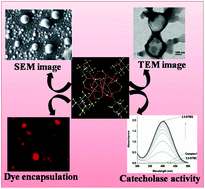
The reaction of Mn(OBz)2·4H2O (OBz = benzoate) with hmt (hmt = hexamethylenetetramine) in CH3CN affords a mixed-valence hexanuclear complex [MnIII2MnII4O2(hmt)4(OBz)10] (1). The complex has been characterized in solid state by elemental analysis, IR spectroscopy and single-crystal X-ray diffraction analysis. X-Ray crystal structure reveals that 1 consists of a [MnIII2MnII4O2] core, co-ordinated to ten bridging benzoates (six syn,-syn-η1:η1:μ2, four η1:η2:μ3) and four terminal hmt groups. In solution state (acetonitrile solvent) 1 spontaneously forms self-assembled vesicular structure with diameter in the range of 100–150 nm. The morphology in solution state has been studied through various microscopic techniques like SEM, TEM, AFM etc. These self-assembled vesicular structures can encapsulate organic dye molecules, which have been confirmed by confocal microscopic imaging. Complex 1 also exhibits substantial catecholase-like activity with 3,5-di-tert-butylcatechol (3,5-DTBC) as the substrate in acetonitrile solvent; the Kcat value is determined to be 2337.9 h−1.