A nanoscale Fe(II) metal–organic framework with a bipyridinedicarboxylate ligand as a high performance heterogeneous Fenton catalyst†
Yue Li,
Huan Liu,
Wen-Juan Li,
Fang-Yao Zhao and
Wen-Juan Ruan*
Department of Chemistry and Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University, Tianjin 300071, China. E-mail: wjruan@nankai.edu.cn; Tel: +86-22-23501717
First published on 8th January 2016
Abstract
With 2,2′-bipyridine-5,5′-dicarboxylate as the ligand, a nanoscale Fe(II) MOF material (Fe-bpydc) was synthesized. Fe-bpydc could activate H2O2 to degrade organic pollutants in near neutral conditions, and its activity and stability are superior to reported results. Interestingly, Fe-bpydc only consumes H2O2 in the presence of organics, leading to high H2O2 utilization efficiency.
The Fenton reaction has attracted persistent attention due to its potential in water purification.1–4 In the Fenton system, environmentally benign H2O2 is used as the oxidant, and cheap Fe2+ ion is used as the catalyst. The reactions between them generate a series of highly oxidative intermediates, such as ˙OH, ˙OOH and high valent iron species, which induce the degradation of organic pollutants. However, the traditional Fenton reaction can only be carried out under acidic conditions (pH < 3), and large amount of ion slurry is produced in the treatment, which hinders its practical application. To overcome these drawbacks, plenty of heterogeneous Fenton systems have been developed.5–7 The reported heterogeneous Fenton catalysts can be largely classified into two kinds. One kind is inorganic iron minerals, such as Fe3O4,8 α-FeOOH,9 FeS,10 FeS2 (ref. 11) and FeVO4.12 Although their performance can be enhanced to some extent by the morphology modulation, the inorganic frameworks of these catalysts determine that the coordination sphere of metal ion, which is extremely important for the redox reactivity and stability of the catalyst, cannot be modified. The other kind of heterogeneous Fenton catalysts are prepared by the immobilization of Fe species (ion or complex) to various supports.13–15 However, the low loading of active components makes these catalysts usually exhibit an unsatisfactory activity. Apparently, the development of novel heterogeneous Fenton catalysts is still in urgent need yet.
Metal–organic frameworks (MOFs) have emerged as a new class of organic–inorganic hybrid materials in recent years and shown potential application in various fields.16–19 Especially, MOF is an ideal platform to construct catalysts, because the limitless choice of organic linker makes the coordination environment easy to be modulated, and the compact arrangement of metal ions is favorable to obtain high catalytic efficiency. Up to now, a series of MOF based Fenton catalysts have been proposed and shown great promise in the treatment of organic pollutants.20–24 For example, Zhao et al. reported that MIL-100(Fe) could be used to the degradation of high concentration azo-dye wastewater.20 Jiang et al. observed that MIL-53(Fe) could completely decompose Rhodamine B in the presence of a certain amount of H2O2.21
The reasonable selection of organic linker is the key to enhance the performance of MOF catalyst. 2,2′-Bipyridine is a widely used ligand to the synthesis of Fenton catalysts,14,25,26 not only because of its strong binding affinity with Fe2+ but also the oxidative resistance of pyridine ring to ˙OH. Some studies showed that inducing carboxyl group to the 2,2′-bipyridine ligand would further stabilize the obtained complex, due to it would bind with the Fe3+ formed in the catalytic cycle to avoid its hydrolyzation. Therefore, carboxylbipyridine type ligands are ideal for the construction of MOF based Fenton catalysts. On the other hand, minimizing the particle size of MOF catalyst to micro/nanoscale is also a feasible way to enhance the activity, since small-sized catalyst would expose more metal sites on the surface relative to the bulk crystal.
In this work, a nanoscale Fe(II) MOF material (Fe-bpydc) was synthesized with bipyridinedicarboxylate ligand. In the presence of H2O2, this MOF material could catalyze the degradation of organic pollutants in near neutral conditions, and exhibited high activity, high stability and high efficiency of H2O2 utilization in the reaction process. These results indicate that Fe-bpydc could be used as an ideal heterogeneous Fenton catalyst in water treatment.
Fe-bpydc was obtained as brown powder from the solvothermal reaction between Fe(ClO4)2 and 2,2′-bipyridine-5,5′-dicarboxylic acid (H2bpydc) in the mixed solvent of DMF and H2O (v/v = 1/1). SEM observation shows that the as-prepared Fe-bpydc sample presents a 2D morphology of irregular shaped plates with thickness of 100–500 nm (Fig. 1a). Sharp edges are shown on the plates, indicating the crystalline structure of Fe-bpydc. The crystalline structure of the sample was also corroborated by the measured PXRD pattern (Fig. 1b), in which well-defined diffraction peaks are shown. All the diffraction peaks in the pattern could be indexed to the reported bulk phase of {[Fe(bpydc)(H2O)]·H2O}n (Fig. S1, ESI†),27 indicating that Fe-bpydc has the same framework structure with it.
Further evidence supporting the structure of {[Fe(bpydc)(H2O)]·H2O}n comes from FT-IR, elemental and thermogravimetric analysis. Fig. S2 (ESI†) gives the FT-IR spectrum of Fe-bpydc. The broad band in the range of 3650–3000 cm−1 is typical for the coordinated H2O molecules with H bonds. The carboxyl group gives intense peaks at 1611 and 1378 cm−1, indicating that the H2bpydc ligand is completely deprotonated to coordinate with metal ions. The peaks at 851, 773 and 571 cm−1 could be assigned to the vibration of Fe–O bonds. Elemental analysis gave the contents of C, H and N as 41.13%, 3.11% and 7.98%, respectively, which are consistent with the theoretical values based on the formula of {[Fe(bpydc)(H2O)]·H2O}n (C 42.59%, H 2.97%, N 8.42%). Two stages of weight loss were shown in the measured TGA curve of Fe-bpydc (Fig. S3, ESI†). The first stage of weight loss occurred from room temperature to 190 °C (obsd. 10.64%, calcd. 10.78%), corresponding to the loss of two H2O molecules. The organic component was lost in the temperature range of 345–445 °C (obsd. 61.39%, calcd. 65.31%). The measured weight losses are in good accordance with the calculated values.
Phenol was used as the model substrate to evaluate the catalytic property of Fe-bpydc, and the reaction was carried out under natural pH conditions. As shown in Fig. 2, phenol did not react remarkably in the solution of H2O2 (curve a). In contrast, the addition of Fe-bpydc sharply accelerated the degradation process of phenol (curve b). Under the catalysis of only 0.01 g L−1 of Fe-bpydc, 0.25 mM phenol could be removed completely (Conv.% > 90) in 2 h, showing the high activity of Fe-bpydc as a heterogeneous Fenton catalyst. The pH value of the media showed a slow decrease from 5.3 to 4.0 during the degradation process due to the formation of carboxyl intermediates. In the absence of H2O2, the decay rate of phenol was negligible (curve c), showing that H2O2 works as the primary oxidant in the catalytic system of Fe-bpydc. After the complete degradation of phenol, the catalyst was separated from the reaction medium. The used catalyst gave nearly identical PXRD pattern (Fig. 1b) and morphology (Fig. S4, ESI†) as those of the as-prepared sample, which indicates the stability of Fe-bpydc under experimental conditions. Additionally, when phenol and H2O2 were supplemented to the reacted solution (without Fe-bpydc) to the initial concentration, no further reaction was observed (curve d), implying that the Fe-bpydc catalyzed degradation is truly heterogeneous in nature and not due to the leaching of active components to the solution. This oxidation system with Fe-bpydc as catalyst and H2O2 as oxidant can also be applied to the degradation of other substrates, such as 4-chlorophenol and methylene blue, and high removal rates were observed in these experiments (Fig. S5 and S6, ESI†). These results show the extensive applicability of Fe-bpydc in the treatment of organic pollutants.
To characterize the reusability of Fe-bpydc, cyclic degradation experiment was carried out. As shown in Fig. 3, Fe-bpydc can realize the complete removal of phenol in three consecutive cycles. Based on the determined formula of {[Fe(bpydc)(H2O)]·H2O}n (MW = 334), the total turnover number (TON) of three cycles is 23. To the best of our knowledge, although a series of MOF based Fenton catalysts have been reported, the TON values obtained in those works were usually much less than unit.20–24 The TON calculated here definitely proves that Fe-bpydc works as a catalyst in the degradation system and its stability is superior to most of the reported MOF catalysts, probably due to the oxidative recalcitrance and versatile coordination ability of the applied carboxylbipyridine type ligand. However, the removal rates of the second and third cycles exhibited remarkable slowdown relative to the first one, indicating the decrease of the efficiency of Fe-bpydc during the reaction. The deactivation of Fe-bpydc may be due to the blockage of its active sites by the intermediates formed in the degradation of phenol.
The acidity of the reaction system is known to be an important factor that affects the efficiency of Fenton catalysts, so we tested the kinetics of the Fe-bpydc catalyzed decomposition of phenol in the medium with different initial pH values. As shown in Fig. 4, the degradation can perform in a wide pH range from 3 to 6, and gives the maximum rate at pH 4–5. Further enhancement of the media pH to 7 would stop the reaction. Compared with the traditional Fenton system which can only work under strongly acidic environment, the near neutral operation condition of Fe-bpydc is more suitable for practical water treatment.
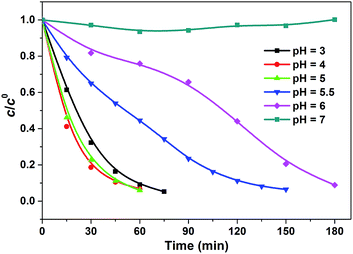 | ||
Fig. 4 Effect of pH on the degradation of phenol in the catalytic system of Fe-bpydc, 0.01 g L−1 Fe-bpydc, c0phenol = 0.25 mM, 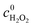 = 1.5 mM. = 1.5 mM. | ||
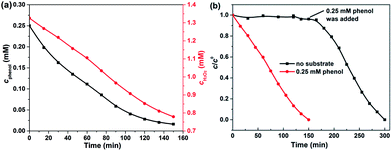
Since H2O2 works as the primary oxidant in the catalytic system of Fe-bpydc, the consumption of H2O2 during the reaction was also monitored. It was observed that the H2O2 decomposition occurred synchronically with the degradation of phenol (Fig. 5a). The consumption ratio between H2O2 to phenol was only 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showing high efficiency of H2O2 utilization in this system. In another experiment, Fe-bpydc was added solely to the solution of H2O2 (without substrate). Interestingly, no remarkable decomposition of H2O2 was observed in 150 min (Fig. 5b). After phenol was added artificially, the consumption of H2O2 happened subsequently. These results clearly show that the presence of organic substrate is necessary for the decomposition of H2O2 in the catalytic system of Fe-bpydc, which is distinct from the substrate independent H2O2 consumption in the traditional Fenton system. Because the invalid decomposition of H2O2 (produce O2 and H2O) is negligible in the Fe-bpydc catalysed system, H2O2 is consumed primarily by the degradation of substrate, which gives a high H2O2 utilization efficiency.
1, showing high efficiency of H2O2 utilization in this system. In another experiment, Fe-bpydc was added solely to the solution of H2O2 (without substrate). Interestingly, no remarkable decomposition of H2O2 was observed in 150 min (Fig. 5b). After phenol was added artificially, the consumption of H2O2 happened subsequently. These results clearly show that the presence of organic substrate is necessary for the decomposition of H2O2 in the catalytic system of Fe-bpydc, which is distinct from the substrate independent H2O2 consumption in the traditional Fenton system. Because the invalid decomposition of H2O2 (produce O2 and H2O) is negligible in the Fe-bpydc catalysed system, H2O2 is consumed primarily by the degradation of substrate, which gives a high H2O2 utilization efficiency.
