Applications of metal N-heterocyclic carbene complexes in olefin polymerizations
Ting
Feng
a,
Le
Zhang
a,
Shang-Xiao
Wu
a,
Xiaochao
Shi
 *b and
Ying-Feng
Han
*b and
Ying-Feng
Han
 *a
*a
aKey Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, Xi'an Key Laboratory of Functional Supramolecular Structure and Materials, College of Chemistry and Materials Science, Northwest University, Xi'an 710127, China. E-mail: yfhan@nwu.edu.cn
bDepartment of Polymer Materials, School of Materials Science and Engineering, Shanghai University, Nanchen Street 333, Shanghai 200444, China. E-mail: xcshi@shu.edu.cn
First published on 20th August 2024
Abstract
Polyolefins play an important role in modern society and are applied as both commodity plastics and high-performance polymers. Nowadays, they are widely used in large-scale industrial production and considered one among the most important polymers in the world. Since the 1950s, the exploration of Ziegler–Natta catalysts has not just brought continuous breakthroughs in the industrialization of polyolefins, but has also promoted the development of research in organometallic chemistry. Obviously, a key and long-standing focus in the polyolefin industry is the investigation of new metal catalysts that possess customizable catalytic performances. Over the past few decades, metal complexes with N-heterocyclic carbene (NHC) ligands have achieved great improvements and found vast applications in diverse research fields, and definitely, they have been also demonstrated to show excellent catalytic performances in olefin polymerization. Numerous reviews have been published on the catalytic performances of NHC-chelated metal complexes; however, a comprehensive overview of them with the central metals across the periodic table in olefin polymerization is currently lacking. This review aims to bridge this gap by providing a comprehensive summary of NHC catalysts and their corresponding structure–activity relationships in the polymerization and copolymerization of different olefins.
1. Introduction
Polyolefins, such as polyethylenes (PEs) and polypropylenes (PPs), are commercially synthetic polymers worldwide due to their cost-effectiveness and excellent properties.1 The majority of these polymers are synthesized via the strategy of transition-metal-catalyzed olefin polymerization. Ever since Karl Ziegler and Giulio Natta discovered titanium-based olefin polymerization catalysts in the early 1950s, an extensive range of transition metal complexes with various central metals and chelating ligand frameworks have been developed as catalysts for olefin polymerization. These advancements have significantly contributed to the development of metal catalysis, organometallic chemistry, and polymer science, which remain the most exciting research fields nowadays. Notably, group 4 metallocene catalysts, rare-earth metal half-sandwich alkyl catalysts, and late transition metal catalysts have exhibited outstanding catalytic performances for olefin copolymerization.2 Given the significance of metal catalysts in the field of polyolefins, researchers from both academia and industry are enthusiastic about designing and synthesizing novel efficient metal catalysts, as well as achieving precise synthesis of polyolefin products.3,4 On the other hand, the research and application of N-heterocyclic carbenes (NHCs) have become a hot topic in chemistry since Arduengo and colleagues first isolated and characterized the free and stable NHCs.5 Various NHCs with distinct structures and their corresponding metal complexes have been synthesized and reported, leading to significant breakthroughs in a wide range of catalytic reactions. Generally, phosphine ligands are among the most important class of ligands to stabilize the metal complexes because of the alterability of their electronic and steric properties in a systematic and predictable way. It has been proved that NHCs have similar chemical properties to phosphine ligands and can partially replace phosphine ligands in catalytic reactions. Furthermore, the strong σ-donating ability of NHCs allows for the formation of more stable metal NHC complexes compared to their phosphine-based counterparts.6,7 This effectively addresses the deactivation issue commonly observed with phosphine-based metal complexes during catalytic processes.8 Consequently, NHCs have emerged as versatile ancillary ligands for constructing metal complexes, which have proved to be effective homogeneous catalysts for various reactions including olefin metathesis, coupling reactions, ring-opening translocation polymerization, and olefin coordination polymerization.8,9 In general, as a soft carbon-donor ligand, NHCs are more prone to coordinate late-transition metals to form thermally and chemically stable complexes than the early transition metals in high oxidation states because of the hard–soft mismatch. This poses a challenge for the applications of NHCs in olefin polymerization, considering that complexes with early-transition metal centers are the predominant industrial catalyst candidates for such reactions. On the other hand, the covalent tethering of coordinating functional groups to NHCs offers a practical approach for enhancing the interactions of NHC ligands with the central metals, especially the early transition metals. This has stimulated the development of numerous metal complexes with hetero-atom-tethered NHC ligands as excellent catalyst candidates for olefin polymerization.This review focuses on the literature published since 1998 regarding various well-defined metal NHC catalysts used in the polymerization of olefins, particularly α-olefins, styrene, norbornene, and others. The synthesis of NHC-ligated complexes and the structure–activity relationship in NHC-complex-catalyzed olefin polymerization are discussed in a comparative manner, and the catalytic data, including the structures of complexes, co-catalysts, monomers, activities and microstructures of resultant polymers and so on, are summarized in Table 1. Notably, the catalyst activities are classified in this review as high (1000–100), moderate (100–10), low (10–1), and very low (<1) for α-olefins, but for norbornene polymerization, the categories are high (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), moderate (10
000), moderate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1000), low (1000–100), and very low (<100) (in unit: kg mol−1 (M) h−1). For a more comprehensive understanding of the coordination chemistry of metal NHC complexes and their reactivity in diverse reactions, it is highly recommended to refer to other excellent overviews.10–13
000–1000), low (1000–100), and very low (<100) (in unit: kg mol−1 (M) h−1). For a more comprehensive understanding of the coordination chemistry of metal NHC complexes and their reactivity in diverse reactions, it is highly recommended to refer to other excellent overviews.10–13
| Complexes | Monomers | Co-catalyst | Activity (103 g/molM−1 h−1) | M n (104 g mol−1) | M w/Mn | Key features in polymerization | Ref. |
|---|---|---|---|---|---|---|---|
| Complexes with monodentate NHC ligands | |||||||

|
α-Olefin | [Ph3C][B(C6F5)4] | 8–2520 | 0.8–63.1 | 1.7–3.0 | Atactic poly(α-olefin), tunable insertion ratio of 1,5-hexadiene | 14 |
| 1-Hexene/1,5-hexadiene | [Ph3C][B(C6F5)4] | 72–216 | 2.2–4.3 | 1.7–2.6 | |||
| o-Methoxystyrene derivatives | [Ph3C][B(C6F5)4]/AliBu3 | 6.9–2160 | 0.5–455.5 | 1.37–2.24 | Syndiotactic (up to rrrr > 99%) | 15 | |
| Sulfur-functionalized α-olefin | [Ph3C][B(C6F5)4] | 0.32–10.3 | 1.53–11.6 | Syndiotactic (rrrr up to 0.95), crystalline materials | 16 | ||

|
α-Olefin | [Ph3C][B(C6F5)4] | 1–285 | 2.8–21.8 | 1.72–2.45 | Atactic poly(α-olefin), pendant vinyl groups in the copolymers (22.1%) | 17 |
| 1-Hexene/1,7-octadiene | [Ph3C][B(C6F5)4] | 2.9–4.9 | 2.06–3.12 | ||||

|
Ethylene/propylene | Et3Al2Cl3 | 7.2–110.0 | Up to 1612 (Mw) | Randomly distributed monomer | 18 and 19 | |

|
Ethylene (6 atm) | MAO or [Ph3C][B(C6F5)4]/AliBu3 | 3–4590 | 28.0–341 | 1.8–2.7 | High catalytic activity | 20 |
| Ethylene/1-hexene | d-MAO | 66–2470 | 4.10–23.5 | 1.9–5.0 | Efficient 1-hexene incorporation | ||

|
Ethylene | MAO | 29–67 | Supported, high stability (300 minutes) | 21 | ||
|
Ethylene (2 atm) | MAO | Low | Dual peaks in GPC curve | 22 | ||

|
Ethylene (1 atm) | MMAO | 12.9–15.1 | 1-Butene produced, fast β-H elimination | 23 | ||

|
Styrene | MAO | 0.94–16.5 | 2.0–4.0 | Atactic polymer | 24 | |
|
Norbornene | MAO | Trace polymer | 25 | |||

|
1,3-Butadiene | 4.54 | 2.9 | Poly(cis-1,4-butadiene) and atactic polystyrene | 26 and 27 | ||
| Styrene | 8.0 | 2.5 | |||||
| Norbornene and derivates | AgSbF6 or AgBF4 | 0.45–13.3 | 1.25–2.89 | Catalytic activity was highly dependent on the counter anion | 28 | ||

|
Norbornene | 24.1–328.0 | 12–41 (Mw) | 2.4–3.3 | Low catalytic activity | 29 | |

|
5-Norbornene-2-methyl acetate | Li[B(C6F5)4] | 2–123 | 5.8–16.6 | 1.73–2.53 | Low catalytic activity | 30 |

|
5-Alkylidene-2-norbornenes | Na[B(3,5-(CF3)2C6H5)4] | 110–140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3–68.7 | 1.4–4.4 | Extremely high activity, performed in an atmosphere of air and in wet solvents | 31 |
| Substituted nadimides | AgSbF6 or AgBF4 | 19.6–188.9 | Proper combination of a cocatalyst and a Pd precatalyst | 32 | |||

|
Norbornene and derivates | Na[B(3,5-(CF3)2C6H5)4] | 0.30–0.68 | 1.23–1.35 | Redox control | 33 | |

|
Ethylene (2 atm) | MAO | 1–75 | Linear polyethylene generated | 34 | ||
|
Ethylene (10 bar), propylene, 1-butene | MAO, AlEtCl2 | 2.7, 13.4 (ethylene) | Predominant dimerization | 35–38 | ||

|
Ethylene (10 bar) | MAO, AlEtCl2 | 0.2–42.8 (ethylene) | Predominant dimerization | 38 and 39 | ||

|
Styrene derivates | 0.86–18.2 | 1.7–3.4 | Atactic polystyrenes, co-catalyst-free | 40 | ||
| Complexes with a bidentate NHC ligands (bidentate NHC ligand with a neutral functionality) | |||||||

|
Ethylene (6.89 atm) | MAO, Et2AlCl | 0.318–1.871 | 41 | |||

|
Ethylene (10 atm) | MAO, AlEtCl2 | 0.15–2.39 (gM−1) | Mainly polymerization in all the cases | 42 | ||

|
Ethylene (10 atm) | MAO, AlEtCl2 | 1.67–8.79 | MAO as cocatalyst: oligomerization AlEtCl2 as cocatalyst: polymerization | 42 | ||
|
Ethylene (1 atm) | MAO | 2–4 | Poor activity | 43 | ||

|
Ethylene (5 atm) | Low activity in ethylene polymerization | 44 | ||||
|
Ethylene (5 atm) | 77% polyethylene and 23% soluble oligomers with rather low activity | 44 | ||||

|
Ethylene | MAO | 40–140 | Linear polyethylene | 45 | ||
| Norbornene | MMAO or Na[B(3,5-(CF3)2C6H5)4] | 32.0–350 (Pd) | Low catalytic activity, C7 linkage in the polynorbornene | 46 | |||

|
Norbornene | MAO | 1300–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
111–420 | 2.3–3.5 | High catalytic activity for norbornene polymerization and high catalytic activity for ethylene polymerization | 47 |
| Ethylene (1 atm) | MAO | 330 | 6.7 | 12.8 | |||

|
Norbornene | MAO | 5200–77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | — | High catalytic activity | 48 |

|
Ethylene (2.5 atm) and styrene | AgPF6 | Predominant dimerization | 49 | |||

|
Ethylene | Predominant dimerization | 50 | ||||

|
Norbornene | MAO | 500–3000 | Moderate catalytic activity | 51 | ||

|
Ethylene (10 atm) | EtAlCl2 | 13.3–15.6 | Predominant dimerization | 39 | ||

|
Ethylene (30 atm) | 29–152 | 0.65–1.3 | 2.0–2.3 | Low incorporation ratio of polar monomers | 52 | |
| Ethylene (30 atm) /MA derivates | 1.7–13 | 0.48–0.94 | 2.1–2.3 | ||||

|
Ethylene (30 atm) | Trace | Very low catalytic activation | 52 | |||

|
Norbornene | MAO | 996–1700 | 24–82 (Mv) | Moderate catalytic activation | 25 | |
|
Norbornene | MAO | 303 | Low catalytic activation | 25 | ||
| Complexes with bidentate NHC ligands (bidentate NHC ligand with an anionic functionality) | |||||||

|
Isoprene | [Ph3C][B(C6F5)4]/AliBu3 | 2.03–3.81 | 1.36–1.40 | 3,4-Regulated (99%) and syndiotactically enriched microstructure | 53 and 54 | |

|
Isoprene | [Ph3C][B(C6F5)4]/AliBu3 | 3.73–39.4 | 1.05–1.38 | |||
| Ethylene (1 atm)/α-olefin | 760–4120 | 0.59–5.52 (Mw) | 1.2–2.9 | Varied comonomer incorporation (2.1%–38.7%) | 55 | ||
| Ethylene (1–6 atm) /styrene | 5–508 | 0.6–18.9 | 1.26–2.41 | Pseudo-random microstructures | 56 | ||
| Ethylene (1–6 atm)/polar styrene derivates | 7–362 | 0.47–3.55 | 1.49–2.55 | Pseudo-alternating microstructures | 57 | ||
| Ethylene (4 atm)/butadiene | 150–1640 | 2.18–20.2 | 1.81–2.74 | trans-1,4 regularity and alternating sequence | 58 | ||

|
Ethylene (8–40 atm) | MMAO-3A | 35.5–636 (gM−1) | Moderate catalytic activity, polyethylene and no oligomerization | 59 | ||
|
Styrene | NaBPh4 | 2.7 | 1.89 | Low catalytic activity, styrene polymerization at 80 °C | 60 | |

|
Ethylene or (6 atm) | MAO | 4.1–29 | 63–370 (Mw) | 1.6–3.4 | Low catalytic activity, linear polyethylene, isotactic polypropylene together with an atactic fraction | 61 |
| Propylene | 0.029–0.038 | 38–73 (Mw) | 1.7–5.7 | ||||
|
Ethylene (6 atm) | MAO | 0.3–7.3 | 64–377 (Mw) | 2.3–3.4 | 62 | |
| Propylene | 0.3–31 | 31–175 (Mw) | 2.3–3.5 | ||||
|
Ethylene (3–27.2 atm) | MAO | 16–259 | 0.446–82.4 | 8.24–85.6 | Extremely high molecular weight polyethylene (Mw) | 63 and 64 |
| Styrene | MAO | 0.48–1.3 | 1.01–1.33 | 2.4–5.5 | Highly syndiotactic polystyrene | 64 | |

|
Ethylene (4 atm) or ethylene/norbornene or ethylene/cyclopentene | MAO | 25–210 | >600 | Extremely high-molecular weight (co-)polymers | 65 | |

|
Ethylene (15 atm) | 0.8–56 | 0.10–0.71 | 1.95–2.5 | Polymerizing ethylene in the absence of co-catalysts, linear polymer | 66 | |

|
Ethylene (15 atm) | ZnEt2 | 1.5–17.5 | 0.126–0.211 | 2.4–20.68 | Highly linear, low molecular-weight polyethylene | 67 |
|
Norbornene | MAO | Up to 100 | Low catalytic activity | 68 | ||

|
Norbornene | MAO | 110–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
29–211 (Mw) | 1.5–3.3 | Moderate to high catalytic activity | 69–71 |

|
Norbornene | MAO | 2690–49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 970 |
High catalytic activity | 72 and 73 | ||

|
Norbornene | MAO | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 640 |
High catalytic activity | 72 | ||

|
Norbornene | MAO | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290–23 290–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
High catalytic activity | 72 | ||
|
Norbornene | MAO | 7800–49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
High catalytic activity | 74 | ||

|
Norbornene | MAO | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230 |
High catalytic activity | 74 | ||
|
Norbornene | MAO | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
High catalytic activity | 74 | ||

|
Norbornene | MAO | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
High catalytic activity | 75 | ||

|
Norbornene | MAO, Et2AlCl, Et3Al, [Ph3C][B(C6F5)4] | 490–49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
High catalytic activity | 75 | ||

|
Norbornene | MAO | 9900–32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 170 |
High catalytic activity | 75 | ||
|
Norbornene | MAO | 1100–58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
High catalytic activity | 76 | ||
|
Norbornene | MAO | 399–2965 | Moderate catalytic activity | 76 | ||

|
Norbornene | MAO | 4450–101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
Moderate to high catalytic activity | 77 | ||
| [Ph3C][B(C6F5)4] | 1270–42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
High catalytic activity | |||||

|
Norbornene | MAO | 1900–42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
High thermal stability | 78 | ||
| Et2AlCl | 900–8500 | Low to high catalytic activity | |||||

|
Norbornene | MAO | 4000–54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
High thermal stability | |||
| Norbornene | Et2AlCl | 1500–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
Moderate to high catalytic activity | ||||

|
Norbornene | MAO | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–60 000–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
High thermal stability | |||
| Et2AlCl | 7400–29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Moderate to high catalytic activity | |||||

|
Norbornene | MAO or Et2AlCl | 2880–49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Moderate to high catalytic activity | 79 | ||
| Norbornene/polar derivates | MAO or Et2AlCl | 290–2080 | 3.66–8.64 | 1.63–2.47 | Moderate catalytic activity and high incorporation of polar monomers | ||

|
Ethylene (4 MPa), propylene and 1-butene | 3.6–1310 | 0.21–8.0 | 1.8–3.3 | High thermal stability of catalyst, moderate to high catalytic activity | 80 | |
| Ethylene (4 MPa)/polar allyl monomers or propylene/polar allyl monomers | 0–10.8 | 0.23–2.3 | 2.0–5.6 | Successful insertion of polar monomer | |||
| Ethylene (1–2 MPa)/1,1-disubstituted ethylenes | 0.16–2.3 | 0.08–2.7 | 1.7–2.9 | Successful incorporation of polar comonomers | 81 and 82 | ||
| Ethylene (3.0 MPa)/3-ethylidene-6-vinyltetrahydro-2H-pyran-2-one | 0.6–48 | 0.07–3.2 | 1.7–4.7 | Copolymer of ethylene/CO2/1,3-butadiene | 83 | ||
| Ethylene (2.0 MPa)/norbornenes functionalized with polar groups | 2.0 | 2.2 | 1.4 | High incorporation of polar comonomers | 84 | ||

|
Ethylene (4.0 MPa) | 240–1190 | 0.26–8.4 | 2.0–2.7 | Linear polyethylene and successful incorporation of polar comonomers | 85 | |
| Ethylene (4.0 MPa)/polar allyl monomers | 0–2.1 | 0.063–1.4 | 2.1–3.9 | ||||

|
Ethylene (4.0 MPa) and propylene | 9.6–347 | 0.31–23.7 | 1.3–1.9 | Moderate catalytic activity, successful incorporation of polar comonomers | 86 | |
| Ethylene (4 MPa)/polar allyl monomers | 1.8–9.5 | 0.22–2.7 | 1.3–2.4 | ||||

|
Ethylene (4.0 MPa) | 3.6, 5.2 | 0.18, 0.25 | 3.6, 2.0 | Branched polyethylene | 87 | |

|
Ethylene (4.0 MPa) | 4.1–5.3 | 0.36–0.7 | 1.7–3.1 | Branched polyethylene | 87 | |
|
Ethylene (4.0 MPa)/methyl acrylate | 0.09–1.17 | 1.9–3.2 | Successful incorporation of polar comonomers | 88 | ||

|
Ethylene (4.0 MPa) | 16.2–127.0 | 0.032–0.07 | Oligomer in all cases, telechelic microstructure of co-oligomer | 89 | ||
| Ethylene (4.0 MPa)/methyl acrylate | 2.8 | 0.035 | |||||
| Complexes with tridentate NHC ligands (tridentate NHC ligand with neutral functionality) | |||||||

|
Ethylene (10 atm) | MAO | 1.7–9.2 (g Ni) | Oligomer in all cases | 90 | ||

|
Ethylene (10 atm) | Et2AlCl | 12.7–14.6 (g Ni) | Oligomer in all cases | 90 | ||

|
Ethylene (1–5 atm) | MAO | 15–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
High catalytic activity (Cr) for oligomerization, high molecular weight for polymer (Ti, V) | 91 and 92 | ||

|
Ethylene (up to 30 atm) | MAO | No activity for oligomerization and polymerization | 93 | |||

|
Ethylene (1 atm) | MAO | 17–35 | Linear polyethylene | 94 | ||

|
Isoprene | [Ph3C][B(C6F5)4] | 1.84–49.2 | 1.03–1.07 | Highly 3,4-regular polyisoprene | 95 | |
| Isoprene/ε-caprolactone | [Ph3C][B(C6F5)4] | 4.9–10.2 | 1.12–1.16 | ||||
| 2-Phenyl-1,3-butadiene | [Ph3C][B(C6F5)4] | 1.84–7.08 | 1.17–1.66 | Highly 3,4-regular | 96 | ||

|
Isoprene | [Ph3C][B(C6F5)4]/AliR3 | 3.9–95.6 | 1.40–3.87 | Highly cis-1,4-regular | 97 and 98 | |
| Complexes with tridentate NHC ligands (tridentate NHC ligand with monoanionic functionality) | |||||||

|
Styrene | NaBPh4 | 1.76 | 1.97 | Low activity | 99 | |

|
Norbornene | Me2AlCl/Me2AlCl/MAO/B(C6F5)3 | 3.67–3210 (Ni) | 5.19–25.89 (Mw) | 1.72–2.80 | Low to moderate catalytic activity | 70, 73 and 100 |
4074–274![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Pd) 000 (Pd) |
3.8–16.4 | 1.64–2.16 | Moderate to high catalytic activity | ||||

|
Norbornene | Me2AlCl/Me2AlCl/MAO/B(C6F5)3 | 0.41–2280 (Ni) | 1.61–36.69 (Mw) | 1.41–2.05 | Low to moderate catalytic activity | 70 and 73 |
4074–59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 (Pd) 990 (Pd) |
8.6–12.5 | 1.66–1.71 | Moderate to high catalytic activity | ||||
|
Norbornene | Me2AlCl | 286![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–274 000–274![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
High catalytic activity | 100 | ||
| Norbornene/norbornene with polar functionality | 18.2–86.1 | 1.57–3.22 | 1.36–2.15 | Low catalytic activity, high incorporation ratio of polar monomer | |||
|
Norbornene | MAO | 1120–6570 | 22.4–102.5 (Mw) | 2.3–2.8 | Moderate catalytic activity | 101 |
| Complexes with tridentate NHC ligands (tridentate NHC ligand with dianionic functionality) | |||||||

|
Ethylene (3.5–9 atm) | MMAO | 9.71–290 | 0.13–3.98 | Moderate catalytic ability, linear polyethylene with low molecular weight | 102 and 103 | |

|
Ethylene | [Ph3C][B(C6F5)4] | 125 | Moderate catalytic ability | 104 | ||

|
1-Hexene | [Ph3C][B(C6F5)4] | Oligo(1-hexenes) formed | 105 | |||

|
1-Hexene | [Ph3C][B(C6F5)4] | Atactic oligomer | 106 | |||
| Complexes with tetradentate NHC ligands | |||||||

|
Norbornene | MAO | 810–1340 | 18.4–25.1 (Mw) | 10.3–11.5 | Moderate catalytic activity | 101 |
2. Complexes with monodentate NHC ligands
The N-heterocyclic carbene ligated Sc trialkyl complexes [(2,6-C6H3R2NCH)2CSc(CH2SiMe3)3] 1 (Fig. 1, left) were synthesized by the reaction of 1.0 equiv. of [Sc(CH2SiMe3)3(THF)2] with the corresponding NHC ligands.14 Upon activation with 2.0 equiv. of [Ph3C][B(C6F5)4], 1 exhibited remarkably high activities for 1-hexene homopolymerization (up to 2.52 × 106 g mol−1 h−1), generating the polymers with moderate to high molecular weights. The bis(2,6-dimethyl)-substituted complex was also shown to be an efficient catalyst in the copolymerization of 1-hexene with 1,5-hexadiene, yielding random atactic copolymers after addition of 2.0 equiv. of [Ph3C][B(C6F5)4]. 1/[Ph3C] [B(C6F5)4] also exhibited good activities in the polymerization of 1-octene or 1-decene. Moreover, in the presence of 2.0 equiv. of [Ph3C][B(C6F5)4] and excess AliBu3, bis(2,6-diisopropylphenyl)-substituted Sc dication active species displayed excellent activity (2.2 × 106 g mol−1 h−1) in the polymerization of polar o-methoxystyrene and its silyloxy- or fluorine-substituted derivatives, yielding ultrahigh molecular weight syndiotactic poly(oMOS) derivatives (up to rrrr > 0.99).15 The density functional theory (DFT) calculation studies provided evidence that the double bonds and heteroatoms of oMOS pass through the σ,π-coordination mode, associated with high oxyphilic Sc center with two empty coordination sites, which reduces the activation energy of monomer insertion. The 1/[Ph3C] [B(C6F5)4] binary system has been found to be capable of catalyzing the polymerization of thioether-substituted olefin monomers. This resulted in the formation of syndiotactic sulfur-functionalized poly(α-olefin)s (rrrr up to 0.95), which formed crystalline materials with Tm = 91 °C and Td = 378 °C. The presence of electron-donating groups greatly influenced the crystallinity of the polymer. Furthermore, factors such as R substituents, spacer length of the sulfur atom and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, and the stereoregularity of the polymer also impacted its crystalline structure significantly.16
C double bond, and the stereoregularity of the polymer also impacted its crystalline structure significantly.16
In another study, Pan et al. investigated the catalytic activities of bis(oxazoline)-derived NHC lanthanide complexes 2 (Fig. 1, right) in the polymerization of α-olefins. By activation with 2.0 equiv. of [Ph3C][B(C6F5)4], the Sc complex showed high activities (2.85 × 105 g mol−1 h−1) towards 1-hexene polymerization. The resulting polymers had molecular weights of 2.8–8.7 × 104 g mol−1 and 2.18 × 105 g mol−1 at room temperature and −20 °C, respectively. However, the Y and Lu analogues were inert for α-olefin polymerization. The Sc-based catalytic system 2/2[Ph3C][B(C6F5)4] was capable of catalysing the copolymerization of 1-hexene with 1,7-octadiene, giving random copolymers with diene incorporation levels varying from 30% to 70% (depending on the co-monomer feed) with about 20% pendant vinyl groups.17
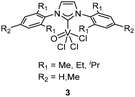
The treatment of equimolar NHCs with VOCl3 in toluene at room temperature smoothly afforded the corresponding NHC V complexes 3 (Fig. 2).18,19 Upon activation with Et3Al2Cl3, these complexes served as efficient catalysts for the copolymerization of ethylene with propylene. The resulting copolymers displayed narrow polydispersity and randomly distributed monomer units. Interestingly, the catalytic activity remained unaffected at increased ratios of cocatalysts, indicating the stability of the active species. The 2,6-iPr2C6H3 substituted complex/Et3Al2Cl3 system displayed the highest activity of 1.1 × 105 g mol−1 h−1, and this catalytic system yielded ultra-high-molecular-weight ethylene–propylene copolymers (Mw = 1.612 × 106 g mol−1) in a living manner. The stabilization and long lifetime of active species in this catalytic system were attributed to the isopropyl substituent in the NHC ligand with bulky and electron-rich phenyl rings.
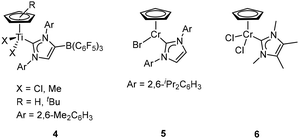
Half-titanocene NHC complexes 4 (Fig. 3, left), which contained anionic NHCs with a weakly coordinating borate B(C6F5)3 group, were synthesized and employed as pre-catalysts for ethylene polymerization. In the presence of AliBu3/[Ph3C] [B(C6F5)4] as the cocatalyst, the tBu-substituted complex 4 exhibited high catalytic activity (4.59 × 106 g mol−1 h−1) for ethylene polymerization. The polymers had high molecular weights and a unimodal molecular weight distribution. The dimethyl complex 4, when used in the copolymerization of ethylene and 1-hexene with a small amount of MAO as a cocatalyst, displayed efficient incorporation ability for 1-hexene. This complex is considered one of the most active catalysts for ethylene copolymerization with 1-hexene and showing considerable incorporation.20
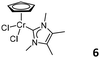
In 2000, an international patent reported the utilization of a metal NHC complex in olefin polymerization.21 The cyclopentadienyl CrII carbene complex 5 (Fig. 3, middle) was activated by the addition of MAO, and the catalytic activities of 5/MAO and the corresponding supported cases on SiO2, MgCl2/SiO2 and polystyrene/divinylbenzene were systematically studied. 5/MAO exhibited an activity of 2.9 × 104 g mol−1 bar−1 h−1, while the activity of the supported catalyst for ethylene polymerization increased to 6.7 × 104 g mol−1 bar−1 h−1. Importantly, the supported catalytic systems maintained high activity and stability for at least about 300 minutes. In the same year, Jolly and colleagues reported a CrIII carbene analogue 6 (Fig. 3, right).22 However, the catalytic activity of 6/MAO was rather low in ethylene polymerization, partially attributed to its low solubility in the polymerization medium, toluene. The resulting polymer was highly branched and melted in two steps, consistent with the low- and high-molecular-weight fractions of the polymer as indicated in GPC measurement. This suggested the presence of more than one active species during ethylene polymerization.
In theory, the soft NHC ligand is much more suitable for stabilizing late transition metals than early transition metals. Shen et al. reported the synthesis of Ni based half-sandwich indene-carbene complexes 7 (Fig. 4, left) via imidazolium salt metathesis and indene elimination. The authors revealed that the distance of the Ni–Ccarbene bond in the solid structure of 7 was shorter than that of the Ni–P bond in (Ind)-(PPh3)NiCl, indicating that the σ-donor ability of 1,3-diisopropylimidazol-2-ylidene is stronger than that of PPh3. Upon activation with modified MAO (MMAO), complex 7 exhibited moderate activity and high selectivity in catalyzing ethylene dimerization into 1-butene.23 This suggested that β-H elimination via the Ni–Bu intermediate was too fast for chain propagation. Subsequently, a series of cyclopentadienyl Ni carbene analogues 8 (Fig. 4, middle) were synthesized and their catalytic activity for styrene polymerization was tested by Pietrzykowski and co-workers.24 Upon the addition of MAO as the cocatalyst, all Ni complexes 8 efficiently catalyzed styrene polymerization (maximum TON was 4450), producing atactic polystyrenes. The study highlighted that the catalytic activity and polystyrene yield were contingent on the number and stability of cationic Ni centers following treatment with MAO.
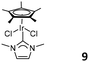
Jin and co-workers reported the pentamethylcyclopentadiene Ir NHC complex 9 (Fig. 4, right).25 However, in the presence of MAO as the cocatalyst, complex 9 only yielded trace amounts of polymer in the polymerization of norbornene.
Cámpora and coworkers reported the synthesis of single NHC ligand and the H2O/CH3CN stabilized cationic allyl-Ni complexes 10 (Fig. 5, top left) through protonating aryloxide-containing precursors.26 The 2,6-iPr2C6H3 substituted Ni complex with an H2O ligand 10 exhibited moderate catalytic activity in the polymerization of 1,3-butadiene (TOF = 400 h−1) and styrene (TOF = 323 h−1), producing poly(cis-1,4-butadiene) and atactic polystyrene. In comparison with the iPr-substituted 10, the Me-analogue displayed much lower catalytic activity in 1,3-butadiene polymerization (TOF = 19 h−1) but a relatively higher catalytic activity in the selective oligomerization of styrene (TOF = 748 h−1), yielding the head-to-tail dimer. Similarly, single NHC allyl-Pd complexes 10 were synthesized and applied in norbornene polymerization.27 After treatment with AgSbF6 and LiB(C6F5)4, the Pd complexes showed high activity in catalyzing polymerization of norbornene and polar norbornene derivatives (the TOF up to 7.5 × 105 h−1), even in water as a solvent. Subsequently, Chung and co-workers reported a series of similar NHC allyl-Pd complexes and studied their catalytic activity for the copolymerization of norbornene and ester-functionalized norbornene derivatives.28 A high catalytic activity was observed in all cases, and the SbF6− counterion-based complexes showed much higher activities than their BF4− – analogues. The polymerization of ester-functionalized norbornenes was slower than that of norbornene, and the catalytic activity was dependent on the counter anion, reaction solvent, and temperature.
As an analogue to the allyl complex, the η3-benzylnickel NHC complexes 11 (Fig. 5, top right) were also successfully prepared, and their catalytic activities for ethylene and norbornene polymerization were investigated.29 In the absence of any activators, 11 exhibited excellent catalytic activity in norbornene homopolymerization, while the highest activity was obtained in the presence of a triflate-coordinated imidazolylidene complex. The catalyst possessed considerable thermal stability, and its activity increased steadily as the temperature increased, reaching 6.5 times higher activity at 130 °C than that at 25 °C. Using a η3-substituted allyl group, the corresponding NHC Pd complexes 12 (Fig. 5, bottom left) were employed as air-stable active catalysts for the polymerization of 5-norbornene-2-methyl acetate, after activation with LiB(C6F5)4.30 The catalytic activity and stability increased with the increase of steric hindrance of the allyl substituents, and the maximum activity reached1.23 × 105 g mol−1 h−1.
Bermeshev and co-workers conducted a study on a series of NHC Pd complexes 13 (Fig. 5, bottom right), by investigating their catalytic activity in selectively catalyzing the addition polymerization of norbornene derivatives, specifically 5-methylene-2-norbornene and 5-ethylidene-2-norbornene. Upon activation with a borate, these complexes showed special selective polymerization performance, involving only endocyclic norbornene double bonds while completely retaining the exocyclic double bond.31 Similar to other allyl-Pd analogues, these catalytic systems exhibited robustness and excellent thermal stability. The smallest five-membered NHC and/or smaller steric hindrance on nitrogen atoms in NHC showed the highest activity (up to 1.4 × 108 g mol−1 h−1). Remarkably, the polymerization reactions could be conducted in air and using wet solvents with very low Pd-catalyst loadings, as low as 50 ppb. Moreover, the authors successfully achieved the vinyl-addition polymerization of polar norbornene with imide-, ether- and cellosolve groups. This resulted in the production of soluble high-molecular-weight polymers featuring high glass-transition temperatures and good thermal stability.32 The study revealed that both the pre-catalyst and cocatalyst played crucial roles in catalytic performances, and high-yield polymers were obtained when AgSbF6 and AgBF4 were used as cocatalysts. Interestingly, NHC Pd complexes in conjunction with only cocatalysts were also capable of producing high-molecular-weight polymers.
Chen and co-workers prepared redox-active naphthoquinone NHC Pd complexes 14 (Fig. 6),33 which could undergo reduction and re-oxidation by CoCp2 and [FeCp2][BAF] (BAF = tetrakis(3,5-bis(trifluoromethyl)phenyl)borate), respectively. In the presence of Na[BAF], the neutral Pd complexes served as effective catalysts in the copolymerization of norbornene, 5-norbornene-2-yl acetate and 1-chloro-1-octyne. However, the reduced analogous catalyst systems exhibited very low activity in these polymerizations. Notably, the addition of the oxidizing agent [FeCp2][BAF] partly restored the monomer conversion rate of the corresponding reduced catalyst systems, demonstrating a redox-controlled property. The electronic effect of the NHC moiety in ligand was identified as the responsible factor for this redox-controlled phenomenon.
Erker et al. synthesized a serial of bis(NHC)-ligated group 4 metal Zr halide complexes 15 (Fig. 7, left). X-ray diffraction revealed that the carbene ligands are trans-coordinated at the Zr center, and DFT calculations indicated that the NHC ligands played the role of σ-donor in these complexes. Upon activation with a large excess of MAO in toluene, all the complexes 15 exhibited moderate activity (7–7.5 × 103 g mol−1 bar−1 h−1) in ethylene polymerization, yielding linear polyethylene.34
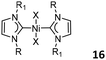
Dicarbene Ni complexes 16 (Fig. 7, middle) with alkyl N-substituents were employed to catalyze the dimerization of propylene and 1-butene in toluene or imidazolium-based ionic liquids.35 While the complexes were inactive to sparingly active in toluene, highly active species were formed in ionic liquids with good stability. The observed results were attributed to the stabilization by the imidazolium-based ionic liquid on the catalyst, as the complexes rapidly decomposed through the reducing elimination of imidazolium cations in toluene. However, a patent demonstrated that high activities of Me-N-substituted complex 16 could be obtained in hydrocarbon solvents when AlEtCl2 was used as the cocatalyst in 1-butene dimerization.36 When the N-substituents were aryl groups, the active species formed by the activation of 16 with MAO were also stable enough in toluene to catalyze the dimerization of ethylene, even though the 2,6-iPr2C6H3 substituted 16/MAO catalyst system was very sluggish.37 Braunstein and Wesolek described a series of bis-NHC Ni halide complexes 17 (Fig. 7, right) with ether- and thioether-groups. Due to the presence of two asymmetric NHC ligands, all the complexes featured syn/trans isomerization, and X-ray diffraction showed that no intra- or intermolecular interaction existed between the Ni center and the (thio)ether group.38,39 With AlEtCl2 as a cocatalyst, all the complexes were active for ethylene oligomerization, with productivities reaching up to 1.81 × 104 gC2H4 gNi−1 h−1. Due to the inherent non-coordination of pendant (thio)ether groups, the steric effect of the N-substituent or the R group attached to the ether or thioether at the NHC ligand was the key factor exerting influence on the catalytic activity.
Cui and co-workers reported the synthesis of the NHC-stabilized rare-earth phosphide Yb complex 18 (Fig. 8) through the reaction of [(THF)4Yb(PPh2)2] with three equivalents of 1,3,4,5-tetramethylimidazol-2-ylidene. Complex 18 exhibited activity in the polymerization of styrene in toluene or THF, yielding atactic polymers. Notably, the polymerization of styrene occurred even more rapidly in a solvent-free environment. When the monomers for polymerization were 1-chloro-4-vinylbenzene and 1-methoxy-4-vinylbenzene, the yields were 43% and 22% within 20 hours, respectively.40
3. Complexes with bidentate NHC ligands
3.1 Bidentate NHC ligand containing neutral functionality
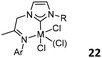
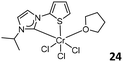
Theopold and co-workers prepared several CrIII complexes 19 (Fig. 9, left).41 Upon activation with MAO or excess Et2AlCl, these complexes catalyzed the polymerization of ethylene with low activity (1.87 × 103 g mol−1 bar−1 h−1) and produced the polymer with a broad molecular weight distribution. Detailed studies revealed that the reaction of mononuclear complexes 19 with commonly used chloride abstraction reagents generated dinuclear, chloride-bridged alkyl cations, suggesting that a binuclear polymerization mechanism could not be ruled out. The bis-carbene CrIII complexes 19 were easily reduced to the CrII analogues, which were inactive for ethylene polymerization under identical conditions. Braunstein and Danopoulos prepared phosphanyl-substituted NHC CrII/CrIII complexes 20 (Fig. 9, middle) and 21 (Fig. 9, right).42 The treatment of phosphanyl- and diphosphanyl-substituted NHC ligands with either CrII or CrIII chlorido precursors formed CrII complexes 20, which could be further transformed to the benzyl CrIII counterpart 21 by reacting with Mg(benzyl)2(THF)2. Upon activation with MAO, the CrIII complexes were active in ethylene oligomerization, displaying catalytic performances superior to those of the CrII precursors. Notably, the oligomers generated by the 2,6-iPr2C6H3 substituted CrII complex 20 were almost exclusively 1-hexene and 1-butene when the reaction was initiated at 30 °C. When AlEtCl2 was used as a cocatalyst, all the Cr complexes predominantly produced polyethylene.A patent reported imine-functionalized NHC Cr, Fe, Co, and Pd complexes 22 (Fig. 10, top left),43 which were tested for ethylene polymerization after treatment with MAO. The activities of Cr complexes were very low, and the Co complex showed activity as low as 2 × 103 g mol−1 bar−1 h−1, while the Fe complex was inactive in ethylene polymerization. The highest activity of the Pd complex was 4 × 103 g mol−1 bar−1 h−1 with a bulky 2,6-diisopropylphenyl substituent in the NHC ligand. McGuinness et al. reported the bidentate carbene-pyridine Cr complex 23 (Fig. 10, top right) and carbene-thiophene Cr complex 24 (Fig. 10, bottom left) with the aim of preparing ethylene oligomer.44 When activated with cocatalysts, 23 did not produce any ethylene oligomers but rather bits of polyethylene with low activity (TON = 710), while 24 produced 77% polyethylene and 23% soluble oligomers with a TON 1030.
Aryl-substituted imine NHC group 4 and 6 transition metal complexes 25 (Fig. 10, bottom right) were synthesized by Lavoie and co-workers. The Ti and Zr complexes were found to be active for ethylene polymerization at room temperature after the activation of MAO, with the Zr complex exhibiting higher activity and a productivity of 1.4 × 105 g mol−1 h−1.45 Analogous imine-NHC Pd complexes 25 with bulky substituents on both the imine and the NHC groups were tested for norbornene polymerization.46 After activation with MMAO, the Pd dichloride complexes and the corresponding methyl-Pd complexes exhibited low activities for the addition polymerization of norbornene. Treatment of the methyl-Pd complexes with Na[BAF] in CH3CN yielded cationic Pd complexes, which also smoothly polymerized norbornene. Moreover, the polymerization experiments demonstrated that bulky substituents on both the imine and NHC sides could suppress the reductive elimination of methyl-Pd active species.
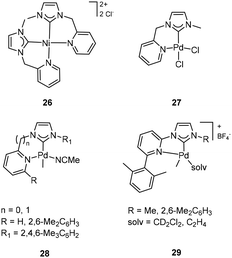
Since the pyridine motif was frequently utilized to construct a chelating ligand, various pyridine modified NHC ligands were also synthesized, and their corresponding transition metal complexes were widely studied in olefin polymerization. Jin and coworkers reported the picolyl-functionalized ligand and its corresponding Ni and Pd complexes. X-ray single-crystal analyses revealed that two NHC ligands chelate the Ni center metal ions in 26 (Fig. 11, top left), forming a square planar configuration,47 while the Pd complexes 27 (Fig. 11, top right) contain one NHC ligand.48 After activation with MAO, the Ni complex 26 catalyzed ethylene polymerization with a moderate activity of 3.3 × 105 g mol−1 h−1, producing linear polyethylene. The 26/MAO catalytic system also promoted norbornene polymerization with a high activity (TOF = 2.44 × 104 h−1).47 Similarly, the Pd complex 27 exhibited ultra-high catalytic activities (up to 7.7 × 107 g mol−1 h−1) for the addition polymerization of norbornene after treatment with MAO.48 By modifying the steric effects of the C,N-bidentate pyridine-functionalized NHC ligand, Albrecht and co-workers found that the catalytic activities of Pd complexes 28 (Fig. 11, bottom left) could be easily regulated for olefin polymerization.49 Interestingly, the flexible methylene-linked pyridine NHC Pd complex was inactive in ethylene polymerization. Compared to complex 27 with moderate catalytic activity, the bulky substituent on the NHC side was supposed to be the main reason that retarded the coordination and insertion of ethylene. When pyridine was directly linked to the NHC motif, the corresponding Pd complexes catalyzed the oligomerization of ethylene in the case of H-group and the dimerization of ethylene in the case of mesityl group on the pyridine ring, respectively. Moreover, all three complexes could catalyze the dimerization of styrene. The same authors later prepared cationic pyridine NHC Pd complexes 29 (Fig. 11, bottom right) that contained bulky substituents on the pyridine motif.50 Ethylene polymerization experiments demonstrated that the large steric substituent of the NHC motif promoted β-H elimination and gave the product as mainly dimers or oligomers.
The cis dihalido-bis-NHC Ni complex 30 (Fig. 12, left) with amine substitution was synthesized and tested for olefin polymerization.51 Both the transformation of ligand lithium and silver-NHC transmetalating methods gave complex 30 with an uncoordinated amine motif. It was inactive in ethylene polymerization but showed moderate catalytic activity (0.5–3.0 × 106 g mol−1 h−1) towards the addition polymerization of norbornene when MAO was used as the cocatalyst. The neutral NHC ligand with a pendant-NMe2 donor group and the corresponding Ni complexes 31 (Fig. 12, right) were also studied in the catalysis of ethylene oligomerization. In the presence of MAO as a cocatalyst, the complexes 31 exhibited good activity for ethylene oligomerization, with the products mainly being C4 and C6 fractions.39 The amine-chelated Ni complex was observed to possibly stabilize and increase the active species’ lifetime.
Bidentate NHC phosphine oxide and imidazo[1,5-a]pyridine-NHC phosphine oxide Pd complexes 32 (Fig. 13, left) and 33 (Fig. 13, right) were studied for ethylene polymerization and ethylene/polar monomer copolymerization by Nozaki and co-workers.52 Complexes 32 exhibited very poor catalytic activity and produced ethylene oligomers. Complexes 33 showed a catalytic activity of up to 1.52 × 105 g mol−1 h−1 for ethylene polymerization at 50 °C, despite with rapid decomposition within 1 hour. Moreover, the complexes 33 were also active in catalyzing the copolymerization of ethylene with polar monomers such as methyl acrylate, allyl acetate, and allyl chloride, albeit with low catalytic activity.
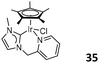
Pyridine and picolyl-functionalized pentamethylcyclopentadiene NHC Ir complexes 34 (Fig. 14, left) and 35 (Fig. 14, right) were used to catalyze the addition polymerization of norbornene.25 In the presence of cocatalyst MAO, complex 34 exhibited a higher catalytic activity of 1.7 × 106 g mol−1 h−1 and complex 35 showed moderate catalytic activity (3.03 × 105 g mol−1 h−1). Detailed studies indicated that the hemilabile coordinated pyridine nitrogen could easily cleave off to form an empty site around the central metal, which was essential for the coordination of monomers.
3.2 Bidentate NHC ligand containing anionic functionality
Theoretically, the introduction of one or more linked anionic functionalities is much more beneficial for stabilizing the metal centers than the cases of NHC carbene solely or neutral functionalities involved because the bond energies of ionic bonds are usually higher than that of coordination bonds. Indenyl and fluorenyl functionalized NHC lanthanide complexes 36 (Fig. 15, left) and 37 (Fig. 15, middle) were reported by Cui and co-workers.53–58 Complex 36 was the first example of NHC-stabilized monomeric rare-earth metal bis(alkyl) complex. Upon treatment with [Ph3C][B(C6F5)4] and AliBu3, all the Sc complexes were inactive for isoprene polymerization, while the complexes with other metal centers exhibited moderate activities, yielding highly 3,4-regular polyisoprene. Generally, the fluorenyl NHC-stabilized rare-earth metal (Y, Lu and Ho) complexes 37 showed higher 3,4-regio-selectivity in isoprene polymerization than their indenyl analogues 36 because the relatively bulkier fluorenyl ligand was more beneficial for regularizing the insertion of isoprene in a determined fashion.53,54 Moreover, the fluorenyl NHC-stabilized complexes 37 were also used in the copolymerization of ethylene with 1-hexene or 1-octene. Compared to the inactivity of Dy and Er complexes, the Sc complex showed high activity (4.12 × 106 g mol−1 h−1 atm−1) towards the copolymerization of ethylene and 1-hexene with a high comonomer insertion ratio (up to 20.2% 1-hexene insertion) after treatment with AliBu3 and [Ph3C][B(C6F5)4]. In addition, this Sc ternary system catalyzed the copolymerization of ethylene and 1-octene with a good catalytic activity (up to 3.64 × 106 g mol−1 h−1 atm−1). By changing the feeding ratio of 1-octene, the contents of the poly(1-octene) sequence in the polymers ranged from 2.1% to 38.7%.55 Also, the Sc complex 37 gave an adjustable ratio of the polystyrene sequence (16.1 mol% to 43.2 mol%) in the copolymerization of ethylene and styrene derivatives, giving the products with a pseudo-random distribution.56 Amazingly, the Sc complex 37 displayed moderate activity towards the direct copolymerization of ethylene with a range of polar and nonpolar styrenes. The N-methyl substituted fluorenyl NHC-Sc complex showed the highest copolymerization activity of 3.19 × 105 g mol−1 h−1, offering pseudo-alternating or perfect alternating copolymers regardless of the polar comonomers used.57 Meanwhile, this Sc complex also showed a high catalytic activity (1.64 × 106 g mol−1 h−1) towards the copolymerization of ethylene and 1,4-butadiene, producing ethylene/1,4-butadiene alternating copolymers with high molecular weights and trans-poly(1,4-PBD)-dominant microstructure. The vulcanized E/BD copolymer rubber had excellent physical and mechanical performance, with some properties even surpassing the commercialized natural or styrene–butadiene rubber.58Hanton and Danopoulos et al. described a family of CrIII and CrII complexes bearing analogous dimethylindenyl-functionalized NHC ligands. In particular, the CrII complexes could convert to dimeric complexes when placed in THF for an extended period of time. In the presence of MMAO, all the CrIII complexes 38 (Fig. 15, right) showed moderate activity for ethylene polymerization.59 Mechanistic studies demonstrated the cationic nature of the active species during the polymerization.
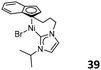
Before the development of indenyl and fluorenyl-functionalized NHC lanthanide complexes 36 and 37, Shen et al. reported the Ind-NHC ligated Ni complex 39 (Fig. 16). Their study showed that 39 was much more thermally stable than the corresponding complex bearing the indenyl and free NHC ligands. This suggested the special advantage of indenyl-functionalized NHC ligands in stabilizing the metal center. After treatment with NaBPh4, complex 39 showed excellent activity for styrene polymerization.60 More importantly, 39 also exhibited higher catalytic activity than its analogue bearing the indenyl and free NHC ligands, highlighting the role of the indenyl-functionalized NHC ligand in stabilizing the Ni-based active species during polymerization.
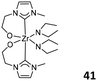
The alkoxide-functionalized NHC Zr complexes 40 (Fig. 17, top left) and 41 (Fig. 17, top right) have been reported to catalyze the polymerization of ethylene and propylene.61,62 Upon the activation of MAO, the cyclopentenyl-containing complex 40 produced linear polyethylene with a high molecular weight and broad polydispersity (Mw/Mn > 2, bimodal). The same catalytic system could also generate highly isotactic polypropylene and an atactic fraction. DFT study of the stability of the catalyst's stereoisomer indicated the presence of more than one active species during the polymerization. In the catalytic systems of cyclohexenyl-containing Zr/MAO, the activity increased steadily with an increasing molar ratio of Al/Zr from 500–3000. However, for the ethenyl alkoxide Zr complex 41, the activity reached a plateau when the molar ratio of Al/Zr was 1000. Nonetheless, the molecular weight of the generated polyethylene always increased in all cases with an increasing Al/Zr ratio, suggesting that β-H elimination had been prevented. Also, more than one active species were formed during the 41-catalyzed ethylene polymerization, similar to the case of 40. Grubbs et al. reported novel Ti and Zr complexes 42 (Fig. 17, bottom left) bearing bidentate phenoxide-functionalized NHC ligands. In the presence of MAO, the Ti complexes 42 were efficient catalysts for ethylene polymerization and its copolymerization with 1-octene and norbornene, generating high-molecular-weight products. However, the analogous Zr catalytic system only gave low-molecular-weight polymers.63 The same Ti complex was also found to serve as an efficient catalyst for the copolymerization of styrene, yielding the copolymers with highly syndiotactic polystyrene sequence.64
Buchmeiser and coworkers reported Ti complexes 43 (Fig. 17, bottom right) with mixed ligands, which contained a bidentate O-chelating NHC and aryloxo ligands.65 Due to the subtle balance of steric and electronic effects within the complex structures, Ti complexes 43 efficiently suppressed β-H elimination in the homopolymerization of ethylene and its copolymerization with cyclic olefins, generating high-molecular-weight copolymers.
A family of special enolate-NHC ligands and their ligated neutral Ni complexes 44 (Fig. 18, left) have been reported by Waymouth and co-workers. In the absence of cocatalysts, 44 catalyzed ethylene polymerization with rapid deactivation via β-H elimination to form an unstable Ni–H species, producing a linear polyethylene with low molecular weight. The neutral complexes 44 also showed activity for propylene oligomerization to give low-molecular-weight atactic oligomers.66 In a subsequent study, similar enolate-NHC allyl complexes 45 (Fig. 18, right) were prepared and tested for ethylene polymerization.67 Different from the neutral Ni analogues 44, the complexes 45 were completely inactive without cocatalysts, and the nitrobenzene-substituted complex showed low activity for ethylene polymerization with the activation of ZnEt2, producing highly linear but low-molecular-weight polymers. It should be noticed that the nitrobenzene-substituted 45/ZnEt2 catalytic system had a much longer lifetime than complex 44 for ethylene polymerization, indicating the good stability of the active species during 45-catalyzed polymerization.
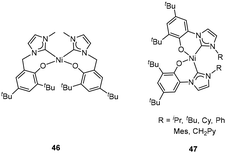
Zhang et al. reported an aryloxy-substituted NHC ligand, and the corresponding Ni complex 46 (Fig. 19, left) showed low activity (105 g mol−1 h−1) towards the addition polymerization of norbornene with MAO as a cocatalyst. Compared to its phosphine analogues, 46 was much less sensitive to air and moisture due to the NHC's strong donor properties.68 Wang and Yan et al. prepared several air- and moisture-stable Ni complexes 47 (Fig. 19, right) bearing o-hydroxyaryl NHC ligands.69–71 X-ray diffraction studies revealed two aryloxide-NHC ligands located in a cis arrangement around the Ni ion. After treatment with MAO, these Ni complexes exhibited moderate to high activities (up to 14.5 × 106 g mol−1 h−1) in the addition polymerization of norbornene, which were much more active than 46 (105 g mol−1 h−1) with a flexible ligand framework. Interestingly, the steric effect of N-substituents at the NHCs slightly influenced the catalytic activities of 47. When R = -CH2Py, complex 47 showed a low activity in norbornene polymerization because of its poor thermal stability on activation with either Et2AlCl or Me2AlCl.
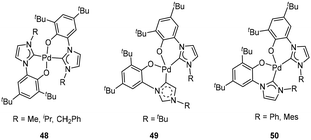
Later, the corresponding bis(aryloxide-NHC) Pd complexes were synthesized by Wang et al. When R = Me, iPr and CH2Ph, trans isomers 48 (Fig. 20, left) were obtained while cis isomers 50 (Fig. 20, right) were isolated for R = Ph and mesityl. But for R = tBu, a cis isomer 49 (Fig. 20, middle) with a normal and an abnormal NHC ligand was obtained.72,73 When MAO was used as the cocatalyst, these bis(aryloxide-NHC) Pd complexes (48, 49 and 50) exhibited excellent catalytic activities (up to 49.97 × 106 g mol−1 h−1) in the addition polymerization of norbornene. Like the case of Ni complexes 47, the N-substituents at the NHCs and the configurations of the complexes 48, 49 and 50 had slight influence on the activity.
By changing the Pd reactants, Wang et al. synthesized and isolated the mono(aryloxide-NHC) palladacycle complexes (51, 52, 53, Fig. 21) successfully.74 Similar to its bis(aryloxide-NHC) analogue 49, complex 52 also bonded to an abnormal NHC ligand when the N-functional group on the NHC was tertbutyl. The N-chelated and o-aryloxide NHC ligated Pd complexes 51 showed excellent catalytic activities in the addition polymerization of norbornene with MAO as the cocatalyst, and the highest activity was 4.95 × 107 g mol−1 h−1. Noticeably, complex 52 (2.72 × 107 g mol−1 h−1) also exhibited high catalytic activity for norbornene polymerization upon the activation of MAO. Meanwhile, the N-chelated C(sp2)–Pd NHC complex 53 displayed slightly lower catalytic activity than 51 under the same polymerization conditions, perhaps because of the higher reactivity of C(sp3)–Pd bond compared to the C(sp2)–Pd bond.
Wang and co-workers further synthesized the analogous σ,π-cycloalkenyl Pd complexes 54–56 (Fig. 22).75 Note that complexes 56 were generated with a free hydroxyl group of the phenol when the N-functional group on the NHC was bulky tert-butyl, by using the similar procedures as for complexes 54 and 55. Although complexes 54 and 55 had similar structures with neutral single-component catalysts, however, the complexes had no catalytic activity for the polymerization of norbornene in the absence of cocatalysts. By treatment with MAO, complexes 54 and 55 showed excellent catalytic activities (the highest was 4.9 × 107 g mol−1 h−1) for norbornene polymerization. Meanwhile, the 56/MAO system also displayed comparable catalytic activity (3.2 × 107 g mol−1 h−1), and it was supposed that the active species might be a “Pd” cation without the o-aryloxide-substituted NHC ligand.
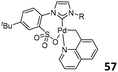
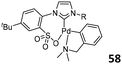
The power of phosphine-sulfonate ligated Ni/Pd complexes for ethylene copolymerization stimulated Wang and coworkers to prepare a family of analogous NHC-arenesulfonate ligands and their corresponding Pd complexes. In the presence of MAO, the N-chelated C(sp3), NHC-sulfonate palladacycles 57 (Fig. 23, left) showed higher catalytic activities (up to 5.84 × 107 g mol−1 h−1) than the corresponding C(sp2) complex 58 (up to 2.97 × 106 g mol−1 h−1; Fig. 23, middle) in the addition polymerization of norbornene. Complex 58 possessed high thermal stability at 100 °C, and the activities increased with temperature increase.76 The lutidine-coordinated analogues 59 (Fig. 23, right) were then prepared, and after treatment with MAO or [Ph3C][B(C6F5)4], all the complexes 59 showed high catalytic activities in norbornene polymerization (up to 1.01 × 108 g mol−1 h−1).77 For complexes 57–59, the steric hindrance effect of N-substituents of NHC ligands had a slight influence on the catalytic activities.
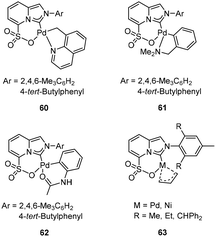
Another kind of NHC-arenesulfonate ligands, namely imidazo[1,5-a]pyridine-sulfonate, were prepared, and their palladacycle complexes 60–62 (Fig. 24) served as excellent catalyst precursors for norbornene polymerization.78 In the presence of MAO or Et2AlCl as a cocatalyst, palladacycle complexes 60–62 exhibited high activities (up to 6.0 × 107 g mol−1 h−1) towards norbornene polymerization. High thermal stability of 60–62/MAO was observed, and the catalytic activity reached the maximum at 100 °C. Various aspects, such as the palladacycle structure, the coordination atom type and the substituent of the ligand, significantly affected the polymerization activity. Later, Wang and coworkers reported the allyl Ni and Pd complexes 63 (Fig. 24, bottom right) chelating similar imidazo[1,5-a]pyridine-sulfonate ligands.79 In the presence of Me2AlCl or MAO, the Ni and Pd complexes displayed high activities (4.5 × 107 g mol−1 h−1) and high thermal stability in norbornene polymerization. Moreover, the Pd complexes could also catalyze the copolymerization of norbornene with butyl vinyl ether (BVE), 10-undecenoate (UA), and methyl acrylate (MA) with high insertion content of polar monomers. However, the corresponding Ni catalysts did not catalyze the copolymerization of norbornene and ester monomers efficiently.
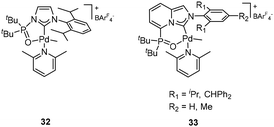
By a rational design to suppress β-H elimination, Nozaki et al. prepared novel tethered NHC ligands (imidazo[1,5-a]quinolin-9-olate-1-ylidene (IzQO)) and their corresponding Pd complexes 64 (Fig. 25, left), which were successfully applied as catalyst precursors for olefin polymerization and olefin/polar monomer copolymerization.80 This study proved that the rigid skeleton and coplanar configuration of the NHC plane with the metal plane were essential for Pd/IzQO complex 64 as efficient catalysts to produce polymers with considerable catalyst durability. The complexes 64 could promote the homopolymerization of ethylene, propylene, and 1-butene, giving high-molecular-weight linear polyethylene, and moderate-molecular-weight polypropylene and poly(1-butene), respectively. The applied high polymerization temperature (80–120 °C) proved the high thermal stability of 64 as polymerization catalysts. Also, 64 successfully realized the copolymerization of ethylene or propylene with polar monomers, such as allyl carboxylate copolymers and methyl acrylate, and so on.
As powerful catalysts, 64 were further utilized to polymerize diverse monomers. Using 64 as catalysts, Nozaki et al. successfully copolymerized ethylene with polar-group-functional 1,1-disubstituted ethylenes, yielding the copolymers in which polar units were non-sequentially incorporated into the main chain for the first time. An analysis of the microstructure of resultant copolymers revealed the poly(methyl methacrylate) (PMMA) sequences mainly in the 1,2-insertion mode and the insensitivity towards steric hindrance of the Pd /IzQO system. Detailed investigation suggested that copolymerization indeed proceeded via a coordination–insertion mechanism.81,82 Nozaki and co-workers further studied the activity of Pd/IzQO complexes 64 in the copolymerization of ethylene with 3-ethylidene-6-vinyltetrahydro-2H-pyran-2-one (EVP), which acted as a CO2/butadiene-derived lactone.83 The coordination/insertion copolymerization of ethylene and EVP proceeded slowly, generating divergent unsaturated lactone-functionalized polyethylenes in the main chain with a considerable EVP incorporation ratio (2.0 mol%) and moderate copolymer molecular weight (Mn = 6000). Michael addition or aminolysis were successfully accomplished to further modify the resultant copolymers. This study provided a novel method to access various polyethylene materials made from ethylene, CO2, and 1,3-butadiene. The complex 64 could also catalyze the copolymerization of ethylene with methyl 5-norbornene-2-carboxylate, giving a copolymer with a 7.9 mol% incorporation ratio of methyl 5-norbornene-2-carboxylate (endo-/exo-mixture). Similar catalytic performance of 64 in the copolymerization of propylene with polar norbornenes was observed.84
Due to the success of Pd/IzQO complexes in olefin copolymerization and the urgent requirement for replacing precious metals in catalysis with readily available metals, the IzQO-ligated Ni complexes 65 (Fig. 25, middle) were successfully synthesized and applied for ethylene polymerization at 50–100 °C without a cocatalyst.85 Addition of Ni(cod)2 was beneficial for increasing the catalytic activity of 65 and the molecular weight of resultant polyethylenes. The copolymerization of ethylene and allyl acetate could also be realized in the catalysis of 65 and produced a copolymer with a low molecular weight, which was increased after the addition of Ni(cod)2 in the catalytic system. It is worth noting that the Ni complexes 65 exhibited relatively lower catalytic activity than their corresponding Pd/IzQO complexes in ethylene/allyl acetate copolymerization under similar conditions. Bulkier substituents on the N atom were highly beneficial for increasing the molecular weight of ethylene/allyl acetate copolymers, but the insertion rate of the polar monomer decreased significantly.
Abnormal imidazo-[1,5-a]pyridine(aImPy)-based NHC-phenolate ligands with a tuneable electronic environment and a rigid tricyclic framework were synthesized, and their Pd complexes 66 (Fig. 25, right) showed good efficiency in catalyzing the homopolymerization of ethylene/propylene and copolymerization of ethylene/polar monomers.86 High-molecular-weight (Mn = 2.37 × 105 g mol−1) linear polyethylene and regio-defect-free moderate-molecular-weights polypropylene were produced. Good tolerance to polar groups enabled these catalytic systems to copolymerize ethylene and polar monomers with good activity, resulting in high incorporation ratios (up to 3.0%) of polar monomers. In addition, MMA was also successfully incorporated into the main chain of polyethylene. DFT calculation studies indicated that the energy of ethylene insertion into a Pd–alkyl bond by aImPy-Pd catalyst 66 was lower than that by a Pd catalyst 64 bearing the corresponding normal NHC ligand.
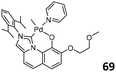
The saturated tetrahydroimidazo[1,5-a]quinolin-9-olate-1-ylidene (SIzQO) NHC-phenolate Pd complexes 67 (Fig. 26, left) and 68 (Fig. 26, middle) were also prepared and applied in the polymerization of ethylene.87 They catalyzed the polymerization of ethylene in the range of 100–120 °C with activities of 3.6–5.3 × 103 g mol−1 h−1. Compared with previously reported Pd/IzQO complexes 64, 68 had catalytic activities lower by more than 100 times in ethylene polymerization. To tune the catalytic property, Nozaki and co-worker synthesized the Pd/IzQO complex 69 (Fig. 26, right) bearing oligoethylene glycol moieties bound to the quinolinolate part.88 Comparing with the reaction without the salt, the addition of LiBArF4 increased the activity of polymerization and decreased the molecular weight with a slight increase in MA incorporation. This was attributed to the fact that the addition of Lewis acid decreased the electron donation ability of the oxygen atom towards Pd ion. Also, the addition of Lewis acid accelerated the chain transfer reaction during polymerization. Notably, the counteranion of lithium also exerted an obvious influence on the catalyst performance: LiB(C6F5)4 showed a similar polymerization result to LiBArF4, while the LiPF6 and LiSbF6 suppressed the MA incorporation.
Nozaki and co-workers also prepared the NHC-sulfonamide Pd complexes 70 (Fig. 27) and tested their catalytic activities in ethylene oligomerization and ethylene/polar monomer copolymerization.89 Complex 70 with a bulky 2,4,6-Me3C6H2-substituent exhibited high activity for ethylene oligomerization up to 1.27 × 105 g mol−1 h−1. After the induction period of oligomerization, the catalyst slowly deactivated during the subsequent oligomerization process. The copolymerization of ethylene and MA catalyzed by 70 also gave a co-oligomer, which was shown to have the telechelic microstructure of the resultant polymer with an MA-moiety at the chain ends.
4. Complexes with tridentate NHC ligands
4.1 Tridentate NHC ligand containing neutral functionality
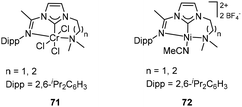
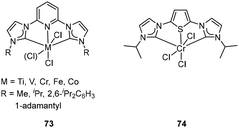
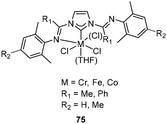
Tridentate NHC pincer ligands with mixed N-imine and N′-amine substituents have been synthesized, and their corresponding Cr/Ni complexes 71 (Fig. 28, left) and 72 (Fig. 28, right) served as efficient catalysts for ethylene oligomerization by the activation of suitable cocatalysts.90 The C2 linked Cr complex 71 in ethylene oligomerization showed moderately activity after treatment with MAO, and the longer C3 linked complex 71 showed higher productivity (up to 9.2 × 103 gC2H4 gNi−1 h−1) and a very high selectivity for α-olefins from C4 to C8. Moreover, the analogous Ni complexes 72 showed a productivity of up to 1.46 × 104 gC2H4 gNi−1 h−1, and the generated oligomer consisted of 65% butenes and 30% hexenes.Bis(carbene)pyridine transition metal complexes 73 (Fig. 29, left) have been studied in ethylene oligomerization and polymerization by Gibson and co-workers.91,92 After treatment with MAO, the 2,6-iPr2C6H3 substituted CrIII complexes 73 showed a high activity (4.044 × 107 g mol−1 bar−1 h−1) for ethylene oligomerization, generating α-olefins as the main product with the 80% to 93% selectivity. Increased steric bulk of ligand increased the K value (K = kprop/(kprop + kch transfer) = mol of Cn+2/mol of Cn) of the resultant oligomer.91 In combination with MAO, bis(carbene)pyridine Ti and V complexes gave rise to highly active ethylene polymerization catalysts, and the V complexes (up to 1.446 × 106 g mol−1 bar−1 h−1) exhibited relatively higher catalytic activity than Ti analogues (up to 7.91 × 105 g mol−1 bar−1 h−1). Both Ti and V catalytic systems produced high-molecular-weight polyethylene with no significant branching. The bis(carbene)pyridine FeII, FeIII and Co complexes were also prepared and tested for ethylene oligomerization/polymerization. However, all these complexes were inactive when treated with MAO. The study revealed that the Fe and Co complexes might decompose via reductive elimination of an alkylimidazolium cation by MAO. This suggested that the bis(carbene)pyridine ligand was less suitable for Fe and Co olefin polymerization catalysts compared to the earlier transition-metal analogues.92 The ethylene oligomerization mechanism with bis(carbene)pyridine Cr complexes has been investigated and suggested that this system produced α-olefins via an extended metallacycle mechanism. When the metallacycle mechanism was applied in a bis(carbene)thiophene Cr complex 74/MAO (Fig. 29, right) catalytic system, no catalytic activity for ethylene oligomerization was observed even at ethylene pressures up to 30 bar.44 Bis(carbene)pyridine Cr complexes 73 also efficiently catalyzed α-olefin oligomerization after activation by MAO, leading mainly to head-to-tail dimers with vinylidene unsaturation. First-order in Cr and zero-order in 1-octene concentration were found in the kinetics investigation of 1-octene dimerization, and the most likely possibility involving metallacycles mechanism was also determined.93
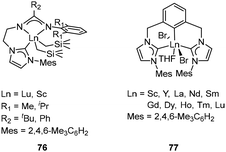
Aryl-substituted imine NHC ligands that could serve as tridentate chelating ligands and their corresponding 1,3-bis(imino)-NHC Cr, Fe, Co complexes 75 (Fig. 30) were prepared by Lavoie and co-workers. X-ray diffraction and NMR spectra showed that the 1,3-bis(imino)-NHC ligands coordinate the central metal ions via a bidentate mode through the Ccarbene and one of the two Niminic atoms in these complexes. After treatment with MAO, the Cr complexes showed moderate activities for ethylene polymerization, giving a linear polymer. However, neither the Fe nor the Co complexes were active for ethylene polymerization. The decrease of electron-donating or the increase of π-accepting ability on the ligand would increase the catalytic activity of the Cr complexes in olefin polymerization.94
4.2 Tridentate NHC ligand containing monoanionic functionality
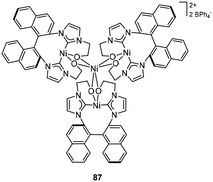
Cui et al. successfully synthesized the amidino-functionalized NHC ligated lanthanide bis(alkyl) complex 76 (Fig. 31, left). In the presence of an organoborate, the Lu complex showed excellent catalytic activity for isoprene polymerization, producing 3,4-regioregular (99.3%) polymer with livingness. Additionally, the Lu-polyisoprene active species could trigger the polar ε-caprolactone ring-opening polymerization, yielding poly(3,4-isoprene)-b-polycaprolactone diblock copolymers with steerable molecular weight (ranging from 4.9 × 104 to 10.2 × 104) and narrow polydispersity.95 Activated by [Ph3C][B(C6F5)4], complexes 76 also catalyzed the polymerization of 2-phenyl-1,3-butadiene with high activities and 3,4-selectivities (96.9%). The resulting polymers represented novel plastic polydienes with high glass-transition temperatures. Both polymerization temperature and solvent polarity impacted the 3,4-selectivity, which decreased accordingly with decreasing solvent polarity and increasing polymerization temperature.96The CCC-pincer 2,6-xylenyl bis(carbene)-ligated lanthanide dibromides 77 (Fig. 31, right) were obtained via dropwise addition of nBuLi to a solution of imidazolium dibromide pro-ligands and LnCl3.97,98 In the presence of AlR3 (R = Me, Et, iBu) and [Ph3C][B(C6F5)4], Y, Nd, Gd, Dy, and Ho complexes catalyzed isoprene polymerization with high activity and cis-1,4 selectivity (99.6%, 25 °C), while Sc, Sm, La, Tm, and Lu complexes were inactive. Notably, these catalytic systems exhibited high thermostability, catalytic activity and high cis-1,4 selectivity (97.4%) even at 80 °C. In situ NMR spectra suggested that the Y hydrido aluminate cation was probably the active species for isoprene polymerization.
The Ni complex 78 (Fig. 32, top left), chelating a salicylaldimine-functionalized tridentate NHC ligand, was successfully synthesized using strategies involving both metal transformation reaction and nickelocene elimination reaction. X-ray analysis revealed a twisted square-planar geometric structure for the complex.99 Following the treatment with NaBPh4 at 80 °C, the system exhibited good activity in styrene polymerization. Wang and co-workers reported tridentate phenoxide-functionalized NHC Ni/Pd complexes 79 (Fig. 32, top right) and 80 (Fig. 32, bottom left).70,73,100 Upon treatment with MAO or Et2AlCl, these Pd complexes displayed excellent catalytic activities (up to 5.99 × 107 g mol−1 h−1) in the addition polymerization of norbornene. Notably, the tridentate Pd complexes 79 and 80 exhibited higher activities compared to the bidentate bis(aryloxide-NHC) Pd analogue 50 in the presence of MAO. Interestingly, polymers produced by 79 or 80/Et2AlCl were soluble in CHCl3, and the polymerization initiated by these Pd complexes followed a vinyl-type addition mechanism.73 The corresponding Ni complexes 79 and 80 were further investigated for norbornene polymerization and copolymerization of norbornene with 1-octene upon activation with suitable cocatalysts.70 When activated with either Me2AlCl or Et2AlCl, these NHC Ni complexes exhibited low activities in norbornene polymerization. However, after treatment with B(C6F5)3, these Ni complexes showed excellent activities in the homopolymerization of norbornene (up to 3.21 × 106 g mol−1 h−1) and its copolymerization with 1-octene at 100 °C, achieving high 1-octene insertion ratios (9%–17%).
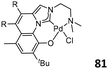
Following the strategy of appending a dimethylaminoethyl sidearm, similar tridentate Pd/IzQO complexes 81 (Fig. 32, bottom right) were developed. These complexes exhibited high activities (up to 2.74 × 108 g mol−1 h−1) in the homo- and copolymerization of norbornene with n-butyl vinyl ether (BVE) and methyl 10-undecanoate (UA) in the presence of Me2AlCl.100 The rigid annulated backbone increased the activities of 81 compared to their non-annulated analogues 79, and 81 also showed high thermal stability (up to 140 °C) during polymerization. The electron-withdrawing groups on the annulated backbone positively impacted the copolymerization activity, and the produced copolymers possessed moderate molecular weight, high comonomer incorporation, and good thermal stability.
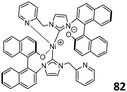
Treatment of the corresponding tridentate imidazolium ligand with Ni acetate resulted in the formation of complex 82 (Fig. 33). X-ray diffraction analysis revealed that Ni2+ is four-coordinated, with one O and one N atom far away from the metal center on two ligands.101 Chiral NHC–Ni complexes 82 showed good activities for norbornene polymerization (106 g mol−1 h−1) upon treatment with MAO. The activity received an increase with increasing the Al/Ni ratios (ranging from 1500–2500) and polymerization temperatures (20–80 °C).
4.3 Tridentate NHC ligand containing dianionic functionality
Kawaguchi and colleagues reported bis(aryloxido)-functionalized NHC Ti complexes 83 (Fig. 34, top left), and X-ray diffraction revealed that the tridentate ligand coordinates to the metal center with two mutually cis chlorine atoms. In the presence of MMAO, 83 showed activity as high as 2.9 × 105 g mol−1 bar−1 h−1 for the polymerization of ethylene.102 Meanwhile, the activity of analogous Ti complexes reached up to 9.7 × 104 g mol−1 bar−1 h−1 for ethylene polymerization upon activation with MAO, producing linear polymers with low molecular weight.103 Aminolysis reactions of the ligand with M(NMe2)4 (M = Zr, Hf) and subsequent alkyl transformation gave rise to complexes 84 (Fig. 34, top right), as reported by Fryzuk and co-workers. Activation of 84 with [Ph3C][B(C6F5)4] yielded cationic complexes that exhibited slight activity for 1-hexene polymerization. However, the cationic Zr complex showed moderate catalytic activity for ethylene polymerization.104 | ||
| Fig. 34 Bis(aryloxido)- and bis(arylamine)-functionalized NHC titanium, zirconium and hafnium complexes. | ||
The protonolysis reaction between bisphenol-functionalized tridentate NHC complexes 85 (Fig. 34, bottom left) and [HNMe2Ph][B(C6F5)4] led to quantitative formation of corresponding anilinium benzyl cations. These cations were active in 1-hexene polymerization. In contrast to the low activity of the Hf-based system, the Zr-based 85 system exhibited moderate activity for 1-hexene oligomerization, generating highly regio-regular poly(1-hexene) containing mainly trimer with vinylene end groups.105 The highly regioselective 2,1-insertion of 1-hexene process was attributed to the coordinated NMe2Ph group, which enhanced the steric hindrance at the Zr center. A similar bisphenol-functionalized tridentate benzimidazolylidene NHC Zr complex 86 (Fig. 34, bottom right) was also reported.106 After the addition of [Ph3C][B(C6F5)4], 86 served as an active catalyst for α-olefin transformations that could be “tuned” from oligomerization to polymerization or inactivity by adding appropriate ligand. In this study, ligand binding seemed to increase catalytic activity, which was attributed to the inherently essential role of ligand in accelerating the initiation of 1-hexene polymerization.
5. Complexes with tetradentate NHC ligands
Zi and Song et al. reported the tetradentate chiral bis-imidazolium ligand precursor and its corresponding complex 87 (Fig. 35) by treating the ligand precursor with 1.0 equiv. of Ni(OAc)2 in the presence of K2CO3 and NaBPh4.101 Upon the activation of MAO, 87 was proved to be an efficient catalyst for norbornene polymerization with good activity under mild conditions, producing polymers with high molecular weight and broad dispersity. It was speculated that the steric hindrance around the metal center and the solid bonding between the NHC ligands and Ni atom hampered the generation of active species.6. Conclusions
While numerous moderately to highly active NHC-based metal catalysts have been developed for the polymerization of olefins in recent decades, they continue to be considered as a promising and interesting topic in catalytic olefin polymerization. This review summarizes such a category of metal catalysts with various NHC ligands and central metals across the periodic table to date. The structure–activity relationships during the polymerization are discussed in detail for all catalytic systems, mainly focusing on the steric and electronic effects of ligands and the inherence of central metals. In general, NHC-ligated late-transition-metal complexes exhibit robust and thermostable characteristics, showcasing impressive catalytic activities in the polymerization of olefins and their copolymerization with polar monomers. In particular, the unprecedented success of NHC-based metal catalysts, in which the NHC-plane is structurally coplanar to the metal plane, can give valuable hints to design more efficient catalysts for olefin polymerization as well as other catalytic applications. It is foreseeable that, in the future, feasible synthetic methodologies and novel NHC ligands will be introduced to prepare well-defined metal complexes, especially those involving early transition metals, with attractive catalytic properties. Exploiting new efficient metal catalysts ligating novel rationally designed NHC ligands for olefin polymerization apparently represents one of the most important directions worth moving. NHC-ligated early-transition metal complexes with high stability during the polymerization are highly expected, and their success in producing polymers with designable microstructures (molecular weight, molecular weight distribution and tacticity and so on) is also a difficult issue that needs to be perfectly resolved. On the other hand, undoubtedly, the copolymerization of ethylene or propylene and polar monomers catalysed by, especially, newly designed NHC-ligated late-transition metal complexes with satisfactory catalytic performances is another hot and continuous issue in this field. The review also covers polymer properties, such as molecular weight, regulation of polymer chain branching, comonomer incorporation (polar and non-polar), tacticity and so on, along with structural illustration of the catalysts. Apparently, the copolymerization of different monomers will provide new polymers, and thus, reasonable design and synthesis of new monomers that are polymerized by the NHC-ligated metal catalytic systems represent another anticipated strategy and valuable direction to prepare new useful polymers. Novel polymeric materials with special microstructures and physical properties will be undoubtedly prepared by utilizing new NHC-based catalysts in the future to satisfy the applications in the fields of manufacturing, construction and so on.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Fund for Distinguished Young Scholars of China (No. 22025107), the Shaanxi Fundamental Science Research Project for Chemistry & Biology (No. 22JHZ003), the National Youth Top-notch Talent Support Program of China, the Key International Scientific and Technological Cooperation and Exchange Project of Shaanxi Province (No. 2023-GHZD-15), Xi'an Key Laboratory of Functional Supramolecular Structure and Materials, and the FM&EM International Joint Laboratory of Northwest University.References
- M. Stürzel, S. Mihan and R. Mülhaupt, From multisite polymerization catalysis to sustainable materials and all-polyolefin composites, Chem. Rev., 2016, 116, 1398–1433 CrossRef PubMed.
- S. Kumar, B. Z. Dholakiya and R. Jangir, Role of organometallic complexes in olefin polymerization: a review report, J. Organomet. Chem., 2021, 953, 122066 CrossRef CAS.
- E. Y.-X. Chen and T. J. Marks, Cocatalysts for metal-catalyzed olefin polymerization: activators, activation processes, and structure-activity relationships, Chem. Rev., 2000, 100, 1391–1434 CrossRef CAS PubMed.
- V. C. Gibson and S. K. Spitzmesser, Advances in non-metallocene olefin polymerization catalysis, Chem. Rev., 2003, 103, 283–316 CrossRef CAS PubMed.
- A. J. Arduengo III, R. L. Harlow and M. Kline, A stable crystalline carbene, J. Am. Chem. Soc., 1991, 113, 361–363 CrossRef.
- D. J. Nelson and S. P. Nolan, Quantifying and understanding the electronic properties of N-heterocyclic carbenes, Chem. Soc. Rev., 2013, 42, 6723–6753 RSC.
- S. Kuwata and F. E. Hahn, Complexes bearing protic N-heterocyclic carbene ligands, Chem. Rev., 2018, 118, 9642–9677 CrossRef CAS PubMed.
- C. Samojłowicz, M. Bieniek and K. Grela, Ruthenium-based olefin metathesis catalysts bearing N-heterocyclic carbene ligands, polymers, Chem. Rev., 2009, 109, 3708–3742 CrossRef PubMed.
- D. McGuinness, Alkene oligomerisation and polymerisation with metal-NHC based catalysts, Dalton Trans., 2009, 6915–6923 RSC.
- M.-M. Gan, J.-Q. Liu, L. Zhang, Y.-Y. Wang, F. E. Hahn and Y.-F. Han, Preparation and post-assembly modification of metallosupramolecular assemblies from poly(N-heterocyclic carbene) ligands, Chem. Rev., 2018, 118, 9587–9641 CrossRef CAS PubMed.
- E. Peris, Smart N-heterocyclic carbene ligands in catalysis, Chem. Rev., 2018, 118, 9988–10031 CrossRef CAS PubMed.
- S. C. Sau, P. K. Hota, S. K. Mandal, M. Soleilhavoup and G. Bertrand, Stable abnormal N-heterocyclic carbenes and their applications, Chem. Soc. Rev., 2020, 49, 1233–1252 RSC.
- T. P. Schlachta and F. E. Kühn, Cyclic iron tetra N-heterocyclic carbenes: synthesis, properties, reactivity, and catalysis, Chem. Soc. Rev., 2023, 52, 2238–2277 RSC.
- Y. Pan, T. Xu, Y.-S. Ge and X.-B. Lu, N-heterocyclic carbene scandium complexes: synthesis, structure, and catalytic performance for α-olefin polymerization and copolymerization with 1,5-hexadiene, Organometallics, 2011, 30, 5687–5694 CrossRef CAS.
- C. Song, J. Chen, Z. Fu, L. Yan, F. Gao, Q. Cao, H. Li, X. Yan, S. Chen, S. Zhang and X. Li, Syndiospecific polymerization of o-methoxystyrene and its silyloxy or fluorine-substituted derivatives by HNC-ligated scandium catalysts: synthesis of ultrahigh-molecular-weight functionalized polymers, Macromolecules, 2021, 54, 10838–10849 CrossRef CAS.
- Z.-X. Liu and T.-Q. Xu, Crystalline sulfur-functionalized poly(α-olefin) synthesized by scandium-catalyzed coordination polymerization, Polym. Chem., 2022, 13, 2477–2483 RSC.
- Y. Pan, A. Zhao, Y. Li, W. Li, Y.-M. So, X. Yan and G. He, Bis(oxazoline)-derived N-heterocyclic carbene ligated rare-earth metal complexes: synthesis, structure, and polymerization performance, Dalton Trans., 2018, 47, 13815–13823 RSC.
- S. Zhang, W.-C. Zhang, D.-D. Shang, Z.-Q. Zhang and Y.-X. Wu, Ethylene/propylene copolymerization catalyzed by vanadium complexes containing N-heterocyclic carbenes, Dalton Trans., 2015, 44, 15264–15270 RSC.
- S. Zhang, W.-C. Zhang, D.-D. Shang and Y.-X. Wu, Synthesis of ultra-high-molecular-weight ethylene-propylene copolymer via quasi-living copolymerization with N-heterocyclic carbene ligated vanadium complexes, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 553–561 CrossRef CAS.
- K. Nomura, G. Nagai, A. Nasr, K. Tsutsumi, Y. Kawamoto, K. Koide and M. Tamm, Synthesis of half-titanocenes containing anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety, Cp'TiX2 (WCA-NHC), and their use as catalysts for ethyle (co)polymerization, Organometallics, 2019, 38, 3233–3244 CrossRef CAS.
- K.-J. Jens, M. Tilset, M. H. Voges, R. Blom and M. Froseth, Catalyst for the polymerization of olefins, WO 00/01739, 2000 (Borealis).
- A. Döhring, J. Göhre, P. W. Jolly, B. Kryger, J. Rust and G. P. J. Verhovnik, Donor-ligand-substituted cyclopentadienylchromium(III) complexes: a new class of alkene polymerization catalyst. 1. amino-substituted systems, Organometallics, 2000, 19, 388–402 CrossRef.
- H. M. Sun, Q. Shao, D. M. Hu, W. F. Li, Q. Shen and Y. Zhang, Indenylnickel(II) N-heterocyclic carbene complexes: synthesis via indene elimination and catalytic activity for ethylene dimerization, Organometallics, 2005, 24, 331–334 CrossRef CAS.
- W. Buchowicz, A. Kozioł, L. B. Jerzykiewicz, T. Lis, S. Pasynkiewicz, A. Pęcherzewska and A. Pietrzykowski, N-Heterocyclic, carbene complexes of cyclopentadienylnickel(II): synthesis, structure and catalytic activity in styrene polymerization, J. Mol. Catal. A: Chem., 2006, 257, 118–123 CrossRef CAS.
- X.-Q. Xiao and G.-X. Jin, Functionalized N-heterocyclic carbene iridium complexes: synthesis, structure and addition polymerization of norbornene, J. Organomet. Chem., 2008, 693, 3363–3368 CrossRef CAS.
- J. Cámpora, L. O. de la Tabla, P. Palma, E. Álvarez, F. Lahoz and K. Mereiter, Synthesis and catalytic activity of cationic allyl complexes of nickel stabilized by a single N-heterocyclic carbene ligand, Organometallics, 2006, 25, 3314–3316 CrossRef.
- B. L. Goodall and L. H. McIntosh, Catalyst complexes for polymerization and co-polymerization of cyclic olefins, US 7041758, 2006 (Rohm and Haas).
- I. G. Jung, J. Seo, Y. K. Chung, D. M. Shin, S.-H. Chun and S. U. Son, Polymerization of carboxylic ester functionalized norbornenes catalyzed by (η3-allyl)palladium complexes bearing N-heterocyclic carbene ligands, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3042–3052 CrossRef CAS.
- S. Sujith, E. K. Noh, B. Y. Lee and J. W. Han, Synthesis, characterization, and norbornene polymerization of η3-benzylnickel(II) complexes of N-heterocyclic carbenes, J. Organomet. Chem., 2008, 693, 2171–2176 CrossRef CAS.
- I. G. Jung, Y. T. Lee, S. Y. Choi, D. S. Choi, Y. K. Kang and Y. K. Chung, Polymerization of 5-norbornene-2-methyl acetate catalyzed by air-stable cationic (η3-substituted allyl) palladium complexes of N-heterocyclic carbene, J. Organomet. Chem., 2009, 694, 297–303 CrossRef CAS.
- E. V. Bermesheva, A. I. Wozniak, F. A. Andreyanov, G. O. Karpov, M. S. Nechaev, A. F. Asachenko, M. A. Topchiy, E. K. Melnikova, Y. V. Nelyubina, P. S. Gribanov and M. V. Bermeshev, Polymerization of 5-Alkylidene-2-norbornenes with highly active Pd-N-heterocyclic carbene complex catalysts: catalyst structure-activity relationships, ACS Catal., 2020, 10, 1663–1678 CrossRef CAS.
- E. V. Bermesheva, I. V. Nazarov, K. D. Kataranova, A. P. Khrychikova, D. P. Zarezin, E. K. Melnikova, A. F. Asachenko, M. A. Topchiy, S. A. Rzhevskiy and M. V. Bermeshev, Cocatalyst versus precatalyst impact on the vinyl-addition polymerization of norbornenes with polar groups: looking at the other side of the coin, Polym. Chem., 2021, 12, 6355–6362 RSC.
- W. Zou, W. Pang and C. Chen, Redox control in palladium catalyzed norbornene and alkyne polymerization, Inorg. Chem. Front., 2017, 4, 795–800 RSC.
- M. Niehues, G. Kehr, G. Erker, B. Wibbeling, R. Fröhlich, O. Blacque and H. Berke, Structural characterization of group 4 transition metal halide bis-Arduengo carbene complexes MCl4L2: X-ray crystal structure analyses and DFT calculations, J. Organomet. Chem., 2002, 663, 192–203 CrossRef CAS.
- D. S. McGuinness, W. Mueller, P. Wasserscheid, K. J. Cavell, B. W. Skelton, A. H. White and U. Englert, Nickel(II) heterocyclic carbene complexes as catalysts for olefin dimerization in an imidazolium chloroaluminate ionic liquid, Organometallics, 2002, 21, 175–181 CrossRef CAS.
- H. Olivier-Bourbigou, D. Commereuc and S. Harry, Catalytic composition and process for the catalysis of dimerization, codimerization and oligomerization of olefins, US 01/0047121, 2001 (Institut Francais du Petrole).
- A. L. MacKinnon and M. C. Baird, The synthesis and X-ray structure of trans-NiCl2(1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene)2; attempts to polymerize olefins utilizing a nickel(II) complex of a sterically demanding N-heterocyclic carbene, J. Organomet. Chem., 2003, 683, 114–119 CrossRef CAS.
- S. Hameury, P. de Frémont, P.-A. R. Breuil, H. Olivier-Bourbigou and P. Braunstein, Bis(ether-functionalized NHC) nickel(II) complexes, trans to cis isomerization triggered by water coordination, and catalytic ethylene oligomerization, Organometallics, 2015, 34, 2183–2201 CrossRef CAS.
- X. Ren, M. Wesolek, C. Bailly, L. Karmazin and P. Braunstein, Silver(I) and nickel(II) complexes with oxygen- or nitrogen- functionalized NHC ditopic ligands and catalytic ethylene oligomerization, Eur. J. Inorg. Chem., 2020, 2020, 1073–1087 CrossRef CAS.
- J. Yuan, H. Hu and C. Cui, N-Heterocyclic, carbene-ytterbium amide as a recyclable homogeneous precatalyst for hydrophosphination of alkenes and alkynes, Chem. – Eur. J., 2016, 22, 5778–5785 CrossRef CAS PubMed.
- K. A. Kreisel, G. P. A. Yap and K. H. Theopold, A chelating N-heterocyclic carbene ligand in organochromium chemistry, Organometallics, 2006, 25, 4670–4679 CrossRef CAS.
- P. Ai, A. A. Danopoulos and P. Braunstein, N-Phosphanyl- and, N,N’-diphosphanyl-substituted N- heterocyclic carbene chromium complexes: synthesis, structures, and catalytic ethylene oligomerization, Organometallics, 2015, 34, 4109–4116 CrossRef CAS.
- M. Tilset, O. Andell, A. Dhindsa and M. Froseth, Compounds, WO 02/49758, 2002 (Borealis).
- D. S. McGuinness, J. A. Suttil, M. G. Gardiner and N. W. Davies, Ethylene oligomerization with Cr-NHC catalysts: further insights into the extended metallacycle mechanism of chain growth, Organometallics, 2008, 27, 4238–4247 CrossRef CAS.
- T. G. Larocque, A. C. Badaj, S. Dastgir and G. G. Lavoie, New stable aryl-substituted acyclic imino-N-heterocyclic carbene: synthesis, characterisation and coordination to early transition metals, Dalton Trans., 2011, 40, 12705–12712 RSC.
- J. Deng, H. Gao, F. Zhu and Q. Wu, Synthesis and structure of imine-N-heterocyclic carbene palladium complexes and their catalytic behavior in norbornene polymerization, Organometallics, 2013, 32, 4507–4515 CrossRef CAS.
- X. Wang, S. Liu and G.-X. Jin, Preparation, structure, and olefin polymerization behavior of functionalized nickel(II) N-heterocyclic carbene complexes, Organometallics, 2004, 23, 6002–6007 CrossRef CAS.
- X. Wang, S. Liu, L.-H. Weng and G.-X. Jin, A trinuclear silver(I) functionalized N-heterocyclic carbene complex and its use in transmetalation: structure and catalytic activity for olefin polymerization, Organometallics, 2006, 25, 3565–3569 CrossRef CAS.
- V. Khlebnikov, A. Meduri, H. Mueller-Bunz, T. Montini, P. Fornasiero, E. Zangrando, B. Milani and M. Albrecht, Palladium carbene complexes for selective alkene di- and oligomerization, Organometallics, 2012, 31, 976–986 CrossRef CAS.
- V. Khlebnikov, A. Meduri, H. Mueller-Bunz, B. Milani and M. Albrecht, Synthesis of a sterically modulated pyridine-NHC palladium complex and its reactivity towards ethylene, New J. Chem., 2012, 36, 1552–1555 RSC.
- D. Zhang, S. Zhou, Z. Li, Q. Wang and L. Weng, Direct synthesis of cis-dihalido-bis(NHC) complex of nickel(II) and catalytic application in olefin addition polymerization: Effect of halogen co-ligands and density functional theory study, Dalton Trans., 2013, 42, 12020–12030 RSC.
- W. Tao, S. Akita, R. Nakano, S. Ito, Y. Hoshimoto, S. Ogoshi and K. Nozaki, Copolymerisation of ethylene with polar monomers by using palladium catalysts bearing an N-heterocyclic carbene-phosphine oxide bidentate ligand, Chem. Commun., 2017, 53, 2630–2633 RSC.
- B. Wang, D. Wang, D. Cui, W. Gao, T. Tang, X. Chen and X. Jing, Synthesis of the first rare earth metal bis(alkyl)s bearing an indenyl functionalized N-heterocyclic carbene, Organometallics, 2007, 26, 3167–3172 CrossRef CAS.
- B. Wang, D. Cui and K. Lv, Highly 3,4-selective living polymerization of isoprene with rare earth metal fluorenyl N-heterocyclic carbene precursors, Macromolecules, 2008, 41, 1983–1988 CrossRef CAS.
- C. Yao, C. Wu, B. Wang and D. Cui, Copolymerization of ethylene with 1-hexene and 1-octene catalyzed by fluorenyl N-heterocyclic carbene ligated rare-earth metal precursors, Organometallics, 2013, 32, 2204–2209 CrossRef CAS.
- S. Li, M. Wang and D. Cui, Copolymerization of ethylene with styrene catalyzed by a scandium catalyst, Polym. Chem., 2018, 9, 4757–4763 RSC.
- S. Li, D. Liu, Z. Wang and D. Cui, Development of group 3 catalysts for alternating copolymerization of ethylene and styrene derivatives, ACS Catal., 2018, 8, 6086–6093 CrossRef CAS.
- K. Zhang, Y. Dou, Y. Jiang, Z. Zhang, S. Li and D. Cui, High-performance elastomer from trans-1,4 copolymerization of ethylene and butadiene, Macromolecules, 2021, 54, 9445–9451 CrossRef CAS.
- S. Conde-Guadano, A. A. Danopoulos, R. Pattacini, M. Hanton and R. P. Tooze, Indenyl functionalized N-heterocyclic carbene complexes of chromium: syntheses, structures, and reactivity studies relevant to ethylene oligomerization and polymerization, Organometallics, 2012, 31, 1643–1652 CrossRef CAS.
- H.-M. Sun, D.-M. Hu, Y.-S. Wang, Q. Shen and Y. Zhang, Synthesis and characterization of indenyl-functionalized N-heterocyclic carbene complex of Ni(II), J. Organomet. Chem., 2007, 692, 903–907 CrossRef CAS.
- C. Bocchino, M. Napoli, C. Costabile and P. Longo, Synthesis of octahedral zirconium complex bearing [NHC-O] ligands, and its behavior as catalyst in the polymerization of olefins, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 862–870 CrossRef CAS.
- C. Costabile, C. Bocchino, M. Napoli and P. Longo, Group 4 complexes bearing alkoxide functionalized N-heterocyclic carbene ligands as catalysts in the polymerization of olefins, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3728–3735 CrossRef CAS.
- A. El-Batta, A. W. Waltman and R. H. Grubbs, Bis-ligated Ti and Zr complexes of chelating N-heterocyclic carbenes, J. Organomet. Chem., 2011, 696, 2477–2481 CrossRef CAS.
- G. M. Miyake, M. N. Akhtar, A. Fazal, E. A. Jaseer, C. S. Daeffler and R. H. Grubbs, Chelating N-heterocyclic carbene group IV complexes for the polymerization of ethylene and styrene, J. Organomet. Chem., 2013, 728, 1–5 CrossRef CAS.
- M. van der Ende, P. M. Hauser, C. Lienert, D. Wang, W. Frey and M. R. Buchmeise, Ti(IV) Complexes with bidentate O-chelating N-heterocyclic carbenes for use in the homopolymerization of ethylene and its copolymerization with cyclic olefins, ChemCatChem, 2019, 11, 744–752 CrossRef CAS.
- B. E. Ketz, X. G. Ottenwaelder and R. M. Waymouth, Synthesis, structure, and olefin polymerization with nickel(II) N-heterocyclic carbene enolates, Chem. Commun., 2005, 5693–5695 RSC.
- S. Benson, B. Payne and R. M. Waymouth, Synthesis and reactivity of allyl nickel(II) N-heterocyclic carbene enolate complexes, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3637–3647 CrossRef CAS.
- D. Zhang and H. Kawaguchi, Deprotonation attempts on imidazolium salt tethered by substituted phenol and construction of its magnesium complex by transmetalation, Organometallics, 2006, 25, 5506–5509 CrossRef CAS.
- L. Li, Y. Feng, W. Yan, H. Ren and B. Wang, Polymerization of norbornene via nickel-based complexes bearing o-hydroxyaryl N-heterocyclic carbenes [C, O] bidentate ligands/MAO, Acta Polym. Sin., 2008, 6, 581–586 CrossRef.
- D. Yang, J. Dong and B. Wang, Homo- and copolymerization of norbornene with tridentate nickel complexes bearing o-aryloxide-N-heterocyclic carbene ligands, Dalton Trans., 2018, 47, 180–189 RSC.
- Y. Kong, M. Cheng, H. Ren, S. Xu, H. Song, M. Yang, B. Liu and B. Wang, Synthesis, structures, and norbornene polymerization behavior of bis(aryloxide-N-heterocyclic carbene) nickel complexes, Organometallics, 2011, 30, 1677–1681 CrossRef CAS.
- Y. Kong, H. Ren, S. Xu, H. Song, B. Liu and B. Wang, Synthesis, structures, and norbornene polymerization behavior of bis(aryloxide-N-heterocyclic carbene) palladium complexes, Organometallics, 2009, 28, 5934–5940 CrossRef CAS.
- D. Yang, Y. Tang, H. Song and B. Wang, Synthesis, structures, and norbornene polymerization behavior of palladium complexes bearing tridentate o-aryloxide-N-heterocyclic carbene ligands, Organometallics, 2016, 35, 1392–1398 CrossRef CAS.
- L. Dang, J. Guo, H. Song, B. Liu and B. Wang, Synthesis, structures, and norbornene polymerization behavior of C(sp3), N-chelated palladacycles bearing o-aryloxide-N-heterocyclic carbene ligands, Dalton Trans., 2014, 43, 17177–17183 RSC.
- L. Dang, H. Song and B. Wang, Synthesis, structures, and norbornene polymerization behavior of o-aryloxide-substituted NHC-ligated σ, π-cycloalkenyl palladium complexes, Organometallics, 2014, 33, 6812–6818 CrossRef CAS.
- M. Li, H. Song and B. Wang, Synthesis, structures, and norbornene polymerization behavior of N-heterocyclic carbene-sulfonate-ligated palladacycles, Organometallics, 2015, 34, 1969–1977 CrossRef CAS.
- M. Li, H. Song and B. Wang, Synthesis, structures, and norbornene polymerization behavior of palladium methyl complexes bearing N-heterocyclic carbene-sulfonate ligands, J. Organomet. Chem., 2016, 804, 118–122 CrossRef CAS.
- J. Dong, M. Li and B. Wang, Synthesis, structures, and norbornene polymerization behavior of imidazo[1,5-a] pyridine-sulfonate-ligated palladacycles, Organometallics, 2019, 38, 3786–3795 CrossRef CAS.
- J. Dong, D. Yang and B. Wang, Homo- and copolymerization of norbornene with allyl palladium and nickel complexes bearing imidazo[1,5-a] pyridine sulfonate ligands, Eur. J. Inorg. Chem., 2021, 2021, 4661–4668 CrossRef CAS.
- R. Nakano and K. Nozaki, Copolymerization of propylene and polar monomers using Pd/IzQO catalysts, J. Am. Chem. Soc., 2015, 137, 10934–10937 CrossRef CAS PubMed.
- H. Yasuda, R. Nakano, S. Ito and K. Nozaki, Palladium/IzQO-catalyzed coordination-insertion copolymerization of ethylene and 1,1-disubstituted ethylenes bearing a polar functional group, J. Am. Chem. Soc., 2018, 140, 1876–1883 CrossRef CAS PubMed.
- K. Nozaki, Copolymerization of ethylene with non-vinyl polar monomers, Proc. Jpn. Acad., Ser. B, 2022, 98, 222–226 CrossRef CAS PubMed.
- S. Tang, Y. Zhao and K. Nozaki, Accessing divergent main-chain-functionalized polyethylenes via copolymerization of ethylene with a CO2/butadiene-derived lactone, J. Am. Chem. Soc., 2021, 143, 17953–17957 CrossRef CAS PubMed.
- K. Tsuge, K. Lau, Y. Hirooka, T. Iwasaki, K. Yokomizo and K. Nozaki, Palladium-catalyzed copolymerization of ethylene or propylene with norbornene carboxylic acids and their esters, Polymer, 2023, 281, 126116 CrossRef CAS.
- W.-J. Tao, R. Nakano, S. Ito and K. Nozaki, Copolymerization of ethylene and polar monomers by using Ni/IzQO catalysts, Angew. Chem., Int. Ed., 2016, 55, 2835–2839 CrossRef CAS PubMed.
- D.-A. Park, S. Byun, J. Y. Ryu, J. Lee, J. Lee and S. Hong, Abnormal N-heterocyclic carbene-palladium complexes for the copolymerization of ethylene and polar monomers, ACS Catal., 2020, 10, 5443–5453 CrossRef CAS.
- S. Akita, R. Nakano, S. Ito and K. Nozaki, Synthesis and reactivity of methylpalladium complexes bearing a partially saturated IzQO ligand, Organometallics, 2018, 37, 2286–2296 CrossRef CAS.
- S. Akita and K. Nozaki, Copolymerization of ethylene and methyl acrylate by palladium catalysts bearing IzQO ligands containing methoxyethyl ether moieties and salt effects for polymerization, Polym. J., 2021, 53, 1057–1060 CrossRef CAS.
- W. Tao, X. Wang, S. Ito and K. Nozaki, Palladium complexes bearing an N-heterocyclic carbene-sulfonamide ligand for cooligomerization of ethylene and polar monomers, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 474–477 CrossRef CAS.
- X. Ren, M. Wesolek and P. Braunstein, Cu(I), Ag(I), Ni(II), Cr(III) and Ir(I) complexes with tritopic NimineCNHCNamine pincer ligands and catalytic ethylene oligomerization, Dalton Trans., 2019, 48, 12895–12909 RSC.
- D. S. McGuinness, V. C. Gibson, D. F. Wass and J. W. Steed, Bis(carbene) pyridine complexes of Cr(III): exceptionally active catalysts for the oligomerization of ethylene, J. Am. Chem. Soc., 2003, 125, 12716–12717 CrossRef CAS PubMed.
- D. S. McGuinness, V. C. Gibson and J. W. Steed, Bis(carbene)pyridine complexes of the early to middle transition metals: survey of ethylene oligomerization and polymerization capability, Organometallics, 2004, 23, 6288–6292 CrossRef CAS.
- D. S. McGuinness, Oligomerization of α-olefins via chromium metallacycles, Organometallics, 2009, 28, 244–248 CrossRef CAS.
- J. A. Thagfi and G. G. Lavoie, Synthesis, characterization, and ethylene polymerization studies of chromium, iron, and cobalt complexes containing 1,3-bis(imino)-N-heterocyclic carbene ligands, Organometallics, 2012, 31, 2463–2469 CrossRef.
- C. Yao, D. Liu, P. Li, C. Wu, S. Li, B. Liu and D. Cui, Highly 3,4-selective living polymerization of isoprene and copolymerization with ε-caprolactone by an amidino N-heterocyclic carbene ligated lutetium bis(alkyl) complex, Organometallics, 2014, 33, 684–691 CrossRef CAS.
- C. Yao, H. Xie and D. Cui, Highly 3,4-selective living polymerization of 2-phenyl-1,3-butadiene with amidino N-heterocyclic carbene ligated rare-earth metal bis(alkyl) complexes, RSC Adv., 2015, 5, 93507–93512 RSC.
- K. Lv and D. Cui, Tridentate CCC-pincer bis(carbene)-ligated rare-earth metal dibromides. synthesis and characterization, Organometallics, 2008, 27, 5438–5440 CrossRef CAS.
- K. Lv and D. Cui, CCC-Pincer bis(carbene) lanthanide dibromides. Catalysis on highly cis-1,4-selective polymerization of isoprene and active species, Organometallics, 2010, 29, 2987–2993 CrossRef CAS.
- W. Li, H. Sun, M. Chen, Z. Wang, D. Hu, Q. Shen and Y. Zhang, Synthesis of salicylaldiminato-functionalized N-heterocyclic carbene complex of nickel(II) and its catalytic activity for styrene polymerization, Organometallics, 2005, 24, 5925–5928 CrossRef CAS.
- J. Dong and B. Wang, Homo- and copolymerization of norbornene using tridentate IzQO palladium catalysts with dimethylaminoethyl as a side arm, Polym. Chem., 2021, 12, 4736–4747 RSC.
- H. Song, D. Fan, Y. Liu, G. Hou and G. Zi, Synthesis, structure, and catalytic activity of nickel complexes with new chiral binaphthyl-based NHC-ligands, J. Organomet. Chem., 2013, 729, 40–45 CrossRef CAS.
- H. Aihara, T. Matsuo and H. Kawaguchi, Titanium N-heterocyclic carbene complexes incorporating an imidazolium-linked bis(phenol), Chem. Commun., 2003, 2204–2205 RSC.
- D. Zhang and N. Liu, Titanium complexes bearing bisaryloxy-N-heterocyclic carbenes: synthesis, reactivity, and ethylene polymerization study, Organometallics, 2009, 28, 499–505 CrossRef CAS.
- L. P. Spencer and M. D. Fryzuk, Synthesis and reactivity of zirconium and hafnium complexes incorporating chelating diamido-N-heterocyclic-carbene ligands, J. Organomet. Chem., 2005, 690, 5788–5803 CrossRef CAS.
- S. Dagorne, S. Bellemin-Laponnaz and C. Romain, Neutral and cationic N-heterocyclic carbene zirconium and hafnium benzyl complexes: highly regioselective oligomerization of 1-hexene with a preference for trimer formation, Organometallics, 2013, 32, 2736–2743 CrossRef CAS.
- E. Despagnet-Ayoub, M. K. Takase, L. M. Henling, J. A. Labinger and J. E. Bercaw, Mechanistic insights on the controlled switch from oligomerization to polymerization of 1-hexene catalyzed by an NHC-zirconium complex, Organometallics, 2015, 34, 4707–4716 CrossRef CAS.
| This journal is © the Partner Organisations 2024 |