Galloborates as ultraviolet nonlinear optical crystals: advances and perspectives
Jing-Yi Lu,
Yangfeifei Ou,
Cong-Cong Jin* and
Jian-Wen Cheng *
*
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Institute of Physical Chemistry, Zhejiang Normal University, Jinhua, Zhejiang 321004, China. E-mail: jincongcong@zjnu.edu.cn; jwcheng@zjnu.cn
First published on 17th June 2024
Abstract
Metal borates are excellent source materials for exploring short-wavelength nonlinear optical (NLO) crystals. Galloborates show rich structural chemistry with various coordination configurations of Ga cation and B–O anionic units and are suitable candidates as ultraviolet NLO crystals. Up to now, the shortest cut-off edge of galloborates was reported to be down to 190 nm in KCs2Ga(B5O10)(OH), while the largest second harmonic generation (SHG) effect of galloborates was reported to be up to 4.6 times that of KH2PO4 (KDP) in Na5Ga[B7O12(OH)]2·2B(OH)3. Herein, we give a detailed summary of the recent progress in NLO inorganic galloborates, where these galloborates are grouped into two types in terms of their compositions: (1) alkali/alkaline earth metal galloborates and (2) alkali/alkaline earth metal galloborate halides. We discuss their structural features, band gaps, and SHG intensities. Finally, we give future perspectives in this field.
1. Introduction
Nonlinear optical (NLO) materials are widely used to convert a specific wavelength of light to half its original.1–17 The discovery of LiB3O5 (LBO), β-BaB2O4 (β-BBO), and KBe2BO3F2 (KBBF) in borate systems greatly accelerated the development of ultraviolet (UV) and deep-UV NLO crystals.18–20 These crystals are used to obtain coherent UV and deep-UV light via the cascaded frequency conversion of Nd:YAG lasers (1064 nm). According to the anionic group theory, [B3O7], [B3O6], and [BO3] are the NLO-active functional units in LBO, β-BBO, and KBBF, while Li, Ba, and K cations contribute less. To date, a large number of alkali and alkaline earth metal borate-based NLO crystals have been found.21–27 One very recent example is Ba4B14O25, which shows a highly polymeric three-dimensional geometry with a closed-loop anionic framework constructed by the fundamental building blocks (FBBs) [B14O33], and it is a deep-UV transparent NLO crystal with strong second harmonic generation (SHG) (3.0 × KH2PO4, KDP).28 Generally, a perfect UV or deep-UV NLO crystal should possess certain characteristics including a wide optical transparency window, large NLO coefficient, and moderate birefringence, and it should be easy to grow large single crystals.The modification of the borate anionic unit is an effective method to find new NLO crystals.29 Fluorooxoborates are considered the best candidates for the next generation of deep-UV NLO materials.30 Fluorinated [BOxF4−x] (x = 1–3) units show larger polarizability anisotropy than that of [BO4], and the higher electronegativity of fluorine ions usually leads to blue-shifted cut-off edges in fluorooxoborates. To date, more than 80 examples of fluorooxoborates have been found, among which more than one-third are crystallized in noncentrosymmetric space groups.31 NH4B4O6F displays a wave-like layer constructed from a corner-sharing anionic structural motif of [B4O8F], and it is an excellent deep-UV NLO crystal with a very short cut-off edge (156 nm) and large NLO effect (3.0 × KDP).32 In addition, the newly discovered sp hybridized linear [BO2] in synthetic borates shows a larger polarizability anisotropy than that of [BO3] and [BO4] units according to the theoretical analyses.33 This functional unit may provide more opportunities to discover other NLO and birefringent materials.
The substitution or partial substitution of alkali and alkaline earth metal cations by other metal cations in borates lead to a new family of borates with excellent properties.34,35 A larger band gap may be achieved by partially eliminating the dangling bond of the terminal O atoms, as evidenced by Zn2BO3(OH),36 Cs3Zn6B9O21,37 β-Rb2Al2B2O7,38 and CsAlB3O6F.39 Zn2BO3(OH) shows a KBBF-like structure by replacing [BeO3F] with [ZnO3(OH)] and exhibits a short UV cut-off edge (204 nm) and a SHG response of 1.5 × KDP.36 Galloborates are suitable candidates for UV NLO crystals due to their rich structures, wide transmittance, and excellent optical properties. To date, 12 NLO inorganic galloborates have been reported, and the shortest cut-off edge of galloborates was reported to be down to 190 nm in KCs2Ga(B5O10)(OH),40 while the largest SHG effect of galloborates was reported to be up to 4.6 × KDP in Na5Ga[B7O12(OH)]2·2B(OH)3.41 In this frontier article, we review the recent progress in NLO galloborates. These galloborates are divided into two types in terms of their chemical compositions: (1) alkali/alkaline earth metal galloborates, and (2) alkali/alkaline earth metal galloborate halides. The chemical formulas, space groups, SHG intensities, and band gaps of these galloborates are summarized in Table 1. We also give the further perspectives and challenges in this field.
| Compounds | Space groups | SHG intensities | Band gaps | Ref. |
|---|---|---|---|---|
| Rb2Ga(B5O10)(H2O)4 | C2221 | 1.0 × KDP | 3.54 eV | 43 |
| KCs2Ga(B5O10)(OH) | I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d 2d |
1.4 × KDP | 6.30 eV | 40 |
| Ba4Ga[B10O18(OH)5](H2O) | Cc | 0.2 × KDP | 4.12 eV | 46 |
| Na4Ga3B4O12(OH) | F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3c 3c |
0.1 × KDP | 4.90 eV | 47 |
| Na5Ga[B7O12(OH)]2·2B(OH)3 | C2 | 4.6 × KDP | 3.90 eV | 41 |
| Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] | Fdd2 | 3.0 × KDP | 5.0 eV | 48 |
| K2Ba4Li2Ga4B6O21 | P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c 2c |
0.5 × KDP | 5.49 eV | 54 |
| Ba2GaB4O9Cl | P42nm | 1.0 × KDP | N/A | 50 |
| NaBa4(GaB4O9)2Cl3 | P42nm | 1.5 × KDP | 3.76 eV | 49 and 53 |
| NaBa4(GaB4O9)2Br3 | P42nm | 1.1 × KDP | 3.71 eV | 53 |
| K3Ba3Li2Ga4B6O20F | P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c 2c |
0.7 × KDP | >6.20 eV | 55 |
| Rb3Ba3Li2Ga4B6O20F | P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c 2c |
0.5 × KDP | >6.20 eV | 55 |
2. Alkali/alkaline earth metal galloborates
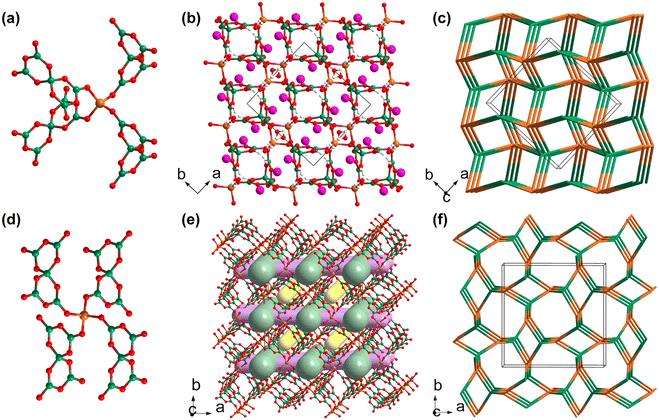
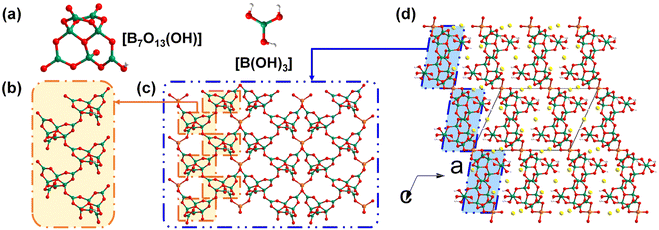
K2Ga(B5O10)(H2O)4 was synthesized by Liu et al. via a mild solvothermal method in 2007. It was the first reported member of borates featuring a three-dimensional zeolite-like [GaB5O10]∞ framework.42 Liu et al. reported the synthesis of K2Ga(B5O10)(H2O)4 and discussed its structural features; however, they did not further study its physical properties. The [GaB5O10]∞ anionic skeletons, comprised of four-fold coordinated [GaO4] tetrahedra and [B5O10] pseudo tetrahedra, are able to encapsulate guest molecules and counter cations ranging from inorganic components to organic amines. Isostructural galloborate Rb2Ga(B5O10)(H2O)4 was discovered later.43 The similar hybrid member (H2EDAP)Ga(B5O10)(H2O) exhibited an SHG response 0.5 times that of KDP.44 The flexibilities of the zeolite-like [GaB5O10]∞ frameworks are reflected in the tuneable sizes of their skeletons for guest encapsulation, with their changeable topologies transforming from dia to und if [GaO4] tetrahedra and [B5O10] units act as 4-connected nodes.40 Rb2Ga(B5O10)(H2O)4 and KCs2Ga(B5O10)(OH) are two galloborates in this category, and they show non-isostructural anionic frameworks with different topologies. Their optical properties have been preliminarily studied.Rb2Ga(B5O10)(H2O)4 synthesized by Mao et al. in 2012 crystallizes in the chiral space group of C2221 (no. 20).43 In the structure of Rb2Ga(B5O10)(H2O)4, the centred Ga cations are four-coordinated with neighbouring [B5O10] FBBs (Fig. 1a), while the [B5O10] FBBs share bridging μ2-O atoms with the epitaxial Ga cations. The alternate connectivity between the [GaO4] tetrahedra and [B5O10] FBBs gives rise to the three-dimensional [GaB5O10]∞ framework with the dia topology (Fig. 1b and c). Rb cations and guest water molecules with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 occupy the vacancies to ensure the charge balance. The band gap of Rb2Ga(B5O10)(H2O)4 is 3.54 eV, which is slightly smaller than that of isostructural (NH4)2Al(B5O10)(H2O)4 (3.96 eV) and K2Al(B5O10)(H2O)4 (4.45 eV). Powder SHG measurements based on Kurtz–Perry rules revealed that Rb2Ga(B5O10)(H2O)4 shows a moderate SHG intensity comparable to that of KDP, while the SHG responses of (NH4)2Al(B5O10)(H2O)4 and K2Al(B5O10)(H2O)4 are two times that of KDP.45 Given that [B5O10] FBBs have two roughly perpendicular [B2O5] dimers, the [BO3] triangles are naturally unable to align. The upper limit of the SHG response is restricted by the geometric configuration of [B5O10] FBBs.
2 occupy the vacancies to ensure the charge balance. The band gap of Rb2Ga(B5O10)(H2O)4 is 3.54 eV, which is slightly smaller than that of isostructural (NH4)2Al(B5O10)(H2O)4 (3.96 eV) and K2Al(B5O10)(H2O)4 (4.45 eV). Powder SHG measurements based on Kurtz–Perry rules revealed that Rb2Ga(B5O10)(H2O)4 shows a moderate SHG intensity comparable to that of KDP, while the SHG responses of (NH4)2Al(B5O10)(H2O)4 and K2Al(B5O10)(H2O)4 are two times that of KDP.45 Given that [B5O10] FBBs have two roughly perpendicular [B2O5] dimers, the [BO3] triangles are naturally unable to align. The upper limit of the SHG response is restricted by the geometric configuration of [B5O10] FBBs.
In 2023, Yang et al. used gallium isopropoxide as a gallium source and synthesized a mixed alkali metal hydrated galloborate KCs2Ga(B5O10)(OH), and then immediately uncovered its potential as an NLO crystal with a deep-UV transparency window.40 It should be noted that large amounts of galloborates and aluminoborates have been obtained under mild hydro/solvothermal condition since Yang et al. first proposed the use of metal isopropoxides as new sources for replacing the traditional inorganic metal oxides in 2009.45 KCs2Ga(B5O10)(OH) crystallizes in the tetragonal space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d (no. 122), and its zeolitic framework [GaB5O10]∞ exhibits a totally different topology to that of Rb2Ga(B5O10)(H2O)4 (Fig. 1c and f). The asymmetric unit of KCs2Ga(B5O10)(OH) contains half a Ga atom, half a [B5O10] cluster, and split alkali metal cations and their disordered coordinated hydroxyl groups. Although the [GaO4] tetrahedra in KCs2Ga(B5O10)(OH) are also coordinated with neighbouring four [B5O10] FBBs, the geometric configuration of the [GaB5O10] fragment in KCs2Ga(B5O10)(OH) is different from that in Rb2Ga(B5O10)(H2O)4 (Fig. 1a and d). As depicted in Fig. 1e, there are two types of open tunnels (the green and yellow channels) along the [001] direction, while there is another type of open tunnel along the [100] direction (the purple channels). Split alkali metal cations and disordered hydroxyl groups fill these channels. The experimental bandgap of KCs2Ga(B5O10)(OH) converted from diffuse reflection data using the Kubelka–Munk equation is as large as 6.30 eV, indicating that KCs2Ga(B5O10)(OH) may have a wide transparency window in the short-wavelength UV spectral region. Powder SHG measurements of KCs2Ga(B5O10)(OH) demonstrated that it exhibits an enhanced SHG response of about 1.4 times that of KDP.
2d (no. 122), and its zeolitic framework [GaB5O10]∞ exhibits a totally different topology to that of Rb2Ga(B5O10)(H2O)4 (Fig. 1c and f). The asymmetric unit of KCs2Ga(B5O10)(OH) contains half a Ga atom, half a [B5O10] cluster, and split alkali metal cations and their disordered coordinated hydroxyl groups. Although the [GaO4] tetrahedra in KCs2Ga(B5O10)(OH) are also coordinated with neighbouring four [B5O10] FBBs, the geometric configuration of the [GaB5O10] fragment in KCs2Ga(B5O10)(OH) is different from that in Rb2Ga(B5O10)(H2O)4 (Fig. 1a and d). As depicted in Fig. 1e, there are two types of open tunnels (the green and yellow channels) along the [001] direction, while there is another type of open tunnel along the [100] direction (the purple channels). Split alkali metal cations and disordered hydroxyl groups fill these channels. The experimental bandgap of KCs2Ga(B5O10)(OH) converted from diffuse reflection data using the Kubelka–Munk equation is as large as 6.30 eV, indicating that KCs2Ga(B5O10)(OH) may have a wide transparency window in the short-wavelength UV spectral region. Powder SHG measurements of KCs2Ga(B5O10)(OH) demonstrated that it exhibits an enhanced SHG response of about 1.4 times that of KDP.
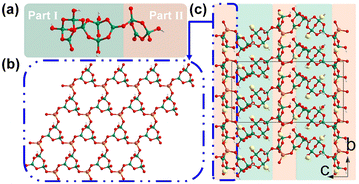
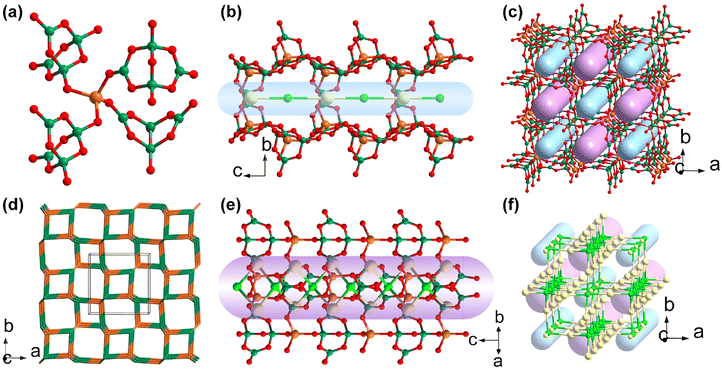
Barium galloborate Ba4Ga[B10O18(OH)5](H2O) was discovered by Mao et al. in 2014.46 Ba4Ga[B10O18(OH)5](H2O) crystallizes in the polar space group Cc (no. 9) and the asymmetric unit of Ba4Ga[B10O18(OH)5](H2O) consists of four Ba cations, one Ga cation, ten boron atoms, twenty-three oxygen atoms, and five hydrogen atoms. Its structure features a three-dimensional {Ga[B10O18(OH)5]}∞ covalent anionic skeleton composed of [B10O18(OH)5] FBBs and [GaO4] tetrahedra joined by Ga–O–B linkages (Fig. 2c). The complex FBB, [B10O18(OH)5], oligomerized from seven [BO4] tetrahedra and three [BO3] triangles, possesses five terminal hydroxyl groups and four exocyclic oxygen atoms for further connections with Ga cations (Fig. 2a). To simplify the structural description, [B10O18(OH)5] FBB is divided into two parts here (Fig. 2a): part 1 [B3O7(OH)] and part 2 [B7O12(OH)4]. As depicted in Fig. 2b, each [GaO4] tetrahedron is coordinated with three [B3O7(OH)] (part 2) via bridging μ2-O atoms to form a two-dimensional {GaO[B3O7(OH)]}∞ layer expanding in the (001) plane. The interlayer [B7O12(OH)4] (part 1) linkers connect the adjacent sheets to form the three-dimensional covalent skeleton {Ga[B10O18(OH)5]}∞ with Ba cations and water molecules filling the vacancies. The diffuse-reflectance absorption spectrum of Ba4Ga[B10O18(OH)5](H2O) indicates that it is transparent in the UV region as its band gap is as large as 4.12 eV. The powder SHG response of Ba4Ga[B10O18(OH)5](H2O) is relatively weak (about 0.2 × KDP@1064 nm), which may be attributed to the low ratio of [BO3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {[BO4] + [GaO4]} and the unfavourable arrangement of [BO3] units.
{[BO4] + [GaO4]} and the unfavourable arrangement of [BO3] units.
Na4Ga3B4O12(OH), another alkali metal galloborate incorporating [BO3] FBBs, was synthesized under surfactant-thermal conditions by Yang et al. in 2017.47 The structure of Na4Ga3B4O12(OH) crystallizes in the noncentrosymmetric cubic space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3c (no. 219) and its structure features a 3D framework composed of octahedral [Ga6(BO3)4] cages. The octahedral [Ga6(BO3)4] cage is constructed by four [BO3] triangles and six Ga cations with the [BO3] triangles capping the four faces and six Ga cations occupying the vertex-sites of octahedron (Fig. 3a). Each [Ga6(BO3)4] cage further connects the neighbouring six cages, thus expanding to an unusual zeolite-topology network [Ga3B4O12(OH)]∞ with disordered Na cations and their coordinated hydroxyl groups filling the cavities (Fig. 3b and c). The experimental band gap (Eg = 4.9 eV) determined from its diffuse-reflectance spectrum indicates that the cut-off edge of Na4Ga3B4O12(OH) is in the short-wavelength UV region. The three principal refractive indices of the crystals belonging to advanced crystal family are equal (n1 = n2 = n3 = n0), even though Na4Ga3B4O12(OH) crystallizes in the noncentrosymmetric space group. This indicates that light waves propagate through crystals in any direction exactly like they would in an isotropic medium, without causing the birefringence phenomenon. This species is not phase-matching and the large dihedral angle between the [BO3] triangles (70.529°) as well as the weak powder SHG response (0.1 × KDP) suggest that Na4Ga3B4O12(OH) may not be a satisfactory NLO crystal.
3c (no. 219) and its structure features a 3D framework composed of octahedral [Ga6(BO3)4] cages. The octahedral [Ga6(BO3)4] cage is constructed by four [BO3] triangles and six Ga cations with the [BO3] triangles capping the four faces and six Ga cations occupying the vertex-sites of octahedron (Fig. 3a). Each [Ga6(BO3)4] cage further connects the neighbouring six cages, thus expanding to an unusual zeolite-topology network [Ga3B4O12(OH)]∞ with disordered Na cations and their coordinated hydroxyl groups filling the cavities (Fig. 3b and c). The experimental band gap (Eg = 4.9 eV) determined from its diffuse-reflectance spectrum indicates that the cut-off edge of Na4Ga3B4O12(OH) is in the short-wavelength UV region. The three principal refractive indices of the crystals belonging to advanced crystal family are equal (n1 = n2 = n3 = n0), even though Na4Ga3B4O12(OH) crystallizes in the noncentrosymmetric space group. This indicates that light waves propagate through crystals in any direction exactly like they would in an isotropic medium, without causing the birefringence phenomenon. This species is not phase-matching and the large dihedral angle between the [BO3] triangles (70.529°) as well as the weak powder SHG response (0.1 × KDP) suggest that Na4Ga3B4O12(OH) may not be a satisfactory NLO crystal.
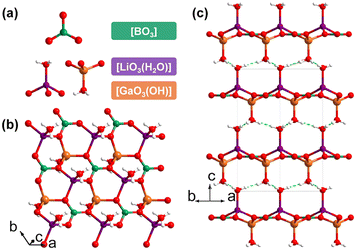
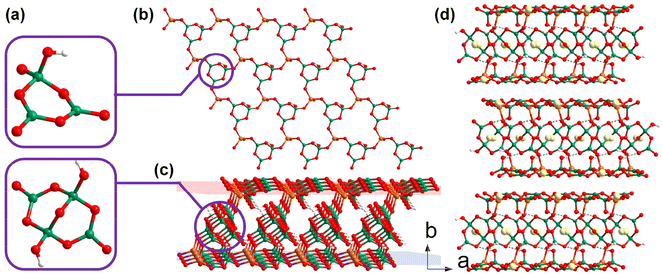
LiGa(OH)(BO3)(H2O) was obtained by Mao et al. in 2012, and it is the sole KBBF-like galloborate constructed from two-dimensional layers via hydrogen bonding interactions.43 LiGa(OH)(BO3)(H2O) crystallizes in the P31c (no. 159) space group and there are three kind of basic building units in its structure: triangular [BO3], tetrahedral hydrated [LiO3(H2O)], and [GaO3(OH)] (Fig. 4a). The assembly of [BO3], [LiO3(H2O)], and [GaO3(OH)] according to the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the (001) plane give rise to the formation of a two-dimensional [Li(H2O)Ga(OH)(BO3)]∞ layer with coordinated water molecules and hydroxyl groups branched out from the sheet (Fig. 4b). It should be noted that the coordinated water molecules and hydroxyl groups locate in the crystallographic 3-fold axes; therefore, all the hydrogen atoms are disordered, obeying the intrinsic geometric configurations of the hydroxyl group and water molecule, and these terminal units may act as both donors and acceptors of hydrogen bonds. The interlayer O–H⋯O hydrogen bonds further induce the [Li(H2O)Ga(OH)(BO3)]∞ layers to stack along the [001] directions (Fig. 4c). The functional [BO3] units are optimally aligned in the lattice and the density of [BO3] unit (8.22 × 10−3 Å−3) is comparable to that of KBBF (9.42 × 10−3 Å−3), which indicates that an SHG response and a birefringence comparable to that of KBBF could be expected. Unfortunately, further detailed optical properties of this crystal were not reported in the literature due to an unremovable unidentified impurity.
1 in the (001) plane give rise to the formation of a two-dimensional [Li(H2O)Ga(OH)(BO3)]∞ layer with coordinated water molecules and hydroxyl groups branched out from the sheet (Fig. 4b). It should be noted that the coordinated water molecules and hydroxyl groups locate in the crystallographic 3-fold axes; therefore, all the hydrogen atoms are disordered, obeying the intrinsic geometric configurations of the hydroxyl group and water molecule, and these terminal units may act as both donors and acceptors of hydrogen bonds. The interlayer O–H⋯O hydrogen bonds further induce the [Li(H2O)Ga(OH)(BO3)]∞ layers to stack along the [001] directions (Fig. 4c). The functional [BO3] units are optimally aligned in the lattice and the density of [BO3] unit (8.22 × 10−3 Å−3) is comparable to that of KBBF (9.42 × 10−3 Å−3), which indicates that an SHG response and a birefringence comparable to that of KBBF could be expected. Unfortunately, further detailed optical properties of this crystal were not reported in the literature due to an unremovable unidentified impurity.
Borates incorporating more than one kind of B–O clusters are rare as they naturally disobey Pauling's 5th rule. The galloborates discussed above contain only one kind of FBB, but there are two cases of hydrated galloborates constructed from two kinds of FBBs.
