outstanding physical and chemical properties, has been the focus of substantial research interests. However, graphene is easy and inevitable to generate irreversible agglomerates in the process of preparation and subsequent production procedures, resulting in the deviation of their specific surface areas from theoretical value.22 Moreover, the synthetic process of graphene is relatively complicated and high-cost, which violates the requirements of easy operation, high yield and high quality in the practical industrial applications.23 Interestingly, 2D porous carbon nanosheets with graphene-like layered structure materials possess more active sites and a more effective surface than 0D and 1D structures, meanwhile, and have some superior properties including less weight, high electrical conductivity and even much larger surface area than graphene materials, possessing shorten paths for fast electrolyte ion diffusion and large exposed surface offering more electron/charge transfer channels, which correspond exactly to the requirements of energy storage/conversion devices, especially supercapacitors.24,25
Currently, various synthetic methods have been reported to obtain 2D porous carbon nanosheets with a graphene-like planar structure, such as template method,26 mechanical/chemical exfoliation,27 chemical vapor deposition,28 molten-salt route,29 ball-milling method,30 blowing strategy31 and so on. Wang et al. synthesized 2D mesoporous carbon sheet-like materials using SiO2 nanosheets as a template and exhibited prominent supercapacitive performance.32 Lei et al. reported the preparation graphene-like carbon nanosheet by a NH4Cl blowing method and the materials presented a relatively high accessible surface area.33 Peng et al. employed a simultaneous urea gasification expansion and CaCl2 activation method to obtain graphene-like carbon nanosheets with excellently electrochemical property.34 The sheet-like carbon materials fabricated by the template route are often mesoporosity, which display higher capacitance retention and excellent rate performance, but the further enhancement in specific capacitance is greatly restricted by the relatively low surface area.35 The blowing and activation methods are the well-known strategies to prepare micropore-enriched carbon materials with a high capacitance at low discharge current density, but the poor mesoporosity hinders the ion/electron transportation at high current density, resulting in the poor rate performance.36 Thus, these as-obtained graphene-like porous carbon nanosheets by conventional approaches usually contain a simplex pore structure of micropores or mesopores, which greatly restrict the further improvement of supercapacitive behavior. Consequently, it is still a high challenge and need for designing 2D graphene-like hierarchically porous carbon nanosheets with tailored micropores and mesopores for better supercapacitor performance.
Herein, we reported a simple and efficient route to fabricate 2D graphene-like hierarchical porous carbon nanosheets (HPCNS) simultaneously containing micropores, mesopores and macropores by the nano-MgO template and the ZnCl2 chemical activation. The synthesized graphene-like porous carbon nanosheets presented a highly crumpled morphology and ultrathin structure, offering a high specific surface area and large numbers of accessible active sites. Moreover, the resultant graphene-like carbon nanosheets possessed a hierarchical and well-developed porosity made up of macropores (ca. 60–80 nm), large proportion of mesopores (ca. 2.7–9.3 nm) fully well-interconnected by narrow micropores (ca. 0.67–1.28 nm). Benefiting from the unique graphene-like and highly crinkly nanosheet morphology with ultrathin thickness and superior hierarchical porous framework, the resulting sheet-like carbon materials exhibited a remarkable capacitive property as electrodes for EDLCs in KOH aqueous electrolytes, including extremely large specific capacitance, satisfactory rate capacity, high power and energy densities and good long-term cycling stability.
2. Experimental section
2.1 Preparation of hierarchical porous carbon nanosheets
Graphene-like hierarchically porous carbon nanosheets were synthesized from glucose using commercial nano-sized MgO spheres (ca. 50 nm in size) as a template and the ZnCl2 chemical activation. In a typical run, 4 g of glucose was mixed with quantified nano-MgO spheres (the mass of MgO is 1 or 2 g) in 40 mL of deionized water, and stirred for 1 h. Then, the mixture was put into a Teflon-sealed autoclave (100 mL) and heated for 10 h at 180 °C. The products were collected by filtration and then washed repeatedly with distilled water and oven-dried at 100 °C.Following, 1 g of the obtained and dried mixture was immersed in 20 mL of ZnCl2 solution (the mass of ZnCl2 is 1 or 2 g). After stirring for 4 h, the mixture was placed in a 110 °C oven until the solvent was completely evaporated. Then the dried material was calcined in a N2 atmosphere at 800 °C for 2 h. The resultant products were washed with 2.0 M HCl solution and deionized water to remove metal oxides or salts. Finally, the materials were dried at 80 °C for 10 h to obtain the final product, which were designed as HPCNS-x-y (x refers to the additive mass of nano-MgO; y represents the additive mass of ZnCl2).
2.2 Characterizations
X-ray diffraction (XRD) patterns were monitored by a Bruker D8 diffractometer using Cu Kα radiation (λ = 0.15418 nm) as an X-ray source. Nitrogen adsorption–desorption isotherms were carried out at −196 °C using a micromeritics ASAP 2020HD88 analyzer. Before adsorption, the samples were out-gassed at 200 °C for 10 h. The specific surface area (SBET) was evaluated using the Brunauer–Emmett–Teller (BET) method, and the pore size distributions were calculated according to the Density-Functional-Theory (DFT) method. The morphology was observed from a FEI Tecnai G2 20 transmission electron microscope (TEM) with an accelerating voltage of 200 kV and a scanning electron microscope (SEM, Quanta 250 FEG). Raman spectra were recorded on a Raman spectrometer (WITEC Spectra Pro 2300I) operating with 532 nm laser. X-ray photoelectron spectra (XPS) were obtained on a VG ESCALAB MK II X-ray photoelectron spectrometer with an exciting source of Mg Kα (1253.6 eV).2.3 Electrochemical measurements
The products were tested via a conventional two-electrode testing device in a 6 M KOH electrolyte solution, which was performed on a CHI660D electrochemical workstation at the room temperature. The working electrodes were prepared by mixing active material, carbon black and polytetrafluoroethylene (PTFE) binder with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After coating the above slurries on foamed Ni grids (1 cm × 1 cm), the electrode was dried overnight at 140 °C before pressing under a pressure of 20 MPa. Cyclic voltammetry (CV) curves were obtained in the potential range of −1.0 to 0 V by varying the scan rate from 5 to 150 mV s−1. Charge–discharge measurements were done galvanostatically at 1–30 A g−1 over a voltage range of −1.0 to 0 V. In a typical two-electrode system, two nearly identical working electrodes were prepared using the previous method, using glass fiber as the separator. The mass of active material loading in signal electrode was about 1–2 mg. Electrochemical impedance spectroscopy (EIS) was measured in a frequency range of 100 kHz to 0.01 Hz at open circuit voltage with an alternate current amplitude of 5 mV in a two-electrode testing device.
1. After coating the above slurries on foamed Ni grids (1 cm × 1 cm), the electrode was dried overnight at 140 °C before pressing under a pressure of 20 MPa. Cyclic voltammetry (CV) curves were obtained in the potential range of −1.0 to 0 V by varying the scan rate from 5 to 150 mV s−1. Charge–discharge measurements were done galvanostatically at 1–30 A g−1 over a voltage range of −1.0 to 0 V. In a typical two-electrode system, two nearly identical working electrodes were prepared using the previous method, using glass fiber as the separator. The mass of active material loading in signal electrode was about 1–2 mg. Electrochemical impedance spectroscopy (EIS) was measured in a frequency range of 100 kHz to 0.01 Hz at open circuit voltage with an alternate current amplitude of 5 mV in a two-electrode testing device.
The gravimetric specific capacitance, Csp (F g−1), for a single electrode was calculated from each galvanostatic charge–discharge curve according to the following equation:
The energy density (E, W h kg−1) was estimated by using the following formula:
The power density (P, W kg−1) was calculated as the following equation:
3. Results and discussion
3.1 Microstructure characterization of as-obtained materials
The fabrication procedure and the possible formation mechanism of HPCNS with well-developed hierarchical porosity are illustrated in Scheme 1. Firstly, during the hydrothermal process, glucose as carbon source is gradually polymerized and carbonized, and meanwhile these MgO particles are embedded into the framework of the yielding oligosaccharides. Importantly, the embedded MgO components can form a carburized phase under the thermal treatment, and the formation–decomposition process of the carburized phase can generate graphene-like nanosheets.37,38 Moreover, these embedded MgO particles also act as templates to bring macropores. After hydrothermal treatment, the as-obtained C/MgO transient phase possesses abundant oxygen-containing and hydrogen-containing functional groups, benefiting the following chemical activation for gaining well-enriched porosity. Secondly, ZnCl2-impregnated transient phase is further calcined and carbonized in a N2 atmosphere at 800 °C. ZnCl2 is usually used as a dehydrating agent to stoichiometrically remove oxygen and hydrogen atoms as water molecule, therefore producing a large number of micropores and mesopores.39 Finally, the MgO, ZnO nanoparticles and other metal salts are eliminated by acid treatment to contribute an additional porosity and subsequently obtain the HPCNS materials. | ||
| Scheme 1 Schematic illustrations of the preparation of HPCNS by using nano-MgO spheres template and the ZnCl2 activation. | ||
The wide-angle XRD patterns of HPCNS samples before and after acid treatment are shown in Fig. 1. For HPCNS-1-0 sample before acid treatment, the strong diffraction peaks at 2θ angles of 37.0°, 43.0°, 62.2°, 74.6° and 78.5° are assigned to MgO (JCPDS no. 04-0829), suggesting the stability of MgO crystal phase during the process of hydrothermal and further carbonization. For HPCNS-1-2 sample before acid treatment, several additional strong diffraction peaks at 2θ = 28.4, 31.7 and 45.4° are found, which should be indexed to the hexagonal phase of zinc oxide that is formed in the process of ZnCl2 activation. After washing by acid and water, only two broad diffraction peaks at ca. 23 and 43° are observed in both HPCNS-1-0 and HPCNS-1-2 samples, which belong to the typical (002) and (100) planes of graphitic carbon material.40 By comparison, the much stronger diffraction in HPCNS-1-2 suggests that the ZnCl2 activation is favorable for the framework graphitization and therefore promoting the electrical conductivity. Raman spectra of HPCNS are shown in Fig. 2a. The peak located at ca. 1340 cm−1 corresponds to D-band, which is related to the defect-induced structure or graphene edges. The other peak at about 1590 cm−1, designated as G-band, is ascribed to the graphitic carbon phase with a sp2 electronic configuration, such as graphene layers. In addition, the graphitic structure can be further characterized with the ratio of the relative intensities of D- and G-band peaks (ID/IG). The narrower G-band and the lower ID/IG value represent that carbon materials own the well-defined graphene-like structure.41 The values of ID/IG for HPCNS-1-0, HPCNS-1-1, HPCNS-1-2 and HPCNS-2-2 materials are 1.07, 0.99, 0.91, and 0.96 (Table 1), respectively, suggesting that HPCNS-1-2 material possess much higher degree of graphitization and a relatively perfect graphite structure among as-obtained HPCNS materials.
 | ||
| Fig. 1 The XRD patterns of as-obtained materials: (a) HPCNS-1-0 before acid treatment; (b) HPCNS-1-2 before acid treatment; (c) HPCNS-1-0; (d) HPCNS-1-2. | ||
 | ||
| Fig. 2 (a) The Raman spectra of HPCNS-1-0, HPCNS-1-1, HPCNS-1-2 and HPCNS-2-2 samples; XPS spectra of HPCNS-1-2: (b) survey; (c) C1s; (d) O1s. | ||
| Sample | SBETa (m2 g−1) | Smicrob (m2 g−1) | Smesoc (m2 g−1) | Vtotald (cm3 g−1) | Vmicroe (cm3 g−1) | ID/IG |
|---|---|---|---|---|---|---|
| a BET surface area.b Micropore surface area calculated using the V–t plot method.c Mesopore surface area calculated using the V–t plot method.d The total pore volume calculated by single point adsorption at P/P0 = 0.9945.e The micropore volume calculated using the V–t plot method. | ||||||
| HPCNS-1-0 | 578.7 | 311.3 | 267.4 | 1.21 | 0.15 | 1.07 |
| HPCNS-1-1 | 1231.1 | 629.4 | 601.7 | 0.95 | 0.31 | 0.99 |
| HPCNS-1-2 | 1415.6 | 382.9 | 1032.6 | 1.57 | 0.38 | 0.91 |
| HPCNS-2-2 | 1218.9 | 118.8 | 1100.1 | 1.28 | 0.09 | 0.96 |
The surface property of carbonaceous materials, including elemental composition and chemical state, was performed by XPS technology (Fig. 2b–d). From the survey spectrum (Fig. 2b), there are only C and O elements existing and their relative contents are examined to be 96.4% and 3.6%, respectively. As shown in Fig. 2c, the C1s spectrum can be approximately divided into three peaks centered at about 283.8, 285.7 and 288.3 eV, respectively related to the sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band of graphitic carbon, the band of sp3 C–C or defective carbon atoms no longer in the regular graphitic structure and the contribution of –C
C band of graphitic carbon, the band of sp3 C–C or defective carbon atoms no longer in the regular graphitic structure and the contribution of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or –COOH bands.42 The O1s spectrum shown in Fig. 2d can also be fitted into three deconvoluted peaks locating at ca. 531.6, 532.7 and 533.5 eV, respectively. The peak at about 531.6 eV corresponds to the contribution of oxygen in carboxyl groups; and the peak at ca. 532.7 eV corresponds to the –C
O or –COOH bands.42 The O1s spectrum shown in Fig. 2d can also be fitted into three deconvoluted peaks locating at ca. 531.6, 532.7 and 533.5 eV, respectively. The peak at about 531.6 eV corresponds to the contribution of oxygen in carboxyl groups; and the peak at ca. 532.7 eV corresponds to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band in ester; the one centered at ca. 533.5 eV should be assigned to the band of –C–O–C.43
O band in ester; the one centered at ca. 533.5 eV should be assigned to the band of –C–O–C.43
Fig. 3 presents SEM images of HPCNS obtained by the different ratio of MgO and ZnCl2. As shown in Fig. 3a, HPCNS-1-0 sample displays a typical crumpled and interconnected sheet-like structure. After further ZnCl2 activation and carbonization, the similar wrinkled sheet structure is preserved in HPCNS-1-1 and HPCNS-1-2 samples, which indicates the initial morphology is not destroyed by the ZnCl2 activation. It is obvious that the thickness in HPCNS-1-1 and HPCNS-1-2 is much thinner than that in HPCNS-1-0, which should be ascribed to the blowing effect of the released gas from ZnCl2 activation to separate carbon layers. The HPCNS-2-2 (Fig. 3d) presents a bad and broken sheet structure with some agglomeration of particles. Therefore, only increasing the dosage of MgO does not benefit the improvement of sheet morphology of material. The crumpled sheet-like morphology and structure were further confirmed by TEM, as shown in Fig. 4. The uniform and wrinkled sheet morphology as well as the macropores on sheet surface of HPCNS-1-0 sample (Fig. 4a) can be clearly observed. Similarly, HPCNS-1-1 and HPCNS-1-2 both display an ultrathin silk-like morphology with highly interconnected and soft wrinkles. Moreover, the trace of wrinkles of the layers folded over each other is clearly visible, suggesting these wrinkles are caused by the crumpling of graphene-like sheet. Such results also prove that the highly crumpled morphology can effectively prevent the aggregation of the sheets.34 Meanwhile, the porous structure with hierarchical pore organization can be discernible in the TEM images (Fig. 4b–d).
