Open Access Article
Open Access ArticleFrom plant to cancer drug: lessons learned from the discovery of taxol
Nadja B.
Cech
 * and
Nicholas H.
Oberlies
* and
Nicholas H.
Oberlies

University of North Carolina Greensboro, USA. E-mail: nadja_cech@uncg.edu; n_oberli@uncg.edu
First published on 14th July 2023
Abstract
Many researchers in the natural product sciences dream of discovering a successful drug. For almost all of us, this dream will never be realized. Among the heroes of our past, though, there is a team whose efforts led to the discovery of not one but two new drugs. Dr Monroe Wall and Dr Mansukh Wani isolated and solved the structures for taxol and camptothecin, plant-based compounds that continue to play a critical role in cancer therapy today. Since the 1960s and 1970s when Wall, Wani and collaborators did their seminal work, there have been tremendous technological advances in the natural product sciences. With access to most sophisticated technology, it might be expected that the rate of discovery of new drugs from plants and other sources would have sped up. However, this has not come to pass. Why is this? Is it that the promise of new drug candidates from plant-based sources has been exhausted? Has our fascination with new technologies and with the promise of the genomics revolution caused us to stop investing effort and resources in the practices that are proven to yield success? With this Viewpoint, we share the story of taxol's discovery, highlighting critical challenges that were overcome and considering their relevance to botanical natural products drug discovery today. We hope that consideration of lessons learned from the past will help fuel success by researchers currently studying plants with the goal of discovering promising therapeutic leads.
In August 1962, a team lead by botanist Dr Arthur Barclay stripped bark off a yew tree (Taxus brevifolia) growing in the Gifford Pinchot National Forest in Washington State. They put the bark in a bag, coded it, and sent it off for cytotoxicity screening. The sample was unremarkable, one of 114
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plant specimens collected by botanists like Barclay under the umbrella of a National Cancer Institute (NCI) program that took place between 1960 and 1981.1 The goal of the program, however, was ambitious and somewhat controversial – find a cure for cancer.
000 plant specimens collected by botanists like Barclay under the umbrella of a National Cancer Institute (NCI) program that took place between 1960 and 1981.1 The goal of the program, however, was ambitious and somewhat controversial – find a cure for cancer.
Yew bark showed promise in initial cytotoxicity screens, but more of it was needed for isolation efforts. In 1964, Barclay returned to the original site and collected more than 30 pounds of material. That sample found its way to the Research Triangle Institute in North Carolina, where Dr Monroe Wall charged his colleague Dr Mansukh Wani the task of purifying it down to a single active compound. As Dr Wani describes it, it was a difficult assignment. “Because the instrumentation that was available for chromatographic separation were not too advanced…it took us 1 and ½ years to isolate…½ gram of taxol, almost 4 thousandths of a percent yield.†”
In honor of the genus Taxus, and because the compound appeared to contain a hydroxy group, Dr Wall named the material that Dr Wani and his colleagues isolated “taxol”. At the time, the structure of taxol was still unknown, and neither Dr Wall nor Dr Wani could have guessed how important the molecule would one day become. Today, taxol is the standard of care in the treatment of multiple malignancies, including breast and ovarian cancer, as described elsewhere in this themed issue [Mize et al., DOI: 10.1039/D2NP00091A]. The impact of this drug on oncology cannot be overstated. Clinicians old enough to remember describe the field in terms of the days before taxol, and the (much brighter) days after.2
The landscape for natural products research has changed dramatically since Dr Wall, Dr Wani and their collaborators isolated and identified taxol. We have at our disposal advanced technologies for isolation and structure elucidation, a wealth of genomic data, new tools for enzymatic manipulation of small molecule structures [Barnum et al., DOI: 10.1039/D2NP00077F], a better understanding of the molecular targets of natural product compounds [Mata et al., DOI: 10.1039/D3NP00007A], and astronomically more powerful computational capabilities for data interpretation. Yet drug discovery from plants (and other organisms) remains a tremendously challenging undertaking with a low rate of success. This viewpoint is written to glean insights from the taxol story about what it takes to turn a plant into a drug.
When the National Cancer Institute initially set out to study plants as a source of anti-cancer agents in the 1960s, not everyone was excited about the idea. As Dr Wani describes it, “There was a lot of public pressure on NCI that they should evaluate the natural products for the discovery of anticancer compounds, and they would always cite examples of countries like India, China, Latin America, Africa, where for centuries herbal products have been used as medicine. The Officers at the National Cancer Institutes were not too excited about exploring the potential of natural products for anticancer agents because maybe they were prejudice, and they said, yeah, nothing is going out of it. It's a waste of money. But because of the public pressure, they decided to start the evaluation of natural products as potential anticancer agents but on a limited basis with limited funding and therefore initially they decided to evaluate the natural products from US flora.”
Those who engage in natural product drug discovery from plants will not find Dr Wani's description particularly surprising. Pervasive in the scientific community in the United States today is the sentiment that natural product drug discovery, particularly plant-based drug discovery, is “old science,” a fishing expedition in overfished seas. Yet plants continue to play a tremendously important role in traditional and modern health care practices around the globe. Given that many of the drugs clinicians rely on today (vinblastine, cardiac glycosides, and aspirin, to name a few) are plant derived compounds, the line that has been drawn between “western” and “traditional” medicine is an artificial one. Further blurring this line, herbal preparations are regularly prescribed by pharmacists in much of Europe, and in the US, the FDA established in 2004 a path to drug approval for botanical preparations where the active ingredient is not known.3 So far, the FDA has approved two complex botanical preparations that showed clinical efficacy; Veregen, from green tea (Camellia sinensis), used topically as a treatment for genital warts, and Fulyzaq, from the South American Tree Croton lechleri, used to address diarrhea in HIV/AIDS patients receiving anti-retroviral therapy.3 As was the case with Fulyzaq, drug discovery efforts from plant sources are often informed by traditional use, so called “ethnobotanical” knowledge. This topic is addressed in more depth by Weathers [DOI: 10.1039/d2np00072e], and Sunmin et al. [DOI: 10.1039/D2NP00090C] in other articles included in this themed issue.
Perhaps Dr Wani is correct when he mentions prejudice as the root of the mismatch between evidence and attitude where plant-based drug discovery is concerned. Today, even with the wealth of scientifically rigorous studies demonstrating the therapeutic value of plant preparations and their isolated constituents, negative biases towards plant-based drug discovery efforts persist. However, bias is not the only issue that makes the pursuit of druggable molecules from plants challenging. Plants are complex, many of them have already been well studied, and thanks to recent court decisions, the likelihood of new intellectual property from plant-based sources is uncertain at best.4 The possibility of multiple compounds contributing to the biological activity of plant extracts, a topic that has been the subject of previous reviews5 and is also addressed in this themed issue [Weathers, DOI: 10.1039/d2np00072e], further complicates matters. Although a potential solution to this last problem is the development of a botanical drug like Veregren or Fulyzaq, such a strategy has many challenges of its own, as discussed elsewhere.3 Ultimately, for all of the reasons outlined here and more, drug discovery from plants is a challenging pursuit. Thus, it is worth considering the lessons we might learn from the discovery of taxol as we contemplate effective strategies to continue leveraging plants in future drug discovery efforts.
More than once along the path towards taxol's eventual development as an effective drug, the quest was nearly abandoned. The first of these crucial junctures was in 1964, when the Pacific yew bark extract that showed cytotoxicity in vitro (against KB cells, a cell line from human epithelial cells isolated from a carcinoma of the mouth) was tested in an in vivo model of leukemia and was found to be inactive.6 This might have been the end of the studies with Pacific yew, had it not been for Dr Wall, who was interested in the sample based on his prior experience with cancer drug discovery from plants. Indeed, taxol was not the only molecule the Wall and Wani team worked on that would eventually become a cancer drug. In 1966 they reported the structure of camptothecin,7 isolated from the plant Camptotheca acuminata. Today, two camptothecin derivatives (irinotecan and topotecan) are used to treat multiple types of cancer,8 including leukemia, pancreatic cancer, colon cancer, and ovarian cancer. As recently as August of 2022, a new antibody-bound camptothecin derivative (trastuzumab deruxtecan) was approved for the treatment of specific types of inoperable or metastatic breast cancers.9
It was that prior experience with camptothecin that helped Wall to recognize the promise of T. brevifolia as an anti-cancer lead. The 30 pounds of yew bark that Barclay collected went first to the laboratory of Dr Morris Kupchan. As Dr Wani describes it, Kupchan, who at the time was more prominent than Wall in the field of natural products research, was not interested in pursuing the sample because of its high cytotoxicity. There was a belief that the anti-cancer leads should be cytotoxic, but not too cytotoxic. Dr Wall, however, did not entirely subscribe to this opinion, and he requested that the sample be sent to his laboratory. According to Dr Wani, “Dr Wall told them [the NCI] that…. we are interested in working with this because prior to that we isolated camptothecin, a promising anti-cancer agent in 1966, under contract with NCI. And, it did become finally an FDA approved drug…so that is how RTI got the plant.” As Dr Wall describes it,6 he considered the Pacific yew extract to be a promising lead because he had previously observed a strong correlation between in vivo efficacy and in vitro cytotoxicity against KB cells. He requested the opportunity to study samples that were active against KB cells, and Pacific yew was one of these. Wall's expectation of in vivo activity was eventually confirmed. Despite the initial disappointing in vivo results with the complex T. brevifolia extract, fractions of the extract showed in vivo activity against P-534 leukemia, P388 leukemia, and Walker 245 carcinosarcoma.6 All of these assays were used at various times to aid in the eventual isolation of pure taxol.6
Isolation of taxol was difficult, but solving its structure proved even more challenging. Dr Wani describes one afternoon more than a year into the project when it almost came to an untimely end. “Dr Wall called me into his office. He addressed me as Dr Wani.…I said…there is some trouble.…When there was some trouble, he used to call me Dr Wani. So as soon as I went into his office, he said, before I even sat down. ‘…Wani, you have been trying to characterize taxol for the last year and a half. You don't know the exact molecular weight. You don't know if the nitrogen is an impurity or if it is actually present in taxol. We don't have unlimited money. We have to show progress every quarter because this is a contract. And NCI also was not interested because they said that, hey, bark is the only source, commercially feasible synthesis would be difficult…’ So Dr Wall told me, ‘forget about taxol’…I did not want to give up…So I told Dr Wall, I told him I understand I'm not doing well. We don't have money. We have to work on camptothecin and other things. Can I work on it on a low priority basis?” Dr Wall agreed, and Wani continued his work to solve the structure of taxol. As he would often tell students, a ‘low priority basis’ meant working on it in the evenings, on weekends, and over holidays, rarely returning home before 8:00 pm. All told, it took Dr Wani another five years to finally publish the structure of taxol. He eventually succeeded after subjecting the compound to Zemplén methanolysis10 (he checked the progress of the reaction using TLC plates that he prepared himself) to break it into two parts for which he could obtain crystals suitable for X-ray crystallography.
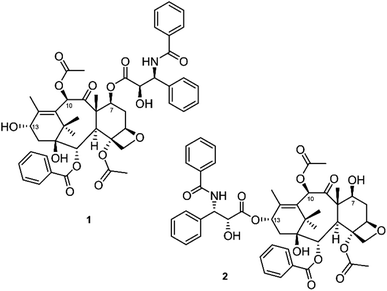
One of the challenging questions in solving the structure of taxol was to decide how the two halves produced by methanolysis should be reconnected. As Wani explains, “…once we got the structures from the crystallographer then we now had to decide with the data that I collected whether taxol is structure A or B. We depended on my expertise on where to put the side chain and the acetate group…We put the side chain on the 7 position and the acetate on the 10 position on the basis of my data that I had suggested and not the other way around. We all agreed and dispersed from the meeting. And Dr Wall immediately said, ‘Wani, we know it is structure A, start writing your publication. We want to publish as soon as possible.”
But Dr Wani didn't just want a publication, he wanted what he called a “good publication”. He had an idea for one more experiment that would strengthen the taxol paper, which was to modify what he thought was the structure of taxol (Fig. 1) to convert the allylic -OH moiety to an α,β-unsaturated ketone, making the molecule more susceptible to biological nucleophiles and therefore more active (i.e., susceptible to a Michael addition).11 In the words of Dr Wani, “…I wanted to get that compound because the OH was free and put it in the publication with the biological data. I struggled for five weeks. One day Dr Wall called really mad. He says ‘Wani we know taxol is structure A. Why are you wasting your time? We don't want to be scooped.’ And he was right. But by the hand of providence, I was not trying to confirm the structure, I was trying to prepare that molecule so that my publication would have more value. I did not succeed. One day, I dreamed … I am not able to prepare the desired ketone because the thirteenth position is occupied by the side chain which will put on the OH on the seven position in structure A. I called Sam Levine‡and others and I told them I said Sam, on the basis of this recent work, I have come to the conclusion that god has assigned neither structure A nor B to taxol. Taxol has structure C. Sam agreed, thank god. That is the structure we published in 1971.”
Solving the structure of taxol was only a first (albeit necessary) step to its eventual development as a cancer therapeutic. The elucidation of its unique biological mechanism of action by Dr Susan Horwitz and colleagues,12 as reviewed in another article in this themed issue [Mize et al., DOI: 10.1039/D2NP00091A], proved to be another important milestone, certainly helping launch taxol into clinical trials. The efforts of synthetic chemists were also tremendously important. Total synthesis13–15 confirmed that the structure published by Wani, all eleven stereocenters of it,16 was correct, and paved the way for the compound to be supplied in sufficient quantities via a semi-synthetic route to satisfy clinical demand [Mize et al., DOI: 10.1039/D2NP00091A]. Nobody could have been more relieved than Wani himself when synthetic studies verified the accuracy of his proposed structure. In his words, “I used to have nightmares because the rest of the world was working on the synthesis of taxol on my structure C assuming it's the structure. I was all the time worried that I hope the structure that I have assigned is correct.”
A few key points stand out from the story of taxol that have relevance to natural product drug discovery today. The first is that taxol was discovered as a promising lead from among 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 samples screened in phenotypic assays (with follow up testing in animal models). The project was not focused on identifying novel chemical structures, but on finding compounds of any structure with promise as anti-cancer agents. It succeeded because of close collaboration between chemists and biologists, with biological activity driving the discovery process. While many researchers in the natural products sciences (including the authors of this article) are chemists, those of us who seek to discover drugs should remember not to become too distracted in the pursuit of tantalizing chemistry, and instead remain grounded in biological reality. While pursuing new biosynthetic mechanisms, developing new instrumental and computational tools, and finding novel chemical scaffolds are all important aspects of advancing the natural products sciences, it is imperative that we continue to invest in large-scale screening of natural product compounds and extracts. The current landscape for funding such initiatives is difficult, but several projects are ongoing. One of these is the NCI funded initiative to identify anticancer agents from tropical rainforests described by De Blanco et al. elsewhere in this themed issue [DOI: 10.1039/D2NP00080F]. The NCI also maintains an extensive library of natural product extracts, many of which are plants, which is available to the community for screening efforts.17 Recently, the lectin griffithsin, isolated by scientists at the NCI from red algae, has shown promising anti-viral activity in clinical studies.18 Red algae are technically not plants (although plants and algae are close relatives), nonetheless, the clinical efficacy of griffithsin suggests the continued relevance of the NCI library, and the future promise it may hold for drug discovery from plants and other organisms.
000 samples screened in phenotypic assays (with follow up testing in animal models). The project was not focused on identifying novel chemical structures, but on finding compounds of any structure with promise as anti-cancer agents. It succeeded because of close collaboration between chemists and biologists, with biological activity driving the discovery process. While many researchers in the natural products sciences (including the authors of this article) are chemists, those of us who seek to discover drugs should remember not to become too distracted in the pursuit of tantalizing chemistry, and instead remain grounded in biological reality. While pursuing new biosynthetic mechanisms, developing new instrumental and computational tools, and finding novel chemical scaffolds are all important aspects of advancing the natural products sciences, it is imperative that we continue to invest in large-scale screening of natural product compounds and extracts. The current landscape for funding such initiatives is difficult, but several projects are ongoing. One of these is the NCI funded initiative to identify anticancer agents from tropical rainforests described by De Blanco et al. elsewhere in this themed issue [DOI: 10.1039/D2NP00080F]. The NCI also maintains an extensive library of natural product extracts, many of which are plants, which is available to the community for screening efforts.17 Recently, the lectin griffithsin, isolated by scientists at the NCI from red algae, has shown promising anti-viral activity in clinical studies.18 Red algae are technically not plants (although plants and algae are close relatives), nonetheless, the clinical efficacy of griffithsin suggests the continued relevance of the NCI library, and the future promise it may hold for drug discovery from plants and other organisms.
Another point that is worth considering related to the discovery of camptothecin and taxol is that both of these molecules led to understanding new mechanisms of action for killing cancer cells. Even if neither of them had become successful drugs, the mechanistic information that was gleaned by having access to the molecules would have been extremely valuable towards the goal of anti-cancer drug discovery. Thus, when a particular lead seems sub-optimal in terms of its likelihood of eventually becoming a drug, it may still be worth pursuing it as a probe to better understand biological processes.
The initial failure of a complex Taxus brevifolia extract to show efficacy in mouse model studies provides another lesson for future studies. It wasn't until the extract was fractionated and partially purified that activity was observed in vivo. Researchers pursing drug discovery from natural products should take note of this observation and consider including fractions as well as complex extracts in activity screens. Consistent with this recommendation, the library that has been made available for screening by the NCI is prepared from pre-fractionated extracts.17
Finally, perhaps the most compelling aspect of the taxol story is that there were multiple moments when naysayers could have tanked the project. Dr Wall spoke up for the promise of taxol even when another highly respected chemist dismissed it, and Dr Wani continued working on its structure elucidation even after Dr Wall suggested his efforts would be better invested elsewhere. Many champions contributed to the eventual success of taxol as a cancer drug, but in the five years while structure elucidation of taxol was a “low priority,” Wani was the sole bearer of the taxol torch. These examples demonstrate how the people behind the science drive it to success (or not), and how important it is for us as scientists not to give up on a project simply because someone says it won't work. Contemplating the story of taxol, one can't help but wonder how many other promising leads have languished on a dusty lab bench, never to see the light of clinical discovery. The road from discovery to implementation is fraught with hurdles, and it is up to all of us to keep the momentum moving forward, to believe in our science and each other, and to lend our support in any way possible to our colleagues who continue to devote themselves to the difficult, but important, pursuit of natural product drug discovery.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
NBC is funded in part from the National Institutes Health National Center for Complementary and Integrative Medicine (NIH NCCIH) and the National Institutes of Health Office of Dietary Supplements (NIH ODS) under grant number U41 AT008178. NHO receives funding from that National Institutes of Health National Cancer Institute (NIH NCI) under grant number P01 CA125066, a project which he had the pleasure of working on with Dr Wani for more than a decade. We are grateful for the perspective, guidance, diligence, inspiration, and generosity of the late Dr‘s Monroe Wall and Mansukh Wani, whose contributions to science were also contributions to humanity.Notes and references
- G. M. Cragg, M. R. Boyd, J. H. Cardellina 2nd, D. J. Newman, K. M. Snader and T. G. McCloud, Ciba Foundation Symposium, 1994, vol. 185, pp. 178–190; discussion 190-176 Search PubMed.
- D. G. I. Kingston, personal communication, Greensboro, NC, September 11, 2015.
- C. Wu, S.-L. Lee, C. Taylor, J. Li, Y.-M. Chan, R. Agarwal, R. Temple, D. Throckmorton and K. Tyner, J. Nat. Prod., 2020, 83, 552–562 CrossRef CAS PubMed.
- C. Harrison, Nat. Biotechnol., 2014, 32, 403–404 CrossRef CAS PubMed.
- L. K. Caesar and N. B. Cech, Nat. Prod. Rep., 2019, 36, 869–888 RSC.
- M. Suffness and M. E. Wall, Taxol: Science and Applications, ed. M. Suffness, CRC Press, London, 1st edn, 1995, ch. 1, pp. 3–25, DOI:10.1201/9780138737368.
- M. E. Wall, M. C. Wani, C. E. Cook, K. H. Palmer, A. T. McPhail and G. A. Sim, J. Am. Chem. Soc., 1966, 88, 3888–3890 CrossRef CAS.
- J. F. Pizzolato and L. B. Saltz, Lancet, 2003, 361, 2235–2242 CrossRef CAS PubMed.
- Press Release, FDA approves first targeted therapy for HER2-low breast cancer, August 5, 2022.
- G. Zemplén, A. Gerecs and I. Hadacsy, Ber, 1936, 69, 1827–1829 CrossRef.
- N. H. Oberlies and D. J. Kroll, J. Nat. Prod., 2004, 67, 129–135 CrossRef CAS PubMed.
- P. B. Schiff, J. Fant and S. B. Horwitz, Nature, 1979, 277, 665–667 CrossRef CAS PubMed.
- K. C. Nicolaou, Z. Yang, J. J. Liu, H. Ueno, P. G. Nantermet, R. K. Guy, C. F. Claiborne, J. Renaud, E. A. Couladouros, K. Paulvannan and E. J. Sorensen, Nature, 1994, 367, 630–634 CrossRef CAS PubMed.
- R. A. Holton, C. Somoza, H. B. Kim, F. Liang, R. J. Biediger, P. D. Boatman, M. Shindo, C. C. Smith and S. Kim, J. Am. Chem. Soc., 1994, 116, 1597–1598 CrossRef CAS.
- R. A. Holton, H. B. Kim, C. Somoza, F. Liang, R. J. Biediger, P. D. Boatman, M. Shindo, C. C. Smith and S. Kim, J. Am. Chem. Soc., 1994, 116, 1599–1600 CrossRef CAS.
- M. C. Wani, H. L. Taylor, M. E. Wall, P. Coggon and A. T. McPhail, J. Am. Chem. Soc., 1971, 93, 2325–2327 CrossRef CAS PubMed.
- C. C. Thornburg, J. R. Britt, J. R. Evans, R. K. Akee, J. A. Whitt, S. K. Trinh, M. J. Harris, J. R. Thompson, T. L. Ewing, S. M. Shipley, P. G. Grothaus, D. J. Newman, J. P. Schneider, T. Grkovic and B. R. O'Keefe, ACS Chem. Biol., 2018, 13, 2484–2497 CrossRef CAS PubMed.
- N. Teleshova, M. J. Keller, J. A. Fernández Romero, B. A. Friedland, G. W. Creasy, M. G. Plagianos, L. Ray, P. Barnable, L. Kizima, A. Rodriguez, N. Cornejal, C. Melo, G. Cruz Rodriguez, S. Mukhopadhyay, G. Calenda, S. U. Sinkar, T. Bonnaire, A. Wesenberg, S. Zhang, K. Kleinbeck, K. Palmer, M. Alami, B. R. O'Keefe, P. Gillevet, H. Hur, Y. Liang, G. Santone, R. N. Fichorova, T. Kalir and T. M. Zydowsky, PloS one, 2022, 17, e0261775 CrossRef CAS PubMed.
Footnotes |
| † When we originally conceived of this themed issue on drug discovery from plants, we planned to introduce it with a viewpoint about lessons learned from the discovery of taxol that we would coauthor with Dr Mansukh Wani. Regrettably, Dr Wani passed away in 2020, before we had the opportunity to work together on this project. Thus, we have elected to provide Dr Wani's perspective with excerpts from a lecture he gave in Greensboro, NC on May 12th, 2017 when he received an honorary Doctorate of Science from the University of North Carolina at Greensboro, and from an interview conducted by Dr Michael Frierson at the University of North Carolina Greensboro on October 20th, 2015. |
| ‡ Dr Samuel G. Levine had worked with Dr Monroe Wall for several years, including at the USDA before they both moved to RTI. He earned his PhD at Harvard under Nobel Laureate R. B. Woodward, and as such, was one of Wall's most trusted colleagues with respect to organic chemistry. Levine's confidence in Wani's reassignment of the structure was assuring to Wall, and the team simply modified the structural assignments before submitting the manuscript for publication. |
| This journal is © The Royal Society of Chemistry 2023 |