Nickel-catalyzed direct synthesis of hyperbranched liquid oligoethylene†
Mengyao
Zhang
a and
Shengyu
Dai
 *ab
*ab
aInstitutes of Physical Science and Information Technology, Anhui University, Hefei, Anhui 230601, China. E-mail: daiyu@ustc.edu.cn; Tel: +86 13955189794
bAnhui Laboratory of Molecule-Based Materials, Key Laboratory of Functional Molecular Solids, Ministry of Education, School of Chemistry and Materials Science, Anhui Normal University, Wuhu 241002, China
First published on 8th October 2024
Abstract
Late transition metal-catalyzed ethylene chain-walking polymerization offers a remarkably convenient method for synthesizing hyperbranched polyethylene. In this study, we created a series of pyridine-imine Ni(II) complexes with axially flexible cycloalkyl substituents, tailored for the production of hyperbranched oligoethylene oils (HBOEOs). These complexes exhibited moderate activity in HBOEO synthesis, reaching rates of up to 4.90 × 105 g mol−1 h−1. The resulting products exhibited low molecular weights (325–523 g mol−1) and high branching densities (110–167/1000C). NMR analysis verified their diverse branching structures, with a significant proportion of hyperbranched motifs. Notably, the activity, structure, and properties of the HBOEOs produced by the catalytic system were significantly influenced by alterations in the catalyst structure and oligomerization conditions. Specifically, when compared to rigid phenyl substituents, flexible cycloalkyl substituents proved more effective in promoting the catalytic system to produce HBOEOs with a higher degree of branching and improved liquefaction properties.
1. Introduction
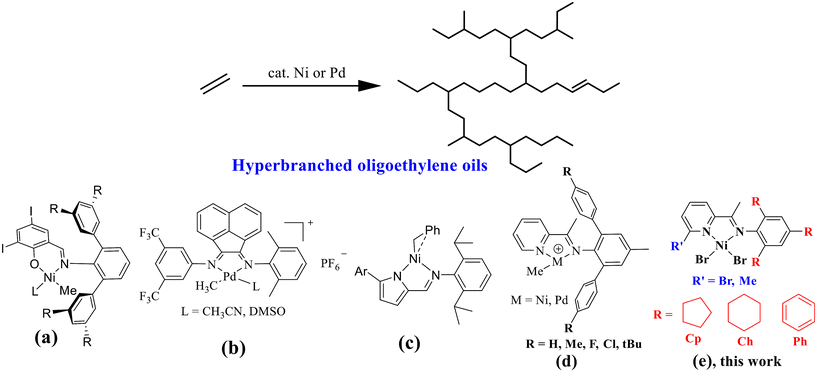
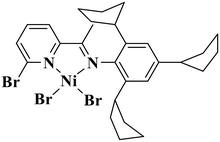
Lately, academic circles have witnessed a profound surge of interest and scrutiny in the field of late-transition-metal-catalyzed chain-walking polymerization of ethylene, aiming to fabricate hyperbranched oligoethylene oils (HBOEOs).1–11 These HBOEOs harbor immense potential as synthetic lubricant base stocks due to their distinctive characteristics.3,5 In particular, HBOEOs with lighter molecular weights exhibit a remarkably low pour point and a significantly high viscosity index, making them highly desirable for commercial utilization. Nevertheless, the synthesis of these lightweight HBOEOs poses a formidable challenge, as it necessitates catalysts that possess exceptional capabilities in chain walking and chain transfer mechanisms. Currently, the number of catalysts that are capable of fulfilling these demanding requirements is relatively limited; examples include electron-rich N-triaryl neutral nickel salicylaldimine catalysts (Chart 1a),5,6 cationic α-diimine palladium catalysts with less steric obstruction (Chart 1b)12–15 and 5-aryl neutral nickel pyrrolimine catalysts (Chart 1c),7–9 which can achieve this challenging synthesis.The nickel and palladium pyridine-imine systems, which constitute a distinct subclass of α-diimine systems, possess a remarkable unilateral steric structure that facilitates efficient chain transfer and chain walking, ultimately allowing for the production of low molecular weight, highly branched polyethylene.16–30 In recent endeavors, our group has developed an array of innovative N-terphenyl pyridine-imine Ni(II) and Pd(II) catalysts (Chart 1d), tailored specifically for the synthesis of HBOEOs and their polar copolymers.31–33 These cutting-edge complexes have exhibited remarkable efficacy in producing HBOEOs and polar HBOEOs, achieving a high polar monomer insertion ratio. Among them, the oligoethylene produced by the nickel system demonstrates a solid–liquid transition phase, exhibiting high viscosity and limited fluidity under ambient conditions. NMR analysis revealed that nearly 40% of the oligoethylene branches are long-chain branches, but they are not the predominant structure, which could primarily explain its reduced fluidity. Previous research has indicated that the introduction of flexible o-aryl substituents elevates the branching density of polyethylene synthesized via nickel α-diimine systems.34–36 Considering this insight, the current study developed a series of nickel pyridine-imine catalysts featuring axially flexible cycloalkyl substituents (Chart 1e). This innovation aims to augment the branching density of the resulting hyperbranched oligoethylene, thereby boosting the fluidity and liquidity of the final product.
2. Results and discussion
2.1 Synthesis and characterization of pyridine-imine Ni(II) complexes
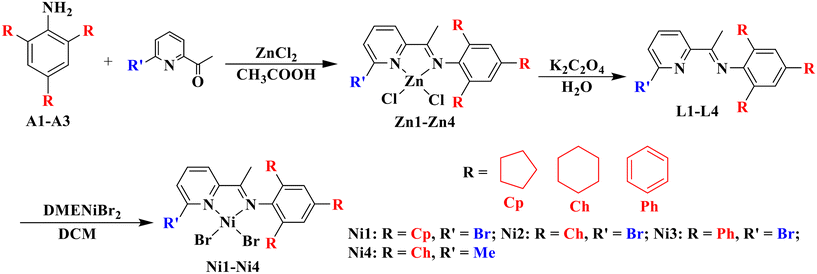
Previously reported methods were employed to prepare 2,4,6-tricycloalkylaniline or 2,4,6-triphenylamine.34,37 Utilizing the template method, these anilines (A1–A3) underwent condensation with 6-substituted 2-acetylpyridines to produce the corresponding pyridine-imine ligands, designated as L1–L4 (Scheme 1). This method boasts an acceptable yield (50–65%) and eliminates the need for column chromatography. To ensure the purity and structure of these ligands, high-resolution mass spectrometry (HRMS) and 1H and 13C NMR were conducted. Subsequently, the target nickel complexes, labeled Ni1–Ni4, were synthesized at room temperature with impressive yields ranging from 70–73% (Scheme 1). These complexes were formed through coordination reactions between the ligands L1–L4 and 1 equivalent of DMENiBr2 (where DME refers to 1,2-dimethoxyethane). The nickel complexes were primarily characterized using high-resolution MALDI-TOF mass spectrometry and elemental analysis. We repeatedly tried to cultivate single crystals of these nickel complexes, but our efforts were unsuccessful. Nevertheless, by chance, we discovered that a solution of nickel complexes, which had been left undisturbed for a considerable duration, had spontaneously formed testable crystals. Upon final analysis, it was revealed that the Ni2 complex encompassed a water molecule (Fig. 1). We postulate that the existence of this water molecule facilitated the development of single crystals of the complex. The complex Ni2 forms a centrosymmetric dimer upon crystallization, in which each nickel atom is bound to a pyridine-imine ligand and linked by two halogen atoms serving as bridges (as illustrated in Fig. 1). The square-pyramidal coordination geometry around the nickel atom is completed by a terminal halide ion. It is worth noting that the cyclohexyl group aligning nearly perpendicular to the five-membered chelate ring inadequately protects the catalytic metal centers, thus rendering them vulnerable to chain transfer during the oligomerization of ethylene.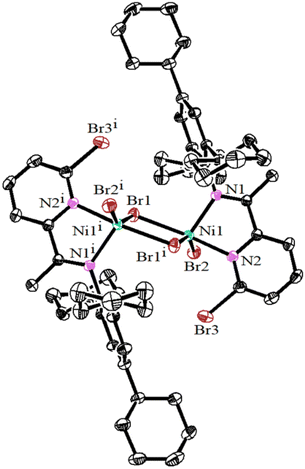 | ||
| Fig. 1 Solid-state molecular structure of Ni2 (2364663) with a 30% probability level, and H atoms and water molecules have been omitted for clarity. | ||
2.2 Synthesis of HBOEOs
Using pyridine-imine nickel catalysts activated by 300 equivalents of methylaluminoxane (MAO), we successfully synthesized HBOEOs via ethylene oligomerization. These catalysts exhibited a moderate oligomerization activity range of 0.58–4.90 × 105 g mol−1 h−1, resulting in HBOEOs with low molecular weights (325–523 g mol−1) and high branching levels (110–167/1000C), as summarized in Table 1. Due to the HBOEOs’ molecular weights falling below the GPC detection limit, we relied on 1H NMR measurements of terminal double bond content as an alternative method for molecular weight determination. Interestingly, increasing the oligomerization temperature significantly enhanced the activity of the HBOEOs, with the exception of Ni4, which showed a slight decrease at 70 °C (Fig. 2a). This observation is in contrast to most previously reported pyridine-imine nickel systems, where activity tends to diminish notably with an increase in oligomerization temperature.18,19 The reason for this divergence could be attributed to the stabilizing effect of the bromine atom at the 6-position pyridine ring on the cationic nickel catalytic center, especially at high temperatures. Concurrently, the molecular weight of the HBOEOs decreased (Fig. 2b), whereas the branching degree increased (Fig. 2c). This shift can primarily be attributed to an increased ratio of chain transfer and chain walking reactions compared to chain growth, aligning with findings in analogous systems.18,19 Among the tested catalysts, Ni4 exhibited the highest activity at 50 °C, while Ni3 yielded HBOEOs with the highest molecular weight at 30 °C, as shown in Fig. 2a and b. This may be due to the fact that the presence of the 6-position methyl substituent facilitates the elimination of the ethylene insertion energy barrier, thereby increasing the oligomerization activity. Meanwhile, the presence of the rigid phenyl group is also beneficial for suppressing the chain transfer during the oligomerization process, thus obtaining a higher molecular weight HBOEO. Furthermore, the HBOEO branching density derived from flexible cyclohexyl Ni2 is conspicuously higher than that attained from the corresponding rigid phenyl Ni3. This underscores the favorable influence of flexible cycloalkyl substituents in significantly augmenting the branching density of the HBOEO. Concurrently, we observed a transition in the HBOEO, shifting from the semi-solid phase associated with the rigid catalyst to the fully liquid phase characteristic of the flexible catalyst (Fig. 3). We selected several representative pyridine-imine nickel catalysts for comparison (Fig. 4).19,22,23,31,38 Despite minor variations in the specific conditions for catalyzing ethylene oligomerization among these catalysts, our new system notably produces oligoethylene with a significantly lower molecular weight and higher branching density compared to previously reported systems, albeit with a substantial reduction in oligomerization activity. The combination of a lower molecular weight and significantly higher branching density is crucial for our system to produce a clear, transparent liquid polyethylene oil, representing the core and innovation of this work. We hypothesize that the presence of substituents at the 6-position of the pyridine framework and the axial cycloalkyl group in the catalyst may facilitate chain transfer and chain walking, leading to the observed phenomena.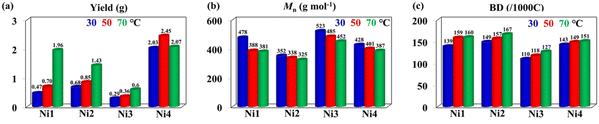 | ||
| Fig. 2 The yield (a), molecular weight (b), and branching density (c) of HBOEOs produced by Ni1–Ni4 at 30–70 °C. | ||
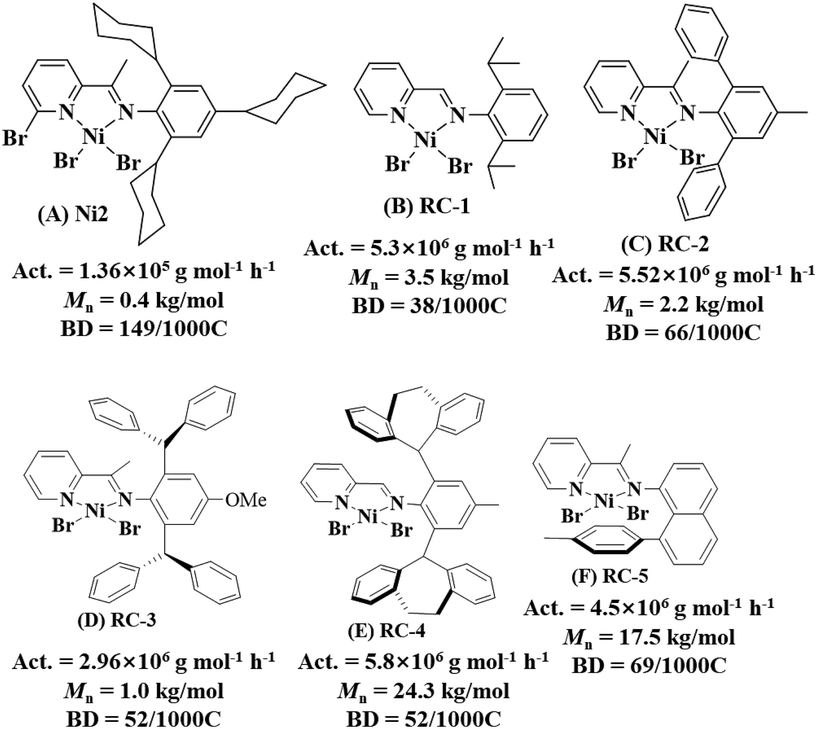 | ||
| Fig. 4 Comparison of activity, polyethylene molecular weight, and polyethylene branching density between Ni2 (A) and representative reported catalysts (B–F). | ||
| Entry | Complex | T (°C) | Yield (g) | Act.b |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
brsd |
|---|---|---|---|---|---|---|
| a Reaction conditions: Ni catalyst (10 μmol), MAO (300 eq.), ethylene (6 atm), oligomerization time (0.5 h), and cyclohexane (40 mL). b Activity is in units of 105 g mol−1 h−1. c M n is in units of g mol−1, as determined by 1H NMR spectroscopy. d brs = Number of branches per 1000C, as determined by 1H NMR spectroscopy and brs = 1000 × 2(ICH3)/3(ICH2+CH + ICH3). | ||||||
| 1 | Ni1 | 30 | 0.47 | 0.94 | 478 | 139 |
| 2 | Ni1 | 50 | 0.70 | 1.40 | 388 | 159 |
| 3 | Ni1 | 70 | 1.96 | 3.92 | 381 | 160 |
| 4 | Ni2 | 30 | 0.68 | 1.36 | 352 | 149 |
| 5 | Ni2 | 50 | 0.85 | 1.70 | 338 | 157 |
| 6 | Ni2 | 70 | 1.43 | 2.86 | 325 | 167 |
| 7 | Ni3 | 30 | 0.29 | 0.58 | 523 | 110 |
| 8 | Ni3 | 50 | 0.36 | 0.72 | 485 | 118 |
| 9 | Ni3 | 70 | 0.60 | 1.20 | 452 | 127 |
| 10 | Ni4 | 30 | 2.03 | 4.06 | 428 | 143 |
| 11 | Ni4 | 50 | 2.45 | 4.90 | 401 | 149 |
| 12 | Ni4 | 70 | 2.07 | 4.14 | 387 | 151 |
A detailed analysis of the microstructure of a representative HBOEO (Table 1, entry 10) was conducted using 1H and 13C NMR spectroscopy, providing insights into its composition (Fig. 2 and 3). The 1H NMR spectrum revealed the presence of a notable C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2 group, a visible 1,2-disubstituted double bond and a large number of terminal methyl groups (Fig. 5). Meanwhile, the 13C NMR spectrum disclosed the presence of a 1,2-disubstituted double bond, a complex branch-on-branch structure, and various lengths of branched chains (Fig. 6). Notably, long-chain branches and chain-end groups are the primary forms of branching in this HBOEO, and there is strong evidence of hyperbranched structures, as indicated by the detection of sec-butyl groups.39–41 We further investigated the effect of temperature on the resulting branching distribution and content and found by quantitative analysis (Table 2) that higher temperatures favored long-chain branching or chain-end groups and branch-on-branch production. In addition, GC testing of the samples from Table 1 indicated that these HBOEOs were composed of isomeric olefin compounds ranging from C18 to C40, and the number of isomers with the same carbon number was uncountable (Fig. S28–30†).
C–CH2 group, a visible 1,2-disubstituted double bond and a large number of terminal methyl groups (Fig. 5). Meanwhile, the 13C NMR spectrum disclosed the presence of a 1,2-disubstituted double bond, a complex branch-on-branch structure, and various lengths of branched chains (Fig. 6). Notably, long-chain branches and chain-end groups are the primary forms of branching in this HBOEO, and there is strong evidence of hyperbranched structures, as indicated by the detection of sec-butyl groups.39–41 We further investigated the effect of temperature on the resulting branching distribution and content and found by quantitative analysis (Table 2) that higher temperatures favored long-chain branching or chain-end groups and branch-on-branch production. In addition, GC testing of the samples from Table 1 indicated that these HBOEOs were composed of isomeric olefin compounds ranging from C18 to C40, and the number of isomers with the same carbon number was uncountable (Fig. S28–30†).
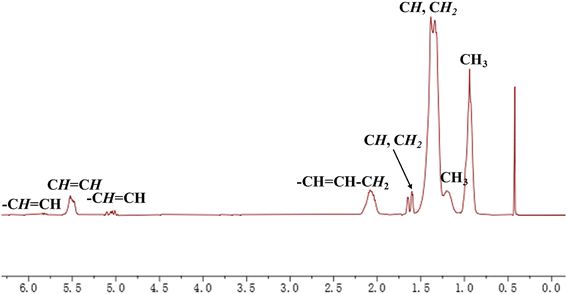 | ||
| Fig. 5 1H NMR spectrum assignment of the HBOEO yielded by Ni4 at 30 °C (Table 1, entry 10). | ||
 | ||
| Fig. 6 13C NMR spectrum assignment of the HBOEO yielded by Ni4 at 30 °C (Table 1, entry 10). | ||
| Ent. | HBOEO | Branches/1000 C | Methyla (%) | Ethyla (%) | Propyla (%) | S, C4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (%) a (%) |
sec-Butyla (%) |
|---|---|---|---|---|---|---|---|
| a Percentages of different branch types can be calculated from the relative intensity ratios of the methyl (1B1, 1B2, 1B3, 1Bn, S1, and sec-butyl) signals of the respective branches in the 13C NMR spectra. b HBOEOs obtained by using Ni4 at 30–70 °C (Table 1, entries 10–12). | |||||||
| 1b | Ni4-30 | 143 | 32 | 4 | 1 | 59 | 4 |
| 2b | Ni4-50 | 149 | 28 | 3 | 2 | 61 | 6 |
| 3b | Ni4-70 | 151 | 23 | 3 | 2 | 64 | 8 |
3. Conclusions
In summary, we have successfully synthesized a series of pyridine-imine Ni(II) complexes modified with axially adaptable cycloalkyl substituents, designed precisely for the fabrication of hyperbranched oligoethylene oils (HBOEOs). These tailored complexes exhibited moderate catalytic activity, ranging from 0.58 to 4.90 × 105 g mol−1 h−1, in the synthesis of HBOEOs, generating products with relatively low molecular weights (325–523 g mol−1) and an impressive branching density (110–167 branches per 1000 carbon atoms). Further analysis using NMR techniques validated the diverse array of branching structures within the HBOEOs, with a substantial fraction exhibiting hyperbranched motifs. Crucially, our findings highlight that subtle modifications in the catalyst's architecture and oligomerization parameters exert profound effects on the activity, structural intricacies, and comprehensive properties of the resulting HBOEOs. Notably, when compared to rigid phenyl substituents, flexible cycloalkyl substituents demonstrate greater efficacy in promoting the catalytic system to yield HBOEOs with an enhanced degree of branching and improved liquefaction characteristics.4. Experimental sections
4.1 Synthesis of ligands L1–L4
In a round-bottom flask, either 2-acetyl-6-bromopyridine or 2-acetyl-6-methylpyridine (1 mmol, 0.5 eq.) was added along with arylamine A1–A4 (2 mmol, 1 eq.). Zinc chloride (1.0 eq.) and acetic acid (4 mL) were then introduced, and the mixture was refluxed for four hours. Once the reaction was complete, the solution was cooled and mixed with a copious amount of diethyl ether, resulting in a turbid solution and the formation of a pale yellow precipitate. This precipitate was then filtered, rinsed with diethyl ether (3 × 10 mL), and dried to produce a powder. Subsequently, the powder was dissolved in dichloromethane (20 mL) in the same flask. An aqueous solution (20 mL) of potassium oxalate (1.0 eq.) was added to the flask, and the mixture was stirred for an hour. The organic layer was then separated, rinsed with water (3 × 20 mL), and dried using anhydrous magnesium sulfate. Following this, the dried filtrate was concentrated under reduced pressure. Ethanol was introduced to precipitate the target solid, resulting in a yellow powder (L1–L4) upon filtration and vacuum drying.
L1 (0.34 g, 50%). 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 7.6 Hz, Ar–H, 1H), 7.66 (t, J = 7.7 Hz, Ar–H, 1H), 7.57 (d, J = 7.7 Hz, Ar–H, 1H), 7.02 (s, Ar–H, 2H), 2.97 (m, –CH–, 1H), 2.79–2.67 (m, –CH–, 2H). 2.18 (s, –CH3, 3H), 2.12–2.03 (m, –CH2–, 2H), 1.96 (m, –CH2–, 2H), 1.86–1.66 (m, –CH2–, 10H), 1.63–1.47 (m, –CH2–, 10H). 13C NMR (101 MHz, CDCl3) δ 166.19 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 157.54, 145.46, 141.26, 140.94, 138.74, 133.14, 129.09, 122.21, 120.05, 45.98 (–CH–), 40.43 (–CH–), 34.74 (–CH2–), 33.84 (–CH2–), 25.68 (–CH2–), 25.66 (–CH2–), 25.46 (–CH2–), 17.40 (–CH3). APCI-MS (m/z): calcd for C52H41N2+: 479.2062, found, 479.2006, [M + H]+.
N), 157.54, 145.46, 141.26, 140.94, 138.74, 133.14, 129.09, 122.21, 120.05, 45.98 (–CH–), 40.43 (–CH–), 34.74 (–CH2–), 33.84 (–CH2–), 25.68 (–CH2–), 25.66 (–CH2–), 25.46 (–CH2–), 17.40 (–CH3). APCI-MS (m/z): calcd for C52H41N2+: 479.2062, found, 479.2006, [M + H]+.
L2 (0.33 g, 63%). 1H NMR (400 MHz, CDCl3) δ 8.30 (d, J = 7.6 Hz, Ar–H, 1H), 7.67 (t, J = 7.7 Hz, Ar–H, 1H), 7.58 (dd, J = 7.9, 1.0 Hz, Ar–H, 1H), 6.95 (s, Ar–H, 2H), 2.52–2.42 (m, –CH–, 1H), 2.23 (m, –CH–, 2H), 2.17 (s, –CH3, 3H), 1.95–1.82 (m, –CH2–, 7H), 1.79–1.69 (m, –CH2–, 5H), 1.67–1.60 (m, –CH2–, 4H), 1.43 (m, –CH2–, 8H), 1.33–1.21 (m, –CH2–, 6H). 13C NMR (101 MHz, CDCl3) δ 166.09 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 157.65, 143.91, 143.14, 141.08, 138.87, 134.48, 129.21, 122.06, 120.02, 44.56 (–CH–), 39.36 (–CH–), 34.83 (–CH2–), 33.71 (–CH2–), 33.38 (–CH2–), 27.23 (–CH2–), 27.18 (–CH2–), 27.16 (–CH2–), 26.39 (–CH2–), 17.42 (–CH3). APCI-MS (m/z): calcd for C52H39F2N2+: 521.2508, found, 521.2468, [M + H]+.
N), 157.65, 143.91, 143.14, 141.08, 138.87, 134.48, 129.21, 122.06, 120.02, 44.56 (–CH–), 39.36 (–CH–), 34.83 (–CH2–), 33.71 (–CH2–), 33.38 (–CH2–), 27.23 (–CH2–), 27.18 (–CH2–), 27.16 (–CH2–), 26.39 (–CH2–), 17.42 (–CH3). APCI-MS (m/z): calcd for C52H39F2N2+: 521.2508, found, 521.2468, [M + H]+.
L3 (0.32 g, 65%). 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 7.7 Hz, Ar–H, 1H), 7.72 (d, J = 7.7 Hz, Ar–H, 2H), 7.66 (s, Ar–H, 2H), 7.51 (m, Ar–H, 9H), 7.39 (d, J = 7.3 Hz, Ar–H, 1H), 7.31 (m, Ar–H, 4H), 7.24 (d, J = 8.9 Hz, Ar–H, 1H), 1.91 (s, –CH3, 3H). 13C NMR (101 MHz, CDCl3) δ 166.07 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.72, 145.54, 140.61, 140.46, 140.02, 138.54, 137.14, 132.27, 129.59, 129.21, 129.01, 128.80, 128.40, 127.97, 127.13, 126.86, 126.83, 120.05, 17.87 (–CH3). APCI-MS (m/z): calcd for C52H39Br2N2+: 503.1106, found, 503.1066, [M + H]+.
N), 156.72, 145.54, 140.61, 140.46, 140.02, 138.54, 137.14, 132.27, 129.59, 129.21, 129.01, 128.80, 128.40, 127.97, 127.13, 126.86, 126.83, 120.05, 17.87 (–CH3). APCI-MS (m/z): calcd for C52H39Br2N2+: 503.1106, found, 503.1066, [M + H]+.
L4 (0.27 g, 59%). 1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 7.8 Hz, Ar–H, 1H), 7.68 (t, J = 7.7 Hz, Ar–H, 1H), 7.23 (d, J = 7.6 Hz, Ar–H, 1H), 6.95 (s, Ar–H, 2H), 2.64 (s, –CH3, 3H), 2.47 (m, –CH–, 1H), 2.35–2.26 (m, –CH–, 2H), 2.18 (s, –CH3, 3H), 1.96–1.81 (m, –CH2–, 6H), 1.79–1.59 (m, –CH2–, 9H), 1.52–1.37 (m, –CH2–, 6H), 1.36–1.18 (m, –CH2–, 9H). 13C NMR (101 MHz, CDCl3) δ 167.39 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 157.24, 156.20, 144.22, 142.56, 136.50, 134.49, 124.05, 121.85, 118.08, 44.44 (–CH), 39.15 (–CH), 34.75 (–CH2), 33.62 (–CH2), 33.33 (–CH2), 27.15 (–CH2), 27.07 (–CH2), 26.34 (–CH2), 26.30 (–CH2), 24.58 (–CH3), 17.37 (–CH3). APCI-MS (m/z): calcd for C52H39Br2N2+: 457.3583, found, 457.3549, [M + H]+.
N), 157.24, 156.20, 144.22, 142.56, 136.50, 134.49, 124.05, 121.85, 118.08, 44.44 (–CH), 39.15 (–CH), 34.75 (–CH2), 33.62 (–CH2), 33.33 (–CH2), 27.15 (–CH2), 27.07 (–CH2), 26.34 (–CH2), 26.30 (–CH2), 24.58 (–CH3), 17.37 (–CH3). APCI-MS (m/z): calcd for C52H39Br2N2+: 457.3583, found, 457.3549, [M + H]+.
4.2 Synthesis of complexes Ni1–Ni4
Under a nitrogen atmosphere, a mixture containing 0.2 mmol of ligand and an equivalent amount of (DME)NiBr2 was introduced into a flask containing 20 ml of dichloromethane. This mixture was stirred at room temperature for a duration of 12 hours. Following the reaction, the solvent was partially evaporated under reduced pressure. Subsequently, the residual mixture was diluted with 20 mL of diethyl ether, resulting in the precipitation of an orange-red solid. The solids were then isolated through filtration, rigorously washed with diethyl ether, and ultimately dried under vacuum conditions, yielding the Ni1–Ni4 complexes.
Ni1 (0.10 g, 70%), MALDI-TOF-MS (m/z): calcd for C28H35Br2N2Ni: 615.0515, found, 615.0486, [M − Br]+. Elemental analysis: calc. for C28H35Br3N2Ni: C, 48.18; H, 5.05; N, 4.01. Found: C, 48.23; H, 5.21; N, 4.14.
Ni2 (0.11 g, 73%), MALDI-TOF-MS (m/z): calcd for C31H41Br2N2Ni: 657.0984, found, 657.0977, [M − Br]+. Elemental analysis: calc. for C31H41Br3N2Ni: C, 50.31; H, 5.58; N, 3.79. Found: C, 50.34; H, 5.61; N, 3.87.
Ni3 (0.22 g, 73%), MALDI-TOF-MS (m/z): calcd for C31H23BrN2Ni: 560.0393, found, 560.0375, [M − 2Br]+. Elemental analysis: calc. for C31H23Br3N2Ni: C, 51.57; H, 3.21; N, 3.88. Found: C, 51.53; H, 3.24; N, 3.71.
Ni4 (0.10 g, 72%), MALDI-TOF-MS (m/z): calcd for C32H44BrN2Ni: 593.2036, found, 593.2047, [M − Br]+. Elemental analysis: calcd for C32H44Br2N2Ni: C, 56.92; H, 6.57; N, 4.15. Found: C, 56.83; H, 6.67; N, 4.31.
4.3 Synthesis of HBOEOs
In a standard experimental procedure, a 350 mL thick-walled glass pressure vessel was dried for 2 hours in a hot air circulation oven kept at 60 °C. Once it cooled down to room temperature, the vessel was filled with cyclohexane (40 mL) and MAO (300 eq.) under a nitrogen atmosphere. Following this, a Ni(II) catalyst (10 μmol) dissolved in CH2Cl2 (2 mL) was carefully injected into the oligomerization system using a syringe. With vigorous stirring at 320 RPM, the reactor was pressurized to maintain a steady 6 atm of ethylene. After an hour, the pressurized reactor was gradually depressurized to atmospheric levels, and the resulting HBOEO was then concentrated under reduced pressure.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Mengyao Zhang: data curation, validation, formal analysis, and investigation. Shengyu Dai: conceptualization, data curation, formal analysis, funding acquisition, investigation, writing – original draft, and writing – review & editing.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the Natural Science Foundation of Anhui Province (2408085MB042 and 2108085Y06).References
- S. D. Ittel, L. K. Johnson and M. Brookhart, Late-metal catalysts for ethylene homo- and copolymerization, Chem. Rev., 2000, 100, 1169–1204 CrossRef CAS PubMed.
- Z. Saki, I. D'Auria, A. Dall'Anese, B. Milani and C. Pellecchia, Copolymerization of ethylene and methyl acrylate by pyridylimino Ni(II) catalysts affording hyperbranched poly(ethylene-co-methyl acrylate)s with tunable structures of the ester groups, Macromolecules, 2020, 53, 9294–9305 CrossRef CAS.
- I. D'Auria, S. Milione, T. Caruso, G. Balducci and C. Pellecchia, Synthesis of hyperbranched low molecular weight polyethylene oils by an iminopyridine nickel(II) catalyst, Polym. Chem., 2017, 8, 6443–6454 RSC.
- M. A. Zuideveld, P. Wehrmann, C. Röhr and S. Mecking, Remote substituents controlling catalytic polymerization by very active and robust neutral nickel(II) complexes, Angew. Chem., Int. Ed., 2004, 43, 869–873 CrossRef CAS PubMed.
- T. Wiedemann, G. Voit, A. Tchernook, P. Roesle, I. Göttker-Schnetmann and S. Mecking, Monofunctional hyperbranched ethylene oligomers, J. Am. Chem. Soc., 2014, 136, 2078–2085 CrossRef CAS PubMed.
- C. J. Stephenson, J. P. McInnis, C. Chen, M. P. Weberski, A. Motta, M. Delferro and T. J. Marks, Ni(II) phenoxyiminato olefin polymerization catalysis: striking coordinative modulation of hyperbranched polymer microstructure and stability by a proximate sulfonyl group, ACS Catal., 2014, 4, 999–1003 CrossRef CAS.
- C. A. Figueira, P. S. Lopes, C. S. Gomes, J. C. Gomes, F. Lemos and P. T. Gomes, New phenyl–nickel complexes of bulky 2-iminopyrrolyl chelates: synthesis, characterisation and application as aluminium-free catalysts for the production of hyperbranched polyethylene, Dalton Trans., 2018, 47, 15857–15872 RSC.
- T. F. Cruz, C. A. Figueira, L. F. Veiros and P. T. Gomes, Benzylnickel(II) complexes of 2-iminopyrrolyl chelating ligands: synthesis, structure, and catalytic oligo-/polymerization of ethylene to hyperbranched polyethylene, Organometallics, 2021, 40, 2594–2609 CrossRef CAS.
- C. A. Figueira, P. S. Lopes, C. S. Gomes, J. C. Gomes, L. F. Veiros, F. Lemos and P. T. Gomes, Neutral mono(5-aryl-2-iminopyrrolyl)nickel(II) complexes as precatalysts for the synthesis of highly branched ethylene oligomers: preparation, molecular characterization, and catalytic studies, Organometallics, 2018, 38, 614–625 CrossRef.
- Q. Li, H. Mu and Z. Jian, Facile access to diverse polyethylenes via neutral salicylaldiminato nickel catalysts, Polym. Chem., 2023, 14, 3196–3202 RSC.
- N. Nie, Y. Wang, C. Tan, M. Chen and D. Peng, The synthesis of hyperbranched ethylene oligomers by nickel catalysts with oxazole structure, J. Catal., 2023, 428, 115164 CrossRef CAS.
- Z. Dong and Z. Ye, Hyperbranched polyethylenes by chain walking polymerization: synthesis, properties, functionalization, and applications, Polym. Chem., 2012, 3, 286–301 RSC.
- H. Wang, Z. Lu and S. Dai, Synthesis of hyperbranched polyethylene and carboxylate functionalized polyethylene using bis(benzocyclopentyl) α-diimine Pd(II) catalysts with axial substituent-constrained strategy, Eur. Polym. J., 2023, 196, 112247 CrossRef CAS.
- Q. H. Tran, X. Q. Wang, M. Brookhart and O. Daugulis, Cationic α-Diimine Nickel and Palladium Complexes Incorporating Phenanthrene Substituents: Highly Active Ethylene Polymerization Catalysts and Mechanistic Studies of syn/anti Isomerization, Organometallics, 2020, 39, 4704–4716 CrossRef CAS.
- A. Meduri, T. Montini, F. Ragaini, P. Fornasiero, E. Zangrando and B. Milani, Palladium-catalyzed ethylene/methyl acrylate cooligomerization: effect of a new nonsymmetric α-diimine, ChemCatChem, 2013, 5, 1170–1183 CrossRef CAS.
- C. Bianchini, G. Giambastiani, L. Luconi and A. Meli, Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands, Coord. Chem. Rev., 2010, 254, 431–455 CrossRef CAS.
- Q. Mahmood, X. Li, L. Qin, L. Wang and W.-H. Sun, Structural evolution of iminopyridine support for nickel/palladium catalysts in ethylene (oligo)polymerization, Dalton Trans., 2022, 51, 14375–14407 RSC.
- L. Guo, S. Li, M. Ji, W. Sun, W. Liu, G. Li, J. Zhang, Z. Liu and S. Dai, Monoligated vs bisligated effect in iminopyridyl nickel catalyzed ethylene polymerization, Organometallics, 2019, 38, 2800–2806 CrossRef CAS.
- C. Wang, Y. Zhang, H. Mu and Z. Jian, Systematic studies on dibenzhydryl and pentiptycenyl substituted pyridine-imine nickel(II) mediated ethylene polymerization, Dalton Trans., 2020, 49, 4824–4833 RSC.
- H. Peng, M. Ji, J. Li, S. Shen, W. Wang, M. Chen, G. He and L. Guo, Investigations of flexible cycloalkyl substituents on pyridine-imine based nickel(II)-and palladium(II)-catalyzed ethylene oligomerization, Polym. Int., 2024 DOI:10.1002/pi.6579.
- X.-L. Chen, J. Gao, H. Liao, H.-Y. Gao and Q. Wu, Synthesis, characterization, and catalytic ethylene oligomerization of pyridine-imine palladium complexes, Chin. J. Polym. Sci., 2017, 36, 176–184 CrossRef.
- Z. Chen, K. E. Allen, P. S. White, O. Daugulis and M. Brookhart, Synthesis of Branched Polyethylene with “Half-Sandwich” Pyridine-Imine Nickel Complexes, Organometallics, 2016, 35(11), 1756–1760 CrossRef CAS.
- T. V. Laine, K. Lappalainen, J. Liimatta, E. Aitola, B. Löfgren and M. Leskelä, Polymerization of ethylene with new diimine complexes of late transition metals, Macromol. Rapid. Commun., 1999, 20, 487–491 CrossRef CAS.
- T. V. Laine, U. Piironen, K. Lappalainen, M. Klinga, E. Aitola and M. Leskelä, Pyridinylimine-based nickel(II) and palladium(II) complexes: preparation, structural characterization and use as alkene polymerization catalysts, J. Org. Chem., 2000, 606, 112–124 CrossRef CAS.
- S. P. Meneghetti, P. J. Lutz and J. Kress, Oligomerization of olefins catalyzed by new cationic palladium(II) complexes containing an unsymmetrical α-diimine ligand, Organometallics, 1999, 18, 2734–2737 CrossRef CAS.
- L. P. Zhang, X. Hao, W. H. Sun and C. Redshaw, Synthesis, Characterization, and Ethylene Polymerization Behavior of 8-(Nitroarylamino)-5,6,7-trihydroquinolylnickel Dichlorides: Influence of the Nitro Group and Impurities on Catalytic Activity, ACS Catal., 2011, 1, 1213–1220 CrossRef CAS.
- C. B. Huang, Y. F. Zhang, T. L. Liang, Z. J. Zhao, X. Q. Hu and W. H. Sun, Rigid geometry 8-arylimino-7,7-dimethyl-5,6-dihydroquinolyl nickel bromides: single-site active species towards ethylene polymerization, New J. Chem., 2016, 40, 9329–9336 RSC.
- F. Huang, Z. L. Sun, S. Z. Du, E. L. Yue, J. J. Ba, X. Q. Hu, T. L. Liang, G. B. Galland and W. H. Sun, Ring-tension adjusted ethylene polymerization by aryliminocycloheptapyridyl nickel complexes, Dalton Trans., 2015, 44, 14281–14292 RSC.
- E. Yue, L. P. Zhang, Q. F. Xing, X. P. Cao, X. Hao, C. Redshaw and W. H. Sun, 2-(1-(2-Benzhydrylnaphthylimino)ethyl)pyridylnickel halides: synthesis, characterization, and ethylene polymerization behavior, Dalton Trans., 2014, 43, 423–431 RSC.
- Z. L. Sun, E. L. Yue, M. N. Qu, I. V. Oleynik, I. I. Oleynik, K. S. Li, T. L. Liang, W. J. Zhang and W. H. Sun, 8-(2-Cycloalkylphenylimino)-5,6,7-trihydro-quinolylnickel halides: polymerizing ethylene to highly branched and lower molecular weight polyethylenes, Inorg. Chem. Front., 2015, 2, 223–227 RSC.
- H. Fan, G. Chang, H. Bi, X. Gui, H. Wang, G. Xu and S. Dai, Facile synthesis of hyperbranched ethylene oligomers and ethylene/methyl acrylate co-oligomers with different microscopic chain architectures, ACS Polym. Au, 2022, 2, 88–96 CrossRef CAS PubMed.
- Z. Yan, H. Bi, B. Ding, H. Wang, G. Xu and S. Dai, A rigid-flexible double-layer steric strategy for ethylene (co)oligomerization with pyridine-imine Ni(II) and Pd(II) complexes, New J. Chem., 2022, 46, 8669–8678 RSC.
- H. Fan, G. Xu, H. Wang and S. Dai, Direct synthesis of hyperbranched ethene oligomers and ethene-MA co-oligomers using iminopyridyl systems with weak neighboring group interactions, J. Polym. Sci., 2022, 60, 1944–1953 CrossRef CAS.
- S. Dai, S. Li, G. Xu, C. Wu, Y. Liao and L. Guo, Flexible cycloalkyl substituents in insertion polymerization with α-diimine nickel and palladium species, Polym. Chem., 2020, 11, 1393–1400 RSC.
- L. Guo, W. Sun, S. Li, G. Xu and S. Dai, Bulky yet flexible substituents in insertion polymerization with α-diimine nickel and palladium systems, Polym. Chem., 2019, 10, 4866–4871 RSC.
- Z. Hai, Z. Lu, S. Li, Z. Cao and S. Dai, The synergistic effect of rigid and flexible substituents on insertion polymerization with α-diimine nickel and palladium catalysts, Polym. Chem., 2021, 12, 4643–4653 RSC.
- B. A. Davis, J. A. Bennett, J. Genzer, K. Efimenko and M. Abolhasani, Intensified hydrogenation in flow using a poly(β-cyclodextrin) network-supported catalyst, ACS Sustainable Chem. Eng., 2022, 10, 15987–15998 CrossRef CAS.
- H. W. Peng, S. K. Li, G. Li, S. Y. Dai, M. J. Ji, Z. Liu and L. H. Guo, Rotation-restricted strategy to synthesize high molecular weight polyethylene using iminopyridyl nickel and palladium catalyst, Appl. Organomet. Chem., 2021, 35, e6140 CrossRef CAS.
- P. M. Cotts, Z. Guan, E. McCord and S. McLain, Novel branching topology in polyethylenes as revealed by light scattering and 13C NMR, Macromolecules, 2000, 33, 6945–6952 CrossRef CAS.
- J. C. Randall, A review of high-resolution liquid 13carbon nuclear magnetic resonance characterizations of ethylene-based polymer, J. Macromol. Sci., Rev. Macromol. Chem. Phys., 1989, 29, 201–317 CrossRef.
- G. B. Galland, R. F. De Souza, R. S. Mauler and F. F. Nunes, 13C NMR determination of the composition of linear low-density polyethylene obtained with [η3-methallyl-nickel-diimine]PF6 complex, Macromolecules, 1999, 32, 1620–1625 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR, single crystal data and mass spectra of synthetic compounds, NMR curves of HBOEO samples. CCDC 2364663. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4py00709c |
| This journal is © The Royal Society of Chemistry 2024 |