 | ||
| Fig. 3 The SEM photographs of as-prepared materials: (a) HPCNS-1-0; (b) HPCNS-1-1; (c) HPCNS-1-2; (d) HPCNS-2-2. | ||
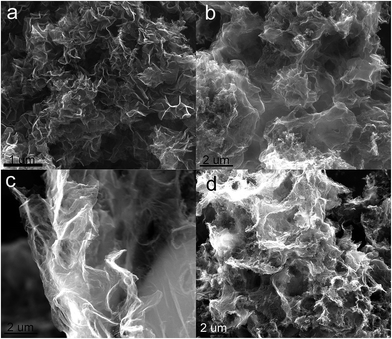
The N2 adsorption–desorption isotherms of HPCNS samples were shown in Fig. 5a. All isotherms of HPCNS are belonged to the type IV in accordance with the IUPAC classification, suggesting the porosity is mainly composed of mesopores.44 Apparently, the HPCNS-1-0 material presents a sharp capillary condensation step at high relative pressure of 0.90–0.99, indicative of the existence of mesopores with a relatively large pore size or even macropores. However, after the ZnCl2 activation, a slope of isotherm with a hysteresis loop at relative pressure of 0.50–0.99 demonstrates the coexistence of mesopores and macropores, which could be constructed from the incompact stacking, entanglement and overlap of carbon sheets. Meanwhile, the slope degree and hysteresis loop gradually enlarge with the enhancement of ZnCl2 dosage, and this indicates the formation of more slit-shaped mesopores. Furthermore, along with the addition of ZnCl2, the steep increase of isotherms in the low pressure area is obviously observed, manifesting the formation of micropores in a large quantity. Thus, it can be concluded that activating agent ZnCl2 is necessary for the construction of hierarchical porous structure, especially in the formation of micropores and mesopores. Fig. 5b displays the pore size distribution of as-made HPCNS samples. With the use of nano-MgO and different dosages of ZnCl2, the pore sizes of materials gradually change from simplex macropore to hierarchical pores (namely, micropore, mesopore and macropore), and the micro/mesopore proportions gradually increases with the improvement of ZnCl2 dosage. The textural properties of all the materials are summarized in Table 1. It can be easily found the influence of activating agent ZnCl2 on the evolution of hierarchical porous structure. As only nano-MgO is used, the pore sizes of HPCNS-1-0 sample are distributed and mainly centered in the rage of 40–85 nm that is from the voids after removing nano-MgO. Compared with HPCNS-1-0, the surface areas and pore volumes of HPCNS-1-1 and HPCNS-1-2 are significantly enlarged with the ZnCl2 activation. And importantly, the micropores of 0.67/1.28 nm and mesopores of 2.7–9.3 nm are generated. However, further raising the usage of nano-MgO in HPCNS-2-2, the porous structure, including BET surface area, pore size distribution and pore volume has no noticeable variation, which reveals that nano-sized MgO plays a negligible role in the further development of hierarchical porous structure. In detail, the optimal HPCNS-1-2 sample displays a highest surface area (1415.6 m2 g−1), a suitable and hierarchical pore size distribution and large pore volume (1.57 cm3 g−1). Meanwhile, these outstanding textural properties of HPCNS-1-2 endow abundant ion adsorption sites and a short ion transmission path, which favor the improvement of electrochemical behaviors.
 | ||
| Fig. 5 (a) N2 adsorption–desorption isotherms and (b) pore size distributions of all as-obtained samples. | ||
3.2 Electrochemical property of as-prepared HPCNS materials
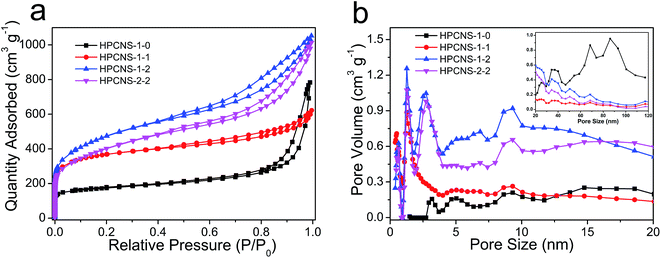
The electrochemical property of HPCNS materials was tested by a two-electrode system in 6 M KOH. Fig. 6a compares the CV curves of all HPCNS-x-y materials at a scan rate of 50 mV s−1. The quasi rectangular CV curves of in a potential range of −1.0 to 0 V suggest the approximately ideal EDLC capacitive behavior. The lowest CV curve area of HPCNS-1-0, indicating the smallest specific capacitance, can be ascribed to the absence of meso/micropores and the low surface area, which hampers the electrolyte ions diffusion and thus leads to an ion sieving effect.45 After the development of hierarchical porous structure by the ZnCl2 activation, an enhancement in CV curve area is achieved. Taking advantage of largely accessible surface area, suitable hierarchical pore channels and ultrathin graphene-like sheet structure, HPCNS-1-2 exhibits a highest specific capacitance among all the resultant HPCNS electrode materials. Fig. 6b shows the CV curves of HPCNS-1-2 material at various scan rates range from 5 to 150 mV s−1. The rectangular-like CV curves are preserved even at an extremely high scan rate of 150 mV s−1, implying HPCNS-1-2 electrode possesses a remarkable rate capability, a quick charge propagation capability and facile ion transport. The galvanostatic charge–discharge measurements are carried out at various current densities ranged from 1 to 30 A g−1 within the potential window of −1 to 0 V to calculate the specific capacitances of the HPCNS-1-2, and the typical results are shown in Fig. 6c. All the charge and discharge curves present approximately symmetric triangular shapes, meaning that HPCNS-1-2 electrode owns typical EDLC behavior and excellent charge–discharge reversibility. The specific capacitance of HPCNS-1-2 is calculated from the discharge curve with value of about 332.8 F g−1 obtained at a current density of 1 A g−1, which is considerably larger than those of HPCNS-2-2 (237.9 F g−1), HPCNS-1-1 (197.1 F g−1) and HPCNS-1-0 (168.7 F g−1), and even greatly remarkable capacitive performance for carbon-base capacitors at the same current density.46–50 The IR drop at the initiation of the discharge with a current density of 2 A g−1 is 0.018 V, indicative of a low internal resistance, which benefits from the superior graphene-like nanosheet structure. Rate capability is a key factor for the practical application of carbon-based electrode materials. To study the capacity for fast energy delivery and storage at high current density, the correlation between the specific capacitance and the various current densities for different electrode materials are shown in Fig. 6d. It is clearly found that specific capacitances of all materials gradually decrease with the raise of current density, which should be related to the increase of diffusion limitation. As displayed in Fig. 6d, HPCNS-1-2 electrode exhibits satisfactory capacitance retention. Even at a high current density of 30 A g−1, the specific capacitance of HPCNS-1-2 still reaches 220.6 F g−1, and an about 66.3% retention of capacitance at 1 A g−1, which is much higher than those of other HPCNS electrodes, demonstrating that a very fast and efficient charge transfer of HPCNS-1-2 electrode. The outstanding capacitive property of HPCNS-1-2 electrode should be ascribed to the following aspects: (i) the extremely high surface area, which provides more electroactive sites for ion accumulation and energy storage; (ii) the well-developed and interconnected porosity with hierarchical pore structure composed of micropores, mesopores and macropores, which offers fast electrolyte diffusion pathways and thus improves electrolyte and electron transport with a reduced resistance; (iii) ultrathin porous graphene-like nanosheet structure facilitates the efficient access of electrolytes; (iv) the relatively dispersed and layered nanosheet as a mini-supercapacitor-unit is connected in parallel or series, which become a relatively large output power of a square supercapacitor.In order to further evaluate the fundamental electrochemical properties of these HPCNS materials as electrodes for supercapacitors, including their resistivity and accessibility for electrolyte ions, electrochemical impedance spectroscopic (EIS) test was conducted. Fig. 7 presents the Nyquist plots of HPCNS-1-2 and HPCNS-1-0 electrodes, and the equivalent circuit for the fitting of the EIS data is also offered (the inset). Apparently, both of plots contains a small semicircle in the high-frequency region and a nearly vertical lines in low-frequency region, which suggests a low charge transfer resistance and an excellent dominance of electrical double-layer capacitors.51 In the high-frequency region, the real axis intercept corresponds to the combined ohmic resistance derived from the electrolyte and the contact between the current collector and the active material and the internal resistance of the electrode,52 corresponding to Rs in the equivalent circuit. The semicircle impedance loop stands for charge-transfer resistance and electronic double-layer capacitor at the interface between the electrode and electrolyte, corresponding to Rct and Cdl in the equivalent, respectively. For the EDLCs, the diameter of semicircle impedance loop represents the equivalent series resistance (ESR), and a smaller semicircle means a smaller ESR.53 As shown in the Nyquist plots, the Rs values of HPCNS-1-2 and HPCNS-1-0 electrodes are 0.52 and 0.69 Ω, respectively, which indicates the high electrical conductivity of the test symmetric cell. Usually, the low Rs value will result in the small electrode-potential drop, which is consistence with observations in charge/discharge experiments. Meanwhile, from Fig. 7, the equivalent series resistance of HPCNS-1-2 is 1.51 Ω, which is much smaller than that of HPCNS-1-0 electrode (3.06 Ω), suggesting the remarkable accessibility of electrolyte ions and charge, which should be benefited from the well-enriched porous framework and ultrathin graphene-like nanosheet structure. In the intermediate frequency region, the short slope of about 45° in the Nyquist plot corresponds to Warburg resistance (W), which is related to the diffusion of the ions into electrode particles interface inside the small pores.54 The significantly decreased length of 45° segment can be found in HPCNS-1-2 electrode, meaning the smaller W value, indicative of reduced resistance encountered by the ions during transporting into the inside of electrode particle, which should be attributed to the developed and well-interconnected porosity in HPCNS-1-2 electrode.
 | ||
| Fig. 7 Electrochemical impedance spectra of HPCNS-1-0 and HPCNS-1-2 electrode materials under the influence of an ac voltage of 5 mV (the insert is the equivalent fitting circuit). | ||
The desirable capacitive property of HPCNS-1-2 electrode material can be further demonstrated by Ragone plot (Fig. 8a), which is calculated from the discharging curves at different current densities. The plot clearly shown that the highest energy density is 45.8 W h kg−1 at a power density of 495.3 W kg−1, and the energy density of HPCNS-1-2 electrode is still maintained at 23.1 W h kg−1 at a high power density of 11.3 kW kg−1. Such results are much higher than most other carbon-based electrode materials previously reported.55–58 Furthermore, in order to efficiently validate the practical application efficiency of HPCNS-1-2 electrode material, the long-term cyclic stability was tested by galvanostatic charge–discharge cycling. Fig. 8b displays the capacitance retention versus cycle number curve of HPCNS-1-2 electrode at a current density of 5 A g−1 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Obviously, no noticeable variation can be found in the capacitance of HPCNS-1-2 electrode during the initial 2000 cycles and then starts to decrease slightly. As a whole, about 93.8% of the initial specific capacitance is maintained even after 10
000 cycles. Obviously, no noticeable variation can be found in the capacitance of HPCNS-1-2 electrode during the initial 2000 cycles and then starts to decrease slightly. As a whole, about 93.8% of the initial specific capacitance is maintained even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, meaning HPCNS-1-2 electrode possesses stable energy-storage process and a high degree of electrochemical reversibility.
000 cycles, meaning HPCNS-1-2 electrode possesses stable energy-storage process and a high degree of electrochemical reversibility.
4. Conclusion
In summary, 2D graphene-like hierarchically porous carbon nanosheets were successfully synthesized by using nano-MgO template and the ZnCl2 activation. This present route is a facile, cost-efficient and scalable preparation of graphene-like carbon materials with high-wrinkled nanosheets morphology and well-developed hierarchical pore structure. Moreover, the crumpled degree and thickness of nanosheets and porosity with different micro/mesopores proportion can be tuned by adjusting the ratio of MgO and ZnCl2. Such well-defined ultrathin sheet-like morphology and superior interconnected porous structure endure accessibly large surface area, more active sites and high-rate transportation of electrolyte ions and electrons throughout the electrode matrix, favoring the excellent electrochemical property. The as-obtained optimal sample of HPCNS-1-2 exhibits a prominent charge storage capacity with a high specific capacitance of 332.8 F g−1 in 6 M KOH at a current density of 1 A g−1 and a satisfactory rate capacity. More importantly, the high energy density, power density and remarkable cycling stability are also displayed. Thus, it has been demonstrated this strategy is a promising and available route to prepare 2D graphene-like hierarchically porous carbon nanosheets for high-efficient energy storage devices.Acknowledgements
The authors gratefully acknowledge the financial support from the program for New Century Excellent Talents in University (NCET-12-0696), the Leading Talents for Zhengzhou Science and Technology Bureau (Grant No. 131PLJRC649), National Natural Science Foundation of China (51472102).References
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC
.
- M. J. Zhi, C. C. Xiang, J. T. Li, M. Li and N. Q. Wu, Nanoscale, 2013, 5, 72–88 RSC
.
- L. Borchardt, M. Oschatz and S. Kaskel, Mater. Horiz., 2014, 1, 157–168 RSC
.
- T. Y. Kim, G. Jung, S. Yoo, K. S. Suh and R. S. Ruoff, ACS Nano, 2013, 7, 6899–6905 CrossRef CAS PubMed
.
