Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Na[Tc(CO)(CNp-F-ArDArF2)4]: an isocyanide analogue of the elusive Na[Tc(CO)5]†
Federico
Salsi
a,
Adelheid
Hagenbach
a,
Joshua S.
Figueroa
 *b and
Ulrich
Abram
*b and
Ulrich
Abram
 *a
*a
aInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Fabeckstr. 34/36, Berlin D-14195, Germany. E-mail: ulrich.abram@fu-berlin.de
bDepartment of Chemistry and Biochemistry, University of California San Diego, 9500 Gilman Drive MC 0358, La Jolla, CA 92193, USA. E-mail: jsfig@ucsd.edu
First published on 9th March 2023
Abstract
The first crystalline technetium complex in a negative oxidation state, [Tc−I(CO)(CNp-F-ArDArF2)4]−, was isolated and structurally characterized as its [Na(Crypt-2.2.2)]+ salt. It mirrors the properties of the textbook organometallic compound Na[Tc(CO)5], which has eluded isolation and structural characterization until today. [Na(Crypt-2.2.2)][Tc−I(CO)(CNp-F-ArDArF2)4] reacts expectedly as a nucleophile, which is demonstrated by reactions with HCl and ClSnMe3. They give the unprecedented monohydrido and trimethylstannyl complexes of technetium.
One century has passed since Walter Hieber noticed that in the reaction between iron pentacarbonyl and ethylenediamine followed by acidification an extremely malodourous and air sensitive compound was released, which was later identified as H2Fe(CO)4.1 He and his coworkers later discovered that this volatile compound was formed by protonation of the corresponding dianion, [Fe(CO)4]2−, the first anionic metal carbonyl derivative.2 Further studies demonstrated that these compounds can be prepared in a variety of ways, of which the most selective is the reaction of metal carbonyls with alkali metals or amalgams in ethereal solvents.3,4 At the end of the 1960's, Hieber isolated the manganese and rhenium pentacarbonyl anions [M(CO)5]− by treating the corresponding [M2(CO)10] species with alkali metals in tetrahydrofuran.5–7 Subsequent acidification resulted in the formation of the monohydrido complexes HM(CO)5 (M = Mn, Re).6,8 Such organometallic compounds are today well investigated. Thus, their vast utility in organometallic synthesis and catalysis has been displayed in innumerable scientific works and textbooks.9–18
Despite the early success in isolating [HM(CO)5] and [M(CO)5]− complexes of manganese and rhenium, the corresponding technetium carbonyls [HTc(CO)5] and [Tc(CO)5]−, have received very little attention and, in fact, have potentially not been previously prepared. The only report about the formation of [Tc(CO)5]− dates back to 1962, when Kaesz and co-workers reacted [Tc2(CO)10] with an excess of sodium amalgam in THF and observed that the IR spectrum of the reaction solution showed two strong absorptions in the region around 1850 cm−1. These values were compatible with those observed for the carbonyl stretching bands of the Na[M(CO)5] complexes (M = Mn, Re).19 Acidification of the solution unexpectedly showed that the hydride formation did not occur in the same straightforward way as for Mn and Re, where [HM(CO)5] were the main products and could easily be isolated.19,20 [HTc(CO)5] was just a minor product of the reaction and it could only be identified by comparison of its IR spectrum in a diluted cyclopentane solution with the Mn and Re analogues. [HTc(CO)5] is, in fact, more prone to CO loss and readily oligomerized in solution upon concentration to give [Tc3(CO)12(μ-H)3].21,22
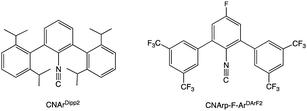
The synthesis of Na[Tc(CO)5] has, to our knowledge, never been reported again in the literature, probably because of the many practical difficulties concerning the manipulation of this radioactive and extremely air-sensitive compound of the rare element technetium. An additional problem results from the radioactivity of technetium, since the protocol for the preparation of [Tc2(CO)10] under high CO pressure is not well compatible with current radiation protection regulations.23m-Terphenyl isocyanides (Fig. 1) revealed in numerous cases to be excellent surrogates for carbon monoxide, which allow the synthesis and structural characterization of several metal carbonyl analogues, which are otherwise intrinsically too unstable to be isolated.24–27
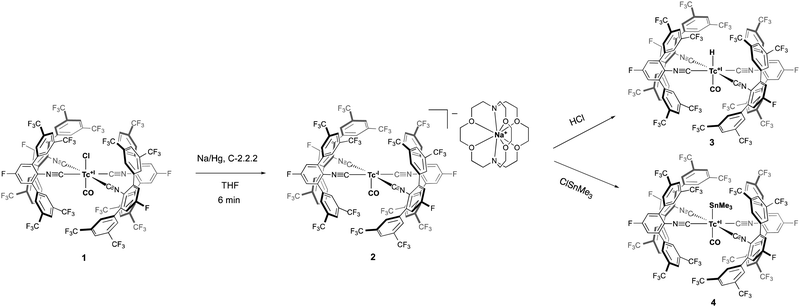
In this work, we show that this holds true also for [HTc(CO)5] and Na[Tc(CO)5]. In fact, trans-[TcCl(CO)(CNp-F-ArDArF2)4] hereby serves as a suitable substitute for the almost unavailable [Tc2(CO)10] (Scheme 1).28
It allows the structural characterization of the first technetium complex in a negative oxidation state: [Tc(CO)(CNp-F-ArDArF2)4]−, which mirrors the properties of the classical, but elusive pentacarbonyltechnetate(-1) anion. Moreover, protonation of the anion forms [HTc(CO)(CNp-F-ArDArF2)4], which is stable and can be structurally characterized, serving as model for the corresponding unstable and extremely volatile pentacarbonyl hydrido complex.
Recently, we could show that the fluorinated meta-terphenyl isocyanide CNp-F-ArDArF2 is able to coordinate with up to four of such ligands in the coordination sphere of technetium giving trans-[TcCl(CO)(CNp-F-ArDArF2)4] (1).28 The fluorinated flanking rings enclose the metal center in a tight organic shell. The steric pressure applied by these ligands favors the formation and stabilization of low-coordinated species. Additionally, CNp-F-ArDArF2 has a high π-acidity, which in some way mimics that of CO.29 Upon treatment with a carefully chosen reagent, it enables the reduction of the metal center down to the formation of anionic carbonyl/isocyanide species. This was already demonstrated for the heavier homolog rhenium; yet in the case of technetium the hard accessibility of a zero-valent carbonyl makes this starting material even more interesting.
Indeed, the treatment of 1 with an excess of diluted sodium amalgam for a prolonged time gives a black solution, that contains a mixture of [Na(Crypt-2.2.2)][Tc(CO)(CNp-F-ArDArF2)4] (2) and insoluble impurities in low yield. Better results are obtained by vigorous stirring for just six minutes. Addition of cryptand-2.2.2 and crystallization from THF/toluene and pentane gives analytically pure 2 in high yields.
While the 1H NMR spectrum of the product is practically identical to that of the starting material, the 19F NMR signal of the fluorine atom in p-position to the isocyanide is low-field shifted by ca. 10 ppm to −110 ppm. The most diagnostic method is 99Tc NMR. Here, a significant high-field shift from −1542 ppm in 1 to −1866 ppm in the anion 2 is observed.
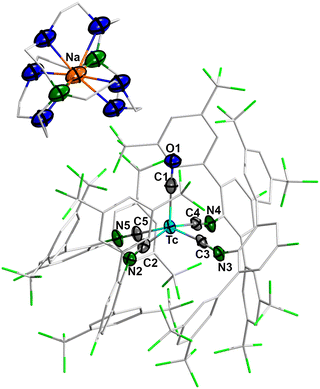
The crystal structure of 2 (Fig. 2) is formally identical to that of [Na(Crypt-2.2.2)][Re(CO)(CNp-F-ArDArF2)4].28 It has a square-pyramidal geometry (Addison-Reedijk parameter τ5 = 0.12)30 with the CO ligand occupying the apical position. The more electron rich Tc(-1) metal center is stabilized by back-donation to the CO and isocyanide ligands. As a consequence, the Tc–C bonds are shortened in comparison to 1, where a Tc–CO bond of 1.94 Å and Tc–CN bonds of 2.016(2) and 2.029(2) Å have been found.28 Accordingly, the CO and CN multiple bonds are elongated compared to 1, where the corresponding values are C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O: 1.17(1) Å and C
O: 1.17(1) Å and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: 1.164(3) and 1.165(3) Å.28
N: 1.164(3) and 1.165(3) Å.28
Moreover, the synthetic utility of the technetium(-1) anion [Tc(CO)(CNp-F-ArDArF2)4]− is shown exemplarily by its reactions with HCl or ClSnMe3. Acidification of 2 with ethereal HCl results in protonation of the metal center and the generation of the two monohydrido complexes cis- and trans-[HTc(CO)(CNp-F-ArDArF2)4] according to the 1H and 19F NMR spectra of the reaction mixture (see ESI†). Upon standing for 12 hours, the cis isomer rearranges, giving the pure trans complex 3. In contrast to [HTc(CO)5], it is stable and can be isolated as a solid. The steric hinderance of the ligands avoids oligomerization through CO loss and allows its structural characterization. The 1H NMR spectrum shows the characteristic hydride signal at −5.26 ppm. No 99Tc NMR signal was found, presumably as consequence of a large line broadening. An unusually large 99Tc NMR line width was also found for the parent compound 1. In the solid state, the complex has a distorted octahedral geometry as a consequence of the lower steric demand of the hydrido ligand compared to the isocyanides. A representation of the molecular structure together with some selected bond lengths and angles are found in the ESI.†
Transition metal hydrides are usually Brønsted–Lowry acids. Despite this, attempts to deprotonate trans-[HTc(CO)(CNp-F-ArDArF2)4] or trans-[HRe(CO)(CNp-F-ArDArF2)4] with non-nucleophilic bases of increasing strengths failed. Even stirring for 1 hour with lithium diisopropyl amide did not lead to changes in the 1H NMR spectra. [HRe(CO)5] has for comparison in acetonitrile a pKa of 21.31 It is known that the exchange of carbonyl ligands by phosphines leads to a decreased acidity of the corresponding monohydrides as a logical consequence of the reduced ability of withdrawing electron density from the conjugated base.32 In the present case, the steric encumbrance of the ligands might be the dominating factor by creating a kinetic barrier for proton transfer.
Manganese and rhenium carbonyl anions are well-behaved metal-centered nucleophiles, which makes them exceptionally useful and versatile starting materials for organometallic synthesis.9–18 The ability of [Tc(CO)(CNp-F-ArDArF2)4]− to behave in the same way, mimicking Na[Tc(CO)5] is demonstrated by the reaction with ClSnMe3, which produces the technetium trimethylstannyl complex trans-[Tc(CO)(SnMe3)(CNp-F-ArDArF2)4] (4). The trimethylsilyl complex is the first example of a technetium complex with a Tc–main group metal bond. The only other known example of a structurally characterized technetium complex containing a Tc–heterometal bond is a high-valent arylimido complex containing a Tc–Hg unit.33
The 1H NMR spectrum of 4 displays a characteristic signal for the trimethylstannyl ligand at −1.79 ppm. The other signals are affected by a severe line broadening. The flanking rings of the CNp-F-ArDArF2 are in most of the hitherto prepared metal complexes free to rotate.28,29,34 But in the presence of the bulky SnMe3 unit, the environment of the metal becomes too crowded to allow free rotation. Thus, the different signals coalesce at room temperature. The same effect is observed for the CF3 signals in the 19F NMR spectra; whereas the perfectly symmetric para-F nuclei give a well resolved sharp triplet. The 99Tc NMR spectrum shows a narrow signal at −2171 ppm, which is at higher field than that of the Tc(-1) anion.
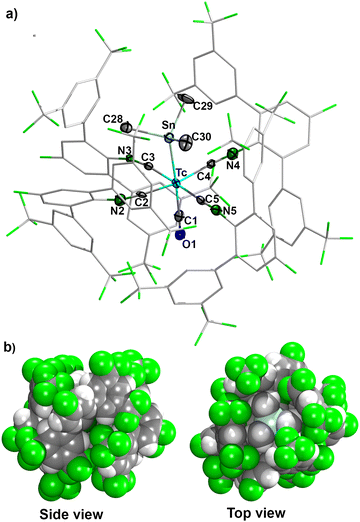
The molecular structure of trans-[Tc(CO)(SnMe3)(CNp-F-ArDArF2)4] (4) displays a distorted octahedral geometry for technetium (Fig. 3). Since compound 4 represents the first example of the structural elucidation of a complex containing a technetium-main group metal bond, its structural features cannot be compared with other technetium complexes. However, there is a number of compounds with alkyl/aryltin-rhenium bonds.35–39 The Tc–Sn bond length of 2.749(1) Å in complex 4 is similar to the Re–Sn bonds found in rhenium complexes with trialkyl- or triarylstannyl ligands (2.761(2)–2.793(1) Å).35,36
In conclusion, we isolated and structurally characterized the first technetium complex containing the metal in a negative oxidation state and the first compound with a Tc–Sn bond. This became possible by the steric protection provided by the coordination of four sterically encumbered m-terphenyl isocyanide ligands (CNp-F-ArDArF2) in the parent technetium(I) complex trans-[TcCl(CO)(CNp-F-ArDArF2)4] (1). Particularly the isolation of a considerable amount of the nucleophilic Tc(-1) complex [Tc(CO)(CNp-F-ArDArF2)4]− (2) opens infinite possibilities for the further development of the organometallic chemistry of this radioactive transition metal; possibilities that remained out of reach until today.
This research was funded by the U.S. National Science Foundation (International Supplement to CHE-1802646) and the Alexander von Humboldt Foundation (Fellowship to J.S.F.). We acknowledge the assistance of the Core Facility BioSupraMol (FU Berlin) sponsored by the DFG.
Conflicts of interest
There are no conflicts to declare.References
- W. Hieber and F. Leutert, Z. Anorg. Allg. Chem., 1932, 204, 145–164 CrossRef CAS.
- W. Hieber, W. Beck and G. Braun, Angew. Chem., 1969, 72, 795–801 CrossRef.
- W. Hieber, in Advances in Organometallic Chemistry, eds. F. G. A. Stone, R. West, Academic Press, 1970, Vol. 8, pp. 1–28 Search PubMed.
- R. B. King and M. B. Bisnette, Inorg. Chem., 1965, 4, 475–481 CrossRef CAS.
- W. Hieber and G. Wagner, Z. Naturforsch. B, 1957, 12, 478–479 CrossRef.
- W. Hieber and G. Braun, Z. Naturforsch. B, 1959, 14, 132–133 CrossRef.
- R. B. King and F. G. A. Stone, Inorg. Synth., 1963, 7, 196–201 CAS.
- W. Hieber and G. Wagner, Z. Naturforsch. B, 1958, 13, 339–347 CrossRef.
- T. C. Farrar, W. Ryan Sr., A. Davison and J. W. Faller, J. Am. Chem. Soc., 1966, 88, 184–185 CrossRef CAS.
- W. F. Edgell, J. W. Fisher, G. Asato and W. M. Risen Jr., Inorg. Chem., 1969, 8, 1103–1108 CrossRef CAS.
- E. J. Moore, J. M. Sullivan and J. R. Norton, J. Am. Chem. Soc., 1986, 108, 2257–2263 CrossRef CAS PubMed.
- C. Li, E. Widjaja and M. Garland, J. Am. Chem. Soc., 2003, 125, 5540–5548 CrossRef CAS PubMed.
- R. L. Sweany and J. Halpern, J. Am. Chem. Soc., 1977, 99, 8335–8337 CrossRef CAS.
- R. M. Bullock and E. G. Samsel, J. Am. Chem. Soc., 1990, 112, 6886–6898 CrossRef CAS.
- R. M. Bullock and J.-S. Song, J. Am. Chem. Soc., 1994, 116, 8602–8612 CrossRef CAS.
- B. H. Byers and T. L. Brown, J. Am. Chem. Soc., 1975, 97, 947–948 CrossRef CAS.
- C. Li, L. Chen and M. Garland, J. Am. Chem. Soc., 2007, 129, 13327–13334 CrossRef CAS PubMed.
- H. Brahim, C. Daniel and A. Rahmouni, Int. J. Quantum Chem., 2012, 112, 2085–2097 CrossRef CAS.
- J. C. Hileman, D. K. Huggins and H. D. Kaesz, Inorg. Chem., 1962, 1, 933–938 CrossRef CAS.
- S. J. La Placa, W. C. Hamilton and J. A. Ibers, Inorg. Chem., 1964, 3, 1491–1495 CrossRef CAS.
- A. E. Miroslavov, A. P. Shishkina, G. V. Sidorenko, V. V. Gurzhiy, D. A. Maltsev and E. V. Kurysheva, Inorg. Chem., 2020, 59, 9239–9243 CrossRef CAS PubMed.
- A. P. Sattelberger, B. L. Scott and F. Poineau, in Comprehensive Organometallic Chemistry III, eds. D. M. P. Mingos and R. H. Crabtree, Elsevier, Oxford, 2005, pp. 833–854 Search PubMed.
- A. E. Miroslavov, H. Braband, G. V. Sidorenko, E. S. Stepanova, A. A. Lumpov and R. Alberto, J. Organomet. Chem., 2018, 871, 56–59 CrossRef CAS.
- B. J. Fox, Q. Y. Sun, A. G. DiPasquale, A. R. Fox, A. L. Rheingold and J. S. Figueroa, Inorg. Chem., 2008, 47, 9010–9020 CrossRef CAS PubMed.
- D. W. Agnew, C. E. Moore, A. L. Rheingold and J. S. Figueroa, Angew. Chem., Int. Ed., 2015, 54, 12673–12677 CrossRef CAS PubMed.
- A. E. Carpenter, A. L. Rheingold and J. S. Figueroa, Organometallics, 2016, 35, 2309–2318 CrossRef CAS.
- L. A. Labios, M. D. Millard, A. L. Rheingold and J. S. Figueroa, J. Am. Chem. Soc., 2009, 131, 11318–11319 CrossRef CAS PubMed.
- F. Salsi, M. Neville, M. Drance, A. Hagenbach, J. S. Figueroa and U. Abram, Organometallics, 2021, 40, 1336–1343 CrossRef CAS.
- G. Claude, J. Genz, D. Weh, M. Roca Jungfer, A. Hagenbach, M. Gembicky, J. S. Figueroa and U. Abram, Inorg. Chem., 2022, 61, 16163–16176 CrossRef CAS PubMed.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC.
- R. A. Morris, Chem. Rev., 2016, 116, 8588–8654 CrossRef CAS PubMed.
- R. G. Pearson and P. C. Ford, Comments Inorg. Chem., 1982, 1, 279–291 CrossRef CAS.
- A. K. Burrell, D. L. Clark, P. L. Gordon, A. P. Sattelberger and J. C. Bryan, J. Am. Chem. Soc., 1994, 116, 3813–3821 CrossRef CAS.
- A. E. Carpenter, C. C. Mokhtarzadeh, D. S. Ripatti, I. Havrylyuk, R. Kamezawa, C. E. Moore, A. L. Rheingold and J. S. Figueroa, Inorg. Chem., 2015, 54, 2936–2944 CrossRef CAS PubMed.
- E. M. Lopez, D. Miguel, J. Perez, V. Riera, C. Bois and Y. Jeannin, Organometallics, 1999, 18, 490–494 CrossRef CAS.
- G. Albertin, S. Antoniutti, J. Castro, S. Garcia-Fontan and G. Zanardo, Organometallics, 2007, 26, 2918–2930 CrossRef CAS.
- R. D. Adams, B. Captain, M. Johansson and J. L. Smith Jr., J. Am. Chem. Soc., 2005, 127, 488–489 CrossRef CAS PubMed.
- R. D. Adams, B. Captain, R. H. Herber, M. Johansson, I. Nowik, J. L. Smith Jr. and M. D. Smith, Inorg. Chem., 2005, 44, 6346–6358 CrossRef CAS PubMed.
- B. T. Huie, S. W. Kirtley, C. B. Knobler and H. D. Kaesz, J. Organomet. Chem., 1981, 213, 45–62 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, Crystal structure data, NMR spectra. CCDC 2238710–2238712. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc00946g |
| This journal is © The Royal Society of Chemistry 2023 |