The polymeric nanofilm of triazinedithiolsilane capable of resisting corrosion and serving as an activated interface on a copper surface†
Yabin Wangab,
Zhong Liub,
Yaping Dongb,
Wu Lib,
Yudong Huang*a and
Yutai Qi*a
aSchool of Chemical Engineering and Technology, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: ydhuang.hit1@aliyun.com; inqdsd@yahoo.com.cn; Fax: +86 451 86221048; Tel: +86 451 86414806
bQinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining, Qinghai 810008, P. R. China
First published on 18th December 2015
Abstract
It seems self-contradictory that a copper surface can resist corrosion and be activated concurrently. On the one hand, an activated surface has a high affinity for water (H2O) and chloride ions (Cl−), which significantly accelerate corrosion; on the other hand, only inert/unactivated/hydrophobic surfaces can exhibit outstanding corrosion resistance. This investigation concentrates on fabricating a novel multifunctional polymeric nanofilm that can resist corrosion and serve as an activated interface on a copper surface simultaneously, as well as revealing the functional mechanism of the nanofilm. A triazinedithiolsilane compound (TESPA) was self-assembled onto a copper surface with subsequent heating to obtain such a multifunctional interface. In order to study its protective ability, octadecyltrichlorosilane (OTS), which can yield substances that are hazardous to copper, was selected to be anchored, forming a bilayer of TESPA-OTS. To confirm the activating ability of the polymeric nanofilm, octyltriethoxysilane (OTES), as a friendly reagent, was grafted onto the surface (TESPA-OTES). Electrochemical tests were applied to determine the corrosion resistance of the bilayers, the contact angle (CA) was measured to monitor changes in the wetting properties/chemical groups, scanning electron microscopy (SEM) was performed to observe the morphologies, and energy-dispersive X-ray spectroscopy (EDS) was used to detect the chemical states. The results from comparative experiments show that OTS and OTES can be successfully anchored to the functionalized copper surface via SiOH groups that originated from the polymeric nanofilm; disulfide units (–SS–) and siloxane networks (SiOSi) efficiently protect the copper surface. In short, the investigation definitely proves that the polymeric nanofilm not only protects the copper, but also serves as an activated interface on the copper surface. This multifunctional interface is expected to open up possibilities for other OH-containing reagents to be anchored onto a copper surface in demanding research or industrial applications such as catalysis and coloring and paint processes that need a protective and activated medium for higher performance.
1. Introduction
In the first part, we propose a novel design route to synthesize new types of inhibitors that can be applied to different metals to resist corrosion.1 By assembling protective triazinedithiol (TDT) and silane groups, triazinedithiolsilane compounds that are capable of protecting copper have been successfully fabricated. 6-(3-Triethoxysilylpropyl)amino-1,3,5-triazine-2,4-dithiol monosodium salt (TESPA-TDT, abbreviated as TESPA) is just one of these triazinedithiolsilane compounds. A polymeric nanofilm prepared by heating a self-assembled monolayer (SAM) of TESPA protects copper from corroding with satisfactory inhibition efficiency. The preparation process of the TESPA polymeric nanofilm is shown in Fig. S1.†In the second part,2 the chemical reactions between the TESPA monomer and the copper substrate, as well as changes in the components during the process of preparing the polymeric nanofilm, were revealed by X-ray photoelectron spectroscopy (XPS). The results show that –SS– units and SiOSi networks act as protective structures. Furthermore, measurement of the contact angle reveals the highly hydrophilic properties of the TESPA-containing copper surfaces, which are due to the silanol groups on top of the nanofilms. In these two studies, a bare copper surface and another surface heated at elevated temperatures in an oven were used as reference samples; a TESPA-treated copper surface and a TESPA-treated surface after heating were compared. For simplification, the abbreviations of the four surfaces are as follows: Cu-Bare, Cu-Heat, Cu-TESPA (the TESPA SAM), and Cu-TESPA-Heat (the TESPA polymeric nanofilm). It should be pointed out that although an activated interface on the copper surface was developed during the process, leading to hydrophilic surfaces of polar SiOH groups in both Cu-TESPA and Cu-TESPA-Heat, the protective structures of disulfide units (–SS–) and siloxane networks (SiOSi) were only developed by heating the TESPA SAM.
The former investigations only emphasized the corrosion resistance and chemical mechanism. As we investigated the unique texture of the TESPA polymeric nanofilm, we supposed that the polymeric nanofilm could in theory resist corrosion and serve as an activated interface on a copper surface concurrently. Consequently, this study will shed light on its multifunctionality. In order to evaluate its activating ability, octadecyltrichlorosilane (OTS) and octyltriethoxysilane (OTES) were chosen to react with the functionalized interface, because these two silanes can be hydrolyzed and produce SiOH groups, which theoretically provide active sites for the subsequent reaction with SiOH groups from the TESPA polymeric nanofilm. In the case of estimating the protective ability, the corrosion resistances of bilayers obtained via grafting OTS and OTES were compared. This design (the choice of OTS and OTES) depends on the chemical characteristics of these two reagents. OTES, which contains an octyl group, belongs to the class of alkoxysilanes and its hydrolytic by-product is alcohol, which makes OTES environmentally friendly and extensively applied as a corrosion inhibitor for metals.3 OTS, which contains an octadecyl group, as a chlorosilane produces hydrogen chloride (HCl) during the grafting procedure if it can be anchored onto SiOH-containing surfaces. It was reported that the concentration of HCl on the substrate surface was very high and the local pH could even reach 0.5–1.4 This abundant HCl would cause serious corrosion on metal substrates by both H+ and Cl−. In this study, we also pre-modified a copper surface directly with OTS (see Fig. S2 in the ESI†). Fig. S2† shows SEM images of a copper surface modified directly with OTS on scales of 5 μm and 1 μm. It is clear that lots of amorphous white particles (Fig. S2b) have emerged and are distributed on the copper surface (Fig. S2a†). The copper surface has been extensively destroyed compared with the bare surface (Fig. S2c and d†). The white particles were also analyzed by EDS (see Fig. S3†). A high content of chlorine is present in these particles and the surface without the white particles contains no Cl, which demonstrates that the white particles are the products of a reaction between copper and OTS. As a result, few studies have utilized OTS as a protector for metals,5–7 which makes grafting OTS on a copper surface worthwhile and meaningful in this study. Moreover, silanes could only be chemically anchored to hydroxyl (–OH)-containing metal surfaces such as stainless steel and aluminium. It is impossible to directly graft silanes onto copper due to the absence of OH groups on the surface.5–7 This study attempts to demonstrate a feasible route to graft this type of compound onto a copper surface.
Assuming that OTS and OTES can be successfully anchored onto TESPA-containing interfaces (i.e., the activating ability is realized), copper surfaces with a TESPA SAM and a TESPA polymeric nanofilm were designed to be treated by the abovementioned silanes, with the purpose of revealing the protective role of disulfide units (–SS–) and siloxane networks (SiOSi). Taking OTS as an example, both Cu-TESPA and Cu-TESPA-Heat were immersed in an OTS solution, leading to Cu-TESPA-OTS and Cu-TESPA-Heat-OTS surfaces. In addition, both Cu-TESPA-OTS and Cu-TESPA-Heat-OTS surfaces were cured in order to study the protective ability of newly formed siloxane networks between the silane reagents and the TESPA-containing surfaces. Thus, four surfaces obtained via treatment with OTS or OTES were prepared in the experiment and are listed in Table 1.
| Experiment | Surface 1 | Surface 2 | Surface 3 | Surface 4 |
|---|---|---|---|---|
| OTS | Cu-TESPA-OTS | Cu-TESPA-OTS-Heat | Cu-TESPA-Heat-OTS | Cu-TESPA-Heat-OTS-Heat |
| OTES | Cu-TESPA-OTES | Cu-TESPA-OTES-Heat | Cu-TESPA-Heat-OTES | Cu-TESPA-Heat-OTES-Heat |
2. Experimental
2.1. Preparing TESPA SAM and TESPA polymeric nanofilm
The solution used for self-assembly of TESPA consisted of 3 mM TESPA monomer dissolved in an ethanol/distilled water (95/5, v/v) mixed solvent with a pH of 5 obtained by adding acetic acid and examined by means of a pH meter. The solution was stirred and hydrolyzed for 15 h at 35 °C. Millipore-Q water (18.2 MΩ cm) was used in all solutions. Unless noted otherwise, all reagents used were of analytical grade. Pure copper plates, which were cut to dimensions of 20 mm by 20 mm by 1 mm thick for electrochemical examinations, were abraded with emery papers of 1000 grit and 800 grit followed by fine polishing with alumina paste with particle sizes of 0.35 mm, 0.2 mm, and 0.1 mm to achieve a mirror finish. The samples were then degreased with acetone and alcohol in an ultrasonic bath for 15 min and finally rinsed with copious Millipore-Q water. After cleaning, the copper specimens were immediately immersed in TESPA solution for 15 min, thoroughly rinsed with ethanol and pure water, and finally dried by cold air from a hair dryer (TESPA SAM), or aged at 100 °C for 15 min in an air oven (the TESPA polymeric nanofilm). Herein, a bare copper surface and another surface heated at 100 °C for 15 min were used as reference samples.The copper samples were then immersed (dip-coating method) into a solution of OTES or OTS for 15 min,8 then rinsed sequentially with ethanol and pure water to remove unbound SAM, and finally dried by cold air. The OTS solution consisted of 3 mM OTS dissolved in anhydrous toluene and stirred for 2 hours. OTES (3 mM) was dissolved in ethanol (95%) and hydrolyzed for 15 h at a pH of ca. 4.9
2.2. Electrochemical measurements
Electrochemical measurements were conducted on an Autolab workstation (Netherlands) using a standard three-electrode system at 25 ± 1 °C. A solution of 3.5 wt% NaCl used as the electrolyte was not deoxygenated and was open to air during each test process. The obtained specimens were successively used as the working electrode with an area of 0.785 cm2 (a circle with a diameter of 1 cm) exposed to the NaCl solution. A saturated calomel electrode (SCE) with a salt bridge in a Luggin capillary served as the reference electrode and a platinum panel (2 cm2) as the counter electrode. All potential values were referred to ESCE. Cyclic voltammetry (CV) was performed by scanning the potential from −0.7 V to 0.6 V with a sweep rate of 10 mV s−1. Electrochemical impedance spectroscopy (EIS) was carried out in the frequency range from 100 kHz to 10 mHz with 100 points at an amplitude of the excitation signal of 10 mV under open-circuit potential (Eocp). All EIS data were collected after immersing the working electrodes in the electrolyte for 50 min to reach a stable situation.1 Potentiodynamic polarization curves (Tafel) were recorded from Eocp − 350 mV to Eocp + 350 mV at a scan rate of 1 mV s−1. Before the data were recorded, the tested specimens were also immersed in the electrolyte for 50 min in an attempt to achieve a steady state.2.3. Equivalent circuits and contact angle
By comparing experimental data with simulated data, the configurations of equivalent circuits (EC) regarding the TESPA-containing surfaces were tested using the ZSimpWin software. The quality of an EC was first judged by a chi-squared (χ2) value and secondly by error distribution. Contact angle measurements were performed on a XG-CAMB contact angle analyzer manufactured by Xuanyichuangxi Industrial Equipment Co., Ltd. (Shanghai, China). Data were collected at five different points on each sample surface, resulting in an average value.2.4. Scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS)
A SEM-EDS study was performed with a JSM-5610LV/INCA (JEOL Ltd., Japan), using a high-resolution environmental scanning electron microscope equipped with an energy-dispersive X-ray spectrometer (EDAX, Japan). Energy-dispersive spectroscopy of each surface was carried out in random areas five times to identify the elements that were present and their contents, which can further confirm the uniform coverage of the nanofilms.3. Results and discussion
3.1. Grafting OTS onto TESPA-containing surfaces
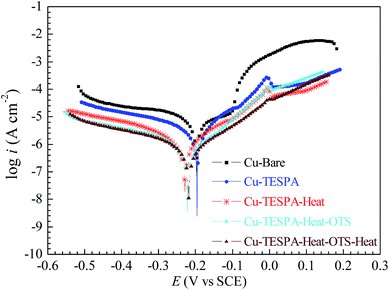
Fig. 1 presents changes in the Tafel plots for the four samples. The anodic polarization curve for bare copper can be split into three main regions: an apparent Tafel region, which is close to the corrosion potential (the formation of a CuCl ad-layer), a limiting-current region with an anodic current peak centred around +0.095 V (the dissolution of the CuCl film), and a mixed-kinetics region above the limiting-current potential (the formation of divalent copper species).11 Cu-TESPA exhibits remarkable declines in the anodic and cathodic currents, implying that the TESPA SAM significantly inhibits anodic dissolution and protects copper. The Tafel plot of Cu-TESPA-OTS is nearly the same as that of Cu-TESPA. The heated OTS-covered TESPA SAM provides better corrosion protection than the OTS-modified TESPA SAM without heating. In general, the Tafel results are in good agreement with the results from CV and the interpretations presented in the earlier section. It should also be mentioned that OTS possesses a long alkyl chain composed of eighteen carbons and a chlorosilane group. The reaction between the chlorosilane moiety and silanol groups from the TESPA SAM gives rise to an outer arrangement of the long chain, i.e., the long alkyl chain is on top of the nanofilms. The hydrophobic properties of TESPA-OTS surfaces act as another contribution to the corrosion resistance of OTS-anchored bilayers. This aspect will be discussed under the contact angle test.
EIS was also performed to understand the electrochemical behaviour of the four samples (see Fig. 2). The Nyquist impedance diagram of bare copper consists of a depressed semicircle at high frequency and a straight line at low frequency. The semicircle, which is caused by the charge-transfer process, is related to the time constant of the charge-transfer resistance (Rct) and the double-layer capacitance (Cdl) at the interface of copper/NaCl solution.1,12 When the semicircle is depressed, this can be attributed to the frequency dispersion that is exerted by the inhomogeneity and roughness of the substrate surface.13 The straight line, which is typically known as the Warburg impedance, can be ascribed to the anodic diffusion process of copper chloride compounds from the electrode surface to the bulk solution,14 the presence of which indicates that diffusion dominates rather than a charge-transfer process under these circumstances. For Cu-TESPA, the Warburg impedance still exists at low frequencies but the capacitive loop becomes large, which indicates that the TESPA SAM is densely packed and hinders diffusion. For OTS-anchored copper surfaces both without and with heating, the Warburg impedances disappear at low frequencies. The observation of only large capacitive loops shows that the processes in the two samples are controlled by charge-transfer instead of diffusion, which is entirely different from the reference and Cu-TESPA samples and reveals that the bilayers are more densely packed and hinder diffusion. The increase in the diameter of the capacitive loop for Cu-TESPA-OTS-Heat compared with Cu-TESPA-OTS indicates better corrosion inhibition; it suggests the formation of disulfide units (–SS–) as the TESPA SAM on the copper surface is heated. The regularity of EIS, which is consistent with the Tafel results, further substantiates that the best protective performance is that of the heated Cu-TESPA-OTS surface.
In order to determine the difference between the bilayers (TESPA-OTS), the equivalent circuits (EC) of OTS-treated Cu-TESPA without/with heating were also derived and are given in Fig. 2. The quality of an EC was first judged by a chi-squared (χ2) value and secondly by error distribution. All the reference EC that are excluded here can be found in Part One.1 Excellent agreement can be observed between the experimental data (points) and the fitted curve (solid line). The value of χ2 for the OTS-treated Cu-TESPA without/with heating calculated by ZSimpWin software is below 10−3. Combined with the observation of only large capacitive loops (charge-transfer dominates), it is reasonable to use the R(Q(R(RQ))) circuit for the two nanofilms. The calculated values of each element in the equivalent circuits are listed in Table 2, where Rs, Rfilm and Rct are the solution resistance, resistance of the coating and charge-transfer resistance and Qfilm and Qdl represent the capacitance of the coating and capacitance of the copper/solution interface, respectively.
| Sample | Cu-TESPA-OTS | Cu-TESPA-OTS-Heat | Cu-TESPA-Heat-OTS | Cu-TESPA-Heat-OTS-Heat |
| Chi-squared (χ2) | 3.72 × 10−3 | 5.36 × 10−3 | 4.66 × 10−3 | 5.19 × 10−3 |
| Circuit | R(Q(R(RQ))) | R(Q(R(RQ))) | R(Q(R(RQ))) | R(Q(R(RQ))) |
| Rs (Ω cm2) | 16.27 | 14.54 | 8.63 | 8.16 |
| Qfilm (Ssn cm−2) | 3.05 × 10−5 | 2.05 × 10−5 | 1.48 × 10−5 | 1.22 × 10−5 |
| nfilm | 0.80 | 0.61 | 0.61 | 0.59 |
| Rfilm (Ω cm2) | 295 | 524 | 1110 | 1661 |
| Qdl (Ssn cm−2) | 1.02 × 10−5 | 6.39 × 10−6 | 9.12 × 10−7 | 6.05 × 10−8 |
| ndl | 0.80 | 0.74 | 0.83 | 0.88 |
| Rct (Ω cm2) | 1.79 × 104 | 1.97 × 104 | 2.34 × 104 | 2.94 × 104 |
In order to clarify these issues about the protective ability of the TESPA polymeric nanofilm, CV, Tafel and EIS tests on the OTS-treated TESPA polymeric nanofilm (Cu-TESPA-Heat) were also carried out. Herein, Cu-TESPA-Heat is added to all the curves as a new reference. In general, the curves for OTS-modified Cu-TESPA-Heat with/without heating from all three measurements can be easily differentiated from those for Cu-TESPA-Heat, which indicates that OTS can react with the TESPA polymeric nanofilm, although the number of SiOH groups is fewer than that of the TESPA SAM. OTS can be successfully anchored onto the TESPA polymeric nanofilm through the residual SiOH groups.
Fig. S5† shows the current for the five samples as a function of the potential in a CV test. It can be seen that the TESPA polymeric nanofilm further protects the copper surface, which is consistent with the result in Part 1. When this surface is modified with OTS, the peak area and peak height in the CV curve of Cu-TESPA-Heat-OTS undergo a slight increase, but are still smaller/lower than those of Cu-Bare. Upon heating the surface, the peak area and peak height appear to be smaller, which suggests a significant enhancement of corrosion resistance. Compared with the OTS-modified TESPA SAM without/with heating in Fig. S4,† the OTS-treated TESPA polymeric nanofilms exhibit a stronger resistance ability. It can be concluded that the formed –SS– units and SiOSi networks preferentially protect the surface.
The changes in the Tafel plots for the five samples are compared in Fig. 3. Cu-TESPA-Heat exhibits remarkable declines in the anodic and cathodic currents, which implies that the polymeric nanofilm significantly inhibits anodic dissolution and protects copper. After coating OTS on the TESPA polymeric nanofilm, the resulting bilayers further resist corrosion. However, the curves for OTS-anchored Cu-TESPA-Heat without/with heating are indistinct, compared with the difference for OTS-anchored Cu-TESPA (see Fig. 1). This subtle variation stems from the amount of OTS that is grafted; the TESPA polymeric nanofilm affords fewer SiOH groups for subsequent reactive sites.
EIS was also performed to understand the electrochemical behaviour of the five samples: see Fig. 4. The increase in the size of the capacitive loops from OTS-treated Cu-TESPA-Heat without heating to the heated sample confirms the increase in protective ability. For the TESPA polymeric nanofilm and OTS-treated Cu-TESPA-Heat surfaces, the appearance of only capacitive loops implies that charge transfer dominates. Therefore, the equivalent circuits of OTS-treated Cu-TESPA-Heat without/with heating are similar to that of OTS-treated Cu-TESPA and can be expressed as R(Q(R(RQ))) as well. The calculated values of each element in the equivalent circuits are also listed in Table 2.
Fig. 5 shows the phase angle plots for the OTS-modified TESPA SAM and TESPA polymeric nanofilms without/with heating. As was observed earlier, one time constant appears in the phase angle plot for bare copper. The TESPA SAM and polymeric nanofilm possess two time constants; the OTS-treated TESPA SAM and polymeric nanofilm have two time constants as well. However, in comparison, the additional time constants of the OTS-treated TESPA SAM and polymeric nanofilm are more evident than those of the TESPA-containing nanofilms alone (see Fig. 7 in Part One1). Herein, when the TESPA polymeric nanofilms are treated with OTS, the angles at high frequencies increase compared with the OTS-modified TESPA SAM, which displays abrupt and distinct angles, giving rise to relatively flattened curves. In general, the appearance of an additional time constant implies the formation of a new, highly water-resistant structure at the interface of the substrate and the nanofilm. A plausible explanation is that the number of SiOH groups determines the behaviour of the phase angle plots; the number of SiOH groups is reduced because they condense with one another as the TESPA SAM is cured.
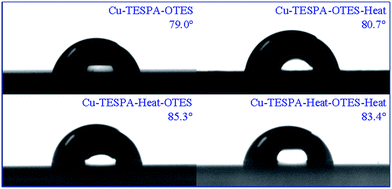
When OTS was coated on TESPA-containing surfaces, the contact angles greatly increased (>90°) and the surfaces became hydrophobic, which is indicative of the successful attachment of OTS to SiOH groups from the TESPA nanofilms. The contact angles for the OTS-anchored TESPA polymeric nanofilms exceed those for the OTS-anchored TESPA SAM, which is in agreement with the change rule, i.e., the contact angle of the TESPA SAM becomes larger when it is heated to yield the TESPA polymeric nanofilm. As is known, the wettability of solid surfaces is governed by their surface geometrical structures and surface free energy.17 When bare copper is heated, the contact angle changes from 63.1° to 84.6°, which indicates that the surface structure substantially varies, as supported by the SEM image in Part Two. As the TESPA SAM is cured, the contact angle changes from 44.7° to 69.3°, which suggests that the SiOH groups condense with each other and their number dramatically decreases, as shown by the XPS results earlier. Thus, these two aspects lead to the relatively large difference between the OTS-modified TESPA SAM and the polymeric nanofilm. Because hydrophobic properties are closely related to the micro- and nanostructure, detailed information about structural changes in OTS-coated surfaces will be studied as part of our upcoming investigations.
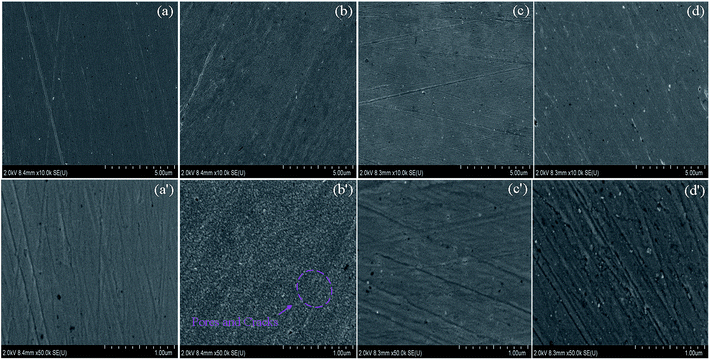
In order to thoroughly understand the origin of the white particles, EDS elemental mapping was carried out on the TESPA- and OTS-modified copper surface (see Fig. S9 in the ESI†). This clearly reveals that there are high contents of C and Cl in the particles. The copper surface without particles is uniformly covered by a bilayer of TESPA and OTS. These observations confirm not only that the white particles are the products of a reaction between TESPA and OTS, but also that the TESPA SAM primarily reacts with OTS and protects the copper substrate. Notably, the morphologies of the white spots (Fig. S7†), the white particles on the copper surface directly modified with OTS (Fig. S3†), and the white particles on the TESPA-covered surface modified with OTS (Fig. S8†) vary, which reveals that they are different compounds. Even on heating the OTS-modified TESPA SAM, the formed bilayer cannot resist corrosion well, because the surface has been damaged already. White particles still cover the surface of the OTS-modified TESPA polymeric nanofilm without heating (Fig. S6g†); however, their number greatly declines and their shape is close to that on the bare surface (Fig. 7g). It can be concluded that the TESPA polymeric nanofilm resists corrosion to some extent; the preformed disulfide units (–SS–) and siloxane networks (SiOSi) act as a protective structure. Upon curing, the white spots in Fig. S6h† are difficult to observe, which indicates that the heated OTS-modified TESPA polymeric nanofilm extensively hinders corrosion (Fig. 7h).
As we know, the difference between the OTS-modified TESPA polymeric nanofilm without heating and the heated film is the formation of SiOSi links (other aspects are the same such as the number of SiOH groups and the octyl chain). This part originates from OTS and the TESPA polymeric nanofilm (another SiOSi part originates from TESPA itself when it is cured). Although the number of SiOH groups in the TESPA polymeric nanofilm decreases compared with that in the TESPA SAM, these active groups play a significant role in grafting new agents and developing siloxane networks (SiOSi) to protect the copper. The SEM images of the reference and studied surfaces offer direct evidence of visual observations that the TESPA polymeric nanofilm not only prevents copper from corroding to some extent, but also serves as an activated interface on the copper surface.
3.2. Grafting OTES onto TESPA-containing surfaces
In order to determine the activating ability of the TESPA SAM and the TESPA polymeric nanofilm, OTES, as an agent that is harmless to copper surfaces, was selected to be coated on these interfaces. Tafel and EIS tests were conducted; the curves for the OTES-modified Cu-TESPA and Cu-TESPA-Heat without/with heating can be easily differentiated, which indicates that OTES can react with TESPA-containing surfaces. In other words, OTES can be successfully anchored onto these interfaces via SiOH groups from both reactants.In order to study the properties of the bilayers, the configurations of equivalent circuits were also derived. On the basis of the equivalent circuits for the OTS-anchored TESPA SAM and the polymeric nanofilms, it is reasonable to use the R(Q(R(RQ))) circuit for OTES-anchored surfaces where charge transfer dominates. The electrochemical parameters are listed in Table 3. As far as the OTES-coated surfaces are concerned, the value of Qfilm changes irregularly without the regularity of the OTS-coated surfaces. To a large extent, this inconsistency is attributed to the length of the alkyl chain (octyl vs. octadecyl). The value of Qdl gradually decreases owing to a decrease in the dielectric constant caused by the adsorption of OTES and structural changes (the formation of SiOSi and SS) in the nanofilms, as OTS-modified surfaces exhibit.
| Sample | Cu-TESPA-OTES | Cu-TESPA-OTES-Heat | Cu-TESPA-Heat-OTES | Cu-TESPA-Heat-OTES-Heat |
| Chi-squared (χ2) | 3.72 × 10−3 | 5.90 × 10−3 | 4.67 × 10−3 | 5.19 × 10−3 |
| Circuit | R(Q(R(RQ))) | R(Q(R(RQ))) | R(Q(R(RQ))) | R(Q(R(RQ))) |
| Rs (Ω cm2) | 25.46 | 13.03 | 23.54 | 25.26 |
| Qfilm (Ssn cm−2) | 1.11 × 10−6 | 9.96 × 10−6 | 3.11 × 10−6 | 9.49 × 10−6 |
| nfilm | 0.81 | 0.57 | 0.81 | 0.58 |
| Rfilm (Ω cm2) | 2756 | 4809 | 2580 | 3716 |
| Qdl (Ssn cm−2) | 3.48 × 10−5 | 1.17 × 10−5 | 1.33 × 10−6 | 3.30 × 10−7 |
| ndl | 0.99 | 0.89 | 0.80 | 0.95 |
| Rct (Ω cm2) | 2.58 × 104 | 4.52 × 104 | 1.76 × 104 | 3.68 × 104 |
By comparing the values of Rfilm and Rct in Tables 2 and 3, it is obvious that the surfaces with the heated OTES-modified TESPA SAM and the TESPA polymeric nanofilm resist corrosion more effectively than any of the OTS-modified surfaces. OTS has a longer alkyl chain than that of OTES; if HCl does not develop during the process of fabricating the bilayer and damage the TESPA nanofilms, it is certain that the protective ability of the bilayer with an octadecyl group is more powerful than that of the bilayer with an octyl group, such as when the second layer is composed of friendly trimethoxyoctadecylsilane or triethoxyoctadecylsilane. From another point of view, the scheme of choosing OTS and OTES as the monomers for the grafting layers is practical and effectual. Furthermore, the values of Qdl for OTES-modified surfaces are smaller than the values for OTS-modified surfaces, which suggest that the dielectric constants decline from the former surfaces to the latter. As a result, the long alkyl chain has a great influence on the dielectric properties of the bilayers.
Phase angle plots for the OTES-modified TESPA SAM and the TESPA polymeric nanofilms without/with heating are displayed in Fig. 11. New time constants appear at low frequency; however, these are indistinct for all the surfaces. The new time constants for the OTS-modified surfaces can be observed easily (Fig. 5). The difference in the additional time constant between the OTS-modified interfaces and the OTES-modified surfaces therefore suggests that the various interfacial phases form as a result of reactions between OTS/OTES and SiOH groups from the TESPA-containing surfaces. The new and original time constants are mixed in the TESPA-OTES system, which indicates that the properties of the formed new phase resemble those of Cu-TESPA and Cu-TESPA-Heat. The XPS and CA results in Part One have proved that SiOH groups and SiOSi plus SiOH groups exist on the above two surfaces. OTES belongs to the class of alkoxysilanes, whereas OTS acts as a chlorosilane. Thus, the chemical difference determines the form of the new time constant.
We also carried out energy-dispersive spectroscopy on each OTES-modified surface in random areas five times. Fig. S10a–d† show one representative of each examination. The elements present, their contents, and the coefficients (σ) are also listed in the inset tables. The results for every surface are identical, which indicates that TESPA-OTES is uniformly distributed on the copper surface. Chlorine cannot be detected, as expected. The reason why the carbon contents of the OTS-treated surfaces exceed those of the OTES-treated surfaces is that OTS possesses a longer alkyl chain.
3.3. Chemical mechanism and prospects
By now, we can confirm that the TESPA polymeric nanofilm not only resists corrosion but also serves as an activated interface on the copper surface concurrently. Moreover, the functionality of the TESPA SAM is incidentally interpreted. Based on the abovementioned results, two models of Cu-TESPA (the TESPA self-assembled monolayer) and Cu-TESPA-Heat (the TESPA polymeric nanofilm) are proposed for use in the following coating applications. If the reagents chosen to be anchored onto TESPA-containing surfaces are detrimental to the substrate, Cu-TESPA-Heat is preferable due to its protective ability; if the reagents are harmless, Cu-TESPA acts as a suitable interface that is full of SiOH activated sites. It should be emphasized that the molar concentration of TESPA was low (only 3 mM) in this investigation; the concentration was purposely chosen to highlight its protective and activating ability (other concentrations bring about an inconspicuous contrast effect). Therefore, it is easy to fabricate a multifunctional interface with excellent corrosion resistance and numerous silicon hydroxyl groups (activated sites) just by increasing the concentration. Application experiments regarding this aspect will be covered in future.4. Conclusions
The multifunctionality of the TESPA polymeric nanofilm has been confirmed by suitably selecting modifiers to prepare bilayers of TESPA-OTS and TESPA-OTES. The functionalized copper surface can resist corrosion and serve as an activated interface on the copper surface simultaneously. Electrochemical tests and SEM show that disulfide units (–SS–) and siloxane networks (SiOSi) from the polymeric nanofilm efficiently protect the surface. The residual silanol groups (SiOH) serve as activated sites for the subsequent chemical reactions. This functional interface could be utilized for further demanding industrial applications such as colouring and paint processes that need a protective and activated medium for higher performance.Acknowledgements
The authors gratefully acknowledge financial supports provided by the International S&T Cooperation Program of China (2013DFR40700), the National Natural Science Foundation of China (No. 21174034, No. 51003019 and No. 51302280), the Natural Science Foundation of Qinghai (2014-ZJ-936Q), and the Young Scholar Project of Qinghai Institute of Salt Lakes, Chinese Academy of Sciences.Notes and references
- Y. Wang, Z. Liu, D. Li, Y. Dong, W. Li and N. Li, Corros. Sci., 2015, 98, 382–390 CrossRef CAS.
- Y. Wang, Z. Liu, Y. Huang and Y. Qi, Appl. Surf. Sci., 2015, 356, 191–202 CrossRef CAS.
- J. Feng, G. Xu, Y. An and X. Zeng, Colloids Surf., A, 2008, 316, 194–201 CrossRef CAS.
- S. Pillai and R. K. Pai, Ultramicroscopy, 2009, 109, 161–166 CrossRef CAS PubMed.
- M. Itoh, H. Nishihara and K. Aramaki, J. Electrochem. Soc., 1995, 142, 1839–1846 CrossRef CAS.
- R. Haneda and K. Aramaki, J. Electrochem. Soc., 1998, 145, 2786–2791 CrossRef CAS.
- M. Itoh, H. Nishihara and K. Aramaki, J. Electrochem. Soc., 1995, 142, 3696–3704 CrossRef CAS.
- S. Hsieh, W. J. Chao, P.-Y. Lin and C. W. Hsieh, Corros. Sci., 2014, 80, 427–433 CrossRef CAS.
- M. C. Brochier Salon and M. N. Belgacem, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 240–254 CrossRef CAS.
- A. V. Benedeti, P. T. A. Sumodjo, K. Nobe, P. L. Cabot and W. G. Proud, Electrochim. Acta, 1995, 40, 2657–2668 CrossRef.
- G. Kear, B. D. Barker and F. C. Walsh, Corros. Sci., 2004, 46, 109–135 CrossRef CAS.
- Y. Feng, W. K. Teo, K. S. Siow, K. L. Tan and A. K. Hsieh, Corros. Sci., 1996, 38, 369–385 CrossRef CAS.
- X. Wu, H. Ma, S. Chen, Z. Xu, A. Sui, X. Wu, S. Chen and A. Sui, J. Electrochem. Soc., 1999, 146, 1847–1853 CrossRef CAS.
- S. Li, Y. Wang, S. Chen, R. Yu, S. Lei, H. Ma and D. Liu, Corros. Sci., 1999, 41, 1769–1782 CrossRef CAS.
- M. D. G. Destreri, J. Vogelsang, L. Fedrizzi and F. Deflorian, Prog. Org. Coat., 1999, 37, 69–81 CrossRef.
- E. Mccafferty and N. Hackerman, J. Electrochem. Soc., 1972, 119, 146–154 CrossRef CAS.
- B. N. Sahoo and B. Kandasubramanian, RSC Adv., 2014, 4, 22053–22093 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19490c |
| This journal is © The Royal Society of Chemistry 2016 |