- S. Bose, T. Kuila, A. K. Mishra, R. Rajasekar, N. H. Kim and J. H. Lee, J. Mater. Chem., 2012, 22, 767–784 RSC
.
- H. Jiang, J. Ma and C. Z. Li, Adv. Mater., 2012, 24, 4197–4202 CrossRef CAS PubMed
.
- W. Xing, S. Z. Qiao, R. G. Ding, F. Li, G. Q. Lu, Z. F. Yan and H. M. Cheng, Carbon, 2006, 44, 216–224 CrossRef CAS
.
- E. Frackowiak and F. Beguin, Carbon, 2002, 40, 1775–1787 CrossRef CAS
.
- L. Zhang, X. Zhao, M. D. Stoller, Y. W. Zhu, H. X. Ji, S. Murali, Y. P. Wu, S. Perales, B. Clevenger and R. S. Ruoff, Nano Lett., 2012, 12, 1806–1812 CrossRef CAS PubMed
.
- Z. S. Wu, Y. Sun, Y. Z. Tan, S. B. Yang, X. L. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 19532–19535 CrossRef CAS PubMed
.
- L. L. Zhang and X. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC
.
- B. B. Chang, Y. Z. Guo, Y. C. Li, H. Yin, S. R. Zhang, B. C. Yang and X. P. Dong, J. Mater. Chem. A, 2015, 3, 9565–9577 CAS
.
- Q. Wang, J. Yan, Y. B. Wang, T. Wei, M. L. Zhang, X. Y. Jing and Z. J. Fan, Carbon, 2014, 67, 119–127 CrossRef CAS
.
- H. Peng, G. F. Ma, K. J. Sun, J. J. Mu and Z. Q. Lei, J. Mater. Chem. A, 2014, 2, 17297–17301 CAS
.
- V. Barranco, M. A. Lillo-Rodenas, A. Linares-Solano, A. Oya, F. Pico, J. Ibanez, F. Agullo-Rueda and J. M. Rojo, J. Phys. Chem. C, 2010, 114, 10302–10307 CAS
.
- Z. Lei, N. Christov and X. S. Zhao, Energy Environ. Sci., 2011, 4, 1866–1873 CAS
.
- K. Lafdi, O. Mesalhy and A. Elgafy, Carbon, 2008, 46, 159–168 CrossRef CAS
.
- D. Bhattacharjya and J. S. Yu, J. Power Sources, 2014, 262, 224–231 CrossRef CAS
.
- H. Jiang, P. S. Lee and C. Li, Energy Environ. Sci., 2013, 6, 41–53 CAS
.
- H. Peng, G. F. Ma, K. J. Sun, J. J. Mu, Z. Zhang and Z. Q. Lei, ACS Appl. Mater. Interfaces, 2014, 6, 20795–20803 CAS
.
- Y. Wang, S. W. Tong, X. F. Xu, B. Ozyilmaz and K. P. Loh, Adv. Mater., 2011, 23, 1514–1518 CrossRef CAS PubMed
.
- J. Yan, J. Liu, Z. Fan, T. Wei and L. Zhang, Carbon, 2012, 50, 2179–2188 CrossRef CAS
.
- B. Wang, K. Ostrikov, T. van der Laan, K. Zheng, J. J. Wang, Y. P. Yan and X. J. Quan, J. Mater. Chem. C, 2013, 1, 7703–7708 RSC
.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246–249 CrossRef CAS PubMed
.
- J. Liu and X. W. Liu, Adv. Mater., 2012, 24, 4097–4111 CrossRef CAS PubMed
.
- Y. Fang, Y. Y. Lv, R. C. Che, H. Y. Wu, X. H. Zhang, D. Gu, G. F. Zheng and D. Y. Zhao, J. Am. Chem. Soc., 2013, 135, 1524–1530 CrossRef CAS PubMed
.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed
.
- W. Liu, T. Dang, Z. Xiao, X. Li, C. Zhu and X. Wang, Carbon, 2011, 49, 884–889 CrossRef CAS
.
- X. Liu, C. Giordano and M. Antonietti, Small, 2014, 10, 193–200 CrossRef CAS PubMed
.
- I. Y. Jeon, H. J. Choi, S. M. Jung, J. M. Seo, M. J. Kim, L. Dai and J. B. Baek, J. Am. Chem. Soc., 2013, 135, 1386–1393 CrossRef CAS PubMed
.
- X. F. Jiang, X. B. Wang, P. Dai, X. Li, Q. Weng, X. Wang, D. M. Tang, J. Tang, Y. Bando and D. Golberg, Nano Energy, 2015, 16, 81–90 CrossRef CAS
.
- Q. Wang, J. Yan, T. Wei, J. Feng, Y. Ren, Z. Fan, M. Zhang and X. Jing, Carbon, 2013, 60, 481–487 CrossRef CAS
.
- H. Lei, T. T. Yan, H. Wang, L. Y. Shi, J. P. Zhang and D. S. Zhang, J. Mater. Chem. A, 2015, 3, 5934–5941 CAS
.
- H. Peng, G. F. Ma, K. J. Sun, Z. G. Zhang, Q. Yang, F. T. Ran and Z. Q. Lei, J. Mater. Chem. A, 2015, 3, 13210–13214 CAS
.
- W. F. Zhang, Z. H. Huang, G. P. Cao, F. Y. Kang and Y. S. Yang, J. Power Sources, 2012, 204, 230–235 CrossRef CAS
.
- X. J. He, N. Zhao, J. S. Qiu, N. Xiao, M. X. Yu, C. Yu, X. Y. Zhang and M. D. Zheng, J. Mater. Chem. A, 2013, 1, 9440–9448 CAS
.
- L. Sun, C. G. Tian, M. T. Li, X. Y. Meng, L. Wang, R. H. Wang, J. Yin and H. G. Fu, J. Mater. Chem. A, 2013, 1, 6462–6470 CAS
.
- L. Wang, C. G. Tian, H. Wang, Y. G. Ma, B. L. Wang and H. G. Fu, J. Phys. Chem. C, 2010, 114, 8727–8733 CAS
.
- B. B. Chang, Y. Z. Guo, Y. C. Li and B. C. Yang, RSC Adv., 2015, 5, 72019–72027 RSC
.
- Z. Q. Li, C. J. Lu, Z. P. Xia, Y. Zhou and Z. Luo, Carbon, 2007, 45, 1686–1695 CrossRef CAS
.
- S. Y. Tao, Y. C. Wang, D. Shi, Y. L. An, J. S. Qiu, Y. S. Zhao, Y. Cao and X. F. Zhang, J. Mater. Chem. A, 2014, 2, 12785–12791 CAS
.
- T. I. T. Okpalugo, P. Papakonstantinou, H. Murphy, J. McLaughlin and N. M. D. Brown, Carbon, 2005, 43, 153–161 CrossRef CAS
.
- D. Hulicova-Jurcakova, M. Seredych, G. Q. Lu and T. J. Bandosz, Adv. Funct. Mater., 2009, 19, 438–447 CrossRef CAS
.
- Z. L. Yang, Y. F. Lu and Z. Z. Yang, Chem. Commun., 2009, 2270–2277 RSC
.
- L. Eliad, G. Salitra, A. Soffer and D. Aurbach, J. Phys. Chem. B, 2001, 105, 6880–6887 CrossRef CAS
.
- W. C. Chen, T. C. Wen and H. Teng, Electrochim. Acta, 2003, 48, 641–649 CrossRef CAS
.
- L. F. Chen, X. D. Zhang, H. W. Liang, M. G. Kong, Q. F. Guan, P. Chen, Z. Y. Wu and S. H. Yu, ACS Nano, 2012, 6, 7092–7102 CrossRef CAS PubMed
.
- H. J. Liu, X. M. Wang, W. J. Cui, Y. Q. Dou, D. Y. Zhao and Y. Y. Xia, J. Mater. Chem., 2010, 20, 4223–4230 RSC
.
- V. G. Pol, L. K. Shrestha and K. Ariga, ACS Appl. Mater. Interfaces, 2014, 6, 10649–10655 CAS
.
- H. Y. Huang, Z. J. Xu, D. H. Cai, Y. T. Hao and L. H. Gan, J. Solid State Electrochem., 2014, 18, 2481–2486 CrossRef CAS
.
- L. Yang, S. Cheng, Y. Ding, X. B. Zhu, Z. L. Wang and M. L. Liu, Nano Lett., 2012, 12, 321–325 CrossRef CAS PubMed
.
- S. P. Wang, R. H. Liu, C. L. Han, J. Wang, M. M. Li, J. Yao, H. R. Li and Y. Wang, Nanoscale, 2014, 6, 13510–13517 RSC
.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed
.
- Y. Chen, X. Zhang, D. Zhang, P. Yu and Y. Ma, Carbon, 2011, 49, 573–580 CrossRef CAS
.
- W. H. Qu, Y. Y. Xu, A. H. Lu, X. Q. Zhang and W. C. Li, Bioresour. Technol., 2015, 189, 285–291 CrossRef CAS PubMed
.
- Y. M. Tan, C. F. Xu, G. X. Chen, Z. H. Liu, M. Ma, Q. J. Xie, N. F. Zheng and S. Z. Yao, ACS Appl. Mater. Interfaces, 2013, 5, 2241–2248 CAS
.
- Z. B. Wen, Q. T. Qu, Q. Gao, X. W. Zheng, Z. H. Hu, Y. P. Wu, Y. F. Liu and X. J. Wang, Electrochem. Commun., 2009, 11, 715–718 CrossRef CAS
.
- H. Q. Li, J. Y. Luo, X. F. Zhou, C. Z. Yu and Y. Y. Xia, J. Electrochem. Soc., 2007, 154, A731–A736 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |