Label-free fluorescence turn-on sensing for melamine based on fluorescence resonance energy transfer between CdTe/CdS quantum dots and gold nanoparticles
Jingjin Zhao*a,
Hong Wub,
Jing Jiangb and
Shulin Zhao*b
aKey Laboratory of Ecology of Rare and Endangered Species and Environmental Conservation of Education Ministry, Guangxi Normal University, Guilin 541004, P. R. China. E-mail: jzhao12@163.com
bKey Laboratory for The Chemistry and Molecular Engineering of Medicinal Resources of Education Ministry, Guangxi Normal University, Guilin, 541004, P. R. China. E-mail: zhaoshulin001@163.com; Fax: +86-773-5832294; Tel: +86-773-5845973
First published on 4th November 2014
Abstract
A label-free fluorescence turn-on sensing system based on fluorescence resonance energy transfer (FRET) between CdTe/CdS quantum dots (QDs) and gold nanoparticles (AuNPs) is developed for the detection of melamine. This FRET process originated from a fluorescent donor–acceptor pair, in which CdTe/CdS quantum dots act as donors and AuNPs as acceptors, owing to the overlap of the fluorescence spectrum of the quantum dots and absorption spectrum of the AuNPs, leading to a decrease in the fluorescence signal. However, the presence of melamine resulted in the aggregation of AuNPs via multiple combinations between melamine and AuNPs, which reduced the spectral overlap and hampered the FRET process by restoring the fluorescence signal. The fluorescence enhancement exhibits a linear relationship to the melamine concentration from 5.0 × 10−8 M to 1.0 × 10−6 M with a detection limit of 3.0 × 10−8 M. Good detection of melamine in raw milk and milk products was also achieved. This sensing system is facile, rapid, easy to implement and may provide a new avenue for the determination of melamine in real samples.
Introduction
Since the very serious milk powder melamine (2,4,6-triamino-s-triazine) contamination scandal broke out, it caused international concern and raised global food safety issues of milk and milk-containing products.1 Melamine is an ordinary chemical used in the manufacturing of plastics, resins, flame-retardants, adhesives and fertilizers. Because of its high nitrogen level (66% by mass), melamine is illegally added to milk products to produce an incorrectly high reading in the measurement of protein content based on total nitrogen.2 Ingestion of melamine at levels above the safety limit can lead to serious or even fatal renal and kidney failure under specific conditions and for specific forms of metabolism.3 Therefore, it is important to develop a reliable and sensitive sensing system for melamine detection in milk products. Up to now, many analytical approaches for sample preparation and determination of residual melamine in biological samples were reported, including enzyme linked immunosorbent assays (ELISA),4 high performance liquid chromatography (HPLC),5 capillary electrochromatography mass spectrometry (CEC-MS),6 liquid chromatography tandem mass spectrometry (LC-MS/MS),7 gas chromatography mass spectrometry (GC-MS),8 surface plasmon resonance (SPR) biosensor assay.9 However, these methods are generally expensive and time-consuming, and may require the use of hazardous materials, complex procedures or bulky instruments. There is still an increasing demand for an alternative technology that can be used for rapid, sensitive and low-cost screening of melamine.In recent years, nanoparticles (NPs) have been developed for sensitive detection of molecules in a variety of optical methods because of their size-dependent physical and chemical characteristics. Due to their unique optical and spectroscopic properties, luminescent semiconductor quantum dots (QDs) have proven to be very effective donor fluorophores in an array of processes and bioassays based on fluorescence resonance energy transfer (FRET).10 In addition, gold nanoparticles (AuNPs) have been of great interest because of their high extinction coefficient and broad absorption spectrum in the visible light that overlaps with the emission wavelengths of usual energy donors.11 This suggests that QDs and AuNPs could provide excellent donor–acceptor pairs and the superiority of the QDs–AuNP system with its high sensitivity has attracted increasing attention.12 For example, Eunkeu Oh et al.13 developed an inhibition assay method for avidin detection based on FRET between biomolecule-conjugated QDs and AuNPs. Tang and coworkers14 designed a simple FRET system formed by thioglycolic acid-modified CdTe quantum dots and citrate-capped AuNPs.
In the present study, we report a label-free, homogeneous and nonenzymatic FRET sensing system between CdTe/CdS quantum dots and AuNPs for the detection of melamine without any melamine-recognizing elements such as antibodies or DNA. In this assay, the CdTe/CdS QDs were used as donors and AuNPs were used as acceptors owing to their excellent spectral overlap. The luminescent semiconductor CdTe/CdS QDs in the excited state transferred their energy to the AuNPs, leading to fluorescence signal quenching via FRET. After introduction of melamine, the aggregation of AuNPs occurred by multiple combinations between melamine and the AuNPs, reducing the spectral overlap. This process turns on the fluorescence signal. According to this signal change, a label-free and homogeneous method for the detection of melamine was developed. This sensing system is facile, rapid and provides a new avenue for the determination of melamine.
Experimental
Materials and instrumentation
Melamine, tellurium powder (99.9%), CdCl2 (99%), Na2S (99%), sodium borohydride (NaBH4), sodium citrate (C6H5Na3O7) and chloroauric acid tetrahydrate (HAuCl4·4H2O) were purchased from Sinopharm Chemical Reagent Co, Ltd. (Shanghai, China). All other chemicals used in this work were of analytical grade. Water was purified with a Milli-Q purification system (Millipore Simplicity, USA) and used throughout the work. Absorption measurements were performed using a TU-1901 UV-visible spectrophotometer (Beijing Purkinje General Instrument Co, Ltd., China). Fluorescence measurements were carried out at room temperature on a LS 55 Fluorescence Spectrometer (PerkinElmer, USA). The fluorescence signal was monitored at 550 nm with excitation at 370 nm, and slits for both excitation and emission were set to 8 nm.Synthesis of CdTe/CdS QDs
Synthesis of the CdTe/CdS QDs was performed according to previous methods with some modifications.15 Firstly, NaHTe was prepared by adding 250 mg NaBH4 to a 50 mL round-bottom flask containing 310 mg of tellurium powder and 6 mL of Milli-Q water under vigorous stirring in an ice–water bath for 1 h. Then, the reaction was continued at 4 °C until all of the tellurium powder was dissolved. CdCl2 (0.4 mmol) and sodium citrate were dissolved in 100 mL of Milli-Q water, followed by adjusting the pH to 9.0 by stepwise addition of 1.0 M NaOH. The precursor solution was bubbled with nitrogen for 30 min. Then, the freshly prepared NaTeH solution was quickly injected into the mixture under vigorous stirring, followed by refluxing under open-air conditions. The molar ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaHTe
NaHTe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6H5Na3O7 was fixed to 2
C6H5Na3O7 was fixed to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8. After reacting for 1 h, sodium sulfide was added as a sulfur source with a constant S
4.8. After reacting for 1 h, sodium sulfide was added as a sulfur source with a constant S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te molar ratio of 1
Te molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the solution. The mixture was heated in an oil bath at 70–80 °C for 4 h. The obtained QDs were stored in a refrigerator at 4 °C.
1 in the solution. The mixture was heated in an oil bath at 70–80 °C for 4 h. The obtained QDs were stored in a refrigerator at 4 °C.
Synthesis of AuNPs
The AuNPs were prepared by reducing HAuCl4 with trisodium citrate.16 Typically, 1.0 mL of 1% trisodium citrate was rapidly injected into a boiling solution of HAuCl4 (100 mL, 1 mM) under stirring, and the solution was further refluxed for another 15 min into a wine-red suspension. The suspension was gradually cooled to room temperature under stirring. The obtained AuNPs were stored in a refrigerator at 4 °C.Melamine assay
Different concentrations of melamine solution were mixed with 1 mL AuNPs for 10 min. After that, 100 μL of the as-prepared original CdTe/CdS QDs aqueous solution was added with gentle shaking, followed by the addition of PBS buffer solution (10 mM pH 7.0) to give a final volume of 10 mL. The fluorescence spectra were recorded 10 min later within the spectral range of 450 to 650 nm.Sample preparation
The pretreatment procedures were similar to a previous report.17 Briefly, 20 g of milk powder was spiked with different weights of melamine, and then 10 mL of 1% aqueous acetonitrile solution was added. After 30 min of sonication, the mixture was centrifuged at 4000 rpm for 10 min. The supernatant was filtered with a filter film and diluted to give a total volume of 10 mL. The control sample was the same milk powder but without addition of melamine. The pretreatment procedures for raw milk and acidophilus milk are the same as for the milk powder.Results and discussion
Principle of sensing system
It is reported that melamine with multiple binding sites, including three exocyclic amino groups and a three-nitrogen hybrid ring, can strongly coordinate to AuNPs via ligand exchange with weakly surface-bound citrate ions. This interaction decreases the electrostatic repulsion between individual AuNPs and finally results in the aggregation of AuNPs.18 On the other hand, the as-prepared CdTe/CdS QDs exhibit strong fluorescence with a maximum emission at 550 nm which overlaps with the absorption spectrum of the dispersed AuNPs, as illustrated in Fig. 1, indicating that they may act as a donor–acceptor FRET pair and fluorescence could be quenched by the AuNPs. Consequently, a label-free fluorescence turn-on sensing system for melamine, based on the modulation of the FRET efficiency between the CdTe/CdS QDs and AuNPs, was proposed, as illustrated in Scheme 1. In the absence of melamine, the AuNPs remained dispersed and displayed an absorption band around 523 nm which overlapped with the emission spectrum of the CdTe/CdS QDs, resulting in fluorescence quenching. However, in the presence of melamine, the AuNPs would aggregate, which was confirmed by TEM images of the AuNPs before and after the addition of melamine in the method reported previously,18,19 causing a red-shift in the absorption band of the AuNPs. That is, the absorbance at 523 nm decreased and a new absorption band around 660 nm appeared (Fig. 2), which hardly overlapped with the emission spectrum of the CdTe/CdS QDs, leading to a weaker FRET efficiency. The corresponding fluorescence signal was therefore restored. | ||
| Scheme 1 Schematic illustration of the melamine assay based on melamine-regulated FRET between CdTe/CdS QDs and AuNPs. | ||
To confirm the feasibility of the present sensing system, the fluorescence emission spectra of the QDs in the presence or absence of AuNPs and/or melamine were investigated (Fig. 3). The spectrum of the CdTe/CdS QDs with melamine (curve b) was similar to that of the CdTe/CdS QDs alone (curve a), indicating that there is no obvious interaction between melamine and the QDs. However, a low fluorescence signal was observed when the CdTe/CdS QDs were mixed with AuNPs, owing to the high fluorescence quenching efficiency of the AuNPs (curve c). However, when melamine was added into the AuNP solution followed by the addition of QDs, the obtained fluorescence signal was increased remarkably (curve d) compared to that of the FRET pair in the absence of melamine. The above results confirmed that the melamine-induced AuNPs aggregation could indeed weaken the FRET efficiency between the QDs and AuNPs, permitting the development of a label-free fluorescence turn-on method for melamine detection.
 | ||
| Fig. 3 Fluorescence emission spectra of CdTe/CdS QDs under different conditions: (a) CdTe/CdS QDs; (b) melamine + CdTe/CdS QDs; (c) AuNPs + CdTe/CdS QDs; (d) melamine + AuNPs + CdTe/CdS QDs. | ||
Optimization of assay conditions
To achieve the best assay performance, some important experimental parameters including the amount of AuNPs and QDs, pH value, and reaction time were optimized.The amount of AuNPs was an important factor for influencing FRET between the CdTe/CdS QDs and AuNPs. It was found that the fluorescence intensity of the CdTe/CdS QDs decreased gradually with augmenting the AuNP amount, and then tended to be constant after addition of about 1 mL AuNP solution (Fig. 4). In addition, since the present method relies on the AuNP aggregation, the sensitivity improvement of the melamine assay depends on decreasing the AuNP amount. Thus, an amount of 1 mL AuNPs was employed to obtain enough FRET efficiency but also enough melamine sensitivity for the assay.
 | ||
| Fig. 4 Effect of AuNP amount on the fluorescence emission spectrum of CdTe/CdS QDs. The volumes of AuNPs added to the system were (from a to i) 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0 and 2.5 mL. | ||
In order to study the effect of CdTe/CdS QD concentration on the fluorescence intensity, different concentrations of CdTe/CdS QDs were investigated. It was found that a too high or too low CdTe/CdS QD concentration is not suitable for increasing the fluorescence intensity for melamine detection. The experiment showed that the most suitable CdTe/CdS QD concentration was 4.0 × 10−5 M.
The concentration of the PBS buffer solution affects directly the ionic strength of the system, which causes a change in the fluorescence intensity. To examine the change, several PBS buffer solutions with concentrations ranging from 0.5 to 15 mM were tested. The results indicate that a maximum fluorescence signal was obtained when the concentration was 10 mM. Therefore, a 10 mM PBS buffer (pH 7.0) was used in this work.
The effect of the pH value of the system on the fluorescence intensity of the CdTe/CdS QDs, in the absence and presence of AuNPs, was also examined in the range of 6.0–8.0 by measuring the peak fluorescence intensity of the QDs with or without AuNPs (Fig. 5). The results demonstrated that a maximum difference in the fluorescence intensity was achieved when the pH value of the system was maintained at 7.0. Therefore, pH 7.0 was selected in the following experiments.
 | ||
| Fig. 5 Effect of pH value of the system on the fluorescence intensity of the CdTe/CdS QDs in the absence (a) and presence of AuNPs (b). | ||
The reaction temperature and time for the FRET between the CdTe/CdS QDs and AuNPs was studied. In order to facilitate the operation, the reaction temperature was fixed at room temperature. The reaction time for the FRET between the CdTe/CdS QDs and AuNPs was studied. The mixture of CdTe/CdS QDs and AuNPs was incubated at room temperature for 2–30 min. It was found that the fluorescence intensity of the CdTe/CdS QDs decreased rapidly with the incubation time for up to 6 min, and then remained constant until 30 min. This indicated that the FRET process between the CdTe/CdS QDs and AuNPs reached an equilibrium at 6 min. Therefore, 10 min was selected as the optimal incubation time.
Melamine detection
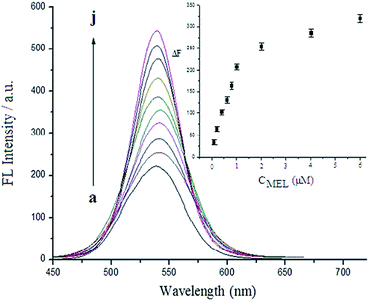
The analytical performance was investigated by testing various melamine concentrations under the optimized assay conditions. Fig. 6 displays the fluorescence spectra of the QDs in the presence of different concentrations of melamine. As can be seen, the fluorescence intensity increased with an enhancing in the melamine concentration. A calibration graph was constructed by plotting the fluorescence intensity increments (ΔF = F − F0, F is the signal in the presence of the target compound and F0 is the signal in its absence) versus the concentration of melamine (Fig. 6, inset). A good linear relationship between the fluorescence signal increments (ΔF) and the melamine concentration in the range from 5.0 × 10−8 M to 1.0 × 10−6 M was achieved. The linear calibration equation was ΔF = 18.2CMEL + 23.6 (CMEL is the concentration of melamine) and the correlation coefficient R = 0.9967. The limit of detection (LOD) was 3.0 × 10−8 M according to the rule of three times the standard deviation. This LOD is lower than that of visual detection based on label-free silver nanoparticles,20 gold nanoparticles,18 and comparable to those of fluorescence assays based on oligonucleotide-stabilized silver nanoclusters21 and the surface enhanced Raman scattering (SERS) method using silver-deposited polystyrene microspheres22 (Table 1). Eleven repetitive measurements of 2.0 × 10−7 M melamine solution showed a reproducible fluorescence response with a deviation coefficient of 5.9%, indicating good reproducibility of the proposed method.| Method | Linear range (μM) | LOD (μM) | Ref. |
|---|---|---|---|
| AuNPs (colorimetric) | 0.2–10 (mg L−1) | 0.48 | 18a |
| AuNPs (colorimetric) | 0.6–1.6 | 0.2 | 18b |
| Silver NPs (colorimetric) | 2.0–250 | 1.83 | 20 |
| Silver nanoclusters (fluorescence) | 0.05–7.0 | 0.010 | 21 |
| Silver deposited polystyrene (SERS) | 0.02–1000 | 0.019 | 22 |
| CdTe/CdS QDs and AuNPs (FRET) | 0.05–1.0 | 0.03 | This work |
Specificity of the sensing system
To study the selectivity of the present sensing system for melamine, some common metal ions, carbohydrates, amino acids and proteins, including Na+, K+, Ca2+, Mg2+, Cl−, glucose, lactose, valine, threonine, lysine, tryptophan, histidine, human serum albumin (HSA), and bovine serum albumin (BSA), were chosen for an interference investigation. Each interferent was mixed with 5.0 × 10−7 M melamine and then analyzed. Table 2 shows the influence of foreign substances on the melamine determination. It can be seen that these foreign substances had negligible effects even at 1000-fold higher concentrations than melamine. This result indicated that the present system possessed an impressive specificity for melamine.| Foreign substances | Added concentration (M) | Relative error (%) |
|---|---|---|
| K+ | 1.0 × 10−4 | −5.2 |
| Ca2+ | 5.0 × 10−4 | +6.2 |
| Mg2+ | 1.0 × 10−3 | +2.5 |
| Cl− | 1.0 × 10−4 | +5.4 |
| SO42− | 1.0 × 10−3 | +3.1 |
| NO3− | 1.0 × 10−3 | +3.5 |
| Glucose | 1.0 × 10−4 | +2.1 |
| Lactose | 3.0 × 10−4 | −2.5 |
| Valine | 2.0 × 10−5 | −3.9 |
| Threonine | 5.0 × 10−5 | +5.4 |
| Cysteine | 2.0 × 10−7 | +4.8 |
| Glutathione | 5.0 × 10−7 | +5.1 |
| Lysine | 1.0 × 10−4 | +2.6 |
| Tryptophan | 1.0 × 10−4 | +5.4 |
| Histidine | 2.0 × 10−4 | −6.2 |
| HSA | 1.0 × 10−4 | +2.4 |
| BSA | 5.0 × 10−4 | +6.5 |
Melamine detection in real samples
The designed method exhibits a good anti-disturbance ability and holds promising potential for the detection of melamine in real samples. Therefore, the sensing system was applied for the determination of the target compound in three real samples, including milk powder, raw milk and acidophilus milk. The concentration of melamine in the samples was determined using the standard addition method and the results are shown in Table 3. As can be seen, satisfactory detection results for the samples were obtained in the range of 95.0–101%. Thus, the proposed sensing system is sufficient for rapid screening of real samples in a quick and simple manner.| Sample | Added (×10−7 M) | Found (×10−7 M) | Detected (%, n = 5) |
|---|---|---|---|
| Milk powder | 2.0 | 1.9 | 95.0 |
| 5.0 | 4.8 | 96.0 | |
| Raw milk | 8.0 | 7.9 | 98.8 |
| 2.0 | 1.9 | 95.0 | |
| 5.0 | 4.9 | 98.0 | |
| Acidophilus milk | 8.0 | 8.1 | 101 |
| 2.0 | 1.8 | 90.0 | |
| 5.0 | 4.8 | 96.0 | |
| 8.0 | 8.1 | 101 |
Conclusions
In summary, we have demonstrated a label-free fluorescence turn-on sensing system for the detection of melamine based on FRET between CdTe/CdS QDs and AuNPs. The excellent quenching efficiency of AuNPs to CdTe/CdS QDs and the subsequent fluorescence recovery caused by melamine-induced aggregation of the AuNPs achieved sensitive and selective detection of melamine. Compared with traditional methods for melamine detection, this sensing system shows multifaceted advantages. First, the method is simple, low-cost and fast with facile experimental operation. Second, it shows high sensitivity and specificity for melamine detection. The detection limit obtained for melamine was 3.0 × 10−8 M, which is lower than those of visual detections based on silver nanoparticles20 and gold nanoparticles.18 Third, the assay is conducted in aqueous solution, without requiring troublesome separation procedures. Moreover, its excellent anti-disturbance ability could be shown and satisfactory results for melamine detection in real milk products were achieved. Thus, this method is expected to be of potential application in the field of food safety.Acknowledgements
This work was supported by the National Natural Science Foundations of China (21305020) and Science Foundations of Guangxi Normal University (2013ZD006).Notes and references
- C. M. E. Gossner, J. Schlundt, P. B. Embarek, S. Hird, D. Lo-Fo-Wong, J. J. Beltran, K. N. Teoh and A. Tritscher, Environ. Health Perspect., 2009, 117, 1803 CrossRef CAS PubMed.
- K. Ai, Y. Liu and L. Lu, J. Am. Chem. Soc., 2009, 131, 9496 CrossRef CAS PubMed.
- X. Pei, A. Tandon, A. Alldrick, L. Giorgi, W. Huang and R. Yang, Food Policy, 2011, 36, 412 CrossRef PubMed.
- (a) E. A. E. Garber and V. A. Brewer, J. Food Prot., 2010, 73, 701 CrossRef CAS PubMed; (b) H. Lei, R. Su, S. A. Haughey, Q. Wang, Z. Xu, J. Yang, Y. Shen, H. Wang, Y. Jiang and Y. Sun, Molecules, 2011, 16, 5591 CrossRef CAS PubMed; (c) W. Yin, J. Liu, T. Zhang, W. Li, W. Liu, M. Meng, F. He, Y. Wan, C. Feng, S. Wang, X. Lu and R. Xi, J. Agric. Food Chem., 2010, 58, 8152 CrossRef CAS PubMed.
- G. Venkatasami and J. R. Sowa Jr, Anal. Chim. Acta, 2010, 665, 227 CrossRef CAS PubMed.
- H. Y. Huang, C. L. Lin, S. H. Jiang, B. Singco and Y. J. Cheng, Anal. Chim. Acta, 2012, 719, 96 CrossRef CAS PubMed.
- (a) Q. R. Cai, Y. Y. Ouyang, Z. J. Qian and Y. F. Peng, Chin. J. Chromatogr., 2008, 26, 339 CAS; (b) W. C. Andersen, S. B. Turnipseed, C. M. Karbiwnyk, S. B. Clark, M. R. Madson, C. M. Gieseker, R. A. Miller, N. G. Rummel and R. Reimschuessel, J. Agric. Food Chem., 2008, 56, 4340 CrossRef CAS PubMed.
- X. L. Zhu, S. H. Wang, Q. Liu, Q. Xu, S. X. Xu and H. L. Chen, J. Agric. Food Chem., 2009, 57, 11075 CrossRef CAS PubMed.
- T. L. Fodey, C. S. Thompson, I. M. Traynor, S. A. Haughey, D. G. Kennedy and S. R. H. Crooks, Anal. Chem., 2011, 83, 5012 CrossRef CAS PubMed.
- (a) H. Peng, L. Zhang, T. H. M. Kjallman, C. Soeller and J. Travas-Sejdik, J. Am. Chem. Soc., 2007, 129, 3048 CrossRef CAS PubMed; (b) X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed; (c) I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS PubMed; (d) L. Shi, V. D. Paoli, N. Rosenzweig and Z. Rosenzweig, J. Am. Chem. Soc., 2006, 128, 10378 CrossRef CAS PubMed.
- (a) C. Burda, X. B. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS PubMed; (b) M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed; (c) B. Dubertret, M. Calame and A. Libchaber, Nat. Biotechnol., 2001, 19, 365 CrossRef CAS PubMed.
- (a) B. Tang, L. H. Cao, K. H. Xu, L. H. Zhuo, J. C. Ge, Q. L. Li and L. J. Yu, Chem.–Eur. J., 2008, 14, 3637 CrossRef CAS PubMed; (b) L. Dyadyusha, H. Yin, S. Jaiswal, T. Brown, J. J. Baumberg, F. P. Booy and T. Melvin, Chem. Commun., 2005, 3201 RSC; (c) H. C. Pan, R. J. Cui and J. J. Zhu, J. Phys. Chem. B, 2008, 112, 16895 CrossRef CAS PubMed.
- E. Oh, M.-Y. Hong, D. Lee, S.-H. Nam, H. C. Yoon and H.-S. Kim, J. Am. Chem. Soc., 2005, 127, 3270 CrossRef CAS PubMed.
- M. Xue, X. Wang, H. Wang, D. Chen and B. Tang, Chem. Commun., 2011, 47, 4986 RSC.
- (a) H. Peng, L. Zhang, C. Soeller and J. Travas-Sejdic, J. Lumin., 2007, 127, 721 CrossRef CAS PubMed; (b) A. L. Rogach, D. Nagesha, J. W. Ostrander, M. Giersig and N. A. Kotov, Chem. Mater., 2000, 12, 2676 CrossRef CAS.
- G. Frens, Nature Phys. Sci., 1973, 241, 20–22 CrossRef CAS.
- N. Ding, N. Yan, C. Ren and X. G. Chen, Anal. Chem., 2010, 82, 5897 CrossRef CAS PubMed.
- (a) L. Li, B. Li, D. Cheng and L. Mao, Food Chem., 2010, 122, 895 CrossRef CAS PubMed; (b) L. Guo, J. Zhong, J. Wu, F. Fu, G. Chen, X. Zheng and S. Lin, Talanta, 2010, 82, 1654 CrossRef CAS PubMed.
- D. Xiang, G. Zeng, K. Zhai, L. Li and Z. He, Analyst, 2011, 136, 2837 RSC.
- H. Ping, M. Zhang, H. Li, S. Li, Q. Chen, C. Sun and T. Zhang, Food Control, 2012, 23, 191 CrossRef CAS PubMed.
- S. Han, S. Zhu, Z. Liu, L. Hua, S. Parveen and G. Xu, Biosens. Bioelectron., 2012, 36, 267 CrossRef CAS PubMed.
- Y. Zhao, W. Luo, P. Kanda, H. Cheng, Y. Chen, S. Wang and S. Huan, Talanta, 2013, 113, 7 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |