Open Access Article
Open Access ArticleExploring metal oxides for the hydrogen evolution reaction (HER) in the field of nanotechnology
Mir Sayed Shah
Danish
 *
*
Energy Systems (Chubu Electric Power) Funded Research Division, IMaSS (Institute of Materials and Systems for Sustainability), Nagoya University, Nagoya, Japan. E-mail: mdanish@nagoya-u.jp
First published on 10th November 2023
Abstract
As the global energy landscape transitions towards a more diversified mix, with electricity and hydrogen constituting half of the final energy consumption by 2050, the focus on efficient and sustainable hydrogen production intensifies. The Hydrogen Evolution Reaction (HER), a critical process for hydrogen production, shows promising potential with metal oxide electrocatalysts. Despite extensive studies on transition metal oxides, non-metal oxides' potential remains relatively underexplored. This exhaustive analysis bridges this knowledge gap, focusing on the classification, strategies, and potential applications of metal oxides in HER catalysis. Aiming to develop efficient, cost-effective hydrogen production methods and green hydrogen energy, future research should also explore combining metal oxides with other materials to enhance HER performance. Parallelly, metal oxide nanomaterials are receiving significant attention due to their diverse applications in sustainable energy and environmental remediation. Green chemistry approaches are indispensable for synthesizing these nanomaterials to minimize environmental impact and promote sustainability. The green synthesis methods, their advantages, challenges, and the role of these nanomaterials in energy storage, batteries, and supercapacitors are discussed. Significant potential has been demonstrated in various sustainable energy applications and environmental remediation technologies, including photocatalytic water splitting, solar cells, and thermoelectric materials. Future research should optimize green synthesis methods, address limitations, and further explore new metal oxide nanomaterials for sustainable energy and environmental applications, thereby advancing the use of these materials in the field of nanotechnology.
Sustainability spotlightThe rapid advancement of sustainable energy technologies necessitates novel solutions, among which the study of green-synthesized metal oxide nanomaterials for Hydrogen Evolution Reaction (HER) catalysis and energy storage stands out. This work makes a pioneering contribution by offering an innovative framework and detailed roadmap for using these materials in clean energy production and storage. The novelty of this study lies in the integrated analysis of metal oxides in HER catalysis, the first of its kind, addressing the underexplored potential of non-metal oxides in this crucial process. It provides a detailed classification of metal oxides, explores various strategies for their application, and presents a clear roadmap for their use as efficient HER catalysts. This comprehensive analysis will significantly bridge gaps in understanding and stimulate further research on the practical application of metal oxides in HER. Additionally, the study proposes a green chemistry approach for synthesizing metal oxide nanomaterials, emphasizing these methods' principles, advantages, and challenges. The exploration of metal oxide nanomaterials' applications in sustainable energy, particularly in energy storage, batteries, and supercapacitors, further adds to the novelty of this study. Ultimately, this work paves the way for more environmentally-friendly production of metal oxide nanoparticles, serving as a foundation for future studies aiming to optimize these green synthesis methods and explore new metal oxide nanomaterials for various applications. This combined approach of HER catalysis and sustainable energy storage using metal oxide nanomaterials will significantly contribute to a more sustainable and energy-efficient future. |
Introduction
Since the 1973 oil crisis, hydrogen has been envisioned as an energy carrier. It can be produced from renewable and nonrenewable sources, ensuring sustainable, zero-emission processes.1 Hydrogen is non-toxic, odorless, colorless, and tasteless, posing no threat to groundwater or air quality. However, it is highly combustible, and leaks can ignite easily. Despite this, hydrogen gas rapidly diffuses into non-flammable concentrations in open environments and is significantly less dense than air.Energy and environmental challenges pose significant threats to the planet and humanity. The world's dependency on fossil fuels has led to the depletion of non-renewable resources, geopolitical tensions, and price volatility. Greenhouse gas emissions contribute to climate change, causing global warming, extreme weather events, sea-level rise, and ocean acidification.2 Air and water pollution from industrial processes, agricultural runoff, and urban sewage severely affect public health and ecosystems.3 Land degradation, deforestation, and unsustainable resource consumption further exacerbate the loss of biodiversity and strain on global food production.4 Inefficient energy production and consumption patterns, coupled with societal challenges such as inequality in access to resources, health risks, and economic vulnerability, demand a comprehensive approach to address these issues.5 Developing and adopting innovative technologies like metal oxide nanomaterials can help promote sustainable energy production, environmental remediation, and resource conservation, offering potential solutions to these complex challenges.6 From a generalized perspective, the energy and environmental challenges are sorted in Table 1.
| Category | Subcategory |
|---|---|
| Fossil fuel dependency | Depletion of non-renewable resources |
| Geopolitical tensions due to uneven distribution of fossil fuels | |
| Price volatility and affordability | |
| Greenhouse gas emissions and climate change | Global warming |
| More frequent and severe extreme weather events | |
| Sea-level rise and coastal flooding | |
| Ocean acidification | |
| Air pollution | Particulate matter (PM) emissions |
| Health risks (respiratory issues, cardiovascular diseases, etc.) | |
| Reduced visibility and air quality | |
| Acid rain | |
| Water pollution and scarcity | Contamination from industrial waste, agricultural runoff, and urban sewage |
| Limited freshwater resources and over-extraction | |
| Waterborne diseases and public health concerns | |
| Ecosystem degradation | |
| Land degradation and deforestation | Soil erosion and loss of arable land |
| Habitat destruction and loss of biodiversity | |
| Desertification and land-use change | |
| Reduced carbon sequestration | |
| Waste generation and management | Increased waste production from industrial processes, urbanization, and consumerism |
| Limited landfill capacity | |
| Inadequate waste disposal and recycling infrastructure | |
| Environmental pollution from waste leakage | |
| Unsustainable resource consumption | Overconsumption and depletion of natural resources |
| Environmental degradation from resource extraction | |
| Loss of biodiversity and ecosystem services | |
| Strain on global food production and supply | |
| Inefficient energy production and consumption | High energy demand and dependence on non-renewable resources |
| Energy loss in transmission and distribution | |
| Inadequate energy infrastructure in developing countries | |
| Low adoption of energy-saving technologies and practices | |
| Societal challenges | Inequality in access to clean energy, water, and sanitation |
| Health risks from environmental pollution and climate change | |
| Economic vulnerability due to resource dependency and climate impacts | |
| Challenges in policy implementation and international cooperation |
Given the ever-increasing global demand for energy, coupled with environmental crises and political instability, the need to transition to sustainable energy systems is more urgent than ever.7 By 2050, it is expected that up to 50% of final energy consumption will be accounted for by electricity and hydrogen, marking a shift in the global energy landscape from traditional renewable and non-renewable resources to a more diverse mix.8 Hydrogen, a carbon-free energy carrier with exceptional energy density, is acknowledged as a vital next-generation clean energy source, making its efficient and environmentally friendly production essential for advancing the hydrogen economy and a sustainable energy future.9
Hydrogen is gaining recognition as a promising clean energy source due to its carbon-free nature and high energy density. However, traditional industrial hydrogen production methods, like steam methane reforming and coal gasification, have significant drawbacks such as high energy consumption, low purity of hydrogen, and greenhouse gas emissions.10 Alternatively, electrochemical water splitting, coupled with renewable energy resources, is emerging as a promising approach for hydrogen production.11 This method offers numerous advantages such as abundant water resources, stable output, high purity, and feasibility of large-scale implementation.
By 2050, the combined share of various fuels and energy carriers in molecular form, such as coal, oil, and natural gas, is expected to constitute 32% of final energy consumption, and could potentially increase to 50%, playing a central and indispensable role in the global energy landscape.12,13 Despite the growing population, energy consumption is projected to rise by only 14%, attributed to advancements in energy efficiency. Electrification in buildings, transportation, and industry is essential for boosting energy efficiency, with the proportion of electricity in final energy consumption anticipated to double by 2050. Moreover, green hydrogen production will significantly contribute to the increasing power demand, particularly in the context of hydroelectric storage and molecules such as coal and natural gas, which offer substantial storage and dispatch capabilities.13 In addition to its use in agricultural applications such as ammonia production for fertilizers and chemicals, low-carbon hydrogen is anticipated to see growth in industrial and transportation sectors. Hydrogen can be produced through various methods, ranging from green to black hydrogen production. According to the studies reports, green hydrogen production research and development is highlighted at the opt of 10 technologies in 2023.14
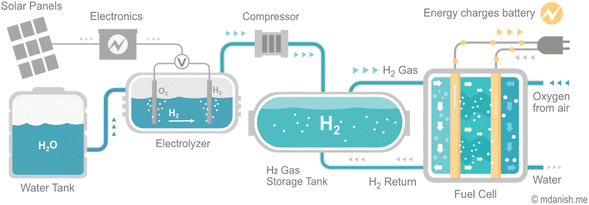
Despite the challenges, hydrogen plays a crucial role in decarbonizing the energy system and economy. As infrastructure investments are made, the optimization of new energy network construction and the repurposing of existing networks become increasingly important for supporting affordable and reliable energy systems. The availability of hydrogen in its molecular form in the natural environment is quite limited. Therefore, it is produced on-demand from hydrogen feedstocks, such as natural gas, to minimize the need for storage and transportation. At present, hydrogen generated from electricity provides less than 1% of global hydrogen production13 (Fig. 1). Nevertheless, the increasing accessibility of low-cost renewable electricity is spurring the advancement and implementation of electrolyzers technologies, which the utilization of metal oxides plays a crucial role in driving this progress.
Hydrogen fuel cells and hydrogen storage play different but equally essential roles in utilizing hydrogen as a clean energy carrier. Fuel cells generate electrical energy from hydrogen gas, while hydrogen storage facilitates the production and storage of hydrogen for later use. Improving the techno-economic efficiency of both technologies is fundamental for the widespread deployment of hydrogen as a clean energy carrier.
According to the World Resources Institute,15 greenhouse gas emissions have increased by 53% globally between 1990 and 2019. The energy sector accounts for 75.6% of emissions, with heat and electricity generation contributing 31.8%, transportation 17%, and manufacturing and construction 12.7%. The top 10 emitters contribute more than two thirds of global emissions, with China leading with 26.4%, followed by the US, India and the EU. Carbon dioxide (CO2) constitutes 74.1% of emissions, with 92% coming from the use of fossil fuels. Methane (CH4) and nitrous oxide (N2O) constitute 17.3% and 6.2% of total emissions, mainly from agriculture and waste treatment. Fluorinated gases from industrial processes account for 2.4% of global emissions and have a high potential for global warming.
However, global emissions remain far from meeting the 1.5 °C Pathway, even if all countries fulfill their current commitments.12 Thus, there is a pressing need for effective policy documentation procedures. The importance of energy and climate data analysis cannot be overstated, as it aids in the development of energy policies.16 Scenario analysis enables decision-makers to make informed choices, leading to a more sustainable and efficient energy future.17 Electrochemical water splitting involves using an electrical current to split water molecules into hydrogen and oxygen gas. Electrocatalysts play a crucial role in this process by reducing the energy required for the reaction to occur.18
Hydrogen evolution reaction (HER)
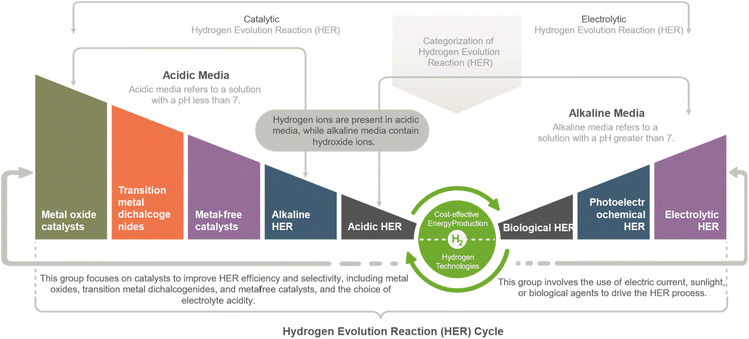
The Hydrogen Evolution Reaction (HER) can be categorized into two main groups. The first group is Electrolytic HER, which employs electricity to drive the reaction through water electrolysis and photoelectrochemical water splitting methods. The second group is Catalytic HER, which involves using catalysts to facilitate the reaction, such as hydrogen fuel cells and various HER catalysts based on metal oxides, metal alloys, metal phosphides/sulfides/sulfides/selenides/carbides/nitrides, single atoms, and functional carbon materials. This study mainly focused on metal oxides-based catalysts applications in HER deployment.The HER is a two-step electron transfer reaction that occurs at the catalyst surface, proceeding via the Volmer–Heyrovsky or Volmer–Tafel mechanisms. The HER in an acidic medium involves the following steps:11,19
| Volmer step: H+ + e− → H* | (1) |
| Heyrovsky step: H* + H+ + e− → H2 | (2) |
| Tafel step: H* + H → H2 | (3) |
In the Volmer step, a hydrogen ion (H+) and an electron (e−) combine to form an adsorbed intermediate species known as an H* atom. The reaction can be represented as H+ + e− → H*. While at the Heyrovsky step, the H* atom combines with another hydrogen ion (H+) and electron (e−) to form a hydrogen molecule (H2). The reaction can be represented as H* + H+ + e− → H2. Tafel's step deals with combining the two adsorbed H* atoms to form a hydrogen molecule (H2). The reaction can be represented as H* + H → H2. Similarly, the HER in an alkaline or neutral medium also follows the Volmer–Heyrovsky or Volmer–Tafel pathway. However, in alkaline or neutral HER, the strong covalent H–O–H bond must be cleaved prior to H adsorption, making the kinetics slower due to the extra water dissociation in the Volmer step compared to acidic media. Mechanism to enhance the initial water dissociation process in alkaline HER have been concentrated on determining the Tafel slope values and theoretical calculations.20
Efficient and cost-effective HER catalysts are critical for hydrogen's widespread adoption as a clean energy carrier. Research in this area focuses on developing new catalyst materials and improving their activity, stability, and selectivity. On the other hand, the development of cost-effective HER catalysts are necessary for the widespread adoption of technologies that improve efficiency, reduce costs, and minimize the environmental impact of the processes involved. The hydrogen evolution reaction (HER) is paramount in various hydrogen-based technologies. The most noteworthy HER-based technologies include hydrogen fuel cells, hydrogen production via water electrolysis, photoelectrochemical water splitting, and hydrogen storage.21 In fuel cells, the HER converts the chemical energy of hydrogen gas into electrical energy through an electrochemical reaction. Water electrolysis produces hydrogen gas from renewable energy sources using an electric current, and photoelectrochemical water splitting uses solar energy to drive water electrolysis and produce hydrogen gas. Hydrogen storage can involve compressed gas or liquid form, but solid-state materials such as metal hydrides or carbon-based materials can also be used. In some cases, the HER is also involved in hydrogen storage, such as when hydrogen is stored as a metal hydride and must be released by reacting with water in the presence of a catalyst.
The hydrogen evolution reaction (HER) offers several advantages as a potential clean energy source. One of the primary advantages is that hydrogen gas is a clean energy source, producing only water vapor as a byproduct. This makes it a promising option for reducing carbon emissions and addressing climate change. Additionally, hydrogen gas has a high energy density, making it a viable option for use as a fuel in transportation (Fig. 2).
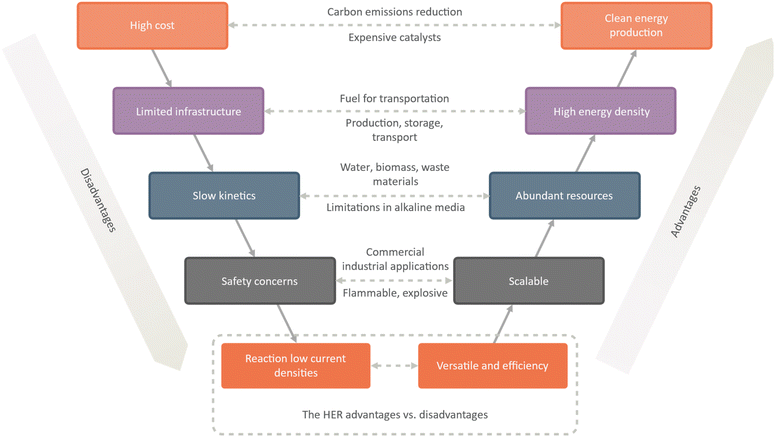 | ||
| Fig. 2 A comprehensive analysis of advantages and disadvantages of the hydrogen evolution reaction (HER). | ||
Another advantage of the HER is that abundant resources, including water, biomass, and waste materials, can power it. This makes it a promising option for reducing dependence on fossil fuels and promoting a more sustainable energy future. Additionally, the scalability of the HER makes it suitable for a range of commercial and industrial applications, from small-scale hydrogen production to large-scale power generation. The versatility of the HER is another crucial advantage, as hydrogen gas can be used as a fuel for fuel cells or for chemical synthesis in a range of industrial processes. Additionally, the HER is highly efficient when powered by renewable energy sources, such as wind or solar power.
However, there are also a number of challenges associated with the HER. One major challenge is the high cost of the catalysts to drive the reaction. Another challenge is the low efficiency of the reaction, particularly in terms of current densities. Additionally, the kinetics of the reaction can be slow, particularly in alkaline media, which can limit its practical applications.
Infrastructure challenges are also associated with the HER, including the production, storage, and transport of hydrogen gas. Safety concerns related to the flammability and explosiveness of hydrogen gas must also be addressed. Finally, there are environmental concerns associated with the production of hydrogen gas from fossil fuels, which can contribute to greenhouse gas emissions and other environmental impacts. While the HER offers significant potential as a clean energy source, it will require ongoing research and development to overcome these challenges and enable widespread adoption.
HER categories
Hydrogen Evolution Reaction (HER) can be achieved through various methods, including electrolytic, photoelectrochemical, and biological HER. Metal oxide catalysts such as ruthenium oxide and molybdenum oxide, as well as transition metal dichalcogenides such as molybdenum disulfide and tungsten disulfide, have shown high activity for the HER. Additionally, metal-free catalysts such as nitrogen-doped carbon nanotubes and graphene can act as HER catalysts without using expensive or rare metals. The HER can be performed in either an alkaline or acidic electrolyte, each with advantages. Alkaline HER can reduce costs and improve the catalysts' stability, while acidic HER can provide high HER activity and selectivity. Regardless of the method used, HER has the potential to play a significant role in the clean energy industry, offering a clean and renewable energy source with high energy density and abundant resources (Fig. 3).18HER strategies
The Hydrogen Evolution Reaction (HER) is a climacteric process for clean energy production and storage. Improving its efficiency and selectivity is a primary research focus in materials science and engineering. Recently, a wide range of strategies has been explored to optimize HER electrocatalysts' catalytic activity and stability. A brief overview of these HER strategies and their potential applications in electrocatalysis and other clean energy technologies are extracted in Table 2.22,23| No. | Strategy | Short definition | Application | Advantages |
|---|---|---|---|---|
| 1 | Catalyst design | Designing catalysts to enhance HER activity | Electrochemical water splitting, hydrogen fuel cells | High efficiency HER, longer lifespan for catalysts |
| 2 | Electrolyte optimization | Optimizing the composition and properties of electrolyte | Electrochemical water splitting, hydrogen fuel cells | Improved HER efficiency, enhanced stability, reduced cost |
| 3 | Nanostructuring | Creating nanostructured materials for catalysts | Electrochemical water splitting, hydrogen fuel cells | High surface area, improved catalytic activity, increased stability |
| 4 | Interface engineering | Modifying the catalyst/electrolyte interface properties | Electrochemical water splitting, hydrogen fuel cells | Enhanced catalytic activity and selectivity, improved stability |
| 5 | Co-catalyst integration | Introducing additional catalysts to the system | Electrochemical water splitting, hydrogen fuel cells | Improved catalytic activity, enhanced stability |
| 6 | Light absorption enhancement | Increasing light absorption of the material | Photoelectrochemical water splitting | Higher photoconversion efficiency, improved stability |
| 7 | Doping | Introducing dopant atoms into the catalyst material | Electrochemical water splitting, hydrogen fuel cells, HER catalysts | Improved catalytic activity and selectivity, enhanced stability |
| 8 | Crystallinity engineering | Modifying the crystal structure of the material | Electrochemical water splitting, hydrogen fuel cells, HER catalysts | Improved catalytic activity and selectivity, enhanced stability |
| 9 | Valence regulation | Controlling the valence state of the catalyst material | Electrochemical water splitting, hydrogen fuel cells, HER catalysts | Improved catalytic activity and selectivity, enhanced stability |
| 10 | Strain engineering | Applying strain to the catalyst material | Electrochemical water splitting, hydrogen fuel cells, HER catalysts | Improved catalytic activity and selectivity, enhanced stability |
| 11 | Hybridization | Combining two or more materials to create a hybrid material with enhanced properties | Electrocatalysis, photocatalysis, energy storage | Improved activity, stability, and selectivity of the catalyst |
| 12 | Oxygen vacancy engineering | Introducing oxygen vacancies into metal oxide catalysts to improve their catalytic activity | Electrocatalysis, photocatalysis | Enhanced activity and stability of the catalyst |
| 13 | Phase-structure engineering | Controlling the crystal structure and phase composition of catalysts to optimize their catalytic properties | Electrocatalysis, photocatalysis | Improved catalytic activity, selectivity, and durability |
| 14 | Morphology engineering | Controlling the size, shape, and surface structure of catalyst particles to optimize their catalytic properties | Electrocatalysis, photocatalysis, energy storage | Improved catalytic activity and selectivity, as well as improved mass transport and charge transfer |
| 15 | Atomic-scale synergy | Enhancing the catalytic properties of catalysts by controlling the interactions between individual atoms or molecules | Electrocatalysis, photocatalysis | Enhanced catalytic activity, selectivity, and stability |
| 16 | Surface reconstruction | Modifying the surface structure and composition of catalysts to improve their catalytic properties | Electrocatalysis, photocatalysis | Enhanced catalytic activity, selectivity, and stability |
Developing efficient and stable HER electrocatalysts is crucial for the practical applications of hydrogen-based technologies such as fuel cells, storage technologies, and water splitting. The strategies discussed here offer promising approaches for improving the catalytic properties of HER electrocatalysts. By combining these strategies and optimizing their implementation, researchers can create electrocatalysts with enhanced activity, selectivity, and stability and help to enable the widespread adoption of clean energy technologies based on hydrogen.
The performance comparison of various metal oxides in the hydrogen evolution reaction (HER) is presented in Table 3. The metrics include overpotential, indicating the extra potential needed for the HER, with values ranging from 135 mV for copper oxide to 150 mV for molybdenum oxide. Current density, representing catalytic activity, shows Nickel Oxide as the top performer at 12.5 mA cm−2. Stability, reflecting the catalyst's active duration, peaks with zinc oxide at 105 hours. Turnover frequency (TOF) measures HER events per catalyst site per second, with values closely ranging from 0.5 s−1 to 0.6 s−1. Lastly, the Tafel slope provides insights into HER kinetics, with cobalt oxide having the lowest value at 54 mV dec−1, suggesting faster reaction kinetics. Overall, the table offers a concise overview of the efficiencies and capabilities of different metal oxides in HER.
| Metal oxide | Overpotential (mV) | Current density (mA cm−2) | Stability (hours) | TOF (s−1) | Tafel slope (mV dec−1) | |
|---|---|---|---|---|---|---|
| Molybdenum oxide | MoOx | 150 | 10 | 100 | 0.5 | 60 |
| Iron oxide | FeOx | 140 | 12 | 95 | 0.6 | 58 |
| Copper oxide | CuOx | 135 | 11 | 90 | 0.55 | 59 |
| Zinc oxide | ZnOx | 145 | 9 | 105 | 0.52 | 57 |
| Titanium oxide | TiOx | 138 | 11.5 | 98 | 0.58 | 56 |
| Tungsten oxide | WOx | 142 | 10.5 | 96 | 0.57 | 58 |
| Nickel oxide | NiOx | 137 | 12.5 | 94 | 0.59 | 55 |
| Cobalt oxide | CoOx | 139 | 11.8 | 97 | 0.56 | 54 |
Hydrogen technologies
Hydrogen technologies are diverse, including electrolytic processes that split water using electricity; chemical methods like steam reforming or coal gasification; biological processes involving microorganisms in fermentation or photosynthesis; photolytic methods utilizing solar energy for photoelectrolysis; thermochemical techniques, such as the sulfur–iodine cycle; and fuel cells that directly convert hydrogen into electricity and heat with high efficiency and zero emissions.1Hydrogen has various applications, including metallurgical processes, electronics, food industry for hydrogenation of fats and oils, glass manufacturing, cooling power generators, and space applications as fuel.29 Additionally, hydrogen is used with oxygen to produce hydrogen peroxide, primarily for paper and pulp bleaching.
Hydrogen production occurs through various methods. Fossil fuels – steam methane reforming (SMR) uses methane and high-temperature steam with a catalyst to produce hydrogen and carbon monoxide, which is purified to obtain hydrogen. Natural gas – steam reforming converts methane and water vapor into hydrogen and carbon monoxide at 700–850 °C and 3–25 bar pressure, with the resulting CO further converted to CO2 and H2:30
| CH4 + H2O + heat → CO + 3H2 | (4) |
| CO + H2O → CO2 + H2 + heat | (5) |
Coal – gasification processes convert carbon into carbon monoxide and hydrogen at high temperatures; coal-derived hydrogen production is established but more complex than from natural gas:30
| C (s) + H2O + heat → CO + H2 | (6) |
Water – electrolysis splits water into hydrogen and oxygen using electrical energy, with hydrogen produced at the cathode and oxygen at the anode:30
| H2O (liquid) + electricity → H2 (gas) + 1/2O2 (gas) | (7) |
The efficiency and performance of an electrolyzer are determined by its voltage (Uanode: potential at the anode, Ucathode: potential at the cathode) and thermal (derived from Gibbs free energy and enthalpy change) efficiencies, which can be calculated using these equations:31
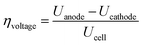 | (8) |
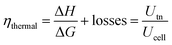 | (9) |
To achieve sustainable, zero-emission hydrogen production, CO2 emitted during the process must be captured and stored through decarbonization. Two methods for reducing CO2 emissions from power plants include using a conventional steam turbine or combined cycle power plant (CCPP) with CO2 removal via the “amine” process, or employing oxyfuel combustion, where fossil fuel is burned with pure oxygen, producing mainly CO2 and water vapor that can be separated and stored in geological formations.32
Alkaline electrolysis is a well-established technology for water electrolysis, accounting for the majority of the global installed capacity, which ranges from 1.8 to 5300 kW. Commercial systems are available in various sizes and offer hydrogen production rates (fH2) from 0.25 to 760 N m3 h−1.33
The cell voltage (Ucell) is affected by overpotential reversible voltage (Urev) due to ohmic losses (Uohm), activation (Uact), and concentration (Ucon) overvoltage:34
| Ucell = Urev + Uohm + Uact + Ucon | (10) |
| 2H2O + 2e− → H2 (g) + 2OH− | (11) |
| 2OH− (aq.) → 12O2 (g) + H2O (l) + 2e− | (12) |
Proton exchange membrane (PEM) electrolysis has higher current densities than typical alkaline electrolyzers, making concentration overvoltage more significant. This overvoltage occurs due to concentration gradients and slow mass transport as the cell reaction proceeds. Commercial PEM electrolyzers usually operate at current densities of 0.6–2.0 A cm−2. Concentration overvoltage occurs when the starting concentration of a reagent at the electrode (C0) changes due to mass transfer (C1, measured in mol m−3):35
Ucon = RT/zF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(C1/C0) ln(C1/C0) | (13) |
Solid oxide electrolyte (SOE) electrolyzers, still in research and development (R&D) have the potential to increase water electrolysis efficiency by operating at high temperatures (700–1000 °C).36 Research focuses on systems operating at 500–700 °C for material stability, with current densities at 0.3–0.6 A cm−2 and cell voltages of 1.2–1.3 V. Considering electricity and heat demands, system efficiencies typically exceed 90%.37 The chemical reactions occurring during SOE electrolysis take place at the cathode and anode, and their specific details are described below:30
| H2O (g) + 2e− → H2 (g) + O2− | (14) |
| O2− → 1/2O2 (g) + 2e− | (15) |
The theoretical minimum energy to produce 1 kg of hydrogen is 39.4 kW h kg−1, but commercial water electrolyzer system efficiency is typically below 80% due to overvoltages and parasitic currents.30 As electrolytic cells age, overvoltages increase due to cell degradation, impacting stack lifetime and efficiency. The electrolyzer stacks are considered at the end of their life when efficiency drops to 10% of the nominal value.33 Voltage degradation rates for alkaline electrolyzers are less than 3 μV h−1, and for PEM electrolyzers, less than 14 μV h−1.38 The effect of dynamic operation on lifetime remains unknown. Commercial electrolyzers typically include auxiliary equipment like power supply and converter, water deionizer, circulation pump, gas purification unit, gas storage, control system, and ventilation system.
Metal oxide nanomaterials
Metal oxide nanomaterials are garnered significant attention due to their diverse applications in sustainable energy and environmental remediation. Green chemistry approaches are essential for synthesizing these materials to minimize environmental impact and promote sustainability. Eco-innovations explores the remarkable potential of metal oxide nanomaterials in advancing sustainable energy and environmental nanotechnologies.39 These nanomaterials, exhibiting exceptional properties such as high surface area, tunable bandgap, and enhanced catalytic activity, have emerged as key components in diverse applications, ranging from energy storage and environmental remediation to electronics, healthcare, and sensing. So far, research efforts have continued to develop novel synthesis and functionalization techniques for metal oxide nanoparticles; their role in revolutionizing various industries and addressing pressing global challenges is increasingly apparent.40–46 This cutting-edge research area holds great promise for a more sustainable and eco-friendly future.Metal oxide nanomaterials play a crucial role in sustainable energy and environmental applications due to their unique properties, such as high surface area and tunable bandgap.46 They enhance energy storage in batteries and supercapacitors, enable photocatalytic water splitting, and improve solar cell efficiency. These nanomaterials also boost thermoelectric performance for waste heat conversion and serve as effective agents in environmental remediation and photocatalytic degradation of pollutants. Furthermore, they act as sensitive materials in environmental sensors for real-time monitoring of contaminants. Employing green chemistry methods for their synthesis promotes sustainability, while their abundance and low cost make them attractive for large-scale deployment.
In,47 the future direction of energy and environmental policies from various perspectives emphasizes the need for further research in crucial areas debated. These include advancing electric vehicles, battery technology, and electricity infrastructure to ensure effective decarbonization; incorporating social dimensions in sustainability evaluations, with a focus on local empowerment and residents' perspectives; and exploring innovative policies and company strategies to facilitate the energy transition, concentrating on technology selection, development, and assessment of social and economic impacts.
This paper deals with the green synthesis methods for metal oxide nanoparticles, their applications in sustainable energy, and the importance of metal oxide nanomaterials in nanotechnology. Focusing on the principles of green chemistry, green synthesis methods for metal oxide nanoparticles, and their advantages and challenges, including the applications of metal oxide nanomaterials in energy storage, batteries, and supercapacitors, were explored. Green synthesis methods are essential for the sustainable production of metal oxide nanoparticles. Metal oxide nanomaterials demonstrated significant potential in various sustainable energy applications, including energy storage, batteries, supercapacitors, and environmental remediation technologies. Metal oxide nanomaterials synthesized through green chemistry approaches offer an excellent opportunity to advance sustainable energy solutions and ecological applications, suggest future research on optimizing green synthesis methods, address limitations and challenges in metal oxide nanomaterial applications, and explore new metal oxide nanomaterials for sustainable energy and environmental applications. Moreover, further investigation into using metal oxide nanomaterials in nanotechnologies, such as photocatalytic water splitting, solar cells, and thermoelectric materials, is necessary to uncover their full potential.
Green chemistry approaches for metal oxide nanomaterial synthesis
Green chemistry approaches have emerged as a sustainable alternative for synthesizing metal oxide nanomaterials, minimizing the environmental impact and reducing the reliance on hazardous chemicals.48 This section explores various green synthesis methods, emphasizing their benefits and potential applications in producing metal oxide nanomaterials for energy and environmental solutions.Principles of green chemistry
The principles of green chemistry serve as a framework for designing products and processes that minimize environmental impact, reduce waste, and promote sustainability. These principles were first introduced by P. Anastas and J. Warner in 1998,49 and have since become widely recognized in the scientific community. Green chemistry principles aim to reduce waste, minimize environmental impact, and promote sustainability. Key aspects include waste prevention, atom economy, less hazardous synthesis, safer chemical design, using safer solvents and auxiliaries, energy-efficient processes, renewable feedstocks, reducing derivatives, employing catalysis, designing for degradation, real-time pollution prevention analysis, and inherently safer chemistry for accident prevention. These principles guide the development of more environmentally friendly and sustainable chemical practices, contributing to a cleaner and safer future.Green synthesis methods for metal oxide nanoparticles
Green synthesis methods have gained prominence as environmentally friendly and sustainable alternatives for producing metal oxide nanoparticles.22 These methods minimize the use of hazardous chemicals, reduce waste generation, and lower energy consumption. The green synthesis techniques, highlighting their advantages, underlying mechanisms, and potential applications in producing metal oxide nanoparticles for energy and environmental purposes, are the key to be discussed.50Plant-mediated and microbe-mediated synthesis are green chemistry approaches for nanoparticle production, including metal oxide nanoparticles. The main difference lies in the biological agents used in the process. Plant-mediated synthesis uses plant extracts for nanoparticle synthesis, leveraging phytochemicals like flavonoids, phenols, and terpenoids to reduce, stabilize, and caress metal ions.41 This cost-effective, eco-friendly, and versatile method requires milder reaction conditions without toxic chemicals. Microbe-mediated synthesis involves microorganisms like bacteria, fungi, and algae that produce enzymes or biomolecules to reduce metal ions and form nanoparticles. This approach offers high scalability, controlled particle size and shape, and potential for simultaneous synthesis and functionalization but may require more complex culturing conditions.48 Both methods contribute to the green chemistry movement by reducing environmental impacts and promoting sustainable practices, offering unique advantages and challenges for various applications and goals.
Advantages and challenges of green synthesis methods
Green synthesis methods, also known as eco-friendly synthesis, have gained considerable attention recently due to their potential to produce sustainable and environmentally friendly materials. These methods use natural, renewable, and non-toxic starting materials, often relying on benign reaction conditions. While these methods offer many advantages, they also present several challenges and potential limitations in the scope and selectivity of the reactions, which is summarized as follows.41,42,48,51–53(2) Cost-effectiveness: green synthesis often uses abundant and inexpensive biological sources, such as plants and microorganisms, reducing the overall cost of production.
(3) Renewable resources: green synthesis methods rely on renewable biological resources, minimizing the depletion of non-renewable raw materials.
(4) Mild reaction conditions: green synthesis typically requires milder reaction conditions, such as lower temperatures and pressures, leading to energy savings and reduced risk of accidents.
(5) Biocompatibility: nanoparticles synthesized using green methods often have better biocompatibility, making them suitable for biomedical applications.
(6) Versatility: a wide range of plant and microbe species can be used for green synthesis, offering diverse options for nanoparticle production with different properties and applications.
(2) Scalability: scaling up green synthesis processes to industrial levels can be challenging, as it may require large amounts of biological materials and optimized growth conditions.
(3) Contamination: the presence of various biomolecules in the biological extracts can lead to contamination, affecting the purity and properties of the synthesized nanoparticles.
(4) Extraction and purification: the separation and purification of nanoparticles from the biological extracts can be complex and time-consuming.
(5) Variability in biological sources: differences in plant or microbe species, growth conditions, and extraction methods can result in variations in the composition and properties of the synthesized nanoparticles.
(6) Research and development: green synthesis is an emerging field, and there is still a need for further research to optimize synthesis protocols and better understand the underlying mechanisms.
Metal oxides catalysts
In recent years, metal oxide-based materials have emerged as promising candidates for catalyzing the HER due to their superior structural and compositional flexibility.27Metal oxide catalysts are one of the most widely studied categories of materials for the hydrogen evolution reaction (HER). These catalysts have shown high activity and stability for the HER, making them attractive for use in various applications such as fuel cells and water splitting.
One of the main challenges in developing metal oxide catalysts for HER is improving their selectivity and stability. Metal oxide catalysts can degrade over time due to factors, e.g., catalyst poisoning, surface oxidation, and dissolution. Efforts are underway to develop new synthesis methods and surface modification techniques to improve the selectivity and stability of metal oxide catalysts for HER. Metal oxide catalysts represent a promising category of materials for the HER, with significant potential to enable the widespread adoption of hydrogen-based technologies for clean energy production and storage.54
Electrocatalysts are materials that lower the activation energy required for electrochemical reactions to occur. In the context of the HER, electrocatalysts catalyze the conversion of protons and electrons into hydrogen gas.27 This process is crucial for improving the efficiency, selectivity, and stability of the HER, and enabling practical applications of hydrogen-based technologies such as fuel cells and water splitting. Some commonly studied electrocatalysts for the HER include transition metals,55 carbon-based materials,56 and metal chalcogenides.57 Electrocatalysts performance is influenced by factors such as chemical composition, crystal structure, morphology, and surface area, as well as the operating conditions of the electrochemical cell. Research is ongoing to optimize electrocatalysts and improve the HER process for clean energy production and storage.
The literature encompasses a range of metal oxide-based HER electrocatalysts, e.g., single transition metal oxides, spinel oxides, perovskite oxides, metal (oxy)hydroxides, uniquely structured metal oxides, and oxide-containing hybrids, with a particular focus on strategies to enhance performance and the relationship between properties and activity.27 Studies on electrocatalysts for the HER covers a diverse range of materials, e.g., metals, alloys, metal phosphides/sulfides/selenides/carbides/nitrides, single atoms, functional carbon materials, and hybrid structures, which have been explored for their potential use in HER electrocatalysis. These materials have been extensively studied and exhibit significant promise for HER electrocatalysis.11
Ruthenium oxide (RuO2) is an efficient but expensive catalyst for HER. Recent research focuses on finding tremendous and cost-effective metal oxides alternatives, such as iron oxide (Fe2O3), nickel oxide (NiO), cobalt oxide (Co3O4), and manganese dioxide (MnO2),58,59 to make tactical and sustainable.
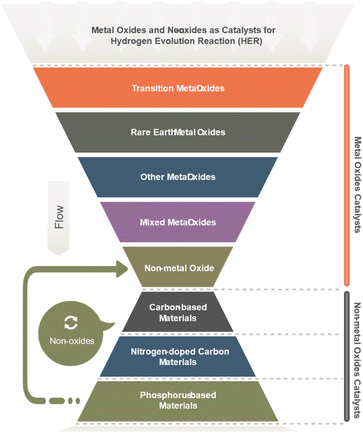
Molybdenum disulfide (MoS2) is a layered material showing excellent HER activity with high intrinsic catalytic activity and tunable electronic properties. Tungsten trioxide (WO3) semiconducting oxide demonstrates a high HER activity, particularly when combined with other metals or metalloids such as cobalt or phosphorus. Cobalt–phosphate (Co–Pi) is a complex metal oxide with high activity for HER, possessing a unique surface structure and electronic properties. Nickel–molybdenum–nitride (Ni–Mo–N), a nitride-based catalyst, has excellent HER activity and stability, even at high current densities. Iron-based oxides such as iron oxide (Fe2O3) and iron oxyhydroxide (FeOOH) have also been explored as HER catalysts due to their low cost and abundance, although their activity and stability can vary depending on their structure and synthesis method. The ongoing research efforts are focused on improving their efficiency, stability, and scalability for practical applications.60 Most metal oxides have been explored (Table 4) as HER catalysts, are depicted in Fig. 4.6,61
| No. | Main category | Sub-category |
|---|---|---|
| 1 | Transition metal oxides | Ruthenium oxide (RuO2) |
| Cobalt oxide (CoO) | ||
| Nickel oxide (NiO) | ||
| Iron oxide (Fe2O3) | ||
| Tungsten oxide (WO3) | ||
| Molybdenum oxide (MoO3) | ||
| Copper oxide (CuO) | ||
| Vanadium oxide (V2O5) | ||
| Chromium oxide (Cr2O3) | ||
| 2 | Rare earth metal oxides | Cerium oxide (CeO2) |
| Lanthanum oxide (La2O3) | ||
| Yttrium oxide (Y2O3) | ||
| 3 | Other metal oxides | Gallium oxide (Ga2O3) |
| Indium oxide (In2O3) | ||
| Lead oxide (PbO2) | ||
| Magnesium oxide (MgO) | ||
| Niobium oxide (Nb2O5) | ||
| Strontium oxide (SrO) | ||
| 4 | Mixed metal oxides | Zinc oxide (ZnO) |
| Tin oxide (SnO2) | ||
| Titanium oxide (TiO2) | ||
| Zinc–titanium oxide (ZnTiO3) | ||
| Zinc–iron oxide (ZnFe2O4) | ||
| 5 | Non-metal oxide | Silicon dioxide (SiO2) |
Metal oxide nanomaterials for sustainable energy applications
Energy storage
Energy storage plays a crucial role in today's society, as it helps balance the supply and demand of electricity, ensuring grid stability and reliability.62,63 It effectively integrates renewable energy sources, such as solar and wind, by storing excess energy produced during peak generation times and releasing it during low power production or high demand.64 Energy storage systems also contribute to power quality management, frequency regulation, and peak shaving, thereby enhancing the efficiency and resilience of the electrical grid. Furthermore, they support the widespread adoption of electric vehicles by providing charging infrastructure and extending the driving range.65Metal oxide nanomaterials have emerged as promising candidates for various energy storage applications due to their unique properties, such as high surface area, tunable electrochemical properties, and enhanced catalytic activity. These nanomaterials can be incorporated into energy storage devices like batteries, supercapacitors, and fuel cells to improve their performance, capacity, and cycling stability.66 Examples of metal oxide nanomaterials used in energy storage include lithium cobalt oxide (LiCoO2) and lithium iron phosphate (LiFePO4) for lithium-ion batteries, manganese dioxide (MnO2) and ruthenium oxide (RuO2) for supercapacitors, and cerium oxide (CeO2) for solid oxide fuel cells. The ongoing research and development of metal oxide nanomaterials aim to enhance energy storage systems' efficiency and capacity, fostering a more sustainable and reliable energy future.
The synthesis and characterization of metal oxide nanomaterials involve various techniques and methodologies to produce and analyze these materials for different applications. Common synthesis methods include the sol–gel process, a wet-chemical technique that forms a gel-like network by reacting metal alkoxides or metal salts with water.67 The hydrothermal synthesis method involves crystallizing materials under high-temperature and high-pressure aqueous conditions. Electrospinning is a fiber production method that uses an electric field to draw charged polymer solutions or melt them into continuous fibers. Lastly, chemical vapor deposition (CVD) is a process that deposits gaseous reactants onto a heated substrate, resulting in the formation of solid thin films or powders.68
Characterization techniques play a crucial role in understanding the properties and behavior of metal oxide nanomaterials. X-ray diffraction (XRD) is a technique used to determine crystalline materials' crystal structure, lattice parameters, and atomic positions.69 Scanning electron microscopy (SEM) is an imaging technique that provides high-resolution, three-dimensional images of the sample surface by scanning it with a focused electron beam. Transmission electron microscopy (TEM) is a high-resolution imaging technique that uses an electron beam to transmit through an ultrathin sample, providing detailed information about the sample's internal structure and morphology. Finally, energy-dispersive X-ray spectroscopy (EDX) is an analytical technique that determines the elemental composition and distribution within a sample by measuring the characteristic X-rays emitted upon interaction with an electron beam.70 These techniques collectively help researchers develop and refine metal oxide nanomaterials for various applications in energy, electronics, and other fields.
Batteries
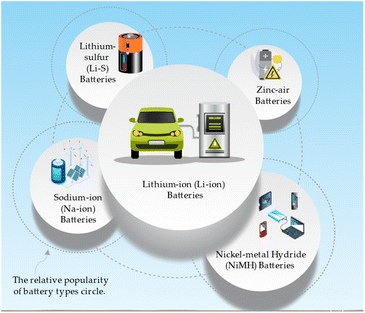
The common battery types that can benefit from nanotechnology and metal oxides and their applications can be directed in Fig. 5.71,72 Lithium-ion (Li-ion) batteries are widely used in electric vehicles, portable electronics, and grid energy storage due to their high energy and power density. Sodium-ion (Na-ion) batteries are a low-cost alternative to Li-ion batteries for grid energy storage and electric vehicles. Lithium–sulfur (Li–S) batteries have gained attention for their potential applications in electric aviation, electric vehicles, and high-energy portable electronics, as they possess very high energy density. These battery types demonstrate with high energy density and low-cost, zinc-air batteries are suitable for grid energy storage, electric vehicles, and remote area power systems.73 Nickel–metal hydride (NiMH) batteries are commonly utilized in hybrid electric vehicles, portable electronics, and backup power systems due to their balanced energy and power density and long cycle life.74A representation of the most common battery types is tentatively drawn in Table 5 and can vary depending on specific battery chemistries and designs.
| Battery type | Energy density [W h kg−1] | Power density [W kg−1] | Cycle life | Cost | Safety | Environmental impact |
|---|---|---|---|---|---|---|
| Lithium-ion | High | High | High | High | Medium | Low |
| Sodium-ion | Medium | Medium | Medium | Low | High | Low |
| Lithium–sulfur | Very high | Medium | Low | High | Low | Low |
| Zinc–air | High | Low | Medium | Low | High | Low |
| Nickel–metal hydride | Medium | Medium | High | Medium | High | Medium |
Supercapacitors
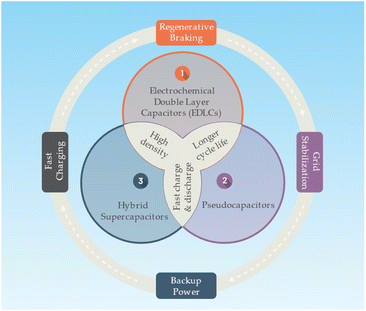
A supercapacitor or an ultracapacitor as an electrochemical capacitor is an energy storage device bridging the gap between conventional capacitors and batteries. Supercapacitors can store and release energy much faster than batteries, and they can endure millions of charge and discharge cycles without significant degradation.75 Supercapacitors store energy in an electric field that separates charges between two electrodes. The electrodes are immersed in an electrolyte, allowing ions to flow between the electrodes. The energy storage mechanism in supercapacitors is mainly based on electrostatic double-layer capacitance and pseudocapacitance (faradaic redox reactions). There is main there types of supercapacitors as follows76,77 (Fig. 6):• Electrochemical Double Layer Capacitors (EDLCs): EDLCs store energy in the electric double layer formed at the interface between the electrode and electrolyte. They usually use high surface area carbon materials as electrodes, and their energy storage is purely electrostatic.
• Pseudocapacitors: pseudocapacitors store energy through fast and reversible faradaic redox reactions occurring on the surface of the electrodes. These electrodes are typically made of metal oxides or conducting polymers.
• Hybrid supercapacitors: hybrid supercapacitors combine the features of EDLCs and pseudocapacitors, using one electrode with a high double-layer capacitance and another with a high pseudocapacitance.
Supercapacitors with a higher power density than batteries, allowing them to charge and discharge much faster. Typically having a lower energy density, meaning they store less energy per unit volume or mass. Supercapacitors also have a longer cycle life, with the ability to undergo millions of cycles without significant capacity loss, whereas batteries may degrade after several thousand cycles.77 Supercapacitors are well-suited for applications requiring rapid charge and discharge, such as regenerative braking in electric vehicles, short-term energy storage in grid systems, or backup power supplies. However, they are not ideal for long-term energy storage or applications requiring high energy density, where batteries are more suitable.
In upcoming research, the focus will be on delving deeper into the applications of metal oxide nanomaterials in photocatalytic water splitting for hydrogen production, solar cells, and thermoelectric materials.26,78 Exploring recent advances, underlying mechanisms, and materials optimization for each application will be conducted to assess potential contributions to sustainable energy production and environmental benefits. For photocatalytic water splitting, the investigation will cover promising metal oxide catalysts, efficiency, and strategies for performance enhancement. In the context of solar cells, the examination will include the role of metal oxide nanomaterials as electron transport layers or light-absorbing materials and their influence on efficiency and stability. Finally, the utilization of metal oxide nanomaterials in thermoelectric materials will be studied, focusing on the ability to convert waste heat into electricity and factors affecting thermoelectric performance.79 These future studies aim to offer a comprehensive understanding of cutting-edge research in these fields and facilitate the development and deployment of sustainable energy solutions.
Finally, the most famous metal oxides application in energy storage system can be briefed in Table 6.
| Metal oxide | Application | Performance boost (%) |
|---|---|---|
| FeOx | Batteries | 20 |
| MoOx | Supercapacitors | 25 |
| MMoO4 | Supercapacitors | 30 |
| MCo2O4 | Batteries | 22 |
| Binary metal oxide | Supercapacitors | 28 |
Nanotechnologies a compound formed of metal oxides
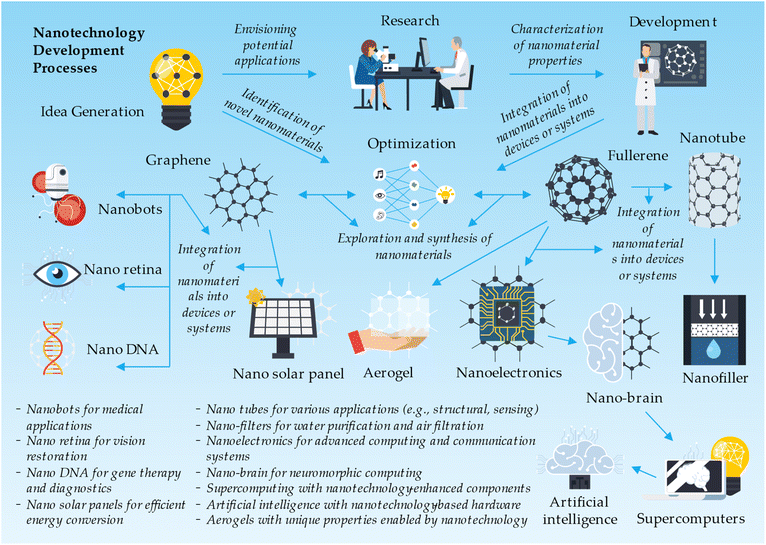
Nanotechnologies, a rapidly evolving field of science and engineering, focus on manipulating and controlling materials at the nanoscale. Among the various nanomaterials, compounds formed of metal oxides have gained significant attention due to their unique properties and versatile applications. Metal oxide nanomaterials, typically ranging from 1 to 100 nanometers in size, exhibit enhanced characteristics such as high surface area, tunable bandgap, and improved catalytic activity.84 These properties make metal oxide nanomaterials suitable for a wide range of applications, including energy storage, environmental remediation, electronics, healthcare, and sensing.39,40 As researchers continue to explore innovative methods for synthesizing and functionalizing metal oxide nanoparticles, the potential of these nanomaterials in revolutionizing various industries and addressing critical global challenges is becoming increasingly evident.46The nanotechnology development process starts with idea generation, where concepts such as nanobots, nano retina, nano DNA, graphene-based nano solar panels, fullerene-derived nanotubes, nano-filters, nanoelectronics, and nano-brain are conceived. Other ideas include nanotechnology applications in supercomputing, artificial intelligence, and aerogels. In the research and development phase, scientists explore and synthesize nanomaterials like graphene and fullerenes, characterizing and modifying them for specific applications. These nanomaterials are then integrated into devices or systems, such as nanobots for medical applications, nano retina for vision restoration, nano DNA for gene therapy and diagnostics, and nano solar panels for efficient energy conversion. Other integrations include nanotubes for various applications, nano-filters for water purification and air filtration, nanoelectronics for advanced computing and communication systems, nano-brain for neuromorphic computing, supercomputing with nanotechnology-enhanced components, artificial intelligence with nanotechnology-based hardware, and aerogels with unique properties enabled by nanotechnology. The development process also involves preclinical and clinical testing, regulatory approval, commercialization, post-market surveillance, and continuous improvement of nanotechnology-based products and applications (Fig. 7).
In conclusion, Table 7 provides a concise summary of specific metal oxides, their primary applications, associated benefits, and challenges. For instance, Titanium oxide (TiO2) is utilized in solar cells due to its high efficiency, though it faces stability concerns. Zinc oxide (ZnO) is employed in sensors, prized for its high sensitivity, but has a limited operational range. Copper oxide (CuO) is used in water treatment as a photocatalyst, proving effective in water purification, but is susceptible to photocorrosion. The table also highlights the emerging class of layered oxide 2D materials with potential applications in supercapacitor electrodes when combined with porous carbon. These materials exhibit notable electrochemical behavior but face challenges related to faradaic proton/alkali metal interactions.
| Metal oxide | Application | Benefits | Challenges |
|---|---|---|---|
| TiO2 | Solar cells | High efficiency | Stability issues |
| ZnO | Sensors | High sensitivity | Limited range |
| CuO | Water treatment with CuO-based photocatalysts | Effective in water purification | Photocorrosion of CuO |
| Various | Layered oxide 2D | Emerging class | Research opportunities |
| Supercapacitor electrode applications with porous carbon | Electrochemical behavior | Faradaic proton/alkali metal |
Why metal oxide nanomaterial and nanotechnology
Metal oxide nanomaterials and nanotechnology have garnered significant interest due to their unique properties at the nanoscale. These properties include high surface area, tunable bandgap, enhanced catalytic activity, and improved electrical, optical, and mechanical performance. Therefore, metal oxide nanomaterials and nanotechnology have numerous applications in various fields, such as energy, environment, healthcare, and electronics.For instance, metal oxide nanomaterials are used to improve the performance of batteries, supercapacitors, and solar cells, enabling more efficient and sustainable energy production and storage. Additionally, they have been employed for environmental applications, such as water purification, air filtration, and pollutant degradation, contributing to cleaner air and water resources. In the health sector, they play a role in drug delivery, diagnostics, and regenerative medicine. All told, metal oxide nanomaterials and nanotechnology offer great potential for addressing some of the most pressing global challenges and promoting sustainable development. The critical role of metal oxide nanomaterials in sustainable energy and environmental applications is summarized as follows:22,90–94
• Enhanced properties: metal oxide nanomaterials exhibit unique properties, such as high surface area, tunable bandgap, and improved catalytic activity, which make them ideal for various energy and environmental applications.
• Energy storage: metal oxide nanomaterials can improve the performance of batteries and supercapacitors, increasing their capacity, rate capability, and cycling stability, which is essential for renewable energy integration and electric vehicles.
• Photocatalytic water splitting: metal oxide nanomaterials can act as efficient photocatalysts for water splitting, generating hydrogen as a clean and renewable energy carrier.
• Solar cells: metal oxide nanomaterials can be employed as electron transport layers or light-absorbing materials in solar cells, enhancing their efficiency and stability.
• Thermoelectric materials: metal oxide nanomaterials can improve the thermoelectric performance of materials, enabling the conversion of waste heat into electricity for sustainable energy generation.
• Environmental remediation: metal oxide nanomaterials can effectively adsorb or degrade various pollutants in water and air, contributing to removing contaminants and improving environmental quality.
• Photocatalytic degradation of pollutants: metal oxide nanomaterials can accelerate the degradation of organic and inorganic pollutants under light irradiation, offering a good water and air purification approach.
• Environmental sensors: metal oxide nanomaterials can serve as sensitive and selective materials in ecological sensors, enabling the real-time monitoring of pollutants and hazardous substances in air, water, and soil.
• Green synthesis: metal oxide nanomaterials can be synthesized using green chemistry approaches, minimizing the environmental impact of their products and promoting sustainable manufacturing practices.
• Cost-effectiveness: many metal oxides nanomaterials, such as TiO2, ZnO, and CuO, are abundant and inexpensive, making them attractive options for large-scale deployment in energy and environmental applications.
The way forward
Some topics, including the applications of metal oxide nanomaterials in environmental contexts, photocatalytic degradation of pollutants with a focus on water treatment and air purification, and the use of metal oxide nanomaterials in environmental sensors such as gas sensors and biosensors, are not covered. However, due to their functionality in improving water and air quality by breaking down pollutants into less harmful substances and their ability to detect and monitor pollutant levels in various environments, these materials are considered essential for improved environmental management.Another area of focus will be environmental remediation techniques, specifically adsorption and membrane-based separation methods. The efficiency and potential of metal oxide nanomaterials in these applications will be examined, highlighting their essential role in removing pollutants from water and other environments. Selected metal oxides, such as TiO2, ZnO, WO3, CuO, and Cu2O, will be the focus of case studies, exploring their synthesis, properties, and applications in sustainable energy and environmental contexts. Recent advances and challenges in utilizing these metal oxides will also be discussed. These future studies will contribute to a comprehensive understanding of metal oxide nanomaterials' role in advancing sustainable solutions for energy and environmental nanotechnology challenges.
Conclusions
The Hydrogen Evolution Reaction (HER) plays a critical role in producing hydrogen as a clean energy source, and metal oxide catalysts have shown promising results in enhancing the efficiency and selectivity of the HER. Despite challenges such as the high cost of catalysts and slow kinetics, the HER offers several advantages, including producing only water vapor as a byproduct and using abundant resources. Metal oxides, including transition metal oxides and rare earth metal oxides, have been extensively studied as HER catalysts, with various strategies being explored to improve their activity, stability, and selectivity. The ongoing research efforts in this field aim to develop more efficient and cost-effective methods for hydrogen production, promoting the widespread adoption of hydrogen as a clean energy source. Future research could further explore using metal oxides in combination with other catalysts or materials to enhance their performance in HER applications.Metal oxide nanomaterials synthesized using green chemistry approaches have demonstrated significant potential for various sustainable energy and environmental applications. These materials offer viable solutions to the lion's share of global energy and environmental challenges. By focusing on optimizing green synthesis methods, overcoming limitations in metal oxide nanomaterial applications, and exploring new materials for sustainable energy, studies can pave the way for a cleaner and more sustainable future. Moreover, the investigation into metal oxide nanomaterials in emerging nanotechnologies, such as photocatalytic water splitting, solar cells, and thermoelectric materials, will be essential to unlock the full potential of metal oxide nanomaterials and contribute to the deployment of innovative, eco-friendly nanotechnologies. Metal oxide nanomaterials exhibit immense potential in addressing the urgent energy and environmental issues confronting our planet.
We can attain a cleaner and more sustainable future by employing eco-friendly synthesis techniques and investigating innovative materials. Utilizing metal oxide nanomaterials in cutting-edge nanotechnologies, including photocatalytic water splitting, solar cells, and thermoelectric materials, offers considerable prospects for implementing inventive, environmentally-conscious solutions. Ongoing research and development in this domain are crucial for effecting transformative changes in the energy and environmental industries, empowering us to establish a greener, more robust future for posterity.
Conflicts of interest
There are no conflicts to declare.References
- L. Barreto, A. Makihira and K. Riahi, Int. J. Hydrogen Energy, 2003, 28, 267–284 CrossRef CAS.
- R. L. Ottinger, Nat. Resour. Forum, 1992, 16, 11–17 CrossRef.
- M. Hafizyar, A. R. Arsallan, N. R. Sabory, M. S. S. Danish and T. Senjyu, in Sustainability Outreach in Developing Countries, ed. M. S. S. Danish, T. Senjyu and N. R. Sabory, Springer Singapore, Singapore, 2021, pp. 65–80 Search PubMed.
- L. N. Proskuryakova, in Handbook of Energy and Environmental Security, Elsevier, 2022, pp. 399–413 Search PubMed.
- B. Shen, Y. Han, L. Price, H. Lu and M. Liu, Energy, 2017, 118, 526–533 CrossRef.
- M. S. S. Danish, L. L. Estrella, I. M. A. Alemaida, A. Lisin, N. Moiseev, M. Ahmadi, M. Nazari, M. Wali, H. Zaheb and T. Senjyu, Metals, 2021, 11, 80 CrossRef CAS.
- J. Chow, R. J. Kopp and P. R. Portney, Science, 2003, 302, 1528–1531 CrossRef PubMed.
- M. S. S. Danish and T. Senjyu, Circ. Econ., 2023, 2, 100040 Search PubMed.
- J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K.-Y. Wong, Chem. Rev., 2020, 120, 851–918 CrossRef CAS PubMed.
- N. Mahmood, Y. Yao, J.-W. Zhang, L. Pan, X. Zhang and J.-J. Zou, Adv. Sci., 2018, 5, 1700464 CrossRef PubMed.
- M. Zeng and Y. Li, J. Mater. Chem. A, 2015, 3, 14942–14962 RSC.
- C. Tryggestad, N. Sharma, O. Rolser, B. Smeets, M. Wilthaner, J. van de Staaij, T. Gruenewald, J. Noffsinger and L. Tiemersma, Global Energy Perspective 2022: Executive Summary, McKinsey & Company, New York, USA, 2022 Search PubMed.
- J. Cochran, A. Burdick, M.-A. Evans, J. Kiviluoma, S. Sergici, P. D. Martini and K. Dragoon, IEEE Power Energy Mag., 2022, 20, 112 Search PubMed.
- P. Fairley, IEEE Spectrum, 2023, 60, 24–27 Search PubMed.
- M. Ge, J. Friedrich and L. Vigna, 4 Charts Explain Greenhouse Gas Emissions by Countries and Sectors, World Resour. Inst., https://www.wri.org/insights/4-charts-explain-greenhouse-gas-emissions-countries-and-sectors Search PubMed.
- Y. Yang, X. Sun, X. Zhu and Y. Xie, Procedia Comput. Sci., 2013, 17, 720–728 CrossRef.
- R. A. Al-Masri, J. Chenoweth and R. J. Murphy, Environ. Sci. Policy, 2019, 100, 192–204 CrossRef.
- The Future of Green Hydrogen and Its Challenges, https://www.geeksforgeeks.org/future-of-green-hydrogen-and-its-challenges/, accessed March 25, 2023 Search PubMed.
- X. Yan, D. Deng, S. Wu, H. Li and L. Xu, Chin. J. Struct. Chem., 2022, 41, 2207004–2207015 CAS.
- J. O. Bockris and E. C. Potter, J. Electrochem. Soc., 1952, 99, 169 CrossRef CAS.
- X. M. C. Ta, R. Daiyan, T. K. A. Nguyen, R. Amal, T. Tran-Phu and A. Tricoli, Adv. Energy Mater., 2022, 12, 2201358 CrossRef CAS.
- M. S. S. Danish, L. L. Estrella-Pajulas, I. M. Alemaida, M. L. Grilli, A. Mikhaylov and T. Senjyu, Metals, 2022, 12, 769 CrossRef CAS.
- M. S. S. Danish, A. Bhattacharya, D. Stepanova, A. Mikhaylov, M. L. Grilli, M. Khosravy and T. Senjyu, Metals, 2020, 10, 1604 CrossRef CAS.
- H. Bian, Z. Chen, T. Chen, M. Humayun, B. Zhou, W. Liao, Z. Li, Z. Zhang, C. Wang and C. Liu, Chem. Eng. J., 2023, 466, 143045 CrossRef CAS.
- X. Liu, W. Liu, M. Ko, M. Park, M. G. Kim, P. Oh, S. Chae, S. Park, A. Casimir, G. Wu and J. Cho, Adv. Funct. Mater., 2015, 25, 5799–5808 CrossRef CAS.
- Y. Yang, S. Niu, D. Han, T. Liu, G. Wang and Y. Li, Adv. Energy Mater., 2017, 7, 1700555 CrossRef.
- Y. Zhu, Q. Lin, Y. Zhong, H. A. Tahini, Z. Shao and H. Wang, Energy Environ. Sci., 2020, 13, 3361–3392 RSC.
- T. Ling, T. Zhang, B. Ge, L. Han, L. Zheng, F. Lin, Z. Xu, W.-B. Hu, X.-W. Du, K. Davey and S.-Z. Qiao, Adv. Mater., 2019, 31, 1807771 CrossRef PubMed.
- R. Ramachandran and R. K. Menon, Int. J. Hydrogen Energy, 1998, 23, 593–598 CrossRef CAS.
- M. B. Syed, in Bioenergy Resources and Technologies, ed. A. K. Azad and M. M. K. Khan, Academic Press, 2021, pp. 157–198 Search PubMed.
- K. Zeng and D. Zhang, Prog. Energy Combust. Sci., 2010, 36, 307–326 CrossRef CAS.
- M. S. S. Danish, Z. Nazari and T. Senjyu, Energy Convers. Manage., 2023, 286, 117063 CrossRef.
- L. Bertuccioli, A. Chan, D. Hart, F. Lehner, B. Madden and E. Standen, Development of Water Electrolysis in the European Union, Clean Hydrogen Partnership, https://www.clean-hydrogen.europa.eu/media/publications/development-water-electrolysis-european-union_en, https://www.clean-hydrogen.europa.eu/document/download/f08530ed-9453-4a50-8f1f-668b5320a468_en?filename=study%20electrolyser_0-Logos_0.pdf Search PubMed.
- A. Ursua, L. M. Gandia and P. Sanchis, Proc. IEEE, 2012, 100, 410–426 CAS.
- F. Marangio, M. Santarelli and M. Calì, Int. J. Hydrogen Energy, 2009, 34, 1143–1158 CrossRef CAS.
- P. Moçoteguy and A. Brisse, Int. J. Hydrogen Energy, 2013, 38, 15887–15902 CrossRef.
- M. Lehner, R. Tichler, H. Steinmüller and M. Koppe, Power-to-Gas: Technology and Business Models, Springer, New York, 2014th edn, 2014 Search PubMed.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- G. Rando, S. Sfameni, M. Galletta, D. Drommi, S. Cappello and M. R. Plutino, Molecules, 2022, 27, 4856 CrossRef CAS PubMed.
- V. C. Hoang, K. Dave and V. G. Gomes, Nano Energy, 2019, 66, 104093 CrossRef CAS.
- J. Jeevanandam, S. F. Kiew, S. Boakye-Ansah, S. Y. Lau, A. Barhoum, M. K. Danquah and J. Rodrigues, Nanoscale, 2022, 14, 2534–2571 RSC.
- F. Khan, A. Shahid, H. Zhu, N. Wang, M. R. Javed, N. Ahmad, J. Xu, Md. A. Alam and M. A. Mehmood, Chemosphere, 2022, 293, 133571 CrossRef CAS PubMed.
- R. A. de Jesus, G. C. de Assis, R. J. de Oliveira, M. Bilal, R. N. Bharagava, H. M. N. Iqbal, L. F. R. Ferreira and R. T. Figueiredo, in Biodegradation and Biodeterioration at the Nanoscale, ed. H. M. N. Iqbal, M. Bilal, T. A. Nguyen and G. Yasin, Elsevier, 2022, pp. 529–560 Search PubMed.
- W. Koo-amornpattana, P. Phadungbut, N. Kunthakudee, W. Jonglertjunya, S. Ratchahat and M. Hunsom, Sci. Rep., 2023, 13, 4705 CrossRef CAS PubMed.
- G. Bhattacharya, S. J. Fishlock, J. A. McLaughlin and S. S. Roy, Int. J. Energy Res., 2021, 45, 8091–8102 CrossRef CAS.
- F. Li, J. Ran, M. Jaroniec and S. Z. Qiao, Nanoscale, 2015, 7, 17590–17610 RSC.
- I. Soares, P. Ferreira and L. Hens, Environ. Dev. Sustainable, 2018, 20, 1–5 CrossRef.
- M. Phour, M. S. S. Danish, N. R. Sabory, M. Ahmadi and T. Senjyu, Sustainability, 2022, 14, 10676 CrossRef CAS.
- P. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, New York, 2000 Search PubMed.
- A. Varughese, R. Kaur and P. Singh, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 961, 012011 CAS.
- M. Huston, M. DeBella, M. DiBella and A. Gupta, Nanomaterials, 2021, 11, 2130 CrossRef CAS PubMed.
- N. E.-A. El-Naggar, W. I. A. Saber, A. M. Zweil and S. I. Bashir, Sci. Rep., 2022, 12, 3515 CrossRef CAS PubMed.
- V. Chandrakala, V. Aruna and G. Angajala, Emergent Mater., 2022, 5, 1593–1615 CrossRef CAS PubMed.
- M. S. S. Danish, T. Senjyu, A. M. Ibrahimi, A. Bhattacharya, Z. Nazari, S. M. S. Danish and M. Ahmadi, J. Sustainable Energy Revolution, 2021, 2, 6–15 CrossRef.
- N. Kaeffer and W. Leitner, JACS Au, 2022, 2, 1266–1289 CrossRef CAS PubMed.
- G. Collins, P. R. Kasturi, R. Karthik, J.-J. Shim, R. Sukanya and C. B. Breslin, Electrochim. Acta, 2023, 439, 141678 CrossRef CAS.
- J. Zhao, J. Wang, Z. Chen, J. Ju, X. Han and Y. Deng, APL Mater., 2021, 9, 050902 CrossRef CAS.
- B. H. R. Suryanto, Y. Wang, R. K. Hocking, W. Adamson and C. Zhao, Nat. Commun., 2019, 10, 5599 CrossRef CAS PubMed.
- Y. Lei, Y. Yang, Y. Liu, Y. Zhu, M. Jia, Y. Zhang, K. Zhang, A. Yu, J. Liu and J. Zhai, Nanoscale Res. Lett., 2019, 14, 329 CrossRef PubMed.
- Y. Zhang, Q. Fu, B. Song and P. Xu, Acc. Mater. Res., 2022, 3, 1088–1100 CrossRef CAS.
- F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet and X. Hu, J. Am. Chem. Soc., 2018, 140, 7748–7759 CrossRef CAS PubMed.
- M. Furukakoi, O. B. Adewuyi, M. S. S. Danish, A. M. Howlader, T. Senjyu and T. Funabashi, Int. J. Electr. Power Energy Syst., 2018, 100, 50–57 CrossRef.
- M. S. S. Danish, A. Yona and T. Senjyu, Int. J. Emerg. Electr. Power Syst., 2015, 16, 107–115 Search PubMed.
- S. H. Mirbarati, N. Heidari, A. Nikoofard, M. S. S. Danish and M. Khosravy, Sustainability, 2022, 14, 15036 CrossRef.
- S. M. S. Danish, M. Ahmadi, M. S. S. Danish, P. Mandal, A. Yona and T. Senjyu, J. Energy Storage, 2020, 32, 101823 CrossRef.
- J. Mei, T. Liao, L. Kou and Z. Sun, Adv. Mater., 2017, 29, 1700176 CrossRef PubMed.
- D. Bokov, A. Turki Jalil, S. Chupradit, W. Suksatan, M. Javed Ansari, I. H. Shewael, G. H. Valiev and E. Kianfar, Adv. Mater. Sci. Eng., 2021, 2021, e5102014 Search PubMed.
- G.-H. Park, K. Nielsch and A. Thomas, Adv. Mater. Interfaces, 2019, 6, 1800688 CrossRef.
- J. Li and J. Sun, Application of X-ray Diffraction and Electron Crystallography for Solving Complex Structure Problems, Acc. Chem. Res., 2017, 50, 2737–2745 CrossRef CAS PubMed.
- K. V. Ivanov and V. E. Ovcharenko, Solid State Phenom., 2020, 303, 59–66 Search PubMed.
- G. Yasin, M. Arif, T. Mehtab, X. Lu, D. Yu, N. Muhammad, M. T. Nazir and H. Song, Energy Storage Mater., 2020, 25, 644–678 CrossRef.
- Y. Sun, J. Sun, J. S. Sanchez, Z. Xia, L. Xiao, R. Chen and V. Palermo, Chem. Commun., 2023, 59, 2571–2583 RSC.
- R. Sun, Q. Liu and W. Deng, Chin. J. Struct. Chem., 2022, 41, 2205085–2205092 CAS.
- L. Dong, W. Yang, W. Yang, Y. Li, W. Wu and G. Wang, J. Mater. Chem. A, 2019, 7, 13810–13832 RSC.
- M. S. Yadav, J. Nanopart. Res., 2020, 22, 367 CrossRef CAS.
- G. M. Tomboc, B. Tesfaye Gadisa, M. Jun, N. K. Chaudhari, H. Kim and K. Lee, Chem. – Asian J., 2020, 15, 1628–1647 CrossRef CAS PubMed.
- Y. Kumar, S. Rawal, B. Joshi and S. A. Hashmi, J. Solid State Electrochem., 2019, 23, 667–692 CrossRef CAS.
- S. Singla, S. Sharma, S. Basu, N. P. Shetti and T. M. Aminabhavi, Int. J. Hydrogen Energy, 2021, 46, 33696–33717 CrossRef CAS.
- A. Kim, P. Kumar, P. K. Annamalai and R. Patel, Adv. Mater. Interfaces, 2022, 9, 2201659 CrossRef.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS PubMed.
- Z.-S. Wu, G. Zhou, L.-C. Yin, W. Ren, F. Li and H.-M. Cheng, Nano Energy, 2012, 1, 107–131 CrossRef CAS.
- Y. Zhang, L. Li, H. Su, W. Huang and X. Dong, J. Mater. Chem. A, 2015, 3, 43–59 RSC.
- B. L. Ellis, P. Knauth and T. Djenizian, Adv. Mater., 2014, 26, 3368–3397 CrossRef CAS PubMed.
- H. I. Abdullah, A. A. Al-Amiery and S. B. Al-Baghdadi, J. Phys.: Conf. Ser., 2021, 1853, 012052 CrossRef CAS.
- V. H. Grassian, J. Phys. Chem. C, 2008, 112, 18303–18313 CrossRef CAS.
- E. Lee, Y. S. Yoon and D.-J. Kim, ACS Sens., 2018, 3, 2045–2060 CrossRef CAS PubMed.
- Metal Oxide Nanomaterials for Chemical Sensors, ed. M. A. Carpenter, S. Mathur and A. Kolmakov, Springer, 2013th edn, 2012 Search PubMed.
- P. Kumbhakar, C. Chowde Gowda, P. L. Mahapatra, M. Mukherjee, K. D. Malviya, M. Chaker, A. Chandra, B. Lahiri, P. M. Ajayan, D. Jariwala, A. Singh and C. S. Tiwary, Mater. Today, 2021, 45, 142–168 CrossRef CAS.
- P. Veerakumar, A. Sangili, S. Manavalan, P. Thanasekaran and K.-C. Lin, Ind. Eng. Chem. Res., 2020, 59, 6347–6374 CrossRef CAS.
- A. M. Huerta-Flores, L. M. Torres-Martínez and E. Moctezuma, Int. J. Hydrogen Energy, 2017, 42, 14547–14559 CrossRef CAS.
- N. Mphuthi, L. Sikhwivhilu and S. S. Ray, Biosensors, 2022, 12, 386 CrossRef CAS PubMed.
- R. Wang, X. Li, Z. Nie, Y. Zhao and H. Wang, J. Energy Storage, 2021, 38, 102479 CrossRef.
- C. Han, Z. Li and S. Dou, Chin. Sci. Bull., 2014, 59, 2073–2091 CrossRef CAS.
- G. M. Nair, T. Sajini and B. Mathew, Talanta Open, 2022, 5, 100080 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |