Open Access Article
Open Access ArticleBionanocomposite materials for electroanalytical applications: current status and future challenges
Gullit Deffo
 *ab,
Ranil Clément Tonleu Temgoua
*ab,
Ranil Clément Tonleu Temgoua
 a,
Evangéline Njanja
a and
Panchanan Puzari
a,
Evangéline Njanja
a and
Panchanan Puzari
 b
b
aDepartment of Chemistry, Electrochemistry and Chemistry of Materials, Faculty of Science, University of Dschang, P. O. Box 67, Dschang, Cameroon. E-mail: gullitdeffo@gmail.com
bDepartment of Chemical Sciences, Tezpur University, Tezpur, Assam 784028, India
First published on 19th August 2024
Abstract
Bionanocomposites are materials composed of particles with at least one dimension in the range of 1–100 nm and a constituent of biological origin or biopolymers. They are the subject of current research interest as they provide exciting platforms and act as an interface between materials science, biology, and nanotechnology and find applications in disciplines such as electrochemistry, biomedicine, biosorption, aerospace, tissue engineering and packaging. They have different properties such as high conductivity, thermal stability, electrocatalytic ability, biocompatibility, adsorption ability and biodegradability, which can be tuned by their preparation methods, functionalities and applications. However, depending on the objective or the goal of a research project, specific preparation and characterization of bionanocomposites can be undertaken to understand the behavior and confirm the applicability of a bionanocomposite in a given field. Like in electroanalysis applications, electrode materials should be porous (meso- and macro-porosities), having large specific area (at least having a Brunauer–Emmett–Teller surface of 200 m2 g−1), higher stability over time with acceptable power recovery between 95% and 105%, good electrocatalytic ability, and be a good absorbent and a good conductor of electricity (that is to say, it facilitates the transfer of electrons from the solution to the surface of the electrode and vice versa). The present review focuses on the most used method of preparation of bionanocomposites with the critical aspect and their physicochemical and electrochemical characterization techniques, and finally, the practical situations of application of bionanocomposite materials as modified electrodes for electroanalysis of several groups of analytes and a comparison with non-bionanocomposite electrodes are discussed. The future scope of bionanocomposites in the field of electroanalysis is also addressed in this review. But before that, a general overview of bionanocomposite materials in relation to other types of materials is presented to avoid any misunderstanding.
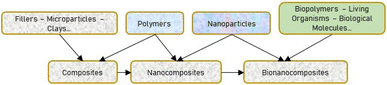
1. Introduction: general information on bionanocomposites
The continuous search for eco-friendly materials that can be used in interdisciplinary fields such as biomedicine, electrochemistry, tissue engineering and packaging has led to the extensive development and use of bionanocomposites by researchers nowadays. Bionanocomposites, where bio stands for biological/biodegradable or biopolymers, nano stands for the small size range (1–100 nm), and composite stands for mixture/assembly of materials, were developed for the first time to design nanocomposites based on layered double hydroxides (LDHs) assembled with alginate and other biopolymers.1,2 Also known as nanobiocomposites, or green composites, bionanocomposites can then be defined as materials that comprise particles with at least one dimension at the nanometric scale (1–100 nm) and a constituent of polymers coming from natural resources (biopolymers) or synthetic biofunctional polymers and inorganic/organic solids.3 Living organisms or biological molecules can also be included in the composition of bionanocomposites, known here as biosensors, which are materials containing enzymes or antibodies used to detect the presence of chemicals.4,5 Moreover, several similar materials have been developed and misleadingly referred to as bionanocomposites. Therefore, the differences between bionanocomposites and other materials have been recapitulated,2 as shown in Fig. 1.From an environmental and industrial point of view, the interest in developing conductive bionanocomposite materials for various applications has gained considerable attention. Their wide application in biomedicine, analytical sciences, agriculture practices, tissue engineering, (bio)sensing, packaging materials, soil conditioning, drug delivery, and water decontaminants, thanks to their unique structure, functionality, conductivity, process ability, responsivity, biocompatibility and biodegradability, make bionanocomposites be a new interdisciplinary field.6 In order to limit the use of non-biodegradable and petroleum-based polymer materials, bionanocomposites consisting of biopolymers such as polylactide, chitosan, chitin, guar-gums, gelatin, proteins, cellulose, etc. and conducting polymers such as polyaniline, polypyrrole, polythiophene along with some fillers including clays, hydroxyapatite, and metal nanoparticles are developed.2,7,8 Bionanocomposites exhibit different properties such as thermal stability, water solubility, biocompatibility and biodegradability, which can be tuned by their preparation methods, functionalities and applications. The majority of bionanocomposites are being prepared for applications in the field of ceramic glasses, energy storage, biosensors, biocatalysts, and disease diagnostics, especially in radiology.9 Several reviews on bionanocomposites have been published previously,9–11 providing exhaustive details on the types of bionanocomposites and their applications in bioplastics, food packaging, and biomedicines. Many other recent and interesting review articles are given dealing with the synthesis and practical applications of biopolymers, bionanocomposites based on clay and lignocellulosic materials.12–15 However, although many electroanalytical research papers are found in the literature survey, there is no review that gives details, with specific examples, of methods for preparing bionanocomposites, characterization and applications in the field of electrochemistry, especially for electroanalysis. Therefore, this review focuses on the preparation and characterization of bionanocomposites used in the field of (bio)sensing. Bionanocomposites can be prepared using different ways with several different characterization methods for application in many fields like biomedical,16 food packaging,17 electrochemical immunosensors,18 and energy storage.19 The novel insight provided by this review is the current status report made on types of bionanocomposites, and the selected method of their preparation and characterization for application in the field of electroanalysis as the only selected field. In this review article, the general procedures for the preparation of bionanocomposites and the physicochemical and electrochemical characterization techniques commonly used are described, and typical examples are given. Before giving some examples of the application of bionanocomposites in electroanalysis, a brief introduction to the electrochemical methods is given. The application section will then focus on the electroanalysis of environmental pollutants (xenobiotics) such as endocrine disruptors (hydroquinone), constituents of pathological samples (glucose, ascorbic acid, dopamine, and uric acid), food additives (cholesterol oleate), pesticides (diuron), heavy metals (Pb2+ ions), and pharmaceutical compounds (diclofenac). An up-to-date critical discussion and comparison is provided at each step for better understanding by any reader and a future scope of bionanocomposites in electroanalysis is also provided.
2. Overview of the preparation and characterization methods of bionanocomposites
Bionanocomposites, which stand for biopolymer-based nanocomposites (abundant, renewable and biodegradable), are increasingly being used to replace petroleum-derived plastics, which are non-renewable, and have a wide range of applications in biomedicine, electronics, environment, membrane separation, sensors and packaging.20 Polymers that are increasingly being used in the bionanocomposite technology to replace non-biodegradable polymers include natural collagen, hydroxyapatite, cellulose, gelatin, chitosan, protein, poly(hydroxyalkanoates), pectin, lignin, and synthetic polyglycolic acid, polyvinyl alcohol, poly(lactic acid), poly(lactic-co-glycolic acids), poly(ε-caprolactone), polyaniline, and polypyrrole.2,21,22 Their preparation, characterization, and applications represent a new interdisciplinary field closely related to important topics such as the chemistry of biodegradable polymers, biomineralization processes, bioinspired materials, and biomimetic systems. Knowing how to prepare them is, therefore, a very important task, as it is also important to characterize them in order to understand their behavior, which is crucial for assessing their effectiveness for any application. For this purpose, several preparation and characterization methods commonly used in the field of electroanalysis have been developed. A corresponding critical discussion, comparison, advantages and disadvantages of these techniques are also presented in Sections 2.1–2.3.2.1 Preparation of bionanocomposites
Bionanocomposites used for electroanalysis are generally prepared by solution coating, in situ polymerization, sol–gel processes, self-assembly, layer-by-layer (LbL) assembling, and melt processing.1,2Solution coating is the simplest and most widely used method for preparing bionanocomposites on the surface of electrodes since it follows the principle of drop or spin coating (the two ways of modifying an electrode). Two methods can be used to prepare a bionanocomposite by solution coating, that is, by direct mixing of individual materials and sonication to form a composite, or by entrapment of one material inside the other, that is, fabrication of nanoparticles inside the polymer solution. For the first procedure, a practical situation is described.23 A bionanocomposite nano-CaCO3/PANI/rGO is obtained by evaporating the solvent after dispersing a mixture of 2.0 mg of nano-CaCO3, 2.0 mg of polyaniline (PANi), and 2.0 mg of reduced graphene oxide in 1 mL of gelatin biopolymer (5%). The practical example of the second procedure consisted of in situ reduction of Cu2+ ions in the PANi solution containing DMF and NaBH4 to form copper nanoparticle entrapped PANi (CuNPs/PANi). Then, aqueous graphene solution is added to the ethanolic solution of CuNPs/PANi followed by sonication to form the bionanocomposite CuNPs/PANi/graphene.24 In these two procedures, both researcher teams have synthesized polyaniline (PANi) according to the known method reported,25 which was used as a matrix for the conductivity of the resulting bionanocomposite. Although the first method is faster, the second seems to be more applicable to electroanalysis because the stability and uniformity of the nanoparticles present here are better when they are entrapped in situ than when they are simply combined to form a composite as it is in the first method. The advantages of the solution coating method are certainly the simplicity of the procedure and the cheap equipment used, and the product obtained retains the original properties of the individual parts. Its main disadvantage, as with other methods, is the increase in complexity and the coating process introduces defects or flaws during preparation and deposition.
The in situ polymerization technique involves the dispersion of nanometer-sized inorganic particles in the monomer followed by polymerization using a simple bulk or solution method in the presence of a small amount of catalyst. This can be done mechanically or electrochemically. Mechanically, for example, Tan et al. reported a bionanocomposite based on multi-walled carbon nanotubes (MWCNTs), polydopamine (PDA), laccase (Lac), and glucose oxide (GOx) for glucose sensing.26 For the preparation of these bionanocomposites, a mixture of 1 mg of MWCNTs in acetate buffer, 20 mM of dopamine (DA), 1 mg mL−1 of Lac, and 1 mg mL−1 of GOx was ultrasonicated to allow the polymerization of the DA monomer in the composite. The resulting bionanocomposite PDA-GOx-Lac-MWCNTs was drop-coated on the surface of bare platinum and used for the sensing of glucose and hydroquinone. Instead of mechanical polymerization, electro-polymerization can also be used as in the work proposed by Deffo et al. (2022).27 This process consisted of immersing a modified glassy carbon electrode with organoclay (matrix in which the polymer will be embedded) in a monomer solution of Alizarin Red S, followed by electropolymerization (25 multiple cycles between the potential windows −1.0 V to 1.2 V at a scan rate of 50 mV s−1), and the bionanocomposite Sa(DODAB)/poly(ARS) obtained was used for the sensing of the herbicide diuron. The advantage of using mechanical polymerization is that the in situ polymer bionanocomposite can be obtained in higher quantities for other purposes such as electronics and packaging, while electropolymerization obtains thin films in small quantities. Moreover, for electroanalysis application, the in situ polymerization for bionanocomposite synthesis seems to be better with the electrochemical method compared to the mechanical method, because electropolymerization leads to controlled film thickness formation with higher stability and uniformity. With the mechanical method, after the synthesis, the amount of dispersion dropping onto the electrode with the micropipette may not be the same for a given volume and some cracking may occur on the surface of the film as it dries. In general, the advantages of in situ polymerization include the use of cost-effective materials, ease of automation, and the ability to integrate with many other heating and curing methods. However, some disadvantages of this preparation method include the limited availability of usable materials and the need for expensive equipment.28
The sol–gel method is a conventional and industrial method for the synthesis of nanoparticles with different chemical compositions. The preparation of a homogeneous sol from the precursors and its conversion into a gel is the basis of the method. A cholesterol sensor based on multi-walled carbon nanotubes/sol–gel-derived silica/chitosan bionanocomposites has been proposed.29 The synthesis involved first preparing a chitosan (CHIT) solution in 5% acetic acid, and then the sol–gel CHIT-SiO2 was prepared by mixing 2 mL of tetraethylorthosilicate (TEOS) and 2 mL of the chitosan solution in the presence of ethanol/water. A dispersion of 5 mg/2 mL of solution of MWCNT/ethanol was added to the mixture and stirred vigorously until the SiO2 was uniformly distributed in the aqueous solution. The hydrolysis reaction occurs with the formation of the opaque and black sol known as SiO2-CHIT/MWCNT, which was then coated on an Indium-Tin-Oxide (ITO) glass plate (0.25 cm2) using a spin coating technique (3000 rpm for 30 s) and dried at room temperature (25 °C). Further treatment was required before the resulting electrode could be used for cholesterol sensing. The resulting product could also be deposited on the surface of the electrode by drop coating. This process has the advantage of producing a uniform nanostructure of the product at low temperatures with high purity, porous structure, high flexibility and good narrow particle size distribution. However this process is inherently complex and time-consuming.30
Self-assembly is also a well-used method for the preparation of bionanocomposites as solution coatings. It is based on the dispersion of nanoparticles, biopolymers, and inorganic materials, which are then assembled into more complex structures, such as clusters and networks, with the possibility of enhancing the composite properties. Li et al. reported the preparation of magnetic polymeric bionanocomposites (MPBNCs) by one-pot self-assembly by stirring a mixture of laccase (Lac) with Fe3O4 magnetic core–shell and dopamine (DA).31 After stirring, to complete the formation of the polymer of dopamine (PDA), the PDA-Lac-Fe3O4 MPBNCs were separated from the rest of the solution using a bar magnet. This prepared bionanocomposite was used for the biosensing of hydroquinone.31 Similarly, the development of bionanocomposites based on mesoporous Au films by using stable block copolymer micelles as templates for the electroanalytical application was well developed by the group of Yamauchi.32–34 The mesoporous gold electrodes (MPGEs) were prepared by electrodeposition in the presence of the self-assembled polymeric micelles containing gold(III) (Au3+) ions as an electrolyte on a sputtered gold electrode (SGE) (Au sputtered on a silicon substrate), as shown in Scheme 1. This method allows the fabrication of materials with structural features on the length scale of several nanometers, not only in two dimensions but also in three dimensions.35
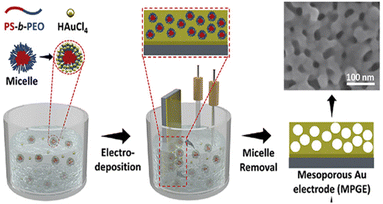 | ||
| Scheme 1 Schematic representation of the preparation of the mesoporous Au electrode (MPGE) by electrodeposition of gold(III)-containing polymeric (block) micelles. The polymeric micelles are formed by dissolving the polystyrene-b-poly(ethylene oxide)) (PS-b-PEO) di-block copolymer in THF, followed by the addition of ethanol and aqueous gold(III) chloride solution. This scheme has been reproduced from ref. 34 with permission from Elsevier B.V., copyright 2020. | ||
In the case of layer-by-layer (LbL) assembly, different layers of components able to establish strong interactions among themselves are deposited alternately with good control of their respective thicknesses. In the work reported by Zhang et al., a method is developed for the renewal of layer-by-layer (LbL) self-assembled inhibition-based enzyme interfaces in multi-walled carbon nanotube (MWCNT) acetylcholinesterase (AChE) biosensors.36 The LbL fabrication consisted of alternating cushion layers of positively charged CNT-polyethylenimine (CNT-PEI) and negatively charged CNT-deoxyribonucleic acid (CNT-DNA) at the surface of a glassy carbon electrode (GCE) used for acetyl thiocholine biosensing. The LbL assembly was also used to elaborate a biosensor based on MWCNTs, palm oil fiber biopolymer, ferrocene, and uricase.37 Thus, after the synthesis of functionalized multi-walled carbon nanotubes (FMWCNTs) and grafting on the surface of palm oil fiber (POF) to form a matrix (POF-FMWCNT) in which the enzyme uricase (UOx) was immobilized by covalent linkage, the ferrocene (Fc) redox mediator was added and the resulting bionanocomposite electrode was used for the biosensing of uric acid in the presence of ferri/ferro cyanide as a redox mediator as shown in Scheme 2. Moreover, the advantage of the Yamauchi method is that the nanoparticles are deposited on the surface of the electrode by electrodeposition, making the method to be more controlled and well stable like molecular imprinted polymers for selective detection. In general, the assembly methods have advantages of the low cost of the equipment used rather than expensive machinery or specialized equipment used for other methods, and it is scalable and flexible. However the biggest challenge is the technical difficulties and complexities involved because it relies on natural interactions between components that can sometimes be difficult to control or predict the outcome.38
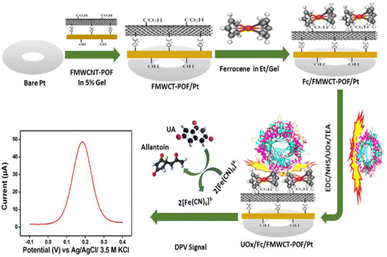 | ||
| Scheme 2 Schematic of the fabrication process of the UOx/Fc/FMWCNT-POF biosensor. This scheme has been reproduced from ref. 37 with permission from The Royal Society of Chemistry, copyright 2023. | ||
Synthesis by melt processing is generally performed in three steps, the first of which consists of flows due to gravity or an external pressure. The second step is the shape definition, which is crucial to determine or to give a desired 3D, 2D, plate or sheet to the resulting material. The third step is shape maintenance, which is achieved by cooling the melt to have the solid form by crystallization or glass transition.39 Moreover, to the best of our knowledge, the bionanocomposites prepared by this method have not yet been used for application in electroanalysis, perhaps due to the fact that the use of higher temperature during the synthesis process may destroy the chemical functions that can promote the sensing of analytes.
From this overview of the above preparation methods, it can be seen that one method is not intended to replace the other. The choice of the method depends essentially on the expected properties of the resulting products. Each method has advantages and disadvantages. Moreover, self-assembly is the simplest one generally used for the preparation of electrochemical sensors.51 Depending on the method used for the preparation of bionanocomposites, in order to be used in electrochemistry, they must be immobilized, which can be done by drop coating or spin coating on the conventional working electrodes. Drop coating is simpler and more widely used than spin coating, which requires a spin coating apparatus for solvent evaporation at high rotation speed. Once the material is obtained several characterization techniques are applied to it before and after immobilization, and the interpretation and understanding of some of them are crucial to understanding the electroanalytical results. Therefore, the various physicochemical characterization techniques commonly used for bionanocomposite materials are discussed in Section 2.2.
2.2 Physicochemical characterization of bionanocomposites
Bionanocomposites prepared by the methods described above and used for electroanalytical applications are generally characterized by physicochemical methods such as Fourier Transform Infra-Red/Attenuated Total Reflection (FTIR/ATR) spectroscopy, Transmission Electron Microscopy/Field Emission Scanning Electron Microscopy/Scanning Electron Microscopy (TEM/FESEM/SEM), Atomic Force Microscopy (AFM), Powder X-ray Diffraction (PXRD) analysis, Energy Dispersive X-ray (EDX) Spectroscopy, Brunauer–Emmett–Teller (BET), Raman, and Thermo-Gravimetry/Differential Scanning Calorimetry (TG/DSC). In this section, we have provided an overview of the main physicochemical characterization techniques commonly used to obtain important information about the electroanalytical application of bionanocomposite materials.FTIR/ATR is the main technique always used to characterize liquid or solid materials applied in electrochemistry as it is very important to know the functional groups present in the electrode materials. This technique is based on the emission of polychromatic radiation in a sample of the material to be analysed so that it absorbs it. The electromagnetic field of the radiation then interacts with the electric charges of the molecule, resulting in a variation of the dipole moment. When the field frequency coincides with the vibrational frequency of the molecule, the resulting interaction leads to excitation of the vibration of certain bonds and causes the energy of the excited wave to be absorbed. The resulting information is returned in the form of an absorption or transmittance band. Sometimes, due to the low sensitivity of FTIR, ATR is used for better magnification, even if it is done in the small/close spectral range. In the work reported by Deffo et al., the FTIR spectra of the samples used/obtained during the different steps of the preparation of the bionanocomposite by solution coating were recorded in transmittance mode and shown in Fig. 2A, where the different functional groups present in the materials were identified.15 A bionanocomposite synthesized by in situ polymerization of β-cyclodextrin on natural hydroxyapatite used for the elaboration of the electrochemical sensor of heavy metal Pb(II) has been reported.40 The FTIR spectra of the different materials used were recorded in absorbance mode in order to identify the different functional groups present in the resulting elaborated material as shown in Fig. 2B. The mode of analysis (absorbance or transmittance) of materials in FTIR would always provide information about the different vibrations (stretching and deformation) of the functional groups present. This technique can therefore help to know how the materials are charged in solution after immobilization on the electrode during electroanalysis. As an example, the presence of a functional group at around 3450 cm−1 in the materials in both cases (Fig. 2A and B) assigned to –OH and or –NH vibrations, constitutes a good functional group in the field of electroanalysis that can be protonated in acidic medium to form positive ions at the surface of the electrode material used for the electrochemical determination of negative analytes. It can also be deprotonated to form a negatively charged surface for the determination of positive analytes since in principle good peak currents are obtained based on electrostatic attraction. Moreover, this technique has some limitations, including its inability to determine the complete chemical structure of a compound, difficulty in identifying complex samples, and does not provide information about the crystallisation of the materials. Therefore, due to the complexity of the materials, several other characterization methods have to be applied to have more information essential for the understanding of the results obtained during the electroanalytical measurements.
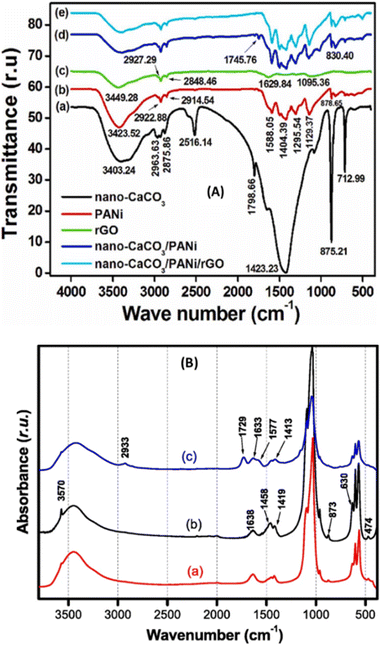 | ||
| Fig. 2 (A) FTIR spectra of (a) nano-CaCO3, (b) PANi, (c) rGO, (d) nano-CaCO3/PANi, and (e) nano-CaCO3/PANi/rGO. This figure has been reproduced from ref. 23 with permission from Elsevier Ltd., copyright 2022. (B) FTIR spectra of (a) NHAP, (b) NHAPP0.5, and (c) NHAPP0.5-CA-β-CD materials. This figure has been reproduced from ref. 40 with permission from Springer-Verlag GmbH Germany, copyright 2021. | ||
PXRD is another important physicochemical technique used to characterize materials applied in electrochemistry. It is based on the constructive interference of monochromatic X-rays and a crystalline sample. The principle consists in sending an X-ray beam from a production tube into the sample, a beam that is diffracted and captured by a detector. The diffraction spectrum obtained is made up of the quantities of diffracted radiation as a function of the angle of reflection θ. This allows access to the interplanar spacing d(h,k,l) characteristic of the crystallized solid studied using Bragg's law. It is well noted in the work reported by Zheng et al., for the characterization of the effect of CuNPs on the structure of PANi (Fig. 3A).24 It can be seen that CuNPs can affect the structure of PANi, since the XRD pattern of PANi shows a broad peak at 20° and can be assigned to (110) crystallographic planes, while with the CuNPs/PANi sample, the broad peak was positioned at 25°, which is assigned to (200) crystallographic planes of PANi. A similar experiment was performed to characterize a bionanocomposite-based MWCNT coupled to palm oil fiber (POF, rich in cellulose and hemi-cellulose), which was used for the immobilization of uricase enzyme for uric acid biosensing.37 The diffractogram they obtained after coupling is shown in Fig. 3B, where it can be seen that the crystallinity of the native structure of MWCNT is not altered, as the characteristic peaks for MWCNT appear at 25.87° and 43.39° respectively for the crystal plane reflections d(002) and d(004), and for cellulose, a plane reflection d(002) is identified at 22.18°. From these two examples, any bionanocomposite newly synthesized should be characterized by XRD, especially powder X-ray diffraction in order to know the crystallinity (average size of nanoparticles elaborated) and the interlayer space available for the diffusion and adsorption of analytes during electroanalysis. This is because the interlayer space of a material increase with the amount of analytes adsorbe and then enhance the sensitivity of the elaborated sensor. Moreover, this technique cannot identify amorphous materials, and does not give any information about the morphology and porosity of the material, which are important aspects to know about an electrode material.
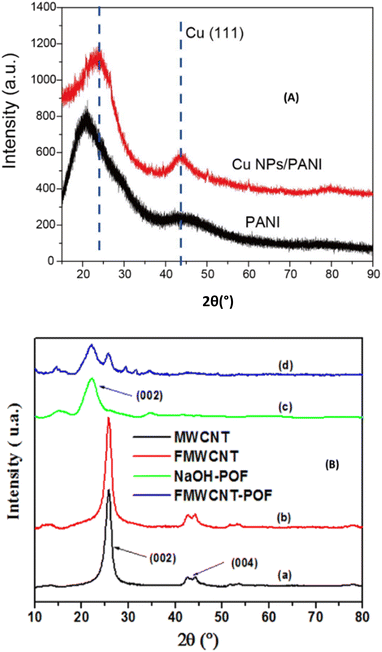 | ||
| Fig. 3 (A) XRD pattern of PANi and CuNPs/PANi. This figure has been reproduced from ref. 24 with permission from Elsevier B.V., copyright 2016. (B) PXRD spectra of (a) MWCNTs, (b) FMWCNTs, (c) NaOH-POF, and (d) FMWCNT-POF. This figure has been reproduced from ref. 37 with permission from The Royal Society of Chemistry, copyright 2023. | ||
TEM/FESEM/SEM are probably the third and most important physicochemical techniques used in the field of electrochemistry after FTIR and PXRD, as they provide information about the morphology and porosity of the film. TEM/FESEM/SEM are methods used to study the surface morphology of materials before and after immobilization on the surface of the electrode. The general principle is that an electron beam scans the surface of the sample to be analyzed, which in response re-emits certain wavelengths resulting from the electron–matter interaction, which are analyzed by different detectors. This screen then emits photons that are captured and imaged by a high-resolution camera, thus making it possible to reconstruct a virtual image of the object from three-dimensional images of the surface. In the work reported by Li et al. for the preparation of bionanocomposites by self-assembly, the SEM was used to study the surface morphology of an Au electrode modified with magnetic polymeric bionanocomposites. The surface of magnetic polymeric bionanocomposites (MPBNCs) was studied with many uniform, loosened and poriferous nanoparticles (Fig. 4A),31 implying that the laccase-catalyzed polymerization of dopamine formed MPBNCs after the adsorption of laccase on nanosized magnetic Fe3O4 nanoparticles. In the absence of Fe3O4 nanoparticles, the polymer of the dopamine-laccase composite film on the Au electrode is composed of aggregated and compact nanoparticles (Fig. 4B). Similarly, FE-SEM surface images of bionanocomposites based on gold nanoparticles/multi-walled carbon nanotubes/chitosan coated on the surface of a glassy carbon electrode (AuNPs/MWCNTs-CS/GCE) and comparative electrodes (MWCNTs/GCE, MWCNTs-CS/GCE, and AuNPs/GCE) prepared by sample/solution coating for the sensing of 2,4-diaminotoluene have been reported.41 From the images obtained, the dimensions of the nanoparticles can be measured, and the fibers that form the native structure of MWCNTs are well observed. TEM which is more sensitive in comparison to SEM and FE-SEM provide more insights into the nanoparticle size as reported by Chaudhari et al. where the comparison has been done between the SEM and TEM images of peroxalate polymer nanoparticles (PLPNs).42 The SEM image (Fig. 4-IA) shows the spherical structure of PLPNs with a size of 450 nm, while the TEM image (Fig. 4-IB) clearly indicates the presence of loaded dye in the nanoparticles (greater contrast) when compared against unloaded plain peroxalate nanoparticles (light contrast).42 Therefore, these microscopy techniques are crucial for the characterization of the film on the electrode surface and at least one of them should be recorded when elaborating a sensor and a biosensor.
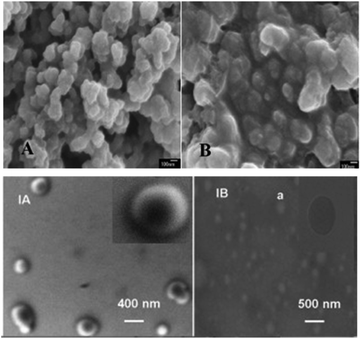 | ||
| Fig. 4 SEM images of PDA-Lac-Fe3O4 (A) and PDA-Lac (B). This figure has been reproduced from ref. 31 with permission from Elsevier B.V., copyright 2012. (IA) SEM and (IB) TEM images of peroxalate polymer nanoparticles. This figure has been reproduced from ref. 42 with permission from Elsevier B.V., copyright 2012. | ||
Like SEM, AFM is generally used to examine the topography (morphology) of bionanocomposites with greater precision/detail. It is based on the cantilever/tip assembly that interacts with the sample; this assembly is also commonly referred to as the probe. The AFM probe interacts with the substrate through a raster scanning motion. In the field of electroanalysis, the AFM is used to study the morphology of films, especially those in which an enzyme has been immobilized. The AFM analysis of the layer-by-layer assembly of the bionanocomposite immobilized on the GCE surface reported by Zhang et al. shows an interlocked network structure of biofunctionalized MWCNTs, and there is an increase in the number of layers and the surface roughness, due to the increase in thickness.36 In the work reported by Verma et al., glass-coated indium-tin oxide (ITO) was used as a support for immobilizing uricase (UOx) enzyme on a gold-reduced graphene oxide matrix (Au-rGO). AFM micrographs of Au-rGO/ITO and UOx/Au-rGO/ITO at the 3.0 μM scale are shown in Fig. 5.43 The image of Au-rGO/ITO exhibited nanostructured surface morphology of the grains with a roughness of 0.74 nm. On the other hand, the image of the UOx/Au-rGO/ITO electrode exhibited modified surface topography with an increased average roughness of 3.19 nm indicating the successful immobilization of enzyme on the Au-rGO electrode surface. Therefore, AFM is a very useful technique which confirms whether the synthesis of materials and the immobilization of enzymes were successfully done before the sensor/biosensor can be applied for the electrochemical studies.
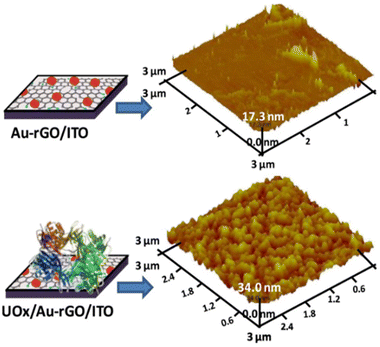 | ||
| Fig. 5 3D AFM images of Au-rGO/ITO and UOx/Au-rGO/ITO. This figure has been reproduced from ref. 43 with permission from Elsevier B.V., copyright 2019. | ||
Apart from the above methods which are generally used for the characterization of bionanocomposites, several other methods can be used such as EDX, BET, TGA/DSC, and Raman spectroscopy. Moreover, they are less used for the characterization of bionanocomposites immobilized on the surface of electrodes for sensing and biosensing studies. Their principles with examples of electroanalytical studies in which they have been applied are given here, to help any reader who wants to know more about them.
EDX sometimes coupled to SEM analysis is based on the generation of X-rays from a specimen through an electron beam. The X-rays are generated according to the characteristics and nature of the elements present in the sample. Therefore, this technique can be used to measure the energy emitted by X-rays, as shown in the reported work.23
Raman spectroscopy consists of sending a monochromatic radiation/beam through a sample so that the radiation may get reflected, absorbed, or scattered. The scattered photons have different frequencies from the incident photons because the vibrational and rotational properties vary. An illustration of the type of result that can be obtained is shown in the reported work.37
BET stands for Brunauer–Emmett–Teller who are the scientists who introduced this technique. It is a surface analysis method based on the formation of a monolayer of gas molecules on the solid surface, which is used to determine the specific surface area, pore volume and surface pore of materials. It provides important information about their physical structure since the surface area of a material determines how that solid will interact with its environment. A good example of this method was reported by Ngaha et al., (2022) consisting of amino alcohol functionalization of alkali palm oil fiber used for electrochemical sensing of 2-nitrophenol.41
TGA/DSC is sometimes used as a characterization technique in the case where the bionanocomposite is obtained by grafting, so that the temperature at which the grafted bond elaborate will break up can be identified. It is a technique in which the mass of the sample is monitored against time or temperature while the temperature of the sample, in a specified atmosphere, is programmed. The principle used includes measurement of a material's thermal stability, filler content in polymers, moisture and solvent content, and the percentage composition of components in a compound. DSC measurements show how much energy a sample absorbs or releases during heating or cooling. When using them together, the bonus information essentially labels what type of reaction produced a given signal as has been reported.41 The physicochemical characterization techniques described above are, of course, not all the techniques used to characterize bionanocomposites, but they are the most commonly used. Another method X-ray photoelectron spectroscopy (XPS) is now more commonly used by researchers to characterize bionanocomposites, especially to confirm the formation of nanoparticles entrapped in a complex composite. Each of them provides specific information that is important to know in order to anticipate the behaviour of the material once in contact with the analyte. Since this review is oriented to the use of bionanocomposites in the field of electrochemistry-electroanalysis, it is important to talk about the two main electrochemical techniques generally used to characterize the material/bionanocomposite after its immobilization on the electrode surface, given in Section 2.3.
2.3 Electrochemical characterization of bionanocomposites
Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS) are two electrochemical methods commonly used to characterize the film present at the surface of the electrode. CV can help to know the permeability and surface charge of the electrode while EIS helps to have information about the charge transfer resistance of the film on the electrode. Two examples of situations where these techniques have been used are shown in Fig. 6.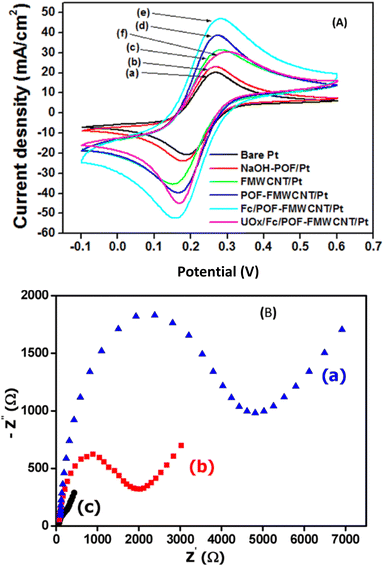 | ||
Fig. 6 (A) Cyclic voltammograms recorded in 0.1 M KCl, 5 mM [Fe(CN)6]3−/4− with bare Pt (a), NaOH-POF/Pt (b), FMWCNT/Pt (c), FMWCNT-POF/Pt (d), Fc/FMWCNT-POF/Pt (e) and UOx/Fc/FMWCNT-POF/Pt (f). Scan rate 100 mV s−1. This figure has been reproduced from ref. 37 with permission from The Royal Society of Chemistry, copyright 2023. (B) Nyquist plots of 1 mM [Fe(CN)6]3−/4− recorded in 0.1 M KCl (pH 7), on CPE (a), POF/CPE (b) and TEA-POF/CPE (c) in the frequency range of 0.01 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz and frequency number 50. This figure has been reproduced from ref. 41 with permission from Wiley-VCH GmbH, copyright 2022. 000 Hz and frequency number 50. This figure has been reproduced from ref. 41 with permission from Wiley-VCH GmbH, copyright 2022. | ||
CV was used to determine which electrode had the best sensitivity for the determination of ferri/ferrocyanide ions (as the redox probe) generally used in electrochemistry to know about the surface charge of the electrode with respect to ruthenium hexamine ions [Ru(NH3)63+], as shown in Fig. 6A.37 It was concluded that the strongest redox peaks for oxidation and reduction of [Fe(CN)6]3−/4− are obtained with Fc/FMWCNT-POF/Pt and the presence of UOx at the surface decreases the peak response due to the non-conducting nature of the enzyme. EIS was used to characterize the various hydrogel–graphene–AOx bionanocomposites synthesized,44 and it was concluded that chitosan and poly-N-isopropylacrylamide (PNIPAAM) had the highest permeability to charged ions compared to fibroin and cellulose nanocrystals (CNC) hydrogels, which indicates that both have a more porous structural network compared to fibroin and CNC hydrogels under the same experimental conditions. A similar conclusion was drawn by Ngaha et al. (2022) when comparing the bionanocomposite based on the aminoalcohol-functionalized palm oil fiber-modified carbon paste electrode (TEA-POF/CPE), CPE, and POF/CPE, with the result presented in the form of Nyquist plots (Fig. 6B).41 In general, CV and ESI are two complementary methods in which the response obtained with the first one is confirmed by the response obtained with the second one. In fact, the electrode showing the highest peak current obtained during the CV of the redox probe ferri/ferrocyanide would surely show the lowest value of charge transfer resistance during the EIS analysis of the same redox probe.
Bionanocomposites that are prepared and intended to be used for electrochemical purposes must have certain properties, as mentioned above. Once the synthesis has been shown to be successful, it can now be applied in the field of electroanalysis, as some of the bionanocomposites mentioned are used for sensing, as described in Section 3 below.
3. Application of bionanocomposites in electroanalysis
3.1 Getting started with electrochemical methods
Among the various methods of analysing samples reported in the literature such as chromatography, fluorescence, mass spectrometry, and electrochemical methods, the latter has become the most popular as it has been established to be a discipline at the interface between the branches of chemistry and many other sciences.45 Electrochemistry, generally referred to as electroanalysis, is a method of analysing dyes, pesticides, heavy metals, food additives, endocrine disruptors, constituents of pathological samples, nucleic acids, and many others. The principle is based on the measurement of the current/potential response when a specific potential/time is applied to allow the oxidation and/or reduction of compounds. The general instrument used consists of an electrochemical cell in which is introduced a supporting electrolyte (SE) with the analyte(s) to be investigated, and the three conventional electrodes (working electrode WE, reference electrode RE, and auxiliary electrode AE) connected to a potentiostat used to allow the passage of the current between the WE and AE, and to establish the potential between the same, it plays an imposing and controlling role. Depending on the information required, several electroanalytical techniques can be used such as cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), differential pulse voltammetry (DPV), square wave voltammetry (SWV), chronoamperometry (CA), normal pulse voltammetry (NPV), linear sweeping voltammetry (LSV), anodic and cathodic stripping voltammetry (ASV and CSV), etc.46 The first two mentioned are used for the characterization as previously shown in Section 2.3, while the others are generally used for the sensitivity studies. The practical situation described in Section 3.2 shows some examples where these methods have been used.3.2 Practical situation of the application of bionanocomposites for electroanalysis
Researchers in the field of electrochemistry are currently developing new electrode materials to enhance the sensitivity of the conventional working electrode such as the glassy carbon electrode (GCE), carbon paste electrode (CPE), platinum (Pt) electrode, gold (Au) electrode, graphite electrode, etc. for the electroanalysis of xenobiotics. These xenobiotics include, among others compounds such as endocrine disruptors (hydroquinone), constituents of pathological samples (ascorbic acid, dopamine, uric acid, and glucose), food additives (cholesterol oleate), pesticides (diuron), heavy metals (Pb2+ ions), pharmaceutical compounds (paracetamol and diclofenac), nucleic acids, etc. In this regard, bionanocomposites are good candidates because they offer good porosity, stability, conductivity, biodegradability, and high specific surface area. Table 1 shows the comparison between the bionanocomposite materials and other types of materials used for the sensing of the same analyte. In this table, the method of preparation of materials, the method used for the sensing, the limit of detection (LOD) in terms of the linear range, and the real medium of applications are given. Detailed experiments of some of the results presented in Table 1 are discussed here to give more inside.| Materials | Methods of preparation | Methods of analysis | Analytes | Linear range (μM) | LOD (μM) | Real media | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a 1stands for bionanocomposite materials and 2for other materials. GCE: Glassy carbon electrode, CPE: carbon paste electrode, DPV: differential pulse voltammetry, SWV: square wave voltammetry, CA: chronoamperometry, DPASV: differential pulse anodic stripping voltammetry, AA: ascorbic acid, DA: dopamine, UA: uric acid, PDA-Lac-MWCNTs: laccase-catalyzed polymerization of dopamine-multiwalled carbon nanotubes, Ba1-Na/L-cyst/GCE: baba sodic clay film amended by L-cysteine, Nano-CaCO3/PANI/rGO: nano-calcium carbonate, polyaniline, and reduced graphene oxide bionanocomposite, ChEt-ChOx/MWCNT/SiO2-CHIT/ITO: cholesterol esterase (ChEt) and cholesterol oxidase (ChOx) immobilized onto sol–gel-derived silica (SiO2)/chitosan (CHIT)/multi-walled carbon nanotubes(MWCNT) deposited onto indium-tin-oxide (ITO) glass, ChEt/ChOx/KMWNTs: co-immobilization of cholesterol esterase and cholesterol oxidase on potassium-doped carbon nanotubes, NHAPP05-CA-β-CD: natural hydroxyapatite functionalization by polymerization of β-cyclodextrin, rGO-β-CD: β-cyclodextrin on chemically reduced graphene oxide, APTES-Amino-AT-Silica: amino-attapulgite-mesoporous silica electrografting with (3-aminopropyl)triethoxysilane, M-Chs NC: magnetic chitosan nanocomposite. | |||||||||||
| 1PDA-Lac-MWCNTs/GCE | In situ polymerization | CA | Hydroquinone | 0.1–48.0 | 0.02 | — | 26 | ||||
| 2Ba1–Na/L-cyst/GCE | Cationic exchange intercalation | DPV | 2.0–10.0 | 0.8 | — | 48 | |||||
| 1Nano-CaCO3/PANI/rGO/GCE | Solution coating | DPV | AA, DA, UA | AA | DA | UA | AA | DA | UA | Human urine and blood serum | 23 |
| 12.0–500.0 | 0.1–14.0 | 0.1–60.0 | 10.8 | 0.01 | 0.04 | ||||||
| 2Chitosan-graphene/GCE | In situ chemical reduction | 50.0–1200.0 | 1.0–24.0 | 2.0–45.0 | 50.0 | 1.0 | 2.0 | — | 47 | ||
| 1ChEt-ChOx/MWCNT/SiO2-CHIT/ITO | Sol–gel | DPV | Cholesterol oleate | 153.58–7679.3 | 9.7 | Blood serum | 29 | ||||
| 2ChEt/ChOx/KMWNTs/GCE | Chemical doping | 0.050–16.0 | 0.01 | Human serum | 49 | ||||||
| 1GCE/NHAPP05-CA-β-CD | Self-assembly | DPASV | Pb2+ | 0.02–0.2 | 0.0005 | Spring water, well water, river water and tap water | 40 | ||||
| 2GCE/rGO-β-CD | 0.001–0.1 | 0.0005 | Industrial waste wastewater | 51 | |||||||
| 2GCE/APTES-amino-AT-silica | Layer-by-layer assembly | SWV | Diclofenac | 0.3–10.0 | 0.053 | Votaren 25 mg, tap water, mineral water and SB water | 52 | ||||
| 1M-Chs NC/CPE | Co-precipitation | DPV | 0.025–4.0 | 0.007 | Voltaren 100 mg and human serum | 53 | |||||
Three constituents of pathological samples ascorbic acid (AA), dopamine (DA), and uric acid (UA) were electroanalyzed using a GCE modified with a bionanocomposite of nano-CaCO3/PANI/rGO obtained by a solution coating method.23 A comparative study was done with a non-bionanocomposite material based on chitosan-graphene obtained by an in situ chemical reduction method. The cyclic voltammetry responses of both prepared electrodes show an irreversible system for AA and UA, while a quasi-reversible system is obtained with DA as shown in Fig. 7. It can be noticed that a better electrocatalytic ability is obtained with the non-bionanocomposite as the lower potentials of AA, DA, and UA are obtained with chitosan-graphene/GCE (Fig. 7B)47 in comparison with the bionanocomposite nano-CaCO3/PANI/rGO/GCE (Fig. 7A).23 Moreover, better peak current and then better limit of detection were obtained with the bionanocomposite as shown in Table 1. As chitosan-graphene belongs to the class of biocomposites, making it become bionanocomposites by introducing nanoparticles would make the resulting sensor to be more efficient in terms of catalysis and sensitivity.
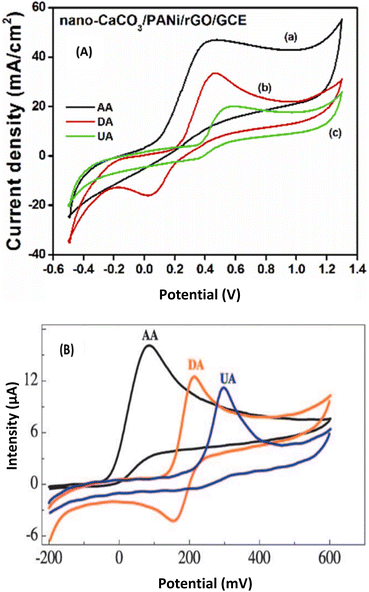 | ||
| Fig. 7 (A) CVs of 3 mM AA (a), 1 mM DA (b) and 1 mM UA (c) at nano-CaCO3/PANi/rGO/GCE (B) in PB (0.1 M, pH 6). Scan rate: 100 mV s−1. This figure has been reproduced from ref. 23 with permission from Elsevier Ltd., copyright 2022. (B) CVs at chitosan-graphene/GCE in 0.05 M PBS (pH 7.0) containing 2 mM AA (black line) or 1 mM DA (red line) or 0.5 mM UA (blue line). Scan rate: 50 mV s−1. This figure has been reproduced from ref. 47 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, copyright 2010. | ||
Great work has been done for the sensing of hydroquinone using a bionanocomposite PDA-Lac-MWCNTs/GCE26 and a non-bionanocomposite baba sodic clay amended by L-cysteine Ba1–Na/L-cyst/GCE48 prepared by in situ polymerization and cation exchange intercalation, respectively. The influence of concentration was determined in chronoamperometry (CA) in the case of PDA-Lac-MWCNTs/GCE, as shown in Fig. 8A and B and in differential pulse voltammetry (DPV) in the case of Ba1–Na/L-cyst/GCE.48 The results show that the better sensitivity of hydroquinone is obtained with the bionanocomposite electrode. It is well known that DPV is more sensitive than CA,46 so it was expected that the best sensitivity would be obtained with the DPV technique. Moreover, the opposite result was obtained here, mainly based on the type of material, i.e., the bionanocomposite used here is better than the non-bionanocomposite.
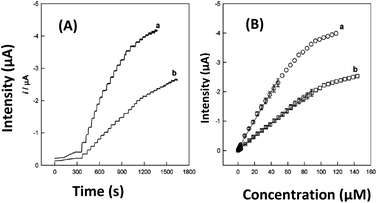 | ||
| Fig. 8 Time-dependent current responses (A) to successive injections of HQ into stirred B–R buffer solution (pH 4.0) under an air atmosphere and calibration curves (B) at PDA-Lac-MWCNTs/GCE (a) and PDA-Lac/GCE (b). Applied potential: −0.05 V vs. SCE. This figure has been reproduced from ref. 26 with permission from the American Chemical Society, copyright 2010. | ||
For the biosensing of cholesterol oleate by the DPV method, a bionanocomposite MWCNT/SiO2-CHIT/ITO was prepared by the sol–gel method followed by the immobilization of the cholesterol esterase (ChEt) and cholesterol oxidase (ChOx) enzymes to have a biosensor ChEt-ChOx/MWCNT/SiO2-CHIT/ITO. This bioelectrode shows a low limit of detection compared to the similar work reported two years later by Li et al.49 In the reported bioelectrode, instead of SiO2-MWCNT prepared by the sol–gel method, they used potassium-doped MWCNT (K-MWCNT) prepared by chemical doping after which they have drop coated on a glassy carbon electrode. Then the enzymes ChEt and ChOx were immobilized and the resulting biocomposite obtained ChEt/ChOx/KMWNTs/GCE was used for the sensing of cholesterol oleate. From the comparison shown in Table 1, the biocomposite ChEt/ChOx/KMWNTs/GCE show more interesting results than the bionanocomposite ChEt-ChOx/MWCNT/SiO2-CHIT/ITO, but the ITO electrode is better than the GCE as it is low cost, has low electrochemical background response, and a wide potential window.50 To improve the bionanocomposite, MWCNTs can be doped with the potassium atom. Moreover, after evaluating the stability, the bionanocomposite lost only 5% of its peak current after three weeks and 20% after 10 weeks while the biocomposite lost 15% of its peak current after 4 weeks. This helps to conclude that the sol–gel method seems to be better for the preparation of the substrate than chemical doping in these two similar reported studies.
Another electrochemical method similar to DPV, namely differential pulse anodic stripping voltammetry (DPASV), has been used as a sensitive method for the sensing of Pb2+ ions using β-cyclodextrin (β-CD) coated on reduced graphene oxide (rGO) to form a non-bionanocomposite GCE/rGO-β-CD by self-assembly.51 Similar work was done few years later using a surface functionalization of natural hydroxyapatite by polymerization of β-cyclodextrin. The bionanocomposite GCE/NHAPP05-CA-β-CD obtained was also used for the sensing of Pb2+ by DPASV.40 What is interesting here is that the two proposed sensors are all based on β-cyclodextrin, and when comparing the results as shown in Table 1, they have the same limit of detection, but it is noticed that the deposition potential (–0.8 V) and electrolysis time (30 s) are better with the bionanocomposite than with the non-bionanocomposite (–1.2 V; 150 s) as shown in Fig. 9. This result shows the higher efficiency of the bionanocomposite as an electrocatalytic material.
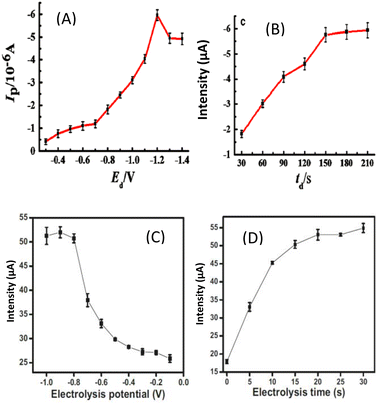 | ||
| Fig. 9 Electrolysis potential (A) and electrolysis time (B) in 10 μM Pb2+ in 0.1 M acetate buffer (pH 5.4) recorded at a β-CD-rGO/GCE. This figure has been reproduced from ref. 51 with permission from Springer, copyright 2016. Electrolysis potential (C) and electrolysis time (D) in 2 μM Pb2+ in 0.1 M acetate buffer (pH 5) recorded at a GCE/NHAPP0.5-CA-β-CD. This figure has been reproduced from ref. 40 with permission from Springer-Verlag GmbH Germany., copyright 2021. | ||
A biocomposite based on amino-attapulgite/mesoporous silica generated by electroassisted self-assembly (GCE/APTES-Amino-AT-Silica) for the voltammetric determination of diclofenac has been proposed.52 When comparing the limit of detection obtained here with the one proposed four years later by Abd-Elsabour et al.,53 using a bionanocomposite based on the chitosan nanocomposite carbon paste electrode (M-ChsNC/CPE) obtained by co-precipitation, it is evident that the bionanocomposite presents good applications. Moreover, the two most sensitive pulse methods46 were used, that is square wave voltammetry (SWV)52 and differential voltammetry (DPV)53 and the good calibration curves obtained with the better sensitivity were found by DPV as shown in Fig. 10.
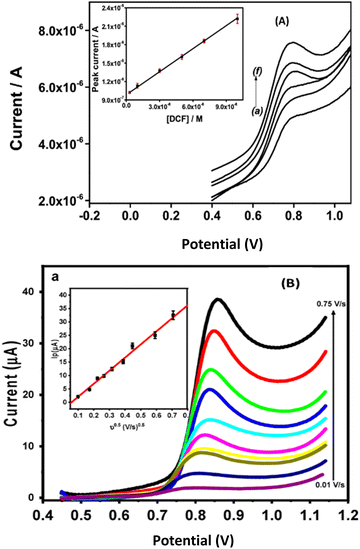 | ||
| Fig. 10 (A) Square-wave voltammograms recorded for DCF in phosphate buffer pH 5.7, using the GCE/APTES-amino-AT silica electrode. Inset: corresponding calibration curve obtained from SWV curves recorded in triplicate. Accumulation time: 3 min. This figure has been reproduced from ref. 52 with permission from Elsevier B.V., copyright 2019. (B) Differential pulse voltammograms for different concentrations of DIC in 0.1 M of BR buffer (pH = 3.0) at M-ChsNC/CPE. Insets: the calibration curve of DIC. This figure has been reproduced from ref. 53 with permission from MDPI, copyright 2023. | ||
From the results presented in Section 3 for the application of bionanocomposites in electroanalysis, it is shown in Table 1 that most of them are applied for real media studies with good recovery percentages. Several of these sensors/biosensors present promising features for the development of point-of-care testing (POCT) devices, which is the expected outcome of all the sensing studies. The development of POCT known as wearable sensors is the new direction ongoing in many research groups around the world now using bionanocomposites,32–34,54–57 which would be subject of another review article.
4. Future scope of bionanocomposites in electroanalysis
The elaboration of wearable (bio)sensors is the next step when developing POCT devices. Wearable sensor technology enables recording and analyzing physical, chemical, and electrophysiological parameters in real-time and in a non-invasive way. Therefore, the use of highly stable materials is mostly required with good electronic conductivity, porosity, and large specific area. Some promising materials are then developed in that sense, such as metal–organic frameworks (MOFs),58 MXenes,59 and core–shells.60 These novel materials have largely proven their application in energy storage, separation, catalysis, and other sectors,61,62 and they are good candidates as sensor materials since limitations such as biocompatibility and hydrophilicity can be overcome through various surface modification techniques and chemical treatments. For instance, facile synthesis of a core–shell Co-MOF with hierarchical porosity at the surface of ZIF-67 frameworks for enhanced electrochemical detection of furaltadone in aquaculture water has been reported by Shi et al., 2023,63 in which they got very nice results of sensitivity (110.40 μA−1 μM−1 cm−2) and detection limit (12 nM). Similarly, a facile synthesis of hierarchical MXene/ZIF-67/CNT composites for electrochemical sensing of luteolin has been proposed.64 These two studies show excellent results and the addition of polymers to these types of materials to make bionanocomposites will surely constitute a near future research field as one promising study has been reported recently by our team concerning it.65 In that work, the self-assembly method has been used to prepare bionanomaterials based on MOF (NH2-MIL-125(Ti)) and core–shells (Ag2S@– and Bi2S3@–) in the presence of the polymer polyaniline (PANi) for the electrochemical sensing of uric acid. It was found that bionanocomposites have higher electronic conductivity and low charge transfer resistance in comparison to the nanocomposites (in the absence of PANi), as shown in Fig. 11. The resulting sensors were able to detect uric acid in its normal range (2.6–8.5 mg dL−1 or 160–500 μmol L−1) and even at a concentration lower than the normal range. Therefore, they can be applied for the checking of patients susceptible to suffer from hyperuricemia and hypouricemia.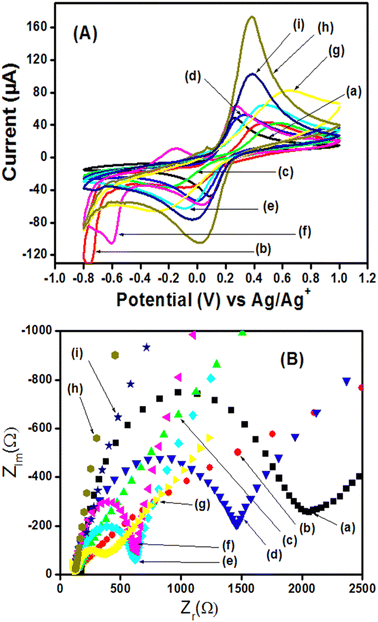 | ||
| Fig. 11 (A) Cyclic voltammograms of 5 mM [Fe(CN)6]3−/4− (0.10 M KCl, pH 7) recorded on the different electrodes: bare GCE (a), Ag2S/GCE (b), Bi2S3/GCE (c), NH2-MIL-125(Ti)/GCE (d), Ag2S@NH2-MIL-125(Ti)/GCE (e), Bi2S3@NH2-MIL-125(Ti)/GCE (f), PANI/GCE (g), Ag2S@NH2-MIL-125(Ti)/PANI/GCE (h), and Bi2S3@NH2-MIL-125(Ti)/PANI/GCE (i). Scan rate = 50 mV s−1. (B) Electrochemical impedance spectra obtained under the same conditions as in cyclic voltammetry with the same electrodes. This figure has been reproduced from ref. 65 with permission from The Royal Society of Chemistry, copyright 2024. | ||
Based on the above, we can anticipate that preparing bionanomaterials with these powerful new materials constitutes then an insightful research topic. Biopolymers, derived from diverse renewable sources, offer a compelling solution as raw materials with great potential for reinforcing composites in a wide range of industrial, commercial, and biomedical applications such as MOFs, Mxenes, and core–shells. By tailoring the properties of resulting bionanocomposites, their mechanical, thermal, and physiological performance can be enhanced to meet the demands of versatile applications.
5. Conclusion
The present review has highlighted the different methods of synthesis/preparation of bionanocomposites generally used for electrochemical purposes, especially for electroanalysis. The different methods of their characterization (physico-chemical and electrochemical) have been investigated. The results of their application for the electroanalysis of xenobiotics demonstrate the feasibility of bionanocomposites to be a promising class of hybrid nanostructured materials (formed by the combination of polymers derived from natural resources (biopolymers) or synthetic biofunctional polymers and inorganic/organic solids at the nanometric scale) in the field of sensing or living organisms/biological molecules in the field of biosensing. They combine the intrinsic properties of natural polymers, biocompatibility and biodegradability, with the typical properties of nanocomposites derived from synthetic polymers, such as improved mechanical properties, conductivity, stability, electrocatalytic ability, and enhanced barrier properties. These novel environmentally friendly materials open new scenarios for biodegradable polymers with potential perspectives for medicine, coatings, automotive, packaging applications, etc. This review has shown one of the major applications of this hybrid nanostructure in the field of (bio)sensing. It can be concluded from this work that depending on the target objective, the choice of the method of preparation of bionanocomposites and their characterization is very important. From the results obtained in the different studies published for the sensing and biosensing of compounds in different media, it is obvious that this new generation of materials can be a promising tool for the development of point-of-care testing (POCT) devices. Their combination with the recently more explored materials such as metal–organic frameworks, MXenes, and core–shells, will surely constitute the future research field more explored.Data availability
We agree that all the data used in this manuscript are available upon request.Author contributions
Gullit Deffo: conceptualization, writing the original draft. Ranil C. T. Temgoua: conceptualization, editing, review. Evangeline Njanja: editing, validation, supervision. Panchanan Puzari: editing, review, validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr Gullit DEFFO thanks DBT-TWAS for the fellowship DBT-TWAS Sandwich Postgraduate Fellowship (2019, FR number: 3240313785) and the contribution of the Alexander von Humboldt Foundation (AvH), the German Ministry for Economic Cooperation and Development (BMZ) and the African-German Network of Excellence in Science (AGNES, 2023). The authors thank the Research Unit of Noxious Chemistry and Environmental Engineering (RUNOCHEE) of the University of Dschang and the Sensor Laboratory (SL) of Tezpur University for the didactic support.References
- M. Darder, M. Lopez-Blanco, P. Aranda, F. Leroux and E. Ruiz-Hitzky, Chem. Mater., 2005, 17, 1969–1977 CrossRef CAS.
- B. Arora, R. Bhatia and P. Attri, New Polymer Nanocomposites for Environmental Remediation, 2018, pp. 699–712 Search PubMed.
- Y. Shchipunov, Pure Appl. Chem., 2012, 84, 2579–2607 CrossRef CAS.
- X. Wang, Q. Liang, Y. Luo, J. Ye, Y. Yu and F. Chen, Bioact. Mater., 2024, 32, 514–529 CAS.
- D. Can, B. Richard, C. Estefanía, F. M. Teresa, M. Arben, M. Andreas, U. G. Anton and G. Firat, Adv. Mater., 2019, 31, 1806739 CrossRef PubMed.
- N. Dubey, C. S. Kushwaha and S. K. Shukla, Int. J. Polym. Mater., 2019, 3–20 Search PubMed.
- J. Puiggalí and R. Katsarava, Clay Polym. Nanocompos., 2017, 7, 239–272 Search PubMed.
- A. Tiwari, A. P. Mishra, S. R. Dhakate, R. Khan and S. K. Shukla, Mater. Lett., 2007, 61, 4587–4590 CrossRef CAS.
- E. Ruiz-Hitzky, P. Aranda, and M. Darder, Kirk-Othmer Encyclopedia of Chemical Technology, 2008, pp. 1–28 Search PubMed.
- S. H. Othman, Agriculture and Agricultural Science Procedia, 2014, pp. 296–303 Search PubMed.
- M. B. Aga, A. H. Dar, G. A. Nayik, P. S. Panesar, F. Allai, S. A. Khan, R. Shams, J. F. Kennedy and A. Altaf, Int. J. Biol. Macromol., 2021, 192, 197–209 CrossRef CAS PubMed.
- A. H. Anwer, A. Ahtesham, M. Shoeb, F. Mashkoor, M. Z. Ansari, S. Zhu and C. Jeong, Adv. Colloid Interface Sci., 2023, 318, 102955 CrossRef CAS PubMed.
- C. I. Idumah, Synth. Met., 2021, 273, 116674 CrossRef CAS.
- G. L. Dico, B. Wicklein, L. Lisuzzo, G. Lazzara, P. Aranda and E. Ruiz-Hitzky, Beilstein J. Nanotechnol., 2019, 10, 1303–1315 CrossRef PubMed.
- B. Ates, S. Koytepe, A. Ulu, C. Gurses and V. K. Thakur, Chem. Rev., 2020, 120, 9304–9362 CrossRef CAS PubMed.
- G. Maduraiveeran, Anal. Methods, 2020, 12, 1688 RSC.
- S. Sanuja, A. Agalya and M. J. Umapathy, Int. J. Biol. Macromol., 2015, 74, 76–84 CrossRef CAS PubMed.
- T. Sarkar, H. B. Bohidar and P. R. Solanki, Int. J. Biol. Macromol., 2018, 109, 687–697 CrossRef CAS PubMed.
- A. Vinod, M. R. Sanjay, S. Suchart and P. Jyotishkumar, J. Clean. Prod., 2020, 258, 120978 CrossRef CAS.
- X. Liu, W. Sun, H. Wang, L. Zhang and J. Y. Wang, Biomaterials, 2005, 26, 109–115 CrossRef CAS PubMed.
- T. Kantaria, T. Kantaria, S. Kobauri, M. Ksovreli, T. Kachlishili, N. Kulikova, D. Tugushi and R. Katsarava, Appl. Sci., 2016, 6, 444 CrossRef.
- A. Díaz, R. Katsarava and J. Puiggalí, Int. J. Mol. Sci., 2014, 15, 7064e7123 Search PubMed.
- G. Deffo, M. Basumatary, N. Hussain, R. Hazarika, S. Kalita, E. Njanja and P. Puzari, Mater. Today Commun., 2022, 33, 104357 CrossRef CAS.
- W. Zheng, L. Hu, L. Lee and K. Wong, J. Electroanal. Chem., 2016, 781, 155–160 CrossRef CAS.
- J. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817–5821 CrossRef CAS PubMed.
- Y. Tan, W. Deng, Y. Li, Z. Huang, Y. Meng, Q. Xie, M. Ma and S. Yao, J. Phys. Chem. B, 2010, 114, 5016–5024 CrossRef CAS PubMed.
- G. Deffo, R. C. T. Temgoua, K. Y. Tajeu, E. Njanja, G. Doungmo, I. K. Tonle and E. Ngameni, J. Chin. Chem. Soc., 2022, 69, 349–358 CrossRef CAS.
- S. G. Advani and K. T. Hsaio, Manufacturing Techniques for Polymer Matrix Composites, Woodhead publishing series in composites science and engineering, 2012, pp. 481–497 Search PubMed.
- P. R. Solanki, A. Kaushik, A. A. Ansari, A. Tiwari and B. D. Malhotra, Sens. Actuators, B, 2009, 137, 727–735 CrossRef CAS.
- D. Bokov, A. T. Jalil, S. Chupradit, W. Suksatan, M. J. Ansari, I. H. Shewael, G. H. Valiev and E. Kianfar, Adv. Mater. Sci. Eng., 2021, 2021, 1–21 Search PubMed.
- Y. Li, C. Qin, C. Chen, Y. Fu, M. Ma and Q. Xie, Sens. Actuators, B, 2012, 168, 46–53 CrossRef CAS.
- C. Li, O. Dag, T. D. Dao, T. Nagao, Y. Sakamoto, T. Kimura, O. Terasaki and Y. Yamauchi, Nat. Commun., 2015, 6, 1–8 CrossRef CAS.
- H. Park, M. K. Masud, J. Na, H. Lim, Y. Kaneti, A. A. Alothman, C. Salomon, H. Phan, N. Nguyen, Md. S. A. Hossain and Y. Yamauchi, J. Mater. Chem. B, 2020, 1–24 Search PubMed.
- M. K. Masud, J. Na, T. E. Lin, V. Malgras, A. Preet, A. A. I. Sina, K. Wood, M. Billah, J. Kim, J. You, K. Kani, A. E. Whitten, C. Salomon, N. T. Nguyen, M. J. A. Shiddiky, M. Trau, M. S. A. Hossain and Y. Yamauchi, Biosens. Bioelectron., 2020, 168, 112429 CrossRef CAS PubMed.
- D. V. Talapin, M. Engel and P. V. Braun, Functional materials and devices by self-assembly, MRS Bull., 2020, 45, 799–806 CrossRef CAS.
- Y. Zhang, M. A. Arugula, J. S. Kirsch, X. Yang, E. Olsen and A. L. Simonian, Langmuir, 2015, 31, 1462–1468 CrossRef CAS PubMed.
- G. Deffo, R. Hazarika, M. C. D. Ngaha, M. Basumatary, S. Kalita, N. Njanja, E. Puzari and E. Ngameni, Anal. Methods, 2023, 1–11 Search PubMed.
- M. A. Ghalia and Y. Dahman, Nanobiomaterials in Solf Tissue Engineering, 2016, pp. 141–172 Search PubMed.
- L. F. Francis, Ceram. Polym., 2016, 105–249 Search PubMed.
- R. Tchoffo, G. B. P. Ngassa, G. Doungmo, A. T. Kamdem, I. K. Tonlé and E. Ngameni, Environ. Sci. Pollut. Res., 2021, 1–14 Search PubMed.
- M. C. D. Ngaha, V. K. Tchieda, A. K. Tamo, G. Doungmo, E. Njanja and I. K. Tonle, Electroanalysis, 2022, 34, 1–14 CrossRef.
- R. D. Chaudhari, A. B. Joshi and R. Srivastava, Sens. Actuators, B, 2012, 173, 882–889 CrossRef CAS.
- S. Verma, J. Choudhary, K. P. Singh, P. Chandra and S. P. Singh, Int. J. Biol. Macromol., 2019, 130, 333–341 CrossRef CAS PubMed.
- S. L. Burrs, D. C. Vanegas, M. Bhargava, N. Mechulan, P. Hendershot, H. Yamaguchi, C. Gomes and E. S. McLamore, Analyst, 2015, 140, 1466–1476 RSC.
- P. H. Rieger, Voltammetry of Reversible Systems, in Electrochemistry, Springer, Dordrecht, 1994, pp. 151–246, DOI:10.1007/978-94-011-0691-7_4.
- G. Deffo, T. F. N. Tene, L. M. Dongmo, S. L. Z. Jiokeng and R. C. T. Temgoua, Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 2023, pp. 1–9 Search PubMed.
- D. Han, T. Han, C. Shan, A. Ivaska and L. Niu, Electroanalysis, 2010, 22, 2001–2008 CrossRef CAS.
- K. Théophile, T. K. Ignas and E. Y. François, Eurasian J. Anal. Chem., 2013, 8, 64–77 Search PubMed.
- X. Li, J. Xu and H. Chen, Electrochim. Acta, 2011, 56, 9378–9385 CrossRef CAS.
- M. R. Akanda, A. M. Osman, M. K. Nazal and M. A. Aziz, J. Electrochem. Soc., 2020, 167, 037534 CrossRef.
- F. Zhan, F. Gao, X. Wang, L. Xie, F. Gao and Q. Wang, Microchim. Acta, 2016, 1–8 Search PubMed.
- Z. L. S. Jiokeng, I. K. Tonle and A. Walcarius, Sens. Actuators, B, 2019, 287, 296–305 CrossRef.
- M. Abd-Elsabour, M. M. Abou-Krisha, S. H. Kenawy and T. A. Yousef, Nanomaterials, 2023, 13, 1079 CrossRef CAS PubMed.
- A. Aldea, E. Matei, R. J. B. Leote, I. Rau, I. Enculescu and V. C. Diculescu, Electrochim. Acta, 2020, 363, 137239 CrossRef CAS.
- D. Botta, I. Enculescu, C. Balan and V. C. Diculescu, Curr. Opin. Electrochem., 2023, 42, 101418 CrossRef CAS.
- R. J. B. Leote, M. Beregoi, I. Enculescu and V. C. Diculescu, Curr. Opin. Electrochem., 2022, 34, 101024 CrossRef CAS.
- M. Li, L. Wang, R. Liu, J. Li, Q. Zhang, G. Shi, Y. Li, C. Hou and H. Wang, Biosens. Bioelectron., 2021, 174, 112828 CrossRef CAS PubMed.
- J. A. Cruz-Navarro, F. Hernandez-Garcia and G. A. A. Romero, Coord. Chem. Rev., 2020, 412, 213263 CrossRef CAS.
- G. Manasa and C. S. Rout, Mater. Adv., 2024, 5, 83–122 RSC.
- S. Bilge, B. Dogan-Topal, M. M. Gürbüz, S. A. Ozkan and A. Sınağ, Microchim. Acta, 2024, 191, 240 CrossRef CAS PubMed.
- N. Makhanya, B. Oboirien, J. Ren, N. Musyoka and A. Sciacovelli, J. Energy Storage, 2021, 34, 102179 CrossRef.
- C. Lamiel, I. Hussain, O. R. Ogunsakin and K. Zhang, J. Mater. Chem. A, 2022, 10, 14247–14272 RSC.
- S. Shi, G. Cao, Y. Chen, J. Huang, Y. Tang, J. Jiang, T. Gan, C. Wan and C. Wu, Anal. Chim. Acta, 2023, 1263, 341296 CrossRef CAS PubMed.
- Q. Xu, S. Chen, J. Xu, X. Duan, L. Lu, Q. Tian, X. Zhang, Y. Cai, X. Lu, L. Rao and Y. Yu, J. Electroanal. Chem., 2021, 880, 114765 CrossRef CAS.
- G. Deffo, C. G. Fotsop, M. C. D. Ngaha, S. G. Fogang, L. A. Vomo, B. W. Nkuigoua, C. A. Shella, A. V. Somba, T. F. N. Tene, I. K. Tchummegne, E. Njanja, I. K. Tonlé, P. Puzari and E. Ngameni, Mater. Adv., 2024, 5, 3683–3695 RSC.
| This journal is © The Royal Society of Chemistry 2024 |