Hydrothermal synthesis and pseudo capacitance behavior of a highly homogeneous dispersed graphene sheets/ruthenium oxide nanocomposite
Xian Leng,
Jianpeng Zou*,
Xiang Xiong* and
Hanwei He
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China. E-mail: zoujp@csu.edu.cn; xiongx@csu.edu.cn; Tel: +86-731-88830376 Tel: +86-731-88836079
First published on 7th November 2014
Abstract
Nanocomposites of reduced graphene oxide (RGO) sheets decorated with amorphous ruthenium oxide nanoparticles are fabricated through a one-pot hydrothermal process without additional dispersants. During the hydrothermal reduction, unilamellar graphene sheets with curved and veil-like morphology are formed and multilamellar graphene sheets are further separated by amorphous RuO2·xH2O with a particle size of 3–8 nm. The nanocomposites exhibit evident pseudo capacitance behavior and the comprehensive electrochemical performance is improved markedly compared with that of the single components. When the precursor ratio of graphene oxide (GO) to RuCl3·xH2O is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the graphene–RuO2 (GR) nanocomposite electrode achieves the highest specific capacitance of 542.5 F g−1 and the minimum ESR value of 1.21 Ω at 0.1 A g−1, retaining 94% after 1000 cycles at 1 A g−1. Thus graphene sheets/ruthenium oxide nanocomposites bode well for a promising candidate in supercapacitor technology.
10, the graphene–RuO2 (GR) nanocomposite electrode achieves the highest specific capacitance of 542.5 F g−1 and the minimum ESR value of 1.21 Ω at 0.1 A g−1, retaining 94% after 1000 cycles at 1 A g−1. Thus graphene sheets/ruthenium oxide nanocomposites bode well for a promising candidate in supercapacitor technology.
1. Introduction
Compared with traditional electrolytic capacitors, electrochemical capacitors (ECs) based on carbonaceous materials, transition metal oxides, and conductive polymers, exhibit two to three orders of magnitude higher energy density. Though most ECs have lower energy density than lithium ion batteries (120–170 Wh kg−1), they increase the power density up to ten times (1000–2000 W kg−1), and have higher cycle efficiency (90–95%), longer cycle life (more than 105 cycles), and a wider working temperature range. ECs based on ruthenium oxide composites have the potential of satisfying the need to apply stable voltage and high-pulse energy for miniaturized electrical devices, and can offer giant power conversion in high voltage operation as well.1,2As a two-dimensional monolayer of carbon atoms bonded in a hexagonal lattice, graphene has ultra-high electrical conductivity (106 S cm−1), prominent surface area (theoretical value of 2630 m2 g−1), and high chemical and thermal stability, showing promising potential in the energy storage field.3,4 Graphene can be prepared by many methods, including mechanical cleavage, redox process, chemical vapor deposition, etc.5–7 Redox process with chemical oxidization and reduction is the most popular approach to obtain abundant graphenes, thus homogeneous dispersed graphene suspension with oxygen-containing functional groups can be produced. The advantage of this graphene suspension is that it can be easily transferred to other materials or apparatuses to form composites by dip or spin coating, pad or ink jet printing, etc. Graphene with highly crumpled and porous morphology can be solely applied in electrochemical devices, such as ECs.8,9 Integrating graphene with other carbonaceous materials of EDLC modifies the surface morphology and the attaching chemical groups, affecting the adsorption/desorption behavior of the electrolyte ions as well as its electrochemical performance.10,11 Graphene can also enhance the electrochemical properties of pseudo capacitance materials (e.g. transient metal oxides, conductive polymers) by triggering synergistic effects and decreasing charging–recharging induced volume change and ion diffusion kinetic barriers.12,13 Recently, graphene/ruthenium oxide composites with varied electrochemical properties have been prepared by different methods.14,15 Hydrothermal process is a good choice to prepare particles with high purity and controllable crystal form, which also could be employed to fabricate graphene/ruthenium oxide composites. Unfortunately, the problem with this technique is that ruthenium oxide becomes easily agglomerated and the particle size of ruthenium oxide and graphene is hard to control; as a result, surfactants or stabilizers are needed to ensure stable dispersion of ruthenium oxide nanoparticles in the composites.16–19
In this work, RuO2·xH2O was deposited on graphene sheets by the hydrothermal method from the mixture of exfoliated GO colloid suspension and ruthenium trichloride solution. As the wet synthesis approach is easy to operate, and could achieve the reduction of graphene oxide and conversion of RuCl3·xH2O to RuO2 simultaneously, resulting in composites with strong interfacial interaction. Interestingly, with the help of intercalation of graphene sheets, the agglomeration problem of ruthenium oxide nanoparticles has been greatly decreased. The pseudo capacitance behavior has been studied by cyclic voltammetry, chronopotentiometry and AC impedance spectroscopy, and optimal electrocapacitive performance of graphene/ruthenium oxide composites prepared at proper precursor ratio has been achieved.
2. Experimental
2.1 Synthesis of composites
GO was obtained by modified Hummers' method from natural graphite (GP, 325 mesh, Alfa Aesar), then the colloidal dispersion of negatively charged GO sheets with solid content of 0.5% was ultrasonicated to form unilamellar graphene oxide layers. Two grams of RuCl3·xH2O (Ru: 37.5–40.0 wt%, Sinopharm Chemical Reagent Co., Ltd) were dissolved in de-ionized water, and one quarter of the solution was adjusted with 0.4 M NaOH solution to pH 7. The remaining three quarters of the RuCl3·xH2O solution were equally divided into three parts and slowly dripped into 10 g, 20 g, and 30 g of GO dispersion, respectively, then 0.4 M NaOH solution was separately added with stirring until pH 7. Thus four samples were prepared with the precursor ratio of GO to RuCl3·xH2O equal to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 2
10, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 3
10, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. The samples were then transferred into stainless hydrothermal autoclaves and kept in a vacuum cabinet drier at 180 °C for 20 h, during which the brown solutions turned into a black aggregated cylinder and colorless liquid, indicating GO was reduced to RGO anchored with RuO2 nanoparticles. The liquid mixture was centrifuged to remove impurity ions and the black aggregates were annealed at 200 °C for 2 h to form GR composites. Energy-dispersive X-ray spectroscopy (EDS) showed that Ru content (at.%) in GR composites (the precursor ratio of GO to RuCl3·xH2O equals to 1
10, respectively. The samples were then transferred into stainless hydrothermal autoclaves and kept in a vacuum cabinet drier at 180 °C for 20 h, during which the brown solutions turned into a black aggregated cylinder and colorless liquid, indicating GO was reduced to RGO anchored with RuO2 nanoparticles. The liquid mixture was centrifuged to remove impurity ions and the black aggregates were annealed at 200 °C for 2 h to form GR composites. Energy-dispersive X-ray spectroscopy (EDS) showed that Ru content (at.%) in GR composites (the precursor ratio of GO to RuCl3·xH2O equals to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 2
10, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 3
10, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) was 12.9, 9.44 and 4.09 accordingly. The electrochemical active materials were blended with black carbon and polyvinylidene difluoride (PVDF) at the ratio of 85
10) was 12.9, 9.44 and 4.09 accordingly. The electrochemical active materials were blended with black carbon and polyvinylidene difluoride (PVDF) at the ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in N-methyl-2-pyrrolidone (NMP). The slurry was coated on tantalum plate to manufacture working electrodes, which usually contain 0.5–2 mg of dry paste.
10 in N-methyl-2-pyrrolidone (NMP). The slurry was coated on tantalum plate to manufacture working electrodes, which usually contain 0.5–2 mg of dry paste.
2.2 Characterization
Scanning electron microscopy (SEM, FEI NOVA Nano230), EDS, transmission electron microscopy (TEM, JEOL 2010II) and selected area electron diffraction (SAED) were used to check the microstructure and element content. X-ray diffraction analysis (Rigaku Ltd., Japan, D/max 2550VB, Cu Kα radiation, U = 40 kV, I = 250 mA) was employed to characterize crystalline information. XPS analysis (K-Alpha 1063, UK) was carried out to identify the chemical environment of as-prepared samples.2.3 Electrochemical measurements
Electrochemical property tests were carried out in 1 M H2SO4 electrolyte using CHI660B electrochemical workstation (Chenhua Corporation, China) and applying three-electrode configuration with Pt plate as counter electrode and saturated calomel electrode (SCE) as the reference electrode. Capacitance, rate capability and equivalent series resistance (ESR) were calculated through cyclic voltammetry (CV), chronopotentiometry (CP) and Nyquist plot in the voltage window of 0–1 VSCE.3. Results and discussion
3.1 Physicochemical properties
Fig. 1a and b illustrate the morphology of starting GP and as-prepared RGO sheets. The former shows distinct orderly linked laminated structure with smooth surface and clear edges. RGO was made through strong oxidation followed by ultrasonic exfoliation and hydrothermal reduction, during which van der Waals force between graphene layers was weakened and laminations were exfoliated to mono-layer graphenes. Highly ruffled appearance helps reduce the high surface free energy and relieves the interlamination stress. Fig. 1c shows that the single RuO2 particle size is very small. Due to the high temperature and high pressure atmosphere during hydrothermal synthesis, the micropores in the RuO2 particles have been compacted and even filled, forming a hard agglomeration. The surfaces of GR (Fig. 1d) with different Ru loading seem similar to pure graphene but show more wrinkled appearance when compared with those in the relevant literature.15,19 This may originate from the larger surface area and mesoporous structure that are highly beneficial in electrode fabrication.20,21 Evenly distributed RuO2 nanodots help separate graphene layers and increase the accessibility of electroactive sites, which facilitates both EDLC and pseudo capacitance.Fig. 2a reveals that dispersed RGO sheets exhibit transparent veil-like appearance with curved morphology similar to what has been shown in previous reports.22,23 The curved surfaces of RGO would increase the wettability of the electrode surface. Furthermore, the disorderly arranged graphenes easily form porous structures, and the porosity would provide proton transferring tunnels that promote the electrocapacitance behavior.24 SAED of RGO (Fig. 2b) confirms the polycrystalline structure that characterizes few layers of graphene, with van der Waals force stronger than the repulsive force between neighboring sheets. As seen from Fig. 2c, RuO2 nanodots show a size of several nanometers and tend to reunite and form agglomerates which may result in decrease of exposed surface. The high resolution image of RuO2 with obvious lattice fringe is shown in Fig. 2c. The faint diffused scattering ring in Fig. 2d is evidence of nanometer-sized crystallites and of the amorphous nature of RuO2·xH2O. Beside electron conduction, the hydration of amorphous RuO2·xH2O can provide proton conduction, which guarantees the high specific capacitance of GR composites. The high-magnification micrographs in Fig. 2e and f show that RuO2 nanocrystallites (marked by circles or arrows) with size of 3–8 nm are homogeneously deposited on graphene layers without visible agglomeration that cannot be clearly identified in Fig. 1d. It is worth noting that the size distribution (3–8 nm) (Fig. 2e) is smaller than RuO2 decoration (3–15 nm) in GR composites prepared by Mishra et al.25 and RuO2 nanorods of 25–35 nm and 12–15 nm in length and diameter respectively reported by Gopiraman.26 A comparison of Fig. 2c with Fig. 2e and f indicates that oxygenic functional groups provide effective anchoring sites (nucleation centers) for RuO2 particles, which assists strong attachment, homogeneous decoration, and avoids agglomeration.25,26 Besides, the network of graphene inhibits the growth and coalescence of RuO2 nanocrystalline particles. Just as Pico reported,27 no diffraction spots of crystalline RuO2 of the GR composite are observed in Fig. 2f. As shown in Fig. 2g, a perfect hexagon confirms restoration of graphene structure through reduction of GO, and multi-layered graphenes are further separated to mono-layer graphenes by RuO2 nanoparticles. Peaks labeled by Miller–Bravais indices in Fig. 2h show that intensities of (0–110) and (−1010) are larger than intensities of (1–210) and (−2110), and indicate the formation of mono-layer graphene, which shows good agreement with Geim's reports.28–30 Thus, RuO2 nanoparticles with small size of 3–8 nm and mono-layer graphene can be formed simultaneously in the GR composites, which suggests advantageous separating effect and synergetic effect of the two phases to each other.
 | ||
| Fig. 2 TEM images and SAED patterns of (a, b) RGO, (c, d) RuO2·xH2O, (e–g) GR (Ru: 9.44 at.%), and (h) diffracted intensity along the (1–210) to (−2110) axis in (g). | ||
As it can be seen from Fig. 3, RGO (002) crystal plane values of 2θ = 24.54° and d002 = 3.6245 Å have slightly changed compared with those of raw GP, which are equal to 2θ = 26.4° and d002 = 3.3726 Å. Since a few functional groups and curved surface structures have been retained after reduction, the interplanar spacing of RGO (3.6245 Å) is a little bit larger than that of GP (3.3726 Å). The broad diffraction peaks in RGO indicate that exfoliation and restacking occur during the oxidation–reduction treatment resulting in lower crystallinity, and abundant mono-layer graphenes may be exist in the RGO products. No sharp peaks were obtained in the XRD pattern of GR (Ru: 9.44 at.%), suggesting that composites are made of highly disordered arranged graphene sheets and nanometer-sized amorphous RuO2 containing hydrated water, which is consistent with nanometer-sized RuO2 lattice fringes in Fig. 2e. The disappearance of the (002) peak of RGO shows agreement with SAED patterns in Fig. 2 and may originate from two reasons. First, the major phase is RuO2·xH2O in the GR composite, since the content of graphene is relatively low. The RuO2·xH2O is amorphous, so the XRD of the GR composite shows an amorphous spectrum without obvious diffraction peaks. Secondly, abundant mono-layer graphenes in RGO show an amorphous spectrum, which is in good agreement with Fig. 2g and h.
From Fig. 4a, the O 1s peak intensity of RGO is much smaller than GO, implying that oxygen content (at.%) of 28% in GO is reduced to 9.32% in RGO during hydrothermal process. The strong O 1s signal of GR comes from remained oxygen groups, ruthenium oxide and textural water. Residual oxygen groups would enlarge interlayer spacing of RGO and offer ion-exchange sites for anchoring metal oxide nanoparticles. Decomposed Gaussian peaks in Fig. 4b show two pairs of Ru 3d3/2,5/2 peaks in the broad C 1s regions, revealing the formation of Ru4+ species; binding energy of 284.32 eV belongs to the C in graphite, 288.11 eV to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O based groups (carboxyl and carbonyl functionalities) and 291.93 eV to π–π* satellite peak that arises from restoration of π–π conjugated structure.29,31,32
O based groups (carboxyl and carbonyl functionalities) and 291.93 eV to π–π* satellite peak that arises from restoration of π–π conjugated structure.29,31,32
 | ||
| Fig. 4 XPS spectra of (a) survey scan, (b) deconvolution of C 1s spectra and Ru 3d spectra of GR (Ru: 9.44 at.%). | ||
3.2 Electrochemical properties
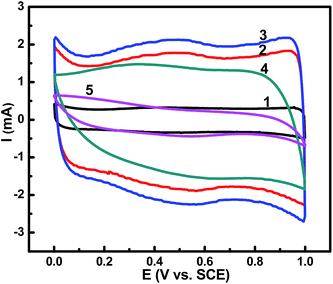
Electrochemical performance of as-prepared pure RuO2 (curve 1), GR electrodes with different Ru content (at.%) of 12.9, 9.44 and 4.09 as obtained by EDS (curves 2, 3 and 4), and RGO (curve 5) is depicted in Fig. 5–9.The capacitive behavior of as-prepared electrodes is featured in Fig. 5, and the rectangular shapes denote ideal capacitive behavior and low contact resistance. Specific capacitance of electrodes in Fig. 5 (1–5) is 78.15 F g−1, 329.29 F g−1, 482.16 F g−1, 315.39 F g−1 and 109.22 F g−1 respectively, based on the integrated area under the CV loops. Previous studies pointed out that the specific capacitance of graphene/RuO2 composites increases with the increase of Ru content.33,34 However, in this paper, the specific capacitance of GR initially increased with decreasing Ru loading and then decreased again. The specific capacitances of GR (Ru: 12.9 and 9.44 at.%) are 329.29 F g−1 and 482.16 F g−1, which are much larger than those of pure RuO2·xH2O or RGO. As shown in Fig. 5, GR electrodes with a couple of redox peaks in 0.4–0.8 V voltage window present highly reversible properties in faradic pseudo capacitance behavior among Ru(IV)/Ru(III)/Ru(II). With the decrease of RuO2 content and the increase of graphene content, graphene shows beneficial separating effect and synergetic effect with the RuO2 nanodots (as shown in Fig. 2e). The proton/electron transferring tunnels have been reconstructed, and more reactive surfaces facilitate the filtration of electrolyte, greatly enhancing the specific capacitances. GR (Ru: 4.09 at.%) and RGO exhibit irregular rectangular shape, indicating bad electrical conductivity. As for the composite, overloading of RGO leads to piling of graphene layers and the separating effect of graphene to RuO2 deteriorate, delaying the proton/electron transfer rate and impairing the overall capacitance. As for pure RGO, without spacers (RuO2), hydrothermal process would intrigue dramatic decrease in available surface for charge storage, and residual surface functionalities and defects may contribute to inferior electrical conductivity. Therefore, the ratio of RGO to RuO2 is of particular importance to provoke better synergistic effects and balance the EDLC and pseudo capacitance.
As shown in Fig. 6, as-prepared electrodes exhibit linear and symmetrical galvanostatic charge–discharge curves, except for GR (Ru: 4.09 at.%) and RGO which have a discernible voltage (IR) drop of large resistance, denoting overall good agreement with the CV curves. The specific capacitances measured at 1 A g−1 of curves 1–5 calculated on the slope of the discharging curve35 are 124.75 F g−1, 338.78 F g−1, 508.35 F g−1, 361.50 F g−1 and 79 F g−1, and are in accordance with the varying rule as in Fig. 5. Compared to the theoretical value of 768 F g−1 of RuO2·xH2O prepared by the sol–gel method,36 the specific capacitance (78.15 F g−1 or 124.75 F g−1) of pure RuO2·xH2O prepared by hydrothermal method is relatively low. The possible reason is that the micropores in the RuO2·xH2O particles tend to close in high temperature and high pressure atmosphere during the hydrothermal process (as shown in Fig. 1c). Therefore, proton/electron transferring tunnels can be hardly constructed and the contact areas between the RuO2·xH2O particles and the electrolyte have been greatly decreased. The electroactivity of RuO2·xH2O has been limited, and low specific capacitance can be achieved consequently. It can be concluded that overloading of aggregated RuO2 or stacked graphene sheets can inhibit the electroactive behavior of graphene and RuO2.
In the recent years, many groups have also been focused on fabricating GR composites for ECs. Rakhi et al.15 reported a GR composite (40 wt% of RuO2) exhibited the specific capacitance of 365 F g−1 at 20 mV s−1 in 30 wt% KOH based on a two-electrode configuration. Chen et al.17 reported GR composites prepared by a sol–gel method using polyvinylpyrrolidone (PVP) as the dispersant and achieved 435 F g−1 at 0.2 A g−1 with 60 wt% of RuO2. Chen et al.19 reported the specific capacitance of 471 F g−1 at 0.5 A g−1 for a GR composite with 45 wt% of RuO2. Wu et al.37 prepared GR composites through a sol–gel synthesis and achieved 570 F g−1 at 1 mV s−1 with 38.3 wt% Ru. Shen et al.38 reported GR composites synthesized using Poly (diallyldimethylammonium chloride) (PDDA) as the reducing agent and stabilizer, and achieved 521.25 F g−1 at 5 mV s−1 with 27.5 wt% of Ru. In the present work, we achieved comparable specific capacitance of 542.5 F g−1 at 0.1 A g−1 and 528.35 F g−1 at 1 mV s−1 with the Ru 9.44 at.% through the eco-friendly hydrothermal process, realizing full exfoliation of graphene layers and enhanced strong interfacial interaction.
Rate capability is evaluated based on specific capacitance calculated under different charge–discharge currents in chronopotentiograms and scan rates in cyclic voltammograms. The specific capacitances of RuO2, GR with Ru content (at.%) of 12.9, 9.44, 4.09 and RGO at 0.1 A g−1 are 128.5 F g−1, 361.8 F g−1, 542.5 F g−1, 377.4 F g−1 and 159.5 F g−1, as shown in Fig. 7a. It is clear from Fig. 7a that capacitance retentions at large discharging current density of 2 A g−1 are 92–96% of those at 0.1 A g−1, except for GR (Ru: 4.09 at.%) and RGO which show a drastic decrease down to the retention ratio of 51% and 36%. According to the previous analysis (CV and chronopotentiometry), GR (Ru: 4.09 at.%) and RGO have much larger resistance and capacitive RC time constant than the others. Large current charge–discharge behavior of GR (Ru: 4.09 at.%) and RGO would be affected more heavily,39 leading to relative sharp drop of specific capacitance at higher current density. More oxygen-containing groups attached on graphene planes, larger electronic resistance and limited ion tunnels might be responsible for such capacitance degradation. As also shown in Fig. 7b, RuO2·xH2O electrode and GR (Ru: 9.44 at.%) electrodes measured at 100 mV s−1 retain 72–82% of its initial capacitance at 1 mV s−1, benefitting from closely bonding inner structure and effective cooperation between graphene and in situ grown RuO2 particles with optimum ratio.40 The possible Ru–C interactions between Ru and pristine or defective graphene (single vacancy, double vacancy, and haeckelite structures, such as 5577, 555 777, and 55 556 777 defects) based on first-principles calculations were illustrated in details by Liu et al.41
Fig. 8 shows that the specific capacitance of all electrodes maintains 91–100% of the original value during the first 300 cycles. Then GR (Ru: 4.09 at.%) gradually decreases to 80% and stabilizes, and pure RGO declines steadily to 76% due to relative high inner resistance, while other electrodes retain 91–100% after 1000 cycles. Compared with pure RGO electrode, GR electrodes demonstrate enhanced cycling stability, mainly due to reversible insertion and expulsion of protons in as-prepared hydrated RuO2 during the energy storage/deliver process. The apparent reduction of retention ratio in GR (Ru: 4.09 at.%) is associated with increased IR drop and slower redox rates in active sites.
Fig. 9 shows the complex capacitance plot of as-prepared electrode materials, which contains kinetic and mechanistic information about the redox process.42,43 It can be seen that Nyquist plots comprise a semicircular feature in high-frequency and a line in lower frequency. According to curves 1, 2, 3, 4, and 5, the intercept at real component (Z′) denotes that ESR are 1.39 Ω, 1.65 Ω, 1.21 Ω, 2.27 Ω and 1.72 Ω, respectively. GR (Ru: 9.44 at.%) displays at the same time the lowest ESR value of 1.21 Ω, according to the impedance measurement method by R. B. Rakhi,15 and the highest capacitance value of 542.5 F g−1. This is due to the contribution of fine-tuned exfoliation of graphene layers separated by amorphous RuO2 nanodots as confirmed by SAED pattern in Fig. 2. The semicircular branch refers to charge-transfer resistance. As shown in curve 4 and 5, the obvious semicircle indicates higher interfacial resistance and poor conductivity of corresponding active materials that may result from poor dispersion and unrecovered sp3 defects and is consistent with its poor rate capability shown in Fig. 7. The nearly vertical portion indicates purely capacitive behavior with fast ion transport at the dc potential of 0.0 V.
4. Conclusions
In this study, GR composites of highly curved graphene dotted with nanoscale RuO2 were in situ prepared through a self-assembly hydrothermal process without additional stabilizers or protective agents. Besides, no rigid expansion condition or toxic reducing agents are required to reduce GO. XRD and TEM patterns reveal that hydrous RuO2 nanodots of 3–8 nm are uniformly distributed on highly curved RGO sheets, acting as spacers that prevent aggregation of graphene sheets, and offering more available interface for EDLC and pseudo capacitance energy storage. RuO2, GR with Ru content (at.%) of 12.9, 9.44 and 4.09, and RGO respectively reach 128.5 F g−1, 361.8 F g−1, 542.5 F g−1, 377.4 F g−1 and 159.5 F g−1 at 0.1 A g−1. Clearly, GR exhibits optimal performance at a certain precursor ratio and the GR (Ru: 9.44 at.%) also shows well-performed cycling durability properties by retaining 94% of initial capacitance after 1000 cycles under 1 A g−1. As mentioned above, excellent overall performance may be ascribed to 3D conductive framework with lower diffusion resistance, more efficient electrochemical active surfaces with less kinetic limitations and smooth exchange pathways in the composite matrix for ion transport of redox process.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 51274248).References
- X. Wang and J. Zheng, The optimal energy density of electrochemical capacitors using two different electrodes, J. Electrochem. Soc., 2004, 151, A1683–A1689 CrossRef CAS PubMed.
- C. Hu and W. Chen, Effects of substrates on the capacitive performance of RuOx·nH2O and activated carbon–RuOx electrodes for supercapacitors, Electrochim. Acta, 2004, 49, 3469–3477 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva and A. Firsov, Electric field effect in atomically thin carbon films, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, The rise of graphene, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- C. Cheng and D. Li, Solvated graphenes: an emerging class of functional soft materials, Adv. Mater., 2013, 25, 13–30 CrossRef CAS PubMed.
- H. Chang and H. Wu, Graphene-Based Nanomaterials: Synthesis, Properties, and Optical and Optoelectronic Applications, Adv. Funct. Mater., 2012, 23, 1984–1997 CrossRef.
- R. Fryczkowski, M. Gorczowska, C. Ślusarczyk, B. Fryczkowska and J. Janicki, The possibility of obtaining graphene/polymer composites from graphene oxide by a one step process, Compos. Sci. Technol., 2013, 80, 87–92 CrossRef CAS PubMed.
- Z. Fan, Q. Zhao, T. Li, J. Yan, Y. Ren, J. Feng and T. Wei, Easy synthesis of porous graphene nanosheets and their use in supercapacitors, Carbon, 2012, 50, 1699–1703 CrossRef CAS PubMed.
- J. Yan, J. Liu, Z. Fan, T. Wei and L. Zhang, High-performance supercapacitor electrodes based on highly corrugated graphene sheets, Carbon, 2012, 50, 2179–2188 CrossRef CAS PubMed.
- L. Wang, L. Sun, C. Tian, T. Tan, G. Mu, H. Zhang and H. Fu, A novel soft template strategy to fabricate mesoporous carbon/graphene composites as high-performance supercapacitor electrodes, RSC Adv., 2012, 2, 8359–8367 RSC.
- M. Wang, Q. Liu, H. Sun, E. A. Stach, H. Zhang, L. Stanciu and J. Xie, Preparation of high-surface-area carbon nanoparticle/graphene composites, Carbon, 2012, 50, 3845–3853 CrossRef CAS PubMed.
- G. Yu, X. Xie, L. Pan, Z. Bao and Y. Cui, Hybrid nanostructured materials for high-performance electrochemical capacitors, Nano Energy, 2013, 2, 213–234 CrossRef CAS PubMed.
- J. Sun and H. Bi, Pickering emulsion fabrication and enhanced supercapacity of graphene oxide-covered polyaniline nanoparticles, Mater. Lett., 2012, 81, 48–51 CrossRef CAS PubMed.
- N. Soin, S. S. Roy, S. K. Mitra, T. Thundat and J. A. McLaughlin, Nanocrystalline ruthenium oxide dispersed Few Layered Graphene (FLG) nanoflakes as supercapacitor electrodes, J. Mater. Chem., 2012, 22, 14944–14950 RSC.
- R. Rakhi, W. Chen, D. Cha and H. Alshareef, High performance supercapacitors using metal oxide anchored graphene nanosheet electrodes, J. Mater. Chem., 2011, 21, 16197–16204 RSC.
- L. Na, J. Tian, Z. Shan, C. Kuan and W. Liao, Hydrothermal synthesis of hydrous ruthenium oxide/graphene sheets for high-performance supercapacitors, Electrochim. Acta, 2013, 99, 219–224 CrossRef PubMed.
- Y. Chen, X. Zhang, D. Zhang and Y. Ma, Increased electrochemical properties of ruthenium oxide and graphene/ruthenium oxide hybrid dispersed by polyvinylpyrrolidone, J. Alloys Compd., 2012, 54, 415–420 CrossRef PubMed.
- J. Kim, K. Kim, S. Yoon, H. Kim, S. H. Park and K. B. Kim, In-situ chemical synthesis of ruthenium oxide/reduced graphene oxide nanocomposites for electrochemical capacitor applications, Nanoscale, 2013, 5, 6804–6811 RSC.
- Y. Chen, X. Zhang, D.-C. Zhang and Y.-W. Ma, One-pot hydrothermal synthesis of ruthenium oxide nanodots on reduced graphene oxide sheets for supercapacitors, J. Alloys Compd., 2012, 511, 251–256 CrossRef CAS PubMed.
- K. Lin, K. Chang, C. Hu and Y. Li, Mesoporous RuO2 for the next generation supercapacitors with an ultrahigh power density, Electrochim. Acta, 2009, 54, 4574–4581 CrossRef CAS PubMed.
- J. F. Oudenhoven, L. Baggetto and P. H. Notten, All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts, Adv. Energy Mater., 2011, 1, 10–33 CrossRef CAS.
- K. Wang, T. Feng, M. Qian, H. Ding, Y. Chen and Z. Sun, The field emission of vacuum filtered graphene films reduced by microwave, Appl. Surf. Sci., 2011, 257, 5808–5812 CrossRef CAS PubMed.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Graphene-based ultracapacitors, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- J. P. Zheng, Ruthenium Oxide-Carbon Composite Electrodes for Electrochemical Capacitors, Electrochem. Solid-State Lett., 1999, 2, 359–361 CrossRef CAS PubMed.
- A. K. Mishra and S. Ramaprabhu, Functionalized Graphene-Based Nanocomposites for Supercapacitor Application, J. Phys. Chem. C, 2011, 115, 14006–14013 CAS.
- M. Gopiraman, S. G. Babu, Z. Khatri, W. Kai, Y. A. Kim, M. Endo, R. Karvembu and I. S. Kim, Dry synthesis of easily tunable nano ruthenium supported on graphene: novel nanocatalysts for aerial oxidation of alcohols and transfer hydrogenation of ketones, J. Phys. Chem. C, 2013, 23582–23596 CAS.
- F. Pico, E. Morales, J. Fernandez, T. Centeno, J. Ibáñez, R. Rojas, J. Amarilla and J. Rojo, Ruthenium oxide/carbon composites with microporous or mesoporous carbon as support and prepared by two procedures. A comparative study as supercapacitor electrodes, Electrochim. Acta, 2009, 54, 2239–2245 CrossRef CAS PubMed.
- J. Meyer, A. Geim, M. Katsnelson, K. Novoselov, D. Obergfell, S. Roth, C. Girit and A. Zettl, On the roughness of single-and bi-layer graphene membranes, Solid State Commun., 2007, 143, 101–109 CrossRef CAS PubMed.
- M. Lotya, Y. Hernandez, P. J. King, R. J. Smith, V. Nicolosi, L. S. Karlsson, F. M. Blighe, S. De, Z. Wang and I. McGovern, Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions, J. Am. Chem. Soc., 2009, 131, 3611–3620 CrossRef CAS PubMed.
- I. Janowska, K. Chizari, O. Ersen, S. Zafeiratos, D. Soubane, V. Da Costa, V. Speisser, C. Boeglin, M. Houllé and D. Bégin, Microwave synthesis of large few-layer graphene sheets in aqueous solution of ammonia, J. Nano Res., 2010, 3, 126–137 CrossRef CAS PubMed.
- C. Mun, J. Ehrhardt, J. Lambert and C. Madic, XPS investigations of ruthenium deposited onto representative inner surfaces of nuclear reactor containment buildings, Appl. Surf. Sci., 2007, 253, 7613–7621 CrossRef CAS PubMed.
- C. Xu, X. Wang and J. Zhu, Graphene– metal particle nanocomposites, J. Phys. Chem. C, 2008, 112, 19841–19845 CAS.
- C. Chuang, C. Huang, H. Teng and J. Ting, Hydrothermally synthesized RuO2/Carbon nanofibers composites for use in high-rate supercapacitor electrodes, Compos. Sci. Technol., 2012, 72, 1524–1529 CrossRef CAS PubMed.
- H. Li, R. Wang and R. Cao, Physical and electrochemical characterization of hydrous ruthenium oxide/ordered mesoporous carbon composites as supercapacitor, Microporous Mesoporous Mater., 2008, 111, 32–38 CrossRef CAS PubMed.
- M. Zheng, J. Cao, S. Liao, J. Liu, H. Chen, Y. Zhao, W. Dai, G. Ji, J. Cao and J. Tao, Preparation of mesoporous Co3O4 nanoparticles via solid– liquid route and effects of calcination temperature and textural parameters on their electrochemical capacitive behaviors, J. Phys. Chem. C, 2009, 113, 3887–3894 CAS.
- C. Hu, W. Chen and K. Chang, How to achieve maximum utilization of hydrous ruthenium oxide for supercapacitors, J. Electrochem. Soc., 2004, 151, A281–A290 CrossRef CAS PubMed.
- Z. S. Wu, D. W. Wang, W. Ren, J. Zhao, G. Zhou, F. Li and H. M. Cheng, Anchoring Hydrous RuO2 on Graphene Sheets for High-Performance Electrochemical Capacitors, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- J. Shen, T. Li, W. Huang, Y. Long, N. Li and M. Ye, One-pot polyelectrolyte assisted hydrothermal synthesis of RuO2-reduced graphene oxide nanocomposite, Electrochim. Acta, 2013, 95, 155–161 CrossRef CAS PubMed.
- G. Cui, X. Zhou, L. Zhi, A. Thomas and K. Müllen, Carbon/nanostructured Ru composites as electrodes for supercapacitors, New Carbon Mater., 2007, 22, 302–306 CrossRef CAS.
- K. Naoi and P. Simon, New materials and new configurations for advanced electrochemical capacitors, J. Electrochem. Soc., 2008, 17, 34–37 CAS.
- X. Liu, Y. Sui, C. Meng and Y. Han, Tuning the reactivity of Ru nanoparticles by defect engineering of the reduced graphene oxide support, RSC Adv., 2014, 4, 22230–22240 RSC.
- J. Rishpon and S. Gottesfeld, Resolution of fast and slow charging processes in ruthenium oxide films: an AC impedance and optical investigation, J. Electrochem. Soc., 1984, 131, 1960–1968 CrossRef CAS PubMed.
- T. Qi, J. Jiang, H. Chen, H. Wan, L. Miao and L. Zhang, Synergistic effect of Fe3O4/reduced graphene oxide nanocomposites for supercapacitors with good cycling life, Electrochim. Acta, 2013, 114, 674–680 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |