 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of microplastic fibres: generation, transport, and vectors for metal(loid)s in terrestrial environments†
H.
Frost
 *a,
T.
Bond
*a,
T.
Bond
 b,
T.
Sizmur
b,
T.
Sizmur
 c and
M.
Felipe-Sotelo
a
c and
M.
Felipe-Sotelo
a
aDepartment of Chemistry, University of Surrey, Guildford, Surrey GU2 7XH, UK. E-mail: h.frost@surrey.ac.uk
bDepartment of Civil and Environmental Engineering, University of Surrey, Guildford, GU2 7XH, UK
cDepartment of Geography and Environmental Science, University of Reading, Reading, RG6 6DW, UK
First published on 24th March 2022
Abstract
The laundering of synthetic fabrics has been identified as an important and diffuse source of microplastic (<5 mm) fibre contamination to wastewater systems. Home laundering can release up to 13 million fibres per kg of fabric, which end up in wastewater treatment plants. During treatment, 72–99% of microplastics are retained in the residual sewage sludge, which can contain upwards of 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microplastics per kg. Sewage sludge is commonly disposed of by application to agricultural land as a soil amendment. In some European countries, application rates are up to 91%, representing an important pathway for microplastics to enter the terrestrial environment, which urgently requires quantification. Sewage sludge also often contains elevated concentrations of metals and metalloids, and some studies have quantified metal(loid) sorption onto various microplastics. The sorption of metals and metalloids is strongly influenced by the chemical properties of the sorbate, the solution chemistry, and the physicochemical properties of the microplastics themselves. Plastic–water partition coefficients for the sorption of cadmium, mercury and lead onto microplastics are up to 8, 32, and 217 mL g−1 respectively. Sorptive capacities of microplastics may increase over time, due to environmental degradation processes increasing the specific surface area and surface density of oxygen-containing functional groups. A range of metal(loid)s, including cadmium, chromium, and zinc, have been shown to readily desorb from microplastics under acidic conditions. Sorbed metal(loid)s may therefore become more bioavailable to soil organisms when the microplastics are ingested, due to the acidic gut conditions facilitating desorption. Polyester (polyethylene terephthalate) should be of particular focus for future research, as few quantitative sorption studies currently exist, it is potentially overlooked from density separation studies due to its high density, and it is by far the most widely used fibre in apparel textiles production.
000 microplastics per kg. Sewage sludge is commonly disposed of by application to agricultural land as a soil amendment. In some European countries, application rates are up to 91%, representing an important pathway for microplastics to enter the terrestrial environment, which urgently requires quantification. Sewage sludge also often contains elevated concentrations of metals and metalloids, and some studies have quantified metal(loid) sorption onto various microplastics. The sorption of metals and metalloids is strongly influenced by the chemical properties of the sorbate, the solution chemistry, and the physicochemical properties of the microplastics themselves. Plastic–water partition coefficients for the sorption of cadmium, mercury and lead onto microplastics are up to 8, 32, and 217 mL g−1 respectively. Sorptive capacities of microplastics may increase over time, due to environmental degradation processes increasing the specific surface area and surface density of oxygen-containing functional groups. A range of metal(loid)s, including cadmium, chromium, and zinc, have been shown to readily desorb from microplastics under acidic conditions. Sorbed metal(loid)s may therefore become more bioavailable to soil organisms when the microplastics are ingested, due to the acidic gut conditions facilitating desorption. Polyester (polyethylene terephthalate) should be of particular focus for future research, as few quantitative sorption studies currently exist, it is potentially overlooked from density separation studies due to its high density, and it is by far the most widely used fibre in apparel textiles production.
Environmental significanceThis paper critically reviews the evidence for microplastic fibres released during home laundering becoming a vector for metal(loid)s from wastewater to terrestrial environments. Quantitative data on fibre shedding from textiles were collated and critically evaluated. A conceptual framework is presented which discusses the transport of microplastic fibres from laundry water, through wastewater treatment plants, to agricultural soils through the application of sewage sludge. Distribution coefficients that quantify the sorption of aqueous metal(loid)s to microplastics were compared and critically analysed. The scarcity of both soil concentration and metal(loid) sorption data for common textiles polymers, such as polyester and nylon, is highlighted as an important knowledge gap. The bioavailability of microplastic-sorbed metal(loid)s to organisms living in agricultural soils is critically discussed. |
1. Introduction
The annual global total production of plastics exceeded 400 million metric tonnes (MMT) per year in 2015.1 In 2015, the seven plastics with the highest demand in the EU (excluding fibres) were polypropylene (PP) > low-density polyethylene (LDPE) > high-density polyethylene (HDPE) > polyvinyl chloride (PVC) > polyurethane (PUR) > polyethylene terephthalate (PET) > polystyrene (PS).2 Plastics offer many advantageous properties such as corrosion resistance, low cost of raw materials and production, and general durability, meaning they have become increasingly favourable over traditional materials (metal, paper, wood) since the 1950s.1 Plastics are now routinely manufactured for a wide variety of end uses, including food packaging, synthetic textile fibres, building insulation, and protective coatings. The most commonly manufactured plastics are not readily biodegradable, and thus accumulate in the environment. The rate of plastic waste recycling to the original product is below 10%.3 These factors, together with the continuously increasing global plastic demand at 8.7% annual growth rate,1 have resulted in a global plastic pollution issue.Microplastics are typically defined as measuring 5 mm or less across their longest dimension, and have become a global pollutant of concern due to their persistence and ubiquity in terrestrial and marine environments.4–6 Microplastics have been detected worldwide in oceans,4,7 lakes and rivers,8,9 Arctic sea ice,10 marine and freshwater sediments,11–13 sewage sludge,14–18 and agricultural soils.19–21 Published literature on the environmental impact microplastics has increased exponentially since 2010. However, the majority of studies have focused on the impacts to marine environments.22 From January 2004 to June 2018, only 4% of published literature on microplastics focused on terrestrial sinks such as soil and sludge.22 Consequently, current knowledge on the scale, environmental fate, and ecological impacts of microplastic pollution in terrestrial environments is limited.
This knowledge gap is concerning as it has been estimated that, in the EU, terrestrial environments could receive 4–23 times more microplastic pollution than oceans.23 It has also been estimated that up to 48% of this microplastic pollution is due to the direct application of contaminated sludge to agricultural soils.24 The shedding of textiles during laundering is thought to be a considerable source of microplastic pollution.25 Synthetic fabrics, such as polyethylene terephthalate (PET), and nylon (polyamide-6,6) shed recalcitrant, non-biodegradable fibres during laundering; the majority of which are 0.20 to 2.75 mm in length.25,26 Between 72% and 99.9% of microplastics (by number) are removed during the sewage treatment process,27 and approximately 78% are retained in the semi-solid sludge fraction.28 This sludge is collected and disposed of in a number of ways, including application to agricultural land, incineration or landfill. Recycling to agricultural land is often a preferred option across much of Europe, USA, and China because treated sludge poses little risk to human and animal health, and contains essential plant nutrients and organic matter which improve soil fertility and physicochemical properties.29 Sludge use in agriculture is heavily regulated. Before application to agricultural land, sludge must be treated to reduce pathogen content, odour, and attraction of potential disease vectors such as rats. This treatment may be biological (e.g. aerobic or anaerobic digestion), chemical (e.g. lime stabilisation), or physical (e.g. thermal drying), or any combination of the three. Sludge that has been treated and stabilised for land application purposes is referred to as biosolids.29,30
In the UK, approximately 79% of municipal sewage sludge is recycled to agricultural soils as biosolids.31 In other European countries, application rates vary from 0–91%.24 Therefore, the application of biosolids may constitute a very significant route for the entry of microplastic fibres derived from laundered fabrics, into agricultural soils. This conceptual pathway is illustrated in Fig. 1 (Section 2). Moreover, during sewage treatment, synthetic fibres are exposed to elevated levels of metals and metalloids,32 and organic contaminants, including antibiotics, endocrine-disruptors and polycyclic aromatic hydrocarbons,33,34 which may sorb to the surfaces of the fibres. The study of the sorption of metals and metalloids to microplastics is still in its infancy. Nevertheless, metals including cadmium (Cd), copper (Cu), nickel (Ni), and lead (Pb) have been shown to sorb to both virgin and beached microplastic pellets.35,36 The bioavailability and environmental fate of microplastic-bound metals and metalloids is poorly understood. However, microplastics may act as vectors for metals and metalloids that would otherwise have been discharged in the effluent. Therefore, metal and metalloid sorption to microplastics may ultimately increase their exposure to important soil organisms such as earthworms.37
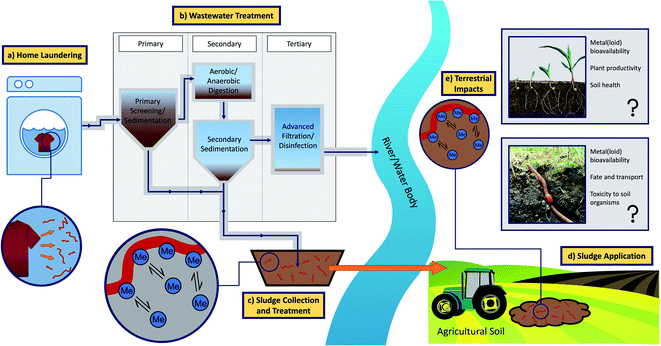 | ||
| Fig. 1 Conceptual pathway visualising the transport of microplastic fibres from laundry effluent to agricultural soils through the production and application of sewage sludge (Me = metal(loid) ion). | ||
Throughout this review, microplastic particles (≤5 mm in length) with fibrous morphologies are referred to as ‘microplastic fibres’, and the uniform adoption of this term is suggested for future research. In textiles science, the term microfibre has been clearly defined as a single thread with a denier of 0.3–1.38–40 A denier is a unit of linear density equal to the mass in grams of 9000 m of fibre.38 In environmental science however, the term ‘microfibre’ has not been appropriately defined and is sometimes used to refer to only synthetic fibres, or to synthetic and natural fibres.7,25,41,42 We suggest that ‘microplastic’ and ‘microfibre’ are not used interchangeably, and that the distinction between natural and synthetic fibres should be retained, given that fibres are likely to have different sources, environmental partitioning behaviours, and environmental impacts than natural fibres or microplastics with other morphologies.
This review aims to (i) synthesise and critically evaluate recent qualitative research on fabric shedding as a source of microplastic fibre pollution; (ii) provide a conceptual framework for, and comment on the environmental significance of, the transfer of microplastic fibres from laundry wastewater to agricultural soils through the application of sewage sludge as a soil amendment, and finally (iii) critically evaluate existing data concerning the sorption of metals and metalloids to microplastics.
