Suzuki–Miyaura coupling of phosphinoyl-α-allenic alcohols with arylboronic acids catalyzed by a palladium complex “on water”: an efficient method to generate phosphinoyl 1,3-butadienes and derivatives†
Teng Liua,
Jie Dong‡
a,
Shu-Jun Cao‡a,
Li-Cheng Guo‡a and
Lei Wu*ab
aJiangsu Key Laboratory of Pesticide Science and Department of Chemistry, College of Sciences, Nanjing Agricultural University, Nanjing 210095, P. R. China. E-mail: rickywu@njau.edu.cn; rickywu@iccas.ac.cn; Fax: +86-25-84396716; Tel: +86-25-84396716
bCAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
First published on 29th October 2014
Abstract
We report here the first palladium-catalyzed Suzuki–Miyaura couplings of phosphinoyl-α-allenic alcohols with arylboronic acids “on water” without phase-transfer catalysts or additives. This new methodology provides a novel approach to generate phosphinoyl 1,3-butadienes and derivatives with medium to excellent yields. A mechanism via initial C(sp3)–OH bond cleavage of the substrate to form π-allyl-palladium intermediates, and transmetallation with arylboronic acid, followed by reductive elimination to afford the product, is proposed.
Introduction
Suzuki–Miyaura cross-coupling1 catalyzed by transition metals is one of the most versatile approaches for constructing biaryl and alkene derivatives towards key moieties of numerous natural products, agrochemicals, pharmaceuticals, and functional polymers etc. Current achievements of the Suzuki–Miyaura coupling have been carried out chiefly by using different ligands and/or metals,1b,2 various reaction media.3 Noticeable advances have been achieved with conducting reactions in water or aqueous solution. From environmental and economic points of view, water as cheap, readily available, non-toxic and non-flammable solvent has been one of the most important choices for organic transformations in green chemistry. In this respect, a number of remarkable results reported by the groups of Glorius, Beller, Herrman, Nolan, Organ and Köhler etc. have been documented conducting the cross-coupling of boronic derivatives with aryl halides in water.2a,4 In order to improve the catalytic efficiency, efforts are also focused on changing the property of organoboronic compounds.1a However, less attention has been paid on exploring novel substrates except conventional electrophiles including alkyl, aryl, alkenyl and alkynyl groups. Currently, alternative allylic partners5 possessing electron-deficient bonds, such as acetates, carbonates, halides and pseudohalides, are emerging into hot topic research. Moreover, the allylic/allenic substrates with electron-rich functionality such as C–OR, C–OH are still particularly attractive and challenging.6 In 2009, Lipshutz et al. disclosed the first Suzuki–Miyaura couplings of functionalized allylic ethers with arylboronic acids in water at room temperature with the presence of phase-transfer catalyst (nonionic amphiphilic PTS).6a Parallel experiments shown the coupling reaction “on water” proceeded sluggishly which further demonstrated the key role of phase-transfer catalyst PTS. On the other hand, the use of allylic alcohols or α-allenic alcohols as electrophiles for Suzuki–Miyaura coupling is more attractive with respect to atom-economy, albeit limited examples have been reported in refluxing organic solvents [Scheme 1a and b].6e,f,8a–e It is noteworthy that the above processes went through π-allyl-palladium intermediates for all of the allylic fragments, no exception to most of the allenic fragments.7 Interestingly, to the best of our knowledge, the coupling reaction of phosphinoyl-α-allenic alcohols with arylboronic acids “on water” has not been reported to date8 [Scheme 1c].Organophosphorus compounds play key roles in catalysis, organic synthesis, biochemistry and materials chemistry, which necessitate their significance in preparation.9 Phosphinoyl 1,3-dienes as one of the functionalized phosphorous compounds are readily be transformed to other valuable compounds, including allylic phosphonates,10 Diels–Alder cyclization products,11 cyclohexa-dienylphosphonates,12 vinyl allenes13 and phosphine-functionalized copolymers14 etc. However, to be noted, the conventional methods for synthesizing phosphinoyl 1,3-dienes are problematic and quite limited. Until recently, Han et al. reported the first nickel-catalyzed generation of phosphinoyl 1,3-butadienes [Scheme 1d], consisting of the metal-catalyzed addition of diphenylphosphine oxide and related P(O)H compounds to propargyl alcohols followed by an acid-catalyzed dehydration.15 Although this method provides quite convenient approach to phosphinoyl 1,3-butadienes, only terminal alkynes can be used, moreover, the adducts suffered from mixtures of Markovnikov and anti-Markovnikov products. Thus, the potential development with diversity and high selectivity/efficiency is highly demanded. In continuing research on synthesis and application of organophosphorus chemistry,16 herein we report, for the first time, palladium-catalyzed Suzuki–Miyaura couplings of phosphinoyl-α-allenic alcohols with arylboronic acids “on water” to generate phosphinoyl 1,3-butadienes and derivatives [Scheme 1c].
Results and discussions
Initially, a number of metal sources and ligands/additives along with solvents were examined for the cross-coupling of phosphinoyl-α-allenic alcohols (1a) with p-methoxylphenyl-boronic acid (2a) under refluxing temperature (Table 1). Nickel and copper catalysts exhibited no activity to this type of couplings (entries 1, 2, 6). Rhodium,8f,17 platinum8b and palladium sources were proven to be superior to afford medium yields in neat water, thus we extensively screened palladium precursors including PdCl2, Pd(OAc)2, Pd(TFA)2, (Ph3P)4Pd and Pd/C. Although phase-transfer catalyst/surfactants were widely used for accelerating Suzuki–Miyaura couplings in water,4l,18 TOABr (Tetraoctylammonium Bromide) and PEG 2000 were shown no improvements, instead of furnishing lower yields (entries 15, 16). Similarly, the presence of organic solvents and bases didn't increase the efficiency. Gratifyingly, the yield was significantly affected by the nature of the ligand and the palladium source. Phosphine ligands were revealed much higher activities than nitrogen ligands such as 1,10-phenanthroline and 2,2′-bipyridine (entries 17, 18). To be noted, mono-dentate phosphine ligands underwent smoother transmetallation with arylboronic acids than bidentate phosphine ligands since the latter tended to form coordinatively saturated π-allyl-palladium intermediates towards slower transmetallation, which has been demonstrated by Lipshutz et al.6a Eventually, bis(triphenyl-phosphine)palladium chloride was found to be the best catalyst to facilitate the coupling of 1a and 2a “on water” under refluxing temperature without phase-transfer catalysts or additives, affording the adduct (3a) with 84% yield (entry 21).| Entry | Catalyst | Solvent/additive/base | Yieldb (%) |
|---|---|---|---|
| a 1 mmol phosphinoyl-α-allenic alcohol, 2 mmol p-methoxyl phenylboronic acid, 10 mL solvent, 5 mol% catalyst, refluxed for 2 h.b Isolated yield based on allenes.c Reaction under room temperature for 24 h. | |||
| 1 | Ni(OTf)2 | H2O | 0 |
| 2 | Ni(PPh3)2Cl2 | H2O | 0 |
| 3 | (PPh3)3RhCl | H2O | 45 |
| 4 | Rh2(OAc)2 | H2O | 40 |
| 5 | PtCl2 | H2O | 42 |
| 6 | CuI | H2O | 0 |
| 7 | PdCl2 | H2O | 45 |
| 8 | Pd/C (10%) | H2O | 0 |
| 9 | (Ph3P)4Pd | H2O | 23 |
| 10 | Pd(TFA)2 | H2O | 35 |
| 11 | Pd(OAc)2 | H2O | 46 |
| 12 | Pd(OAc)2 | H2O/K2CO3 | 46 |
| 13 | Pd(OAc)2 | H2O/KOH | 45 |
| 14 | Pd(OAc)2 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dioxane = 9 dioxane = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/K2CO3 1/K2CO3 |
30 |
| 15 | Pd(OAc)2 | H2O/TOABr/K2CO3 | 30 |
| 16 | Pd(OAc)2 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG 2000 = 3 PEG 2000 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
29 |
| 17 | Pd(OAc)2/1,10-phenanthroline | H2O | Trace |
| 18 | Pd(OAc)2/Bipy | H2O | Trace |
| 19 | Pd(OAc)2(DPPE) | H2O | Trace |
| 20 | Pd(TFA)2(DPPE) | H2O | 58 |
| 21 | Pd(PPh3)2Cl2 | H2O | 84 |
| 22 | Pd(PPh3)2Cl2 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF = 9 THF = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
59 |
| 23 | (BINAP)PdCl2 | H2O | 56 |
| 24 | (Xantphos)PdCl2 | H2O | 54 |
| 25 | Pd(PPh3)2Cl2 | H2O | 0c |
The optimized conditions from the data in Table 1 (5 mol% Pd(PPh3)2Cl2, refluxing water) were generally applicable to several phosphinoyl-α-allenic alcohols and arylboronic acids (Table 2). The coupling of phosphinoyl-α-allenic alcohols (1a) with arylboronic acids bearing electron-rich or electron–neutral groups were effective to afford phosphinoyl 1,3-butadienes with medium to excellent yields (entries 1–5). However, arylboronic acids with electron-deficient groups, including –Br, –F and –NO2, led to decrease of coupling yields dramatically even after longer reaction time (entries, 6–8). This phenomenon might be attributed to the easier polymerization of electron-deficient conjugated dienes especially under heterogeneous high concentration, indicated by the isolation of oligmers. With respect to substituents on phosphinoylallenes, the substrates with groups ranged from alkyl to aromatic, electron-rich to electron-deficient (entries 9–22) were shown medium to excellent yields of phosphinoyl 1,3-butadiene derivatives. For unsymmetrical phosphinolyallenes 1e, 1h, the couplings gave exclusively E-isomers of 3o, 3p and 3u, respectively. This improved selectivity can be explained by relative differences in the stabilities and reactivities of intermediates affected by the electron-deficient groups on allenes. Notably, substrates with high steric substituents did not impair the catalytic efficiency, remaining yield up to 94% (entries 19–22). It is worth to mention that all of these substrates afforded comparable yields with terminal phosphinoylallenes (1a) even in the case of coupling with electron-deficient arylboronic acids (entries 14, 16, 22).
| Entry | R1 | R2 | R3 | Yieldb of 3 |
|---|---|---|---|---|
a 1 mmol phosphinoyl-α-allenic alcohol, 2 mmol arylboronic acid, 10 mL solvent, reflux.b Isolated yield based on allenes.c E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratios and relative stereochemistries were determined by 31P-NMR and NOESY experiments, respectively. Z ratios and relative stereochemistries were determined by 31P-NMR and NOESY experiments, respectively. |
||||
| 1 | H | H (1a) | p-MeO | 84 (3a) |
| 2 | H | H (1a) | p-Me | 82 (3b) |
| 3 | H | H (1a) | o-Me | 96 (3c) |
| 4 | H | H (1a) | H | 61 (3d) |
| 5 | H | H (1a) | Naphthalene | 60 (3e) |
| 6 | H | H (1a) | p-Br | 44 (3f) |
| 7 | H | H (1a) | p-F | 25 (3g) |
| 8 | H | H (1a) | m-NO2 | 25 (3h) |
| 9 | CH3 | CH3 (1b) | p-MeO | 78 (3i) |
| 10 | CH3 | CH3 (1b) | H | 61 (3j) |
| 11 | –C5H10– (1c) | o-Me | 90 (3k) | |
| 12 | –C5H10– (1c) | H | 73 (3l) | |
| 13 | p-MeOC6H4 | H (1d) | H | 69 (3m) [2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]c 1]c |
| 14 | p-MeOC6H4 | H (1d) | p-Br | 52 (3n) [7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]c 1]c |
| 15 | p-CF3C6H4 | CH3 (1e) | H | 85 (3o) [Only E]c |
| 16 | p-CF3C6H4 | CH3 (1e) | p-F | 84 (3p) [Only E]c |
| 17 | CH3CH2 | H (1f) | H | 73 (3q) [12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]c 1]c |
| 18 | CH3CH2 | H (1f) | p-Me | 95 (3r) [12.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]c 1]c |
| 19 | Ph | Ph (1g) | p-MeO | 92 (3s) |
| 20 | Ph | Ph (1g) | H | 94 (3t) |
| 21 | CH3CH2 | p-ClC6H4 (1h) | H | 72 (3u) [Only E]c |
| 22 | CH3CH2 | p-ClC6H4 (1h) | m-NO2 | 60 (3v) [2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]c 1]c |
Although the mechanism of this reaction is still under exploration, we proposed a catalytic cycle based on the preliminary results and previous studies6e,f,19 for the cross-coupling of phosphinoyl-α-allenic alcohol with arylboronic acids “on water” (Scheme 2).20 Firstly, the hydroxy group and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of phosphinoyl-α-allenyl alcohol 1 is activated by arylboronic acids 2 and in situ formed palladium(0) species, respectively. Subsequently, cleavage of allenic carbon–oxygen bond leads to form π-allyl-palladium species 5, which undergoes transmetallation with arylboronic acids to give intermediates 6 and 6′. No observation of product 7 indicates the priority of forming intermediate 6. Eventually, reductive elimination of 6 affords the final phosphinoyl 1,3-butadienes 3 and concurrently regenerates palladium(0) species.
C bond of phosphinoyl-α-allenyl alcohol 1 is activated by arylboronic acids 2 and in situ formed palladium(0) species, respectively. Subsequently, cleavage of allenic carbon–oxygen bond leads to form π-allyl-palladium species 5, which undergoes transmetallation with arylboronic acids to give intermediates 6 and 6′. No observation of product 7 indicates the priority of forming intermediate 6. Eventually, reductive elimination of 6 affords the final phosphinoyl 1,3-butadienes 3 and concurrently regenerates palladium(0) species.
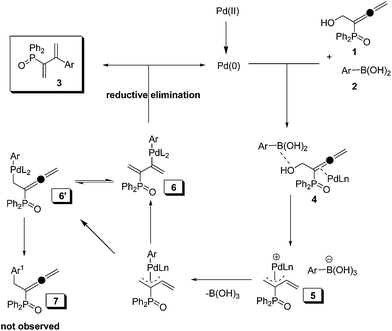 | ||
| Scheme 2 Plausible mechamism for palladium-catalyzed Suzuki–Miyaura coupling of phosphinoyl-α-allenic alcohol with arylboronic acids on water. | ||
Conclusions
In conclusion, we have developed a novel palladium-catalyzed Suzuki–Miyaura coupling of phosphinoyl-α-allenic alcohols with arylboronic acids “on water” without surfactants or additives. In the presence of 5 mol% Pd(PPh3)2Cl2, varieties of phosphinoyl-α-allenic alcohols smoothly coupled with arylboronic acids to afford phosphinoyl 1,3-butadienes and derivatives in medium to excellent yields. This work suggests a new transformation of allenes to phosphinoyl multi-substituted conjugated dienes. Further application of this methodology with tandem reactions to build bioactive compounds is under way.Experimental
To a 10 mL flask equipped with condenser under nitrogen was added phosphinoyl-α-allenic alcohol (1 mmol), arylboronic acid (2 mmol), Pd(PPh3)2Cl2 (5 mol%), and 10 mL degassed water. The reaction mixture was then heated to reflux for several hours until the complete consuming of starting materials monitored by TLC. After being cooling down, the reaction was extracted with ethyl acetate (5 mL × 2). The crude product was purified on flash chromatography, with ethyl acetate/petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluents to afford product.
1) as eluents to afford product.
Acknowledgements
This project is supported by the National Natural Science Foundation of China (no. 21002019), the Foundation Research Project of Jiangsu Province (The Natural Science Found no. BK20141359), the Fundamental Research Funds for the Central Universities (NJAU, no. KYRC201211) and “QinLan Project” of JiangSu Province.Notes and references
- (a) A. J. J. Lennox and G. C. LIoyd-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC; (b) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270 RSC; (c) N. Kambe, T. Iwasaki and J. Terao, Chem. Soc. Rev., 2011, 40, 4937 RSC; (d) C. M. So and F. Y. Kwong, Chem. Soc. Rev., 2011, 40, 4963 RSC; (e) G. C. Fu, Acc. Chem. Res., 2008, 41, 1555 CrossRef CAS PubMed; (f) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed; (g) N. Miyaura, in Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, New York, 2004, ch. 2 Search PubMed; (h) A. Suzuki, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E. Negishi, Wiley-Interscience, New York, 2002, ch. 3, Pt. 2.2 Search PubMed; (i) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS; (j) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (k) T. Moriya, T. Furuuchi, N. Miyaura and A. Suzuki, Tetrahedron, 1994, 50, 7961 CrossRef CAS.
- (a) C. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314 CrossRef CAS PubMed; (b) T. M. Shaikh, C.-M. Weng and F.-E. Hong, Coord. Chem. Rev., 2012, 256, 771 CrossRef CAS PubMed; (c) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS PubMed; (d) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed.
- For selected Suzuki–Miyaura coupling in neat water, aqueous solution, PEG, ionic liquids and supercritical carbon dioxide, see: (a) F. Rajabi and W. R. Thiel, Adv. Synth. Catal., 2014, 356, 1873 CrossRef CAS; (b) G.-F. Zhang, Y.-X. Luan, X.-W. Han, Y. Wang, X. Wen, C.-R. Ding and J.-R. Gao, Green Chem., 2013, 15, 2081 RSC; (c) V. Polshettiwar, A. Decottignies, C. Len and A. Fihri, ChemSusChem, 2010, 3, 502 CrossRef CAS PubMed; (d) A. D. Silva, J. D. Senra, L. C. S. Aguiar, A. B. C. Simas, A. L. F. de Souza, L. F. B. Malta and O. A. C. Antunes, Tetrahedron Lett., 2010, 51, 3883 CrossRef CAS PubMed; (e) T. M. Razler, Y. Hsiao, F. Qian, R. L. Fu, R. K. Khan and W. Doubleday, J. Org. Chem., 2009, 74, 1381 CrossRef CAS PubMed; (f) L.-Y. Li, J.-Y. Wang, T. Wu and R.-H. Wang, Chem.–Eur. J., 2012, 18, 7842 CrossRef CAS PubMed; (g) R. R. Fernandes, J. Lasri, M. F. C. G. da Silva, A. M. F. Palavra, J. A. L. da Silva, J. J. R. F. da Silva and A. J. L. Pombeiro, Adv. Synth. Catal., 2011, 353, 1153 CrossRef CAS.
- Glorius: (a) G. Altenhoff, R. Goddard, C. W. Lehmann and F. Glorius, J. Am. Chem. Soc., 2004, 126, 15195 CrossRef CAS PubMed ; Beller:; (b) S. Harkal, F. Rataboul, A. Zapf, C. Fuhrmann, T. Riermeier, A. Monsees and M. Beller, Adv. Synth. Catal., 2004, 346, 1742 CrossRef CAS; (c) A. Zapf and M. Beller, Chem.–Eur. J., 2004, 10, 1830 Search PubMed; (d) A. Zapf, A. Ehrentraut and M. Beller, Angew. Chem., Int. Ed., 2000, 39, 4153 CrossRef CAS ; Herrmann:; (e) C. W. K. Gstöttmayr, V. P. W. Böhm, E. Herdtweck, M. Grosche and W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1363–1365 CrossRef; (f) S. K. Schneider, P. Roembke, G. R. Julius, H. G. Raubenheimer and W. A. Herrmann, Adv. Synth. Catal., 2006, 348, 1862–1873 CrossRef CAS ; Nolan:; (g) N. Marion, O. Navarro, J.-G. Mei, E. D. Stevens, N. M. Scott and S. P. Nolan, J. Am. Chem. Soc., 2006, 128, 4101 CrossRef CAS PubMed; (h) S. D. González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef PubMed; (i) O. Diebolt, V. Jurcík, R. Correa da Costa, P. Braunstein, L. Cavallo, S. P. Nolan, A. M. Z. Slawin and C. S. J. Cazin, Organometallics, 2010, 29, 1443 CrossRef CAS; (j) C. Zhang, J. Huang, M. L. Trudell and S. P. Nolan, J. Org. Chem., 1999, 64, 3804 CrossRef CAS ; Organ:; (k) E. A. B. Kantchev, C. J. O’Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768 CrossRef CAS PubMed ; Köhler:; (l) C. Röhlich, A. S. Wirth and K. Köhler, Chem.–Eur. J., 2012, 18, 15485 CrossRef PubMed; (m) S. S. Soomro, C. Röhlich and K. Köhler, Adv. Synth. Catal., 2011, 353, 767 CrossRef CAS; (n) C. Röhlich and K. Köhler, Adv. Synth. Catal., 2010, 352, 2263 CrossRef.
- Selected examples for coupling of allylic fragments: (a) C. Nájera, J. Gil-Moltó and S. Karlström, Adv. Synth. Catal., 2004, 346, 1798 CrossRef; (b) G. W. Kabalka and M. Al-Masum, Org. Lett., 2006, 8, 11 CrossRef CAS PubMed; (c) G. W. Kabalka, E. Dadush and M. Al-Masum, Tetrahedron Lett., 2006, 47, 7459 CrossRef CAS PubMed; (d) H. Tsukamoto, T. Uchiyama, T. Suzuki and Y. Kondo, Org. Biomol. Chem., 2008, 6, 3005 RSC; (e) R. Matsubara and T. F. Jamison, J. Am. Chem. Soc., 2010, 132, 6880 CrossRef CAS PubMed; (f) H. Ohmiya, N. Yokokawa and M. Sawamura, Org. Lett., 2010, 12, 2438 CrossRef CAS PubMed; (g) H. Ohmiya, Y. Makida, D. Li, M. Tanabe and M. Sawamura, J. Am. Chem. Soc., 2010, 132, 879 CrossRef CAS PubMed; (h) C.-G. Li, J.-X. Xing, J.-M. Zhao, P. Huynh, W.-B. Zhang, P.-K. Jiang and Y. J. Zhang, Org. Lett., 2012, 14, 390 CrossRef CAS PubMed; (i) J. M. Zhao, J. Ye and Y. J. Zhang, Adv. Synth. Catal., 2013, 355, 491 CAS.
- (a) T. Nishikata and B. H. Lipshutz, J. Am. Chem. Soc., 2009, 131, 12103 CrossRef CAS PubMed; (b) T. Mino, T. Kogure, T. Abe, T. Koizumi, T. Fujita and M. Sakamoto, Eur. J. Org. Chem., 2013, 1501 CrossRef CAS; (c) J. K. Park, H. H. Lackey, B. A. Ondrusek and D. T. McQuade, J. Am. Chem. Soc., 2011, 133, 2410 CrossRef CAS PubMed; (d) S. Asako, L. llies and E. Nakamura, J. Am. Chem. Soc., 2013, 135, 17755 CrossRef CAS PubMed; (e) H.-B. Wu, X.-T. Ma and S.-K. Tian, Chem. Commun., 2014, 50, 219 RSC; (f) H. Tsukamoto, T. Uchiyama, T. Suzuki and Y. Kondo, Org. Biomol. Chem., 2008, 6, 3005 RSC; (g) J. Ye, J. M. Zhao, J. Xu, Y. X. Mao and Y. J. Zhang, Chem. Commun., 2013, 49, 9761 RSC.
- For employing α-allenic esters, phosphates or alcohols as initial π-allyl-palladium precursors (cleavage of C–OR, C–OH bonds by palladium complexes), see: (a) D. Djahanbini, B. Cazes and J. Gore, Tetrahedron Lett., 1984, 25, 203 CrossRef CAS; (b) J. Nokami, A. Maihara and J. Tsuji, Tetrahedron Lett., 1990, 31, 5629 CrossRef CAS; (c) Z. Ni and A. Padwa, Synlett, 1992, 869 CrossRef CAS; (d) W. Li and M. Shi, J. Org. Chem., 2008, 73, 6698 CrossRef CAS PubMed . In some cases, α-allenic alcohols are depicted undergo hydroarylation pathway before forming π-allyl-palladium intermediates, see ref. 8b–d.
- Transition metal-catalyzed additions of arylboronic acids to α-allenic alcohols in organic solvents are rare. Palladium: (a) M. Yoshida, T. Gotou and M. Ihara, Chem. Commun., 2004, 1124 RSC; (b) M. Yoshida, K. Matsuda, Y. Shoji, T. Gotou, M. Ihara and K. Shishido, Org. Lett., 2008, 10, 5183 CrossRef CAS PubMed; (c) M. Yoshida, Y. Shoji and K. Shishido, Org. Lett., 2009, 11, 1441 CrossRef CAS PubMed; (d) M. Yoshida, Y. Shoji and K. Shishido, Tetrahedron, 2010, 66, 5053 CrossRef CAS PubMed; (e) M. Yoshida, T. Kasai, T. Mizuguchi and K. Namba, Synlett, 2014, 25, 1160 CrossRef PubMed ; rhodium:; (f) T. Miura, H. Shimizu, T. Igarashi and M. Murakami, Org. Biomol. Chem., 2010, 8, 4074 RSC.
- (a) L. D. Quin, A Guide to Organophosphorus Chemistry, Wiley-Interscience, New York, 2000 Search PubMed; (b) K. V. L. Crepy and T. Imamoto, Top. Curr. Chem., 2003, 229, 111 CrossRef; (c) C. S. Demmer, N. Krogsgaard-Larsen and L. Bunch, Chem. Rev., 2011, 111, 7981 CrossRef CAS PubMed.
- S. D. Darling, F. N. Muralidharan and V. B. Muralidharan, Tetrahedron Lett., 1979, 20, 2761 CrossRef.
- (a) C. E. Griffin and W. M. Daniewski, J. Org. Chem., 1970, 35, 1691 CrossRef CAS; (b) E. Villemin, K. Robeyns, R. Robiette, M.-F. Herent and J. Marchand-Brynaert, Tetrahedron, 2013, 69, 1138 CrossRef CAS PubMed.
- S. D. Darling and N. Subramanian, J. Org. Chem., 1975, 40, 2851 CrossRef CAS.
- Z.-F. Xi, W.-X. Zhang, Z.-Y. Song, W.-X. Zheng, F.-Z. Kong and T. Takahashi, J. Org. Chem., 2005, 70, 8785 CrossRef CAS PubMed.
- N. Ajellal, C. M. Thomas and J.-F. Carpentier, Polymer, 2008, 49, 4344 CrossRef CAS PubMed.
- L.-B. Han, Y. Ono and H. Yazawa, Org. Lett., 2005, 7, 2909 CrossRef CAS PubMed.
- (a) L. Wu, B.-L. Li, Y.-Y. Huang, H.-F. Zhou, Y.-M. He and Q.-H. Fan, Org. Lett., 2006, 8, 3605 CrossRef CAS PubMed; (b) L. Wu, Z.-W. Li, F. Zhang, Y.-M. He and Q.-H. Fan, Adv. Synth. Catal., 2008, 350, 846 CrossRef CAS; (c) L. Wu, J. Ling and Z.-Q. Wu, Adv. Synth. Catal., 2011, 353, 1452 CrossRef CAS; (d) L. Wu, X. Zhang and Z.-M. Tao, Catal. Sci. Technol., 2012, 2, 707 RSC; (e) L. Wu, X. Zhang, Q.-Q. Chen and A.-K. Zhou, Org. Biomol. Chem., 2012, 10, 7859 RSC.
- For rhodium-catalyzed asymmetric hydroarylation of di-phenylphosphinylallenes with arylboronic acids, see: (a) T. Nishimura, S. Hirabayashi, Y. Yasuhara and T. Hayashi, J. Am. Chem. Soc., 2006, 128, 2556 CrossRef CAS PubMed; (b) X.-X. Guo, T. Sawano, T. Nishimura and T. Hayashi, Tetrahedron: Asymmetry, 2010, 21, 1730 CrossRef CAS PubMed.
- L.-F. Liu, Y.-H. Zhang and Y.-G. Wang, J. Org. Chem., 2005, 70, 6122 CrossRef CAS PubMed.
- (a) F. K. Sheffy, J. P. Godshalx and J. K. Stille, J. Am. Chem. Soc., 1984, 106, 4833 CrossRef CAS; (b) C. G. Frost, J. Howarth and J. M. J. Williams, Tetrahedron: Asymmetry, 1992, 3, 1089 CrossRef CAS.
- Another alternative mechanism involving palladium-catalyzed hydroarylation of phosphinoyl-α-allenic alcohols with arylboronic acids ref. 8b described as below is also conceivable. However, it is difficult to rationalize this mechanism since the aliphatic substitutions on allenes would inevitably lead to by-product D via beta-hydride elimination, which are not observed. In addition, the hydrolysis of intermediate B under current conditions towards by-product allylic alcohol (C) should be detected
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and products characterizations. See DOI: 10.1039/c4ra12251h |
| ‡ Undergraduate participants. |
| This journal is © The Royal Society of Chemistry 2014 |




