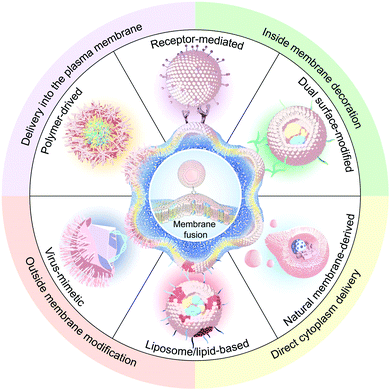
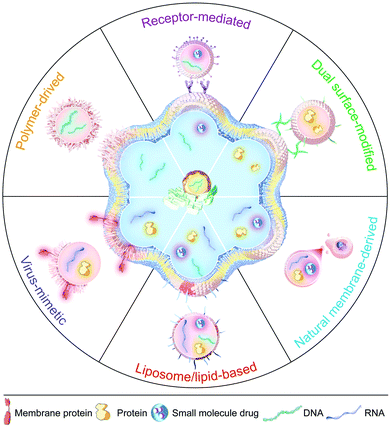
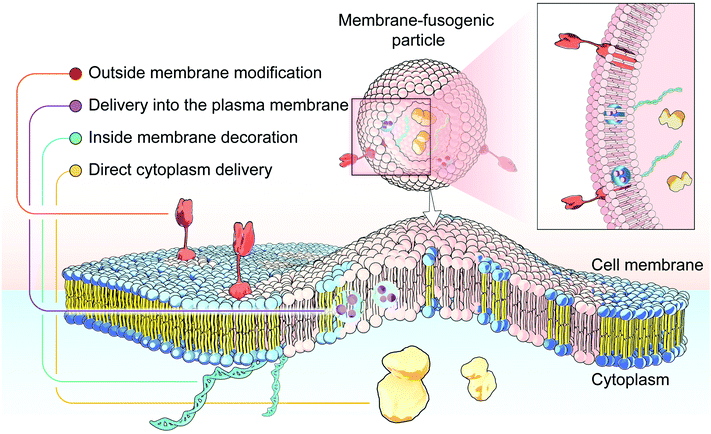
Membrane-fusogenic biomimetic particles: a new bioengineering tool learned from nature
Huimin
Kong
a,
Ke
Yi
a,
Chunxiong
Zheng
a,
Yeh-Hsing
Lao
b,
Huicong
Zhou
c,
Hon Fai
Chan
d,
Haixia
Wang
 a,
Yu
Tao
a,
Yu
Tao
 *a and
Mingqiang
Li
*a and
Mingqiang
Li
 *ae
*ae
aLaboratory of Biomaterials and Translational Medicine, Center for Nanomedicine, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou 510630, China. E-mail: taoy28@mail.sysu.edu.cn; limq567@mail.sysu.edu.cn
bDepartment of Pharmaceutical Sciences, University at Buffalo, The State University of New York, Buffalo, NY 14214, USA
cCollege of Science, Changchun Institute of Technology, Changchun 130012, China
dInstitute for Tissue Engineering and Regenerative Medicine, School of Biomedical Science, The Chinese University of Hong Kong, Hong Kong 999077, China
eGuangdong Provincial Key Laboratory of Liver Disease Research, Guangzhou 510630, China
First published on 6th June 2022
Abstract
Membrane fusion, a fundamental biological process of the fusion of the membrane composition between cells, is vital for cell–cell communication and cargo transport between living cells. This fusion interaction achieves the transportation of the inner content to the cellular cytosol as well as the simultaneous blending of foreign substances with the cell membrane. Inspired by this biological process, emerging membrane-fusogenic particles have been developed, opening a new area for bioengineering and biomedical applications. Especially, membrane-fusion-mediated transfer of inner cargoes can bypass endosomal entrapment to maximize the transportation efficiency, emerging as a unique cytoplasmic delivery platform distinct from those depending on conventional endocytosis-based pathways. In addition, the membrane fusion enables cell surface modification through lipid diffusion and mixing, providing a tool for direct cell membrane engineering. In this review, we focus on the development of membrane-fusogenic particles and their up-to-date progress. We briefly introduce the concept of membrane fusion, elaborate inspiring strategies of membrane-fusogenic particles, and highlight the recent advances and the promising applications of membrane-fusogenic particles as a next-generation bioengineering tool. In the end, we conclude with the present challenges and opportunities, providing insights in the future research of membrane-fusogenic particles.
1. Introduction
Membrane fusion is the fundamental biological process of the fusion of membrane components between cells, which occurs during the whole lifetime of the body.1–3 In this process, the targeted cell is primely tethered by its membrane to contact donor cells. Subsequently, a fusion reaction is facilitated to initiate the membranous mixing to form a merged continuous lipid bilayer. Lastly, a fusion pore is formed with the concomitant transfer of inner contents. As a result, not only is the composition of the membrane blended to promote the component exchange between cells, but also the internal contents are directly mixed to further enhance the message transmitting into the cytoplasm. Hence, such a “two-into-one” process can be considered as a both-side approach for extracellular and intracellular information transfer and cargo sharing between living cells. This kind of fusion between the plasma membranes has an overarching impact on cellular events in natural organisms, especially cell–cell communication and cargo transport.1Inspired by this natural membrane-fusion mechanism, material/chemical scientists have recently developed artificial membrane-fusion systems, mainly particles, to manipulate cell behaviors for broad bioengineering and biomedical applications. With rational and elaborate designs, these emerging systems can perform a biomimetic membrane-fusion process, rapidly fusing with the targeted cellular plasma membrane to transport the inner content to the cellular cytosol as well as simultaneously blending foreign substances with the cell membrane. In particular, membrane-fusion-mediated transfer of inner cargoes can bypass endosomal entrapment to maximize the transportation efficiency, emerging as a unique cytoplasmic delivery platform distinct from those depending on traditional endocytosis-based pathways.4 Furthermore, this could be a cell membrane engineering approach, as the membrane fusion would allow the lipid diffusion and mixing between particles and cells. Based on these features, membrane fusion-based particles have been advanced into various applications, particularly efficient drug/gene delivery and novel cell engineering.
In this review, we first commence with an overview of the natural membrane fusion processes and their occurring mechanism, which inspires the development of membrane-fusogenic particles for available biomedical applications (Fig. 1). Next, we highlight and give a detailed summary of the most recent advancements in strategies to induce fusion-based reactions as follows: (1) particle-cell surface modification to drive fusion; (2) virus-mimetic membrane fusion; (3) receptor-mediated membrane fusion; (4) natural cell membrane-fusion; (5) liposome/lipid-based membrane fusion; and (6) polymer-based membrane fusion. Furthermore, we share our prospects on these emerging membrane-fusogenic particles for various biomedical applications, mainly consisting of cargo delivery and membrane engineering. At the end, we highlight the opportunities and challenges of these emerging membrane-fusogenic particles, followed by summarizing current attempts and future potentials to develop the next-generation tools of membrane fusion.
2. The origin and mechanism of membrane-fusion effect
2.1 Membrane fusion in natural organisms
As a universal biological reaction in most natural organisms, the process of membrane fusion shares elementary processes, such as membrane attachment, merging of the lipid bilayers, and the formation of a fusion input pore for substance exchange.1 Generally, these biological fusion reactions occur variously in space and time within the body, tightly associated with the transport of molecules between and inside the living cells for communication.1 Based on this, natural membrane fusion processes can be distinguished into three types: (1) extra- and intracellular fusion, which are mainly mediated by invading viruses; (2) extracellular fusion; and (3) intracellular fusion. The first type is initiated by virus invasion that can induce the recognition and fusion between lipid-wrapped viral pathogens and the host cell plasma membranes. Such virus-triggered extra- and intracellular fusion are mainly dependent on the viral envelope glycoprotein on surfaces.2 The second type, extracellular fusion is existing between eukaryotic cells, such as the fusion of muscle cells into syncytia, the fusion for synaptic transmission, sperm-egg fusion to form fertilized eggs, etc. This extracellular recognition and the later fusion are catalyzed by a series of fusogenic proteins at the cell surface and are responsible for mediating biological processes of exocytosis, fertilization, and so on.1,5 The third type, intracellular fusion takes place in organelles, including the fusion between Golgi membranes and vesicles transported from the endoplasmic reticulum. With the distinct catalysts of regulatory molecules, these intracellular fusion events facilitate molecular trafficking inside the cells.2 Herein, the topic of “membrane fusion” discussed in this review is mainly referring to the fusion with the cell surface membrane with the concomitant inner content release.For the mechanism and process, there have been studies identifying that the biological fusion proceeds by an ordered sequence of steps, which can be embodied as a “kiss-and-run” strategy.1–3,5 The prime step is the aggregation between the membranes that are likely to close together and fuse. In this step, the highly specified fusogenic transmembrane proteins play a direct role in executing the fusion command.1 Next, the trigger of appropriate physical conditions and interactions enables two membranes to perform proximity of their lipid bilayers, accelerating further membrane attachment. Then, the local disruption hits the contacted cell membranes with transient destabilization to open the fusion pore.6 The last step is the membrane merging of mixed components in lipid layers, which can accompany the transfer of inner substances. Importantly, this membrane-fusion effect greatly impacts the biological conditions, deserving further studies from more precise scrutiny for the related delivery route and concomitant information exchange.
2.2 Inspired by this phenomenon: membrane-fusogenic particles
Since membrane fusion is quite a characteristic biological process, a series of membrane-fusogenic particles have been developed with potential benefits for various biomedical applications. These emerging biomimetic particles, with advanced designs of material components and decoration strategies, can mimic the route of membrane fusion in the cells, which is promising for fitting different biomedical needs to solve tough problems in challenging diseases. Briefly, given that the “kiss-and-run” membrane fusion process could directly transport the inner content to the cellular cytosol as well as insert foreign substances into the cell membrane synchronously, these biomimetic particles are mainly designed as a novel bioengineering tool for cytoplasmic cargo delivery and membrane editing.To date, there are generally two types of cellular uptake routes, including endocytosis and non-endocytosis (membrane fusion, direct translocation, intermembrane transfer, and so on).7 Currently, most particle-mediated delivery systems are internalized via endocytosis. However, their efficiency has been greatly criticized by endosomal entrapment and adverse degradation. It should be noticed that in the unique intracellular mechanism of membrane fusion, the inner contents are directly released into the cellular cytosol, which can entirely bypass the endocytic pathway. Thus, the cytoplasmic delivery using biomimetic membrane-fusogenic particles is expected to achieve higher efficiency without being trapped in the endo-lysosomes. This utilization is promising to be applied to a wide range of bio-functional and bioactive molecules.
More importantly, membrane-fusogenic particles can be not limited to just serving as carriers of inner cargos but can be also applied for membrane engineering. When the membrane fusion occurs, the merging of lipid bilayers provides access to substance exchange inside the membranes. As a result, functional cargos can be simply inserted into the cell membranes from fusion-based delivery without other complicated steps. Predominantly, this shining feature of the fusion-based strategy can be utilized for membrane editing to attain some specific therapeutic goals, such as intramembranous cargo transfer, exterior and interior membrane engineering, etc. Furthermore, the approach to induce membrane fusion is also potential for membrane engineering, in which more and more clear studies are needed to excavate this field.
3. Emerging strategies for membrane fusion
The natural process of membrane fusion has gained the structural and mechanistic insights from many studies. Learning from nature, biologists and material scientists have taken great efforts to develop more and more membrane-fusogenic systems under the assistance of bioactive materials and biotechnologies for various applications and potentials in biomedicines. Here, we give a thorough description of the emerging membrane-fusogenic strategies mediated by various biomaterials and biomimetic particles (Fig. 2). With the summary of these current strategies for artificial membrane fusion, we discuss their strengths or shortcomings, giving an outlook on both the development opportunities and the challenges (Table 1).| Fusogenic strategies | Advantages | Disadvantages | Triggers for fusion | Applications | Ref. |
|---|---|---|---|---|---|
| Dual surface modification to drive fusion | • Easy modification in vitro | • Limited for in vivo applications | Surface modification of complementary (KIAALKE)4 and (EIAALEK)4 peptides to form the coiled-coil structure | Intracellular delivery of fluorescent dyes and DOX | 8 |
| • Wide suitability in cell types | • Insufficient engineering efficiency | Intracellular delivery of upconversion nanoparticles and DOX | 10 | ||
| Intracellular delivery of cytochrome-c (cytC) in the core of mesoporous silica nanoparticles | 11 | ||||
| Surface modification of complementary (KIAALKE)3 and (EIAALEK)3 peptides to form the coiled-coil structure | Improved fusion of both homogeneous cell–cell fusion and heterogeneous cell–cell fusion | 9 | |||
| Surface modification of complementary DNA sequences to form the zipper-like structure | Intracellular delivery of protein cargos | 12 | |||
| Controlling specific vesicle fusion for parallel biological reactions | 13 | ||||
| Virus-mimetic membrane fusion | Good fusion efficiency | • Insufficient delivery efficiency in vivo | Surface modification of VSVG fusogen to facilitate virus-like fusion | Delivering functional membrane proteins into the plasma membrane | 14 |
| • Absent tissue or cellular specificity | Membrane engineering with pathogen-associated molecular pattern molecules | 15 | |||
| Membrane engineering of N3 groups for selective tumor labeling | 16 | ||||
| Intracellular delivery of CRISPR/Cas9 RNPs | 17 | ||||
| Surface modification of HA fusogen to facilitate virus-like fusion | Intracellular delivery of mRNA in the core of PLGA | 18 | |||
| Receptor-mediated membrane fusion | • Reliable safety | Few in vivo employments | Surface expression of full-length monoclonal antibodies to facilitate fusion | Intracellular delivery of DOX and antibody-dependent immunotherapy | 19 |
| • Good delivery targeting ability | |||||
| Surface modification of ICAM1 antibodies to induce tumor-specific fusion | Intracellular delivery of CRISPR/Cas9 plasmids in the core of alginate hydrogel | 20 | |||
| Natural cell membrane-derived fusion | • Reliable safety | • Insufficient delivery efficiency | Coating of cell-derived membranes with natural membranous fusogens to drive fusion | Intracellular delivery of DOX and poly(ADPribose) in the core of mesoporous silica nanoparticles | 21 |
| • Good biocompatibility | • Costing | ||||
| • Complicate production | Natural cell-secreted exosomes and vesicles with fusogen proteins to initiate fusion | Membrane engineering with HER2 for enhanced anti-HER2 therapy | 22 | ||
| Intracellular delivery of protein cargos | 23 | ||||
| Liposome/lipid-induced fusion | Flexibility | Concerns of serum stability | Appropriate lipid components to mimic the regulators to induce non-physiological fusion | Intracellular delivery of the TRAIL gene | 24 |
| Intracellular delivery of USP22 shRNA and sorafenib delivery into the plasma membrane | 25 | ||||
| Intracellular delivery of Irf5 siRNA | 26 | ||||
| Intracellular delivery of REV3L siRNA and PI3K-γ siRNA | 27 | ||||
| Intracellular delivery of microRNA-21 | 28 | ||||
| Intracellular delivery of microRNA1, 133, 208, and 499 | 29 | ||||
| Intracellular delivery of anti-S100A4 antibody and DOX | 30 | ||||
| Intracellular delivery of DOX | 31 | ||||
| Delivering photosensitizers into the plasma membrane | 32 and 33 | ||||
| Intracellular delivery of VEGF siRNA and DiR into the plasma membrane | 34 | ||||
| Membrane engineering on both the external and internal sides | 35 | ||||
| Polymer-mediated fusion | Flexibility | Needing optimizations | Rationally-designed polymeric structure to induce fusion-like behaviors | Intracellular delivery of DNA | 36 |
3.1 Dual surface modification for artificial membrane fusion
One of the earliest established systems for artificial membrane fusion is down to the dual modification of fusion-regulatory molecules on the particle and cell surfaces, allowing the particles to reach attachment to the targeted cells and then facilitate fusion.1,2 This strategy is primarily inspired by complementary soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex-mediated fusion in the process of neuronal exocytosis. The complementary SNARE protein subunits expressed on the opposing membrane surfaces can form a coiled-coil structure to drive the two membranes into close proximity, bringing the final lipid mixing, pore formation, and the concomitant content transfer.1 Learning from this SNARE machinery, Kros and coworkers have identified a pair of complementary synthetic peptides, (KIAALKE)4 (K4) and (EIAALEK)4 (E4), that can interact to form a SNARE-like coiled-coil structure to mediate liposome–cell membrane fusion (Fig. 3(A)).8 By conjugation of these two peptides on cholesterol-polyethylene glycol (PEG), these two lipidic peptides were able to insert onto the lipid bilayer surfaces of liposomes and cells, respectively, followed by the formation of a dimeric coiled-coil to promote liposomal and cellular membranes to contact and fuse. Similarly, Yoshihara et al. modified three repeated units of the EIAALEK and KIAALKE peptides on the membranes of two types of cells via lipid-PEG tails.9 The interaction of two peptides could induce a firm attachment between cells, which significantly improved homogeneous and heterogeneous cell–cell fusion. However, this coiled-coil-derived membrane fusion occurs spontaneously, distinct from the membrane fusion in the natural process, which is highly regulated in both time and space to ensure correct biological function. To further optimize this membrane fusion system, Huang et al. proposed a temporally-controllable membrane-fusion strategy via a light-triggered formation of a coiled-coil-based structure.10 They inserted a photolabile PEG, cholesterol-ortho-nitrobenzyl-PEG, on the lipid layer of K4-modified liposomes, which can shield the interaction of K4 with E4 decorated on cells to prevent the membrane-fusion between liposomes with cells. After exposure to the UV light irradiation, the nitrobenzyl groups were cleft to remove PEG shielding blocks from liposomes, thereby recovering the interactions between K4 and E4 peptides to mediate temporally-controllable liposome–cell membrane fusion. These coiled-coil-derived systems can achieve rapid fusion-based delivery of encapsulated cargos in liposomes into cells bypassing the endocytosis. Given this feature, Kros's group also explored the potential of dual modification of these complementary peptides as a membrane-fusion-mediated cytoplasmic drug delivery platform for therapeutic purposes.11 Using cytochrome-c-loaded mesoporous silica nanoparticles as the cores, the peptide E4-modified liposomes could deliver encapsulated cargoes into peptide-K4-decorated cancer cells via membrane fusion.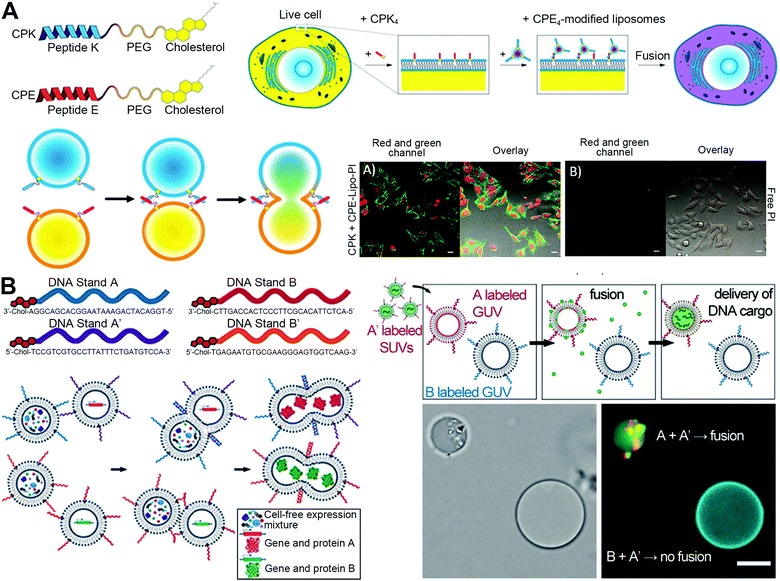 | ||
| Fig. 3 Strategies of particle-cell dual modification to facilitate membrane attachment and induce fusion. (A) Developed lipopeptides for membrane fusion. The coiled-coil structure formed by the complementary lipopeptides can induce fusion, which was verified by confocal analysis of PI delivery mediated by this coiled-coil-triggered fusion. Scale bar, 25 μm. Reproduced with permission under an ACS AuthorChoice License (Creative Commons License).8 Copyright 2016, American Chemical Society. (B) DNA hybridization with complementary oligonucleotides to induce fusion and thus achieve parallel biological reactions. Scale bar, 10 μm. Reproduced with permission.13 Copyright 2019, Wiley-VCH. | ||
This improved delivery system directly released therapeutic cargos in the cytoplasm, resulting in nearly 50% tumor apoptosis in vitro. In total, the hybridization of peptides anchored on dual cell membranes can induce recognition and membrane attachment, which promotes the fusion with specific cell membranes.
Besides these peptide-based molecules, another dual-surface modification strategy based on DNA hybridization has been applied to trigger artificial membrane integration. For example, Sun et al. designed DNA–lipid hybrids for surface modification to perform targeted and programmable fusion with cellular plasma membranes.12 They found that the hybridization of 3′ cholesterol-functionalized single-stranded DNA and the complementary 5′ cholesterol-functionalized single-stranded DNA modified on cell membranes and liposomes respectively could form a zipper-like structure to achieve membrane tethering and facilitate fusion. The cellular uptake results also showed that the protein delivery using these DNA–lipid hybrids was dependent on the fusion pathway, not the endocytosis pathway. Recently, Kamat and coworkers reported complementary DNA oligonucleotides to induce membrane attachment and facilitate fusion between vesicles (Fig. 3(B)).13 They utilized diverse sets of DNA oligonucleotides to control membrane fusion between specific vesicle populations for parallel biological reactions. Such DNA-mediated orthogonal vesicle fusion can mediate content mixing to induce cell-free protein synthesis, expanding the potential of vesicle-based materials.
The strategy of dual-surface modification of fusion-regulatory molecules on both particles and cell membranes is one of the earliest inspiring ideas for membrane fusion from nature. However, the dual modification on cell–cell or liposome–cell surfaces for artificial membrane fusion is limited for in vivo applications due to the difficulty of in vivo modification. Additionally, with these dual-modification strategies it is still hard to engineer the targeted cells with sufficient efficiency, and requires further improvement.
3.2 Virus-mimetic membrane fusion
Membrane fusion of viruses with host cells is a typical pathophysiological process.37 Naturally, this process is mediated by glycoproteins expressed on the virus, such as spike vesicular stomatitis virus G-protein (VSVG) and hemagglutinin (HA) that has been proved to trigger membrane fusion at acidic pH.14–16 As for VSVG, when exposed to the acidic environment, they can dramatically bind the targeted cell surfaces and facilitate membrane fusion due to the electrostatic repulsion and solvation energy gained from protonated histidine in protein sequences. For HAs that are usually expressed in the influenza A virus, the acid environment after endocytic uptake will trigger a conformational change of the HA subunit to facilitate the fusing process between the viral envelope and the endosomal membrane. Promisingly, these specific viral proteins have been fabricated onto the surface of particles, usually genetically-engineered cell-derived vesicles and exosomes, forming virus-like particles to facilitate membrane fusion for plasma editing and cargo delivery. For example, Yang and coworkers constructed VSVG-expressed exosomes derived from 293T cells to modify membrane proteins on the target cells (Fig. 4(A)).14 These engineered exosomes with viral fusion components on the surfaces could achieve a pH-mediated fusion reaction with the targeted cellular membrane, directly delivering the co-expressed functional membrane proteins (CD63 and GLUT4) into the targeted plasma membranes. This fusogenic exosome could be applicable with further modifications of targeting moieties for future membrane-protein therapy. Besides, Kim et al. used VSVG-engineered exosomes to fuse with tumor cells to achieve tumor xenogenization for enhanced antitumor immunity (Fig. 4(B)).15 The VSVG proteins could facilitate the fusion of exosomes with tumors to present themselves on the tumor surfaces, subsequently serving as pathogen-associated molecular pattern molecules to mediate the recognition and engulfing by dendritic cells for immune activation. This tumor xenogenization strategy mediated by VSVG-modified exosomes performed effective reactions of immunogenicity to inhibit tumor growth, which was identified in multiple tumor mouse models. Furthermore, Ren et al. utilized vesicles anchored with both VSVG and N3 groups to achieve selective tumor labeling via membrane fusion for diagnosis.16 The low-pH activated property of VSVGs enabled vesicles to selectively fuse with tumor cells under the acidic environment, presenting N3 groups on tumor surfaces to bind with dibenzocyclooctyne (DBCO)-modified molecules for cancer-targeted diagnosis.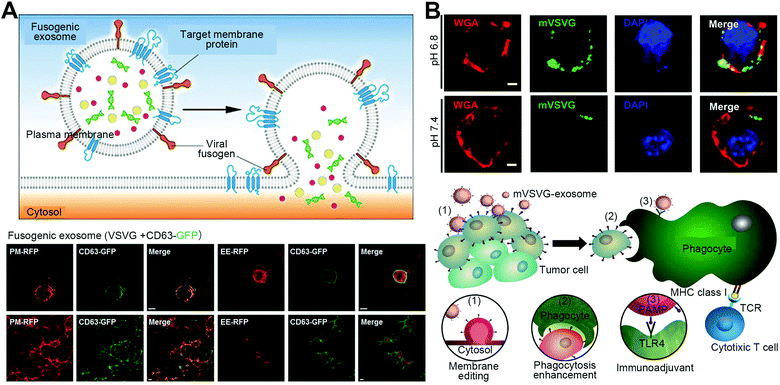 | ||
| Fig. 4 Strategies of virus-mimetic fusion. (A) VSVG-expressed fusogenic exosomes to deliver target membrane proteins into the plasma membrane. Here is the schematic illustration of fusogenic exosomes mediated by viral fusogens and confocal images showing the localization of the exosomes (CD63-GFP), the plasma membrane (PM-RFP), and early endosomes (EE-RFP). Scale bars, 10 μm. Reproduced with permission.14 Copyright 2017, Wiley-VCH. (B) VSVG-expressed fusogenic exosomes to achieve tumor xenogenization. Here is the confocal analysis showing the fusion at pH 6.8 and a schematic summary of the VSVG-mediated tumor xenogenization strategy. Scale bar, 50 μm. Reproduced with permission under a Creative Commons CC BY-NC License.15 Copyright 2020, American Association for the Advancement of Science. | ||
These VSVG/HA-engineered fusogenic exosomes and vesicles have also been developed as platforms for intracellular delivery of the inner therapeutic agents. For example, Montagna et al. reported a VSVG-decorated vesicle carrying CRISPR/Cas9 ribonucleoproteins (RNPs) with high gene editing efficiency via fusion-based transport.17 With the VSVG modification on membranes and encapsulation of RNP cargos inside, this vesicle-based delivery system exhibited membrane fusion for direct cytosol release of inner contents, achieving effective gene editing in pluripotent stem cells and cardiomyocytes. Recently, Park et al. designed an HA-displayed cell membrane-coated nanoparticle for the membrane-fusion-mediated cytosolic delivery of mRNA.18 This nanoparticle was formulated by coating an HA-expressed cell membrane on the poly(lactic-co-glycolic acid) (PLGA) cores carrying mRNA. Through HA-mediated membrane fusion with tumor cells in the acidic environment, these developed virus-mimicking particles successfully transfected model mRNA payloads (EGFP and Cypridina luciferase) both in vitro and in vivo.
It is an elegant strategy to utilize the existing transmembrane viral fusion proteins in particle construction to induce the events of viral fusion. However, two large challenges pertain to the in vivo delivery efficiency and the absence of tissue or cellular specificity. It is also worrying whether the introduction of foreign viral fusogens would induce undesired immune responses.
3.3 Receptor-mediated membrane fusion
The receptor-mediated membrane fusion is another strategy to exploit fusogenic particles. In the case where two cell membranes are destined to be fused, there should be some driving factors for the initial recognition, especially the interactions with a specific receptor on the cell surface.5 Inspired by this trait, the integration of particles with receptor-stimulating factors (mainly antibodies) can mimic the specific tethering factors to allow cell membranes for close apposition and downstream fusion. Recently, Liu et al. constructed nanovesicles with the expression of full-length monoclonal antibodies (hGC33/KM3934 antibodies) to selectively deliver cytotoxic drugs via the antibody-mediated energy-dependent membrane fusion for combining anti-tumor chemotherapy and immunotherapy (Fig. 5(A)).19 The antibodies on the surfaces could mediate the binding to responding receptors on the cell membranes and then facilitate the fusion (Fig. 5(B)). With receptor-mediated membrane fusion, not only were the internalized drugs from nanovesicles directly released into the cell for chemotherapy, but the transferred antibodies also mediated the activation of NK cells for antibody-dependent cellular cytotoxicity (Fig. 5(C)). This multifunctional platform had an excellent tumor-targeting ability and antitumor effect. Furthermore, Guo et al. constructed a tumor-specific nanolipogel system with intercellular adhesion molecule 1 (ICAM1) antibody-guided targeting and fusion for CRISPR/Cas9 delivery.20 By modification of ICAM1 antibodies on the surfaces, the system exhibited receptor-mediated membrane fusion with triple-negative breast cancer cells to deliver plasmids loaded in the cores directly in the cytosol for specific and anti-tumor gene editing. The in vivo results showed more than 80% CRISPR gene knockout of Lipocalin 2 (the oncogene of breast cancer) in orthotopic tumor models, exerting significant inhibition of tumor growth.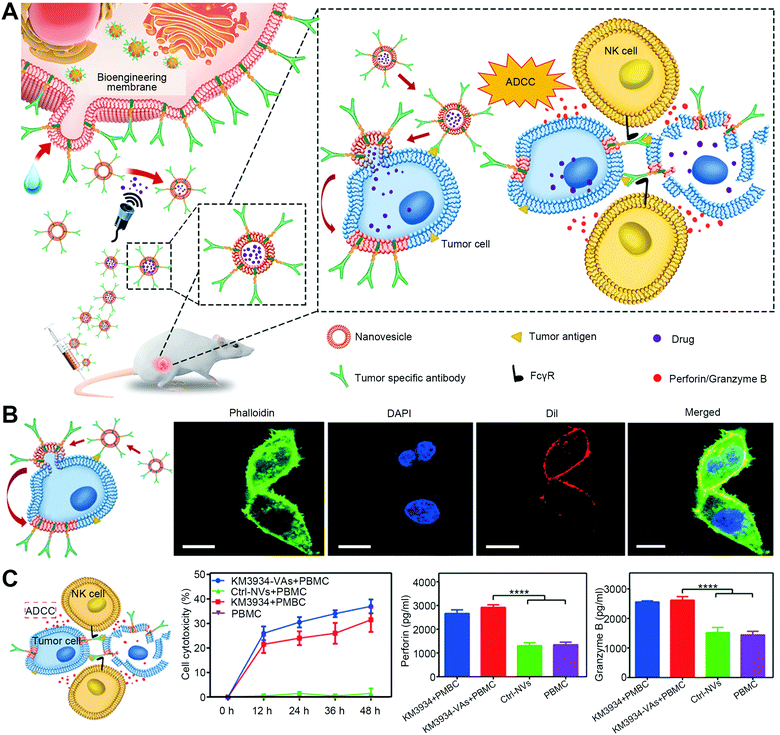 | ||
| Fig. 5 The strategy of receptor-mediated fusion via tumor-specific antibody-expressed nanovesicles (VAs). (A) Schematic illustration of bioengineering VAs for anti-tumor combination therapy. (B) Antibody-mediated fusion verified by confocal images. Scale bar, 10 μm. (C) ADCC-mediated immunotherapy induced by KM3994-VAs with the measurements of cell toxicity, NK activation (granzyme B), and proinflammatory cytokine (perforin). PBMC, human peripheral blood mononuclear cell. Reproduced with permission.19 Copyright 2019, Wiley-VCH. | ||
Fusogenic particles with receptor-mediated membrane fusion have broadened the range of biomedical applications, being safer than the virus-mimetic membrane fusion that is derived from infectious viruses. Besides, the rational receptor modifications of particles can not only possess the ability for efficient membrane fusion but also enhance the targeting ability, which is the key point for further in vivo applications.
3.4 Natural cell membrane-derived fusion
For the natural cell–cell fusion, the authentic fusogenic proteins expressed on the natural cell membranes have a great impact on tethering membranes for fusion.5 To date, a variety of surface proteins have been identified with the ability to promote cell–cell membrane fusion, including the Caenorhabditis elegans anchor cell fusion failure-1 (AFF-1) and epithelial fusion failure-1 (EFF-1) proteins on epithelial cells, syncytins on the trophoblast cells in the placenta, proteins with immunoglobulin (Ig)-like domains, such as CD9 and CD47, the highly conserved family of SNARE proteins, multiple-C2-domain proteins, etc.5 These fusogenic proteins expressed on cell membranes generally involve the processes of inducing close proximity and destabilization of membranes, bringing about high membrane curvature for the subsequent lipid merging and fusion. Therefore, the coating of natural cell membranes for the particle surfaces can be used as a simple but direct strategy to construct the biomimetic membrane-fusogenic systems due to the intact preservation of these authentic fusogenic proteins For example, Nie et al. synthesized a yolk-shell-structure nanoparticle with the coating of cancer cell membranes to induce fusion-based delivery of therapeutic agents for cancer therapy (Fig. 6(A)).21 With natural membranous fusogens on the particle surface, nanoparticles could target the homologous sites and exhibit a direct cellular fusion (Fig. 6(B)). As a result, efficient internalization and a 23.3-fold increased tumor penetration were observed in vivo (Fig. 6(C)). Besides, co-encapsulation of doxorubicin (DOX) and the poly(ADPribose) polymerase inhibitor in the nanoparticle yolks also had significant anti-tumor activities, highlighting the promising future of cancer-cell-membrane-coated particles for cancer therapy.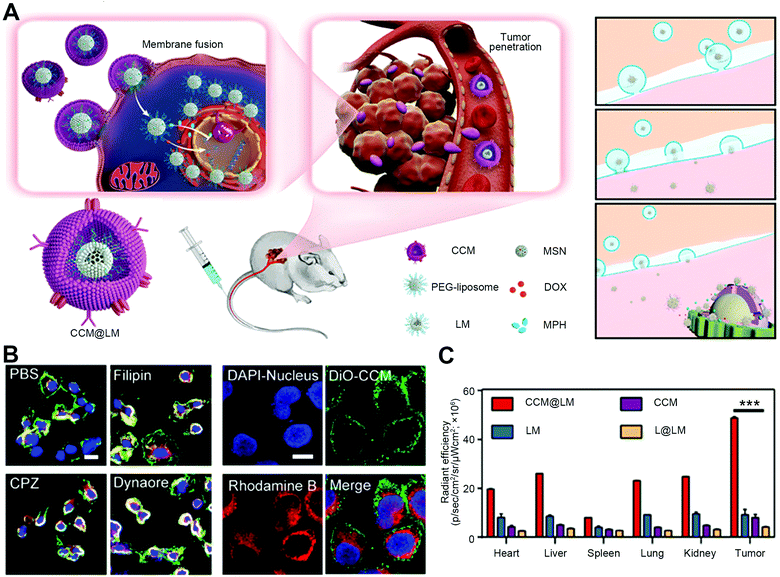 | ||
| Fig. 6 The strategy of natural cell membrane-derived fusion that is mediated by the coating of cancer cell membranes. (A) Schematic illustration of CCM@LM to induce fusion-based delivery for enhanced cancer chemotherapy. (B) Confocal analysis of the cellular uptake of inhibition treatments and rhodamine-B-loaded CCM (DiO). Scale bar, 10 μm. (C) Tumor targeting assay of different nanoparticles after 24 hour injection. Reproduced with permission.21 Copyright 2019, American Chemical Society. | ||
Similarly, the endogenous cell-secreted exosomes and vesicles that are associated with intercellular communication and manipulation of biological behaviors by shuttling genetic information and proteins or other biomacromolecules to exchange information and give certain orders,38–40 for which they have the potential to be biocompatible delivery systems and regulators for interesting biological behaviors. Interestingly, exosomes have been demonstrated with potential cellular uptake via direct membrane fusion with the plasma membrane.23,41,42 Both exosomes and vesicles derived from natural cells have been proven with abundant fusogenic proteins that can activate binding and initiate fusion in the active site.2 For example, Quinn et al. reported an extracellular vesicle derived from human epidermal growth factor receptor 2 (HER2)-overexpressed BT-474 cells that could fuse with triple-negative breast cancer (TNBC) to transfer sufficient HER2 on their surfaces as anti-HER2 targeting domains.22 Combined with anti-HER2 antibody-conjugated paclitaxel-loaded liposomes, this extracellular vesicle-based anchoring strategy greatly improved their therapeutic efficacy both in vitro and in vivo. Despite the promise of natural exosomes and vesicles as membrane fusion-based delivery tools, their cargo-loading rate and efficiency, especially for biomacromolecules, are really limited. This is because traditional loading methods, such as electroporation, are dependent on the passive loading of biomacromolecules into isolated exosomes or vesicles. To optimize this, Yim et al. developed an active protein-loading method in exosomes via optically-reversible protein–protein interaction for more effective delivery of proteins into the cytoplasm.23 They designed two fusion proteins including a cargo protein fused with a photoreceptor cryptochrome 2 (CRY2) and a protein conjugate of CRY-interacting basic-helix-loophelix 1 (CIB1) protein with an exosome-associated tetraspanin protein CD9. After transduction in the exosome-producing cells, these two proteins will bind together under blue light illumination due to the binding of CRY2 with CIB1, connecting the cargo proteins with CD9. With the assistance of CD9 tetraspanin proteins, the cargoes were actively introduced into the exosomes efficiently. Exosomes generated using these optically-regulated protein–protein interactions could achieve a more efficient intracellular delivery of cargo proteins, like mCherry, Bax, super-repressor IκB protein, and Cre enzyme, into the target cells via membrane fusion in vitro, and into brain parenchymal cells in vivo.
Despite the insufficient efficiency, natural cell membrane-derived membrane fusion has the excellent advantage of good biocompatibility for biosafety since this manner of membrane fusion might be the most similar process to the biological one. However, the preparation of these systems might have a considerable cost outlay and require a complex and laborious procedure, which hinders further progress. An improved method of natural cell membrane-derived fusion, such as combining with the viral-mimetic or receptor-mediated membrane-fusion strategies, will have great potential for in vivo medicine and clinical translation.
3.5 Fine-tuned liposomes: controlling formulation properties to drive membrane fusion
To establish a membrane fusion-based platform depending on artificial materials, liposomes and lipid-based particles are suitable to mimic the membrane fusion process with the lipid merging for a direct cytosolic influx of therapeutic biologics in target cells or compartments. It is conceivable that lipid materials have a chemical structure, fluidity, and phase-transition behavior similar to the cell membrane lipid layers, for which appropriate lipid components are expected to mimic the regulators to induce non-physiological fusion.5,43 For example, Shen's group developed a fusogenic lipo-polyplex coating with lipids to trigger membrane fusion-mediated DNA delivery.24 The fusogenic lipid envelope of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-polyethylene glycol (DSPE-PEG), and negatively charged cholesteryl hemisuccinate (CHEMS) could be tuned to a fusion-based internalization, thereby allowing DNA-loaded polyplex cores to eject into the cytosol. With the further fabrication of RGDK ligands for enhanced targeting, these fusogenic lipo-polyplexes could be applied for anti-tumor TRAIL gene delivery in vivo, resulting in both significant tumor accumulation and powerful anticancer activities. Recently, such established membrane-fusogenic vectors were switched to be decorated with galactose to co-deliver sorafenib and ubiquitin-specific protease 22 (USP22) short hairpin RNA (shRNA) for specific and synergetic treatment of hepatocellular carcinoma (HCC).25 With HCC-targeting membrane fusion-mediated transport, this therapeutic nanoplatform potently inhibited the tumor cell viability and exhibited effective tumor inhibition in sorafenib-insensitive patient-derived xenograft models.Besides, Kim's group developed another lipid-based fusion system: porous silicon particle shedding with fusogenic liposomes to introduce an oligonucleotide payload into the cytosol via membrane fusion.26 This fusogenic lipid layer was constructed by the composites of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), and DSPE-PEG with a specific ratio. The large portion of DMPC components endows the lipid layer with a moderately low phase transition point near room temperature, maintaining a fluidity similar to cell membranes. The positive charged DOTAP allows the liposome to interact with cell membranes via electrostatic absorption. Meanwhile, PEG or an equivalent dehydrating agent presented in the lipid formulation excludes the disturbance to lipid–membrane interaction by other molecules in the environment. By anchoring appropriate target motifs on surfaces and loading cargoes in the silicon cores, this established fusogenic liposome could be utilized to deliver a variety of molecules to desirable sites. For example, they established a fusogenic liposome with the decoration of macrophage-homing peptides and the encapsulation of oligonucleotide payloads in the core of porous silicon particles. Benefiting from the fusogenic and macrophage-targeting coating, the system was proved to deliver siRNAs through membrane fusion but not endocytosis, significantly knock-downing the proinflammatory macrophage marker Irf5 to restart the clearance ability of macrophages. Such fusogenic liposome-based reprogramming of macrophages could achieve an efficient elimination of Staphyloccocus aureus pneumonia in mouse models. More recently, they also improved the formula of this type of fusogenic lipid coating (DMPC:DOTAP:DSPE-PEG) for siRNA delivery for cancer therapy (Fig. 7(A)).27 To confirm the membrane fusion, the fusogenic nanoparticles and non-fusogenic nanoparticles were used to determine cellular uptake with endocytosis inhibition (Fig. 7(B)) and intracellular trafficking (Fig. 7(C)). Then, they applied the optimized fusogenic particles modified with tumor cell-targeting iRGD and tumor-associated macrophage (TAM)-targeting Lyp-1 peptide to deliver REV3L siRNA and PI3K-γ siRNA, to the cancer cell and TAM, respectively (Fig. 7(D)). The results indicated that this therapy showed efficient REV3L silence and TAM reprogramming, achieving significant anti-cancer effects (Fig. 7(E)).
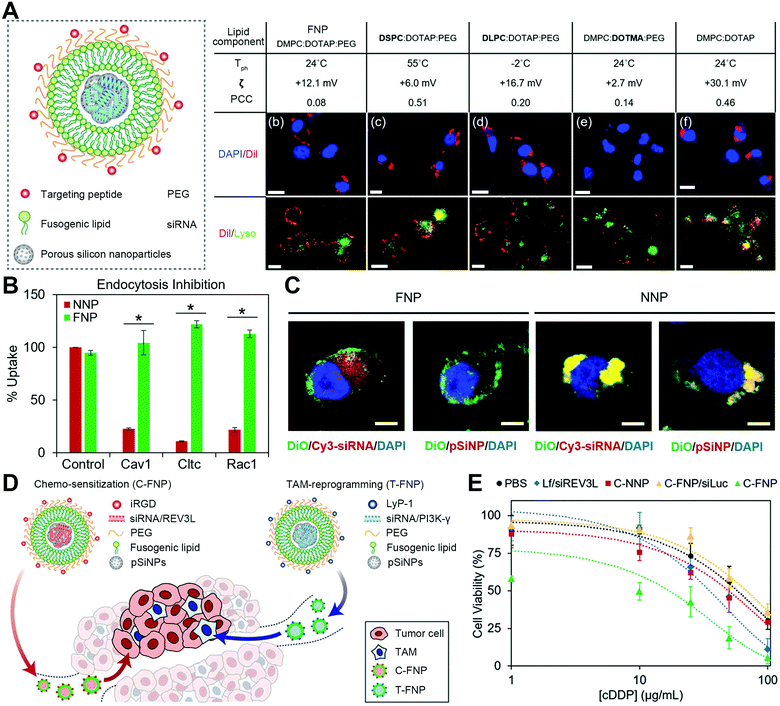 | ||
| Fig. 7 The strategy of designing liposomes with optimized lipid components and ratios to induce membrane fusion. (A) Schematic illustration of optimized fusogenic nanoparticles (FNPs) to achieve fusion with the cellular plasma membrane. (B) Cellular uptake of DiI-loaded FNPs and non-fusogenic nanoparticles (NNPs) in CAOV-3 cells that were pre-treated with different endocytosis inhibitors. (C) Confocal analysis of CAOV-3 cells incubated with FNPs and NNPs. FNPs and NNPs were labeled with DiO (green) in the lipid shell. Scale bar, 5 μm. (D) Schematic showing C-FNPs and T-FNPs for gene silence in cancer chemotherapy. (E) The chemosensitivity analysis in CAOV-3 cancer cells. Reproduced with permission.27 Copyright 2019, Wiley-VCH. | ||
Furthermore, this type of fusogenic liposome (DMPC/DOTAP/DSPE-PEG) was also reported to be hybridized with natural cell membranes or membrane proteins for enhanced targeting of some specific lesions. Ge's team modified these membrane-fusogenic liposomes with additional hybridization of platelet cell membranes to deliver the anti-inflammatory microRNA-21 for myocardial remodeling in cardiac healing.28 Owing to the aggregation of circulating platelet-monocytes in post-myocardial ischemia-reperfusion injury, these platelet cell membrane-integrated fusogenic liposomes could specifically accumulate in inflammatory monocytes in blood circulation, efficiently delivering microRNA-21 speedily into their cytoplasm for improved myocardial remodeling. Likewise, this group hybridized neutrophil-derived membrane proteins on fusogenic liposome surfaces to mimic the homing ability of neutrophils to the injured heart after myocardial infarction.29 The neutrophil-modified fusogenic liposomes could achieve specificity through the interaction with chemokine ligands expressed on injured endothelium and myocardium. With this specific targeting ability, the fusogenic liposomes could precisely deliver a combination of microRNAs (microRNA1, 133, 208, and 499) to cardiac fibroblasts via a membrane-fusion manner, resulting in effective cardiac reprogramming with cardiac function recovery and alleviative fibrosis in vivo.
Overall, the similar structure of lipid bilayers and appropriate preparations of selective lipid materials are significant for these flexible fusion-based systems with profound functional optimizations. Besides, lipid or liposomal particle also possess the advantages of broad cargo-loading capacity and feasible surface modification. Therefore, lipid/liposome membrane-fusogenic particles can be designed and engineered to fuse with the targeted cell membrane and applied to achieve quick and efficient drug delivery and membrane engineering. Currently, with the advantages of great loading capacity, the developing fusogenic lipid particles have been applied to deliver therapeutic nucleus acids, proteins, and chemical drugs, bypassing the endocytosis-endosome pathway to directly release cargos into the cytoplasm. Hence, these fusion-based delivery systems are promising to improve drug efficiency to a higher extent for a more effective efficacy. Furthermore, some studies have reported that fusogenic liposomes could load hydrophobic agents in the lipid bilayers, fusing with the plasma membrane to achieve cargo transfer to cell membranes for labelling or therapeutic goals. Therefore, fine-tuning liposomes and lipid-based particles to induce membrane fusion are worth further exploration, in which decent lipid formulation properties and specific additions are vital for further improvements in the stability and delivery efficiency.
3.6 Rationally-designed polymers: pore-mediated fusion
Quite recently, an artificial polymer-based nanosystem has exhibited its promising potential in fusion-based delivery to facilitate the release of encapsulated cargos. With elaborate designs, polymer materials can mimic the viral behaviors to stick onto the cell membrane and induce a fusion pore to eject inner cargos to the cytoplasm. Representatively, Shen and coworkers innovatively developed virus-mimetic polyplexes for gene delivery (Fig. 8(A)).36 This polyplex is composed of a quaternized linear polyethyleneimine whose ammonium moieties have N-(p-acyloxy benzyloxycarbonyl)ethyl groups (Fig. 8(B)). Such polyplexes could condense DNA to form a nanoparticle due to the electrostatic interaction and hydrophobic blocks. Upon binding to cell membranes, the phenol ester bonds would be hydrolyzed by esterase in the cytoplasm to turn the polymer from cation to zwitterion, reducing its interaction with DNA to release them into the inner sides of membranes. Meanwhile, the residual polymer would still be retained on the cell membrane due to the membrane-insertion of the long acyl chain and the protein adsorption-resistance of zwitterion blocks. With this process, these polyplexes could deliver DNA directly into the cytoplasm in a pore-mediated fusion manner. Furthermore, these DNA-injecting polyplex-based nanoparticles could be also coated with a poly(γ-glutamic acid) (γPGA) layer to prolong their blood retention (Fig. 8(C)). This polymer-mediated membrane fusion is hoped to open a new area for the design of fusogenic particles, allowing more diverse utilizations.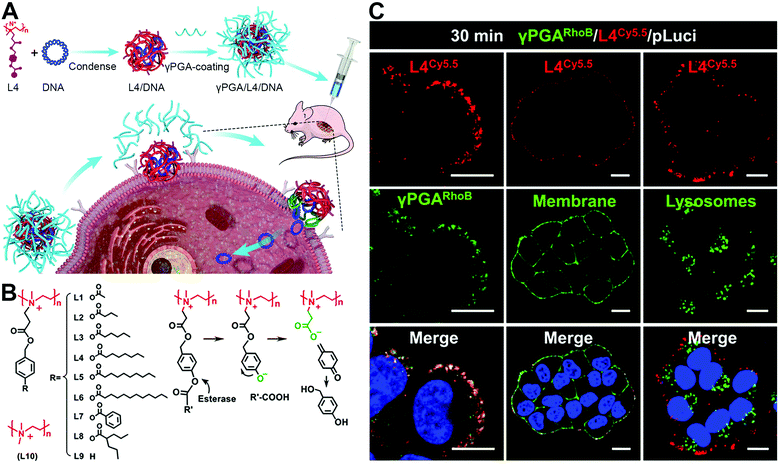 | ||
| Fig. 8 The strategy of specially designed polymer nanocarriers with pore-mediated fusion transport. (A) Schematic illustration of γPGA/L4/DNA to induce a fusion pore for cargo direct injection into the cells. (B) The structures of quaternized polymers with different acyl chain lengths. (C) Confocal analysis of γPGA/L4/DNA with the co-localization of L4, γPGA, the plasma membrane, and lysosomes. Scale bar, 25 μm. Reproduced with permission.36 Copyright 2021, Elsevier. | ||
Polymeric materials have been widely used in particle constructions with great adaptability,44–46 while there is currently a gap in polymer-based fusogenic systems. The aforementioned γPGA/L4/DNA system represents a milestone for promoting the development of polymer-mediated fusion, expanding more possibilities. Promisingly, well-designed polymers have the potential to exploit alternative strategies to accomplish a pore-mediated fusion manner, providing a wider range of opportunities for their use in different biomedical applications with convenient and tunable synthetic processes.
4. Biomedical applications of membrane-fusogenic particles
With the rapid development of membrane fusion-mediated strategies, the emerging fusogenic particles have been developed, accomplishing efficient and controllable membrane fusion for various applications in the field of medicine with enhanced effects. Since the membrane-fusogenic particles have been developed to mimic the fusion process, these undertaken systems possess valuable merits, with which they can not only directly deliver inner cargos to the targeted cellular cytoplasm, but also achieve membrane editing via the synchronous plasma membrane insertion. In the following section, we discuss different developing directions of membrane-fusogenic particles, including delivery directly into the cytoplasm, delivery into the plasma membrane, and engineering of extra- and intra-cellular membranes (Fig. 9).4.1 Delivery directly into the cytoplasm
In the field of rapidly-developing medicine, it is vital to gain highly efficient delivery of biologic agents towards intracellular targets for intended functions. However, current nanocarrier-mediated delivery is still hindered by physiological barriers, especially due to the clearance and degradation risks via inefficient endocytosis.7,47,48 Traditional endosomal uptake is prone to only reach less than 10% cytosolic release of payloads that remain active.4,49 Although there have been materials constructed for endosome escape, these methods have the possibility to cause undesired toxicity. Therefore, direct penetration and cytosol delivery strategies that avoid endosomal elimination are attracting more attention.50–52 In particular, increasing membrane fusion-based nanocarriers have been energized to provide a direct and fast route for inner cargo delivery without endosomal entrapment. With appropriate and individual designs, membrane-fusogenic particles can encapsulate inner cargos of nucleic acids, proteins, small molecule drugs, and so on, which is promising for a novel and efficient delivery.Taking a recent study as an example, Ge's group established a fusogenic system to deliver miRNAs via membrane fusion-based transport to treat fibroblast features (Fig. 10(A)).29 This system showed efficient fusion-mediated gene delivery by avoiding the obstacles of endosome-mediated degradation (Fig. 10(B)). In the mouse models of myocardial damage, the efficient miRNA delivery of this system promoted significant cardiac regeneration (Fig. 10(C)). Besides, Qin's team established a tumor microenvironment-sensitive membrane-fusogenic liposome to transfer both anti-S100A4 antibodies and DOX into the metastatic tumors.30 This system performed fast delivery based on the efficient fusion-like uptake, thereby achieving great synergistic cancer therapy in both 4T1 cells and tumor xenograft mouse models. Moreover, Pitchaimani et al. engineered a natural killer cell membrane-infused fusogenic liposome (NKsome) for targeted drug delivery directly into the cytoplasm to treat tumors.31 The results indicated that these engineered NKsomes retained great biocompatibility, fusogenic characteristics, and NK cell membrane-associated specific proteins for tumor targeting efficiency. Thus, the DOX-loaded NKsomes achieved effective therapeutic activity for cooperative drug delivery both in vitro and in MCF-7 tumor cell-bearing mice. These membrane-fusogenic carriers have been successfully and widely used in the cytoplasmic delivery of various reagents.
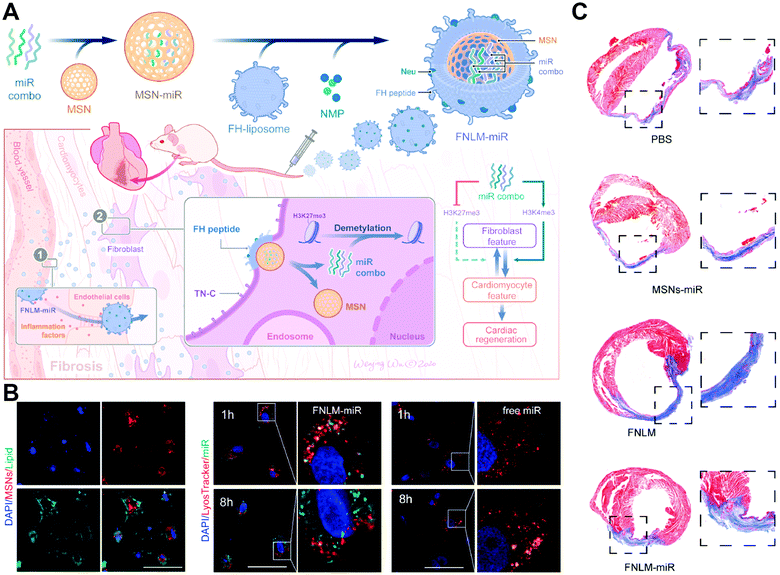 | ||
| Fig. 10 The application of membrane fusion-based transport for inner cargo delivery directly into the cytoplasm, avoiding endosome-induced degradation. (A) Schematic illustration of fusogenic nanoparticles to deliver miRNAs for cardiac regeneration. (B) Confocal analysis of the colocation of MSNs, lipids, endosomes, and miRNAs. Scale bar, 50 μm.(C) Representative images of myocardium after different treatments. Reproduced with permission.29 Copyright 2021, Elsevier. | ||
The developing membrane-fusogenic particles acting as nanocarriers can perform in an effective manner fusion with the targeted cellular plasma membrane, smoothly and speedily delivering the inner cargos into the cytosol while bypassing endocytosis. More than that, membrane-fusogenic nanocarriers are tolerant to a wide range of cargos. This delivery strategy has the prominent advantage of low degradation risk but high efficiency, for which these fusogenic nanocarriers have the potential for diverse clinical translations.
4.2 Delivery into the plasma membrane
Besides intracellular delivery, delivering into the plasma membrane is another direction for exploration. However, workable approaches for cargo transfer into the targeted lipid layers of cell membranes are limited. With agents packaged inside the lipid bilayers, the fusion-dependent manner can be a novel cargo transport tool for inserting cargos into membranous lipid layers, allowing different ways for delivery and achieving special applications.For example, the exosome-based fusogenic delivery with “membrane editing” developed by Kim et al. achieved the transfer of biomedical membrane proteins into the targeted cell membranes.14
By honoring VSVG fusogen proteins and interested proteins on the fusogenic exosomes, the cargoes could be successfully transported into the plasma membrane via the fusion pathway both in vitro and in vivo. This study offers a novel delivery strategy to insert bioactive membrane proteins in cell membranes, providing a reliable solution to membrane protein defects in human disorders. Similarly, Park and coworkers developed membrane-fusogenic liposomes for the delivery of photosensitizers into plasma membrane.32 The photosensitizers, ZnPc, were loaded inside the lipid layer of membrane-fusogenic liposomes, which were demonstrated to be inserted into the plasma membrane via the fusion reactions. The in vitro results indicated that this selective membrane location of ZnPc with irradiation could rapidly disrupt cellular membranes and effectively perform photodynamic therapy. Additionally, they also reported a liposome-mediated membrane vesicle engineering strategy via the selective delivery of hydrophobic compounds to the tumor cell plasma membranes (Fig. 11(A)).33 The developed membrane-fusogenic liposomes loaded with hydrophobic cargos could be incorporated into recipient cell membrane and subsequently transported to secreted vesicles for deep penetration (Fig. 11(B) and (C)). This delivery strategy of photosensitizers significantly enhanced the therapeutic effects in both spheroids and in vivo tumors. More recently, Xue's group reported worm-like nanocell mimics constructed by coating erythrocyte membranes on a worm-like nanoparticle for in situ tumor cell engineering via membrane fusion.34 With targeted membrane fusion, 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indotricarbocyanine iodide (DiR) inside the lipid bilayers of the nanocell mimics could be inserted into the plasma membranes of primary and circulating tumor cells (CTCs). Then the engineered cells could secrete extracellular vesicles to enhance the tumor penetration as well as capture and cluster homologous CTCs, inducing NIR-mediated photothermal therapy in both primary tumors and metastatic sites. The in vivo results illustrated that the worm-like nanocell mimics performed excellent penetration, achieving great anti-tumor and anti-metastasis efficacy.
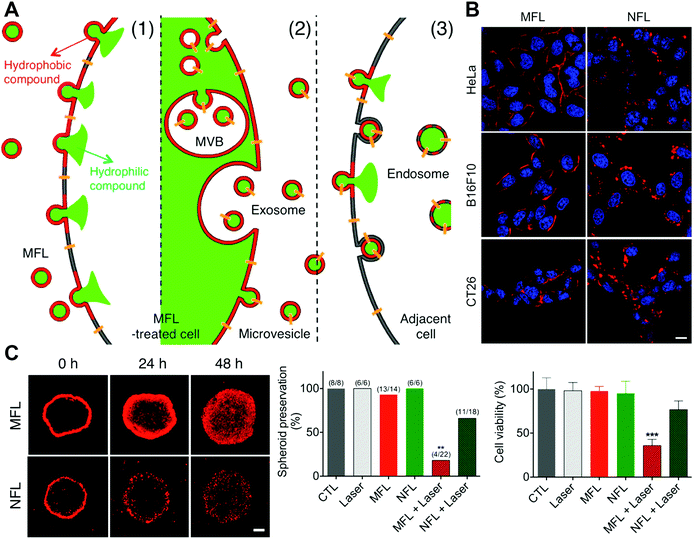 | ||
| Fig. 11 The application of membrane fusion-based delivery into the cellular plasma membranes. (A) Schematic illustration of MFL-delivered hydrophilic and hydrophobic compounds into the membrane vesicles and adjacent cells. (B) Confocal analysis of cells incubated with fusogenic and non-fusogenic liposomes carrying the ZnPc photosensitizer (red). Scale bar, 5 μm. (C) Confocal microscopic images of tumor spheroids treated with ZnPc-loaded liposomes and the analysis of preservation and cell viability. Scale bar, 200 μm. Reproduced with permission.33 Copyright 2015, American Chemical Society. | ||
Utilizing membrane-fusogenic nanocarriers for delivery into plasma membrane is a potential direction for biomedical applications. Researchers have exploited these nanocarriers for the transport of membrane proteins and hydrophobic photosensitizers to facilitate the therapeutic benefits. Yet, there is room for further development in this membrane fusion-based membrane engineering.
4.3 Cellular membrane engineering of the outer-membrane and inner-membrane
Except for transferring cargos inside the cytoplasm or plasma membrane, the manner of membrane fusion can be used for cellular membrane engineering with expected decoration on the outside or inside membranes. That is to say, the membrane-fusogenic particles modified with molecules on the external and internal surface of the lipid layers can provide the potential for spatially-controlled cell membrane engineering. For example, Ren and coworkers developed VSVG-immobilized vesicles to deliver azide motifs (–N3) onto the targeted tumor cell membranes for cancer diagnosis.16 These mimovirus vesicles presented tumor microenvironment-responsive fusion to allow the –N3 groups anchored onto the plasma membranes of targeted tumor cells, allowing the subsequent conjugation of diagnostic moieties for tumor visualization. These N3-modified vesicles successfully transported –N3 to the tumor region with reduced tumor heterogeneity for in vivo tumor diagnosis of the tumor xenograft models with HepG2, MCF-7, and 4T1 cells. More interestingly, Lin et al. reported a liposome fusion-based transport approach to engineer both the external and internal cell plasma membrane (Fig. 12(A) and (B)).35 They developed the spherical fusogenic liposomes with DNA–cholesterol conjugates loaded in the lipid layer, which could achieve two orthogonal DNA functions on cell surfaces after membrane fusion: the outer surface-anchored DNA was for regulating cell–cell communication, while the inner surface-anchored DNA was for intracellular monitoring. This work unprecedentedly provided an initial fundamental study to engineer both sides of the targeted cell membrane and manipulate the biochemical functions of the plasma membrane.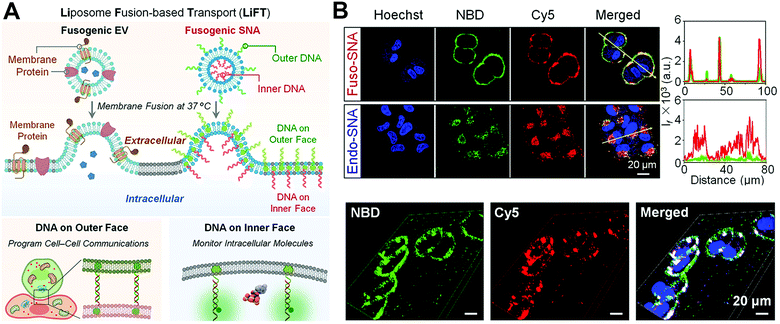 | ||
| Fig. 12 The application of membrane fusion-based transport for engineering external and internal sides of the plasma membrane. (A) Schematic illustration of DNA-anchored fusogenic nanoparticles fusing with cells on both the outer face and inner face. (B) Confocal analysis of verifying external and the internal membrane engineering mediated by the membrane fusion via Fuso-SNA. Reproduced with permission.35 Copyright 2022, Wiley-VCH. | ||
Taking advantage of membrane fusion for cell membrane engineering is such a potential approach, which can be applied in multiple significant fields. In this way, the engineering of both outer-membrane and inner-membrane can be achieved, which has great potential for theranostics.
5. Perspectives and future directions
With the rapid advancements in technology, membrane-fusogenic systems are promising for future advanced biomedicine. However, challenges and opportunities always co-exist, for which these particles meet their limitations, including unclear mechanisms in the complex biological environment, safety concerns, non-ideal efficiency, and defective scalability for clinic translations. One of the most crucial obstacles of fusogenic particles remains the indistinctness of the internalization process when applied in the complex biological milieu of the body. Recently, Liang and coworkers indicated that the protein corona (formed by cloaking biological components) could decide the transportation pathway of fusogenic particles to induce membrane fusion or endocytosis.53 They utilized aggregation-induced-emission-visualized fusogenic nanoliposomes to determine their intracellular access routes. Results indicated that the formed corona onto nanoliposomes under only a 0.3% serum concentration could switch the transportation mechanism from membrane fusion to endocytosis. From this report, it is clarified that membrane-fusogenic particles might have a reverse effect in vivo, losing their characteristic fusion manner and changing into the traditional endocytosis-mediated uptake impacted by the protein corona. Meanwhile, the methods and techniques to characterize the membrane fusion process are rare, limited in confocal or fluorescence microscopy and some bioassays which are usually used for in vitro studies. Direct observation or determination of the membrane fusion process in vivo is still challenging. These two obstacles of membrane-fusion particles, uncertain internalization pathway in complex biological milieu and difficulty for in vivo determination, are the principal issues that urgently need materialists to devote efforts.Since membrane-fusogenic particles are promising tools for advanced medicine, strategies for combating the current challenges are on the way. Firstly, for the improvement of membrane-fusogenic particles, the system should be carefully designed with the control of the composition, size, surface charge, shape, and other physicochemical properties to meet the goal with membrane fusion-based interactions.47,54–56 The insightful attempts of fusogenic liposomes and polymers with exclusive constructions have encouraged the expansion of the library of membrane-fusogenic particles from advanced materials with easier tunability and variability. Secondly, the efficiency of fusion reactions is crucial to biomedical applications, and there is considerable room to enhance the current efficiency. On the premise of ensuring safety, particles to trigger a more efficient membrane fusion are capable of dramatically increasing the potential for therapeutics. Thirdly, the strategies, such as ligand-mediated functionalization and tissue/cell-specific peptide decoration, are mostly recommended to enhance the targeting ability and specificity, especially for the in vivo application.57–61
To promote the improvement of membrane-fusogenic systems, more precise and convincing approaches are expected to be established to evaluate the fusion manner. Currently, methods to verify the membrane fusion manner are limited to the use of endocytosis/fusion inhibitors and endosome trackers to determine the in vitro cellular uptake.19,27,53 However, these inhibitors are still difficult to apply for in vivo verification, while endosome trackers lack convincing statistical results. The delivery process of membrane fusion, especially the intracellular internalization and subsequent route, requires more detailed data to be verified. Using methods like dynamic monitoring may give a deeper analysis of the fusion events.
Membrane fusion-based delivery technologies would hold great prospects in a material/medical laboratory and clinical applications for personalized cell behaviors. There might be the greatest concern whether membrane-fusogenic particles can be still practical in the complicated physical environment. Nevertheless, as developing technology evolves to meet the desires, there is the potential that advanced membrane-fusogenic particles can have enormous impacts in various fields.
6. Conclusions
Development of membrane-fusogenic particles is emerging as a new and promising area in medicine. With rapid development, more and more potential particles have been constructed to induce membrane fusion, including the strategies as follows: (1) particle-cell dual modification, (2) introducing virus fusogens and receptors, (3) bio-mimicking nature cells, and (4) excavating specifical-structured liposomes, polymers, and so on. In the past 10 years, studies on membrane-fusogenic particles have been extraordinarily brought up and achieved remarkable progress. These particles have been gradually developed to be not only applied for inner payload delivery to dodge endosomes completely for direct cytosolic access, but also used for inter-membrane delivery, cell engineering, and other innovative applications. However, this emerging field is in a great need of collaborative efforts with researchers in multiple disciplines, especially materials, chemistry, and biology, to enable membrane-fusogenic particles to be improved with more effective effects. Overall, we are optimistic and confident to foresee that this exciting field will keep moving forward at a staggering rate, by overcoming the challenges effectively and open a new avenue in biomedicine in the near future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2019YFA0111300), the National Natural Science Foundation of China (51903256, 21907113, 32001012, 52103197), the Guangdong Provincial Science and Technology Program (International Scientific Cooperation, 2018A050506035), the Science and Technology Program of Guangzhou (202102010225, 202102010217), the China Postdoctoral Science Foundation (2020M680133), the Thousand Talents Plan, and the Guangdong Provincial Pearl River Talents Program (2019QN01Y131).References
- R. Jahn, T. Lang and T. C. Südhof, Cell, 2003, 112, 519–533 CrossRef CAS PubMed
.
- W. Wickner and R. Schekman, Nat. Struct. Mol. Biol., 2008, 15, 658–664 CrossRef CAS PubMed
.
- H. R. Marsden, I. Tomatsu and A. Kros, Chem. Soc. Rev., 2011, 40, 1572–1585 RSC
.
- S. Du, S. S. Liew, L. Li and S. Q. Yao, J. Am. Chem. Soc., 2018, 140, 15986–15996 CrossRef CAS PubMed
.
- S. Martens and H. T. McMahon, Nat. Rev. Mol. Cell Biol., 2008, 9, 543–556 CrossRef CAS PubMed
.
- W. Shin, L. Ge, G. Arpino, S. A. Villarreal, E. Hamid, H. Liu, W.-D. Zhao, P. J. Wen, H.-C. Chiang and L.-G. Wu, Cell, 2018, 173, 934–945 CrossRef CAS PubMed
.
- W. He, X. Xing, X. Wang, D. Wu, W. Wu, J. Guo and S. Mitragotri, Adv. Funct. Mater., 2020, 30, 1910566 CrossRef CAS
.
- J. Yang, A. Bahreman, G. Daudey, J. Bussmann, R. C. Olsthoorn and A. Kros, ACS Cent. Sci., 2016, 2, 621–630 CrossRef CAS PubMed
.
- A. Yoshihara, S. Watanabe, I. Goel, K. Ishihara, K. N. Ekdahl, B. Nilsson and Y. Teramura, Biomaterials, 2020, 253, 120113 CrossRef CAS PubMed
.
- F. Huang, R. Duan, Z. Zhou, M. Vazquez-Gonzalez, F. Xia and I. Willner, Chem. Sci., 2020, 11, 5592–5600 RSC
.
- J. Yang, J. Tu, G. E. M. Lamers, R. C. L. Olsthoorn and A. Kros, Adv. Healthcare Mater., 2017, 6, 1700759 CrossRef PubMed
.
- L. Sun, Y. Gao, Y. Wang, Q. Wei, J. Shi, N. Chen, D. Li and C. Fan, Chem. Sci., 2018, 9, 5967–5975 RSC
.
- J. A. Peruzzi, M. L. Jacobs, T. Q. Vu, K. S. Wang and N. P. Kamat, Angew. Chem., Int. Ed., 2019, 58, 18683–18690 CrossRef CAS PubMed
.
- Y. Yang, Y. Hong, G.-H. Nam, J. H. Chung, E. Koh and I.-S. Kim, Adv. Mater., 2017, 29, 1605604 CrossRef PubMed
.
- B. Kim, Gi, G.-H. Nam, Y. Hong, J. Woo, Y. Cho, C. Kwon Ick, Y. Yang and I.-S. Kim, Sci. Adv., 2020, 6, eaaz2083 CrossRef PubMed
.
- E. Ren, C. Chu, Y. Zhang, J. Wang, X. Pang, X. Lin, C. Liu, X. Shi, Q. Dai, P. Lv, X. Wang, X. Chen and G. Liu, Small Methods, 2020, 4, 2000291 CrossRef CAS
.
- C. Montagna, G. Petris, A. Casini, G. Maule, G. M. Franceschini, I. Zanella, L. Conti, F. Arnoldi, O. R. Burrone, L. Zentilin, S. Zacchigna, M. Giacca and A. Cereseto, Mol. Ther.–Nucleic Acids, 2018, 12, 453–462 CrossRef CAS PubMed
.
- J. H. Park, A. Mohapatra, J. Zhou, M. Holay, N. Krishnan, W. Gao, R. H. Fang and L. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202113671 CAS
.
- X. Liu, C. Liu, Z. Zheng, S. Chen, X. Pang, X. Xiang, J. Tang, E. Ren, Y. Chen, M. You, X. Wang, X. Chen, W. Luo, G. Liu and N. Xia, Adv. Mater., 2019, 31, e1808294 CrossRef PubMed
.
- P. Guo, J. Yang, J. Huang, D. T. Auguste and M. A. Moses, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18295–18303 CrossRef CAS PubMed
.
- D. Nie, Z. Dai, J. Li, Y. Yang, Z. Xi, J. Wang, W. Zhang, K. Qian, S. Guo, C. Zhu, R. Wang, Y. Li, M. Yu, X. Zhang, X. Shi and Y. Gan, Nano Lett., 2019, 20, 936–946 CrossRef PubMed
.
- Z. Quinn, W. Mao, Y. Xia, R. John and Y. Wan, Bioact. Mater., 2021, 6, 749–756 CrossRef CAS PubMed
.
- N. Yim, S. W. Ryu, K. Choi, K. R. Lee, S. Lee, H. Choi, J. Kim, M. R. Shaker, W. Sun, J. H. Park, D. Kim, W. D. Heo and C. Choi, Nat. Commun., 2016, 7, 12277 CrossRef CAS PubMed
.
- X. Liu, J. Xiang, D. Zhu, L. Jiang, Z. Zhou, J. Tang, X. Liu, Y. Huang and Y. Shen, Adv. Mater., 2016, 28, 1743–1752 CrossRef CAS PubMed
.
- S. Xu, S. Ling, Q. Shan, Q. Ye, Q. Zhan, G. Jiang, J. Zhuo, B. Pan, X. Wen, T. Feng, H. Lu, X. Wei, H. Xie, S. Zheng, J. Xiang, Y. Shen and X. Xu, Adv. Sci., 2021, 8, 2003042 CrossRef CAS PubMed
.
- B. Kim, H. B. Pang, J. Kang, J. H. Park, E. Ruoslahti and M. J. Sailor, Nat. Commun., 2018, 9, 1969 CrossRef PubMed
.
- B. Kim, S. Sun, J. A. Varner, S. B. Howell, E. Ruoslahti and M. J. Sailor, Adv. Mater., 2019, 31, e1902952 CrossRef PubMed
.
- H. Tan, Y. n Song, J. Chen, N. Zhang, Q. Wang, Q. Li, J. Gao, H. Yang, Z. Dong, X. Weng, Z. Wang, D. Sun, W. Yakufu, Z. Pang, Z. Huang and J. Ge, Adv. Sci., 2021, 8, e2100787 CrossRef PubMed
.
- Q. Wang, Y. Song, J. Chen, Q. Li, J. Gao, H. Tan, Y. Zhu, Z. Wang, M. Li, H. Yang, N. Zhang, X. Li, J. Qian, Z. Pang, Z. Huang and J. Ge, Biomaterials, 2021, 276, 121028 CrossRef CAS PubMed
.
- H. Deng, K. Song, X. Zhao, Y. Li, F. Wang, J. Zhang, A. Dong and Z. Qin, ACS Appl. Mater. Interfaces, 2017, 9, 9315–9326 CrossRef CAS PubMed
.
- A. Pitchaimani, T. D. T. Nguyen and S. Aryal, Biomaterials, 2018, 160, 124–137 CrossRef CAS PubMed
.
- J. Kim, O. A. Santos and J.-H. Park, J. Controlled Release, 2014, 191, 98–104 CrossRef CAS PubMed
.
- J. Lee, J. Kim, M. Jeong, H. Lee, U. Goh, H. Kim, B. Kim and J. H. Park, Nano Lett., 2015, 15, 2938–2944 CrossRef CAS PubMed
.
- X. Ji, Y. Ma, W. Liu, L. Liu, H. Yang, J. Wu, X. Zong, J. Dai and W. Xue, ACS Nano, 2020, 14, 7462–7474 CrossRef CAS PubMed
.
- M. Lin, Y. Chen, S. Zhao, R. Tang, Z. Nie and H. Xing, Angew. Chem., Int. Ed., 2022, 61, e202111647 CAS
.
- G. Wang, S. Chen, N. Qiu, B. Wu, D. Zhu, Z. Zhou, Y. Piao, J. Tang and Y. Shen, Nano Today, 2021, 39, 101215 CrossRef CAS
.
- F. Vigant, N. C. Santos and B. Lee, Nat. Rev. Microbiol., 2015, 13, 426–437 CrossRef CAS PubMed
.
- H. Valadi, K. Ekström, A. Bossios, M. Sjöstrand, J. J. Lee and J. O. Lötvall, Nat. Cell Biol., 2007, 9, 654–659 CrossRef CAS PubMed
.
- L. Lyu, M. Hu, A. Fu and B. Xing, Bioconjugate Chem., 2018, 29, 2715–2722 CrossRef CAS PubMed
.
- J. Jiang, J. Mei, Y. Ma, S. Jiang, J. Zhang, S. Yi, C. Feng, Y. Liu and Y. Liu, Exploration, 2022, 2, 20210144 CrossRef
.
- C. P. Lai, E. Y. Kim, C. E. Badr, R. Weissleder, T. R. Mempel, B. A. Tannous and X. O. Breakefield, Nat. Commun., 2015, 6, 7029 CrossRef CAS PubMed
.
- G. van Niel, G. D'Angelo and G. Raposo, Nat. Rev. Mol. Cell Biol., 2018, 19, 213–228 CrossRef CAS PubMed
.
- R. Kolašinac, C. Kleusch, T. Braun, R. Merkel and A. Csiszár, Int. J. Mol. Sci., 2018, 19, 346 CrossRef PubMed
.
- A. I. S. van den Berg, C. O. Yun, R. M. Schiffelers and W. E. Hennink, J. Controlled Release, 2021, 331, 121–141 CrossRef CAS PubMed
.
- H. Kong, E. Ju, K. Yi, W. Xu, Y. H. Lao, D. Cheng, Q. Zhang, Y. Tao, M. Li and J. Ding, Adv. Sci., 2021, 8, e2102051 CrossRef PubMed
.
- Y. Guan, W. Yao, K. Yi, C. Zheng, S. Lv, Y. Tao, Z. Hei and M. Li, Small, 2021, 17, 2007727 CrossRef CAS PubMed
.
- W. Poon, B. R. Kingston, B. Ouyang, W. Ngo and W. C. W. Chan, Nat. Nanotechnol., 2020, 15, 819–829 CrossRef CAS PubMed
.
- D. C. Luther, R. Huang, T. Jeon, X. Zhang, Y. W. Lee, H. Nagaraj and V. M. Rotello, Adv. Drug Delivery Rev., 2020, 156, 188–213 CrossRef CAS PubMed
.
- M. P. Stewart, A. Lorenz, J. Dahlman and G. Sahay, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 465–478 Search PubMed
.
- F. Scaletti, J. Hardie, Y.-W. Lee, D. C. Luther, M. Ray and V. M. Rotello, Chem. Soc. Rev., 2018, 47, 3421–3432 RSC
.
- R. Goswami, T. Jeon, H. Nagaraj, S. Zhai and V. M. Rotello, Trends Pharmacol. Sci., 2020, 41, 743–754 CrossRef CAS PubMed
.
- X. Qin, C. Yu, J. Wei, L. Li, C. Zhang, Q. Wu, J. Liu, S. Q. Yao and W. Huang, Adv. Mater., 2019, 31, 1902791 CrossRef CAS PubMed
.
- Y.-F. Wang, C. Zhang, K. Yang, Y. Wang, S. Shan, Y. Yan, K. A. Dawson, C. Wang and X.-J. Liang, Natl. Sci. Rev., 2021, 8, nwab068 CrossRef PubMed
.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed
.
- J. Bourquin, A. Milosevic, D. Hauser, R. Lehner, F. Blank, A. Petri-Fink and B. Rothen-Rutishauser, Adv. Mater., 2018, 30, e1704307 CrossRef PubMed
.
- R. K. Singh, J. C. Knowles and H.-W. Kim, J. Tissue Eng., 2019, 10, 2041731419877528 Search PubMed
.
- G.-Z. Jin, A. Chakraborty, J.-H. Lee, J. C. Knowles and H.-W. Kim, J. Tissue Eng., 2020, 11 DOI:10.1177/2041731419897460
.
- C. Zheng, M. Li and J. Ding, BIO Integr., 2021, 2, 57–60 CrossRef
.
- C. Zheng, J. Zhang, H. F. Chan, H. Hu, S. Lv, N. Na, Y. Tao and M. Li, Small Methods, 2021, 5, 2001191 CrossRef CAS PubMed
.
- Y. Jin, H. Wang, K. Yi, S. Lv, H. Hu, M. Li and Y. Tao, Nano-Micro Lett., 2021, 13, 25 CrossRef PubMed
.
- C. Zhuo, J. Zhang, J.-H. Lee, J. Jiao, D. Cheng, L. Liu, H.-W. Kim, Y. Tao and M. Li, Signal Transduct. Target. Ther., 2021, 6, 238 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2022 |