 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
First heterometallic GaIII–DyIII single-molecule magnets: implication of GaIII in extracting Fe–Dy interaction†
Sihuai
Chen
*ab,
Valeriu
Mereacre
*a,
Christopher E.
Anson
a and
Annie K.
Powell
*ac
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology, Engesserstrasse15, 76131 Karlsruhe, Germany. E-mail: valeriu.mereacre@kit.edu; annie.powell@kit.edu
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: chensihuai@fjirsm.ac.cn
cInstitute of Nanotechnology, Karlsruhe Institute of Technology, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 10th May 2016
Abstract
The compounds of the system [M4M′2(μ3-OH)2(nbdea)4(C6H5CO2)8]·MeCN, where M = GaIII, M′ = DyIII (2), M = FeIII, M′ = YIII (3) are isostructural to the known [Fe4Dy2] compound (1). Those of the system [M4M′4(μ3-OH)4(nbdea)4(m-CH3C6H4CO2)12]·nMeCN, where M = GaIII, M′ = DyIII, n = 4 (5), M = FeIII, M′ = YIII, n = 1 (6) are isostructural to the [Fe4Dy4] compound (4). This allows for comparisons between single ion effects of the paramagnetic ions. The structures were determined using single crystal analysis. Magnetic susceptibility measurements reveal that the GaIII–DyIII compounds 2 and 5 are SMMs. The energy barrier for 2 is close to that for the known isostructural Fe4Dy2 compound (1), but with a significantly increased relaxation time.
Introduction
Single molecule magnets (SMMs) are a class of coordination clusters displaying the phenomena of slow magnetic relaxation at low temperature.1–3 In order to observe such behaviour, it is usually necessary to have a high spin ground state S and magnetic anisotropy, normally of an easy axis type as quantified by the zero-field splitting parameter, D. This implies using a strategy to increase the total spin quantum number S via the synthesis of high-nuclearity clusters,4–8 and/or increasing the magnetic anisotropy, with the idea of incorporating rare earth metal ions with their large single-ion magnetic anisotropies seen as an attractive proposition in the preparation of new generations of SMMs.9–12 This works well for many of the heterometallic 3d–4f SMMs which have been investigated and reported in recent years.13–16In terms of 3d ions in general, although the high spin FeIII ion is isotropic in its ground state, the presence of nearby excited states in many FeIII systems17 means that SMM properties are observed for a number of examples.18–23 Although in pure FeIII systems, the exchange interactions are predominantly antiferromagnetic in nature, the combination of FeIII ions with highly anisotropic LnIII spin carriers can lead to ferromagnetically coupled FeIII–4f coordination clusters exhibiting SMM behaviour.24–34
Up to now, N-substituted diethanolamine or triethanolamine ligands have been widely used for the synthesis of the FeIII–4f coordination clusters because of their chelating and bridging capabilities.35–39 Recently, we reported the synthesis, structures and magnetic properties of [Fe4Dy2(μ3-OH)2(nbdea)4 (C6H5CO2)8]·MeCN (1) and [Fe4Dy4(μ3-OH)4(nbdea)4(m-CH3C6H4CO2)12]·MeCN (4) complexes by employing N-butyldiethanolamine (nbdeaH2) as ligand.40 Both compounds displayed ferromagnetic interactions and the [Fe4Dy2] compound (1) showed SMM behaviour. In order to study the magnetic contributions of FeIII or DyIII spin carriers within both compounds we have extended the work by replacing either FeIII ions with diamagnetic GaIII centres or the DyIII ions with diamagnetic YIII centres. Herein, we described the synthesis, structures and magnetic properties of two series of heterometallic complexes, namely [Ga4Dy2(μ3-OH)2(nbdea)4(C6H5CO2)8]·MeCN (2) and [Fe4Y2(μ3-OH)2(nbdea)4(C6H5CO2)8]·MeCN (3), which are isostructural to the [Fe4Dy2] compound (1), and [Ga4Dy4(μ3-OH)4(nbdea)4(m-CH3C6H4CO2)12]·4MeCN (5) and [Fe4Y4(μ3-OH)4(nbdea)4(m-CH3C6H4CO2)12]·MeCN (6), which are isostructural to the [Fe4Dy4] compound (4).
Experimental
General procedures
Unless otherwise stated, all chemicals and solvents were obtained from commercial sources and were used without further purification. All reactions were carried out under aerobic conditions. [Fe3O(C6H5CO2)6(H2O)3]·(O2CC6H5), [Ga3O(C6H5CO2)6(H2O)3]·(O2CC6H5)·2H2O and [Fe3O(m-CH3C6H4COO)6(H2O)(C2H5OH)2](NO3)·2H2O were prepared according to the literature procedure.41,42 Compound 5 was synthesised by sealing the reaction mixture in transparent 20 mL Biotage Microwave Reaction Kits (http://www.biotage. com) and placing the vials in an oven at 120 °C under normal solvothermal conditions, rather than under microwave conditions. Elemental analyses for C, H, N were performed using an ElementarVario EL analyzer and were carried out at the Institute of Inorganic Chemistry, Karlsruhe Institute of Technology. IR spectra were measured on a Perkin-Elmer Spectrum One spectrometer using KBr pellets and the X-ray powder diffraction patterns were measured at room temperature using a Stoe STADI-P diffractometer with a Cu-Kα radiation. The synthetic procedures for compounds 1 and 4 were previously reported.40Preparation of [Ga4Dy2(μ3-OH)2(nbdea)4(C6H5CO2)8]·MeCN (2)
A mixture of [Ga3O(C6H5CO2)6(H2O)3]·(O2CC6H5) (0.063 g, 0.06 mmol), Dy(NO3)3·6H2O (0.030 g, 0.07 mmol) and nbdeaH2 (0.081 g, 0.50 mmol) in MeCN (20 ml) was stirred at room temperature for 10 minutes. This mixture was heated to 80 °C and became clear. The resulting solution was further stirred for 3 h at 80 °C and then left undisturbed in air. Colorless blocks were crystallized after one day. Yield: 15% (based on Dy). Anal. Calc. for C90H113N5O26Ga4Dy2: C, 47.31; H, 4.99; N, 3.07; found C, 47.36; H, 4.71; N, 3.37%. IR (KBr)/cm−1: 3437 (br), 2959 (s), 1962 (w), 1643 (s), 1598 (s), 1558 (s), 1493 (m), 1449 (s), 1399 (vs), 1322 (s), 1301 (s), 1174 (m), 1135 (m), 1084 (s), 1026 (s), 908 (m), 824 (m), 721 (s), 690 (m), 677 (s), 632 (w), 590 (m), 537 (mw).Preparation of [Fe4Y2(μ3-OH)2(nbdea)4(C6H5CO2)8]·MeCN (3)
To a solution of Y(NO3)3·6H2O (0.096 g, 0.25 mmol) and nbdeaH2 (0.322 g, 2.00 mmol) in MeCN (10 ml) was dropwise added a solution of [Fe3O(C6H5CO2)6(H2O)3]·(C6H5CO2) (0.250 g, 0.23 mmol) in MeCN (10 ml) during the stirring. The mixture was stirred at room temperature for one hour. The resulting clear solution was left undisturbed in air. Yellow blocks were crystallized after three hours. Yield: 38% (based on Y). Anal. Calc. for C90H113N5O26Fe4Y2 (·2H2O): C, 51.03; H, 5.57; N, 3.31; found C, 50.86; H, 5.18; N, 3.19%. IR (KBr)/cm−1: 3431 (br), 2960 (m), 1643 (m), 1598 (s), 1558 (s), 1493 (w), 1449 (m), 1401 (vs), 1322 (s), 1302 (m), 1174 (mw), 1135 (w), 1085 (m), 1026 (m), 905 (mw), 824 (mw), 719 (s), 690 (mw), 676 (m), 590 (m), 544 (w).Preparation of [Ga4Dy4(μ3-OH)4(nbdea)4(m-CH3C6H4O2)12]·4MeCN (5)
A mixture of Ga(NO3)3·xH2O (0.030 g, 0.12 mmol), Dy(NO3)3·6H2O (0.054 g, 0.12 mmol), nbdeaH2 (0.161 g, 1.00 mmol) and m-CH3C6H4CO2H (0.067 g, 0.49 mmol) in MeCN (10 ml) was sealed in a 20 mL microwave reaction vial. The reaction mixture was kept at 120 °C for 24 h. After cooling, the colourless crystals were collected, washed with MeCN and dried in the air. Yield: 9% (based on Dy). Anal. Calc. for C136H168N8O36Ga4Dy4: C, 47.77; H, 4.95; N, 3.28; found C, 47.36; H, 4.71; N, 3.57%. IR (KBr)/cm−1: 3546 (m), 3436 (br), 2958 (m), 1625 (s), 1612 (s), 1600 (s), 1567 (s), 1404 (vs), 1285 (m), 1225 (m), 1162 (w), 1110 (m), 1041 (w), 984 (w), 901 (m), 786 (m), 755 (s), 687 (mw), 672 (m), 585 (m), 455 (m).Preparation of [Fe4Y4(μ3-OH)4(nbdea)4(m-CH3C6H4CO2)12]·MeCN (6)
Compound 6 was obtained using the similar procedure as for 3, but using [Fe3O(m-CH3C6H4CO2)6(H2O)(C2H5OH)2](NO3)·2H2O in place of [Fe3O(C6H5CO2)6(H2O)3]·(C6H5CO2). After one day yellow needles were crystallized. Yield: 43% (based on Y). Anal. Calc. for C128H156N4O36Fe4Y4 (loss of lattice MeCN): C, 52.91; H, 5.41; N, 1.93; found C, 52.41; H, 5.20; N, 1.90%. IR (KBr)/cm−1: 3551 (m), 3438 (br), 2958 (m), 1627 (s), 1599 (s), 1568 (s), 1405 (vs), 1286 (mw), 1226 (mw), 1163 (w), 1111 (s), 1042 (w), 986 (w), 901 (m), 786 (m), 755 (s), 686 (w), 672 (m), 584 (m), 456 (m).Physical measurements
Crystallographic and structure refinement data are summarised in Table 1. Crystallographic data (excluding structure factors) for the structures of 2 and 5 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 1460736 and 1460737. The previously published structures of compounds 1 and 4 have deposition numbers CCDC 1000674 and 1000675.40
| 1 (ref. 40) | 2 | 3 | 4 (ref. 40) | 5 | 6 | |
|---|---|---|---|---|---|---|
| a The structures of compounds 3 and 6 were not fully refined. The unit cells are included here for comparison: either 3 with 1 from ref. 40 and 2, or 6 with 4 from ref. 40 and 5. | ||||||
| Formula | C90H113N5O26Fe4Dy2 | C90H113N5O26Ga4Dy2 | C90H113N5O26Fe4Y2 | C130H159N5O36Fe4Dy4 | C136H168N8O36Ga4Dy4 | C130H159N5O36Fe4Y4 |
| M r | 2229.25 | 2284.73 | 2082.10 | 3241.02 | 3419.65 | 2946.71 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic | Triclinic | Triclinic |
| Space group | P21/c | P21/c | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| T (K) | 298(2) | 180(2) | 298(2) | 180(2) | 150(2) | 298(2) |
| a (Å) | 15.3144(16) | 15.0554(5) | 15.318 | 14.0948(11) | 15.4662(4) | 14.3970 |
| b (Å) | 29.190(4) | 29.0734(11) | 29.220 | 16.8520(13) | 16.8729(5) | 16.7858 |
| c (Å) | 22.497(2) | 22.3017(8) | 22.524 | 29.6754(19) | 29.1650(8) | 29.7649 |
| α (°) | 90 | 90 | 90 | 89.624(6) | 87.415(2) | 89.148 |
| β (°) | 108.001(12) | 106.594(4) | 107.88 | 87.037(6) | 87.793(2) | 86.314 |
| γ (°) | 90 | 90 | 90 | 73.680(6) | 68.609(3) | 73.756 |
| V (Å3) | 9564.5(18) | 9355.2(6) | 9594 | 6755.4(9) | 7077.4(4) | 6883.5 |
| Z | 4 | 4 | 4 | 2 | 2 | 2 |
| D calc (g cm−3) | 1.662 | 1.605 | ||||
| F (000) | 4608 | 3432 | ||||
| μ (mm−1) | 2.790 | 2.911 | ||||
| Reflections collected | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 641 641 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 399 399 |
||||
| Unique reflections | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158 158 |
||||
| R int | 0.0597 | 0.0454 | ||||
| Parameters | 1148 | 1704 | ||||
| R 1 (I > 2σ(I)) | 0.0660 | 0.0653 | ||||
| wR2 (all data) | 0.1196 | 0.1195 | ||||
| S (all data) | 1.103 | 1.122 | ||||
| CCDC | 1000674 | 1460736 | 1000675 | 1460737 | ||
Results and discussion
Synthesis and crystal structures
The reaction of Dy(NO3)3 with the [Ga3O]7+ benzoate triangle and nbdeaH2 at 80 °C in MeCN afforded the hexanuclear GaIII4DyIII2 (2), while the octanuclear GaIII4DyIII4 (5) was synthesized at 120 °C under solvothermal conditions for 24 h using Ga(NO3)3/Dy(NO3)3/nbdeaH2/m-CH3C6H4COOH in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. Compounds 3 and 6 can be obtained through the identical procedure as that reported for compound 1 or 4.40 The reactions of [FeIII3O]7+/Y(NO3)3/nbdeaH2 in a molar ratio of 1
8. Compounds 3 and 6 can be obtained through the identical procedure as that reported for compound 1 or 4.40 The reactions of [FeIII3O]7+/Y(NO3)3/nbdeaH2 in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 in MeCN at room temperature produced compounds 3 and 6, respectively. By comparison of their X-ray powder diffraction patterns (Fig. S1†) and unit cells, compounds 1–3 were found to crystallise isotypically. Compound 6 was shown to be crystallise isotypically with compound 4, with both having one lattice MeCN per cluster. Although compound 5 has by contrast four lattice MeCN per cluster, the unit cell is in fact rather similar to that of 4 and 6, apart from a corresponding increase in volume, and the packing is also not disimilar.
8 in MeCN at room temperature produced compounds 3 and 6, respectively. By comparison of their X-ray powder diffraction patterns (Fig. S1†) and unit cells, compounds 1–3 were found to crystallise isotypically. Compound 6 was shown to be crystallise isotypically with compound 4, with both having one lattice MeCN per cluster. Although compound 5 has by contrast four lattice MeCN per cluster, the unit cell is in fact rather similar to that of 4 and 6, apart from a corresponding increase in volume, and the packing is also not disimilar.
Single-crystal X-ray diffraction studies reveal that compounds 1–3 crystallise in the monoclinic space group P21/c and compounds 4–6 in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) .
.
The structure of GaIII4DyIII2 (2) is shown in Fig. 1. The hexanuclear core of 2 exhibits a curved 2Ga:2Dy:2Ga arrangement similar with the previously discussed compound 1.40 Each GaIII centre is chelated by a doubly-deprotonated (nbdea)2− ligand, forming a cationic metalloligand, [Ga(nbdea)]+, which is bridged to another [Ga(nbdea)]+ and the central dimeric [Dy2(μ3-OH)2]4+ unit. Both DyIII ions are eight-coordinate with approximate square antiprismatic geometry, while all the GaIII ions are six-coordinate, exhibiting a distorted octahedral coordination geometry. The central core of compound 5 is based on an approximate square of four coplanar DyIII ions (Fig. 2, top). Each pair of adjacent Dy centres is bridged by a (μ3-OH)− ligand to a GaIII ion, forming an octanuclear core possessing a “Dy4-square-within-a-Ga4-square”. All four Ga centres are nearly coplanar, displaced alternatively slightly above and below the {Dy4} plane; 0.346, 0.281, 0.397 and 0.450 Å for Ga1, Ga2, Ga3 and Ga4, respectively (Fig. 2, bottom).
 | ||
| Fig. 1 Molecular structure of compound [Ga4Dy2(μ3-OH)2(nbdea)4 (C6H5CO2)8]·MeCN (2). Organic hydrogen atoms apart from in the core have been omitted for clarity. | ||
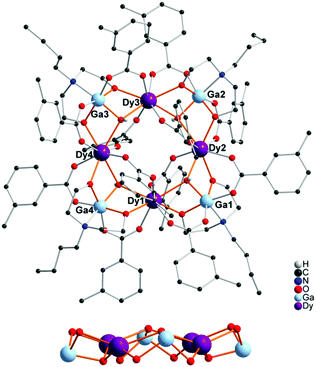 | ||
| Fig. 2 Molecular structure of compound [Ga4Dy4(μ3-OH)4(nbdea)4(m-CH3C6H4O2)12]·4MeCN (5) (top) and a side view of the core in 5 (bottom). Organic hydrogen atoms have been omitted for clarity. | ||
Magnetic properties
The dc magnetic susceptibility data for compounds 2, 3, 5 and 6 were measured in the temperature range 1.8–300 K at 1000 Oe, respectively. For 2 (Fig. 3), the χT product is 27.0 cm3 K mol−1 at 300 K, which is in relatively good agreement with the value (28.4 cm3 K mol−1) corresponding to two uncoupled DyIII (S = 5/2, L = 5, 6H15/2, g = 4/3) and four GaIII (S = 0) ions. On lowering temperature the χT product continuously decreases, reaching the minimum value of 18.8 cm3 K mol−1 at 1.8 K. This type of behaviour suggests that the magnetic interaction between the central DyIII2 unit is weakly antiferromagnetic. The thermal depopulation of the DyIII excited states could also contribute to the decrease of the χT product. In the case of compound 3 (Fig. 3), the χT product of 14.5 cm3 K mol−1 at 300 K is lower than the expected value of 17.5 cm3 K mol−1 for four FeIII (S = 5/2, g = 2) and two YIII (S = 0) non-interacting ions, which indicates the presence of antiferromagnetic interactions between spin carriers. The χT value decreases gradually with decreasing temperature, reaching the minimum value of 0.3 cm3 K mol−1 at 1.8 K, suggesting that the dominant antiferromagnetic interactions between FeIII ions lead to a spin ground state of zero. | ||
| Fig. 3 Temperature dependence of the χT product at 1000 Oe for 1 (blue),402 (red) and 3 (black). | ||
However, the magnetic behaviour of the reported isostructral Fe4Dy2 compound 1 indicates the presence of ferromagnetic interactions between spin centres at very low temperature (Fig. 3).40 Since the Dy–Dy and the Fe–Fe exchange interactions are all antiferromagnetic, it can be concluded that ferromagnetic Fe–Dy interactions are revealed at low temperature. This could be one of the origins for the slow relaxation observed in the bulk magnetic data and the Mössbauer spectra for FeIII containing compounds with antiferromagnetically coupled Fe–Fe pairs as seen in compound 140 and in the similar Fe4Dy2 compound recently reported.30,34 It is worth to mention that, using CoIII as a diamagnetic ion, recent studies have proved the strong magnetic exchange between the CrIII and DyIII ions.44
The field dependences of the magnetisation at low temperatures for compounds 2 and 3 are shown in Fig. S2.† For the Ga4Dy2 compound 2 (Fig. S2,† left), the magnetisation increases rapidly at low fields below 10 kOe, followed by an almost linear increase till 70 kOe. The lack of saturation even up to 70 kOe suggests the thermally and/or field-induced population of low lying excited states, as well as the presence of significant magnetic anisotropy. However, the very low value of 10.1μB at 2 K and 70 kOe is in good agreement with that expected for two DyIII single ions in polycrystalline samples (each ∼5–6μB). The magnetisation measurements with varying scan rate did not show hysteresis. The field dependence of the magnetisation for the Fe4Y2 compound 3 shows a very slow increase with the applied fields and at 2 K only reaches 0.25μB at 70 kOe (Fig. S2,† right), which confirms the antiferromagnetic coupling between FeIII ions.
To investigate the dynamics of the relaxation, ac susceptibility measurements were performed under a zero dc applied field in the 1–1500 Hz frequency range between 1.8 and 20 K. Both temperature- and frequency-dependent in-phase and out-of-phase signals were observed for the Ga4Dy2 compound 2 (Fig. 4 and 5), revealing slow relaxation of magnetization expected for a SMM. In the χ′′ vs. T plot, no maximum is observed at lower frequencies. However, clear peaks and some shoulders are observed at higher frequencies (Fig. 4, right). In addition, there is evidence for one peak which develops at temperatures below 1.8 K. This phenomenon suggests the presence of quantum tunnelling effects and at least two additional relaxation processes in this system. The linear fitting of Arrhenius plots (Fig. S3†) of the data from frequency dependent measurements (Fig. 5, right) give an extracted energy barrier Ueff of 20.9 K, which is very close to the value of 21.4 K for the previously reported isostructural Fe4Dy2 compound 1.40 However, the relaxation time increases significantly from τ0 = 2.7 × 10−8 s (for 1)40 to τ0 = 1.5 × 10−5 s (for 2). This behaviour was also observed in the cases of the reported {CrIII2DyIII2} and {CoIII2DyIII2} systems,45,46 indicating possible suppression of the quantum tunnelling. The Cole–Cole plots in Fig. S4† show nearly symmetric semicircles and were fitted to a generalised Debye function. The resulting α parameter ranges from 0.17 to 0.26 in the temperature range between 1.9 and 7.9 K, indicating a wider distribution of the relaxation time in comparison to the value (α = 0.04–0.13 between 2.4 and 2.9 K) for 1,40 and the presence of multi-relaxation processes in the system.
 | ||
| Fig. 4 Temperature dependence of the in-phase (χ′) (left) and out-of-phase (χ′′) (right) ac susceptibility components at different frequencies in zero dc field for 2. | ||
 | ||
| Fig. 5 Frequency dependence of the in-phase (χ′) (left) and out-of-phase (χ′′) (right) ac susceptibility components at different temperatures in zero dc field for 2. | ||
A dc field of 1500 Oe was applied to further investigate the relaxation dynamic in compound 2 (Fig. 6 and 7). Clear shoulders are observed in the χ′′ vs. T plot (Fig. 6, right). The data from frequency dependent measurements (Fig. 7) were analysed using an Arrhenius law, which gives a characteristic energy barrier Ueff of 41.2 K and a relaxation time τ0 of 2.2 × 10−6 s (Fig. S5†). As shown in Fig. S6,† the Cole–Cole plots for 2 at 1500 Oe can be fitted to a generalised Debye function at high temperature, giving large α parameters in the range 0.31–0.38. At low temperature between 1.9 and 5.5 K, the Cole–Cole plots cannot be fitted well, confirming that more than one relaxation process occurs in this system.
 | ||
| Fig. 6 Temperature dependence of the in-phase (χ′) (left) and out-of-phase (χ′′) (right) ac susceptibility components at different frequencies under 1500 Oe dc field for 2. | ||
 | ||
| Fig. 7 Frequency dependence of the in-phase (χ′) (left) and out-of-phase (χ′′) (right) ac susceptibility components at different temperatures under 1500 Oe dc field for 2. | ||
The χT values for the Ga4Dy45 and Fe4Y46 with the “square-in-square” core topology are 55.7 and 18.4 cm3 K mol−1 at 300 K, which are close to the expected values (56.7 and 17.5 cm3 K mol−1) for non-interacting spin centres: four DyIII (S = 5/2, L = 5, 6H15/2, g = 4/3) and four GaIII (S = 0) ions in 5 or four FeIII (S = 5/2, g = 2) and four YIII (S = 0) ions in 6, respectively. On lowering the temperature from 300 to 50 K, the χT products for both compounds remain almost constant and then rapidly drop to 33.8 and 14.9 cm3 K mol−1 with further cooling to 1.8 K, respectively (Fig. 8, top). The overall behaviour suggests very weak antiferromagnetic interactions between DyIII centres in 5 or between FeIII centres in 6. As shown in Fig. 8 (top), the increase of the χT vs. T curve on decreasing the temperature in the 4–16 K temperature range suggests the presence of weak ferromagnetic interactions as observed in the reported isostructral FeIII4DyIII4 compound 4.40 If both Fe–Fe and Dy–Dy interactions are antiferromagnetic within 4, then the shape of χT vs. T plot for compound 4 at low temperature (Fig. 8, top) leads to the conclusion that the interactions between FeIII and the adjacent DyIII centres must be weakly ferromagnetic. The orientation of the anisotropy axis for each DyIII ion in the Ga4Dy4 compound 5 was calculated using the program, MAGELLAN,47 and shown in Fig. 8 (bottom). All four axes are nearly perpendicular to the Dy4 plane and nearly parallel to each other, similarly to the situation reported for [CrIII4DyIII4], for which the directions of main anisotropy axes were determined from ab initio calculations.48
 | ||
| Fig. 8 (top) Temperature dependence of the χT product at 1000 Oe for 4 (blue),405 (red) and 6 (black); (bottom) the orientations of the anisotropy axes for DyIII ions in compound 5, calculated by MAGELLAN.47 | ||
The field dependent magnetisation measurements at low temperature for 5 show that the magnetisation increases steadily with the application of the external field without saturation even at 70 kOe (Fig. S7,† left). This behaviour indicates the presence of magnetic anisotropy and/or low lying excited states in this system. However, the value of 22.6μB at 2 K and 70 kOe is in good agreement with the expected saturation value for four DyIII isolated ions in polycrystalline samples (each ∼5–6μB). For 6, the magnetisation at 2 K under a field of 70 kOe is 20.7μB (Fig. S7,† right), which is in very good agreement with the presence of four isolated S = 5/2 FeIII ions aligned parallel to the dc field suggesting a possible S = 10 ground state for 6. Although the ac susceptibilities for the FeIII4DyIII4 compound 4 did not show any sign of slow relaxation of the magnetisation,40 the ac susceptibility measurement for 5 in zero dc field shows weak out-of-phase ac signal with no maximum is observable above 1.8 K (Fig. S8†). In order to check any quantum tunnelling effects above 1.8 K, the frequency dependence of the ac susceptibility was measured under different applied dc fields at 1.8 K (Fig. S9†). As shown in Fig. S9† (right), the maximum in the frequency dependent out-of-phase plot is only slightly moved to lower frequency, indicating the absence of quantum tunnelling effects above 1.8 K.
To study the system further, an external dc field of 1000 Oe was applied and both the in-phase and out-of-phase signals show temperature and frequency dependence (Fig. 9 and 10). Although there is no peak observed in the χ′′ vs. T plot (Fig. 9, right), clear peaks are observed in the χ′′ vs. v data (Fig. 10, right). Fitting the data using an Arrhenius law leads to an estimation of the energy gap Ueff = 5.4 K and relaxation time τ0 = 4.1 × 10−5 s (Fig. S10†).
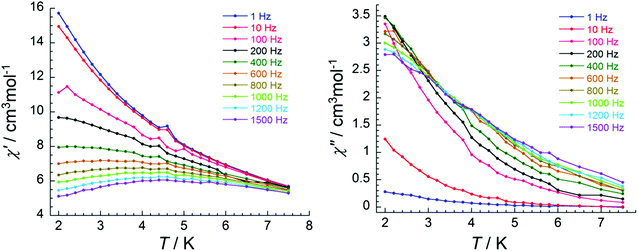 | ||
| Fig. 9 Temperature dependence of the in-phase (χ′) (left) and out-of-phase (χ′′) (right) ac susceptibility components at the indicated frequencies under 1000 Oe dc field for 5. | ||
 | ||
| Fig. 10 Frequency dependence of the in-phase (χ′) (left) and out-of-phase (χ′′) (right) ac susceptibility components at the indicated temperatures under 1000 Oe dc field for 5. | ||
Conclusions
Two series of {M4Ln2} and {M4Ln4} (M = FeIII or GaIII; Ln = DyIII and YIII) complexes have been synthesised in order to study the magnetic contribution of FeIII or DyIII centres within 3d–4f heterometallic systems. Direct current magnetic susceptibility measurements revealed that DyIII–DyIII interactions within 2 or 5 and FeIII–FeIII interactions within 3 or 6 are all weakly antiferromagnetic. Alternating current magnetic susceptibility measurements did not show any SMM behaviour for both the {Fe4Y2} compound 3 and the {Fe4Y4} compound 6 containing diamagnetic YIII ions, while both the {Ga4Dy2} compound 2 and the {Ga4Dy4} compound 5 exhibit out-of-phase ac susceptibility signals, indicating that 2 and 5 are both SMMs. The characteristic SMM energy barrier of 20.9 K for {Ga4Dy2} compound 2 under zero dc field is very close to the value of 21.4 K for the reported isostructural {Fe4Dy2} compound 1. However, the relaxation time increases significantly from 2.7 × 10−8 s for {Fe4Dy2} compound 1 to 1.5 × 10−5 s for {Ga4Dy2} compound 2, indicating the QTM is likely to be suppressed by replacing paramagnetic FeIII ions with diamagnetic GaIIIcentres. Furthermore, although the {Fe4Dy4} compound 4 did not show any sign of magnetisation slow relaxation, the {Ga4Dy4} compound 5 did show slow relaxation of magnetisation. This result suggests that the weak ferromagnetic FeIII–DyIII exchanges quench the magnetic anisotropy of the DyIII ions and the SMM behaviour.Acknowledgements
We acknowledge the Karlsruhe Institute of Technology for financial support.Notes and references
- G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 25, 66 CrossRef CAS.
- S. M. J. Aubin, M. W. Wemple, D. M. Adams, H.-L. Tsai, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1996, 118, 7746 CrossRef CAS.
- D. Ruiz-molina, G. Christou and D. N. Hendrickson, Mol. Cryst. Liq. Cryst., 2000, 343, 17 CrossRef CAS.
- A. M. Ako, I. J. Hewitt, V. Mereacre, R. Clérac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 4926 CrossRef CAS PubMed.
- S. K. Langley, B. Moubaraki and K. S. Murray, Polyhedron, 2013, 64, 255 CrossRef CAS.
- A. Baniodeh, I. J. Hewitt, V. Mereacre, Y. Lan, G. Novitchi, C. E. Anson and A. K. Powell, Dalton Trans., 2011, 40, 4080 RSC.
- S. K. Langley, B. Moubaraki and K. S. Murray, Dalton Trans., 2010, 39, 5066 RSC.
- C. Papatriantafyllopoulou, T. C. Stamatatos, C. G. Efthymiou, L. Cunha-Silva, F. A. Almeida Paz, S. P. Perlepes and G. Christou, Inorg. Chem., 2010, 49, 9743 CrossRef CAS PubMed.
- R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS PubMed.
- F. Habib and M. Murugesu, Chem. Soc. Rev., 2013, 42, 3278 RSC.
- P. Zhang, Y.-N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728 CrossRef CAS.
- M. Andruh, J.-P. Costes, C. Diaz and S. Gao, Inorg. Chem., 2009, 48, 3342 CrossRef CAS PubMed.
- G. E. Kostakis, I. J. Hewitt, A. M. Ako, V. Mereacre and A. K. Powell, Philos. Trans. R. Soc. London, Ser. A, 2010, 368, 1509 CrossRef CAS PubMed.
- J. W. Sharples and D. Collison, Coord. Chem. Rev., 2014, 260, 1 CrossRef CAS PubMed.
- K. Liu, W. Shi and P. Cheng, Coord. Chem. Rev., 2015, 289, 74 CrossRef.
- M. Gerloch, J. Lewis and R. C. Slade, J. Chem. Soc. A, 1969, 1422 RSC.
- A. M. Ako, V. Mereacre, Y. Lan, W. Wernsdorfer, R. Clérac, C. E. Anson and A. K. Powell, Inorg. Chem., 2010, 49, 1 CrossRef CAS PubMed.
- C. Delfs, D. Gatteschi, L. Pardi, R. Sessoli, K. Wieghardt and D. Hanke, Inorg. Chem., 1993, 32, 3099 CrossRef CAS.
- J. C. Goodwin, R. Sessoli, D. Gatteschi, W. Wernsdorfer, A. K. Powell and S. L. Heath, J. Chem. Soc., Dalton Trans., 2000, 1835 RSC.
- R. F. Saalfrank, A. Scheurer, I. Bernt, F. W. Heinemann, A. V. Postnikov, V. Schünemann, A. X. Trautwein, M. S. Alam, H. Rupp and P. Müller, Dalton Trans., 2006, 2865 RSC.
- G. W. Powell, H. N. Lancashire, E. K. Brechin, D. Collison, S. L. Heath, T. Mallah and W. Wernsdorfer, Angew. Chem., Int. Ed., 2004, 43, 5772 CrossRef CAS PubMed.
- M. Murugesu, R. Clérac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2005, 44, 6678 CrossRef CAS PubMed.
- D. Schray, G. Abbas, Y. Lan, V. Mereacre, A. Sundt, J. Dreiser, O. Waldmann, G. E. Kostakis, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 5185 CrossRef CAS PubMed.
- S. Schmidt, D. Prodius, V. Mereacre, G. E. Kostakis and A. K. Powell, Chem. Commun., 2013, 49, 1696 RSC.
- S. Nayak, O. Roubeau, S. J. Teat, C. M. Beavers, P. Gamez and J. Reedijk, Inorg. Chem., 2010, 49, 216 CrossRef CAS PubMed.
- J.-P. Costes, F. Dahan, F. Dumestre, J.-M. Clemente-Juan, J. Garcia-Tojal and J.-P. Tuchagues, Dalton Trans., 2003, 46 Search PubMed.
- J.-P. Costes, A. Dupuis and J.-P. Laurent, Eur. J. Inorg. Chem., 1998, 1543 CrossRef CAS.
- M. Murugesu, A. Mishra, W. Wernsdorfer, K. A. Abboud and G. Christou, Polyhedron, 2006, 25, 613 CrossRef CAS.
- V. Mereacre, F. Klöwer, Y. Lan, R. Clérac, J. A. Wolny, V. Schünemann, C. E. Anson and A. K. Powell, Beilstein J. Nanotechnol., 2013, 4, 807–814 CrossRef CAS PubMed.
- Y.-F. Zeng, G.-C. Xu, X. Hu, Z. Chen, X.-H. Bu, S. Gao and E. C. Sañudo, Inorg. Chem., 2010, 49, 9734 CrossRef CAS PubMed.
- M. N. Akhtar, V. Mereacre, G. Novitchi, J.-P. Tuchagues, C. E. Anson and A. K. Powell, Chem. – Eur. J., 2009, 15, 7278 CrossRef CAS PubMed.
- A. Baniodeh, Y. Lan, G. Novitchi, V. Mereacre, A. Sukhanov, M. Ferbinteanu, V. Voronkova, C. E. Anson and A. K. Powell, Dalton Trans., 2013, 42, 8926 RSC.
- S. Chen, V. Mereacre, C. E. Anson and A. K. Powell, Dalton Trans., 2016, 45, 98 RSC.
- S. Schmidt, D. Prodius, G. Novitchi, V. Mereacre, G. E. Kostakis and A. K. Powell, Chem. Commun., 2012, 48, 9825 RSC.
- V. Mereacre, D. Prodius, Y. Lan, C. Turta, C. E. Anson and A. K. Powell, Chem. – Eur. J., 2011, 17, 123 CrossRef CAS PubMed.
- J. Bartolomé, G. Filoti, V. Kuncser, G. Schinteie, V. Mereacre, C. E. Anson, A. K. Powell, D. Prodius and C. Turta, Phys. Rev. B: Condens. Matter, 2009, 80, 014430 CrossRef.
- A. M. Ako, V. Mereacre, R. Clérac, I. J. Hewitt, Y. Lan, C. E. Anson and A. K. Powell, Dalton Trans., 2007, 5245 RSC.
- G. Abbas, Y. Lan, V. Mereacre, W. Wernsdorfer, R. Clérac, G. Buth, T. Sougrati, F. Grandjean, G. J. Long, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 9345 CrossRef CAS PubMed.
- S. Chen, V. Mereacre, D. Prodius, G. E. Kostakis and A. K. Powell, Inorg. Chem., 2015, 54, 3218 CrossRef CAS PubMed.
- R. F. Weinland and A. Herz, Ber. Dtsch. Chem. Ges., 1912, 45, 2662–2680 CrossRef.
- A. Baniodeh, Cooperative Effects in Non-cyclic and cyclic FeIII/4f Coordination Clusters, Ph.D. Thesis, Cuvillier Verlag, Göttingen, 2013, p. 247 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- S. K. Langley, C. Le, L. Ungur, B. Moubaraki, B. F. Abrahams, L. F. Chibotaru and K. S. Murray, Inorg. Chem., 2015, 54, 3631 CrossRef CAS PubMed.
- S. K. Langley, D. P. Wielechowski, V. Vieru, N. F. Chilton, B. Moubaraki, L. F. Chibotaru and K. S. Murray, Chem. Sci., 2014, 5, 3246 RSC.
- S. K. Langley, D. P. Wielechowski, V. Vieru, N. F. Chilton, B. Moubaraki, B. F. Abrahams, L. F. Chibotaru and K. S. Murray, Angew. Chem., Int. Ed., 2013, 52, 12014 CrossRef CAS PubMed.
- N. F. Chilton, D. Collison, E. J. L. Mclnnes, R. E. P. Winpenny and A. Soncini, Nat. Commun., 2013, 4, 2551 Search PubMed.
- J. Rinck, G. Novitchi, W. Van den Heuvel, L. Ungur, Y. Lan, W. Wernsdorfer, C. E. Anson, L. F. Chibotaru and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 7583 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1460736 and 1460737. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt01364c |
| This journal is © The Royal Society of Chemistry 2016 |
