Tribological behavior of molybdenum disulfide bonded solid lubricating coatings cured with organosiloxane-modified phosphate binder
Yulong Jiaab,
Lei Chen*a,
Xiaozhen Fengc,
Huidi Zhou*a and
Jianmin Chen*a
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, PR China. E-mail: chenlei@lzb.ac.cn; hdzhou@lzb.ac.cn; chenjm@lzb.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, PR China
cAviation Industry Corporation of China South Aviation Industry Co., Ltd, Zhuzhou 412002, PR China
First published on 7th August 2015
Abstract
Hydroxyl-polysiloxane prepolymers were prepared via refluxing methyltriethoxysilane (METS) and dimethoxydimethylsilane (MSDS) at 75 °C in the presence of hydrochloric acid as the reactant. As-obtained prepolymers were used to modify aluminum chromium phosphate binder; and the modified aluminum chromium phosphate binder was adopted to fabricate bonded solid lubricating coatings with MoS2 and Sb2O3 as the solid lubricants. The structure of the modified aluminum chromium phosphate binder was investigated by infrared spectrometry and X-ray photoelectron spectroscopy. The corrosion behavior of the organosiloxane-modified phosphate solid lubricating coatings was examined by salt spray test. Moreover, an MM-200 friction and wear tester and an Optimol SRV-IV oscillating friction and wear tester were performed to evaluate the tribological behavior of the solid lubricating coatings at room temperature and 500 °C. The worn surfaces of the solid lubricating coatings were observed and analyzed with a scanning electron microscope. It was found that P–O–Si bond was formed in the organosiloxane-modified aluminum chromium phosphate binder, which confirmed that there occurs chemical reaction between hydroxyl-polysiloxane prepolymer and the aluminum chromium phosphate binder. As a result, the flexible organic structure bonded in the aluminum chromium phosphate binder favored to improve the toughness, corrosion resistance and tribological behavior of the molybdenum disulfide bonded solid lubricating coatings.
1. Introduction
Bonded solid lubricating coating is a major type of solid lubricating material, and it is widely used in the fields of aviation, spaceflight and weaponry, due to its advantages of simple preparation technology, controllable coating layer thickness, excellent friction-reducing and anti-wear performance and high commercial value.1–5 Also known as dry lubricating film, bonded solid lubricating coating relies on solid lubricants and anti-wear additives bonded by adhesives to reduce friction and wear of mechanical parts. Epoxy resin, phenolic resin, and polyimide resin are traditional organic resin adhesives suitable to bonded solid lubricating coating, while phosphate and silicate are inorganic adhesives.1 Among various inorganic binders phosphate binder is of particular significance, because it exhibits strong adhesion, good impact resistance and low curing temperature.6,7 In the meantime, phosphate-bonded materials as ideal carriers have good strength, excellent dielectric behavior, inoxidizability, and low coefficient of thermal expansion, which also adds to the significance of phosphate binder.7–11 As a kind of inorganic binder, aluminum chromium phosphate is able to resist to temperature as high as 1200 °C,12 which possesses its natural characteristics of high-temperature chemical stability, thermal shock resistance, thermal insulation and oxidation resistance.6,7,13 As a result, aluminum chromium phosphate binder can satisfy lots of special and different requirements and has been widely used in the fields of refractory materials, wave-transmitting materials, composite materials and so on.14–16 However, just as other inorganic materials, phosphate binder has high brittleness, strong hygroscopicity and fair load-bearing capacity.17 Dachuan Chen18 reported a refractory and wear-resistant coating of SiC reinforced aluminum phosphate composites. Shifeng Deng17 used methyltriethoxysilane precursor and aluminum phosphate to prepare inorganic–organic hybrid materials and glass fiber reinforced composites, methyltriethoxysilane improved the brittleness of aluminum phosphate binder and enhanced the flexural strength of glass fiber reinforced composites. Daqing Chen19 utilized silicone resin to modify aluminum chromium phosphate wave-transmitting material, which improved the mechanical properties of tensile strength, shear strength and bending strength of material.Viewing the abovementioned, we pay special attention to inorganic–organic hybrid materials as potential binders of bonded solid lubricating coatings, hoping to acquire lowered brittleness and increased mechanical strength by combining the flexible organic phase with hard inorganic component. In the present research, therefore, we prepared hydroxyl-polysiloxane prepolymers (denoted as HPPS), a category of siloxane with a low degree of polymerization, via refluxing methyltriethoxysilane (METS), dimethoxydimethylsilane (MSDS) and hydrochloric acid at 75 °C. As-obtained prepolymers were used to modify aluminum chromium phosphate binder, and the modified binder was finally adopted to fabricate bonded solid lubricating coatings consisting of MoS2 and Sb2O3 solid lubricants (the incorporation of Sb2O3 into MoS2 bonded solid lubricating coating helps to improve both the endurance life and antioxidant ability of the coating). Such a fabrication strategy, hopefully, may provide bonded solid lubricating coatings with significantly improved tribological properties. The reason lies in that the as-synthesized siloxane possesses good thermal and thermo-oxidative stability, excellent moisture resistance, low surface energy, good flame retardancy and good hydrophobicity, meanwhile it contains Si–OH bond which can chemically react with aluminum chromium phosphate binder, which makes the free rotation of chains about Si–O–Si bonds with limited length connect up to the rigid chains of P–O–P bonds in aluminum chromium phosphate binder. In consequence, the new molecular structure of modified aluminum chromium phosphate binder can bear compressive and tensile force better than original binder, and the toughness, corrosion resistance and load-bearing capacity of phosphate coatings can be enhanced.20–23 This article reports the preparation of aluminum chromium phosphate binder modified by HPPS and the bonded solid lubricating coatings with MoS2 and Sb2O3 as the lubricant fillers, and it also deals with the tribological properties of the as-fabricated solid lubricating coatings in relation to the effect of additive HPPS. In this solid lubricating coating system MoS2 is the main solid lubricant filler, and Sb2O3 is an effective additive instead of a lubricant itself whose dominating roles are acting synergistically with MoS2 to improve friction and wear properties, providing a mechanically hard surface to increase load-carrying capacity and endurance, preventing crack growth, and serving as an oxidation barrier.24–27
2. Experimental details
2.1 Preparation of HPPS
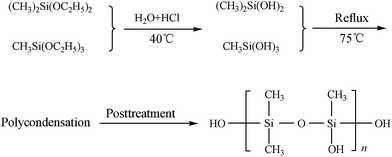
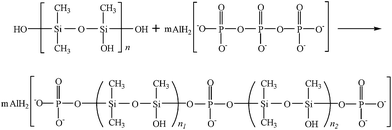
The mixture of METS and MSDS was placed in a round-bottomed flask heated to 40 °C with a water bath and refluxed. Then a proper amount of hydrochloric acid was added to the solution, and the reactant mixture was heated to 75 °C and held there for 4 h to allow the reaction to be completed. At end of the reaction, colorless, transparent and viscous liquid product was obtained by extraction, washing, filtration and evaporation. The schematic plot for synthesizing HPPS is shown in Fig. 1.2.2 Preparation and modification of ACP binder by HPPS
A proper amount of orthophosphoric acid (H3PO4) was placed in a three-necked-round-bottomed flask and diluted with deionized water to 65% (mass fraction; the same hereafter). Resultant solution was heated to 80 °C, and then a proper amount of chromium oxide (CrO3) was dissolved in the solution. When the mixed solution became clear, a proper amount of aluminum hydroxide (Al(OH)3) was added and heated to 100 °C and held there for 3 h to afford ACP binder. Upon completion of the reaction, HPPS was added to the solution at a concentration of 5%, 10% and 15% and stirred for 12 h to yield HPPS-modified ACP binders. As the reaction progressing, the organic phase and inorganic phase merged, accompanied by gradual variation of the solution color from rufous to viscous atrovirens.2.3 Preparation of bonded solid lubricating coatings
MoS2 and Sb2O3 were homogeneously dispersed in ACP binder or HPPS-modified ACP binder, which with a MoS2/Sb2O3 molar ratio of 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixed paint was ground and then sprayed onto 45# steel (30 mm × 7 mm × 6 mm) block and GH4169 steel disc (24 mm in diameter and 7.8 mm in thickness) with a spray gun operating with 1.5 MPa N2 gas. Corresponding bonded solid lubricating coatings were denoted as SI0, SI5, SI10 and SI15, where the numeral suffixes (0%, 5%, 10% and 15%; see Table 1) refer to the content of HPPS in HPPS-modified ACP binders. Prior to coating preparation, the steel substrates were roughened by sandblasting to a surface roughness (Ra) of 2.00 ± 0.20 μm and ultrasonically cleaned with acetone for 15 min. The coating specimens were sequentially cured at 120 °C for 2 h and at 310 °C for 1 h in a muffle furnace to affording as-fabricated bonded solid lubricating coatings with a thickness of 25–30 μm (measured with a coating thickness gauge).
1. The mixed paint was ground and then sprayed onto 45# steel (30 mm × 7 mm × 6 mm) block and GH4169 steel disc (24 mm in diameter and 7.8 mm in thickness) with a spray gun operating with 1.5 MPa N2 gas. Corresponding bonded solid lubricating coatings were denoted as SI0, SI5, SI10 and SI15, where the numeral suffixes (0%, 5%, 10% and 15%; see Table 1) refer to the content of HPPS in HPPS-modified ACP binders. Prior to coating preparation, the steel substrates were roughened by sandblasting to a surface roughness (Ra) of 2.00 ± 0.20 μm and ultrasonically cleaned with acetone for 15 min. The coating specimens were sequentially cured at 120 °C for 2 h and at 310 °C for 1 h in a muffle furnace to affording as-fabricated bonded solid lubricating coatings with a thickness of 25–30 μm (measured with a coating thickness gauge).
| Coating | Thickness (μm) | Ra (μm) | Adhesion strength (grade) | Flexibility (mm) | Impact resistance (cm) |
|---|---|---|---|---|---|
| SI0 | 25–30 | 2.00 ± 0.20 | 0 | 1 | 50 |
| SI5 | 25–30 | 2.00 ± 0.20 | 0 | 1 | 50 |
| SI10 | 25–30 | 2.00 ± 0.20 | 0 | 1 | 50 |
| SI15 | 25–30 | 2.00 ± 0.20 | 0 | 1 | 50 |
2.4 Friction and wear tests
An MM-200 friction and wear tester was performed to evaluate the room temperature tribological behavior of the bonded solid lubricating coatings in a ring-on-block contact configuration. The counterpart GCr15 bearing steel ring (outer diameter Ø40 mm, inner diameter Ø16 mm; wall thickness 10 mm) was driven by an electric motor to slide against the coated 45# steel blocks at a normal load of 980 N, a rotary speed of 200 rpm (rpm; linear speed 0.42 m s−1) and a sliding time of 60 min. An Optimol SRV-IV oscillating friction and wear tester was used to evaluate the high temperature tribological behavior of the coatings in a ball-on-disc contact configuration. A commercially available Al2O3 ball (diameter 9.8 mm; hardness 1650 HV) as the counterpart was driven to slide against the coatings on the GH4169 steel disc at a reciprocating distance of 1 mm, an oscillatory frequency of 5 Hz, a normal load of 10 N, a sliding duration of 30 min, and an elevated temperature of 500 °C. The friction coefficients were automatically measured and recorded by the computer connected to the test rig. A Nano Map 500LS contact surface mapping profiler was performed to measure the cross-section profile of the worn surface of the tested coating specimens. The wear volume loss is determined as ΔV = AL (A refers to the cross-section area (unit: mm2) of the wear track and L (unit: mm) refers to the length of the wear track). The wear rate (unit: mm3 (N−1 m−1)) is calculated as Ws = ΔV/SF, where S refers to the total sliding distance in m and F is the applied load in N. The friction and wear tests of each coating specimen were repeated three times under the same condition to obtain reliable data with minimized scattering. The averages of the repeat tests are cited in this article.A Nicolet NEXUS FTIR spectrometer was performed to record the Fourier transform infrared (FTIR) spectrum of HPPS in the wave number range of 4000–400 cm−1 (KBr pellets were used). A PHI-5702 multifunctional X-ray photoelectron spectroscope (XPS) was employed to identify the chemical structure of HPPS-modified ACP binder (monochromated Al Kɑ radiation; pass energy 29.4 eV), and the binding energy of carbon contaminant (C1s: 284.8 eV) was adopted as the reference. Thermogravimetric-differential scanning calorimetric (TG-DSC) analysis of the ACP binder from room temperature to 1000 °C under air environment was performed with a Netzsch STA-409 PG/PC Jupiter Analyst (heating rate: 10 °C min−1).
The morphologies of surface and worn surface of the coatings were analyzed with a JSM-5600LV scanning electron microscope (SEM; acceleration voltage: 20 kV). The corrosion resistance of the coatings on the stainless steel substrates (50 mm × 120 mm × 0.2 mm) was examined with an SST-9NL salt spray cabinet. The aqueous solution of 3.5% NaCl was used as the corrosive medium. The coating specimens were fixed on the holder at an angle of (65 ± 5)°; and temperature and pressure of the cabinet were fixed at (35 ± 2) °C and 70–170 kPa). Prior to the corrosion tests, all the coating specimens were cleaned with acetone and dried in air.
3. Results and discussion
3.1 Effect of HPPS on the chemical structure of ACP binder
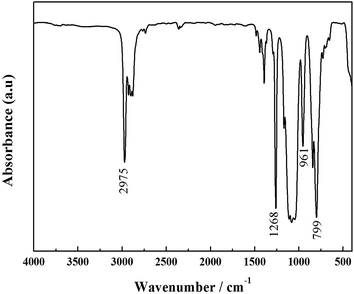
Fig. 2 displays the FTIR spectra of HPPS. The absorption band at 2975 cm−1 is assigned to C–H stretching vibration, the sharp peak at 1268 cm−1 is due to CH3 bending vibration of Si–CH3 group, and the band at 799 cm−1 is attributed to Si–CH3 rocking vibration.28 The broad band at 1000–1130 cm−1 corresponds to Si–O–Si stretching vibration, and the peak at 961 cm−1 belongs to Si–OH bending vibration.29 These FTIR data suggest that polycondensation reaction takes place between METS and MSDS to afford HPPS during the refluxing process.Fig. 3 presents the FTIR spectra of unmodified ACP binder and HPPS-modified ACP binder. Unmodified ACP binder shows a strong band of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching at 1243 cm−1 and a weak band of P
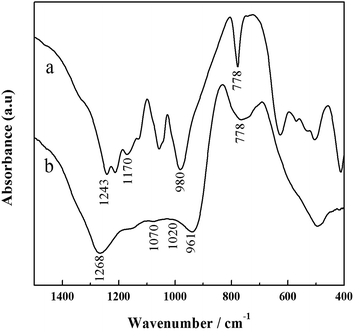
O asymmetric stretching at 1243 cm−1 and a weak band of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching at 1170 cm−1 (Fig. 3a), which could be related to the phase transition from Al(H2PO4)3 to AlH2P3O10·2.5H2O during curing process. In the meantime, the asymmetric stretching band of linear P–O–P chain at 950 cm−1 shifts to 980 cm−1 in association with the decrease of the content of hydroxyl, also due to the effect of curing.30 The board and overlapped band at 1000–1250 cm−1 in Fig. 3b is assigned to Si–O–Si, P–O–P, P–O–Si and P
O symmetric stretching at 1170 cm−1 (Fig. 3a), which could be related to the phase transition from Al(H2PO4)3 to AlH2P3O10·2.5H2O during curing process. In the meantime, the asymmetric stretching band of linear P–O–P chain at 950 cm−1 shifts to 980 cm−1 in association with the decrease of the content of hydroxyl, also due to the effect of curing.30 The board and overlapped band at 1000–1250 cm−1 in Fig. 3b is assigned to Si–O–Si, P–O–P, P–O–Si and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.31 Besides, the bands at 1268 cm−1 and 961 cm−1, corresponding to CH3 bending vibration in Si–CH3 and Si–OH bending vibration, remain unchanged after the ACP binder is modified by HPPS. The absorption band at 778 cm−1 becomes boarder, due to overlapping of cyclic P–O–P symmetric stretching and Si–CH3 rocking vibration. The peak nears 1070 cm−1 is assigned to P–O stretching vibration in P–O–P and P–O–Si linkages, whereas the band near 1020 cm−1 arises from Si–O stretching vibration in Si–O–Si and P–O–Si linkages.32 These consequences demonstrate that ACP binder and HPPS react chemically, thereby forming P–O–Si bond during modification of ACP binder by HPPS.
O.31 Besides, the bands at 1268 cm−1 and 961 cm−1, corresponding to CH3 bending vibration in Si–CH3 and Si–OH bending vibration, remain unchanged after the ACP binder is modified by HPPS. The absorption band at 778 cm−1 becomes boarder, due to overlapping of cyclic P–O–P symmetric stretching and Si–CH3 rocking vibration. The peak nears 1070 cm−1 is assigned to P–O stretching vibration in P–O–P and P–O–Si linkages, whereas the band near 1020 cm−1 arises from Si–O stretching vibration in Si–O–Si and P–O–Si linkages.32 These consequences demonstrate that ACP binder and HPPS react chemically, thereby forming P–O–Si bond during modification of ACP binder by HPPS.
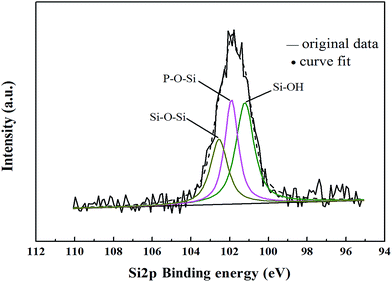
In order to further approve the presence of P–O–Si bond in HPPS-modified ACP binder, we conducted relevant XPS analysis. Fig. 4 shows the XPS Si2p spectrum of the modified ACP binder. The XPS Si2p spectrum can be decomposed into three portions. Namely, the Si2p peak at 101.2 eV belongs to Si–OH, the peak at 101.9 eV corresponds to P–O–Si, and the one at 102.5 eV refers to Si–O–Si.33 The binding energy of Si2p in P–O–Si is higher than it in Si–OH, possibly because P exhibits a higher electronegativity than H, the bonding of P–O–Si results in that the superficial electron cloud of Si atom occurs excursion to one end of P atom, the electron density around Si atom decreases and the shielding effect between electrons of Si reduces, thereby leading to increased binding energy of P–O–Si bond, which is higher than the binding energy of Si–OH.34,35 These XPS data further confirm that there is P–O–Si bond in HPPS-modified ACP binder.
Combining the abovementioned FTIR data with XPS data, we can conclude that HPPS is chemically bonded with the ACP binder to generate HPPS-bonded ACP binder. Namely, the modification of ACP binder by HPPS is a kind of chemical modification, and it is based on the embedding of the flexible organic group (Si(CH3)2OSiCH3O)n of HPPS in the ACP binder as follows:
3.2 Mechanical properties and surface morphology of bonded solid lubricating coatings
The mechanical properties of the as-fabricated bonded solid lubricating coatings are listed in Table 1, where the adhesion between the coating and substrate were tested according to “paints and varnishes-cross cut test for films (Chinese National Standard GB/T 9286)”, the flexibility of the coating was measured according to “determination of flexibility of films (Chinese National Standard GB/T 1731)”, and the impact-resistance ability of the coating was evaluated according to “determination of impact resistance of films (Chinese National Standard GB/T 1732)”.From Table 1, it can be found that the series of coatings are kept consistence in thickness and roughness, which is for the sake of the comparability of experimental results. According to the inspection standards of films, all of the as-fabricated bonded solid lubricating coatings reach the highest standards, as a result the as-fabricated coatings can be used as solid lubricating coatings.
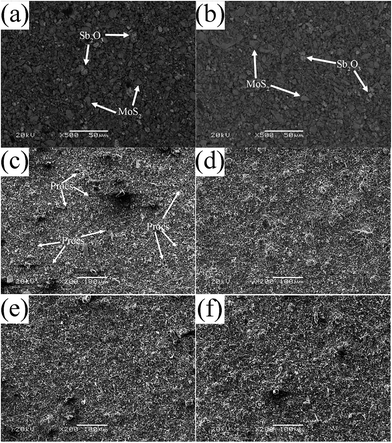
The SEM images of various as-fabricated bonded solid lubricating coatings after curing are displayed in Fig. 5. It is seen that the coatings are mainly composed of gray and deep gray lumps (Fig. 5a and b). The gray lump refers to Sb2O3, since Sb element has a high atomic number and looks bright in the back electron image (BEI). The deep gray lump refers to lamellar MoS2, and it seems that Sb2O3 and MoS2 are uniformly dispersed in both unmodified ACP binder and HPPS-modified ACP binder.
A close observation of Fig. 5c–f indicates that there are many pores on the surface of SI0 coating, and this coating has a loose microstructure (Fig. 5c). Besides, fewer pores are present on the surface of SI5 coating, and the compactness of this coating is better than that of SI0 coating (Fig. 5d). As the content of HPPS in the HPPS-modified ACP binders rises, the SI10 and SI15 coatings tend to be nearly free of pores on the surfaces, and their compactness rise therewith (Fig. 5e and f). Thus it can be inferred that the chemical reaction between ACP binder and HPPS facilitates the formation of a three-dimensional network structure, thereby resulting in better dispersion of the lubricant fillers in the binder as well as increased compactness of the bonded solid lubricating coatings.
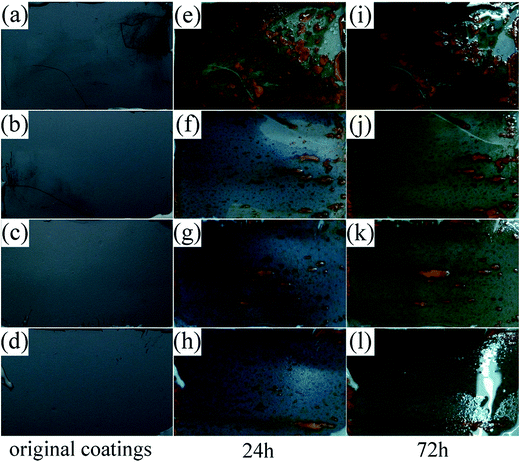
Fig. 6 shows the photographs of the bonded solid lubricating coatings after salt spray test for different duration. It can be seen that, along with the increase of the content of HPPS in HPPS-modified ACP binder, the corroded area of the coatings reduces gradually, which indicates that modifying ACP binders with HPPS helps to improve the corrosion resistance of the bonded solid lubricating coatings to some extent. This is because, on the one hand, increasing content of HPPS in the modified ACP binder helps to increase the compactness of the bonded solid lubricating coatings thereby affording improved corrosion resistance. On the other hand, HPPS is hydrophobic36 and can promote the hydrophobicity of the coatings, which also favors to improve the corrosion resistance of the coatings.
3.3 Effect of HPPS on the tribological properties of the bonded solid lubricating coatings at room temperature
Fig. 7 presents the friction coefficients and wear rates of a series of as-fabricated bonded solid lubricating coatings at room temperature. It can be seen that the friction coefficients reduce firstly and then increase with the increase of HPPS content of HPPS-modified ACP binder, and the largest value of 0.049 is observed for SI15 coating. As to those coatings cured with the modified binders containing more than 5% HPPS, the friction coefficient is higher than that of the coating cured with unmodified ACP, and SI5 coating exhibits the lowest friction coefficient value of 0.043. Moreover, the wear rate of the coatings varies with varying HPPS content in similar manner, but the wear rate of the coatings cured with HPPS-modified ACP binder is lower than that of the coating cured with unmodified ACP binder. Similarly, as shown in Fig. 8 2D wear track of SI5 coating is smoother than that of other three coatings. The profile of worn surfaces become rugged with the HPPS content increasing. The reason may lie in that the modified ACP binder possesses flexible inorganic–organic structure and good load-bearing capacity, thereby providing better wear resistance of the coatings. Particularly, SI5 coating exhibits the lowest wear rate among various tested coatings, which means that the optimum volume fraction of HPPS in the HPPS-modified ACP binder should be 5%.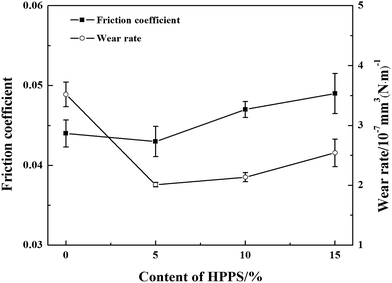 | ||
| Fig. 7 Friction coefficients and wear rates of various bonded solid lubricating coatings which were tested on the MM-200 friction and wear tester at room temperature. | ||
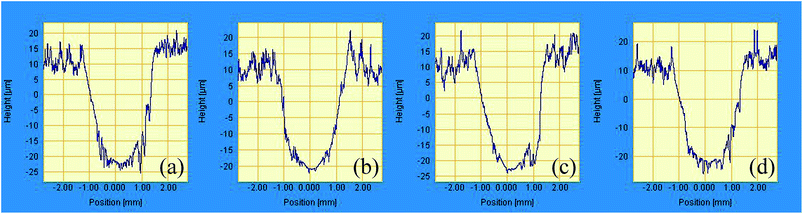 | ||
| Fig. 8 2D contact profile of worn surfaces of (a) SI0, (b) SI5, (c) SI10 and (d) SI15 solid lubricating coatings tested on the MM-200 friction and wear tester at room temperature. | ||
Fig. 9 shows SEM micrographs of the worn surfaces of various bonded solid lubricating coatings after friction tests at room temperature. It can be seen that lubricating film is formed on the worn surface of SI0 coating due to grind of the soft MoS2 solid lubricant (Fig. 9a), but the lubricating film in this case shows sings of brittle fracture and delamination as well as slight plastic deformation, which suggests that the main wear mechanism of SI0 coating is dominated by adhesive wear and plastic deformation. As a consequence the wear rate of SI0 coating is the highest. Similarly, lubricating films are observed on the worn surfaces of various coatings cured with HPPS-modified ACP binders (Fig. 9b–d), but few signs of brittle fracture, delamination or plastic deformation are detected for these coatings. It indicates that appropriate additive amount of HPPS promotes a better capacity of HPPS-modified ACP binders to disperse fillers than pure ACP binder, which makes MoS2 form lubricating film more easily as shown in Fig. 9b. Whereas, due to the additive amount of HPPS increasing, the capacity of lubricating film formation is impeded and lubricating films decrease in the wear tracks of SI10 and SI15 which are displayed in Fig. 9c and d. Therefore, SI5 coating has the lowest friction coefficient and friction coefficients of SI10 and SI15 coatings are higher, evenly exceed SI0 coating. More importantly, reduction of brittle fracture, delamination and plastic deformation of the HPPS-modified coatings owes to the structure and mechanical properties of HPPS-modified ACP binders which improve compactness, toughness and load-bearing capacity of the solid lubricating coatings. Therefore, HPPS-modified ACP binders are favorable for inhibiting the brittle exfoliation and delamination of the bonded solid lubricating coatings. The bonded solid lubricating coatings cured with HPPS-modified ACP binder are dominated by adhesion wear, and their degree of damage caused by adhesion wear seems to rise with increasing content of HPPS in the modified ACP binder, which explains that the wear rates of HPPS-modified coatings are lower than SI0 and with the HPPS amount increasing the wear rate rises.
3.4 Effect of HPPS on the thermal stability and tribological properties of the bonded solid lubricating coatings at 500 °C
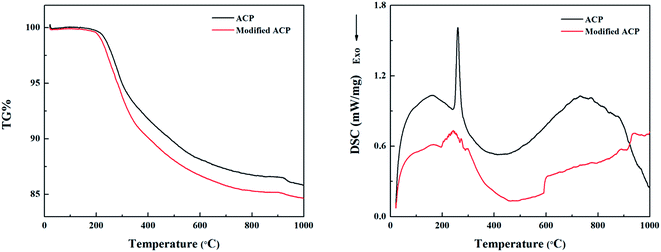
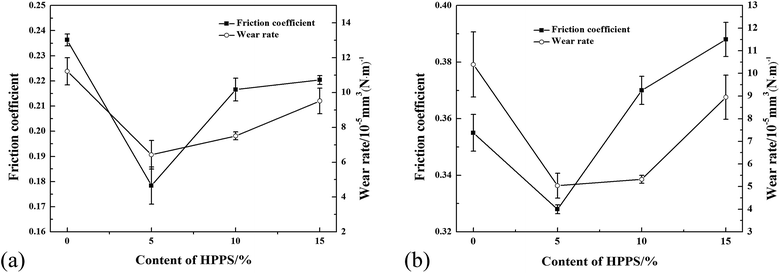
The thermogravimetric-differential scanning calorimetric (TG-DSC) curves of unmodified ACP binder and the ACP binder modified with 5% HPPS are shown in Fig. 10. It can be seen that unmodified ACP binder and the modified ACP binder exhibit similar TG and DSC curves. Namely, they both show an endothermic peak near 200 °C, due to the dehydrolytic condensation of monoaluminum phosphate (Al(H2PO4)3) generating aluminum hydrogen phosphate (AlH3(PO4)2). Besides, they undergo a transition to type B aluminum metaphosphate (Al(PO3)3) near 250 °C, which corresponds to an endothermic peak thereat. The transition to type A aluminum metaphosphate (Al(PO3)3) begins at 700 °C and completes at 900 °C, which is in accordance with that reported elsewhere.37 Differing from neat ACP binder, the modified ACP binder shows a small exothermic peak near 300 °C, and this exothermic peak corresponds to the oxygenolysis of CH3 in HPPS. Moreover, the modified ACP binder provides slightly fewer residue than the neat ACP binder, which is possibly also attributed to the oxygenolysis of CH3 in HPPS.38,39 Therefore, it can be concluded that the HPPS modifier almost has no influence on the thermal stability of ACP binder.Fig. 11 shows the typical friction coefficient curves of a series of solid lubricating coatings at 500 °C. The lifetime of the coatings cured with HPPS-modified ACP binder is longer than that of the coating cured with neat ACP binder by about 50%, which demonstrates that the HPPS modifier is favorable for improving the wear resistance of the bonded solid lubricating coatings at 500 °C. The average friction coefficients and wear rates are determined for three repeated measurements of 20 min duration at the same test condition at room temperature and 500 °C, as shown respectively in Fig. 12. Similar to that shown in Fig. 12a, SI5 coating exhibits the lowest friction coefficient of 0.326 at 500 °C, and the friction coefficient rises with increasing content of HPPS and even exceeds the value of SI0 coating. Moreover, SI0 coating has the largest wear rate at 500 °C, and SI5 coating has the lowest wear rate (5.04 × 10−5 mm3 (N−1 m−1)) thereat. From Fig. 13, it can be seen that the 2D profile of wear track of SI5 coating is the glossiest and the cross-sectional area of it is the smallest, which demonstrates SI5 coating has the lowest wear rate form another side. These friction and wear test data prove that the bonded solid lubricating coating cured with ACP binder modified by 5% HPPS exhibits the best wear resistance at room temperature and elevated temperature of 500 °C as well.
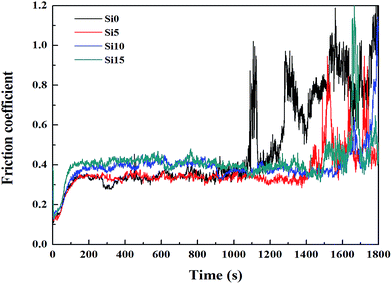 | ||
| Fig. 11 Friction coefficient-sliding time curves of a series of SI coatings which were tested on the Optimol SRV-IV oscillating friction and wear tester at 500 °C. | ||
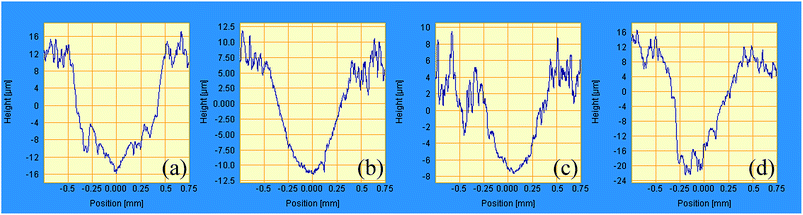 | ||
| Fig. 13 2D contact profile of worn surfaces of (a) SI0, (b) SI5, (c) SI10 and (d) SI15 solid lubricating coatings tested on the Optimol SRV-IV oscillating friction and wear tester at 500 °C. | ||
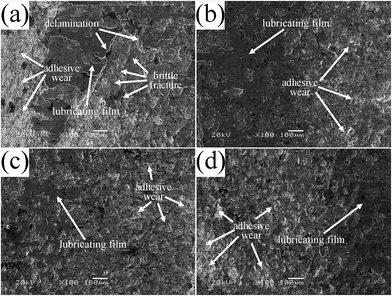
Fig. 14 shows the SEM images of the worn surfaces of various bonded solid lubricating coatings after sliding for 20 min at 500 °C. It can be observed that discontinuous lubricating film is formed on the worn surface of SI0 coating, and this coating is dominated by delamination in association with the generation of a large amount of wear debris (Fig. 14a), which is related to the damage of the lubricating film and the oxidation of MoS2 forms hard oxide particles11,40 generating wear debris, causing delamination of brittle oxides and three-body abrasion wear of the coating. As to the bonded solid lubricating coating cured with 5% HPPS-modified ACP binder, a continuous lubricating film is formed on the greatly smoothened worn surface, and the coating is dominated by slight abrasive wear and micro-ploughing (Fig. 14b), which well corresponds to its lowest friction coefficient and wear rate. When the HPPS content of the modified ACP binder increases to 10% and 15%, corresponding bonded solid lubricating coatings show deep grooves and pits on worn surfaces (Fig. 14c and d), which corresponds to the decrease of the continuity of the lubricating films as well as the slight increase of the friction coefficient and wear rate as compared with SI5 coating. Obviously, brittle fracture and delamination of HPPS-modified coatings decrease heavily as same as the situation at room temperature. It can be concluded that at 500 °C HPPS-modified binders play roles of enhancing the toughness, compactness and load-bearing capacity similarly, which improves the wear resistance of the coatings. Moreover, it seems that the damage of the bonded solid lubricating coatings caused by abrasive wear at elevated temperature of 500 °C becomes more severe as the HPPS content of the modified ACP binder rises from 10% to 15%. Thus it can be concluded that, adding HPPS to ACP binder helps to enhance the friction-reducing and anti-wear properties of the bonded solid lubricating coatings at 500 °C. The optimal HPPS content of the modified ACP binder is suggested as 5%, and increasing HPPS content above this level worsens the tribological properties of the bonded solid lubricating coatings to some extent owing to the decrease of the continuity of the lubricating film on worn coating surface as well as the enhancement of the abrasive wear damage.
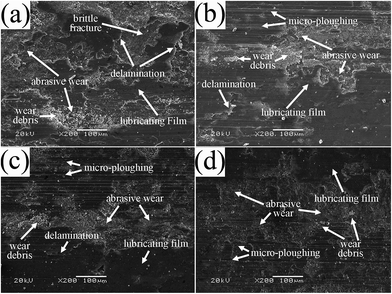 | ||
| Fig. 14 SEM micrographs of the worn surfaces of bonded solid lubricating coatings cured with HPPS-modified ACP binders with different content of HPPS at 500 °C (a: SI0, b: SI5, c: SI10 and d: SI15). | ||
4. Conclusions
Hydroxyl-polysiloxane prepolymers were synthesized and used to modify aluminum chromium phosphate binder. Resultant HPPS-modified ACP binders were adopted to fabricate bonded solid lubricating coatings on mild steel substrate and high-temperature steel with MoS2 and Sb2O3 as the solid lubricants. The tribological properties of the bonded solid lubricating coatings sliding against GCr15 steel ring and Al2O3 ball at room temperature and 500 °C were evaluated. The main conclusions are drawn as follows:(1) HPPS and ACP binder react chemically to generate flexible organic specie (Si(CH3)2OSiCH3O)n, thereby forming network-like structure of HPPS-modified ACP binder through P–O–Si linkage.
(2) The bonded solid lubricating coatings cured with HPPS-modified ACP binder are more compact than the one cured with neat ACP binder, because the HPPS-modified ACP binder is capable of forming three-dimensional inorganic–organic network structure and allows enhanced dispersion of the lubricant fillers.
(3) Because of the increased coating compactness and the hydrophobicity of HPPS itself, the bonded solid lubricating coatings cured with HPPS-modified ACP binder exhibit enhanced corrosion resistance than the coating cured with neat ACP binder.
(4) The bonded solid lubricating coating cured with 5% HPPS-modified ACP binder exhibits the best friction-reducing and anti-wear properties at room temperature and 500 °C, which means that the optimal HPPS content of the modified ACP binder should be 5%. When the HPPS content increases to 10% and 15%, the tribological properties of the bonded solid lubricating are worsened to some extent as compared with SI5 coating, due to enhanced abrasive wear damage therewith.
Acknowledgements
This research is financially supported by National Natural Science Foundation of China (Grant no. 51175491).References
- J. M. Chen, Y. P. Ye and H. X. Dang, Tribology, 1994, 14, 180–189 CAS.
- T. Endo, T. Iijima, Y. Kaneko, Y. Miyakawa and M. Nishimura, Wear, 1995, 190, 219–225 CrossRef CAS.
- K. A. Ravindran and P. Ramasamy, Wear, 1984, 93, 291–297 CrossRef CAS.
- C. J. Beall, Met. Finish., 2000, 98, 513–517 CrossRef.
- K. Miyoshi, Wear, 2001, 251, 1061–1067 CrossRef.
- E. Leivo, M. Vippola, P. Sorsa, P. Vuoristo and T. Mäntylä, J. Therm. Spray Technol., 1997, 6, 205–210 CrossRef CAS.
- M. Vippola, J. Keranen, X. Zou, S. Hovmöller, T. Lepistö and T. Mäntylä, J. Am. Ceram. Soc., 2000, 83, 1834–1836 CrossRef CAS PubMed.
- W. D. Kingery, J. Am. Ceram. Soc., 1950, 33, 239–241 CrossRef CAS PubMed.
- J. Alongi and G. Malucelli, RSC Adv., 2015, 5, 24239–24263 RSC.
- L. P. He, D. C. Chen and S. P. Shang, J. Mater. Sci., 2004, 39, 4887–4892 CrossRef CAS.
- C. C. Liu, L. Chen, J. S. Zhou, H. D. Zhou and J. M. Chen, Appl. Surf. Sci., 2014, 300, 111–116 CrossRef CAS PubMed.
- L. Y. Hong, H. J. Han, H. Ha, J. Y. Lee and D. P. Kim, Compos. Sci. Technol., 2007, 67, 1195–1201 CrossRef CAS PubMed.
- D. P. Kim, H. G. Woo, H. Ha, F. Cao, H. S. Myung, J. S. Rho and K. S. Han, Compos. Sci. Technol., 2003, 63, 493–499 CrossRef CAS.
- D. D. L. Chung, J. Mater. Sci., 2003, 38, 2785–2791 CrossRef CAS.
- N. Chen, S. Gao, J. Huo, H. Wang, J. He and Y. Zhu, J. Therm. Anal. Calorim., 2014, 116, 875–879 CrossRef CAS.
- S. Hoshii, A. Kojima, T. Tamaki and S. Otani, J. Mater. Sci. Lett., 2000, 19, 557–560 CrossRef CAS.
- S. F. Deng, C. F. Wang, Y. Zhou, F. Huang and L. Du, Mater. Sci. Eng., A, 2007, 477, 96–99 CrossRef PubMed.
- D. C. Chen, L. P. He and S. P. Shang, Mater. Sci. Eng., A, 2003, 348, 29–35 CrossRef.
- D. Q. Chen, H. B. Wang, J. C. Huo, Y. L. Huo and S. Z. Lv, Funct. Mater., 2013, 44, 1217–1220 Search PubMed.
- M. Cazacu, A. Vlad, M. Alexandru and P. Budrugeac, Polym. Bull., 2010, 64, 421–434 CrossRef CAS.
- H. Ni'mah, W. F. Chen, Y. C. Shen and P. L. Kuo, RSC Adv., 2011, 1, 968–972 RSC.
- H. Maciejewski, J. Karasiewicz, M. Dutkiewicz, M. Nowickid and L. Majchrzyckic, RSC Adv., 2014, 4, 52668–52675 RSC.
- Y. Chen, C. Zhou, J. Chang, H. Zou and M. Liang, RSC Adv., 2014, 4, 60685–60693 RSC.
- J. S. Zabinski, J. E. Bultman, J. H. Sanders and J. J. Hu, Tribol. Lett., 2006, 23, 155–163 CrossRef CAS.
- J. J. Hu, J. E. Bultman and J. S. Zabinski, Tribol. Lett., 2006, 21, 169–174 CrossRef.
- T. W. Scharf, P. G. Kotula and S. V. Prasad, Acta Mater., 2010, 58, 4100–4109 CrossRef CAS PubMed.
- G. J. Dudder, X. Zhao, B. Krick, W. G. Sawyer and S. S. Perry, Tribol. Lett., 2011, 42, 203–213 CrossRef CAS.
- N. B. Colthup, L. H. Daly and S. E. Wiberley, Introduction to Infrared and Raman Spectroscopy, Academic, San Diego, CA, USA, 3rd edn, 1990 Search PubMed.
- A. Bertoluzza, C. Fagnano, M. Antonietta Morelli, V. Gottardi and M. Guglielmi, J. Non-Cryst. Solids, 1982, 48, 117–128 CrossRef CAS.
- H. J. Han and D. P. Kim, J. Sol-Gel Sci. Technol., 2003, 26, 223–228 CrossRef CAS.
- K. A. Andrianov, T. V. Vasil'eva and L. V. Kozlova, Russ. Chem. Bull., 1965, 14, 366–369 CrossRef.
- I. N. Chakraborty and R. A. Condrate, Phys. Chem. Glasses, 1985, 26, 68–73 CAS.
- P. Massiot, M. A. Centeno, I. Carrizosa and J. T. Odriozola, J. Non-Cryst. Solids, 2001, 292, 158–166 CrossRef CAS.
- F. Tian, L. Pan, X. Wu and F. Wu, J. Non-Cryst. Solids, 1988, 104, 129–134 CrossRef CAS.
- C. L. Yang, K. Cheng, W. J. Weng and C. Y. Yang, J. Mater. Sci.: Mater. Med., 2009, 20, 667–672 CrossRef CAS PubMed.
- Y. Huang, W. Liu and X. Zhou, J. Appl. Polym. Sci., 2012, 125, E282–E291 CrossRef CAS PubMed.
- J. M. Chiou and D. D. L. Chung, J. Mater. Sci., 1993, 28, 1435–1446 CrossRef CAS.
- M. J. Michalczyk, W. E. Farneth and A. J. Vega, Chem. Mater., 1993, 5, 1687–1689 CrossRef CAS.
- M. A. Schiavon, S. U. A. Redondo, S. R. O. Pina and I. V. P. Yoshida, Macromol. Chem. Phys., 2006, 207, 627–635 CrossRef PubMed.
- B. C. Windom, W. G. Sawyer and D. W. Hahn, Tribol. Lett., 2011, 42, 301–310 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |