Open Access Article
Open Access ArticleSynthesis and characterization of a Schiff base crosslinked hydrogel based on hyperbranched polyglycerol†
Kyriakos
Karakyriazis
abc,
Vanessa
Lührs
abc,
Sebastian
Stößlein
c,
Ingo
Grunwald
cd and
Andreas
Hartwig
 *ab
*ab
aFraunhofer Institute for Manufacturing Technology and Advanced Materials, Wiener Straße 12, 28359 Bremen, Germany. E-mail: andreas.hartwig@ifam.fraunhofer.de
bUniversity of Bremen, Department 2 Biology/Chemistry, Leobener Straße 3, 28359 Bremen, Germany
cPurenum GmbH, Fahrenheitstraße 1, 28359 Bremen, Germany
dIndustrial and Environmental Biology, Hochschule Bremen-City University of Applied Sciences, Neustadtswall 30, 28199 Bremen, Germany
First published on 7th March 2023
Abstract
Schiff base hydrogels have attracted much attention in recent years in the field of biomedical applications, due to the reversibility of the imine bond, granting the gels degradation properties. Commonly used multifunctional amines include proteins and polysaccharides like bovine serum albumin and chitosan, that exhibit low water solubility. One of the most commonly used dialdehydes, utilized for Schiff base formation, is glutaric aldehyde, that is known to be toxic. We are presenting a new two component Schiff-base-crosslinked hydrogel based on an aminoterminated hyperbranched polyglycerol (hPG-NH2) and a polyethyleneglycol dialdehyde (PEG-DA), that shows promising mechanical properties. The hPG-NH2 is synthesized by a photoinitiated thiolene coupling reaction, following a previously established copolymerization of glycidol and allyl glycidyl ether. The PEG-DA was synthesized in a one-pot synthesis, by the addition of isophorone diisocyanate (IPDI) and hydroxymethylfurfural (HMF). The hydrogels were characterized regarding their mechanical properties with a texture analyzer. With this method, the impact of the solid content, the ratio of the components and the pH on the gel properties was studied.
Introduction
Hyperbranched polyglycerols (hPGs) are multifunctional polyethers that have attracted high scientific interest in recent years, due to their dendrimer-like structure, their biocompatibility and their multifunctionality.1–3 The facile synthetic nature and functionalization of hPGs is an enormous advantage compared to dendritic polymers that require complex multi-step synthesis.1,4 hPGs are usually synthesized by the ring opening multibranching polymerization (ROMBP) of glycidol under slow monomer addition (SMA) conditions.1,5,6 However the main point for the increasing attention on hPGs is their excellent biocompatibility, allowing them to be used in different biomedical applications.1,4,5In the field of biomedical applications, hydrogels have attracted much interest in the past years. The three-dimensional crosslinked networks are utilized in wound healing, in tissue engineering as well as in drug delivery systems, and more.7 Hydrogels based on hPGs became of high interest in the past decade, as they show huge potential as drug delivery systems and for applications in tissue engineering.7 The biocompatible polyether backbone and the high number of hydrophilic –OH-groups make hPGs excellent candidates for the formation of biomedical hydrogels. Many approaches have been made in the past years to synthesize hPG-based hydrogels.8–10 Oudshoorn et al. derivatized hPGs with glycidyl methacrylate to obtain hydrogels by crosslinking with various radical initiators and catalysts.8 Dey et al. synthesized a polyanionic hydrogel by in situ crosslinking dendritic polyglycerol sulfate azide with PEG-cyclooctyne to be used in cartilage tissue engineering.9,10 Further hydrogel formulations based on polyglycerols have been made, which were used to encapsulate, cultivate and deliver living human cells.9,11,12
A second topic that got a lot of attention in the field of biomedical hydrogels are Schiff base crosslinks.13,14 Schiff bases are formed by the reaction between primary amines and carbonyl groups via nucleophilic addition. Due to the reversibility of the bond, hydrogels crosslinked this way, exhibit degradability, self-healing abilities and a wide spectrum of tunable properties.13,14 Hydrogels based on Schiff base linkages have been utilized for various biomedical applications, including drug delivery,15–17 tissue engineering18,19 and wound healing.20,21 A well-known example is bovine serum albumin crosslinked with glutaraldehyde which has been introduced as a wound adhesive by CryoLife Inc. under the name BioGlue.22
The motivation of this work is a combination of the two topics named, by synthesizing a hydrogel based on hPGs, baring Schiff base crosslinks, by utilizing the simple synthesis and functionalization of hPGs to synthesize an aminoterminated-hPGs (hPG-NH2). In the past years, different approaches have been made to synthesize hPG-NH2. Song et al. synthesized a glycidylether derivative bearing a protected primary amine group, which was deprotected after completion of the copolymerization with glycidol.23 Further approaches by copolymerizing glycidol with various glycidyl ethers were reviewed by Verkoyen and Frey.24 Another way to get to hPG-NH2 is via postpolymerization modification. Sunder et al. presented the copolymerization of glycidol and allyl glycidyl ether (AGE) already in 2000, introducing allyl groups in the hPG.25 The allyl groups provide easily accessible double bonds that can be utilized to introduce new functional groups. Koyama et al. functionalized a copolymer of ethylene oxide and AGE via a thiolene coupling reaction with cysteamine hydrochloride.26 This polyether amine was further investigated and used for peptide conjugation27 and as a coating promoting cell adhesion of human fibroblasts.28
In this work we propose a combination of the copolymerization of glycidol and AGE as established by Sunder et al.25 and the previously reported postpolymerization modification of AGE with cysteamine via thiolene coupling,26–28 to synthesize hPG-NH2. The synthesized hPG-NH2 were used to form hydrogels based on Schiff base linkages via reaction with a polyethylene glycol dialdehyde (PEG-DA). The PEG-DA was synthesized in a one-pot synthesis as an alternative to the toxic glutaraldehyde,29 while the hPG-NH2 synthesized, serves as an alternative for animal origin BSA. The hydrogels formed, were investigated regarding their mechanical properties by measuring the gel stability of various formulations.
Experimental
All chemicals were purchased by Sigma-Aldrich (Steinheim, Germany) unless stated otherwise.Characterization
Synthesis of hyperbranched polyglycerol allyl glycidyl ether copolymer (hPG-co-AGE)
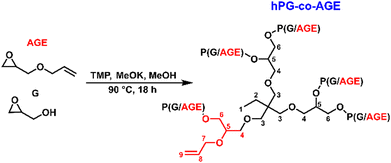
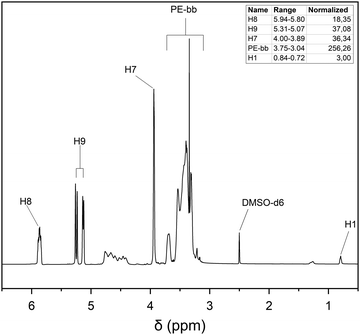
hPG-co-AGE was synthesized by anionic ring-opening polymerization, according to a method previously reported by Sunder et al.25 (Scheme 1) The polymerization was carried out in a 100 mL three-neck round bottom flask equipped with a mechanic stirrer under argon atmosphere. 1,1,1-Tris(hydroxylmethyl)-propane (TMP) (0.541 g, 4.032 mmol, 1 eq.) was partially deprotonated with a 25% potassium methoxide solution in methanol (MeOH) (450 μL, 1.21 mmol, 0.3 eq.) for 30 minutes. The excess methanol was removed under vacuum at 90 °C leaving only the initiator in the flask. A mixture of glycidol (12.26 g, 165.5 mmol, 41.0 eq.) and allyl glycidyl ether (11.68 g, 102.3 mmol, 25.4 eq.) was added dropwise over 12 h using a NE-1000 syringe pump (New Era Pump Systems Inc., Farmingdale, USA). After the complete addition, the reaction mixture was left stirring for additional 4 h. The reaction mixture was dissolved in methanol and precipitated in cold tert-butyl methyl ether (Carl Roth, Karlsruhe, Germany) twice. The precipitate was dried at 90 °C in vacuum for 12 h (overnight), yielding 22.79 g (93.1%) of a lightly yellow, highly viscous fluid.1H NMR (DMSO-d6) δ = 0.76–0.82 (3H, s, H1); 1,23–1.29 (2H, m, H2); 3.21–3.75 (≈250H, m, H3-6); 3.90–3.98 (2H, s, H7); 5.10–5.28 (2H, dd, H8); 5.80–5.92 (1H, m, H9) ppm.
Synthesis of hPG-NH2via thiolene coupling of cysteamine
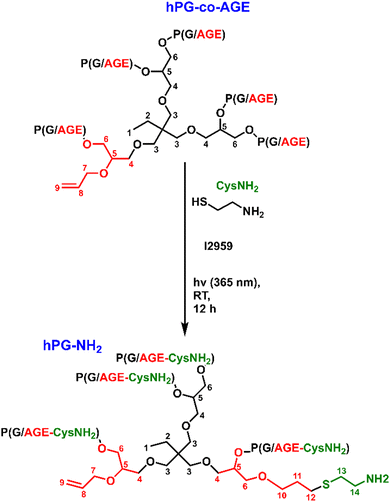
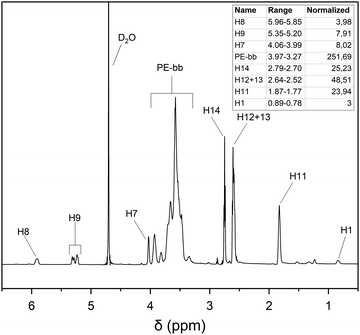
The previously synthesized hPG-co-AGE (10.07 g, 2.13 mmol) was dissolved in 200 mL of MeOH in a 500 mL three-neck round bottom flask. After addition of of cysteamine (3.93 g, 49.04 mmol) and α-hydroxy-4-(2-hydroxyethoxy)-α-methyl-propiophenon (Irgacure 2959) (0.125 g, 0.54 mmol, 9 w%), as a photoinitiator, the solution was degassed with argon for an hour. Through a septum, the light diode of an OmniCure® S-1000 UV-Lamp (EXFO, Mississauga, Canada) was centered over the solution at a distance of approximately 7 cm. The reaction mixture was exposed to UV-Light at λ = 365 nm and stirred for 12 h under argon atmosphere overnight (Scheme 2). After removing the methanol from the crude product, it was dissolved in 10 mL of double-deionized water (ddH2O) and dialyzed against ddH2O with a molecular weight cut-off (MWCO) of 1 kDa for three days. The dialysate was transferred into a round bottom flask and lyophilized until the hPG-NH2 was dry, obtaining the highly viscous honey-like yellow hPG-NH2.1H-NMR (D2O) δ 0.76–0.82 (s, 3H, H-1); 1.23–1.29 (m, 2H, H-2); 1.78–1.88 (s, 2H, H-11); 2.54–2.63 (m, 4H, H-12, H-13); 2.71–2.79 (m, 2H, H-14), 3.27–3.97 (m, ≈250H H-3-6, H-10); 3.99–4.07 (s, 2H, H-7); 5.19–5.34 (dd, 2H, H-8); 5.85–5.95 (m, 1H, H-9).
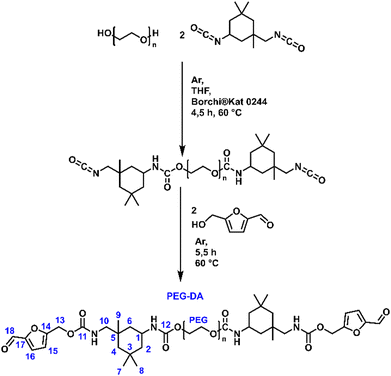
One-pot synthesis of PEG-DA
A 250 mL three-neck round bottom flask equipped with a mechanical stirrer a cooler and a septum with pre-dried PEG-3000 (Merck KGaA, Darmstadt, Germany) (22.00 g, 7.25 mmol, 1 eq.) was heated to 70 °C in vacuum for 1 h, until complete removal of moisture. After cooling to 60 °C 12 mL dry tetrahydrofuran (THF) were added under argon atmosphere. The Borchi®Kat-0244 (Borchers Americas Inc., West Lake, USA) (9.1 mg, 0.036 w%) was dissolved in 8 mL dry THF and was added to the flask as well. After the addition of isophorone diisocyanate (IPDI) (3.35 g, 15.07 mmol, 2.08 eq.) the mixture was stirred for 4.5 h at 60 °C (Scheme 3). The reaction mixture was left overnight to cool down. No work up was done to the isocyanate-functionalized PEG, as it was directly used for the next step. The crude product was melted at 60 °C under argon atmosphere. After cooling down to 50 °C dry hydroxymethylfurfural (HMF) (Fluorochem Ltd, Hadfield, UK) (2.10 g, 16.65 mmol) was added. The reaction mixture was stirred at 50 °C for 5.5 h under argon, yielding a brown product mixture. The crude product was worked up by flash chromatography to remove low-molecular compounds with ethyl acetate as eluent. The desired PEG-DA was then removed from the silica by eluting with dichlormethane:MeOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Determination of the aldehyde (CHO)-content
Due to formation of oligomers of the type (HMF-IPDI-(PEG-IPDI)n-HMF) a further analysis to calculate the products CHO-content is necessary. Due to the UV-active HMF-ring, the determination could be carried out using UV/Vis-spectroscopy. The measurements were performed with a SPECORD210 PLUS Spektralphotometer (Analytik Jena GmbH, Jena, Germany) in a range from 190–400 nm. The absorption maximum of HMF is located at 284 nm (ESI†). Three aqueous solutions of the PEG-DA were prepared (β = 0.1–0.25 mg mL−1) and measured without any further dilution. The recording and evaluation of the spectra was carried out using the software WinASPECT PLUS (version 4.2.0.0)Hydrogel formation
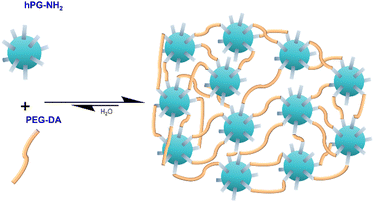
For the formation of the hydrogels, two aqueous prepolymer-solutions of hPG-NH2 and PEG-DA, were prepared, according to the desired polymer concentrations in the gel. After the addition of 1 mL of the PEG-DA solution in a glass vial containing 1 mL of the hPG-NH2 solution, the mixture was vortexed for 20 s. The mixture was then poured in a well of a 24-well-plate and left airtight for 22 h to ensure complete curing, as the gels showed different curing times, depending on the formulation (Fig. 1).Measurement of the hydrogel stability
The gel stability was measured using a Texture Analyser TA.XTplusC (Stable Micro Systems, Godalming, UK) equipped with a 5 kg loadcell and a P/0.5 plunge. After curing for 22 h, the gels were measured directly in the well-plate. The measurement parameters were based on the Bloom test: after contacting the gel and exceeding a trigger-load of 40.2 mN the plunge penetrates the gel with a speed of 0.5 mm s−1 for a distance of 4 mm. The maximum-recorded load was considered as a reference for the gel-stability.Results and discussion
Synthesis of hPG-co-AGE
The synthesis of the precursor hPG-co-AGE was verified via1H-NMR. The structure analysis and the Mn calculation was based on the 1H-NMR data, by integrating the spectrum against the methyl group signal (H1) of the TMP (Fig. 2). The amount of integrated AGE was determined by the distinctive signals H8, H9 and H7, exhibiting 18 AGE units in the hPG-co-AGE. The number of glycidol building blocks was calculated by eqn (1),‡ based on the method of Sunder et al.25 Through the structural analysis the synthesized hPG-co-AGE were found to bare 32 glycidol and 18 AGE units, which correspond to a conversion of approximately 80%.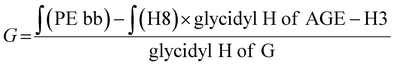 | (1) |
Synthesis of hPG-NH2
The synthesis of hPG-NH2 was confirmed by 1H-NMR experiments (Fig. 3). The depletion of the signals for H7–9 as well as the new signals H11–14 confirm the thiolene coupling of the cysteamine to the allyl groups. The signals H7–9 were still present, indicating that the allyl ether groups were not completely converted. Even when increasing the cysteamine and the photoinitiator concentrations, the unreacted allyl-groups still remained. This can be attributed to the hyperbranched molecule architecture, making some of the allyl-groups inaccessible.The structural analysis conducted, was similar to the proposed way by Sunder et al. for the hPG-co-AGE, via1H-NMR-analysis. The number of coupled cysteamine units could be calculated by integrating the distinctive signals H11, H12 + 13 and H14 against the methyl group H1. The number of unreacted AGE units can be calculated by the integrals of the distinct signals H8, H7 or H9, while the number of reacted AGE units equals the number of coupled cysteamine units.
The number of glycidol units can be calculated similar to the reported method for hPG-co-AGE. It can be calculated using eqn (2).§
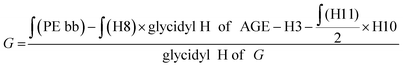 | (2) |
Through the structural analysis, the hPG-NH2 was found to average 28 glycidol, 4 unreacted AGE and 12 coupled AGE-Cys-NH2 groups. The discrepancies with the previously synthesized hPG-co-AGE, regarding the overall number of AGE-moieties can be attributed to the inaccuracy of the 1H-NMR. The Mn of the hPG-NH2 was calculated to approximately 5000 g mol−1. The amine content was further verified via titration against hydrochloric acid, with a concurring result of 12 NH2-groups per molecule.30
Synthesis of PEG-DA
The synthesis of the PEG-DA was verified by the absence of the characteristic absorption bands for the NCO- and the OH-groups in the ATR-FTIR spectra at 2259 cm−1 and 3390 cm−1 respectively. The appearance of the two carbonyl-bands at 1680 cm−1 and 1717 cm−1 prove the formation of both an aldehyde and a carbamate group. Further analysis was conducted via1H and 13C NMR experiments. The isomer mixture of commercially available IPDI (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, cis
30, cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans) makes the assignment of the corresponding signals complicated, unlike the distinct HMF- and PEG-signals (ESI†). Some signals though, are present more than two times, indicating there is a product mixture.
trans) makes the assignment of the corresponding signals complicated, unlike the distinct HMF- and PEG-signals (ESI†). Some signals though, are present more than two times, indicating there is a product mixture.
For this reason, MALDI-ToF end-group analysis experiments were conducted. After end group analysis in the range of 2–5 kDa (ESI†), the synthesis of the desired product (HMF-IPDI-PEG-IPDI-PEG) was verified. In the MALDI-ToF measurements with a range of 3–16 kDa additional PEG distributions could be identified: one around 7.2 kDa and another with much lower intensity at 10.5 kDa (ESI†). As the PEG calibrations used for the experiments did not cover these mass ranges, no exact end group analysis could be conducted for those distributions. Due to the mass ranges though, it could be assumed, that they are PEG di- and trimers linked with IPDI. This indicates a product mixture in the form of HMF-IPDI-(PEG-IPDI)n-HMF with n = 1, 2, 3.
For a quantitative analysis of the product mixture, GPC experiments were performed. The results showed a fourth distribution belonging to the quatrimer, which didn’t fly in the MALDI-ToF experiments performed before. The ratio of the four different species was 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (n = 1, 2, 3, 4) for the oligomers (ESI†). The theoretical molar mass of the product mixture (HMF-IPDI-(PEG-IPDI)n-HMF; n = 1, 2, 3, 4), is 6925 g mol−1.
12 (n = 1, 2, 3, 4) for the oligomers (ESI†). The theoretical molar mass of the product mixture (HMF-IPDI-(PEG-IPDI)n-HMF; n = 1, 2, 3, 4), is 6925 g mol−1.
The oligomer ratio in the product mixture stayed in the same range for all syntheses carried out, resulting to a reproducible synthesis route. Due to the bifunctionality of the IPDI, oligomer-formation cannot be completely excluded. It is assumed, that all the products can form Schiff bases with primary amines, as there were no isocyanate or alcohol groups present in the ATR-FTIR-spectra (ESI†) indicating a successful coupling of the HMF and the PEG-IPDI-oligomers.
Calculation of the CHO –content
To be able to calculate the molar ratios of the PEG-DA to the hPG-NH2, the HMF content of the PEG-DA product mixture was calculated. The aldehyde content can be derived from the HMF content and allows the calculation of a theoretical molar mass of the product mixture. The molar mass calculated over the aldehyde content (6 kDa), shows a similar value to the theoretical molar mass calculated by the GPC results (6.9 kDa). (Table 1) For further calculations, the medium molar mass calculated via the aldehyde content was used, as the aldehyde content is the decisive attribute for the following experiments.| Method | Molar mass [g mol−1] |
|---|---|
| GPC absolute | 8870 |
| GPC theoretical | 6925 |
| UV-Vis (CHO-content) | 6000 |
Hydrogel formation and characterization
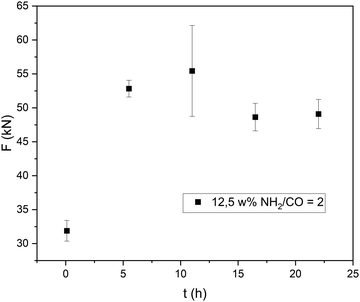
The sol–gel transition can be observed two minutes after mixing the two components. The visible transition is completed after one more minute. During the sol–gel transition the opaque yellowish color of the mixture turns clear and darker, resulting in a clear gold-brown hydrogel. To verify the hydrogel formation ATR-IR experiments were conducted, showing a complete depletion of the aldehyde carbonyl signal, as well as the appearance of a new signal for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (ESI†). It was shown, that the maximum strength is already achieved after 6 h at a solid content of 12.5% and a ratio of NH2/CO = 2 (Fig. 4). However, formulations at unfavourable conditions achieve their maximum strength later. Thus, all of the following experiments were performed with a curing time of 22 h.
N bond (ESI†). It was shown, that the maximum strength is already achieved after 6 h at a solid content of 12.5% and a ratio of NH2/CO = 2 (Fig. 4). However, formulations at unfavourable conditions achieve their maximum strength later. Thus, all of the following experiments were performed with a curing time of 22 h.
Three different experiments were carried out, to investigate the properties of the hydrogels:
1. A variation of the solid content, to determine the optimal solid content for further investigations, as well as the minimum solid content required for the formation of a gel.
2. A variation of the amine to aldehyde ratio, to optimize the gel stability.
3. A variation of the pH-value, to investigate the effect of buffer systems on the hydrogel formation.
Effect of the solid content on the hydrogels
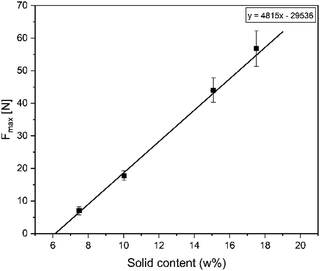
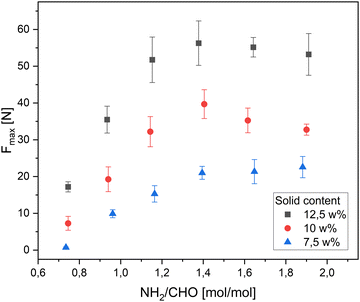
First, the solids content was varied to study the hydrogel behaviour (Fig. 5). As expected higher solid contents lead to an increase of the gel stability. The maximum load recorded was facilitated by the samples with 17.5 w% achieving values around 55 N. The linear trend allowed the calculation of the minimum solid content required to allow hydrogel formation, being just over 6 w%. It can be observed, that a 5 w% formulation shows only a viscosity increase, but it never reached a fully transitioned hydrogel, confirming the calculated assumption.Effect of the amine to aldehyde ratio
The amine to aldehyde ratio, was varied in this part, to investigate the effect on the gel stability for different solid contents (Fig. 6). An amine deficit lead to lower gel stabilities. The excess aldehyde cannot react with any amine groups, as all of the amine groups are already converted into Schiff bases. The remaining unreacted PEG-DA molecules remain in solution, while an equimolar gel with a lower solid content is formed, explaining the lower values measured.On the other hand, an amine excess leads to higher gel stabilities. As the hPG-NH2 bares an average of 12 amines per molecule, there does not seem to be a problem for the aldehyde to find a reaction partner. The higher gel stability can be attributed to the higher affinity to form hydrogen bonds, due to the free amines. The hydrogel system exhibits a maximum at an amine to aldehyde ratio of 1.4. Beneath that point a plateau is reached showing only statistical fluctuations. The plateau is expected to decline while the amine excess increases, as the crosslink density will decrease drastically at higher amine contents (NH2/CHO = 4). At an even higher amine excess there is no hydrogel expected at all, as the reaction would lead to a linear polymer (NH2/CHO > 6).
Effect of the pH on the hydrogels formation
In DI water the hPG-NH2 solution showed a pH of 10.55, while the PEG-DA solution a pH of 7. The combined pH was calculated to be 10.25 as it could not be measured, due to the fast curing of the gels after mixing the solutions. To study the effect of the pH on the gel formation, different buffers were used to form hydrogels.At acidic pH-values no gels could be formed in either of the two amine to aldehyde ratios. The ammonium-groups, formed by the protonation of the primary amines cannot react to hemiaminals, inhibiting the formation of Schiff bases. The formation of gel is thus prevented, as the number of crosslinks formed is very low, depending on the degree of protonation of the amines (Table 2).
| Aqueous system | pH | NH2/CHO | Gel stability [N] |
|---|---|---|---|
| DI water | 10.25* | 1 | 45.2 |
| McIlvaine buffer | 3 | No gel | |
| 5 | No gel | ||
| 7 | 1.37 | ||
| 8 | 22.1 | ||
| 3 | 1.4 | No gel | |
| 5 | No gel | ||
| 7 | 13.2 | ||
| 8 | 32.4 |
In the buffered solutions at a pH of 7 and 8 gels were formed, showing an enhancement in their mechanical stability with increasing pH. The superiority of the gels in DI–water can be attributed to the very high pH-value of the hPG-NH2 solutions compared to the buffered solutions. This indicates that is of high importance, that the amines remain in their free form and not be protonated.
Self-healing
In compliance to literature reporting Schiff base hydrogels to show self-healing properties,13 the synthesized gels have shown some first indications that they exhibit a self-healing character. All tested hydrogel formulations were removed from the well plate after testing and stored in airtight vials. The damage caused to the samples by the plunger of the texture analyser disappeared, and the gels formed the shape of the glass vials in which they where stored. As our focus does not lie on this property of the gels, only a qualitative assessment has been performed.Conclusions
A new hydrogel was synthesized, by combining aqueous solutions of hPG-NH2 and PEG-DA. The novel hydrogel shows a promising alternative to the widely used BSA-GLA-hydrogel, while getting rid of the toxic GLA and providing an animal-free substitute to BSA. The mechanical analysis showed a linear dependency between the hydrogel stability and the solid content, exhibiting a 6 w% solid content minimum required for gel formation. The gels showed a maximum stability at an amine to aldehyde ratio of 1.4. At acidic pH values no hydrogels could be formed, due to the protonation of the amines interfering with the Schiff base formation. At pH values above 7, gels were formed, showing increasing mechanical properties with rising pH values, indicating the importance of the amines not being protonated. The hydrogel system is degradable, due to the reversible Schiff base crosslinks.13–15 The polyether-based hydrogel could be a potential candidate for biomedical applications in tissue engineering, drug delivery, etc.For the formation of the hydrogel, a simple way to obtain aminoterminated hyperbranched polyglycerols via photoinitiated thiolene coupling of cysteamine to the double bonds of the already established hPG-co-AGE was reported. The simple work up by dialyzing the hPG-NH2 allows higher gram-scale synthesis in the lab, without the need of high solvent-consuming procedures like column chromatography and precipitation. The successful synthesis was verified via1H NMR studies, which simultaneously were used to calculate the amine content of the hPG-NH2.
Additionally a new one-pot synthesis for functionalizing PEG with aldehyde groups has been reported. In a first step PEG reacted with a small excess of IPDI, forming urethane bonds on both PEG chain ends. The free isocyanate groups of the IPDI reacted in a second step with the hydroxyl groups of HMF under urethane bond forming, introducing aldehyde groups to the PEG. The synthesis of the PEG-DA was verified via1H, 13C and 2D NMR studies. MALDI-ToF end group analysis confirmed the formation of the desired product, but also showed the formation of oligomers in the form of HMF-IPDI-(PEG-IPDI)n-HMF. The ratio of the oligomers is stable, which allows result comparison between different batches. The IR-experiments showed no remaining isocyanate or hydroxyl groups, meaning that all of the products were aldehyde-terminated and can be utilized to form Schiff bases.
Author contributions
Kyriakos Karakyriazis: conceptualization, investigation, visualization, writing – original draft. Vanessa Lührs: conceptualization and Investigation of the PEG-DA synthesis. Sebastian Stößlein: supervision, writing – review & editing, Ingo Grunwald: review & editing, Andreas Hartwig: supervision, writing – review & editing, basic concept of the system.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the German Ministry for Education and Research (BMBF) in the program GO-Bio (project mediGLUE), grant number 161B0393. The support is gratefully acknowledged.Notes and references
- S. Abbina, S. Vappala, P. Kumar, E. M. J. Siren, C. C. La, U. Abbasi, D. E. Brooks and J. N. Kizhakkedathu, Hyperbranched polyglycerols: recent advances in synthesis, biocompatibility and biomedical applications, J. Mater. Chem. B, 2017, 5, 9249–9277 RSC.
- L. C. Solano-Delgado, C. A. Bravo-Sanabria, C. Ardila-Suárez and G. E. Ramírez-Caballero, Stimuli-Responsive Hydrogels Based on Polyglycerol Crosslinked with Citric and Fatty Acids, Int. J. Polym. Sci., 2018, 2018, 1–8 CrossRef.
- A. L. Sisson and R. Haag, Polyglycerol nanogels: highly functional scaffolds for biomedical applications, Soft Matter, 2010, 6, 4968 RSC.
- M. Gosecki, M. Gadzinowski, M. Gosecka, T. Basinska and S. Slomkowski, Polyglycidol, Its Derivatives, and Polyglycidol-Containing Copolymers-Synthesis and Medical Applications, Polymers, 2016, 8, 227 CrossRef PubMed.
- D. Wilms, S.-E. Stiriba and H. Frey, Hyperbranched polyglycerols: from the controlled synthesis of biocompatible polyether polyols to multipurpose applications, Acc. Chem. Res., 2010, 43, 129–141 CrossRef CAS PubMed.
- A. Sunder, R. Hanselmann, H. Frey and R. Mülhaupt, Controlled Synthesis of Hyperbranched Polyglycerols by Ring-Opening Multibranching Polymerization, Macromolecules, 1999, 32, 4240–4246 CrossRef CAS.
- M. Kumari, S. Prasad, L. Fruk and B. Parshad, Polyglycerol-based hydrogels and nanogels: from synthesis to applications, Future Med. Chem., 2021, 13, 419–438 CrossRef CAS PubMed.
- M. H. M. Oudshoorn, R. Rissmann, J. A. Bouwstra and W. E. Hennink, Synthesis and characterization of hyperbranched polyglycerol hydrogels, Biomaterials, 2006, 27, 5471–5479 CrossRef CAS PubMed.
- P. Dey, T. Schneider, L. Chiappisi, M. Gradzielski, G. Schulze-Tanzil and R. Haag, Mimicking of Chondrocyte Microenvironment Using In Situ Forming Dendritic Polyglycerol Sulfate-Based Synthetic Polyanionic Hydrogels, Macromol. Biosci., 2016, 16, 580–590 CrossRef CAS PubMed.
- P. Dey, S. Hemmati-Sadeghi and R. Haag, Hydrolytically degradable, dendritic polyglycerol sulfate based injectable hydrogels using strain promoted azide–alkyne cycloaddition reaction, Polym. Chem., 2016, 7, 375–383 RSC.
- R. Randriantsilefisoa, Y. Hou, Y. Pan, J. L. C. Camacho, M. W. Kulka, J. Zhang and R. Haag, Interaction of Human Mesenchymal Stem Cells with Soft Nanocomposite Hydrogels Based on Polyethylene Glycol and Dendritic Polyglycerol, Adv. Funct. Mater., 2020, 30, 1905200 CrossRef CAS.
- C. Wu, C. Strehmel, K. Achazi, L. Chiappisi, J. Dernedde, M. C. Lensen, M. Gradzielski, M. B. Ansorge-Schumacher and R. Haag, Enzymatically cross-linked hyperbranched polyglycerol hydrogels as scaffolds for living cells, Biomacromolecules, 2014, 15, 3881–3890 CrossRef CAS PubMed.
- J. Xu, Y. Liu and S.-H. Hsu, Hydrogels Based on Schiff Base Linkages for Biomedical Applications, Molecules, 2019, 24, 3005 CrossRef CAS PubMed.
- Z. Zhang, C. He and X. Chen, Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications, Mater. Chem. Front., 2018, 2, 1765–1778 RSC.
- E. Jalalvandi, L. R. Hanton and S. C. Moratti, Schiff-base based hydrogels as degradable platforms for hydrophobic drug delivery, Eur. Polym. J., 2017, 90, 13–24 CrossRef CAS.
- J. Huang, Y. Deng, J. Ren, G. Chen, G. Wang, F. Wang and X. Wu, Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery, Carbohydr. Polym., 2018, 186, 54–63 CrossRef CAS PubMed.
- X. Zhou, Y. Li, S. Chen, Y.-N. Fu, S. Wang, G. Li, L. Tao, Y. Wei, X. Wang and J. F. Liang, Dynamic agent of an injectable and self-healing drug-loaded hydrogel for embolization therapy, Colloids Surf., B, 2018, 172, 601–607 CrossRef CAS PubMed.
- F.-Y. Hsieh, L. Tao, Y. Wei and S.-H. Hsu, A novel biodegradable self-healing hydrogel to induce blood capillary formation, NPG Asia Mater., 2017, 9, e363–e363 CrossRef CAS.
- T.-C. Tseng, L. Tao, F.-Y. Hsieh, Y. Wei, I.-M. Chiu and S.-H. Hsu, An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System, Adv. Mater., 2015, 27, 3518–3524 CrossRef CAS PubMed.
- X. Zhao, H. Wu, B. Guo, R. Dong, Y. Qiu and P. X. Ma, Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing, Biomaterials, 2017, 122, 34–47 CrossRef CAS PubMed.
- L. Han, Y. Zhang, X. Lu, K. Wang, Z. Wang and H. Zhang, Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness, ACS Appl. Mater. Interfaces, 2016, 8, 29088–29100 CrossRef CAS PubMed.
- L. Gallagher, A. Smith, K. Kavanagh, M. Devereux, J. Colleran, C. Breslin, K. Richards, M. McCann and A. Rooney, Preparation and Antimicrobial Properties of Alginate and Serum Albumin/Glutaraldehyde Hydrogels Impregnated with Silver(I) Ions, Chemistry, 2021, 3, 672–686 CrossRef CAS.
- S. Song, J. Lee, S. Kweon, J. Song, K. Kim and B.-S. Kim, Hyperbranched Copolymers Based on Glycidol and Amino Glycidyl Ether: Highly Biocompatible Polyamines Sheathed in Polyglycerols, Biomacromolecules, 2016, 17, 3632–3639 CrossRef CAS PubMed.
- P. Verkoyen and H. Frey, Amino-functional polyethers: versatile, stimuli-responsive polymers, Polym. Chem., 2020, 11, 3940–3950 RSC.
- A. Sunder, H. Türk, R. Haag and H. Frey, Copolymers of Glycidol and Glycidyl Ethers: Design of Branched Polyether Polyols by Combination of Latent Cyclic AB 2 and ABR Monomers, Macromolecules, 2000, 33, 7682–7692 CrossRef CAS.
- Y. Koyama, M. Umehara, A. Mizuno, M. Itaba, T. Yasukouchi, K. Natsume and A. Suginaka, Synthesis of novel poly(ethylene glycol) derivatives having pendant amino groups and aggregating behavior of its mixture with fatty acid in water, Bioconjugate Chem., 1996, 7, 298–301 CrossRef CAS PubMed.
- B. Obermeier and H. Frey, Poly(ethylene glycol-co-allyl glycidyl ether)s: a PEG-based modular synthetic platform for multiple bioconjugation, Bioconjugate Chem., 2011, 22, 436–444 CrossRef CAS PubMed.
- S. Heinen, S. Rackow, J. L. Cuellar-Camacho, I. S. Donskyi, W. E. S. Unger and M. Weinhart, Transfer of functional thermoresponsive poly(glycidyl ether) coatings for cell sheet fabrication from gold to glass surfaces, J. Mater. Chem. B, 2018, 6, 1489–1500 RSC.
- T. Takigawa and Y. Endo, Effects of glutaraldehyde exposure on human health, J. Occup. Health, 2006, 48, 75–87 CrossRef CAS PubMed.
- DIN 53176:2002-11, Bindemittel für Beschichtungsstoffe_- Bestimmung der Aminzahl von wasserverdünnbaren Bindemitteln, Beuth Verlag GmbH, Berlin.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma01050j |
‡ Calculation of the glycidol repeating units (G) in the hPG-co-AGE from 1H-NMR spectra. The 5 glycidyl protons of AGE (H4-6) are multiplied by the amount of AGE (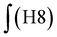 ) and subtracted from the integral of the polyether backbone ( ) and subtracted from the integral of the polyether backbone (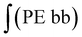 ). The six methylene protons (CH2–O; H3) of the TMP are also subtracted from the PE-bb and the result is divided by 5 (protons of glycidol H4-6). ). The six methylene protons (CH2–O; H3) of the TMP are also subtracted from the PE-bb and the result is divided by 5 (protons of glycidol H4-6). |
§ Calculation of the glycidol repeating units (G) in the hPG-NH2 from 1H-NMR spectra. The 5 glycidyl protons of AGE (H4-6) are multiplied by the amount of AGE (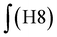 ) and subtracted from the integral of the polyether backbone ( ) and subtracted from the integral of the polyether backbone (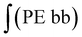 ). The two methylene protons of the allyl group (CH2–O; H10) are multiplied by the amount of coupled cysteamine ( ). The two methylene protons of the allyl group (CH2–O; H10) are multiplied by the amount of coupled cysteamine (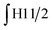 ). The six methylene protons (CH2–O; H3) of the TMP are also subtracted from the PE-bb and the result is divided by 5 (protons of glycidol H4-6). ). The six methylene protons (CH2–O; H3) of the TMP are also subtracted from the PE-bb and the result is divided by 5 (protons of glycidol H4-6). |
| This journal is © The Royal Society of Chemistry 2023 |