2. Microplastic fibres from synthetic textiles
The laundering of synthetic textiles was first evidenced as a diffuse source of microplastic fibre pollution by Habib et al. (1998),14 who used polarised light microscopy to qualitatively identify synthetic fibres in dewatered sewage sludge, biosolid pellets, and wastewater effluent from a wastewater treatment plant (WWTP) in Long Island, New York. These fibres were hypothesised to come from the shedding of apparel textiles during laundering (Fig. 1a).14 Shedding refers to the detachment and release of fibres from the surface of the fabric and primarily occurs during laundering, where the rotational force and mechanical action of the washing machine drum cause fibres to break and enter the water.42 Zubris and Richards (2005)19 detected synthetic fibres in soil samples up to 15 years after the application of biosolids, implicating sludge disposal to soil as an important pathway for the terrestrial transport of fibres. Browne et al. (2011)4 found that sediments from sewage sludge disposal sites and wastewater treatment plant effluent contained proportions of synthetic fibres which resembled those used in apparel textiles (78% polyester, 9% polyamide, 7% polypropylene, and 5% acrylic),43 suggesting that the fibres were largely derived from the shedding of clothing during laundering.Several factors may influence the propensity of fabrics to shed during laundering, including the physicochemical properties of the fibres,25 yarns,44,45 and fabric.41,42,46–48 Fabric shedding is also influenced by laundering conditions, such as washing machine type (front-loading or top-loading), water temperature, and the presence of surfactants and fabric softeners.49,50
We highlight 25 research papers, published between 2011 and 2021, that report quantitative data on fabric shedding and summarise key data in Table 1.4,25,41,42,46–66 Quantifying the number of fibres shed from a fabric during laundering is practically difficult due to the small size and vast numbers of fibres generated, and the lack of standardised methodologies. Moreover, the fabric types and construction, reported units, laundering apparatus, laundering conditions, and fibre characterisation techniques all differ between studies, making comparison of the results difficult. Hernandez et al. (2017),50 Almroth et al. (2018),42 De Falco et al. (2018)56,57 Jönsson et al. (2018),59 Zambrano et al. (2019),25 Haap et al. (2019),58 Kelly et al. (2019),61 Frost et al. (2020),48 Raja Balasaraswathi and Rathinamoorthy (2021),65 Cai et al. (2020),52 and Özkan and Gündoğdu (2021),63 all used standard laundry testing apparatus, while the remaining studies used commercially available washing machines to generate fibres. Fabric shedding varied from 900–110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fibres per garment,4,42 although the sizes and masses of the garments were unspecified. On a number per mass basis, fibre shedding ranged from 8809–72
000 fibres per garment,4,42 although the sizes and masses of the garments were unspecified. On a number per mass basis, fibre shedding ranged from 8809–72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per kg of fabric,52,66 and on a mass per mass basis, 7–1507 mg fibres per kg of fabric.54,65
000 per kg of fabric,52,66 and on a mass per mass basis, 7–1507 mg fibres per kg of fabric.54,65
| Reference | Fibres | Laundering | Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fabric type | Fabric mass (g) | Fibres shed | Units | Type/apparatus | Experimental/washing conditions | Filter pore-size (μm) | Fibre characterisation | LOD (μm) | Chemical confirmation method | |
| Browne et al. (2011)4 | 100% polyester blankets, fleeces and shirts | NS | >1900 | Fibres per garment | Conventional: Bosch WAE24468GB, John Lewis JLWM1203 and Siemens Extra Lasse XL 1000 washing machines | Speed 600 rpm; time unspec.; 40 °C | NS | Number: counted manually | NS | None |
| Bruce et al. (2016)49 | 100% polyester; 100% nylon; 97% polyester; 3% Spandex fleece and non-fleece jackets | NS | 100.5–1081.9 | mg per garment | Conventional: Whirlpool WET3300XQ1 washing machine | Speed unspec.; 30 min; temp. Unspecified; 43 L capacity, front-load and top-load machines; artificially aged and new garments; ‘regular warm wash cycle’ | 333 and 20 | Mass: gravimetric analysis | NS | None |
| Napper and Thompson (2016)46 | 100% polyester jumper | NS | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 671 |
Fibres per kg fabric | Conventional: Whirlpool WWDC6400 washing machine | 1400 rpm; 75 min; 30 and 40 °C; 20 × 20 cm swatches; no detergent; with bio/non-bio detergent; with/without conditioner | 25 | Mass: gravimetric analysis; number: estimated; length: light microscope and image analysis; width: SEM and image analysis | NS | None |
| 100% acrylic jumper | 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465 465 |
Fibres per kg fabric | ||||||||
| 65% polyester; 35% cotton jumper | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 992 992 |
Fibres per kg fabric | ||||||||
| Pirc et al. (2016)47 | 100% polyester fleece blanket | NS | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
Fibres per kg fabric | Conventional: Bosch model Maxx7 VarioPerfect | 600 rpm; 15 min; 30 °C; 120 × 70 cm blankets; without additives; with detergent; with detergent and fabric softener | 200 | Mass: gravimetric analysis; relative release: estimated | NS | None |
| 12 | mg per kg fabric | |||||||||
| Sillanpää and Sainio (2017)41 | 100% polyester blankets and T-shirts; 100% cotton jeans and shirts | NS | 211![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–13 000–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per fabric | Conventional: Bosch WAE28477SN washing machine | 1200 rpm; 75 min; 40 °C; 50 mL liquid detergent; 27–39 L of washing water | 0.7 | Number: counted manually, light microscopy; mass: gravimetric analysis | NS | None |
| Belzagui et al. (2019)51 | Commercial garments: 100% polyester; 80% polyester, 20% elastane; 70% acrylic, 30% nylon | 74–728 | 166![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667–1 667–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 083![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 333 |
Fibres per kg of fabric | Conventional: FAGOR innovation F-2810, Spain | 1000 rpm; 15 min; ambient temp.; 22 L water, commercially available detergent used as specified; water hardness of 349 mg L−1 as CaCO3 | 20 | Number: counted manually, light microscopy; mass: estimated; morphology: SEM | NS | None |
| De Falco et al. (2019)55 | 100% polyester T-shirts and blouses | 2000–2500 | 48.6–307.6 | mg per kg fabric | Conventional: Bosch WLG24225i | 1200 rpm; 107 min; 40 °C; liquid detergent | 400; 60; 20 and 5 | Mass: gravimetric analysis; number: estimated; length and width: light microscopy, SEM and image analysis | NS | FTIR |
641![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1 000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | |||||||||
| McIlwraith et al. (2019)62 | 100% polyester fleece blanket | 545 | 233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 395 395 |
Fibres per fabric | Conventional: SDL Atlas M6 Vortex washing machine | 660 rpm; 30 min; 16 °C; 26.5 L capacity | 10 | Mass: gravimetric analysis; number: light microscopy and image analysis; length: light microscopy and image analysis | 100 | None |
| Dalla Fontana et al. (2020)53 | 100% polyester knit | 2000 | 32.5–38.6 (sum of 5 consecutive washes) | mg per kg fabric | Conventional: Bosch WAW286H8 | 600, 1400 rpm; 43, 90 min; 30 °C, 40 °C; with commercial detergent (45 mL) | 40 | Mass: gravimetric | NS | FTIR |
| Dalla Fontana et al. (2021)54 | 100% polyester | NS | 7–19.5 | mg per kg fabric | Conventional: Bosch WAW286H8 | 1400 rpm; 90 min; 40 °C; with commercial detergent (45 mL) | 40 | Mass: gravimetric | NS | None |
| Kärkkäinen and Sillanpää (2021)60 | Various polyester, nylon, and acrylic commercially available garments | 184–1052 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–6 000–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Conventional: Bosch WAE28477SN | 1200 rpm; 75 min; 40 °C; with commercial detergent (50 mL) | 0.7 | Number: counted manually, light microscopy; mass: gravimetric; length: light microscopy and image analysis | NS | None |
| Periyasamy (2021)64 | Jeans, 97% polyester, 3% lycra; 70% polyester, 27% cotton, 3% lycra; 50% polyester, 50% cotton | 453–462 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–4 000–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Conventional: Whirlpool FRESH CARE | 1200, 1400 rpm; 60, 75, 90 min; 30 °C, 45 °C, 60 °C; with and without 40 mL of fabric conditioner | 200; 5 | Number: counted manually, light microscopy; length and width: SEM and image analysis | NS | FTIR |
| Vassilenko et al. (2021)66 | Fabrics of various compositions, constructions, treatments and finishes | 500 | 8809–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 877 877![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Conventional: SDL Atlas Vortex M6 | 645 rpm; 18 min; 41 °C; 34.1 L capacity | 20 | Mass: gravimetric; number: estimated, light microscopy | NS | FTIR |
| 9.6–1240 | mg per kg fabric | |||||||||
| Hernandez et al. (2017)50 | 100% polyester knitted interlock, 98% polyester and 2% Spandex single knit Jersey fabrics | 7 | 25–100 | mg per kg fabric | Accelerated: Washtex-P Roaches laboratory washing machine | 40 rpm; 45 min; 40 °C; 7 g of fabric, with/without liquid or powder detergent (4 g L−1); 200 mL liquid; 10 × 6 mm diameter metal beads | 0.45 | Number: counted manually, light microscopy; mass: estimated; length normalised distribution: light micros and image analysis | 40 | None |
| Almroth et al. (2018)42 | 100% polyester, acrylic and nylon knits | NS | 900 | Fibres per garment | Accelerated: Gyrowash one bath 815/8 standardised laboratory washer | Speed unspec.; 30 min; 60 °C; 10 × 10 cm fabric swatches, 125 mL water; 0.375 mL liquid detergent; 25 × 6 mm diameter metal beads; with/without detergent | 1.2 | Number: counted manually, light microscopy; length: light microscopy, image analysis | NS | None |
| 100% polyester fleece | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per garment | ||||||||
| De Falco et al. (2018)57 | 100% polyester weave and double-knit Jersey, and 100% polypropylene weave | 1.05–1.67 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Accelerated: Linitest apparatus | Speed unspec.; 45 min; 40 °C; 9.3 × 9 cm swatches; with/without household and industrial detergent; 10 steel beads | 5 | Number: count. Manual SEM; length: SEM and image analysis; diameter: SEM and image analysis; mass: estimated | NS | None |
| Jönsson et al. (2018)59 | Recycled 100% polyester tricot | NS | 9318 | Fibres per 17 × 9 cm fabric swatch | Accelerated: Gyrowash standardised laboratory washer | Speed unspec.; 60 min; 40 °C; 17 × 9 cm fabric swatches; edge welded to form a bag with 25 steel beads inside; 75 mL water | 0.65 | Number, length and width: counted manually, light microscopy and image analysis | 100 length, 5 number | None |
| Virgin 100% polyester tricot | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886 886 |
|||||||||
| Ultrasonic cut recycled 100% polyester tricot | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 618 618 |
|||||||||
| Scissor cut recycled 100% polyester tricot | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
|||||||||
| Recycled 100% polyester fleece | 7075 | |||||||||
| Virgin 100% polyester fleece | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 489 489 |
|||||||||
| Haap et al. (2019)58 | 50% polyester, 50% cotton woven fabric | 6 | 368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Accelerated: Labomat lab-scale washing machine | 40 rpm; 30 min; 40 °C; 8.5 × 16.5 edge-sealed fabric pillows; 50 steel beads; 160 mL liquid containing 2 mL liquid detergent | 8 | Number, length and width: SEM, dynamic image analysis; morphology: SEM | NS | Cotton removal by sulphuric acid digestion |
| Zambrano et al. (2019)25 | 100% cotton, 100% rayon, 100% polyester and 50%/50% polyester–cotton weft knitted interlock fabrics | 2–2.5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15 000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Accelerated: SDL Atlas LaunderOmeter | Speed unspec.; 16 min; 25 and 44 °C; 10 × 10 cm fabric swatches; with/without AATCC standard reference liquid detergent (1.47 g L−1); 150 mL liquid; 25 × 6 mm diameter metal beads | 1.2 | Number: FQA; mass: gravimetric analysis; length and width normalised distributions (FQA) | 25 | None |
| Frost et al. (2020)48 | 100% cotton knit (virgin, 20% recycled and 40% recycled) | 2.1–3.6 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–11 000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Accelerated: SDL Atlas LaunderOmeter | Speed unspec.; 16 min; 20 °C; 10 × 10 cm fabric swatches; with AATCC standard reference liquid detergent (1.47 g L−1); 150 mL liquid; 25 × 6 mm diameter metal beads | 1.2 | Number: FQA; mass: gravimetric analysis; length and width normalised distributions (FQA) | 25 | None |
| 100% polyester knit (virgin, 40% recycled and 70% recycled) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–32 000–32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||||||
| 30% polyester 70% cotton twill weave (virgin, 7% recycled cotton, 21% recycled cotton) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–4 000–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||||||||
| Cai et al. (2020)52 | 100% polyester, various knitted and woven fabrics | 1.5–2.3 | 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–72 000–72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Accelerated: Gyrowash standardised laboratory washer | Speed unspec.; 45 min; 40 °C; with surfactant (linear alkylbenzene sulfonic acid; 0.75 g L−1; 0, 10 and 20 metal beads | 0.45 | Number: counted manually, light microscopy; mass: estimated; length and width: light microscope and image analysis | 90 | None |
| Özkan and Gündoğdu (2021)63 | 100% virgin polyester | NS | 167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 437 437 |
Fibres per kg fabric | Accelerated: Gyrowash standardised laboratory washer | Speed unspec.; 45 min; 40 °C; 4 × 10 cm swatches; 150 mL water; 10 metal beads | 0.45 | Number: counted manually, light microscopy; length and width: unspec | 0.45 | None |
| 100% recycled polyester | 368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094 094 |
|||||||||
| Raja Balasaraswathi and Rathinamoorthy (2021)65 | 100% polyester weft-knitted fabrics | 3.15–6.3 | 905![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–5 000–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 675 675![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Accelerated: SDL Atlas LaunderOmeter | Speed unspec.; 45 min; 30 °C; 15 × 15 cm fabric swatches; with detergent (4 g L−1); 150 mL liquid; 10 metal beads | 11 | Number: estimated; mass: estimated, light microscopy | NS | None |
| 131.9–1507.2 | mg per kg fabric | |||||||||
| De Falco et al. (2018)56 | 100% polyester knit T-shirt | 2500 | 549![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 913 913 |
Fibres per kg fabric | Conventional: Bosch WLG24225 | 1200 rpm; time unspecified; 40 °C; liquid detergent | 400; 60; 20 | Mass: gravimetric analysis; number: estimated; length and width: light microscopy, SEM and image analysis | NS | FTIR |
| NS | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 733 733![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fibres per kg fabric | Accelerated: Gyrowash standardised laboratory washer | Speed unspec.; 45 min; 40 °C; 9 × 9 cm fabric swatches, edges thermally sealed; 10 steel beads; 150 mL liquid | 5 | |||||
| Kelly et al. (2019)61 | 100% polyester knit T-shirts | NS | 86.63–206.49 | mg per kg fabric | Conventional: Miele front-loading washing machine W3622 | 600 or 1600 rpm; 30, 59 or 85 min; 13–15 or 30 °C; various wash cycles including cotton short cycle, cold express cycle and delicate cycle; 30–69 L; with/without 35 mL of commercial liquid detergent; 1.5 kg of T-shirts | 22 | Mass: gravimetric analysis; number: estimated; length: light microscopy and image analysis; width: SEM and image analysis | NS | None |
618![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724–1 724–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 474![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 793 793 |
Fibres per kg fabric | |||||||||
| 53.84–119.83 | mg per kg fabric | Accelerated: Tergotometer benchtop washing simulation device | 100 or 200 rpm; 15 or 60 min; 15 or 30 °C; 5 × 5 cm laser-cut fabric swatches; no steel beads | |||||||
384![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545–855 545–855![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 884 884 |
Fibres per kg fabric | |||||||||
The extreme range in literature values highlights the need for standardisation in the quantification of fabric shedding during laundering to make meaningful comparisons between fabrics. Pirc et al. (2016)47 reported among the lowest number of fibres per mass of fabric, but also used the largest filter size (200 μm). Zambrano et al. (2019)25 reported that shed fibres 25–200 μm in length were more numerous than fibres 200 μm–2.75 mm, shed from PET, cotton, and PET-cotton blend fabrics, suggesting that the shedding data from Pirc et al. (2016)47 may have underestimated total fibre release, due to smaller fibres passing through the filter. Raja Balasaraswathi and Rathinamoorthy (2021)65 and Vassilenko et al. (2021)66 found shedding propensity significantly increases with fabric thickness. This is thought to be due to an increased density of fibre ends per unit of surface area.65 A higher stitch density (number of stitches per unit area) results in less fibre release, as friction between constituent fibres is increased.65 Dalla Fontana et al. (2021)54 compared the shedding of two 100% polyester fabrics with differing constructions, and observed significantly different fibre release during conventional laundering experiments. Differences were attributed to the differing linear densities of the constituent fibres, which influences tensile properties, and the stitching used to finish the fabric edges.54 Fabrics composed of natural fibres, such as cotton, generally have a higher shedding propensity then fabrics constructed with synthetic fibres, such as polyester.25 This may be due to the lower tensile strength of natural fibres, or the shorter fibre length of cotton, resulting in more fibre breakages overall.25,66 It is important to note that chemical identification of the shed fabrics with spectroscopic techniques (IR/Raman), was only performed in 5 of the 25 studies (Table 1). In the remaining studies, fibres were counted and/or weighed without confirming their composition. 10 studies investigated the shedding of fabrics with mixed fibre compositions, and of these, only 3 confirmed the chemical composition of the shed fibres with FTIR. Chemical confirmation of shed fibres is of particular importance in studies using mixed composition fabrics, so that the relative proportions of shed fibres can be assigned to each fibre composition. Haap et al. (2019)58 investigated the shedding of fibres from a 50% polyester, 50% cotton woven fabric. After quantification, chemical separation was performed by using sulphuric acid to digest the cotton fibres. 86% by mass, of fibres released from the fabric were cotton and 14% were polyester, which can be attributed to the higher tensile strength and abrasion resistance of polyester, and the shorter constituent fibre lengths of cotton.25,58,66 Kelly et al. (2019)61 found that increasing the water volume in accelerated laundering experiments, from 300 mL to 600 mL, resulted in an increase in fibre shedding, from 54 mg kg−1 to 120 mg kg−1. Several studies in Table 1 do not report the total volume of water used, and the fabric weight, density and surface area. It is recommended in future that these parameters are quantified and reported, as they also influence shedding propensity.
Microscopy and manual or computational counting from micrographs was by far the most common method for fibre characterisation, used in 18 of the 25 studies included in Table 1. However low spatial resolution of optical microscopes and image analysis techniques mean that underestimations are likely. McIlwraith et al. (2019)62 used ImageJ (image processing software) to quantify fibres from a series of micrographs. However, the limit of detection was 100 μm in length, resulting in the exclusion of fibres <100 μm. Hernandez et al. (2017)50 also analysed micrographs with ImageJ; ascertaining a 40 μm limit of detection from the minimum visible number of covered pixels in each micrograph (between 2 and 5 pixels). In this study, it was found that shed fibres from a PET single-knit jersey and interlock fabrics were typically 100–800 μm in length. However, size distributions revealed a general increase in fibre frequency as fibre length decreased. Moreover, fibres above 1 mm in length represented only 2–5% of the total shed fibre mass. Size distributions constructed by Zambrano et al. (2019),25 for fibres shed from PET, cotton, and PET-cotton blend fabrics also revealed this trend – fibre frequency increased as fibre length decreased, until the lower limit of detection for fibre length (200 μm) was reached.25 Since the widths of synthetic fibres are usually very uniform and have a typical mean diameter of 11–16 μm,25,46 it could be reasonably assumed that the majority of shed fibres would be captured by a filter with a pore-size of 10 μm or below. In Table 1, 9 of the 25 studies used filters with a pore-size above 10 μm, meaning fibres may have been lost even before analysis. Furthermore, it is important to highlight that only six studies provided a lower limit of detection for the measurement of fibre length. This is an essential parameter to evaluate the suitability of the methodology, and to contextualise results in ecotoxicology, since fibre size influences environmental fate and transport,67 ingestion rates by organisms,68 and specific surface area.69
The laundering method and apparatus used also likely contributed to the variation in shedding rates reported in the literature. Laundering speed varied from 40–1600 rpm,50,61 and was unspecified in 10 of the 25 studies listed in Table 1. Laundering speed is thought to greatly influence fibre shedding because it determines the mechanical action exerted on the fabric.42,49 Accelerated laundering methods were used in 9 studies, whereas commercial, or laboratory-scale washing machines were used in the other 8 studies. Accelerated laundering refers to the laboratory-scale simulation of home laundering, by placing the fabric and water/detergent solution under continuous agitation, often with the addition of metal beads to increase mechanical abrasion. This allows multiple experimental treatments and replicates to be performed simultaneously and reduces the total volume of water to be filtered and analysed. Cai et al. (2020)52 reported that up to 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fibres per kg of fabric could be released, but accelerated laundering was used in their study. Moreover, Zambrano et al. (2019)25 found accelerated laundering generated approximately 40 times more fibres per unit mass of fabric, compared to conventional washing machines. This likely accounts for some variation in shedding rates between studies adopting conventional and accelerated laundering, and highlights that results from accelerated laundering studies should not be used to estimate fibre emissions during home laundering.
000 fibres per kg of fabric could be released, but accelerated laundering was used in their study. Moreover, Zambrano et al. (2019)25 found accelerated laundering generated approximately 40 times more fibres per unit mass of fabric, compared to conventional washing machines. This likely accounts for some variation in shedding rates between studies adopting conventional and accelerated laundering, and highlights that results from accelerated laundering studies should not be used to estimate fibre emissions during home laundering.
Control strategies at various levels of intervention have been proposed to reduce microplastic fibre emissions (Ramasamy and Subramanian, 2021).70 At the individual level, commercially available capture devices may be used during laundering to reduce microplastic fibre emissions at the source. McIlwraith et al. (2019)62 compared the microplastic fibre reduction efficiencies of two such devices; the Cora ball (a plastic ball with hooked arms) and the Lint LUV-R (retrofitted filter), when laundering a 100% polyester fleece. Where no device was used, 4800 fibres per litre were released. Fibre release decreased to 3580 fibres per litre with the use of the Cora ball, and only 648 fibres per litre with the Lint LUV-R filter,62 representing fibre capture rates of 25.4% and 86.5% respectively. Napper et al. (2020)71 tested the efficiency of several capture devices, finding the XFiltra retrofitted filter to be the most effective, reducing fibre emissions by 78% compared to a control where no devices were used. This was attributed to the fine pore-size of the filter (60 μm). However, two mesh bags, the Guppyfriend and the Fourth Element washing bag, resulted in reductions of 54% and 21% respectively, despite both having a mesh pore-size of 50 μm, so other design variables appear to influence the efficiency of these devices.71 Using lower water volume laundering cycles has also been shown to significantly reduce the mass of shed fibres.61 Fabric production may also be altered to reduce fibre shedding propensity. Generally, fibres with higher tensile strength and tenacity result in yarns with a lower hairiness (number of protruding loops and ends), meaning the final fabric has a higher abrasion resistance and therefore a lower shedding propensity.25,44 Increasing the number of yarn twists per unit length, and the stitch density of fabrics can also decrease their shedding propensity.42,65 Fleece fabrics, which are mechanically cut after construction, have an increased shedding propensity compared to similar, non-fleece knitted fabrics.42 Current legislation aimed at reducing microplastic fibre emissions is non-existent in most countries.70,72 Laws implemented in New York, and California, state that clothing containing more than 50% synthetic fibres must be labelled as a contributor to microplastic fibre pollution, aim to impart consumer knowledge to facilitate gradual consumer behavioural changes.70
3. Microplastics in sewage sludge
Shed fibres are typically carried through municipal drainage systems to a wastewater treatment plant (WWTP) (Fig. 1b). Processes of wastewater treatment vary between facilities, however they typically begin with primary screening and sedimentation to remove coarse grit and suspended solids. This stage is followed by secondary treatment, which involves aerobic or anaerobic microbial incorporation to remove suspended or dissolved organic matter, often aided by the addition of flocculants in a secondary sedimentation tank. Secondary treatment usually also involves a disinfection stage to remove pathogens.17 Tertiary treatment involves additional specialised mechanisms to improve effluent quality before discharge into the environment, such as additional filtration or the removal of nitrates and phosphates.73 The solid residue, or sewage sludge, is collected and typically dewatered to reduce its volume, before being chemically, aerobically or anaerobically stabilised.74,75 In the UK, the majority of sewage sludge is then utilised as a fertiliser (biosolids) in agriculture (79%), incinerated for energy recovery (18.4%), or disposed of in landfill (0.6%).32 Throughout Europe, biosolids application rates to land vary greatly between countries, from over 90% in Ireland and Lithuania, to less than 5% in the Netherlands, Slovenia and Malta.76 In the USA and China, 49–60% and 14–45% of sludge produced respectively, is applied to agricultural land as biosolids.77–80Several studies have quantified microplastic contamination through the different stages of the wastewater treatment process at specific WWTPs. Comparison of microplastic concentrations in the influent to the effluent has revealed that 72–99.9% of microplastics (by number) are removed during the wastewater treatment process.81,82 For example, Murphy et al. (2016)16 sampled concentrations of microplastics at four progressive stages of wastewater treatment, at a WWTP in Scotland, finding that microplastic concentrations decreased from 15.7 microplastics L−1 in the influent, to 0.25 microplastics L−1 in the final effluent; a reduction of 98%. Microplastics were identified visually using a dissection microscope and characterised using FT-IR (Fourier Transform Infrared) spectroscopy, although specific details of spatial resolution and limits of detection for size were not reported. It has been estimated that approximately 78% of microplastics (by number) entering a WWTP are removed during wastewater treatment and are present in the sewage sludge.29 Primary wastewater treatment has been shown to remove 92–93% of textiles fibres (both natural and synthetic fibres), with secondary treatment resulting in only a further 0.2% reduction.83 In the same study, microplastics removal after primary treatment was 32%, with 76% of remaining microplastics being removed after secondary treatment.
While there is some published data on the fate of microplastics in WWTPs, there is a lack of information concerning the fate of microplastic fibres specifically. The differing behaviours of textiles fibres and microplastics may be explained by differences in density, which affects the settling velocity of the particles.84 Polyethylene terephthalate (polyester) is the most widely used synthetic polymer in the manufacturing of apparel textiles,44 and has a density of 1.32–1.41 g mL−1,85 which is higher than that many other polymer types commonly identified in sewage, including polyethylene (ρ = 0.89–0.97 g mL−1) and polypropylene (ρ = 0.85–0.92 g mL−1).86 It is yet unclear how microplastic morphology influences wastewater separation efficiency, although for spherical microplastics, a larger diameter will increase settling velocity.84 Nonetheless, methods of sampling, microplastic extraction from sludge, and characterisation are still currently in development and often differ widely between studies.
Chemical oxidation is often employed to digest organic matter, allowing an easier separation of microplastics from the solid sewage sludge fraction.27 It also aids the removal of any organic substances impregnating/coating the surfaces of the microplastics, which would hinder the spectral characterisations and chemical classification with techniques such as FT-IR or Raman spectroscopy. Mason et al. (2016)17 used 30% hydrogen peroxide (H2O2) to digest sludge samples from 17 WWTPs, before analysing microplastics visually under a dissection microscope. The dominant shape fraction (fibres, fragments, films, pellets, foams) in this study was found to be fibres; accounting for 46% and 80% of total microplastics in the 125–355 μm and >355 μm size fractions respectively.17 However, sampling bias and misidentification of microplastics can occur where only visual identification is employed.27 Talvitie et al. (2017)28 found that only 34% of fibres separated from sludge were composed of synthetic polymers (PET – 33%; polyacrylic – 1%) when analysed with FT-IR, with the remainder being natural fibres such as cotton, or regenerated fibres such as rayon. Eriksen et al. (2013)87 and Lenz et al. (2015)88 found 20% and 32% of particles were visually identified erroneously as microplastics, after verification with scanning electron microscopy (SEM) and Raman microscopy, respectively. Mason et al. (2016)17 may therefore have overestimated the presence of microplastic fibres in sewage sludge samples, as only visual identification based on morphology was employed. The use of H2O2 may also result in the oxidation and subsequent destruction of polymers such as nylon in the digested sample.89 Nylon is a common synthetic fibre used in apparel textiles,44 and the shedding propensity of nylon garments during laundering is comparable to that of polyester (Table 1).42,49,51 It is possible, therefore, that the relative proportion of nylon fibres is underestimated when H2O2 is used as a chemical oxidant in sludge processing. Other methods used for processing of microplastics from environmental or biological samples include ultrasonic extraction and the use of alkaline (e.g. KOH or NaOH) dissolution and/or acid (e.g. HNO3 or HCl) digestion of samples.90–94 However, these methods have not been tested for the analysis microplastic fibres in sewage sludge.
Microplastic extraction from sludge in more recent studies typically involves a density separation step to remove inorganic debris such as sand, grit and aluminosilicate minerals.27 This separation involves the agitation and prolonged settling of the sludge matrix in a high density, saturated salt solution, such as sodium chloride (NaCl) (ρ = 1.2 g mL−1) or zinc chloride (ZnCl) (ρ = 1.6 g mL−1),85,94 or mixtures of water, sucrose, and ethanol.95 Plastics typically have a density of 0.89–1.2 g mL−1. However, PET and PVC can have densities up to 1.41 and 1.70 g mL−1, respectively.86 Li et al. (2018)96 extracted microplastics by density separation from sewage sludge from 28 WWTPs in China using saturated NaCl solution, followed by H2O2 oxidation to digest remaining organic matter. Microplastics were visually sorted by morphology and subsamples were qualitatively analysed by FT-IR and SEM microscopy. Li et al. (2018)96 identified between 1565 and 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386 microplastics per kg of sludge (37 μm–5 mm in size), 62.5% of which were fibres. The total number, and proportion of fibrous microplastics were potentially underestimated in this study due to the higher density of PET (ρ = 1.32–1.41 g mL−1) than the NaCl solution (ρ = 1.2 g mL−1).85 This underestimation is a significant limitation of the methodology because PET is by far the most widely used synthetic fibre in apparel textiles production.43 During quality control experiments by Liu et al. (2018),20 soils were spiked with microplastics including polypropylene (PP), polyethylene (PE), nylon (polyamide), PET and PVC, before density separation with NaCl. Recovery of both PET and PVC (ρ = 1.32–1.7 g mL−1)86 from the soil samples was 0%, highlighting the importance of using higher density salts such as ZnCl (ρ = 1.6 g mL−1).94
386 microplastics per kg of sludge (37 μm–5 mm in size), 62.5% of which were fibres. The total number, and proportion of fibrous microplastics were potentially underestimated in this study due to the higher density of PET (ρ = 1.32–1.41 g mL−1) than the NaCl solution (ρ = 1.2 g mL−1).85 This underestimation is a significant limitation of the methodology because PET is by far the most widely used synthetic fibre in apparel textiles production.43 During quality control experiments by Liu et al. (2018),20 soils were spiked with microplastics including polypropylene (PP), polyethylene (PE), nylon (polyamide), PET and PVC, before density separation with NaCl. Recovery of both PET and PVC (ρ = 1.32–1.7 g mL−1)86 from the soil samples was 0%, highlighting the importance of using higher density salts such as ZnCl (ρ = 1.6 g mL−1).94
There is general agreement between studies investigating the mass balance of microplastics entering WWTPs that microplastics are removed from wastewater very effectively during treatment. Carr et al. (2016),15 Magnusson and Norén (2014),82 Gies et al. (2018)97 and Leslie et al. (2017)81 reported removal rates of 99.9%, 99.9%, 97–99%, and 72%, respectively, by quantifying the number of microplastics in the influent, effluent and sludge. The majority of microplastics are retained in the collected sewage sludge (Fig. 1c) where they are exposed to elevated concentrations of metals and metalloids (Table S1 in ESI materials†).98–106 Despite practical limitations of microplastic extraction from solid media such as sludge and soils, reported concentrations in sewage sludge samples have ranged from 1–56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386 microplastics per kg of sludge.17,19,82,96,107 Their small size and high specific surface area, coupled with the consistently elevated concentrations of metals and metalloids in sludge, means that sorption of metals and metalloids on microplastic surfaces may occur during the wastewater or sludge treatment processes.
386 microplastics per kg of sludge.17,19,82,96,107 Their small size and high specific surface area, coupled with the consistently elevated concentrations of metals and metalloids in sludge, means that sorption of metals and metalloids on microplastic surfaces may occur during the wastewater or sludge treatment processes.
4. Metal and metalloid sorption onto microplastics
The presence of metals and metalloids such as arsenic (As), copper (Cu), chromium (Cr), mercury (Hg), nickel (Ni), lead (Pb), and zinc (Zn) in sewage sludge has been well studied, and regulations for sludge use in agriculture are enforced worldwide, including in the UK, EU and USA (Table S2 in ESI material†), to reduce their potential harm to organisms.29,108–111 Many studies have quantified the sorption of a variety or organic pollutants such as polycyclic aromatic hydrocarbons (PAHs),112 antibiotics,113 and phthalate esters114 onto microplastic surfaces, and this topic has been extensively reviewed.115,116 However, the sorption of metals and metalloids to microplastic surfaces is far less studied. Furthermore, the sorption of metals and metalloids to microplastic fibres has rarely been studied, and data are extremely scarce. As a result, inferences on the sorption of metals and metalloids to microplastic fibres need to be made, based on observations made on the sorption of metals and metalloids to microplastic pellets (Table 2). There are several limitations to this inference that should be acknowledged. The surface area to mass ratio of microplastic fibres is likely to be greater than pellets. Therefore, a fairer comparison of the sorption of metals and metalloids to microplastics should be based on mass of adsorbate to surface area of adsorbent. However, because the surface area of microplastics is rarely reported in sorption experiments, this is currently not possible with the data available in Table 2.| Metal | Polymer | K d (mL g−1) | Matrix | Type of microplastic | Experimental conditions (solid to liquid ratio, S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L; agitation; contact time L; agitation; contact time |
Ref. |
|---|---|---|---|---|---|---|
| Ag | PE | 1.18 | Freshwater (river Plym, UK) | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 |
| PE (>90%) and PP | 96.3 | Aged (beached) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 (S 86 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
|||
| Cd | PE | 1.24 | Seawater | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (S 75 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Holmes et al. (2012)35 |
| 7.94 | Aged (beached) | |||||
| PE | 0.347 | Freshwater (river Plym, UK) | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 | |
| HDPE | 242 | Distilled water | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–1 500–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4000 (S 4000 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 20 rpm; <24 h L); 20 rpm; <24 h |
Zou et al. (2020)117 | |
| LDPE | 345 | |||||
| PVC | 1748 | |||||
| CPE | 7485 | |||||
| Co | PE | 0.834 | Seawater | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (S 75 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Holmes et al. (2012)35 |
| 4.03 | Aged (beached) | |||||
| PE | 0.304 | Freshwater (river Plym, UK) | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 | |
| Cu | PE | 45.0 | Seawater | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (S 75 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Holmes et al. (2012)35 |
| 45.2 | Aged (beached) | |||||
| PE | 3.62 | Freshwater (river Plym, UK) | Virgin pellets | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 | |
| PE (>90%) and PP | 61.0 | Aged (beached) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 (S 86 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
|||
| PS | 659 | Seawater (Madeira, Portugal) | Virgin, with anti-fouling paint | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500 (S 2500 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); no agitation; 336 h L); no agitation; 336 h |
Brennecke et al. (2016)118 | |
| PVC | 849 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (S 1000 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); no agitation; 336 h L); no agitation; 336 h |
||||
| HDPE | 385 | Distilled water | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–1 500–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4000 (S 4000 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 20 rpm; <24 h L); 20 rpm; <24 h |
Zou et al. (2020)117 | |
| LDPE | 56 | |||||
| PVC | 431 | |||||
| CPE | 3868 | |||||
| Nylon | 172–432 | Unspecified | Aged (aquaculture) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 (S 333 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 185 rpm; 72 h L); 185 rpm; 72 h |
Tang et al. (2021)119 | |
| PS | 394.8 | Seawater (Shenzhen, China) | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 (S 2000 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 140 rpm; 24 h L); 140 rpm; 24 h |
Chen et al. (2021)120 | |
| 761.3 | Aged (1 month in seawater) | |||||
| 591.3 | Aged (3 months in seawater) | |||||
| PE | 331.2 | Virgin | ||||
| 755 | Aged (1 month in seawater) | |||||
| 448.4 | Aged (3 months in seawater) | |||||
| Cr | PE | 11.6 | Seawater | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (S 75 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Holmes et al. (2012)35 |
| 221 | Aged (beached) | |||||
| PE | 1.01 | Freshwater (river Plym, UK) | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 | |
| PE (>90%) and PP | 4.60 | Aged (beached) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 (S 86 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
|||
| Cs | HDPE | 80.3 | Freshwater (NSW, Australia) | Gamma-irradiated, aged | Unspecified (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 50 rpm; 48 h L); 50 rpm; 48 h |
Johansen et al. (2018)121 |
| 9.30 | Estuarine water (NSW, Australia) | |||||
| Hg | PE | 5.84 | Freshwater (river Plym, UK) | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 |
| PE (>90%) and PP | 31.8 | Aged (beached) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 (S 86 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
|||
| Ni | PE | 1.60 | Seawater | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (S 75 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Holmes et al. (2012)35 |
| 8.87 | Aged (beached) | |||||
| PE | 0.491 | Freshwater (river Plym, UK) | Virgin pellets | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 | |
| Nylon | 48–183 | Unspecified | Aged (aquaculture) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 (S 333 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 185 rpm; 72 h L); 185 rpm; 72 h |
Tang et al. (2021)119 | |
| Pb | PE | 0.245 | Seawater | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (S 75 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Holmes et al. (2012)35 |
| 149 | Aged (beached) | |||||
| PE | 5.80 | Freshwater (river Plym, UK) | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 (S 96 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
Turner and Holmes (2015)36 | |
| PE (>90%) and PP | 217 | Aged (beached) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 (S 86 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 150 rpm; 48 h L); 150 rpm; 48 h |
|||
| HDPE | 2316 | Distilled water | Virgin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–1 500–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4000 (S 4000 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 20 rpm; <24 h L); 20 rpm; <24 h |
Zou et al. (2020)117 | |
| LDPE | 590 | |||||
| PVC | 2518 | |||||
| CPE | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 306 306 |
|||||
| Sr | HDPE | 6.90 | Freshwater (NSW, Australia) | Gamma-irradiated, aged | Unspecified (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 50 rpm; 48 h L); 50 rpm; 48 h |
Johansen et al. (2018)121 |
| 5.00 | Estuarine water (NSW, Australia) | |||||
| Zn | PS | 304 | Seawater (Madeira Island, Portugal) | Virgin, with anti-fouling paint | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500 (S 2500 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); no agitation; 336 h L); no agitation; 336 h |
Brennecke et al. (2016)118 |
| PVC | 195 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (S 1000 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); no agitation; 336 h L); no agitation; 336 h |
||||
| Nylon | 87–191 | Unspecified | Aged (aquaculture) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 (S 333 (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L); 185 rpm; 72 h L); 185 rpm; 72 h |
Tang et al. (2021)119 |
Reported plastic–water partition coefficients (Kd values), representing the distribution of the respective metal or metalloid between the plastic-bound phase and the aqueous phase, range widely between metal or metalloid type, plastic type, plastic aging, and aqueous matrix type (Table 2). For example, the partitioning of aqueous Cu to virgin plastics decreases in the order PVC > PS > PE,36,118 and is higher in seawater than in freshwater.35,36 Cr is sorbed more strongly to aged, rather than virgin, PE microplastics by an order of magnitude.35 Cu sorption was not significantly affected by plastic age in seawater whereas, in freshwater, sorption was greater on the aged, rather than virgin, PE microplastics.35 Tang et al. (2021)119 investigated the sorption of Cu, Ni and Zn onto nylon microplastics collected from the environment. Langmuir modelling of isotherm data revealed maximum sorption capacities for Cu, Ni and Zn were 16.7 μg g−1, 10.6 μg g−1 and 12.7 μg g−1 respectively, however, the data are not reported for the virgin nylon microplastics. Chen et al. (2021)120 compared the sorption of Cu onto virgin and aged PS and PE microplastics, finding aged microplastics to have a higher sorption capacity (Table 2). This was partly attributed to the increased specific surface area, and more negative zeta potentials of the aged microplastics.120
The sorption of metals and metalloids onto microplastic surfaces is strongly influenced by the chemical properties of the metal or metalloid and the physicochemical properties of the microplastic surfaces.35,36,118–120 Previous studies investigating metal and metalloid sorption onto microplastics have mainly focussed on microplastic types commonly found in marine environments, such as PE, PS and PVC. Data for metal and metalloid sorption onto PET microplastic surfaces are scarce in the literature in comparison with other polymer types. Cojocariu et al. (2017)122 quantified the sorption of Pb and Cu to recycled PET fibres, reporting Pb and Cu sorption to be 48.9 mg g−1 and 30.7 mg g−1 respectively. These sorption values are several orders of magnitude higher than others reported thus far in the literature,35,36,118–120 although it should be noted that a physicochemical characterisation of the PET fibres was not provided in this study, so fibre size and specific surface area are not known. Recently, Han et al. (2021)123 investigated the sorption of Pb, Cu and Cr onto PET microplastics of varying size fractions (<0.9 mm; 0.9–2 mm; 2–5 mm). Sorption increased in the order Cr < Cu < Pb, and decreased with increasing microplastics size. Kinetics experiments revealed that at equilibrium the sorbed concentrations of Pb, Cu and Cr on the PET fibres, calculated by fitting the pseudo-second order model, were 1.04, 0.488 and 0.385 μg g−1 respectively. These values are similar to those previously reported for virgin and beached PE pellets.35,36 PET is often underrepresented in density separations due to its high density,85 yet is the most commonly used material in the manufacturing of apparel textiles,43 which have been shown to shed large quantities of fibres into the wastewater system (Table 1). The laundering of synthetic textiles therefore represents a considerable diffuse source of PET microplastics into sewage sludge where they are exposed to elevated concentrations of metals and metalloids (Table S1†). Unlike the previously investigated plastic types (PE, PVC and PS), PET contains hydroxyl (–OH) groups at the ends of the polymer chains, which are approximately 100 monomer units in length.124 These hydroxyl groups may increase the sorption capacity of PET for metals and metalloids, as they become deprotonated at circumneutral pH, which is often characteristic of sewage sludges.125 Deprotonated hydroxyl groups (–OH–) are negatively charged and therefore may facilitate the sorption of cationic metals, such as Cu2+, Pb2+, and Cd2+.126,127 Zou et al. (2020)117 found that the sorption capacity of chlorinated-PE for Cd, Cu and Zn, was at least one order of magnitude higher than HDPE and LDPE microplastics, which was partly attributed to the high electronegativity of chlorine. The high electronegativity the oxygen atoms in PET may therefore increase its sorption capacity, particularly for cationic metals. Further study of PET is required to understand the role of its unique functional groups in the sorption of metals and metalloids.
Environmental water samples often contain humic substances; high molecular weight organic macromolecules, with heterogeneous branching structures and oxygen-containing functional groups,36,126 which may also influence the sorption of metals and metalloids onto microplastics. Li et al. (2019)128 reported decreased sorption of Cd to PP and PE microplastics with increasing concentrations of humic substances. This observation was attributed to the complexation of Cd by the acidic functional groups of the humic substances, and the subsequent decrease in free Cd2+ ions in solution.128 Turner and Holmes (2015),36 however, hypothesised that the observed increase in the sorption capacity of beached PE pellets over virgin PE pellets could be in part due to the gradual accumulation of organic matter on the surfaces of the beached pellets. It was suggested that this organic matter provided an increased number of charged functional groups, and therefore increased the sorption capacity of the plastic surface.36 These conflicting results are difficult to resolve, because the concentrations of humic substances in the water samples used by Turner and Holmes (2015)36 were not quantified. Biofilm formation may also alter the sorption capacity of microplastics over time. Biofilms are formed by the extracellular polymeric substances, such as polysaccharides, of colonising bacteria on surfaces. Biofilms may change the surface properties, and therefore sorption capacity of microplastics by introducing new functional groups, such as amines, hydroxyls, and carboxylic acids.129 Biofilms may also enrich the microplastic surfaces with particular elements, for example strontium and sulphur, present in the radiolarian protozoa Acantharea.130,131
The accumulation of metals and metalloids to microplastics represents an important knowledge gap, and more work is necessary in order to provide a more systematic quantification of the retention of metals and metalloids onto microplastic surfaces in general, and microplastic fibres in particular. Nonetheless, the current data show that microplastics are capable of sorbing considerable amounts of metals and metalloids which may in turn influence the biogeochemical cycling of these metals and metalloids in agricultural soils when the microplastics are applied in biosolids.
5. Sewage sludge application and impacts to terrestrial environments
Sewage sludge, the solid residual product of wastewater treatment, contains elevated concentrations of microplastics, and is widely applied to agricultural soils as biosolids. Using density separation, followed by filtration and visual identification with a stereo microscope, Corradini et al. (2019)21 quantified microplastic concentrations in agricultural soils with differing biosolids application rates. The median estimated mass of microplastics in the soil increased with each successive biosolids application, from 1.37 to 4.38 mg kg−1 in soils that had received 1 and 5 biosolids applications, respectively, and 97% of these microplastics were categorised as fibres. Fibres were also found to be the dominant morphology of microplastics in agricultural soils in north-western and south-western China, accounting for 49% and 92% of all microplastics respectively.132,133 As highlighted by Corradini et al. (2019)21 microplastic morphologies were categorised at the operator's discretion, further necessitating a need for a strict and appropriate definition of microplastic fibres. Nevertheless, Corradini et al. (2019)21 provide strong in situ evidence that the application of sewage sludge derived biosolids provides an important pathway for the transfer of microplastics, particularly microplastic fibres, to agricultural soils (Fig. 1d).Like most other widely manufactured polymers, PET is highly resistant to degradation under typical environmental conditions, however, is susceptible to hydrolytic degradation due to its ester bonds, particularly at extreme pH values.86,134 Over time, water reacts with the ester bonds of the PET backbone, forming two shorter polymer chains ending in alcohol and carboxylic acid groups. UV radiation from sunlight exposure will also initiate photooxidation of PET, where the ester bonds are cleaved, leaving carboxylic acid groups on the polymer surface. Degradation products include CO, CO2, terephthalic acid and other carboxylic acids.134 Environmental exposure of PET can significantly decrease tensile strength after approximately one year, depending on UV intensity, temperature, and precipitation.135 Shape also influences degradation rate, as highlighted by Chamas et al. (2020),134 who estimated that HDPE films, fibres and spheres weighing 2.75 g each, will take approximately 1.8, 465 and 2000 years respectively to completely degrade. Microplastic fibres are likely to degrade in the environment more rapidly than films, but let rapidly than spheres, as degradation is controlled largely by the size of the surface area exposed.134 Biological degradation of PET fibres is typically slow. Zambrano et al. (2019)25 assessed the biodegradation of various yarns, by measuring the total oxygen demand in a closed respirometer over 243 days, after inoculation with aerobic microorganisms from activated sludge. Biodegradation of the polyester yarn was only 4.1% compared with 75.9% for the cotton yarn, revealing that PET is a relatively unavailable carbon source for microorganisms.25 Yoshida et al. (2016)136 isolated a bacterium, Ideonella sakaiensis 201-F6, that was capable of biodegrading PET by adhering to its surface and secreting two hydrolytic enzymes. The bacterium was capable of almost completely degrading a 60 mg PET film after 60 days, and catabolised 75% of the total carbon after 15 days.136 The main biodegradation product was mono(2-hydroxyethyl)terephthalic acid, which was rapidly metabolised further into the two monomers of PET, terephthalic acid and ethylene glycol.136,137 The co-polymer poly(ethylene terephthalate-co-lactate) can be effectively degraded under laboratory composting conditions,138 although further research is needed before bioremediation strategies are implemented for PET fibres in sludge and soils.
The impacts of microplastics on agroecosystems are still relatively unknown. However, emerging research is indicating that microplastic contamination may influence the physical, chemical, and biological properties of soils. Machado et al. (2018)139 reported a reduction in bulk density, and an increase in water holding capacity for soils spiked with polyester fibres, that was not observed in soils spiked with polyethylene fragments and polyamide beads. This observation is thought to be due to the more efficient entanglement of soil aggregates by the fibres, creating more air-filled pore spaces in the soil. Kim and An (2019)140 observed marked behavioural changes and a significant reduction in mobility of springtails in soils due to the presence of PS microplastics, even at relatively low concentrations (8 mg kg−1). PS nanoplastics at concentrations of 10 μg L−1 were shown to induce toxicity in nematodes (Caenorhabditis elegans).141 Earthworms (Lumbricus terrestris) were observed ingesting PE microplastics (150–2800 μm in diameter) in mesocosm experiments,67,142,143 however the ecotoxicological implications of this were not studied.
Sewage sludge contains elevated concentrations of metals and metalloids (Table S1†), which have been shown to sorb onto microplastic surfaces (Table 2). In the UK and EU, legislation stipulates the maximum concentrations of metals and metalloids such as Cd, As, Hg, Pb and Zn in agricultural soils and maximum permissible loadings in sludge applications (Table S2†). However, there are currently no regulatory limits on microplastic additions to agricultural soils. As a result, the occurrence and accumulation of microplastics in agricultural soils has gone largely unmonitored in recent years. In the EU, it is estimated that up to 4.7 times more microplastics per year are released into agricultural soils than to surface waters,24 despite terrestrial microplastics research continuing to lag behind marine and freshwater microplastics research.22
Metals and metalloids sorbed onto the surfaces of ingested microplastics may have an altered bioavailability and bioaccessibility to soil organisms, compared to dissolved ions. Pollutants sorbed onto the surfaces of ingested microplastics may readily desorb in acidic gut environments.37,144,145 Microplastics may therefore act as a vector, facilitating an increased exposure of the sorbed pollutant to the organism (Fig. 1e).144 For example, desorption of persistent organic pollutants from PE and PVC microplastics was shown to be up to 30 times greater under simulated gut conditions, than in seawater alone.145 Synthetic earthworm gut extraction tests of HDPE microplastics and soil aggregates by Hodson et al. (2017),37 revealed that Zn desorption was 4–30 times greater from the microplastics than from the soils. Despite this, in the same study, Zn-loaded microplastics induced no statistically significant effects in Zn bioaccumulation, weight or mortality on earthworms. Exposure studies have shown that ingestion can facilitate the fragmentation of microplastics, creating smaller particles. In a study by Kwak and An (2021),146 earthworms (Eisenia andrei) were exposed to polyethylene microplastics (180–300 μm diameter) for 21 days, before microplastics were extracted from earthworm intestines and casts. Using SEM, the researchers identified smaller microplastics on the surfaces of the ingested and excreted microplastics, measuring as small as 182 nm in diameter.146 This is thought to be primarily due to microbial degradation by bacteria in the gut microbiome of earthworms.147 Similar results were reported by Dawson et al. (2018),148 who observed that microplastics ingested by Antarctic krill (Euphausia superba) were on average 78% smaller in diameter (7.1 μm) than the original microplastics (31.5 μm). Ingestion of microplastics by soil organisms may therefore result in an increase in the specific surface area of the microplastics as they are fragmented. Experiments by Khan et al. (2017),144 using polyethylene microplastics loaded with silver (Ag), revealed that desorption of Ag was significantly higher under lower pH conditions, reaching 98% after 3 hours at a pH of 4.1. However, such experiments have not been performed with polymers commonly used in textiles, such as PET, nylon, and polyurethanes. These polymers are likely to contribute significantly to the microplastics entering agricultural soils that receive municipal sewage sludge.
6. Conclusions
Microplastics are ubiquitous, highly recalcitrant emerging contaminants of concern. Current data suggest microplastics, particularly fibres, are pervasive in sewage sludge and agricultural soils. Further work is required to develop more robust, efficient, and consistent analytical procedures to quantify their abundance. The laundering of synthetic fibres provides an important diffuse source of microplastic fibres, which are transported into wastewater systems. Microplastic fibres from laundering have a typical diameter of approximately 20 μm. An inverse relationship between frequency and length is often observed. Values reported in the literature for the number of fibres shed during conventional laundering experiments are 1900–110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fibres per garment, 22
000 fibres per garment, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600–13
600–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fibres per kg of fabric, and 7–1240 mg of fibres per kg of fabric. During wastewater treatment, 78% of microplastics are retained in the sewage sludge. Municipal biosolids can reportedly contain up to 56
000 fibres per kg of fabric, and 7–1240 mg of fibres per kg of fabric. During wastewater treatment, 78% of microplastics are retained in the sewage sludge. Municipal biosolids can reportedly contain up to 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microplastics per kg, although values reported in the literature vary greatly. This sludge is commonly applied to agricultural land as a soil amendment, creating a pathway for microplastic fibres generated during laundering to contaminate agricultural soils. Therefore, microplastic fibres may cause deleterious effects to the soil biophysiochemical environment which require further investigation.
000 microplastics per kg, although values reported in the literature vary greatly. This sludge is commonly applied to agricultural land as a soil amendment, creating a pathway for microplastic fibres generated during laundering to contaminate agricultural soils. Therefore, microplastic fibres may cause deleterious effects to the soil biophysiochemical environment which require further investigation.
The surfaces of microplastics may also be loaded with elevated concentrations of metals and metalloids due to sorption during the wastewater treatment process. Research on the sorptive properties of microplastics is still in its infancy. However, available data reveals that sorption of metals and metalloids is largely dependent on solution chemistry (pH, ionic strength, presence of humic substances), and the physicochemical properties of the microplastics (functional groups, degree of surface oxidation, specific surface area, point of zero charge). Therefore, the application of sewage sludge to agricultural land may represent an important vector for metals and metalloids, sorbed on the surfaces of microplastics, to soil organisms.
Microplastic fibres are an important subcategory of microplastics, due to their prevalence in sludge and agricultural soils, and high specific surface area. However, the sample processing method used to isolate microplastics from soils and sludge should be carefully considered, particularly during density separation to avoid excluding high-density microplastics, such as PET, from being quantified. Research on the sorptive properties of synthetic polymers commonly used in textiles, such as PET and poly(amide), is extremely scarce, representing an important knowledge gap. This gap requires immediate attention because textiles-derived microplastic fibres are likely to represent a significant proportion of microplastics applied to agricultural soils, in biosolids produced from municipal sewage sludge.
Author contributions
H. Frost: conceptualisation, investigation, writing – original draft, writing – review & editing, visualisation; T. Bond: conceptualisation, writing – review & editing, supervision, funding acquisition; T. Sizmur: conceptualisation, writing – review & editing, supervision, funding acquisition; M. Felipe-Sotelo: conceptualisation, writing – review & editing, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Harrison Frost is supported by a NERC SCENARIO PhD studentship (NE/S007261/1).References
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- PlasticsEurope, Plastics – the Facts 2016: an Analysis of European Plastics Production, Demand and Waste Data, Plastics Europe, Belgium. 2016 Search PubMed.
- W. Wang, N. J. Themelis, K. Sun, A. C. Bourtsalas, Q. Huang, Y. Zhang and Z. Wu, Current influence of China's ban on plastic waste imports, Waste Dispos. Sustain. Energy, 2019, 1(1), 67–78 CrossRef.
- M. A. Browne, P. Crump, S. J. Niven, E. Teuten, A. Tonkin, T. Galloway and R. Thompson, Accumulation of microplastic on shorelines woldwide: sources and sinks, Environ. Sci. Technol., 2011, 45(21), 9175–9179 CrossRef CAS PubMed.
- A. L. Andrady, Microplastics in the marine environment, Mar. Pollut. Bull., 2011, 62(8), 1596–1605 CrossRef CAS PubMed.
- A. A. de Souza Machado, W. Kloas, C. Zarfl, S. Hempel and M. C. Rillig, Microplastics as an emerging threat to terrestrial ecosystems, Global Change Biology, 2018, 24(4), 1405–1416 CrossRef PubMed.
- J. Gago, O. Carretero, A. V. Filgueiras and L. Viñas, Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments, Mar. Pollut. Bull., 2018, 127, 365–376 CrossRef CAS PubMed.
- A. K. Baldwin, S. R. Corsi and S. A. Mason, Plastic debris in 29 Great Lakes tributaries: relations to watershed attributes and hydrology, Environ. Sci. Technol., 2016, 50(19), 10377–10385 CrossRef CAS PubMed.
- S. Rech, V. Macaya-Caquilpán, J. F. Pantoja, M. M. Rivadeneira, D. J. Madariaga and M. Thiel, Rivers as a source of marine litter–a study from the SE Pacific, Mar. Pollut. Bull., 2014, 82(1–2), 66–75 CrossRef CAS PubMed.
- R. W. Obbard, S. Sadri, Y. Q. Wong, A. A. Khitun, I. Baker and R. C. Thompson, Global warming releases microplastic legacy frozen in Arctic Sea ice, Earth's Future, 2014, 2(6), 315–320 CrossRef.
- H. K. Imhof, N. P. Ivleva, J. Schmid, R. Niessner and C. Laforsch, Contamination of beach sediments of a subalpine lake with microplastic particles, Curr. Biol., 2013, 23(19), R867–R868 CrossRef CAS PubMed.
- A. A. Horton, C. Svendsen, R. J. Williams, D. J. Spurgeon and E. Lahive, Large microplastic particles in sediments of tributaries of the River Thames, UK–Abundance, sources and methods for effective quantification, Mar. Pollut. Bull., 2017, 114(1), 218–226 CrossRef CAS PubMed.
- M. Di and J. Wang, Microplastics in surface waters and sediments of the Three Gorges Reservoir, China, Sci. Total Environ., 2018, 616, 1620–1627 CrossRef PubMed.
- D. Habib, D. C. Locke and L. J. Cannone, Synthetic fibers as indicators of municipal sewage sludge, sludge products, and sewage treatment plant effluents, Water, Air, Soil Pollut., 1998, 103(1), 1–8 CrossRef CAS.
- S. A. Carr, J. Liu and A. G. Tesoro, Transport and fate of microplastic particles in wastewater treatment plants, Water Res., 2016, 91, 174–182 CrossRef CAS PubMed.
- F. Murphy, C. Ewins, F. Carbonnier and B. Quinn, Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment, Environ. Sci. Technol., 2016, 50(11), 5800–5808 CrossRef CAS PubMed.
- S. A. Mason, D. Garneau, R. Sutton, Y. Chu, K. Ehmann, J. Barnes, P. Fink, D. Papazissimos and D. L. Rogers, Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent, Environ. Pollut., 2016, 218, 1045–1054 CrossRef CAS PubMed.
- X. Lv, Q. Dong, Z. Zuo, Y. Liu, X. Huang and W. M. Wu, Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies, J. Cleaner Prod., 2019, 225, 579–586 CrossRef CAS.
- K. A. Zubris and B. K. Richards, Synthetic fibers as an indicator of land application of sludge, Environ. Pollut., 2005, 138(2), 201–211 CrossRef CAS PubMed.
- M. Liu, S. Lu, Y. Song, L. Lei, J. Hu, W. Lv, W. Zhou, C. Cao, H. Shi, X. Yang and D. He, Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China, Environ. Pollut., 2018, 242, 855–862 CrossRef CAS PubMed.
- F. Corradini, P. Meza, R. Eguiluz, F. Casado, E. Huerta-Lwanga and V. Geissen, Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal, Sci. Total Environ., 2019, 671, 411–420 CrossRef CAS PubMed.
- D. He, Y. Luo, S. Lu, M. Liu, Y. Song and L. Lei, Microplastics in soils: Analytical methods, pollution characteristics and ecological risks, TrAC, Trends Anal. Chem., 2018, 109, 163–172 CrossRef CAS.
- A. A. Horton, A. Walton, D. J. Spurgeon, E. Lahive and C. Svendsen, Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities, Sci. Total Environ., 2017, 586, 127–141 CrossRef CAS PubMed.
- L. Nizzetto, M. Futter and S. Langaas, Are Agricultural Soils Dumps for Microplastics of Urban Origin?, Environ. Sci. Technol., 2016, 50(20), 10777–10779 CrossRef CAS PubMed.
- M. C. Zambrano, J. J. Pawlak, J. Daystar, M. Ankeny, J. J. Cheng and R. A. Venditti, Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation, Mar. Pollut. Bull., 2019, 142, 394–407 CrossRef CAS PubMed.
- K. K. Leonas, Textile and apparel industry addresses emerging issue of microfiber pollution, J. Text. Appar. Technol. Manag.t, 2018, 10(4), 1–6 Search PubMed.
- G. Gatidou, O. S. Arvaniti and A. S. Stasinakis, Review on the occurrence and fate of microplastics in Sewage Treatment Plants, J. Hazard. Mater., 2019, 367, 504–512 CrossRef CAS PubMed.
- J. Talvitie, A. Mikola, O. Setälä, M. Heinonen and A. Koistinen, How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant, Water Res., 2017, 109, 164–172 CrossRef CAS PubMed.
- S. R. Smith, A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge, Environ. Int., 2009, 35(1), 142–156 CrossRef CAS PubMed.
- H. Rigby, B. O. Clarke, D. L. Pritchard, B. Meehan, F. Beshah, S. R. Smith and N. A. Porter, A critical review of nitrogen mineralization in biosolids-amended soil, the associated fertilizer value for crop production and potential for emissions to the environment, Sci. Total Environ., 2016, 541, 1310–1338 CrossRef CAS PubMed.
- DEFRA (Department for Environment, Food and Rural Affairs), Waste Water Treatment in the United Kingdom – 2012: Implementation of the European Union Urban Waste Water Treatment Directive – 91/271/EEC, Crown Copyright, London. 2012 Search PubMed.
- A. Ronda, A. Gómez-Barea, P. Haro, V. F. de Almeida and J. Salinero, Elements partitioning during thermal conversion of sewage sludge, Fuel Process. Technol., 2019, 186, 156–166 CrossRef CAS.
- X. Yu, J. Xue, H. Yao, Q. Wu, A. K. Venkatesan, R. U. Halden and K. Kannan, Occurrence and estrogenic potency of eight bisphenol analogs in sewage sludge from the US EPA targeted national sewage sludge survey, J. Hazard. Mater., 2015, 299, 733–739 CrossRef CAS PubMed.
- S. Yang, F. I. Hai, W. E. Price, J. McDonald, S. J. Khan and L. D. Nghiem, Occurrence of trace organic contaminants in wastewater sludge and their removals by anaerobic digestion, Bioresour. Technol., 2016, 210, 153–159 CrossRef CAS PubMed.
- L. A. Holmes, A. Turner and R. C. Thompson, Adsorption of trace metals to plastic resin pellets in the marine environment, Environ. Pollut., 2012, 160, 42–48 CrossRef CAS PubMed.
- A. Turner and L. A. Holmes, Adsorption of trace metals by microplastic pellets in fresh water, Environ. Chem., 2015, 12(5), 600–610 CrossRef CAS.
- M. E. Hodson, C. A. Duffus-Hodson, A. Clark, M. T. Prendergast-Miller and K. L. Thorpe, Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates, Environ. Sci. Technol., 2017, 51(8), 4714–4721 CrossRef CAS PubMed.
- G. Jerg and J. Baumann, Polyester microfibers-a new generation of fabrics, Text. Chem. Color., 1990, 22(12), 12–14 CAS.
- K. L. Hatch, The use of classification systems and production methods in identifying manufactured textile fibers, Identification of Textile Fibers, Woodhead Publishing, 2009, pp. 111–130 Search PubMed.
- J. Liu, Y. Yang, J. Ding, B. Zhu and W. Gao, Microfibers: a preliminary discussion on their definition and sources, Environ. Sci. Pollut. Res., 2019, 26(28), 29497–29501 CrossRef PubMed.
- M. Sillanpää and P. Sainio, Release of polyester and cotton fibers from textiles in machine washings, Environ. Sci. Pollut. Res., 2017, 24(23), 19313–19321 CrossRef PubMed.
- B. M. Carney Almroth, L. Åström, S. Roslund, H. Petersson, M. Johansson and N. K. Persson, Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment, Environ. Sci. Pollut. Res., 2018, 25(2), 1191–1199 CrossRef CAS PubMed.
- A. Engelhardt, The Fibre Year 2008/09: A World Survey on Textile and Nonwovens Industry, Oerlikon Textiles, Pfäffikon, Switzerland, 2009 Search PubMed.
- Y. S. Koo, Yarn hairiness affecting fluff generation, Fibers Polym., 2003, 4(3), 119–123 CrossRef.
- A. Telli and N. Özdil, Effect of recycled PET fibers on the performance properties of knitted fabrics, J. Eng. Fibers Fabr., 2015, 10(2), 155892501501000206 Search PubMed.
- I. E. Napper and R. C. Thompson, Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions, Mar. Pollut. Bull., 2016, 112(1–2), 39–45 CrossRef CAS PubMed.
- U. Pirc, M. Vidmar, A. Mozer and A. Kržan, Emissions of microplastic fibers from microfiber fleece during domestic washing, Environ. Sci. Pollut. Res., 2016, 23(21), 22206–22211 CrossRef CAS PubMed.
- H. Frost, M. C. Zambrano, K. Leonas, J. J. Pawlak and R. A. Venditti, Do Recycled Cotton or Polyester Fibers Influence the Shedding Propensity of Fabrics During Laundering?, AATCC J. Res., 2020, 7(1), 32–41 CrossRef.
- N. Bruce, N. Hartline, S. Karba, B. Ruff and S. Sonar, Microfibre Pollution and the Apparel Industry, Bren School of Environmental Science and Management, Santa Barbara, 2016 Search PubMed.
- E. Hernandez, B. Nowack and D. M. Mitrano, Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing, Environ. Sci. Technol., 2017, 51(12), 7036–7046 CrossRef CAS PubMed.
- F. Belzagui, M. Crespi, A. Álvarez, C. Gutiérrez-Bouzán and M. Vilaseca, Microplastics' emissions: Microfibers' detachment from textile garments, Environ. Pollut., 2019, 248, 1028–1035 CrossRef CAS PubMed.
- Y. Cai, T. Yang, D. M. Mitrano, M. Heuberger, R. Hufenus and B. Nowack, Systematic study of microplastic fiber release from 12 different polyester textiles during washing, Environ. Sci. Technol., 2020, 54(8), 4847–4855 CrossRef CAS PubMed.
- G. Dalla Fontana, R. Mossotti and A. Montarsolo, Assessment of microplastics release from polyester fabrics: the impact of different washing conditions, Environ. Pollut., 2020, 264, 113960 CrossRef CAS PubMed.
- G. Dalla Fontana, R. Mossotti and A. Montarsolo, Influence of sewing on microplastic release from textiles during washing, Water, Air, Soil Pollut., 2021, 232(2), 1–9 CrossRef.
- F. De Falco, E. Di Pace, M. Cocca and M. Avella, The contribution of washing processes of synthetic clothes to microplastic pollution, Sci. Rep., 2019, 9(1), 1 CAS.
- F. De Falco, G. Gentile, E. Di Pace, M. Avella and M. Cocca, Quantification of microfibres released during washing of synthetic clothes in real conditions and at lab scale, Eur. Phys. J. Plus, 2018, 133(7), 1–4 Search PubMed.
- F. De Falco, M. P. Gullo, G. Gentile, E. Di Pace, M. Cocca, L. Gelabert, M. Brouta-Agnésa, A. Rovira, R. Escudero, R. Villalba and R. Mossotti, Evaluation of microplastic release caused by textile washing processes of synthetic fabrics, Environ. Pollut., 2018, 236, 916–925 CrossRef CAS PubMed.
- J. Haap, E. Classen, J. Beringer, S. Mecheels and J. S. Gutmann, Microplastic fibers released by textile laundry: a new analytical approach for the determination of fibers in effluents, Water, 2019, 11(10), 2088 CrossRef CAS.
- C. Jönsson, O. Levenstam Arturin, A. C. Hanning, R. Landin, E. Holmström and S. Roos, Microplastics shedding from textiles—Developing analytical method for measurement of shed material representing release during domestic washing, Sustainability, 2018, 10(7), 2457 CrossRef.
- N. Kärkkäinen and M. Sillanpää, Quantification of different microplastic fibres discharged from textiles in machine wash and tumble drying, Environ. Sci. Pollut. Res., 2021, 28(13), 16253–16263 CrossRef PubMed.
- M. R. Kelly, N. J. Lant, M. Kurr and J. G. Burgess, Importance of water-volume on the release of microplastic fibers from laundry, Environ. Sci. Technol., 2019, 53(20), 11735–11744 CrossRef CAS PubMed.
- H. K. McIlwraith, J. Lin, L. M. Erdle, N. Mallos, M. L. Diamond and C. M. Rochman, Capturing microfibers–marketed technologies reduce microfiber emissions from washing machines, Mar. Pollut. Bull., 2019, 139, 40–45 CrossRef CAS PubMed.
- İ. Özkan and S. Gündoğdu, Investigation on the microfiber release under controlled washings from the knitted fabrics produced by recycled and virgin polyester yarns, J. Text. Inst., 2021, 112(2), 264–272 CrossRef.
- A. P. Periyasamy, Evaluation of microfiber release from jeans: the impact of different washing conditions, Environ. Sci. Pollut. Res., 2021, 28(41), 58570–58582 CrossRef CAS PubMed.
- S. Raja Balasaraswathi and R. Rathinamoorthy, Effect of fabric properties on microfiber shedding from synthetic textiles, J. Text. Inst., 2021, 1–21 Search PubMed.
- E. Vassilenko, M. Watkins, S. Chastain, J. Mertens, A. M. Posacka, S. Patankar and P. S. Ross, Domestic laundry and microfiber pollution: Exploring fiber shedding from consumer apparel textiles, PLoS One, 2021, 16(7), e0250346 CrossRef CAS PubMed.
- M. C. Rillig, L. Ziersch and S. Hempel, Microplastic transport in soil by earthworms, Sci. Rep., 2017, 7(1), 1–6 CrossRef CAS PubMed.
- M. Lehtiniemi, S. Hartikainen, P. Näkki, J. Engström-Öst, A. Koistinen and O. Setälä, Size matters more than shape: Ingestion of primary and secondary microplastics by small predators, Food Webs, 2018, 17, e00097 CrossRef.
- C. Goedecke, U. Mülow-Stollin, S. Hering, J. Richter, C. Piechotta, A. Paul and U. Braun, A first pilot study on the sorption of environmental pollutants on various microplastic materials, J. Environ. Anal. Chem., 2017, 4(1), 1000191 CrossRef.
- R. Ramasamy and R. B. Subramanian, Synthetic textile and microfiber pollution: a review on mitigation strategies, Environ. Sci. Pollut. Res., 2021, 28(31), 41596–41611 CrossRef CAS PubMed.
- I. E. Napper, A. C. Barrett and R. C. Thompson, The efficiency of devices intended to reduce microfibre release during clothes washing, Sci. Total Environ., 2020, 738, 140412 CrossRef CAS PubMed.
- D. M. Mitrano and W. Wohlleben, Microplastic regulation should be more precise to incentivize both innovation and environmental safety, Nat. Commun., 2020, 11(1), 1–2 CrossRef PubMed.
- M. R. Michielssen, E. R. Michielssen, J. Ni and M. B. Duhaime, Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed, Environ. Sci.: Water Res. Technol., 2016, 2(6), 1064–1073 RSC.
- M. Jaskulak, A. Grobelak, A. Grosser and F. Vandenbulcke, Gene expression, DNA damage and other stress markers in Sinapis alba L. exposed to heavy metals with special reference to sewage sludge application on contaminated sites, Ecotoxicol. Environ. Saf., 2019, 181, 508–517 CrossRef CAS PubMed.
- S. Ledakowicz, P. Stolarek, A. Malinowski and O. Lepez, Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification, Renewable Sustainable Energy Rev., 2019, 104, 319–327 CrossRef CAS.
- M. C. Collivignarelli, A. Abbà, A. Frattarola, M. Carnevale Miino, S. Padovani, I. Katsoyiannis and V. Torretta, Legislation for the reuse of biosolids on agricultural land in Europe: Overview, Sustainability, 2019, 11(21), 6015 CrossRef CAS.
- North East Biosolids Residuals Association (NEBRA), A National Biosolids Regulation, Quality, End Use & Disposal Survey, NEBRA, Tamworth, NH, 2007 Search PubMed.
- T. E. Seiple, A. M. Coleman and R. L. Skaggs, Municipal wastewater sludge as a sustainable bioresource in the United States, J. Environ. Manage., 2017, 197, 673–680 CrossRef CAS PubMed.
- L. Cheng, L. Wang, Y. Geng, N. Wang, Y. Mao and Y. Cai, Occurrence, speciation and fate of mercury in the sewage sludge of China, Ecotoxicol. Environ. Saf., 2019, 186, 109787 CrossRef CAS PubMed.
- J. Wang, Z. W. Lu, S. Tian and J. J. Tao, Existing state and development of sludgy researches in domestic and foreign, Municipal Engineering Technology, 2006, 24(3), 140–142 Search PubMed.
- H. A. Leslie, S. H. Brandsma, M. J. Van Velzen and A. D. Vethaak, Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota, Environ. Int., 2017, 101, 133–142 CrossRef CAS PubMed.
- K. Magnusson and F. Norén, Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant, IVL Swedish Environmental Research Institute, Stockholm, 2014 Search PubMed.
- J. Talvitie, M. Heinonen, J. P. Pääkkönen, E. Vahtera, A. Mikola, O. Setälä and R. Vahala, Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea, Water Sci. Technol., 2015, 72(9), 1495–1504 CrossRef CAS PubMed.
- P. U. Iyare, S. K. Ouki and T. Bond, Microplastics removal in wastewater treatment plants: a critical review, Environ. Sci.: Water Res. Technol., 2020, 6(10), 2664–2675 RSC.
- S. Klein, E. Worch and T. P. Knepper, Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany, Environ. Sci. Technol., 2015, 49(10), 6070–6076 CrossRef CAS PubMed.
- T. Bond, V. Ferrandiz-Mas, M. Felipe-Sotelo and E. Van Sebille, The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: A review, Crit. Rev. Environ. Sci. Technol., 2018, 48(7–9), 685–722 CrossRef.
- M. Eriksen, S. Mason, S. Wilson, C. Box, A. Zellers, W. Edwards, H. Farley and S. Amato, Microplastic pollution in the surface waters of the Laurentian Great Lakes, Mar. Pollut. Bull., 2013, 77(1–2), 177–182 CrossRef CAS PubMed.
- R. Lenz, K. Enders, C. A. Stedmon, D. M. Mackenzie and T. G. Nielsen, A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement, Mar. Pollut. Bull., 2015, 100(1), 82–91 CrossRef CAS PubMed.
- K. Munno, P. A. Helm, D. A. Jackson, C. Rochman and A. Sims, Impacts of temperature and selected chemical digestion methods on microplastic particles, Environmental toxicology and chemistry, 2018, 37(1), 91–98 CrossRef CAS PubMed.
- M. Cole, H. Webb, P. K. Lindeque, E. S. Fileman, C. Halsband and T. S. Galloway, Isolation of microplastics in biota-rich seawater samples and marine organisms, Sci. Rep., 2014, 4(1), 1–8 Search PubMed.
- J. Wagner, Z. M. Wang, S. Ghosal, C. Rochman, M. Gassel and S. Wall, Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices, Anal. Methods, 2017, 9(9), 1479–1490 RSC.
- Z. M. Wang, M. Parashar, S. Ghosal and J. Wagner, A new method for microplastic extraction from fish guts assisted by chemical dissolution, Anal. Methods, 2020, 12(45), 5450–5457 RSC.
- L. Lv, X. Yan, L. Feng, S. Jiang, Z. Lu, H. Xie, S. Sun, J. Chen and C. Li, Challenge for the detection of microplastics in the environment, Water Environ. Res., 2021, 93(1), 5–15 CrossRef CAS PubMed.
- S. M. Mintenig, I. Int-Veen, M. G. Löder, S. Primpke and G. Gerdts, Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging, Water Res., 2017, 108, 365–372 CrossRef CAS PubMed.
- S. Barnett, R. Evans, B. Quintana, A. Miliou and G. Pietroluongo, An environmentally friendly method for the identification of microplastics using density analysis, Environ. Toxicol. Chem., 2021, 40(12), 3299–3305 CrossRef CAS PubMed.
- X. Li, L. Chen, Q. Mei, B. Dong, X. Dai, G. Ding and E. Y. Zeng, Microplastics in sewage sludge from the wastewater treatment plants in China, Water Res., 2018, 142, 75–85 CrossRef CAS PubMed.
- E. A. Gies, J. L. LeNoble, M. Noël, A. Etemadifar, F. Bishay, E. R. Hall and P. S. Ross, Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada, Mar. Pollut. Bull., 2018, 133, 553–561 CrossRef CAS PubMed.
- J. Antonkiewicz, A. Baran, R. Pełka, A. Wisła-Świder, E. Nowak and P. Konieczka, A mixture of cellulose production waste with municipal sewage as new material for an ecological management of wastes, Ecotoxicol. Environ. Saf., 2019, 169, 607–614 CrossRef CAS PubMed.
- C. Barca, M. Martino, P. Hennebert and N. Roche, Kinetics and capacity of phosphorus extraction from solid residues obtained from wet air oxidation of sewage sludge, Waste Management, 2019, 89, 275–283 CrossRef CAS PubMed.
- I. Bartkowska, P. Biedka and I. A. Tałałaj, Analysis of the quality of stabilized municipal sewage sludge, J. Ecol. Eng., 2019, 20(2), 200–208 CrossRef.
- F. Bastida, N. Jehmlich, J. Martínez-Navarro, V. Bayona, C. García and J. L. Moreno, The effects of struvite and sewage sludge on plant yield and the microbial community of a semiarid Mediterranean soil, Geoderma, 2019, 337, 1051–1057 CrossRef CAS.
- M. Černe, I. Palčić, I. Pasković, N. Major, M. Romić, V. Filipović, M. D. Igrc, A. Perčin, S. G. Ban, B. Zorko and B. Vodenik, The effect of stabilization on the utilization of municipal sewage sludge as a soil amendment, Waste Management, 20191, 94, 27–38 CrossRef PubMed.
- A. Kicińska, J. Gucwa and B. Kosa-Burda, Evaluating potential for using municipal sewage sludge in the rehabilitation of ground degraded by the sodium processing industry, Bull. Environ. Contam. Toxicol., 2019, 102(3), 399–406 CrossRef PubMed.
- P. Regkouzas and E. Diamadopoulos, Adsorption of selected organic micro-pollutants on sewage sludge biochar, Chemosphere, 2019, 224, 840–851 CrossRef CAS PubMed.
- A. Turek, K. Wieczorek and W. M. Wolf, Digestion procedure and determination of heavy metals in sewage sludge—An analytical problem, Sustainability, 2019, 11(6), 1753 CrossRef CAS.
- J. Urra, I. Alkorta, I. Mijangos, L. Epelde and C. Garbisu, Application of sewage sludge to agricultural soil increases the abundance of antibiotic resistance genes without altering the composition of prokaryotic communities, Sci. Total Environ., 2019, 647, 1410–1420 CrossRef CAS PubMed.
- A. M. Mahon, B. O'Connell, M. G. Healy, I. O'Connor, R. Officer, R. Nash and L. Morrison, Microplastics in sewage sludge: effects of treatment, Environ. Sci. Technol., 2017, 51(2), 810–818 CrossRef CAS PubMed.
- G. B. Parliament, The Sludge (Use in Agriculture) Regulations, 1989 (Statutory Instruments 1989 No. 1263), Her Majesty’s Stationary Office, London, 1989 Search PubMed.
- Department of the Environment (DoE), Code of Practice for the Agricultural Use of Sewage Sludge, Department of the Environment, UK, 1996 Search PubMed.
- EU, Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, when Sewage Sludge Is Used in Agriculture, 1986 Search PubMed.
- N. Abdu, A. A. Abdullahi and A. Abdulkadir, Heavy metals and soil microbes, Environ. Chem. Lett., 2017, 15(1), 65–84 CrossRef CAS.
- A. Batel, F. Borchert, H. Reinwald, L. Erdinger and T. Braunbeck, Microplastic accumulation patterns and transfer of benzo [a] pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos, Environ. Pollut., 2018, 235, 918–930 CrossRef CAS PubMed.
- J. Li, K. Zhang and H. Zhang, Adsorption of antibiotics on microplastics, Environ. Pollut., 2018, 237, 460–467 CrossRef CAS PubMed.
- F. F. Liu, G. Z. Liu, Z. L. Zhu, S. C. Wang and F. F. Zhao, Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry, Chemosphere, 2019, 214, 688–694 CrossRef CAS PubMed.
- W. Mei, G. Chen, J. Bao, M. Song, Y. Li and C. Luo, Interactions between microplastics and organic compounds in aquatic environments: a mini review, Sci. Total Environ., 2020, 736, 139472 CrossRef CAS PubMed.
- L. Fu, J. Li, G. Wang, Y. Luan and W. Dai, Adsorption behavior of organic pollutants on microplastics, Ecotoxicol. Environ. Saf., 2021, 217, 112207 CrossRef CAS PubMed.
- J. Zou, X. Liu, D. Zhang and X. Yuan, Adsorption of three bivalent metals by four chemical distinct microplastics, Chemosphere, 2020, 248, 126064 CrossRef CAS PubMed.
- D. Brennecke, B. Duarte, F. Paiva, I. Caçador and J. Canning-Clode, Microplastics as vector for heavy metal contamination from the marine environment, Estuarine, Coastal Shelf Sci., 2016, 178, 189–195 CrossRef CAS.
- S. Tang, L. Lin, X. Wang, A. Yu and X. Sun, Interfacial interactions between collected nylon microplastics and three divalent metal ions (Cu (II), Ni (II), Zn (II)) in aqueous solutions, J. Hazard. Mater., 2021, 403, 123548 CrossRef CAS PubMed.
- C. C. Chen, X. Zhu, H. Xu, F. Chen, J. Ma and K. Pan, Copper Adsorption to Microplastics and Natural Particles in Seawater: A Comparison of Kinetics, Isotherms, and Bioavailability, Environ. Sci. Technol., 2021, 55(20), 13923–13931 CrossRef CAS PubMed.
- M. P. Johansen, E. Prentice, T. Cresswell and N. Howell, Initial data on adsorption of Cs and Sr to the surfaces of microplastics with biofilm, J. Environ. Radioact., 2018, 190, 130–133 CrossRef PubMed.
- B. Cojocariu, A. M. Mocanu, D. Bulgariu, G. Nacu and L. Bulgariu, Equilibrium isotherm study of metal ions adsorption on PET recycled fibers, E-health and Bioengineering Conference (EHB), IEEE, 2017, pp. 85–88 Search PubMed.
- X. Han, S. Wang, X. Yu, R. D. Vogt, J. Feng, L. Zhai, W. Ma, L. Zhu and X. Lu, Kinetics and Size Effects on Adsorption of Cu (II), Cr (III), and Pb (II) onto Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles, 2021 Search PubMed.
- S. G. Venkatachalam, S. G. Nayak, J. V. Labde, P. R. Gharal, K. Rao and A. K. Kelkar, Degradation and recyclability of poly (ethylene terephthalate), Polyester, InTech, Rijeka, Croatia, 2012, pp. 75–98 Search PubMed.
- A. Pathak, M. G. Dastidar and T. R. Sreekrishnan, Bioleaching of heavy metals from sewage sludge: a review, J. Environ. Manage., 2009, 90(8), 2343–2353 CrossRef CAS PubMed.
- D. Kulikowska, Z. M. Gusiatin, K. Bułkowska and K. Kierklo, Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil, Chemosphere, 2015, 136, 42–49 CrossRef CAS PubMed.
- T. Sizmur, T. Fresno, G. Akgül, H. Frost and E. Moreno-Jiménez, Biochar modification to enhance sorption of inorganics from water, Bioresour. Technol., 2017, 246, 34–47 CrossRef CAS PubMed.
- X. Li, Q. Mei, L. Chen, H. Zhang, B. Dong, X. Dai, C. He and J. Zhou, Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process, Water Res., 2019, 157, 228–237 CrossRef CAS PubMed.
- J. Wang, X. Guo and J. Xue, Biofilm-developed microplastics as vectors of pollutants in aquatic environments, Environ. Sci. Technol., 2021, 55(19), 12780–12790 CAS.
- J. Decelle, N. Suzuki, F. Mahé, C. De Vargas and F. Not, Molecular phylogeny and morphological evolution of the Acantharia (Radiolaria), Protist, 2012, 163(3), 435–450 CrossRef PubMed.
- Z. M. Wang, J. Wagner, S. Ghosal, G. Bedi and S. Wall, SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts, Sci. Total Environ., 2017, 603, 616–626 CrossRef PubMed.
- L. Ding, S. Zhang, X. Wang, X. Yang, C. Zhang, Y. Qi and X. Guo, The occurrence and distribution characteristics of microplastics in the agricultural soils of Shaanxi Province, in north-western China, Sci. Total Environ., 2020, 720, 137525 CrossRef CAS PubMed.
- G. S. Zhang and Y. F. Liu, The distribution of microplastics in soil aggregate fractions in southwestern China, Sci. Total Environ., 2018, 642, 12–20 CrossRef CAS PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, Degradation rates of plastics in the environment, ACS Sustainable Chem. Eng., 2020, 8(9), 3494–3511 CrossRef CAS.
- L. D. Suits and Y. G. Hsuan, Assessing the photo-degradation of geosynthetics by outdoor exposure and laboratory weatherometer, Geotext. Geomembr., 2003, 21(2), 111–122 CrossRef.
- S. Yoshida, K. Hiraga, T. Takehana, I. Taniguchi, H. Yamaji, Y. Maeda, K. Toyohara, K. Miyamoto, Y. Kimura and K. Oda, A bacterium that degrades and assimilates poly (ethylene terephthalate), Science, 2016, 351(6278), 1196–1199 CrossRef CAS PubMed.
- H. P. Austin, M. D. Allen, B. S. Donohoe, N. A. Rorrer, F. L. Kearns, R. L. Silveira, B. C. Pollard, G. Dominick, R. Duman, K. El Omari and V. Mykhaylyk, Characterization and engineering of a plastic-degrading aromatic polyesterase, Proc. Natl. Acad. Sci., 2018, 115(19), E4350–E4357 CrossRef CAS PubMed.
- M. Vaverková, D. Adamcová, L. Kotrchová, J. Merna and S. Hermanová, Degradation of pet copolyesters under real and laboratory composting conditions, J. Mater. Cycles Waste Manage., 2018, 20(1), 414–420 CrossRef.
- A. A. de Souza Machado, C. W. Lau, J. Till, W. Kloas, A. Lehmann, R. Becker and M. C. Rillig, Impacts of microplastics on the soil biophysical environment, Environ. Sci. Technol., 2018, 52(17), 9656–9665 CrossRef CAS PubMed.
- S. W. Kim and Y. J. An, Soil microplastics inhibit the movement of springtail species, Environ. Int., 2019, 126, 699–706 CrossRef PubMed.
- L. Zhao, M. Qu, G. Wong and D. Wang, Transgenerational toxicity of nanopolystyrene particles in the range of μg L−1 in the nematode Caenorhabditis elegans, Environmental Science: NanoEnviron. Sci.: Nano, 2017, 4(12), 2356–2366 RSC.
- E. Huerta Lwanga, H. Gertsen, H. Gooren, P. Peters, T. Salánki, M. Van Der Ploeg, E. Besseling, A. A. Koelmans and V. Geissen, Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae), Environ. Sci. Technol., 2016, 50(5), 2685–2691 CrossRef CAS PubMed.
- E. Huerta Lwanga, J. Mendoza Vega, V. Ku Quej, J. D. Chi, L. Sanchez del Cid, C. Chi, G. Escalona Segura, H. Gertsen, T. Salánki, M. van der Ploeg and A. A. Koelmans, Field evidence for transfer of plastic debris along a terrestrial food chain, Sci. Rep., 2017, 7(1), 1–7 CrossRef CAS PubMed.
- F. R. Khan, D. Boyle, E. Chang and N. R. Bury, Do polyethylene microplastic beads alter the intestinal uptake of Ag in rainbow trout (Oncorhynchus mykiss)? Analysis of the MP vector effect using in vitro gut sacs, Environ. Pollut., 2017, 231, 200–206 CrossRef CAS PubMed.
- A. Bakir, S. J. Rowland and R. C. Thompson, Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions, Environ. Pollut., 2014, 185, 16–23 CrossRef CAS PubMed.
- J. I. Kwak and Y. J. An, Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability, J. Hazard. Mater., 2021, 402, 124034 CrossRef CAS PubMed.
- E. H. Lwanga, B. Thapa, X. Yang, H. Gertsen, T. Salánki, V. Geissen and P. Garbeva, Decay of low-density polyethylene by bacteria extracted from earthworm's guts: A potential for soil restoration, Sci. Total Environ., 2018, 624, 753–757 CrossRef PubMed.
- A. L. Dawson, S. Kawaguchi, C. K. King, K. A. Townsend, R. King, W. M. Huston and S. M. Bengtson Nash, Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill, Nat. Commun., 2018, 9(1), 1–8 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1em00541c |
| This journal is © The Royal Society of Chemistry 2022 |