The intermediates formed during the degradation of phenol were determined by HPLC method. Besides the peak of residue substrate, two additional peaks were shown in the UV chromatograms of the reacted solutions (Fig. S7, ESI†). By comparing the retention time with authentic compounds, these peaks could be assigned respectively to o- and p-benzenediols, the hydroxylated products of phenol. These two intermediates accumulated with the conversion of phenol initially, and reached their maximum concentrations in 30 min (Fig. S8, ESI†). The concentrations of benzenediols decreased with the further prolongation of reaction time due to secondary reactions, and these intermediates were consumed nearly completely in 210 min. To monitor the selectivity of these two benzenediols, an experiment with high substrate concentration (to minimize the secondary consumption of intermediates) was carried out (Fig. 6). The total formation of these two intermediates in 180 min was 0.32 mM, which accounts for 58% of the consumption of substrate (0.56 mM). This result indicates that o- and p-benzenediol are the main primary oxidation intermediates of phenol. It is expected that, in the catalytic oxidation system of Fe-bpydc, phenol undergoes a hydroxylation process initially, and then the further oxidation of the formed hydroxylated intermediates leads to the ring-opening and even mineralization of the substrate, so no other intermediates (without UV absorption) were detected in our experiments.
 | ||
Fig. 6 Consumption of substrate and accumulation of hydroxylated intermediates during the degradation of phenol with Fe-bpydc as catalyst. 0.01 g L−1 Fe-bpydc, c0phenol = 1.25 mM,  = 1.5 mM. = 1.5 mM. | ||
The formation of hydroxylated products as main intermediates illustrates that the catalytic oxidation is via a ˙OH pathway. To confirm the participation of ˙OH, ESR experiment was carried out with DMPO as spin trapper. After the addition of H2O2, the characteristic fourfold peaks of DMPO–˙OH adduct with the intensity ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was recorded (Fig. 7). This finding clearly proves that ˙OH works as the dominant active species in the catalytic degradation system of Fe-bpydc.
1 was recorded (Fig. 7). This finding clearly proves that ˙OH works as the dominant active species in the catalytic degradation system of Fe-bpydc.
 | ||
| Fig. 7 The ESR signals of the system of Fe-bpydc/phenol before and after the addition of H2O2 (1.5 mM). 0.01 g L−1 Fe-bpydc, c0phenol = 0.25 mM, 20 mM DMPO was added as spin-trapper. | ||
Conclusions
In summary, we have synthesized an Fe(II) bipyridinedicarboxylate based MOF catalyst, which presents a 2D morphology of nanoplate. This catalyst exhibited high efficiency towards the activation of H2O2 to induce the degradation of organic pollutants, and maintained good stability in the reaction system. The TON of Fe-bpydc can reach 23 in three consecutive degradation cycles, much exceeding previously reported MOF based Fenton systems. The degradation reaction can be carried out in the wide pH range of 3–6. The measurement of the concentration change of H2O2 indicates that Fe-bpydc can only consume H2O2 in the presence of organic substrates, which prevents the invalid decomposition of H2O2 and enhances its utilization efficiency. Above results show that Fe-bpydc could be used as an ideal heterogeneous Fenton catalyst to the water purification. This work also indicates that the reasonable selection of organic linker and the minimization of particle size are viable methods to enhance the performance of MOF catalyst.Acknowledgements
This work is supported by the National Natural Science Foundation of China (21307062, 20671053) and Tianjin Research Program of Application Foundation and Advanced Technology (14JCQNJC08000).Notes and references
- Z. Wang, C. Chen, W. Ma and J. Zhao, J. Phys. Chem. Lett., 2012, 3, 2044–2051 CrossRef CAS.
- A. D. Bokare and W. Choi, J. Hazard. Mater., 2014, 275, 121–135 CrossRef CAS PubMed.
- Venny, S. Gan and N. K. Ng, Chem. Eng. J., 2012, 213, 295–317 CrossRef CAS.
- E. Brillas, I. Sires and M. A. Oturan, Chem. Rev., 2009, 109, 6570–6631 CrossRef CAS PubMed.
- P. V. Nidheesh, RSC Adv., 2015, 5, 40552–40577 RSC.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, ChemSusChem, 2011, 4, 1712–1730 CrossRef CAS PubMed.
- S. Navalon, M. Alvaro and H. Garcia, Appl. Catal., B, 2010, 99, 1–26 CrossRef CAS.
- L. Xu and J. Wang, Appl. Catal., B, 2012, 123–124, 117–126 CrossRef CAS.
- Y. Zhao and J. Hu, Appl. Catal., B, 2008, 78, 250–258 CrossRef CAS.
- H. Chen, Z. Zhang, Z. Yang, Q. Yang, B. Li and Z. Bai, Chem. Eng. J., 2015, 273, 481–489 CrossRef CAS.
- Y. Zhang, K. Zhang, C. Dai, X. Zhou and H. Si, Chem. Eng. J., 2014, 244, 438–445 CrossRef CAS.
- J. Deng, J. Jiang, Y. Zhang, X. Lin, C. Du and Y. Xiong, Appl. Catal., B, 2008, 84, 468–473 CrossRef CAS.
- H. Ji, W. Song, C. Chen, H. Yuan, W. Ma and J. Zhao, Environ. Sci. Technol., 2007, 41, 5103–5107 CrossRef CAS PubMed.
- Z. Han, J. Guo and W. Li, Chem. Eng. J., 2013, 228, 36–44 CrossRef CAS.
- S. A. Messele, O. S. G. P. Soares, J. J. M. Orfao, F. Stueber, C. Bengoa, A. Fortuny, A. Fabregat and J. Font, Appl. Catal., B, 2014, 154, 329–338 CrossRef.
- M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782–835 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- V. Stavila, A. A. Talin and M. D. Allendorf, Chem. Soc. Rev., 2014, 43, 5994–6010 RSC.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- H. Lv, H. Zhao, T. Cao, L. Qian, Y. Wang and G. Zhao, J. Mol. Catal. A: Chem., 2015, 400, 81–89 CrossRef CAS.
- L. Ai, C. Zhang, L. Li and J. Jiang, Appl. Catal., B, 2014, 148, 191–200 CrossRef.
- S. E.-d. H. Etaiw and M. M. El-bendary, Appl. Catal., B, 2012, 126, 326–333 CrossRef CAS.
- S. de Rosa, G. Giordano, T. Granato, A. Katovic, A. Siciliano and F. Tripicchio, J. Agric. Food Chem., 2005, 53, 8306–8309 CrossRef CAS PubMed.
- T. A. Vu, G. H. Le, C. D. Dao, L. Q. Dang, K. T. Nguyen, P. T. Dang, H. T. K. Tran, Q. T. Duong, T. V. Nguyen and G. D. Lee, RSC Adv., 2014, 4, 41185–41194 RSC.
- M. Cheng, W. Ma, C. Chen, J. Yao and J. Zhao, Appl. Catal., B, 2006, 65, 217–226 CrossRef CAS.
- J. Li, W. H. Ma, Y. P. Huang, M. M. Cheng, J. C. Zhao and J. C. Yu, Chem. Commun., 2003, 2214–2215 RSC.
- R. C. Finn and J. Zubieta, Solid State Sci., 2002, 4, 83–86 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, FT-IR spectrum and TGA curve of the catalyst, intermediate concentration change and typical HPLC chromatogram during phenol decay, degradation kinetics of 4-CP and MB, and the SEM image of the used catalyst. See DOI: 10.1039/c5ra22779h |
| This journal is © The Royal Society of Chemistry 2016 |