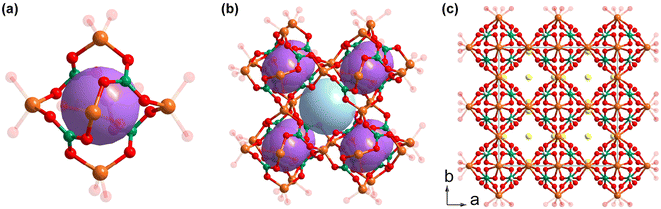
Na5Ga[B7O12(OH)]2·2B(OH)3 was identified as a new NLO crystal by Yang et al. in 2022.41 Na5Ga[B7O12(OH)]2·2B(OH)3 crystallizes in the polar C2 (no. 5) space group and there are two kinds of FBBs in the lattice: the [B7O13(OH)] FBB and the isolated [B(OH)3] cluster (Fig. 5a). The polymerization of [B7O13(OH)] FBBs along the b axis leads to the formation of a one-dimensional [B7O12(OH)]∞ chain (Fig. 5b). The [GaO4] units connect the as-formed chains to form a {Ga[B7O12(OH)]}∞ layer expanding in the bc plane (Fig. 5c). The isolated [B(OH)3] FBBs are not involved in the {Ga[B7O12(OH)]}∞ layer, as they as well as the Na cations are located in the voids between adjacent layers and stabilize the whole structure by hydrogen bonds and ionic bonds (Fig. 5d). The experimental data of Na5Ga[B7O12(OH)]2·2B(OH)3 showed a relatively small band gap as large as 3.90 eV but a remarkable powder SHG intensity. The extremely large powder SHG response of Na5Ga[B7O12(OH)]2·2B(OH)3 (as large as 4.6 times that of KDP) is a record in galloborate systems. Na5Ga[B7O12(OH)]2·2B(OH)3 is phase-matching according to the Kurtz–Perry rules.
Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] is another NLO-active galloborate constructed by two kinds of FBBs (Fig. 6a).48 This species was found and identified by Yang et al. in 2013 for the first time. Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] crystallizes in the polar space group Fdd2 (no. 43), and it features a sandwich {Ga2[B3O6(OH)]2[B4O7(OH)2]}∞ layer. Each sandwich {Ga2[B3O6(OH)]2[B4O7(OH)2]}∞ layer can be divided into two [GaOB3O6(OH)]∞ single layer and [B4O7(OH)2] linkages (Fig. 6c). As shown in Fig. 6b, only [B3O6(OH)] FBBs as well as the [GaO4] tetrahedra are involved in constructing the [GaOB3O6(OH)]∞ single layer with embedded eighteen-membered rings (18-MRs). A pair of [GaOB3O6(OH)]∞ single layers are assembled into one sandwich layer through the bridging [B4O7(OH)2] FBBs. Apart from the Ga–O ionic bonding interaction, the hydrogen bonds between two kinds of FBBs also help to stabilize the whole sandwich layer. As shown in Fig. 6d, Ba cations are located within and between the sandwiched layers to balance the charge and expand the two-dimensional layer to a three-dimensional framework. Interestingly, Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] is isostructural with its Al counterpart. The Ba cations in the structure of Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] are disordered (Fig. 6d) while the Ba cations in the structure of Ba3Al2[B3O6(OH)]2[B4O7(OH)2] are ordered. The large SHG response (3.0 × KDP@1064 nm) and the band gap (Eg = 5.0 eV) are comparable to Ba3Al2[B3O6(OH)]2[B4O7(OH)2] (3.0 × KDP@1064 nm, Eg = 5.4 eV), as can be inferred from their highly similar structures. The excellent optical properties indicate that Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] may be a potential NLO crystal in the UV region.
3. Alkali/alkaline earth metal galloborate halides
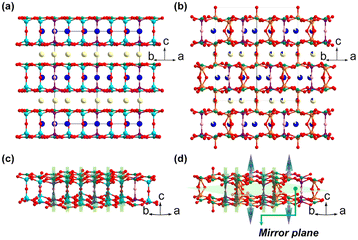
The first galloborate halide NaBa4(GaB4O9)2Cl3 was synthesized by Li et al. in 2006, and its structure was determined by powder X-ray diffraction data.49 NaBa4(GaB4O9)2Cl3 is a salt-inclusion galloborate, which shows a [GaB4O9] network featuring the dia topology. It is formed by the interconnection of [B4O9] FBBs and [GaO4] tetrahedra with Ba cations, Cl ions, and NaCl salt filling the tunnels. Soon after the discovery of NaBa4(GaB4O9)2Cl3, Barbier reported three isostructural borate halides, namely Ba2TB4O9Cl (T = Al and Ga) and Ba2GaB4O9Br, whose structures are similar with NaBa4(GaB4O9)2Cl3.50 Noncentrosymmetric NaBa4(AlB4O9)2X3 (X = Cl and Br) was synthesized by Wang et al. and Pan et al. in 2012 to 2013.51,52 In 2016, the single crystals of NaBa4(GaB4O9)2X3 (X = Cl and Br) were successfully obtained by Pan et al.53 Moreover, Pan et al. also identified [GaO4] as an NLO-active unit for the first time. The mentioned tetraborates show the same [GaB4O9] anionic skeleton featuring the dia topology with salt components incorporated.Since Ba2GaB4O9Cl and Ba2GaB4O9Br are isostructural, only the structure of Ba2GaB4O9Cl is discussed here. Ba2GaB4O9Cl crystallizes in the P42nm space group (no. 102). All the [GaO4] tetrahedra in the structure are four-coordinated (Fig. 7a), and the alternative arrangement of Ga cations and B–O clusters gives rise to a zeolite-like anionic framework with the dia topology (Fig. 7c and d). There are two kinds of channels incorporated in the [GaB4O9]∞ anionic skeleton for encapsulation of the salt components (see the blue and purple pipes in Fig. 7b, c and e). Ba2GaB4O9Cl has a moderate SHG effect (about 1.0 × KDP@1064 nm), while the powder SHG response of Ba2GaB4O9Br has not yet been reported.
Both NaBa4(GaB4O9)2Cl3 and NaBa4(GaB4O9)2Br3 crystallize in the P42nm space group (no. 102), and their structures are similar to that of Ba2GaB4O9Cl with different included salts. The Ba cations, Cl ions, and NaCl salt make up a ionic bonding network filling the tunnels in the [GaB4O9]∞ anionic skeleton (Fig. 7c and f). Although Ga3+ and Al3+ cations have similar outer electronic configurations, the 3d electrons of Ga3+ cations as well as the reduced ability of dangling bond elimination narrows the band gaps of galloborates compared with isostructural aluminoborates. NaBa4(GaB4O9)2Cl3 and NaBa4(GaB4O9)2Br3 show narrowed band gaps (3.76 and 3.71 eV) compared with the band gaps of their aluminium counterparts (3.93 and 3.95 eV). Whereas NaBa4(GaB4O9)2Cl3 and NaBa4(GaB4O9)2Br3 show enhanced powder SHG responses as large as 1.5 times and 1.1 times that of KDP under 1064 nm fundamental laser radiation, the SHG responses of NaBa4(AlB4O9)2X3 (X = Cl and Br) are 0.9 times and 0.8 times that of KDP, respectively. Theoretical calculations illustrated that the Ga–O hybrid orbitals also contribute to the optical properties and thus verify that the [GaO4] non-π conjugated is also an NLO-active chromophore.
In 2013, Li et al. synthesized a mixed metal galloborate K2Ba4Ga4Li2B6O21 by spontaneous crystallization with K2O–B2O3–LiF flux.54 K2Ba4Ga4Li2B6O21 can be regarded as the result of chemical co-substitution from the parental K2Al2B2O7 and its formula is consistent with the triple K2Al2B2O7 (K6Al6B6O21). Covalent [LiO4] and [GaO4] tetrahedra act as 4-connected linkages, like [AlO4] in K2Al2B2O7. The polymerization of [LiO4], [GaO4], and [BO3] in the (001) plane leads to the formation of the three-dimensional [Ga4Li2B6O21]∞ framework. The UV–Vis diffuse-reflectance spectrum shows that the cut-off edge of K2Ba4Ga4Li2B6O21 is about 226 nm. The structure of K2Ba4Ga4Li2B6O21 is seriously disordered (Fig. 8), and the powder SHG response of K2Ba4Ga4Li2B6O21 is as large as only half that of KDP.
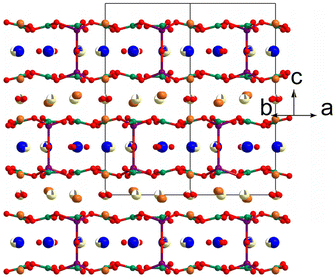 | ||
| Fig. 8 Structure of K2Ba4Li2Ga4B6O21. K, Ba, Li, Ga, B, and O atoms are shown in blue, light yellow, purple, orange, olive, and red, respectively. | ||
Inspired by the discovery of K2Ba4Ga4Li2B6O21, a series of aluminoborate fluorides and galloborate fluorides (A3Ba3Li2T4B6O20F, A = K or Rb, T = Al or Ga) with similar formulas were synthesized and identified as NLO crystals.55–57 K3Ba3Li2Ga4B6O20F and Rb3Ba3Li2Ga4B6O20F were synthesized by Xia et al. in 2018, after the discovery of K3Ba3Li2Al4B6O20F and Rb3Ba3Li2Al4B6O20F. Although galloborate fluorides A3Ba3Li2Ga4B6O20F (A = K and Rb) possess a similar formula to the aluminoborate fluorides A3Ba3Li2Ga4B6O20F (A = K and Rb), they cannot be regarded as the results of a simple chemical substitution of [AlO4] by [GaO4] tetrahedra. A3Ba3Li2Al4B6O20F (A = K and Rb) features a double-layered [Li2Al4B6O20F]∞ sheet (Fig. 9a) while the anionic skeleton of A3Ba3Li2Ga4B6O20F (A = K and Rb) is the three-dimensional [Ga4Li2B6O20F]∞ framework (Fig. 9b). The different orientations of covalent [LiO4] and [AlO4]/[GaO4] are responsible for the different dimensionalities of [Li2Al4B6O20F]∞ and [Ga4Li2B6O20F]∞. The [LiO4] and [AlO4] tetrahedra marked with yellow arrows face the same positions and are coordinated with [BO3] triangles to form the [Li2Al4B6O20F]∞ double layer, whereas the [LiO4] and [AlO4] tetrahedra marked with yellow and blue arrows face opposite positions to expand the layered structure to a three-dimensional framework, as depicted in Fig. 9c and d. The weakened powder SHG responses of A3Ba3Li2Ga4B6O20F (A = K and Rb) were 0.7 and 0.5 times that of KDP, which are smaller than that of A3Ba3Li2Al4B6O20F (A = K and Rb) (1.5 × KDP@1064 nm). The calculated birefringence values were 0.0416 and 0.0434@800 nm, respectively.
4. Summary and perspectives
This frontier article focused on the recent progress of NLO inorganic galloborates. These galloborates are divided into two types, namely alkali/alkaline earth metal galloborates and alkali/alkaline earth metal galloborate halides, in terms of their compositions. Their chemical formulas, space groups, anionic structural features, SHG intensities, and band gaps are summarized. New raw materials and synthetic methods are used to obtain new galloborates. For example, gallium isopropoxide and the surfactant–thermal method are used in the synthetic process. In these galloborates, KCs2Ga(B5O10)(OH) shows the shortest cut-off edge of 190 nm, while Na5Ga[B7O12(OH)]2·2B(OH)3 shows the largest SHG effect of 4.6 × KDP. Although some progress has been achieved, the development of NLO galloborates is still in the primary stage, and some aspects need further study.It is noteworthy that only the [GaO4] configuration has been observed in these NLO galloborates. Ga cations have several other coordination geometries, such as square pyramid, trigonal bipyramidal, and octahedron. It is expected that noncentrosymmetric galloborates could be obtained by the combination of [GaO5]/[GaO6] with borate anionic units. The fluorination strategy has been widely used to obtain new NLO crystals. Two NLO alkali/alkaline earth metal galloborate fluorides with enlarged band gaps have been reported, where the introduction of fluoride ions greatly widens the transparency windows in the short-wavelength spectral region. In these two compounds, fluorine ions are only coordinated with alkali/alkaline earth metal cations. Neither [GaOmFn] (m + n = 4, 5, 6) nor [BOxF4−x] (x = 1, 2, 3) units are observed in these NLO galloborates. Galloborates with larger SHG effects, and shorter cut-off edges are expected if these NLO-active units can be realized in the future. It should be noted that emergent boron-based compounds with other anionic units, like [NO3], [CO3], [SO4], [PO4], [GeO4], and [SiO4], will accelerate the exploration of new NLO crystals.58–63 Finally, it is important to grow large single crystals for practical applications. Compared with aluminoborates, the general raw material of Ga2O3 possesses a lower melting point, and the viscosity of the corresponding melts are also reduced, which are favourable to crystal growth. We believe the structural diversity of galloborates will inspire the design of more functional NLO crystals in future work.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 21975224).References
- M. Mutailipu, K. R. Poeppelmeier and S. L. Pan, Chem. Rev., 2021, 121, 1130–1202 CrossRef CAS PubMed.
- P. S. Halasyamani and W. G. Zhang, Inorg. Chem., 2017, 56, 12077–12085 CrossRef CAS PubMed.
- J. Chen, C. L. Hu, F. Kong and J. G. Mao, Acc. Chem. Res., 2021, 54, 2775–2783 CrossRef CAS PubMed.
- Y. Q. Li, J. H. Luo and S. G. Zhao, Acc. Chem. Res., 2022, 55, 3460–3469 CrossRef CAS PubMed.
- L. Kang and Z. S. Lin, Light: Sci. Appl., 2022, 11, 201 CrossRef CAS PubMed.
- M. Mutailipu, Z. H. Yang and S. L. Pan, Acc. Mater. Res., 2021, 2, 282–291 CrossRef CAS.
- Q. X. Liu, Q. Wu, T. Y. Wang, L. Kang, Z. S. Lin, Y. G. Wang and M. J. Xia, Chin. J. Struct. Chem., 2023, 42, 100026 CrossRef.
- H. A. Liu, H. P. Wu, Z. G. Hu, J. Y. Wang, Y. C. Wu and H. W. Yu, J. Am. Chem. Soc., 2023, 145, 12691–12700 CrossRef CAS PubMed.
- C. C. Jin, H. Zeng, F. Zhang, H. T. Qiu, Z. H. Yang, M. Mutailipu and S. L. Pan, Chem. Mater., 2022, 34, 440–450 CrossRef CAS.
- J. H. Liu, M. H. Lee, C. X. Li, X. H. Meng and J. Y. Yao, Inorg. Chem., 2022, 61, 19302–19308 CrossRef CAS PubMed.
- X. Y. Li, J. H. Li, J. W. Cheng and G. Y. Yang, Inorg. Chem., 2023, 62, 1264–1271 CrossRef CAS PubMed.
- Y. N. Zhang, Q. F. Li, B. B. Chen, Y. Z. Lan, J. W. Cheng and G. Y. Yang, Inorg. Chem. Front., 2022, 9, 5032–5038 RSC.
- H. T. Qiu, F. M. Li, C. C. Jin, Z. H. Yang, J. J. Li, S. L. Pan and M. Mutailipu, Angew. Chem., Int. Ed., 2024, 63, e202316194 CrossRef CAS PubMed.
- X. Liu, Y. C. Yang, M. Y. Li, L. Chen and L. M. Wu, Chem. Soc. Rev., 2023, 52, 8699–8720 RSC.
- J. J. Li, W. F. Chen, Y. Z. Lan and J. W. Cheng, Molecules, 2023, 28, 5068 CrossRef CAS PubMed.
- W. F. Zhou and S. P. Guo, Acc. Chem. Res., 2024, 57, 648–660 CAS.
- H. X. Fan, N. Ye and M. Luo, Acc. Chem. Res., 2023, 56, 3099–3109 CrossRef CAS PubMed.
- C. T. Chen, Y. C. Wu, A. D. Jiang, B. C. Wu, G. M. You, R. K. Li and S. J. Lin, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef CAS.
- C. T. Chen, B. C. Wu, A. D. Jiang and G. M. You, Sci. Sin., Ser. B, 1985, 28, 235–243 Search PubMed.
- B. C. Wu, D. Y. Tang, N. Ye and C. T. Chen, Opt. Mater., 1996, 5, 105–109 CrossRef CAS.
- Q. Wei, J. W. Cheng, C. He and G. Y. Yang, Inorg. Chem., 2014, 53, 11757–11763 CrossRef CAS PubMed.
- W. F. Chen, J. Y. Lu, J. J. Li, Y. Z. Lan, J. W. Cheng and G. Y. Yang, Chem. – Eur. J., 2024, 30, e202400739 CrossRef CAS PubMed.
- J. H. Huang, C. C. Jin, P. L. Xu, P. F. Gong, Z. S. Lin, J. W. Cheng and G. Y. Yang, Inorg. Chem., 2019, 58, 1755–1758 CrossRef CAS PubMed.
- W. Z. Zhao, Y. N. Zhang, Y. Z. Lan, J. W. Cheng and G. Y. Yang, Inorg. Chem., 2022, 61, 4246–4250 CrossRef CAS PubMed.
- Q. Wei, J. J. Wang, C. He, J. W. Cheng and G. Y. Yang, Chem. – Eur. J., 2016, 22, 10759–10762 CrossRef CAS PubMed.
- F. H. Ding, K. J. Griffith, W. G. Zhang, S. X. Cui, C. Zhang, Y. R. Wang, K. Kamp, H. W. Yu, P. S. Halasyamani, Z. H. Yang, S. L. Pan and K. R. Poeppelmeier, J. Am. Chem. Soc., 2023, 145, 4928–4933 CrossRef CAS PubMed.
- T. Ouyang, Y. G. Shen and S. G. Zhao, Chin. J. Struct. Chem., 2023, 42, 100024 CrossRef CAS.
- W. J. Xie, R. L. Tang, S. N. Yan, N. Ma, C. L. Hu and J. G. Mao, Small, 2023, 2307072 Search PubMed.
- M. Cheng, X. L. Hou, Z. H. Yang and S. L. Pan, Mater. Chem. Front., 2023, 7, 4683–4692 RSC.
- M. Mutailipu and S. L. Pan, Angew. Chem., Int. Ed., 2020, 59, 20302–20317 CrossRef CAS PubMed.
- H. K. Su, Z. T. Yan, X. L. Hou and M. Zhang, Chin. J. Struct. Chem., 2023, 42, 100027 CrossRef CAS.
- G. Q. Shi, Y. Wang, F. F. Zhang, B. B. Zhang, Z. H. Yang, X. L. Hou, S. L. Pan and K. R. Poeppelmeier, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef CAS PubMed.
- C. M. Huang, M. Mutailipu, F. F. Zhang, K. J. Griffith, C. Hu, Z. H. Yang, J. M. Griffin, K. R. Poeppelmeier and S. L. Pan, Nat. Commun., 2021, 12, 2597 CrossRef CAS PubMed.
- J. H. Jiao, M. Zhang and S. L. Pan, Angew. Chem., Int. Ed., 2023, 62, e2022170 Search PubMed.
- Q. F. Li, W. F. Chen, Y. Z. Lan and J. W. Cheng, Chin. J. Struct. Chem., 2023, 42, 100036 CrossRef.
- X. F. Wang, F. F. Zhang, L. Gao, Z. H. Yang and S. L. Pan, Adv. Sci., 2019, 6, 1901679 CrossRef CAS PubMed.
- H. W. Yu, H. P. Wu, S. L. Pan, Z. H. Yang, X. L. Hou, X. Su, Q. Jing, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2014, 136, 1264–1267 CrossRef CAS PubMed.
- T. T. Tran, N. Z. Koocher, J. M. Rondinelli and P. S. Halasyamani, Angew. Chem., Int. Ed., 2017, 56, 2969–2973 CrossRef CAS PubMed.
- H. K. Liu, Y. Wang, B. B. Zhang, Z. H. Yang and S. L. Pan, Chem. Sci., 2020, 11, 694–698 RSC.
- W. F. Liu, C. A. Chen, X. Y. Li and G. Y. Yang, Cryst. Growth Des., 2023, 23, 3556–3561 CrossRef CAS.
- C. A. Chen, W. F. Liu and G. Y. Yang, Chem. Commun., 2022, 58, 8718–8721 RSC.
- Z. H. Liu, P. Yang and P. Li, Inorg. Chem., 2007, 46, 2965–2967 CrossRef CAS PubMed.
- T. Hu, C. L. Hu, F. Kong, J. G. Mao and T. C. W. Mak, Inorg. Chem., 2012, 51, 8810–8817 CrossRef CAS PubMed.
- L. Cheng and G. Y. Yang, Inorg. Chem., 2018, 57, 13505–13512 CrossRef CAS PubMed.
- C. Rong, Z. W. Yu, Q. Wang, S. T. Zheng, C. Y. Pan, F. Deng and G. Y. Yang, Inorg. Chem., 2009, 48, 3650–3659 CrossRef CAS PubMed.
- H. Yang, C. L. Hu, J. L. Song and J. G. Mao, RSC Adv., 2014, 4, 45258–45265 RSC.
- S. J. Yu, X. Y. Gu, T. T. Deng, J. H. Huang, J. W. Cheng and G. Y. Yang, Inorg. Chem., 2017, 56, 12695–12698 CrossRef CAS PubMed.
- L. Cheng, Q. Wei, H. Q. Wu, L. J. Zhou and G. Y. Yang, Chem. – Eur. J., 2013, 19, 17662–17667 CrossRef CAS PubMed.
- R. K. Li and Y. Yu, Inorg. Chem., 2006, 45, 6840–6843 CrossRef CAS PubMed.
- J. Barbier, Solid State Sci., 2007, 9, 344–350 CrossRef CAS.
- J. X. Zhang, S. F. Zhang, Y. C. Wu and J. Y. Wang, Inorg. Chem., 2012, 51, 6682–6686 CrossRef CAS PubMed.
- H. W. Yu, S. L. Pan, H. P. Wu, Z. H. Yang, L. Y. Dong, X. Su, B. B. Zhang and H. Y. Li, Cryst. Growth Des., 2013, 13, 3514–3521 CrossRef CAS.
- M. Wen, X. Su, H. P. Wu, J. J. Lu, Z. H. Yang and S. L. Pan, J. Phys. Chem. C, 2016, 120, 6190–6197 CrossRef CAS.
- X. Gao and R. K. Li, Opt. Mater., 2014, 36, 2026–2029 CrossRef CAS.
- X. H. Meng, F. Liang, M. J. Xia and Z. S. Lin, Inorg. Chem., 2018, 57, 5669–5676 CrossRef CAS PubMed.
- S. G. Zhao, L. Kang, Y. G. Shen, X. D. Wang, M. A. Asghar, Z. S. Lin, Y. Y. Xu, S. Y. Zeng, M. C. Hong and J. H. Luo, J. Am. Chem. Soc., 2016, 138, 2961–2964 CrossRef CAS PubMed.
- H. P. Wu, H. W. Yu, S. L. Pan and P. S. Halasyamani, Inorg. Chem., 2017, 56, 8755–8758 CrossRef CAS PubMed.
- L. Y. Zhang, S. B. Wang, F. F. Zhang, Z. H. Yang and X. L. Hou, Dalton Trans., 2023, 52, 13492–13496 RSC.
- S. Bai, D. Q. Yang, B. B. Zhang, L. Li and Y. Wang, Dalton Trans., 2022, 51, 3421–3425 RSC.
- W. F. Chen, M. J. Zou, J. J. Li, Y. N. Zhang, Y. Z. Lan, J. W. Cheng and G. Y. Yang, Inorg. Chem., 2024, 63, 9026–9030 CrossRef CAS PubMed.
- J. Bruns, H. A. Höppe, M. Daub, H. Hillebrecht and H. Huppertz, Chem. – Eur. J., 2020, 26, 7966–7980 CrossRef CAS PubMed.
- R. Pan, J. W. Cheng, B. F. Yang and G. Y. Yang, Inorg. Chem., 2017, 56, 2371–2374 CrossRef CAS PubMed.
- W. J. Xie, C. L. Hu, Z. Fang, M. Y. Cao, Y. Lin and J. G. Mao, Inorg. Chem., 2022, 61, 10629–10633 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |