Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Origin of luminescence properties and synthetic methods for gold- and bimetallic gold-based nanomaterials
Kanika
Bharti†
,
Jitendra K.
Sahu†
and
Kalyan K.
Sadhu
 *
*
Department of Chemistry, Indian Institute of Technology Roorkee, Roorkee – 247667, Uttarakhand, India. E-mail: sadhu@cy.iitr.ac.in
First published on 15th June 2022
Abstract
Organic emissive dyes, which were first synthesized in the mid-19th century, have played a major role in the field of sensing and biological imaging for several decades. The extensive use of π conjugation in organic dyes has shown their red-shifted emission from the ultraviolet (UV) to near infrared (NIR) region. The emission property modulation is not only restricted to π conjugation, but also affects the rotation of their bonds, functional group modification and intermolecular interactions. The photobleaching property of organic dyes makes it necessary to develop alternative emissive molecules and materials. Among the alternative emissive sources, gold-based nanomaterials showed 10−4 to 10−5 quantum efficiency for the first time in 1998. The quantum yields of these systems are mostly low except for a few exceptional cases in comparison to organic dyes. However, the high extinction coefficient values of these gold-based nanomaterials overcome the issue of overall brightness. The high stability of gold-based nanomaterials has gained attention in sensing and biological applications. The origin of the emission in these gold-based nanomaterials varies significantly among bimetallic nanoclusters (BMNCs) and metal oxide nanoparticles (NPs). In this review article, we deliberate on the foundation of luminescence properties among gold-based nanomaterials and compare their synthetic methods in detail.
1. Introduction
The emission property of organic dyes is important for studies on biosensing and bioimaging.1 Small organic fluorophores have been widely developed2 for this purpose since their first synthesis more than 150 years ago.3 Currently, for the synthesis of novel organic fluorophores, a few important parameters should be considered in terms of their physical properties.4 Among the photophysical properties, the absorption wavelength maximum (λabs), emission wavelength maximum (λem), molar extinction coefficient (ε) and quantum yield (Φf) are the four important parameters to be determined. The fluorogenicity of the quenched fluorophore is also important for sensing and imaging to avoid the background emission, which is a common artifact in imaging studies.5 This fluorogenicity in the organic fluorophore is commonly achieved through chemical reactions involving bioanalytes.6 In a few cases, the chemical reactions are controlled by external light irradiation to achieve a better fluorescence response.7 A molecular-level correlation between the chemical structure of an organic fluorophore and its photophysical properties has been found to be useful for the molecular design of novel fluorescent bioprobes.8In the last two decades, the use of organic fluorophores with high temporal and spatial resolution in bioimaging experiments has been found to be useful in living systems.9 However, to avoid strong autofluorescence by biomolecules in the ultraviolet region, a recent review article discussed the importance of the development of NIR fluorophores for bioimaging experiments.10
The photoluminescent property of metal nanoclusters is significantly different from that of organic fluorophores (Fig. 1A) and dependent on metal–metal, metal–ligand and ligand–ligand interactions. As shown in Fig. 1B, the metal core and Au(I)–S surface contribute to the luminescent property in nanoclusters. The surface-like excited states are affected by the rotational and vibrational motion in surface ligands, where restricted rotational and vibrational motion decreases the non-radiative relaxation and enhances the fluorescence intensity.11 However, organic fluorophores with high quantum yields suffer from photobleaching with time and brightness in aqueous medium as two major drawbacks.12 The brightness of an emissive molecule or material is often defined as ε·Φf.13 To overcome these limitations, metal-based NPs are considered an alternative probe.14 The poor Φϕ properties of metal-based NPs are generally balanced with a very high value of ε to produce brightness. The advantages and disadvantages of organic fluorophores vs. metal-based nanoclusters are summarized in Table 1.15–18 Hence, recently, review articles and perspectives on metal-based nanomaterials have attracted significant attention for a wide range of applications.19–28 Among the applications of metal-based NPs, hypoxic tumor radiotherapy is one of the most important for clinical cancer treatment.19 An integrated “metallomics” approach for metal-based NP activities in a review article was published almost a decade ago to give insight into the in vivo behavior and biological effects of nanomaterials, specifically metal-based nanomaterials (MNMs).23 Robust non-Pt noble metal-based nanomaterials are also well known for their electrocatalytic activity in the hydrogen generation reaction.25 Noble metal (Au, Ag and Pd) NPs and their composites together with transition metal (Mn, Fe, Co, Ni, Cu, Zn, Cd, Mo and W) nanomaterials and main group metallic (Bi) nanocomposites act as catalysts in CO-selective CO2 reduction.27
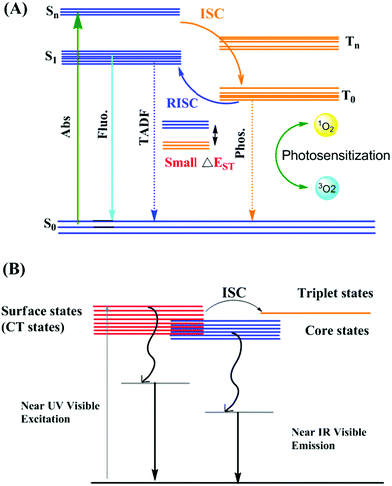 | ||
| Fig. 1 Schematic Illustration of Jablonski diagram (A) representing photophysical properties and ROS generation mechanism using organic dyes; adapted from ref. 18. (B) Proposed mechanism for the origin of luminescence in metal nanoclusters; adapted from ref. 11. | ||
| Advantages | Disadvantages | |
|---|---|---|
| Organic dyes | • AIE probes are highly luminescent, which is a useful property for bioimaging | • Conventional organic dyes showed limited retention time, photobleaching, small Stokes shift, small lifetime (ns), poor quantum yield in NIR region |
| • Interaction with biomolecules used to trace analyzing the changes in intensity in hydrophilic bioenvironment after aggregation | • Pretreatment required to overcome limitation such as photostability for biological application | |
| • AIE-organic molecules have resolved the limitation observed in case of conventional organic dyes | • Lower internalization due to hydrophobic nature, low ROS for PDT and PTT applications, less biocompatibility | |
| • AIE organic molecules have longer lifetimes, good quantum efficiency, large stoke shift, enhanced brightness. Efficient energy transfer causes increase in reactive oxygen species generation (ROS) | • Sterically hindered bulky group results in low emissive twisted intramolecular charge transfer (TICT) and low quantum yield. | |
| Luminescent nanoparticles | • Enhanced permeability and retention (EPR) effect as compared to organic dyes | • Emission can be quenched due to several factors such as pH, buffers, biothiols, metal ions |
| • Efficient excretion and high biocompatibility | • In order to overcome Luminescence quenching due aggregation, surface functionalisation needed | |
| • Surface engineering possible for improving cellular interaction and organ distribution | • Rapid renal clearance and short circulation time decrease tumor target efficiency | |
| • Higher lifetime (μs) increase the probability of singlet oxygen species for disease therapy | ||
| • Photobleaching resistant | ||
| • NIR based luminescent nanomaterials allows deeper tissue penetration | ||
Gold-based nanomaterials are highly fascinating for wide research interest in several fields such as catalysis, photonics, sensing of normal cells and bioanalytes due to their stimulating optical properties.29–33 Gold-based nanomaterials can mainly be grouped into gold-based nanomaterials and bimetallic nanomaterials. The characteristic optical property of gold NPs with a high extinction coefficient is known as surface plasmon resonance (SPR).34
Synthetic methods to introduce luminescent properties in gold nanomaterials have been considerably developed within the last one and half years. The photoluminescence (PL) properties of gold nanoclusters (AuNCs) have been altered significantly via the traceless removal of two kernel atoms through thermal treatment.35 Rod-shaped icosahedral AuNCs have been reported with bright NIR-II PL from the suppression of non-radiative transitions by the central gold atom in AuNCs to a state with near-zero oscillator strength in the ground-state geometry.36 In 2021, opposite to the classical concept of the heavy atom effect, the NIR lifetime of photoluminescence from AuNCs has been enhanced in the presence of iodine.37 Rare earth-doped luminescent microparticles were transformed to an air-stable single crystal with the help of plasmonic gold nanoislands.38
T. Pradeep's group reported bright-pink luminescence under UV light by synthesizing AuNCs through the electricity from a triboelectric generator.39 The same group also prepared luminescent silver–gold nanocomposites by combining silver nanoclusters (AgNCs) and gold nanorods (AuNRs).40 Pavelka et al. combined AuNCs and AuNRs in a compact core–shell nanostructure with a tunable geometry and plasmon-enhanced luminescence properties.41 Emission enhancement is also possible through a hybrid hydrogel as a cross-linker on AuNCs.42 Biomolecules, such as enzymes, proteins and polysaccharides, have been explored employing the luminescence from gold nanomaterials.43–45 In 2020, we explored an assembly of gold nanoparticles (AuNPs) with cucurbit[8]uril to develop a supra-pyramid for drug delivery.46 Recently, Huang et al. used an assembly of AuNCs with cucurbit[n]uril for luminescence switching.47
Mechanistic models through experiments48 and theory49 have been considered to determine the origin of the visible and NIR luminescence in AuNCs and BMNCs. Recent developments on the luminescent properties of gold nanomaterials involve two-photon excited luminescence,50,51 light-emitting diodes,52 prodrug activation,53 manipulation of surface charge effect,54 nanothermometry,55 molecular recognition,56 encapsulation57 and optical fibers.58
The most common applications of luminescent gold nanomaterials have been observed in sensing applications by varying their luminescence intensities in the presence of target analytes. Metal ions59,60 such as Cu2+, Hg2+, and Fe3+, small molecules61–67 such as protons, CN−, H2O2, H2S, pyrophosphate, dicofol, and ciprofloxacin, and biomolecules68–70 such as alkaline phosphatase, uric acid, glucose and glucose oxidase are important classes of targets reported since 2021. Another notable detection is the hepatitis B virus through the luminescence properties of AuNCs.71
The most challenging application of any luminescence system is the imaging of the probe or material in biological cells and living systems. This challenge has been successfully overcome in cancer cell lines72,73 such as HeLa and MCF-7 cells, in the tumor imaging of mice74 and in zebrafish models.75 Besides sensing and imaging applications of gold nanomaterials due to their SPR peak or luminescence properties, they have several applications including real-time observation of the temperature variation in targeted cancer cells,76 luminescence resonance energy transfer,77 screening of synthetic cannabinoids78 and antibacterial applications.79,80
The atomic-level synthetic approach for the preparation of noble metal NPs and the complexity in their synthetic process have been discussed in recent reviews (Table 2).34,81–93 Simultaneously, the correlation of the atomic-level modification of the surface of nanomaterials with their corresponding catalytic activity is important for understanding the role of metal doping in these NPs. The sensing applications of these metal-incorporated nanomaterials depend on their inherent luminescent property. In the current review, we focus on the mechanistic aspects of the origin of the luminescence in gold-based nanomaterials with a greater emphasis on gold-based nanomaterials and also discuss different parameters affecting their luminescence intensity. Also, the role of heterometals on the luminescence intensity of gold-based nanocomposites is illustrated. We attempt to discuss in detail the different synthetic routes for gold-based nanomaterials by classifying them based on their surface capping agents (Scheme 1).
| S. No. | Material | Luminescence properties | Synthesis discussed | Ref. |
|---|---|---|---|---|
| 1. | Metal nanocluster-based hybrid nanomaterials | Ligand-to-metal charge transfer mechanism, FRET, increasing ligand rigidification, AIE, and confinement, Electrochemiluminescence (ECL) | Hybridizing metal nanoclusters with organic molecules, 1D nanomaterials such as nanorods, nanowires, nanotubes and nanofibers, 2D nanostructures such as graphene, metal hydroxides, metal oxides, boron nitride and carbon nitride, 3D nanostructure such as mesoporous silica zeolites, metal–organic frameworks (MOFs), nanogels and mesoporous metal oxides | 81 |
| 2. | Gold nanoclusters | FRET, PET, disaggregation/aggregation, and change of ligand conformation, AuNCs based sensor advantages such as photostability, large Stokes shift, biocompatibility | Bottom up approach involving such as electrochemical reduction, sono-reduction, photoreduction, chemical reduction using template synthesis and ligand protected AuNCs; top down approach using solvent and ligand induced etching | 82 |
| 3. | Metal nanoclusters | Enhancement in luminescent properties dependent on composition, structure, oxidation state of metal nanoclusters metal core, structure and steric hindrance of metal nanocluster ligand shell | Synthetic approaches for highly luminescent metal nanoclusters using metal doping, surface motif engineering, ligand engineering, structure modulation of metal nanoclusters | 83 |
| 4. | Metal nanoclusters | Aggregation induced emission | Brief discussion of synthetic strategies for aggregation induced emission active nanoparticles | 84 |
| 5. | Alkynyl protected metal nanoclusters | Ligand effect on luminescence property, thermally activated delayed fluorescence, NIR emission | Synthetic method such as comproportionation, anion-templated, solvothermal, post-synthetic ligand exchange method and one pot synthesis; synthesizing alkynyl protected AuNCs via nuclear transformation and reassembly | 85 |
| 6. | Metal nanoclusters | Luminescence mechanism based on quantum confinement, LMMCT/LMCT, aggregation induced emission, factors affecting luminescence properties such as size and composition of metal core, surface ligand, temperature, pH, and solvent viscosity. | Top down and bottom up synthesis | 86 |
| 7. | Metal nanoclusters | Absorption and fluorescence properties, two-photon absorption, electrochemiluminescence, solvatochromic effect, polarized emission and fluorescence lifetimes | Top down and bottom up synthesis | 87 |
| 8. | Chiral gold based nanoassemblies | CD and luminescence based biosensing application | Different biomolecule modulated chiral gold assemblies | 88 |
| 9. | DNA-template metal nanoclusters | Fluorescence based ion detection, enzyme detection and biomolecule detection, bioimaging | Synthesis of ss-DNA, dsDNA, triplex DNA, quadruplex DNA, higher-order DNA, | 89 |
| 10. | Metal–metal oxide nanoparticles | — | Vaccum sputtering method for luminescent gold nanoclusters | 90 |
| 11. | Noble metal nanostructure–serum albumin interaction | Luminescence dependent on the binding process and plasmonic NPs – serum albumin interactions | — | 91 |
| 12. | DNA templated silver nanoclusters | Impact of DNA sequence and Ag nanoclustres structure on the optical properties | Synthetic methodologies based on interaction of Ag+ with homobase DNA strand, helix duplexes via interplanar H-bonds, homobase and heterobase Ag+-mediated duplexes | 92 |
| 13. | Protein protected metal nanoclusters | Fluorescence enhancement strategies, structure dependent optoelectronic properties | One pot synthesis | 93 |
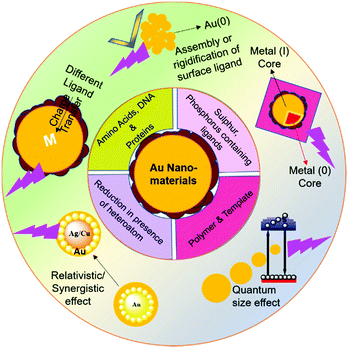 | ||
| Scheme 1 Schematic illustration of ligand-based synthesis and origin of luminescence in gold-based nanomaterials. | ||
2. Origin of luminescence in gold nanomaterials
The synthesis of luminescent nanomaterials and their application in real life are possible by acquiring clear insight into the origin of the luminescence from different nanomaterials at the molecular and electronic levels. This section of the review discusses the mechanisms behind the origin of the luminescence in gold-based nanomaterials and bimetallic nanoclusters (BMNCs).Noble metal NPs basically constitute two types of particles based on their size and intrinsic properties. The larger particles (∼size > 2 nm) behave as plasmonic nanomaterials possessing the fascinating surface plasmon absorption band, which originates due to the collective oscillation of the surface free electrons, while absorbing the incident light of wavelength, similar to the frequency of oscillation properties.94,95 Given that these plasmonic NPs possess a larger particle size, their electronic states involve a greater number of gold atoms and result in the disappearance of their molecular bandgap. Due to the absence of this band gap, the electron hole recombination process is absent in these NPs, leading to the disappearance of their emission properties.96
Alternatively, when the size of the particle decreases (∼size < 2 nm), the properties associated with the bulk metal readily disappear. When the size of the particle finally approaches the Fermi wavelength of electrons, the electronic motion gets severely restricted due to the confinement of the electrons. Consequently, the continuous electronic energy band structure transforms into discrete energy states, which are the origin of molecule-like behavior in these ultrasmall NPs. These changes in electronic structure yield fascinating optoelectronic properties such as PL, molecular chirality, redox behavior and intrinsic magnetism.94,97–99 Due to the quantum confinement effect, the photo-induced excited electrons jump to a higher energy level and get radiatively relaxed in discrete energy levels. This radiative energy appears in the form of light, leading to ultrabright luminescence.
The proposed energy band structure for the origin of the emission in gold NCs was first reported by A. Mooradian, who demonstrated PL in gold NCs by exciting them with a high-energy laser.100 The direct recombination of electrons and holes via interband transitions between the valence band (d band) and conduction band (sp band) is mainly responsible for the origin of their emission properties. It has been fifty years since the first proposed theory on AuNCs, but their legacy continues to date given that several new observations appeared in the emission properties by applying different influencing parameters.
2.1 Key and influencing factors for the origin of luminescence in AuNCs
The above-mentioned observations are solely based on the size of AuNCs, which is considered as the key factor for the origin of their luminescence. The corresponding wavelength for excitation and emission is highly dependent on the size (quantum size effect) of the MNCs. A decrease in size leads to an increase in the relaxation energy gap of electrons, which eventually causes a hypochromic shift in the emission wavelength.101 However, the emphasis on the optical properties of AuNCs during the last two decades revealed several exceptions in their luminescence properties.For example, an explanation for the dual emission in Au28(SG)16 NCs (corrected as Au25 in later works) could be presented after Link et al. proposed their energy band structure by considering the solid-state model and molecular model for the origin of the two luminescence bands in Au28(SG)16 NCs.102 It was reported that both the radiative intraband transition (sp–sp) and the radiative interband recombination (sp–d) in the HOMO–LUMO gap contribute to the low and high energy luminescence bands, respectively. Thiolated gold NCs are being extensively studied given that they possess several fascinating size-independent emission properties. For example, with the same core size in Au25 NCs but different ligands, their emission and excitation spectra are modified.103,104
Alternatively, the discovery of aggregation-induced emission (AIE) led to the nanoscale community identifying the major factors that can influence the luminescence properties of nanomaterials.105 Besides particle size, this review summarizes the major influencing factors that affect their luminescence properties in detail.
Further, Jin and coworkers profoundly studied the effect of ligands on the luminescence properties of Au25 NCs. The Au25 NCs were composed of an Au13 core surrounded by six dimeric Au2(SR)3 staple motifs. It was observed that the luminescence intensity depends on the alkyl chain length and the charge-donating ability of the ligands (Fig. 2A). The charge transfer from the ligands to the metal core via S–Au bonds and the electropositive nature of the metal core support the remarkable increase in luminescence intensity (Fig. 2B).104
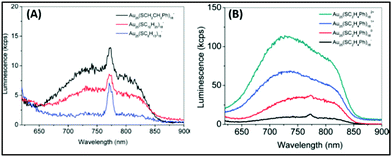 | ||
| Fig. 2 Emission spectra of (A) [Au25(SR)18]− with R groups (1) –C2H4Ph (black), (2) –C12H25 (red), and –C6H13 (blue). (B) [Au25(SC2H4Ph)18]q (q = −1, 0, +1, +2). λex = 514 nm. Note: spike at ∼770 nm in (A) is an artifact from the quartz cell. Reproduced from ref. 104 with permission from the American Chemical Society, Copyright 2010. | ||
Aiken's group provided theoretical insights into the origin of luminescence in Ag25(SR)18− NCs.110 They utilized density functional theory (DFT) and time dependent DFT to calculate the geometric and electronic structural changes during the excitation. The emission from Au25(SR)18− nanoclusters involves several excited states, where the core-based transitions involve superatomic P orbitals and D orbitals. In the lowest energy excited states of Au25(SR)18− nanoclusters, the energy levels of the frontier orbitals are significantly influenced because of the geometric relaxation factors. This phenomenon generates a Stokes shift for Au25(SH)18−, which can exhibit a larger value in the presence of a longer ligand.
Recently, Zhou et al. proposed the mechanism behind transient absorption spectroscopy analysis.111 The Au13 core of the NCs is mainly associated with UV-vis absorption. The luminescence is observed due to the rapid relaxation of the excited electrons from higher core states to a lower core state and surface state. When excited by UV light (350 nm), the multiexponential decay from the surface state leads to the generation of visible PL (750 nm), whereas the mono exponential decay from the core state results in NIR PL (1100 nm), as shown in Fig. 3. However, lower energy NIR light can only excite the lower core state, preventing the core to surface charge transfer. The visible PL of NCs depends on their surface ligands given that the origin of the PL is associated with core–shell charge transfer. Recent review and reports also shed some light on the ligand-dependent luminescence in AuNCs.112–114 These interesting observations explain how the surface-stabilizing ligands in MNCs play a significant role in enhancing their luminescence properties via LMCT (ligand to metal charge transfer), LMMCT (ligand to metal–metal charge transfer) or LMCCCT (ligand to metal cluster core charge transfer).
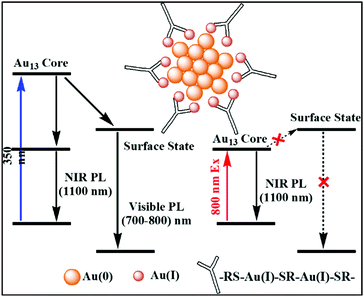 | ||
| Fig. 3 Schematic diagram of Au(0) core and Au(I)–thiolate shell [Au25(SR)18]− and the mechanism behind the visible and NIR emissions; adapted from ref. 111. | ||
Hence, after excitation, the probability of nonradiative relaxation is significantly reduced, which eventually assists in the luminescence enhancement. By selective reduction and controlled aggregation, highly emissive Au(0)@Au(I)–thiolate NCs can be prepared (Fig. 4).
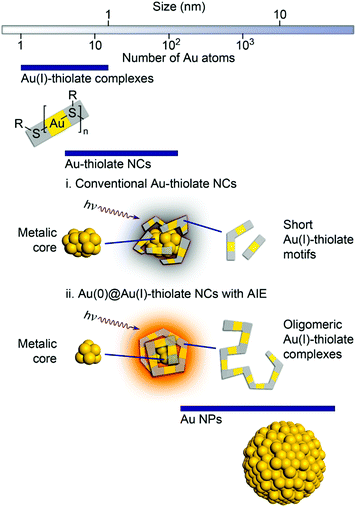 | ||
| Fig. 4 Schematic illustration of the structures of (i) conventional Au–thiolate NCs with short Au(I)–thiolate motifs and (ii) luminescent Au NCs with AIE. Reproduced from ref. 116 with permission from the American Chemical Society, Copyright 2010. | ||
In 2016, the same group prepared a highly luminescent nanogel by utilizing the electrostatic attraction between chitosan and thiolate ligands.117 This interaction hampered the intramolecular motions of the surface ligands on the Au nanoclusters, which led to an enhancement in luminescence. The AIE origin and the emission energy also depend on the length of the Au(I)–SR motif. Recently, Heyon and coworkers synthesized a luminescent gold cluster assembly using mercaptocarboxylic acid and Zn2+ ions as the coordinating metal.118 The rigidification of the ligands through coordination with Zn2+ and the aurophilic interaction among the Au4 clusters were mainly responsible for the bright greenish-blue fluorescence. Recently, several reports have demonstrated the AIE effect in AuNPs, which were utilized as fluorescent sensors and in bioimaging.119–125 Recent reviews on the aggregation and self-assembly of nanoclusters shed light on the nanoscale forces/interactions such as dipolar attraction, van der Waals interactions, electrostatic interactions, π–π stacking, and metallophilic interactions, which can initiate the aggregation or assembly process in nanoclusters.11,126,127
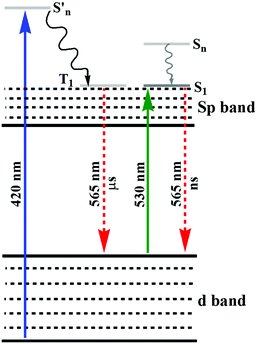
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio to synthesize glutathione-stabilized AuNPs.128 The synthetic process resulted in two different NPs with orange and yellow emission and their lifetime changed from micro- to nanoseconds when the excitation wavelength changed from 420 to 530 nm, as shown in Fig. 5. The XPS spectra of these NPs indicated the presence of a significant amount of Au(I). A strong reducing agent induced quenching of the emission, demonstrating the important role of the oxidation state of Au towards PL. The authors hypothesized that the generation of luminescence is due to the LUMO (sp band) to HOMO (d band) transitions. The microsecond emission lifetime originated from the triplet excited states in the sp band, where gold was mixed with the p orbitals of sulfur. When excited near 530 nm, the nanosecond lifetime suggested the resultant emission was correlated with the transition between singlet excited states and ground states. The degeneracy in the singlet and triplet excited states was responsible for the different lifetimes under different excitation wavelengths. Similar observations have also been reported in other reports.129,130 Tang and co-workers demonstrated that the small energy gap between the singlet and triplet excited states resulted in long luminescence decay components in Au25 NCs.131 Recently, we also reported the role Au(I)/methionine metallic bonds.132 Another interesting observation, which deals with the interaction towards the generation of emissive properties in nucleated Au(0)/Au(I) NPs in aqueous medium, was reported.132 The interaction of methionine with Au(I) led to the inhibition of secondary nucleation during the growth reaction of AuNPs. At a lower concentration of Au salt, this inhibition resulted in the generation of luminescent NPs (2.8 nm). The emission properties were quenched during the growth reaction with a high concentration of Au salt, which led to the generation of larger nucleated particles. Other than AIE properties,133,134 interesting observations were reported for bimetallic NCs, where either of the monometallic NCs emitted weakly under illumination.
Au ratio to synthesize glutathione-stabilized AuNPs.128 The synthetic process resulted in two different NPs with orange and yellow emission and their lifetime changed from micro- to nanoseconds when the excitation wavelength changed from 420 to 530 nm, as shown in Fig. 5. The XPS spectra of these NPs indicated the presence of a significant amount of Au(I). A strong reducing agent induced quenching of the emission, demonstrating the important role of the oxidation state of Au towards PL. The authors hypothesized that the generation of luminescence is due to the LUMO (sp band) to HOMO (d band) transitions. The microsecond emission lifetime originated from the triplet excited states in the sp band, where gold was mixed with the p orbitals of sulfur. When excited near 530 nm, the nanosecond lifetime suggested the resultant emission was correlated with the transition between singlet excited states and ground states. The degeneracy in the singlet and triplet excited states was responsible for the different lifetimes under different excitation wavelengths. Similar observations have also been reported in other reports.129,130 Tang and co-workers demonstrated that the small energy gap between the singlet and triplet excited states resulted in long luminescence decay components in Au25 NCs.131 Recently, we also reported the role Au(I)/methionine metallic bonds.132 Another interesting observation, which deals with the interaction towards the generation of emissive properties in nucleated Au(0)/Au(I) NPs in aqueous medium, was reported.132 The interaction of methionine with Au(I) led to the inhibition of secondary nucleation during the growth reaction of AuNPs. At a lower concentration of Au salt, this inhibition resulted in the generation of luminescent NPs (2.8 nm). The emission properties were quenched during the growth reaction with a high concentration of Au salt, which led to the generation of larger nucleated particles. Other than AIE properties,133,134 interesting observations were reported for bimetallic NCs, where either of the monometallic NCs emitted weakly under illumination.
However, a remarkable enhancement in the luminescence intensity of several folds was obtained from their corresponding bimetallic counterparts after controlled doping of a particular metal. For example, Jin and co-workers reported the significant enhancement of the quantum yield (QY) of weakly emissive Au25 nanorods (Au25(PPh3)10(SC2H4Ph)5Cl2]2+) from 0.1% to 40.1% by simply replacing 13 Au atoms methodically by Ag atoms (Ag13Au12, species II) in the metal kernel without affecting their structural architecture (Fig. 6a–c).135
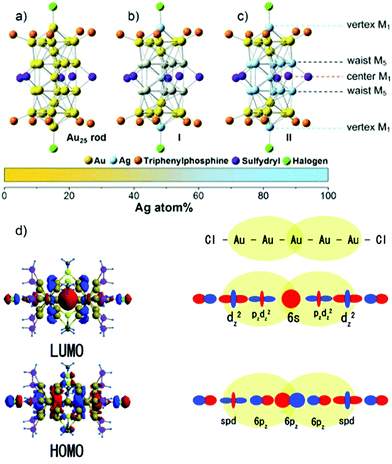 | ||
| Fig. 6 Crystal structures of (a) Au25 NCs and (b) and (c) AgxAu25−x with a probability of (I) 12 and (II) 13 doping Ag atoms, M = metal atom. (d) HOMO and LUMO of species II together with metal orbital participation for absorbance. Spheres with color green, red, yellow, pink and grey represent Ag, Au, S, P and C, respectively. H atoms are omitted for simplicity. (a)–(c) reproduced with permission.135 Copyright 2014. Wiley-VCH. (d) Reproduced from ref. 136 with permission from the American Chemical Society, Copyright 2007. | ||
The authors also experimentally demonstrated that the doping of 12 silver atoms (Ag12Au13, species I) in the parent NC did not result in similar absorption and emission properties even after maintaining an analogous ligand environment. They successfully correlated the HOMO–LUMO energy gap with different experimental techniques. It has been reported that the 6s and 6p atomic orbitals of the ten Au waist atoms (Fig. 6c) are deeply associated with the HOMO in Au25 NC (Fig. 6d), whereas the LUMO is mainly contributed by the central Au atom.136,137 The 13th doping with silver atoms in the parent NC involves the replacement of the central Au atom by an Ag atom, and hence the large perturbation of electronic states remarkably influenced the LUMO→HOMO transition. This is mainly due to the relativistic effect in gold involving the dominant sd hybridization. After doping with 13 Ag atoms, the LUMO energy shifted due to the involvement of the 5s orbital of Ag and the LUMO→HOMO band gap increased, and hence the recombination of excited electrons involves highly intense emission. A comparatively similar observation was also reported experimentally for Ag29(BDT)12(TPP)4 NC (BDT: 1,3-benzenedithiol and TPP: triphenylphosphine), where gold doping was the origin of the anomalous behavior in the absorption and luminescence signal in Ag29−xAux(BDT)12(TPP)4 by applying density electronic relaxation, and the electronic modulation in Ag29−xAux(BDT)12(TPP)4 (x = 1–5) induced a similar enhancement in luminescence and structural stability.138 This is mainly possible due to the doping-induced perturbation in the structures.138
By considering the importance of the relativistic effect, Dolg and co-workers also theoretically established density functional theory (DFT) and time-dependent density functional theory (TD-DFT) approaches towards the geometric and electronic structures.139 The replacement of Ag by Au atoms significantly alters the recombination path of excited electrons through effective orbital contributions. After considering the relativistic effect, the theoretical calculations also indicated that the controlled increase in the Au doping inside the Ag29−xAux(BDT)12(TPP)4 NCs remarkably lowered the centroid of charge of the excited electrons and holes. These phenomena overall enhanced the luminescence intensities of Ag29−xAux(BDT)12(TPP)4 NCs (x = 3–5). It was also reported that the sensitization of QY and PL enhancement factor are highly dependent on the number of possible hetero annular d10–d10 metallic bonds.140 Another interesting observation, which deals with the synergistic effect originating from the presence of two different d10 metals in the close proximity, has been reported for cooperatively sensitizing their luminescence and altering their basic properties such as stability and catalytic activity.138,141 During the last decade, several reports have been reported on this co-operative luminescence enhancement effect in the case of bimetallic NCs. The synergist effect of silver particularly plays an important role through the doping of Ag in AuNCs.142–144
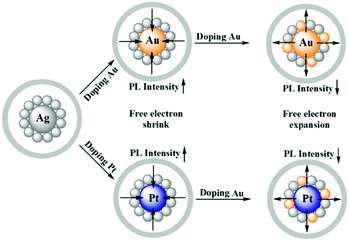
Zhu and co-workers experimentally demonstrated the anomalous blue-shifting followed by broadening in the absorption spectra and enhancement in PL intensity with limited Au and Pt doping in Ag25(SPhMe2)18PPh4 NCs.145 The PL intensity enhanced during the replacement of the central Ag atom of the icosahedral Ag core by an Au or Pt atom. However, further Ag atom substitution exceptionally quenched the PL intensity.
The origin of the selective PL intensity enhancement can be attributed to the high electron affinity of Au or Pt atoms. These metal atoms contribute more towards the super atomic orbitals of the NCs and force the free valence electrons to shrink towards the central atom (Fig. 7). In contrast, more substitutions on the periphery of the icosahedral core result in a significant change in the electron density inside the M13 core, which expands the core size, leading to the emission quenching process. Recently, Antoine and coworkers demonstrated the controlled doping of silver atoms in Au10SG10 catenane NCs, which yielded silver-doped Au10−xAgxSG10 NCs with different extents of doping (e.g., x = 0–2 and x = 1–4). Au10−xAgxSG10 with x = 1–4 showed a blue shift in absorption and a significant red shift in the two-photon emission spectrum in contrast to Au10SG10. In the case of Au10−xAgxSG10 (x = 1–4), the improved relaxation of the first excited state (S1) of silver-doped AuNCs may be the prime reason behind the shifting of the two-photon excited fluorescence (TPEF) towards a longer wavelength.146
3. Synthetic methods for luminescent gold nanomaterials
In the 21st century, the scientific attention has been shifted partially from the conventional organic fluorophores towards alternative novel fluorescent metal NCs to fabricate different fluorogenic systems. Several advantages such as excellent stability, good biocompatibility, and high luminescence intensity collectively make metal NCs competitive fluorescent probes aimed at possible applications in different fields.147–151 Compared to metal NCs comprised of a single metal, BMNCs comprised of heterometals in their system possess better cooperative electronic, optical, sensing and biological properties.102,138,152–155 This section discusses the synthetic procedures available to synthesize metal-based luminescent nanomaterials.3.1 Gold nanoclusters (AuNCs)
Gold nanoclusters exhibit unique photoluminescence properties, biocompatibility and high renal clearance. Recently, synthetic approaches for water-soluble AuNCs have been explored for various biological applications such as biosensing of metal ions, small molecules, protein and DNA sensors, cell environment sensors and biological imaging.148 Developing synthetic methodologies can be useful for overcoming the shortcoming observed for the practical applications of AuNCs. The review by Khan et al. highlighted the evolution of AuNCs and highlighted the importance of modifying synthetic methods for various biological applications in detail.84 Surface functionalization is crucial for catalysis given that it protects nanoclusters from aggregation, improves their stability and alters their electronic structure. Recent progress in the surface-modified gold nanoclusters such as thiolate-protected AuNCs, phosphine-protected AuNCs, alkynyl-protected AuNCs and their role in the catalysis and selectivity for oxidation, reduction, cycloisomerization, hydrolysis, heterocoupling and electrocatalysis reactions has been summarized in the review article by Li et al. The steric hindrance of ligands, Au active sites, and lattice oxygen atoms are a few factors affecting their catalytic activity.156 The ongoing research has found that the kernel structure and atomic packing leading to various crystallographic structures, i.e., fcc, decahedral kernels, or NCs with icosahedral kernels, can tune the optical properties and energy gap. Synthetic strategies can be used to control the crystal structure of AuNCs, and hence their electronic and absorption properties.157Xie et al. developed a synthetic approach, where BSA sequesters and reduces the Au3+ ions, forming BSA–Au25 NC bioconjugates with red fluorescence.159 Their synthesis depends on NaOH, reaction temperature and ratio of BSA and Au concentration. The post-synthesis modification of BSA-stabilized AuNCs can be used for enhancing their photoluminescence property by rigidification and increasing their aurophilic interaction. Wong et al. modified the methodology utilizing a thermomixer for mixing BSA and HAuCl4 solution, which is more advantageous compared to the earlier reported methods because of its short reaction time, higher quantum yield and mild conditions.160 AuNCs were also synthesized using ligands besides the traditional ligands such as anti-Flt1 peptide (CGNQWFI, AF), as shown in Fig. 8. Li et al. reported the one-pot synthesis of AF@AuNCs, where the template plays an important role both as a stabilizer and reductant because of the presence of amino acids such as tyrosine and cysteine.
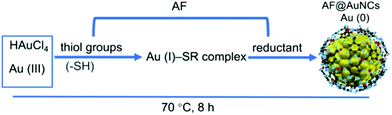 | ||
| Fig. 8 Schematic illustration of the synthesis of anti-Flt1 peptide (CGNQWFI, AF)-stabilized AuNCs. Reproduced from ref. 161 with permission from the American Chemical Society, Copyright 2021. | ||
This template-based synthetic methodology is unique compared to earlier reported methods given that it does not require additional reducing agents.161 Jain et al. reported the synthesis of BSA-coated AuNCs with bright red fluorescence in the dark overnight. The gold nanoclusters synthesized using this method showed a good quantum yield and brightness. The cysteine residues present in BSA stabilized Au3+ and reduced under alkaline pH.162 A scheme for the synthesis of BSA-stabilized AuNCs is shown in Fig. 9.
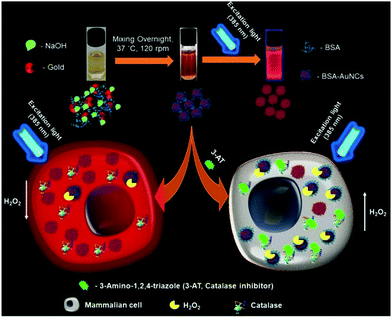 | ||
| Fig. 9 Schematic illustration of the synthesis of red fluorescent BSA-stabilized AuNCs and changes in the fluorescence intensity in the presence of hydrogen peroxide after treating cells with 3-amino-1,2,4-triazole (catalase inhibitor). Reproduced with permission from ref. 162, Copyright Elsevier (2021). | ||
Hada et al. reported the preparation of BSA-stabilized AuNCs, which showed temperature-dependent (in the range of 25 °C to 70 °C) and excitation-induced tunable red PL. The shift in the photoluminescence wavelength with different excitation is considered to be due to the temperature-activated delayed fluorescence (TADF) emission. The temperature-induced conformational changes in BSA led to a decrease in the emission intensity and the change was found to be irreversible in the temperature range of 52–60 °C.148,163 The gold nanoclusters synthesized using this method exhibited stable reversible photoluminescence with temperature.164
Ungor et al. reported lysozyme (LYZ), HSA, BSA and gamma globulin (γG) protein-stabilized AuNCs using the template-assisted method. The AuNCs showed a large lifetime in the microsecond range and average QY% values in the range of 3.8–5.4%.165 Li et al. reported BSA-stabilized AuNCs embedded in self-assembled N-fluorenylmethoxycarbonyl diphenylalanine (Fmoc-FF) together with horseradish peroxidase (HRP).166 Although AuNCs in general do not show pH-dependent fluorescent property, the Fmoc-FF/AuNCs/HRP film showed pH dependency in the pH range of 9.0 to 5.0 due to the structural changes in Fmoc-FF.
Niu et al. reported the preparation of BSA-stabilized AuNCs by mixing aqueous HAuCl4 solution and BSA solution in the presence of NaOH.167 The changes in fluorescence intensity were monitored using Cys and Cu2+. The fluorescence was enhanced when Cys was added to the AuNCs by filling the surface defects and fluorescence quenching occurred due to the aggregation in the presence of Cu2+. The changes in fluorescence spectra with the addition of metal ions such as Cu2+ and cysteine are illustrated in Fig. 10. Although there are several reports on the protein-templated synthesis of gold nanoclusters, Chakraborty et al. reported the preparation of HSA-stabilized Au25NCs168 with a high quantum yield and exceptionally stable photoluminescence property for over a year. AuNCs was encapsulated compactly within the bulky HSA structure via reduction by the tyrosine group at a pH greater than the pKa of tyrosine with red emission. The nanoclusters exhibited no change in PL property in the range pH range of 3–12 and were highly thermally stable, with a long lifetime (>100 ns) as a result of their triplet–singlet intra-band transitions. Temperature-dependent PL spectra changes were observed due to the conformational changes in the protein structure, as in the case of earlier reported methods for BSA-stabilized AuNCs.
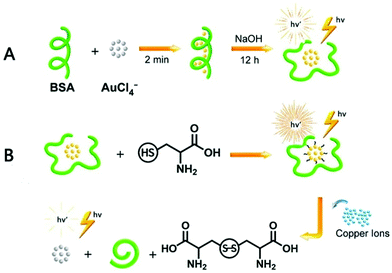 | ||
| Fig. 10 Schematic illustration of the synthesis of BSA-stabilized AuNCs and effect of Cu2+ ions and cysteine on their fluorescent property. Reproduced with permission from ref. 167, Copyright Elsevier (2021). | ||
Yan et al. reported the microwave-assisted synthesis of BSA-stabilized AuNCs with red emission. HSA-protected AuNCs were synthesized using a similar methodology, as shown in Fig. 11.169 This methodology is significant for reducing the reaction time but has certain limitations given that the reaction time needs to be strictly monitored considering that continuous MW irradiation leads to the formation of larger NPs with no fluorescence. Protamine–AuNCs with strong fluorescence were synthesized via a one-step process.170 This methodology required mild conditions for the synthesis of protamine-stabilized AuNCs, which can be used for gene delivery and provide a scope for the utilization of AuNCs in the field of biological applications. Zhang et al. reported the preparation of cytidine (also known as ribofuranose where cytosine is attached to a ribose sugar)-stabilized green fluorescent AuNCs, as shown in Fig. 12.171 This green emission is attributed to the intraband transitions of the free electrons in AuNCs. The emission property was found to be red-shifted from 490 to 560 nm and enhanced significantly in the presence of AgNO3 due to the Au–Ag metallic bonds. Similarly, the fluorescence was quenched in the presence of Hg2+ due to the high-affinity metallophilic Hg2+–Ag+ interaction. The enhancement in the emission due to metal doping is expected according to literature reports because of the changes in the nature of the electronic state and orbitals.158
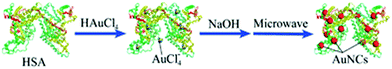 | ||
| Fig. 11 Scheme for the microwave-assisted synthesis of gold nanoclusters. Reproduced from ref. 169 with permission from The Royal Society of Chemistry, Copyright 2012. | ||
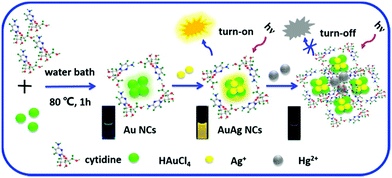 | ||
| Fig. 12 Schematic illustration of the synthesis of cytidine-stablised AuNCs and changes in the luminescence property with the addition of Ag+ and Hg2+. Reproduced with permission from ref. 171, Copyright Elsevier (2015). | ||
Egg shell membrane (ESM) is composed of water-insoluble glycoproteins such as collagen and amino acids such as glycine alanine and uranic acid. Devi et al. reported the preparation of Au–ESM–NCs, where ESM acts as a template and interacts with Au3+, resulting in change in a color from deep yellow solution to colorless, and the subsequent appearance of a pink/blue color was observed with a broad peak in the visible range (500–750 nm), indicating the reduction to Au(0).172 This method led to the formation of both Au8 and Au25 clusters. Both broad UV-visible spectra and excitation-dependent emission spectra confirmed the formation of AuNCs of variable size. Blue and red emissive AuNCs were obtained due to the electronic transitions between the sp band and d band and electron–hole recombination. The prepared AuNCs were found to be stable for only up to 15–20 days.
3.1.1.1 Amino acid-stabilized AuNCs. Au25(Cys)18 NCs were synthesized via a simple one-pot method in the presence of L-Cys as a stabilizing agent. This methodology enabled the size-controlled synthesis of Au25(SR)18 NCs on a large scale using CO as a reducing agent.173 Different stages of the growth and formation of Au10–15 NCs (20 min), Au10–15 to Au16–25 NCs (20–90 min), and Au16–25 to Au25 NCs were finally illustrated using UV-Visible spectroscopy and MALDI-TOF analysis.
Gran et al. reported the synthesis of Au25(AcCys)18 using gold salts with acylated Cys in methanol in the presence of different bases.174 Yang et al. reported the synthesis of 6-aza-2-thiothymine (ATT)-protected AuNCs and Arg/ATT–AuNC in the presence of NaOH, involving two steps, as shown in the Table 3. An enhancement in luminescence observed with Arg/ATT–AuNCs as rigid host–guest assemblies formed via reduced non-radiative relaxation.175
| Reagent added | Surface capping agent | Reaction condition | λ ex (nm) | λ em (nm) | size(nm) | QY% | Brightness (M−1 cm−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| NaOH (pH 12) | BSA | 37 °C, 12 h | 480 | 640 | 0.8 | 6% | 1.26 × 103 | 159 |
| NaOH | BSA | 60 °C,6 h | 365 | 630 | <2.0 | 10.62% | 3.80 × 106 | 160 |
| — | Anti-Flt1(AF) peptide | 70 °C, 8 h | 328 | 620 | 1.9 | 9.92% | 5.39 × 106 | 161 |
| NaOH | BSA | 37 °C, dark, overnight | 511 | 651 | 3.18 | 95.84% | 1.26 × 106 | 162 |
| Ascorbic acid NaOH | BSA | 37 °C, 36 h | 530 | 670 | 2–3 | NA | — | 164 |
| Lyzozyme | 40 °C, 24 h | 645 | 1.3 | 3.8% | ||||
| NaOH (pH 12) | HSA | 40 °C, 24 h | 350 | 650 | 1.4 | 4.1% | — | 165 |
| yG protein | 40 °C, 24 h | 645 | 1.5 | 4.4% | ||||
| BSA | 40 °C, 24 h | 660 | 1.4 | 5.4% | ||||
| NaOH | BSA | 37 °C, 12 h | 500 | 660 | 3.7 | — | — | 166 |
| Fmoc-FF, HRP, BSA–AuNCs | — | 4 °C, 12 h | 365 | 660 | — | — | — | 166 |
| NaOH | BSA | 37 °C, 12 h | 495 | 660 | 3.0 | — | — | 167 |
| NaOH | HSA | 40 °C, 18 h | 505 | 660 | 1.7 | 12% | 1.20 × 105 | 168 |
| NaOH | BSA | MW radiation (300 W) | 645 | 510 | 5 | — | — | 169 |
| HSA | 6 min | 640 | 514 | |||||
| NaOH | Protamine | 37 °C, 12 h | 280 | 600 | 1.65 | — | — | 170 |
| Citrate–citric acid buffer (pH 6) | Cytidine | 80 °C, 1 h | 370 | 490 | 1.50 | — | — | 171 |
| Dilute acetic acid | Egg shell membrane | RT | 335 | 440 | <20 | — | — | 172 |
| 585 | 630 | |||||||
| NaOH (pH 11) | Cysteine | Sealed reaction vessel, CO, RT, 24 h RT | — | — | — | — | — | 173 |
| Tributylamine, triethylamine, NH4OH | N-Acetyl-L-cysteine(methanol) | RT | — | — | — | — | — | 174 |
| NaOH (pH 8.0) | 6-Aza-2-thiothymine (ATT) | Dark, 1 h, RT | 423 | 532 | 3.0 | 0.03% | 1.10 × 10 3 | 175 |
| ATT—AuNcs (pH 11) | L-Arginine | 37 °C, 24 h | 423 | 532 | 3.0 | 67.02% | 2.49 × 10 4 | 175 |
| NaOH (pH 11) | Aq. glutathione | CO, RT, 24 h | 808 | 1120 | <2.0 | 0.05% | 6.55 × 10 2 | 176 |
| — | Aq. glutathione | 70 °C, 24 h | 365 | 580 | 2.5 | — | 1.65 × 103 | 177 |
| NaOH, NH4OH, Methanol, glacial acetic acid | Glutathione (methanol & triethylamine) | RT, overnight | 420 | 510 | — | 1.2 × 103 | 1.32 × 10 | 178 |
| Tetrabutylammonium borohydride | Glutathione (methanol & tributylamine) | Mixed at −10 °C, 1 h and strirred overnight at RT | 435 | 820 | — | 3 × 10−3 | 3.6 × 103 | 179 |
| Tetrabutylammonium borohydride, tetramethylammonium borohydride | Glutathione (methanol & tributylamine, water) | Ice bath 3 h | Two-photon excited at 780 | 600 | — | — | — | 180 |
| NaBH4 | Glutathione (methanol) | 0 °C, 1.5 h | 514.5 | 700 | 0.7 | — | 8.8 | 103 |
| — | Aq. glutathione | 90 °C, 6.5 h | 370 | 615 | 1.71 | 0.22% (pH 7) | 2.60 × 102 | 181 |
| 0.15% (pH 11) | 1.80 × 102 | |||||||
| NaBH4 | 8-Mercapto-9-propyladenine | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
365 | 510 | 2 | 1.2% | 1.44 × 105 | 182 |
| Trisodium tetraborate (pH 9.2) | THPC (alkaline solution), 11-mercaptoundecanoic acid | Dark, RT, 72 h | 675 | 520 | 2.0–2.9 | 3.1 × 10−2 | 5.58 × 104 | 183 |
| NaOH | 11-Mercaptoundecanoic acid | RT, 5 h | 285 | 608 | 1.8 | 2.4% | 2.14 × 104 | 184 |
| NaOH, NaBH4 | LA–PEG–OCH3 | RT, 15 h | 365 | 750 | 1.2 | 14% | 8.8 × 104 | 185 |
| TOAB & HAuCl4 (THF), NaBH4 | PhC2H4SH (added two times) | RT, total reaction time 28 h | 400 | 650 | 1.32 ± 0.66 | — | — | |
| BM–Au NCs (THF), water | DSPE–PEG5000 (THF) | Ultrasound, evaporation done reduced pressure at 40 °C | 400 | 650 | 62 ± 18 | — | — | 186 |
| Me2SAuCl, NaBH4(in ethanol) | Tris(2-carboxyrthyl)phosphine hydrochloride L HCI | CH2Cl2, MeOH, dark, overnight | — | — | — | — | — | 187 |
| Au(tht)Cl, triethylamine, AgNO3(CH3CN), NaBH4 | 9-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-closo-1,2-C2B10H11(CH2Cl2) C-closo-1,2-C2B10H11(CH2Cl2) |
RT, 12 h, dark | 550 | 964 | 2.0 | — | — | 188 |
| Au28(methanol) | — | RT, 48 h, recrystallization (layering methanol on DMF) | 470 | 1095 | 2.0 | — | — | 188 |
| Ph3PAuCl (CHCl3) & AgSbF6 (methanol) | — | Dark, RT, 25 h | 380 | 925 | — | 0.12 | 9.7 × 107 | 189 |
PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAu, NaBH4 Me2SAuCl (CHCl3), NaOH CAu, NaBH4 Me2SAuCl (CHCl3), NaOH |
1,2,3-Ph3(CN3H2), tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH |
Dark, RT, 12 h | 250–600 | 665 CH2CL2 | — | 0.004 | 3.11 × 103 | 190 |
| 550 | 625 | — | 0.15 | 1.17 × 105 |
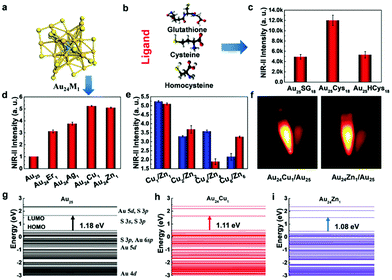
3.1.1.2 Thiol-stabilized AuNCs:. There are several reports in the literature on the synthesis of GSH-stabilized AuNCs. Liu et al. reported the synthesis of GSH-stabilized AuNCs as Au25(SG)18 with emission in the NIR region ranging from 1100–1350 nm in a reaction vessel saturated with CO at room temperature. The contribution of ligand stabilization (Fig. 13b and c) and metal doping (Fig. 13a, d and e) for enhancing the fluorescence in the NIR II region for Au25 nanoclusters with a HOMO–LUMO band gap (1.18 eV) was studied in detail. NIR fluorescence images were observed for Cu- and Zn-doped AuNCs (Fig. 13f). The different electronic states for the Au atom and ligand contributing to the HOMO–LUMO energy levels are shown in Fig. 13(g), (h) and (i). The maximum NIR II intensity was found in the case of cysteine. The changes in LUMO energy levels due to S 3p and S 3s electronic states using different surface ligands modulate the fluorescence intensity. Metal doping resulted in a decrease in the band gap to 1.11 eV and 1.08 eV for Cu and Zn, respectively, and the LUMO split into sub-energy levels.176 Pan et al. reported GSH-stabilized AuNCs via the reaction of gold chloride salt and GSH at 70 °C under gentle stirring for 24 h.177
Gran et al. synthesized Au10SG10, Au15SG13, Au18SG14, Au25SG18, Au15PEG and Au25PEG (PEG represents polyethylene glycol) using the earlier reported methods.174 Au10(SG)10 was prepared at ambient temperature by mixing aqueous gold chloride solution in a methanolic mixture containing GSH and trimethylamine. NaOH solution was added to the reaction solution, and then centrifuged. Centrifugation was done repeatedly by dissolving the resulting product in aqueous NH4OH, and then again precipitating in methanol. The resultant powder was dissolved in water followed by glacial acetic acid and left undisturbed for 1 h.178 Au15(SG)13 was synthesized using the same procedure with slight modification at −10 °C and tetrabutylammonium borohydride (TBA–BH4) as a reducing agent.179
Au18(SG)14 was synthesized by dissolving GSH in methanol, tributylamine and water followed by gold solution and diethyl ether. The NCs were purified initially by dissolving in basic solution, precipitated in MeOH, and further through centrifugation. Tetrabutylammonium borohydride and tetramethylammonium borohydride were added in two parts under strong agitation and stirring in an ice bath. The synthesized nanoclusters after purification were modified to form TBA– and TOA–Au NCs using tetrabutylammonium hydroxide and tetraoctylammonium bromide, respectively. The addition of bulky counterions has been reported to enhance the fluorescence, as shown in Fig. 14, and the solvent-dependent changes in the two-photon excited fluorescence spectra are illustrated in Fig. 15.180
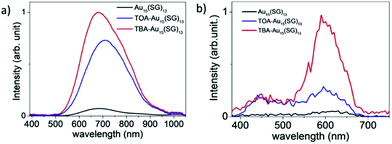 | ||
| Fig. 14 One-photon excited fluorescence spectra of Au15(SG)13 in (a) water and (b) presence of bulky ammonium cations, namely, tetrabutylammonium (TBA) and tetraoctylammonium (TOA) for (TOA–Au15(SG)13 and TBA–Au15(SG)13 in methanol). Reproduced with permission.180 Copyright 2018. Wiley-VCH. | ||
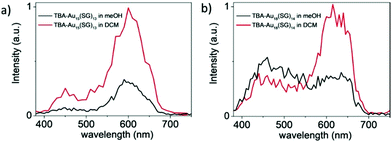 | ||
| Fig. 15 Two-photon excited fluorescence spectra for (a) TBA-Au15(SG)13 and (b) TBA-Au18(SG)14 at the excitation wavelength of 780 nm. Reproduced with permission.180 Copyright 2018. Wiley-VCH. | ||
Au25(SG)18 was synthesized similarly by stirring gold salt and GSH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) in a methanolic solution, followed by the addition of NaBH4 as a reducing agent, resulting in a dark-brown precipitate.103 Huang et al. reported GSH-capped AuNCs using a similar procedure.181 Venkatesh et al. reported the preparation of green fluorescent 8-mercapto-9-propyladenine-stabilized AuNCs.182 During the synthesis, a white precipitate was obtained with the formation of an Au(I)–thiolate complex, which was further reduced to AuNCs in the presence of NaBH4.
4) in a methanolic solution, followed by the addition of NaBH4 as a reducing agent, resulting in a dark-brown precipitate.103 Huang et al. reported GSH-capped AuNCs using a similar procedure.181 Venkatesh et al. reported the preparation of green fluorescent 8-mercapto-9-propyladenine-stabilized AuNCs.182 During the synthesis, a white precipitate was obtained with the formation of an Au(I)–thiolate complex, which was further reduced to AuNCs in the presence of NaBH4.
Au@MUA NCs were synthesized via the method reported by Huang et al.183 Tetrakis(hydroxymethyl)-phosphonium chloride (THPC) in alkaline solution was added as a reducing agent to gold salt followed by MUA stock solution and sodium tetraborate. The reaction mixture was kept in the dark at room temperature for about 72 h and purified by a centrifugal filter and resuspended in sodium borate buffer of pH 9. Au@MUA NCs were further stabilized by mixing with ligand stock solution from the same n-alkanethiolate family with different carbon chain lengths. Sun et al. reported a one-pot approach using water-soluble MUA-stabilized AuNCs.184 In this synthetic approach, MUA was added to an aqueous solution of gold salt in the presence of NaOH at room temperature, where MUA acts as both a capping and reducing agent.
3.1.1.3 Polymer-stabilized AuNCs. Aldeek et al. reported the preparation of bidentate LA-functionalized AuNCs with fluorescence in the red to near-infrared region with an emission centered at ∼750 nm.185 LA was first modified with PEG short chain LA–PEG750–OCH3, LA–PEG550–OCH3, LA–PEG600–COOH, LA–PEG600–NH2 and LA–PEG600–N3 (PEG molecular weights are 750, 550, and 600) or a zwitterion group followed by the synthesis in the Au
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio of 1
ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in the presence of NaOH. This methodology is useful for synthesizing polymer-stabilized AuNCs, exhibiting a long lifetime and excellent colloidal stability over a wide pH range and NaCl and glutathione concentration.
3 in the presence of NaOH. This methodology is useful for synthesizing polymer-stabilized AuNCs, exhibiting a long lifetime and excellent colloidal stability over a wide pH range and NaCl and glutathione concentration.
Recently, the preparation of DSPE–PEG [(1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)]-encapsulated benzyl mercaptan-stabilized AuNCs was reported by Li et al.186 The synthesis involves two steps, firstly the synthesis of benzylmercaptan (BM)-stabilized AuNCs, and then their encapsulation in amphiphilic polymer using the solvent evaporation method. Tetraoctylammonium bromide (TOAB), a phase-transfer reagent, and gold chloride salt were stirred in the presence of PhC2H4SH and NaBH4. PhC2H4SH was added to the reaction mixture again after centrifugation and stirred at room temperature to obtain BM–AuNCs. DSPE–PEG encapsulated BM-stabilized AuNCs showed enhanced PL due to aggregation-induced emission.
3.1.1.4 Phosphorous-stabilized AuNCs. [Au25(SR)18]− is also known as magic clusters because of its well-defined molecular structure, good stability, photoluminescence and electrochemiluminescence in the visible to near-infrared region and chiral and magnetic properties. Lei et al. developed the cluster from cluster approach to synthesize Au25 nanoclusters from Au13, as shown Fig. 16. This synthetic approach consisted of a two-step reaction.
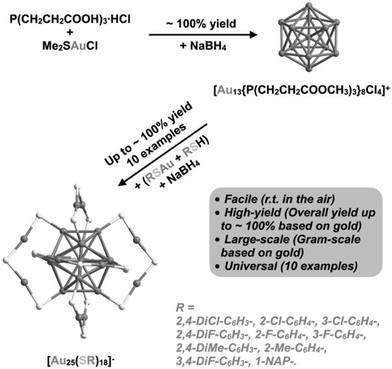 | ||
| Fig. 16 Schematic illustration of cluster from cluster approach for the synthesis of AuNCs. Reproduced with permission.187 Copyright 2021. Wiley-VCH. | ||
Firstly, [Au13{P(CH2CH2COOH3)3}8Cl4]+ was synthesized by dissolving tris(2-carboxyethyl)phosphine hydrochloride, L·HCl, and Me2SAuCl in a CH2Cl2 and MeOH mixture, and then sodium borohydride was added when the reaction mixture turned colorless. Further, in the second step, Au13 nanoclusters and polymeric (AuSR)x complexes were directly reduced in the presence of sodium borohydride and RSH at room temperature in the air, resulting in the high-yield and large-scale synthesis of [Au25(SR)18]−.187
3.1.1.5 Alkynyl-stabilized AuNCs:. Recently, the synthesis of alkynyl-protected AuNCs, their structural determination and optical properties have gained much attention. Wang et al. reported the preparation of the carboranealkynyl-protected gold nanocluster Au28 using the self-reduction method.188 The detailed reaction conditions are presented in Table 3, and as shown in the scheme in Fig. 17(a), yellow-colored [Au7·Au]n) crystals were formed, which after remaining undisturbed, converted into red block crystals of Au28. The dissociated B atom was converted to BO33−, as confirmed by the 11B NMR spectrum, and the Au cation was reduced to Au0 together with the formation of deboronated species and coupling diyne. The yield of Au28 could be improved by adding a strong reducing agent such as sodium borohydride. Given that Au28 is labile in solution, it recrystallized and transformed to Au23 in methanol at room temperature, as shown in Fig. 17(b).
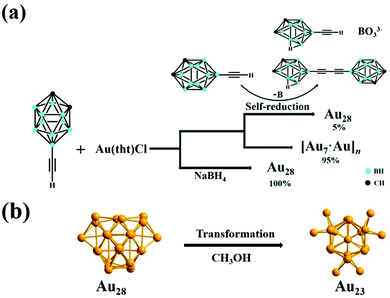 | ||
| Fig. 17 Synthesis of (a) Au28 nanoclusters via the self-reduction method and in the presence of sodium borohydride and (b) Au23 nanoclusters from Au28 in methanol. Reproduced with permission.188 Copyright 2021. Wiley-VCH. | ||
Wan et al. reported NIR-emissive alkynyl-stabilized AuNCs having the formula [Au24(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)14(PPh3)4](SbF6)2.189 Han et al. reported alkynyl-protected Au22(tBuC
CPh)14(PPh3)4](SbF6)2.189 Han et al. reported alkynyl-protected Au22(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18 AuNCs using NaOH, showing a temperature-dependent emissive property.190 Emission and excitation were available both in solution and solid state.
C)18 AuNCs using NaOH, showing a temperature-dependent emissive property.190 Emission and excitation were available both in solution and solid state.
3.1.1.6 Brightness of AuNCs. Gold nanoclusters have been used for biomedical applications for a long time for fluorescence imaging because of their advantageous properties such as biocompatibility, photostability, long lifetime, and enhanced permeability and retention time. However, their poor quantum yield and low molar absorption coefficient are important issues that impact their brightness.191 Thus, it is necessary to develop new synthetic approaches in the field of nanotechnology to obtain gold nanoclusters with improved brightness especially for fluorescence-based bioimaging purposes.158
Most of the synthesized gold nanoclusters have low brightness compared to conventional organic fluorophores. In earlier reports by Cantelli et al., their compared the photophysical properties of different glutathione-stabilized gold nanoclusters including Au10(SG)10, Au15(SG)13, Au18(SG)14, Au22(SG)16, Au22(SG)17, Au25(SG)18, Au29(SG)20, Au33(SG)22, and Au39(SG)24. They reported Aun(SG)m NCs (n < 15, n > 29) with a low molar absorption coefficient in the order of 104 M−1 cm−1 compared to organic dyes.158 Fluorophore organic dyes are reported with brightness of 105 M−1 cm−1 or more.192 We have calculated the brightness of gold nanoclusters using the formula (brightness = ελΦ), as given in the Table 3, where the molar absorption coefficient, ελ, was either calculated using the absorbance intensity/nanoparticle molar concentration or taken from the literature for glutathione-stabilized gold nanoclusters by Cantelli et al.158 The nanoparticle concentration was calculated using the theoretically reported method by Lewis et al.193 The brightness values were found to vary approximately in the range of 10−3 to 107. In most cases, the brightness values were found to be either very low or comparable to that of organic dyes.
3.2 Emissive Au nanoparticles (AuNPs)
In general, larger AuNPs do not show emission properties. However, within the last two decades, a few attempts successfully showed emission from AuNPs. In 2005, Luong and coworkers compared the fluorescence intensity of cetyltrimethylammonium bromide (CTAB)-stabilized longer nanorods (aspect ratio = 13) with a shorter GNR (aspect ratio = 2–3) prepared via an electrochemical technique. They demonstrated that the PL intensity increases several folds by increasing the aspect ratio.194 In 2008, Ren and coworkers synthesized typical citrate-stabilized emissive AuNPs (16–55 nm), where the emission intensity at 610 nm (λex = 532 nm) is directly proportional to the particle size.195 The synthetic procedure involves the addition of citrate solution of different concentrations to a refluxing solution of HAuCl4. A few years later, Negro and coworkers demonstrated lithographically engineered Au nanocylinders in a planar arrangement with both monomeric and dimeric forms on a fused silica substrate. It was observed that the PL position and the line width are related to both AuNP size and the inter-particle separation between in the fabricated system.196In 2017, our group reported a simple synthetic procedure for the development of AIE from AuNPs in the NIR range in the absence of an organic fluorophore molecule (Fig. 18A).197 We synthesized AuNPs via different procedures and treated them with diluted aqua regia, which induced the aggregation of the AuNPs (Fig. 18B), leading to the generation of an NIR luminescence peak at 916 nm upon excitation at 560 nm (Fig. 18C). In another trial, we utilized non-emissive porous-based cryptands as the aggregation source of AuNPs. The treatment of AuNPs separately with regioisomeric cryptands resulted in the generation of spherical and elongated dodecahedron gold suprastructures, which also possessed a similar emission in the NIR region.198
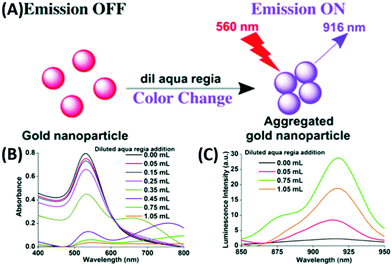 | ||
| Fig. 18 (A) Synthetic scheme for luminescent AuNPs. Changes in (A) absorption and (B) increase followed by decrease in the PL intensities at 916 nm (λex = 560 nm) due to the addition of diluted aqua regia to 3 mL of 0.6 nM AuNPs. Reproduced from ref. 197 with permission from The Royal Society of Chemistry, Copyright 2017. | ||
In our recent report, we also showed the room temperature in situ synthesis of luminescent gold–zinc oxide nanocomposites (Fig. 19A) in aqueous medium from the growth of AuNPs as seeds, sodium citrate as the stabilizing agent and Zn powder as the reducing agent for Au3+ ions added to the growth solution.199 The broad emission (Fig. 19B) is due to the presence of ZnO and freshly generated 5–6 nm AuNPs on the surface of the nanocomposite.
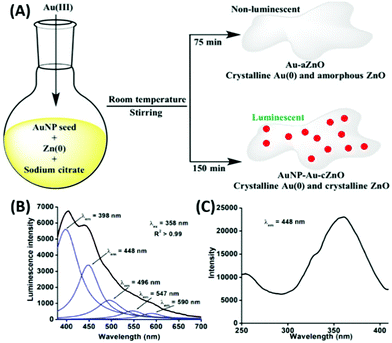 | ||
| Fig. 19 (A) Synthetic route of Au–aZnO (aZn1–aZn8) and AuNP–Au–cZnO (cZn1–cZn4) nanocomposites in aqueous medium. (B) Emission and (C) excitation spectra of cZn2. Reproduced from ref. 199 with permission from Frontiers, Copyright 2021. | ||
3.3 Gold-based bimetallic or alloy nanoclusters
The most popular synthetic methodologies for NPs involve the top-down and bottom-up approaches.98The luminescent properties in BMNCs appear due to their distinct synergistic145 and relativistic139 effects, which diversify their utility in numerous fields. Hence, controlled doping of one metal is very challenging in the presence of another metal. Mostly, the chemical reduction methods have been utilized to synthesize BMNCs. Other classification parameters, which involve the synthesis of BMNC, are based on the type of synthetic procedures, type of metals, protecting ligands used or based on metal precurssors.109,200,201 Another way to specifically classify the bimetallic system is based on the type of chemical reduction utilized in the procedure. If both metal precursors get reduced via one-pot synthesis, it is known as co-reduction synthesis, which generally originates from their monometallic procedures.201 In contrast, the other method includes post-synthetic modification constituting two steps. Firstly, the procedure involves the synthesis of intermediary monometallic NCs followed by post-synthetic treatment to get BMNCs by introducing the second metal ion.201,202 In this review, we classify gold-based BMNCs firstly based on the metal precursors utilized in the system, followed by the ligands utilized to separate them. The preparation of gold-based BMNCs typically involve the combination of gold with the other two coinage metals.
| Reagents added | Surface capping agent | Reaction condition | Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag Ag |
λ ex (nm) | λ em (nm) | Size (nm) | QY% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Indicates hydrodynamic diameter. 1, 2 and 3 corresponds to 3 different NCs ↑ represents increase in PL intensity. The brightness could not be calculated for BMNCs due to the difficulties faced during the calculation of the concentration oh NCs which involve radius of both metals. | ||||||||
| NaOH, NaBH4 | BSA | RT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
370 | 707 | 1.2 | 2.8% | 142 |
| NaOH | BSA | 37 °C, 12 h | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
370 | 620 | 4.5a | 10.5% | 143 |
| NaOH, NaBH4 | BSA | 37 °C, 20 h | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
490 | 718 | ∼2 | 42% | 203 |
| NaOH | BSA | 30–70 °C & 30 to 300 min | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
420 | 645 | ∼2.35 | 17.6% | 205 |
| NaOH | BSA | 37 °C, 6 h | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
325 | 405 | 2.1∼4.2 | NA | 206 |
| NaOH | BSA | 37 °C, 12 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
390 | 630, 470 | ∼3–5 | 6.37 | 207 |
| NH3·H2O | BSA | 700 W, 3 min | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
365 | 577 | ∼3–5 | 6.8% | 208 |
| NaOH | Lipoic acid | 70 °C, 8 h | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
385 | 630 | 1.9 ± 0.4 | 6.4% | 209 |
| NA | GSH | 70 °C, 24 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
335 | 605 | ∼2 nm | 7.2% | 211 |
| NA | GSH | 65 °C, 48 h | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
400 | 600 | 1.78 ± 0.20 | 13% | 212 |
| NA | GSH | RT, 12 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
320 | 515, 630 | 1.7 ± 0.4 | 26% | 213 |
| NaOH | GSH | 80 °C, 3 h | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
360 | 616, 412 | ∼1 | NA | 214 |
| NaOH | GSH | RT, 1.5 h, inert atm. | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
370 | 610 | <2 | 9.6% | 215 |
| NaBH4 | GSH | 20 °C, 15 h | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
520 | 6801, 7102, 8153 | 0.431, 0.652, 0.763 | NA | 216 |
| Ag NC | MSA | RT, 5 h | NA | 390 | 650 | NA | 0.035 | 217 |
| NA | 11-MUA | RT, 5 h | variable | 285–355 | 607–635 | ∼1.5 | 6.18% | 218 |
| NaOH | 11-MUA | RT, 20 min | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
320 | 610 | 1.56 | NA | 219 |
| NaOH, NaBH4 | 11-MUA | 15 min | variable | 250–330 | 608–640 | 1.8–2.1 | NA | 220 |
| PEG | MUTB | 70 °C, 5 h | variable | 353–397 | 440–455, 620–665 | < 2 min | NA | 221 |
| NA | DAMP | 70 °C, 5 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
473 | 640 | 1.63 ± 0.4 | 42.4 | 222 |
| Dopamine | Chondroitin sulfate | 30 °C, 4 h | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
450 | 510 | 0.99 | 1.41 | 223 |
| AuNP, AA | CTAC | 60 °C, 1 h | NA | 820 | 650–700 (2PPL) | 20–25 | NA | 152 |
| DNA-AgNCs, NaBH4 | 5′-CCCTTAATCCCC-3′, 5′-CCCCCCCCCCCC-3′, and 5′-CCCTCTTAACCC-3′ | Ice bath, 30 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
460 | 630 | NA | 4.5 | 223 |
| NaBH4 | 5′-CGCCCCCCTTGGCGT-3′ | Ice bath, 15 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
260 | 605 | 2.75 | NA | 224 |
| NA | C4-ATAT-C4 | 80 °C, 1 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
290 | 535 | 8.3 | 0.78 | 225 |
| NA | Cytidine | 80 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
370 | 560 | 1.50 ± 0.31 | 9% | 226 |
| Sodium citrate | AMP | 120 °C, 30 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
354 | 550 | 2.25 | 8.46% | 227 |
| Sodium citrate | AMP | 80 °C, 6 h | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
356 | 475 | 1.25 | 9.42% | 228 |
| NaOH | Lysozyme | 37 °C, 22 h | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
420 | 660 | 1.75 | 4.5% | 229 |
| NA | L-Tryptophan | 120 °C, 4 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
370 | 455 | 2.7 | 20% | 230 |
| NaOH | Methionine | 37 °C, 10 h | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
526 | 756 | 5 | NA | 231 |
| NaOH | Egg white protein | 250 W, 90 °C, 250 psi, 30 min | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
360 | 600 | 4.4 | 5.4% | 232 |
| NA | Egg shell membrane | RT, 3/7 days | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
340 | 435–445 | 5–100 | NA | 233 |
| p(NIPAM–AA–AAM)-Ag hybrid microgels | Ascorbic acid | Ice bath, 38 min | NA | 236 | 370 | NA | NA | 234 |
| Au@PNIPAM microgels | CTAB, ascorbic acid | RT | Variable | 405 | 570–580 | ∼57–102 | NA | 236 |
| [Au11(PPh3)8Cl2]+NaSbF6 | PhC2H4SAg | RT, 6 h | NA | 370 | 680 | NA | 40.1% | 135 |
| C18H15AuCIP | BDT, TPP, NaBH4 | Dark, 12 h | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
∼445 | ∼660 | NA | 24% | 136 |
| Ag25(SPhMe2)18 | AuCLPPh3 | RT, 4 h | NA | 467 | ∼810 | NA | NA | 237 |
| NaBH4 | Lipoic acid | RT, 3–5 h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
485 | ∼680 | NA | 7.9% | 202 |
| Ag25(SPhMe2)PPh4 | AuCLPPh3 | RT, 4 h | NA | 405 | 810 | NA | NA | 145 |
3.3.1.1 BSA-mediated Synthesis:. During the last decade, BSA protein remains the most frequent choice during the synthesis of BMNCs. This is due to the presence of residues such as cysteine, which not only can reduce Au3+ but also stabilize the Au(0) clusters by the formation of a strong Au–S bond.
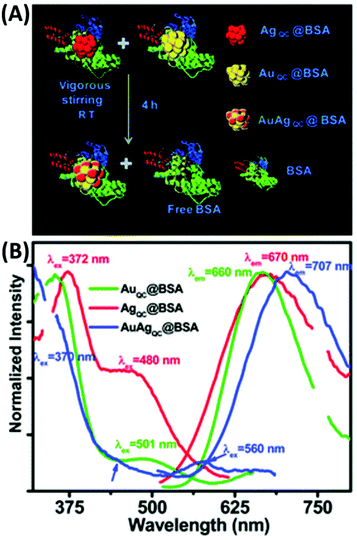
T. Pradeep and co-workers first introduced BSA for the preparation of luminescent Au/Ag alloy NCs (1.2 nm), which were obtained by gently mixing and stirring the separately synthesized BSA-capped Au38 and Ag31 quantum clusters (QC) (Fig. 20). In another galvanic exchange method, different concentrations of aq. Au3+ were introduced in the as-prepared AgQC@BSA under vigorous stirring and the reaction continued for 8 h to yield Au/Ag BMNCs.142 Upon excitation 370 nm, the λem of these BMNCs red-shifted to 707 nm, unlike the AuQCs and AgQCs, having λem at 660 and 670 nm, respectively. Inspired by this, K. Chattopadhyay and co-workers also synthesized red-emissive Au–Ag@BSA NCs by simply adding Au3+ and Ag+ to BSA solution and stirring at 37 °C for 20 h. However, increasing the dopant (Ag) concentration caused a continuous red shift in the emission maximum, which is mainly attributed to the modulation of the electronic state with silver doping. Another possible explanation is either the presence of MLCT (M→BSA) or MMCT (Ag→Au) in the BMNCS. The molar ratio of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag was optimized to 2.3
Ag was optimized to 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 at an effective pH of 11 to obtain enhanced emissive Au–Ag@BSA, which was subsequently utilized for the cellular detection of toxic heavy metals (Pb2+).203
2.0 at an effective pH of 11 to obtain enhanced emissive Au–Ag@BSA, which was subsequently utilized for the cellular detection of toxic heavy metals (Pb2+).203
 | ||
| Fig. 20 (A) Synthetic scheme of AuAg@BSA alloy clusters through QC–QC interaction and (B) emission spectra of AuQC, AgQC and AUAgQC@BSA indicated by green, red, and blue line, respectively. λex = 370 nm. Reproduced from ref. 142 with permission from The Royal Society of Chemistry, Copyright 2012. | ||
It was reported that the tyrosine residues present in BSA can reduce Au3+ to Au(0) in basic medium with pH > 9. Hence, the basic medium was utilized to synthesize BSA-mediated AuNCs.204 For instance, Zhang et al. reported the synthesis of bimetallic alloying Au–AgNCs by utilizing metal precursor HAuCl4 and AgNO3 with BSA functioning as both the reducing and stabilizing agent in basic medium.143 By maintaining the molar ratio of Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag precursors to 25
Ag precursors to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in the reaction medium, this silver effect exceptionally enhanced the red emission (620 nm) intensity of the Au–AgNCs by several folds compared to that of conventional AuNCs and core shell Au@AgNCs.
6 in the reaction medium, this silver effect exceptionally enhanced the red emission (620 nm) intensity of the Au–AgNCs by several folds compared to that of conventional AuNCs and core shell Au@AgNCs.
XPS studies confirmed not only the presence of Au(0) and Ag(0)/Ag(I) but also confirmed the Au–S interaction, which suggest that the formation and attachment of bimetallic Au–AgNCs in the S-containing site of the protein scaffold is the probable path of nucleation in the protein scaffold. BMNPs initiate with the reduction of HAuCl4 and Ag+ ions by 21 tyrosine residues in the protein scaffold. The competitive nucleation rate is faster in Au than Ag. Hence, after nucleation, these Au atoms catalytically enhance the Ag deposition in the protein matrix and their co-deposition leads to the formation of bimetallic Au–AgNCs. The origin of luminescence was attributed to the presence of LMCT in the BMNCs. In addition, the silver effect specifically enhanced the sensing performance of the Au–AgNCs towards different metal ions such as Cu2+ and Hg2+. There are a few other reports available on the synthesis of Au–AgNCs following a similar method with minor modifications.205,206
In another discovery, K. Huang and co-workers reported BSA-protected dually emissive Au–AgNCs through a green synthetic procedure, which involved the addition of an aqueous solution of HAuCl4 and AgNO3 to a BSA solution with a gentle stirring for 2 min, followed by the addition of NaOH solution at 37 °C.207 As shown in Fig. 21, the BSA-stabilized Au–AgNCs possess dual emission peaks at 470 nm (Ag NCs) and 630 nm (Au NCs) under 390 nm excitation. These emissions may originate from the different interactions of the metal core with the surface ligand.
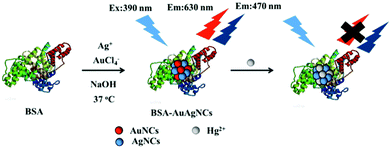 | ||
| Fig. 21 Schematic illustration of the synthesis of dual-emitting BSA–Au/Ag NCs and their application towards Hg2+ sensing. Reproduced with permission from ref. 207, Copyright Elsevier (2018). | ||
The origin of the emissive properties is attributed to the perturbation of the electronic state in the quantum confinement region. Upon interaction with Hg2+, the luminescence intensity at 630 nm quenched due to the high-affinity of Hg2+ towards Au+via d10–d10 metallophilic interaction. BSA-protected Au–Ag luminescent BMNCs were also synthesized by Zheng et al. via a simple microwave (MW)-assisted method. The BSA solution was added to a mixture containing an aqueous solution of HAuCl4 and AgNO3 under stirring condition followed by the addition of NH3·H2O. Heating by MW radiation yielded yellow emissive (577 nm) bimetallic Au/Ag NCs.208
3.3.1.2 Sulfur-containing Ligand-mediated Synthesis:. The interaction of thiol with noble metals is well established due to their soft–soft interactions. Researchers across the world utilized this fact to explore different synthetic methods for noble metal NCs by simply employing sulfur-containing ligands in the reaction medium. For example, Huang et al. utilized the strong Au and Ag interaction with sulfur to synthesize fluorescent Au–AgNCs by employing lipoic acid as a capping agent.209 Lipoic acid is dissolved in a strongly basic solution followed by the addition of HAuCl4 and AgNO3 solution in a molar ratio of 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under constant stirring at elevated temperature (Fig. 22A). Further centrifugation yielded red-emitting (630 nm) Au–Ag BMNCs, where the luminescence intensity was highly dependent on temperature and decreased with an increase in temperature from 20 °C to 65 °C (Fig. 22B).209 This phenomenon is attributed to the increase in the rate of collision frequency, which eventually results in increased nonradiative transition with an increase in temperature without affecting the radiative transition rate. The as-prepared Au–Ag BMNCs were reported as a chemosensor for Fe3+ through aggregation-induced quenching of the parent BMNPCs.
1 under constant stirring at elevated temperature (Fig. 22A). Further centrifugation yielded red-emitting (630 nm) Au–Ag BMNCs, where the luminescence intensity was highly dependent on temperature and decreased with an increase in temperature from 20 °C to 65 °C (Fig. 22B).209 This phenomenon is attributed to the increase in the rate of collision frequency, which eventually results in increased nonradiative transition with an increase in temperature without affecting the radiative transition rate. The as-prepared Au–Ag BMNCs were reported as a chemosensor for Fe3+ through aggregation-induced quenching of the parent BMNPCs.
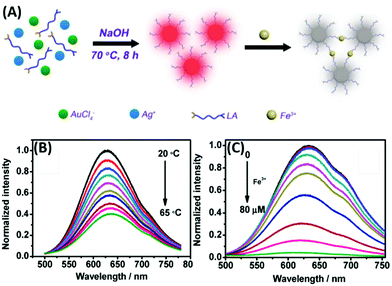 | ||
| Fig. 22 Schematic illustration of the (A) synthesis of lipoic acid-stabilized Au/Ag BMNCs and their Fe3+ sensing and (B) temperature- and (C) Fe3+-dependent fluorescence quenching. Reproduced with permission from ref. 209, Copyright Elsevier (2016). | ||
The origin of the aggregation is attributed to the interaction of Fe3+ with the carboxylate group of lipoic acid (Fig. 22C). Hikosou et al., Brach et al. and Ye et al. separately synthesized GSH-stabilized bimetallic luminescent Au–AgNCs by modifying the Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag ratio in the synthetic procedure.210–212 Liu et al. also synthesized dual-emitting GSH-stabilized Au–AgNCs by reacting GSH, AgNO3 and HAuCl4 in basic medium for 6 h at 80 °C.213 The as-synthesized NCs exhibited emission bands at 412 nm and 616 nm upon excitation at 360 nm. This nanosystem was demonstrated as a potential ratiometric fluorescence probe for the detection of Arg and Cys amino acids. Yin et al. also followed a similar procedure to obtain orange-emitting Au–AgNCs by maintaining the Au:Ag molar ratio at 9
Ag ratio in the synthetic procedure.210–212 Liu et al. also synthesized dual-emitting GSH-stabilized Au–AgNCs by reacting GSH, AgNO3 and HAuCl4 in basic medium for 6 h at 80 °C.213 The as-synthesized NCs exhibited emission bands at 412 nm and 616 nm upon excitation at 360 nm. This nanosystem was demonstrated as a potential ratiometric fluorescence probe for the detection of Arg and Cys amino acids. Yin et al. also followed a similar procedure to obtain orange-emitting Au–AgNCs by maintaining the Au:Ag molar ratio at 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.214 Yao and co-workers synthesized GSH-stabilized Au–Ag BMNCs by reacting a 3
1.214 Yao and co-workers synthesized GSH-stabilized Au–Ag BMNCs by reacting a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Au:Ag with excess GSH in methanol. The reduction of the metal precursors was carried out using sodium borohydride under an inert atmosphere.215
1 molar ratio of Au:Ag with excess GSH in methanol. The reduction of the metal precursors was carried out using sodium borohydride under an inert atmosphere.215
T. Pradeep and co-workers introduced mercaptosuccinic towards the synthesis of emissive BMNCs. The detail multi-step procedure involves the synthesis of MSA-stabilized AgNPs, which produced AgNCs through interfacial etching. The next step included the addition of HAuCl4 to the pre-synthesized Ag7/8 cluster with gentle stirring to get the resultant emissive Ag7Au6 alloy NCs.216 Jin and co-workers employed MUA as another thiol-containing capping agent to synthesize luminescent MUA–Ag/Au BMNCs with an emission peak at 630 nm.217 It was observed that the emission wavelength varied with the amount of Ag doping, but was independent of the excitation wavelength. While a similar reaction was performed by Yang et al. in basic medium,218 Ristig et al. utilized NaBH4 to complete the reduction process to get the desired product.219
T. Yonezawa and co-workers utilized the double-target sputtering method to synthesize bimetallic Au/Ag nanoclusters by utilizing 11-mercaptoundecyl-N,N,N-trimethylammonium bromide (MUTAB) as the surface-protecting ligand. Both Au and Ag metal targets were employed in the usual sputtering process and etched simultaneously by ionized Ar gas under high vacuum. The targets were set to face each other at a certain angle, enabling the generated unstable Au and Ag particles to have a higher collision probability, which resulted in the formation of Au–Ag BMNCs.220 By introducing a new sulfur-containing capping agent, 4,6-diamino-2-mercaptopyrimidine (DAMP), Yu et al. synthesized luminescent Au–Ag NCs in a one-pot synthetic method and utilized them for the detection of Hg2+ (Fig. 23A–C).221
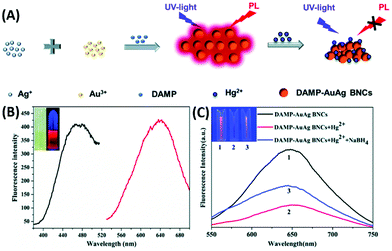 | ||
| Fig. 23 (A) Schematic illustration of synthetic process of DAMP–AuAg BMNCs and mercury ion-induced fluorescence quenching. (B) Excitation (black line) and emission (red line) spectra of the DAMP–AuAg BMNCs. Inset: DAMP–AuAg BMNC solution under visible light and UV at 365 nm. (C) Fluorescence spectra (λex = 473 nm). Inset: Picture of DAMP–Au–Ag BMNCs under UV light in the (1) absence and (2) presence of Hg2+ ions (50 μM), and (3) solution (2) after the addition of aqueous NaBH4 (10 mM). Reproduced from ref. 221 with permission from The Royal Society of Chemistry, Copyright 2021. | ||
3.3.1.3 Templated synthesis. Q. Liu et al. utilized chondroitin sulphate as new sulfur-containing template for the synthesis of novel fluorescent bimetallic Au/Ag NCs by simply reacting AgNO3 and HAuCl4 with a chondroitin sulphate solution. After 30 min of stirring, a dopamine solution was introduced in the reaction mixture, followed by stirring, resulting in a reddish-brown color solution containing Au/Ag NCs with an emission at 610 nm, which is attributed to the synergistic effect arising in the presence of silver.222 P. Yuan et al. synthesized bimetallic core–shell Au@Ag NPs possessing two-photon PL by utilizing cetyltrimethylammonium chloride solution (CTAC) as the template. They successfully demonstrated a two-photon PL (2PPL) enhancement with an increase in the Ag shell thickness.152
3.3.1.4 Biomolecule-mediated synthesis. Biomolecules such as DNA, peptides and amino acids assisting the synthesis of luminescent bimetallic Au/Ag NCs have attracted special attention given that the complex structural property of biomolecules induces the resultant shape during the growth of NCs. For example, in 2011 Chang and co-workers synthesized DNA (5′-CCCTTAATCCCC-3′ or 5′-CCCCCCCCCCCC-3′ or 5′-CCCTCTTAACCC-3′)-assisted Au/Ag BMNCs by reacting the DNA molecule with both metal precursors (DNA
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au
Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag = 1
Ag = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) in an ice bath for 15 min in the presence of NaBH4 as a reducing agent.223
6) in an ice bath for 15 min in the presence of NaBH4 as a reducing agent.223
Similarly, Pang and co-workers replaced the DNA sequence to 5′-CGCCCCCCTTGGCGT-3′ in the previous procedure to get red-emissive DNA-assisted Au/Ag BMNCs.224 Deng and co-workers synthesized DNA-mediated emissive Au–Ag BMNCs by adding HAuCl4 and AgNO3 to DNA molecules in citrate–citric acid buffer.225 Wang and co-workers introduced the cytidine-template synthesis of luminescent bimetallic cytidine–AuAg NCs. Specifically, to a cytidine solution in PBS buffer, an aqueous solution of HAuCl4 was introduced, followed by citrate buffer (pH 6). The reaction mixture was heated in a water bath at 80 °C followed by the addition AgNO3 to yield bimetallic cytidine–AuAg NCs with an emission centered at 560 nm.226
In 2017, Wu and co-workers introduced for the first time for the hydrothermal synthesis of fluorescent bimetallic Au–AgNCs@AMP using adenosine monophosphate (AMP) as the surface-protecting ligand. The AMP (solid) in deionized water together with HAuCl4, AgNO3 and sodium citrate solution (Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag: AMP to 0.2
Ag: AMP to 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) was autoclaved at 120 °C to get the emissive BMNCs. Similar BMNCs were also prepared by two other methods involving heating and stirring via seed-mediated hydrothermal synthesis.227 By modifying the Au
5) was autoclaved at 120 °C to get the emissive BMNCs. Similar BMNCs were also prepared by two other methods involving heating and stirring via seed-mediated hydrothermal synthesis.227 By modifying the Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag to 9
Ag to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Z. Suo et al. also synthesized AMP–Au/Ag NCs and incorporated them in pre-synthesized right and left handed G-quartet nanofibers to obtain enantiomeric circularly polarized light emission (Fig. 24).228
1, Z. Suo et al. also synthesized AMP–Au/Ag NCs and incorporated them in pre-synthesized right and left handed G-quartet nanofibers to obtain enantiomeric circularly polarized light emission (Fig. 24).228
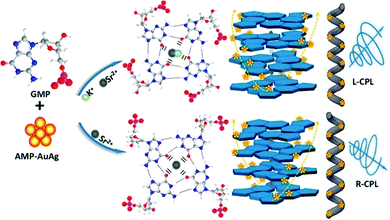 | ||
| Fig. 24 Schematic Illustration of the self-assembly of g-fiber–AuAg NCs exhibiting R-/L-CPL emission. Reproduced from ref. 228 with permission from the American Chemical Society, Copyright 2020. | ||
S. Pang et al. carried out the reduction reaction of Au and Ag precursors in basic medium by introducing lysozymes as a stabilizing agent to get the luminescent Lys–Au/Ag BMNCs.229
Meng et al. synthesized L-Trp@AuAgNCs by utilizing tryptophan as a reducing and capping agent in the reaction mixture involving Au and Ag metal precursors.230 Recently, Zou and co-workers synthesized methionine (Met)-stabilized Au–Ag BMNCs via a doping growth strategy.
The brief synthetic procedure involves mixing of AgNO3, HAuCl4 and Met in water under ultrasonication with a pH of 12.0. The orange-colored mixture was sealed at 37 °C for 10 h followed by the addition of a drop of H2SO4 to precipitate the Au–Ag BMNCs.231
Chicken eggs, which are enriched with several proteins, have also been considered for the synthesis of metallic NCs. For example, Li and co-workers utilized the chicken egg white protein matrix to synthesize orange-emitting Au–Ag BMNCs via a microwave-assisted facile green one-pot synthetic method.232 Briefly, to the egg white protein obtained after centrifugation, aqueous HAuCl4 solution followed by AgNO3 solution (varying ratio with Au) was added under vigorous stirring. Then, NaOH solution was added to the mixture under continuous shaking, and finally put in a microwave at 250 W 90 °C and 250 Psi for 30 min under stirring, yielding Au–Ag BMNCs.232 Similarly, Pramanik et al. synthesized bimetallic alloy Au–Ag NCs in the absence of any reducing agent via the ESM-induced reduction of AgNO3 and HAuCl4 solutions at room temperature.233
3.3.1.5 Polymer-mediated synthesis. Zhou and co-workers demonstrated the in situ synthesis of luminescent bimetallic Ag–Au NPs by performing the reduction reaction of Ag+ in the presence of poly(N-isopropylacrylamide–acrylic acid–acrylamide) [p(NIPAM–AA–AAm)] microgel followed by treatment with HAuCl4 solution under stirring in an ice bath.234 After the color change, L-AA was added to complete the reduction process to get p(NIPAM–AA–AAm)–Ag/Au. Rubio-Retama and co-workers also synthesized luminescent bimetallic Au@Ag@PNIPAM having a variable silver thickness.235 The detailed procedure includes the growth reaction of the as-prepared Au@PNIPAM NPs in the presence of CTAB as the template followed by AA-mediated reduction of Ag+.
| [Ag25(SPhMe2)18]− + Au+ → [Ag24Au(SPhMe2)18]− + Ag+ |
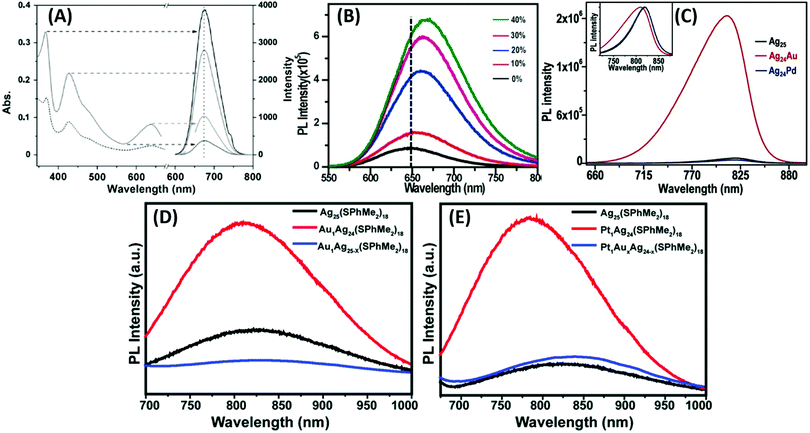 | ||
| Fig. 25 (A) Enhancement in PL intensity of product NC II [Ag13Au12(PPh3)10(SR)5Cl2]2+. PL (–), UV/Vis (…), UV/Vis and excitation spectra (left), and emission spectra (right) at arrow-indicated excitation wavelengths. (B) PL spectra of undoped Ag29 and different percentages (mmol%) of Au-doped Ag29 clusters. (C) PL spectra of [Ag25(SPhMe2)18]−, [Ag24Au(SPhMe2)18]−, and [Ag24Pd(SPhMe2)18]2− clusters. Inset represents the normalized spectra. PL spectra of (D) Ag25(SPhMe2)18, Au1Ag24(SPhMe2)18, and AuxAg25−x(SPhMe2)18 and (E) Ag25(SPhMe2)18, Pt1Ag24(SPhMe2)18, and Pt1AuXAg24−x(SPhMe2)18 with λex = 405 nm. (A)–(C) Reproduced with permission.135,138,237 Copyright 2014. Wiley-VCH. (D) and (E) reproduced from ref. 143 with permission from The Royal Society of Chemistry, Copyright 2014. | ||
Briefly, for the synthesis of Au1Ag24(SPhMe2)18PPh4 alloy NCs, the pre-synthesized Ag25(SPhMe2)18PPh4 was treated with AuClPPh3 in DCM under vigorous stirring for 4 h at room temperature to get a green-coloured solution. AgCl was removed by centrifugation and washing. The final cluster precipitate was dissolved in DCM and crystalized in a DCM/hexane mixture.237 By following a previously reported procedure for the synthesis of Ag25(SPhMe2)18 NCs,238 the same group also tried to dope more than one atom of Au and Pd inside the Ag25 clusters by performing the sodium borohydride-mediated reduction reaction of AgNO3 and AuClPPh3/Pd(OAc)2 together with HSPhMe2 and PPh4Br.237 The doping of a single Au atom remarkably enhanced the luminescence signal up to 25-fold against Pd-doped or pure Ag25 NCs (Fig. 25C). The blue shift in λem is attributed to the Ag atom, which modulates the HOMO–LUMO gap and/or the surface states after its incorporation in the crystal structure.237
Three years later, Groot and co-workers demonstrated mono Au atom doping in Ag29 NCs by modifying the procedure previously reported for the synthesis of Ag29 NCs.202,239 The synthetic procedure involves the reduction reaction of AgNO3 followed by HAuCl4 in the presence of LA and NaBH4 at room temperature overnight. In another post-synthetic method, Ag29 NCs were prepared first, followed by treatment with gold chloride salt and NaBH4 under stirring overnight to get the desired product. The Au-doped AgNCs possessed enhanced PL properties, which is attributed to the feasible radiative decay.202
For the synthesis of mono Pt atom-doped Pt1Ag24(SPhMe2)18PPh4 NCs, Yuan et al. performed the NaBH4-mediated reduction reaction of AgNO3 and H2PtCl6 in solution. The precipitation of the NCs was accomplished by the treatment with excess PPh4Br.145 Mono Au-doped AgNCs were prepared using the reported procedure described in ref. 237. For the replacement of more Ag atoms by Au or Pt atoms, the previously synthesized single-atom substituted NCs, M1Ag24(SR)18 (M = Au or Pt) were reacted with Me2PhSAu under stirring for 1 h. By replacing the innermost atom of Ag25(SR)18 by Au or Pt, there was a remarkable increase in the PL intensity of the mono-doped NCs compared to the undoped or multi atom doped NCs (Fig. 25D and E), respectively.145
It was observed that with an increase in the copper ratio, a bathochromic shift in the emission maximum occurred from 947 nm to 1067 nm. Chang and co-workers proposed a one-pot synthetic method for the preparation of penicillamine-capped luminescent bimetallic Au–Cu NCs having λem at 625 nm.241 Briefly, an HAuCl4 solution was introduced in the PA solution under vigorous stirring. Subsequently, Cu(NO3)2 dissolved in nitric acid solution was added to the reaction mixture under vigorous stirring. The color of the solution was changed from brown to milky white, showing the generation of PA–AuCu NCs. The pure product was obtained after centrifugation. They successfully demonstrated the enhancement in the emission intensity by varying either the amount of Cu or Au and keeping other parameters in the system fixed.
The restriction in intramolecular rotation through chemical bonds can also induce luminescence in bimetallic NPs having different oxidation states. The enhancement factor in bimetallic fluorescence dominates the fluorescence of the individual metallic system.242 For example, reducing Au(I) to Au(0) by NaBH4 can induce a significant enhancement in fluorescence in the CuISR1 (R1 = C10H15) system in bimetallic Au2Cu6 NCs, but it was observed that the same reaction without Au resulted in the absence of the emission band. The brief synthetic procedure for Au2Cu6 involves the room-temperature reaction of CuCl in CH3CN and CH3OH and adamantanethiol in toluene under vigorous stirring. Then ice-cold [Au(PPh2Py)Cl] and ice-cold water containing NaBH4 were introduced dropwise in the previous reaction mixture with vigorous stirring under an N2 atmosphere, and stirring was continued for 60 h. After the reaction, the pure product was obtained after centrifugation, washing and crystallization. The enhancement in the emission intensity was highly triggered by the restricted movement of the CuISR system inside the crystal structure of Au2Cu6(PPh2Py)2(SC10H15)6. A similar strategy was also extended to another system containing t-butyl mercaptan as the ligand system.243 By modifying a previously reported procedure for introducing hetero-metallic atoms in the core of Au25 NCs,244 Kazan et al. prepared emissive Au38Cu1(2-PET)24, (PET–2-phenylethanethiol) adduct nanoclusters by reacting as-prepared Au38(2-PET)24 in toluene with Cu(I)–(SR) at room temperature for 1 h followed by a few purification steps.153 They successfully demonstrated the two-fold enhancement in the luminescence intensity by the introduction of CuI in the reaction system. Bazán-Díaz et al. synthesized 2D-nanoribbons comprised of Au/Cu NPs by controlling the assembly and growth of the metal precursors inside a soft template.152 The flexible nanoribbons were synthesized by utilizing hexadecyl amine (HDA) as the surface protectant and self-assembly initiator. Dual-emitting nanoribbons were synthesized by reacting an aqueous solution of the metal precursors (CuCl2 and HAuCl4) with HDA in water under magnetic stirring at 60 °C until its color changed to mint green. Finally, an aqueous glucose solution was introduced in the reaction mixture.
The reaction was continued with another 30 min stirring, followed by centrifugation to get the desired product with narrow emission peaks at 700 and 813 nm. Nie et al. synthesized luminescent GSH–Cu/Au BMNCs at room temperature by reacting Cu(NO3)2 solution with GSH solution.245 The reaction proceeded via the formation of a white hydrogel. The dropwise addition of NaOH solution resulted in the formation of a transparent light-yellow solution at approximately pH 5. Finally, the reaction mixture containing CuNCs was added with aqueous HAuCl4 at room temperature with stirring. The desired products were obtained after a few rounds of purification. The GSH–Cu/Au BMNCs could be utilized as a potential candidate for chromium ion sensing and temperature sensing.
Bagheri and co-workers utilized the well-explored protein template BSA towards the synthesis of luminescent bimetallic Au–Cu NCs in aqueous medium.246 This procedure involved the treatment of equimolar HAuCl4 and Cu(NO3)2 with the BSA solution under constant stirring for 15 min, followed by the introduction of NaOH to maintain pH 12. After 12 h stirring, the pure product was obtained after dialization. The Cu doping in the Au–BSA system enhanced the electrochemical catalytic properties via the synergistic effect. The authors investigated their properties towards determination of the bisphenol A.
Besides Ag and Cu, recently platinum has also been utilized for the doping of AuNCs. For example, in a typical hydrothermal synthesis, Wu and coworkers employed guanosine monophosphate (GMP) as the protecting ligand to synthesize luminescent Au–Pt bimetallic nanoclusters, which showed an emission band at 415 nm with λex = 330 nm (Fig. 26A and B).247 One year later, the same group proposed another method for the hydrothermal synthesis of GSH-stabilized Au–Pt BMNCs, which possessed both visible emission at 625 nm and NIR emission band at 805 nm, and finally utilized them towards the detection of Ag(I). An aqueous solution containing HAuCl4 and K2PtCl4 (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was mixed with GSH and sodium citrate, and finally autoclaved with varying temperature and time to get pure BMNCs. When the reaction time was fixed to 1 h or 2 h, the corresponding BMNCs showed an emission band in the visible or NIR range.248
1) was mixed with GSH and sodium citrate, and finally autoclaved with varying temperature and time to get pure BMNCs. When the reaction time was fixed to 1 h or 2 h, the corresponding BMNCs showed an emission band in the visible or NIR range.248
 | ||
| Fig. 26 (A) Schematic illustration of the synthetic procedure for Au–PtNCs–GMP and (B) excitation (red dotted line) and emission (red) spectra and UV-vis (black line) absorption spectrum of the Au–PtNCs–GMP BMNCs. Reproduced from ref. 247 with permission from the American Chemical Society, Copyright 2020. | ||
4. Summary and outlook
Luminescent AuNCs can emit from the blue to near-IR (NIR) spectral region depending on the number of atoms in the cluster. In recent years, various approaches have been developed to prepare highly photoluminescent AuNCs with emission in the UV-vis region, for example: (1) engineering the particle surface by using different ligands, DNA, peptides, and proteins including bovine serum albumin (BSA), (2) controlling the metal core size, (3) aggregation-induced emission and (4) rigidification of the Au(I)–thiolate shell.11,43,44,112,114,127,249,250 Recent advances in the synthesis of AuNCs enabled the development of NIR-photoluminescent (NIR PL) AuNCs functionalized with a variety of thiol-containing ligands.251These ligands exhibit a significant enhancement in the emission efficiency of AuNCs, e.g., the optimized quantum yield (QY) of glutathione (GSH)-stabilized AuNCs reached about 15%, which can truly overcome the QY of less than 0.1% obtained from the classic Burst method for the synthesis of AuNCs.252 The tunable emission of the protein-stabilized Au nanoclusters (AuNCs) over a large spectral range makes them a useful platform in biomedical studies. AuNCs can harness photon energy in the field of light harvesting materials by virtue of their discrete energy levels, which can extend the lifetime of excited electrons up to the microsecond regime, which is 106 times larger than the ultrafast relaxation of hot electrons in the case of plasmonic nanoparticles.253
In general, the luminescence properties in these nanoclusters originate from the typical modulation of their HOMO–LUMO energy gap within discrete gold nanoclusters. These variations in energy gaps can also be introduced by incorporating another metal in the system. Bimetallic nanoclusters have been shown to possess better luminescence properties compared to their monometallic nanomaterials. The location of specific substituted metal atoms in the nanoclusters has a significant contribution in the enhancement of their luminescence properties. The synthetic aspects involving small molecules or biomolecules or their combinations as stabilizing agents in these metal-based nanomaterials were covered in this current review.
Scientists across the globe have recently focused on the applications of luminescent gold nanomaterials. The chemical and biological applications of these materials in analysis and sensing applications have drawn significant interest.216,254 Increasing attempts have already been made to achieve AIE in highly luminescent metal nanocluster assemblies using hierarchical functional materials.127 Luminescent gold nanomaterials have also been tested in devices and cellular imaging.255
Still, in comparison to the traditional highly promising emissive materials such as quantum dots (QDs) and organic dyes, most of the noble metal nanoclusters cannot show a higher fluorescence quantum yield although they are comparatively less toxic and safely renal-clearable. In some cases, aggregation-induced emission has demonstrated a promising enhancement in fluorescence from nanoclusters, but in this case, the limited hydrophilicity of the as-obtained products becomes an issue, which restricts their applications in living systems.64 An improvement in the water solubility of these aggregation-induced emission based NCs can be done by encapsulating the aggregates with some additional water-soluble coating and engineering them with a controllable size to make them very impactful for future applications in therapeutics.
Author contributions
K. B., J. K. S. and K. K. S. have contributed in all the topics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
K. K. S. acknowledges DST Nanomission (DST/NM/NB/2018/237), SERB-DST Grants (EMR/2014/000714 and CRG/2018/000269) and CSIR Grant No. 01(2989)/19/EMR-II for funding to carry out research in this topic.Notes and references
- K. M. Dean and A. E. Palmer, Nat. Chem. Biol., 2014, 10, 512–523 CrossRef CAS.
- E. Kim, Y. Lee, S. Lee and S. B. Park, Acc. Chem. Res., 2015, 48, 538–547 CrossRef CAS.
- T. F. G. G. Cova, A. A. C. C. Pais and J. S. S. de Melo, Sci. Rep., 2017, 7, 6806 CrossRef PubMed.
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS PubMed.
- K. K. Sadhu, S. Mizukami, Y. Hori and K. Kikuchi, ChemBioChem, 2011, 12, 1299–1308 CrossRef CAS.
- J. Zhang, X. Chai, X.-P. He, H.-J. Kim, J. Yoon and H. Tian, Chem. Soc. Rev., 2019, 48, 683–722 RSC.
- J. Tian, N. Cheng, Q. Liu, W. Xing and X. Sun, Angew. Chem., Int. Ed., 2015, 54, 5493–5497 CrossRef CAS PubMed.
- A. N. Bismillah and I. Aprahamian, Chem. Soc. Rev., 2021, 50, 5631–5649 RSC.
- H. Xiao, W. Zhang, P. Li, W. Zhang, X. Wang and B. Tang, Angew. Chem., Int. Ed., 2020, 59, 4216–4230 CrossRef CAS.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- S. Kolay, D. Bain, S. Maity, A. Devi, A. Patra and R. Antoine, Nanomaterials, 2022, 12, 544 CrossRef CAS.
- T. A. Tsunoyama, Y. Watanabe, J. Goto, K. Naito, R. S. Kasai, K. G. N. Suzuki, T. K. Fujiwara and A. Kusumi, Nat. Chem. Biol., 2018, 14, 497–506 CrossRef CAS.
- L. Y. Mironov, P. S. Parfenov, A. V. Shurukhina, Y. I. Lebedev and A. A. Metlenko, J. Phys. Chem. C, 2017, 121, 19958–19965 CrossRef CAS.
- R. D. Mehlenbacher, R. Kolbl, A. Lay and J. A. Dionne, Nat. Rev. Mater., 2018, 3, 17080 CrossRef CAS.
- X. Luo and J. Liu, Adv. Sci., 2022, 9, 2103971 CrossRef CAS.
- Y. Zhou, F. Mazur, Q. Fan and R. Chandrawati, View, 2022, 20210008 CrossRef.
- C.-H. Chuang, W.-Y. Chen, W.-B. Tseng, A. Lin, C.-Y. Lu and W.-L. Tseng, ACS Sustainable Chem. Eng., 2022, 10, 2461–2472 CrossRef CAS.
- D. Barman, K. Narang, R. Parui, N. Zehra, M. N. Khatun, L. R. Adil and P. K. Iyer, Aggregate, 2022, e172 Search PubMed.
- C. Zhang, L. Yan, Z. Gu and Y. Zhao, Chem. Sci., 2019, 10, 6932–6943 RSC.
- W. Zhao, A. Li, A. Zhang, Y. Zheng and J. Liu, ChemMedChem, 2018, 13, 2134–2149 CrossRef CAS PubMed.
- C.-N. Lok, T. Zou, J.-J. Zhang, I. W.-S. Lin and C.-M. Che, Adv. Mater., 2014, 26, 5550–5557 CrossRef CAS PubMed.
- C. F. Markwalter, A. G. Kantor, C. P. Moore, K. A. Richardson and D. W. Wright, Chem. Rev., 2019, 119, 1456–1518 CrossRef CAS PubMed.
- B. Wang, W. Feng, Y. Zhao and Z. Chai, Metallomics, 2013, 5, 793–803 CrossRef CAS.
- Z. Xia and S. Guo, Chem. Soc. Rev., 2019, 48, 3265–3278 RSC.
- J. Yu, Y. Dai, Q. He, C. Cheng, Z. Shao and M. Ni, Appl. Phys. Rev., 2020, 7, 041304 CAS.
- J. Balach, J. Linnemann, T. Jaumann and L. Giebeler, J. Mater. Chem. A, 2018, 6, 23127–23168 RSC.
- V. C. Hoang, V. G. Gomes and N. Kornienko, Nano Energy, 2020, 78, 105311 CrossRef CAS.
- J. Mao, J. Li, J. Pei, Y. Lu, D. Wang and Y. Li, Nano Today, 2019, 26, 164–175 CrossRef CAS.
- L. Wang, W. Zheng, S. Li, L. Zhong and X. Jiang, Nano Lett., 2022, 22, 3576–3582 CrossRef CAS PubMed.
- H. Huang, J. Zhao, B. Weng, F. Lai, M. Zhang, J. Hofkens, M. B. J. Roeffaers, J. A. Steele and J. Long, Angew. Chem., Int. Ed., 2022, e202204563 Search PubMed.
- C.-C. Chang, C.-F. Li, Z.-H. Yang, P.-Y. Lin, H.-C. Chang and C.-W. Yang, Sens. Actuators, B, 2022, 364, 131823 CrossRef CAS.
- Z. Tang, I. Surin, A. Rasmussen, F. Krumeich, E. V. Kondratenko, V. A. Kondratenko and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2022, 61, e20220077 Search PubMed.
- Y. A. Hong and J. W. Ha, Sci. Rep., 2022, 12, 6983 CrossRef CAS.
- L. Priest, J. S. Peters and P. Kukura, Chem. Rev., 2021, 121, 11937–11970 CrossRef CAS PubMed.
- Y. Zhou, L. Liao, S. Zhuang, Y. Zhao, Z. Gan, W. Gu, J. Li, H. Deng, N. Xia and Z. Wu, Angew. Chem., Int. Ed., 2021, 60, 8668–8672 CrossRef CAS.
- Q. Li, C. J. Zeman IV, Z. Ma, G. C. Schatz and X. W. Gu, Small, 2021, 17, 2007992 CrossRef CAS PubMed.
- G. Pramanik, K. Kvakova, M. A. Thottappali, D. Rais, J. Pfleger, M. Greben, A. E. Zoka, S. Bals, M. Dracinsky, J. Valenta and P. Cigler, Nanoscale, 2021, 13, 10462–10467 RSC.
- T. Kong, C. Zhang, X. Gan, F. Xiao, J. Li, Z. Fu, Z. Zhang and H. Zheng, J. Mater. Chem. C, 2020, 8, 4338–4342 RSC.
- V. Kumar, P. Srikrishnarka, J. S. Mohanty, M. P. Kannan, R. Nagarajan and T. Pradeep, ACS Sustainable Chem. Eng., 2021, 9, 7431–7436 CrossRef CAS.
- A. Chakraborty, H. Dave, B. Mondal, Nonappa, E. Khatun and T. Pradeep, J. Phys. Chem. B, 2022, 126, 1842–1851 CrossRef CAS PubMed.
- O. Pavelka, K. Kvakova, J. Vesely, J. Mizera, P. Cigler and J. Valenta, Nanoscale, 2022, 14, 3166–3178 RSC.
- G. Wu, T. Jiang, W. Li, T. Liu and X. Ma, Dyes Pigm., 2021, 188, 109211 CrossRef.
- M. Wang, X. Zhou, X. Wang, M. Wang and X. Su, Sens. Actuators, B, 2021, 345, 130407 CrossRef CAS.
- M. Wactawska, H. Niezanska and W. Dzwolak, J. Mater. Chem. C, 2022, 10, 3775–3783 RSC.
- T. Zhang, C. Tang, Y. Wang, C. Wang, Y. Zhang, W. Qi, R. Su and Z. He, Langmuir, 2022, 38, 4147–4155 CrossRef CAS PubMed.
- M. Saini, S. Ghosh, V. Kumar, P. Roy and K. K. Sadhu, Chem. – Eur. J., 2020, 26, 15150–15158 CrossRef CAS PubMed.
- K. Huang, Q. Fang, W. Sun, S. He, Q. Yao, J. Xie, W. Chen and H. Deng, J. Phys. Chem. Lett., 2022, 13, 419–426 CrossRef CAS PubMed.
- B. Peng, L.-X. Zheng, P.-Y. Wang, J.-F. Zhou, M. Ding, H.-D. Sun, B.-Q. Shan and K. Zhang, Front. Chem., 2021, 9, 756993 CrossRef CAS.
- S. Havenridge and C. M. Aikens, J. Chem. Phys., 2021, 155, 074302 CrossRef CAS PubMed.
- Z. Wei, Y. Pan, G. Hou, X. Ran, Z. Chi, Y. He, Y. Kuang, X. Wang, R. Liu and L. Guo, ACS Appl. Mater. Interfaces, 2022, 14, 2452–2463 CrossRef CAS PubMed.
- A. Pniakowska and J. O. Banska, Molecules, 2022, 27, 807 CrossRef CAS PubMed.
- M. Wang, B. Duan, Y. Li, S. Jiang, Z. Huang and W. Yang, ACS Appl. Nano Mater., 2021, 4, 7486–7492 CrossRef CAS.
- D. Cheng, R. Liu, L. Tian, Q. Zhou, F. Niu, Y. Yue and K. Hu, ACS Appl. Nano Mater., 2021, 4, 13413–13424 CrossRef CAS.
- A. R. Ziefuss, T. Steenbock, D. Benner, A. Plech, J. Gottlicher, M. Teubner, B. Grimm-Lebsanft, C. Rehbock, C. C. Zerbino, R. Antoine, D. Amans, I. Chakraborty, G. Bester, M. Nachev, B. Sures, M. Rubhausen, W. J. Parak and S. Barcikowski, Adv. Mater., 2021, 33, 2101549 CrossRef CAS PubMed.
- J. Valenta, M. Greben, G. Pramanik, K. Kvakova and P. Cigler, Phys. Chem. Chem. Phys., 2021, 23, 11954–11960 RSC.
- J. Liu, Y. Yu, C. Wang, J. Shen, J. Feng and W. Qi, Chem. Commun., 2021, 57, 10202–10205 RSC.
- B. Casteleiro, J. M. G. Martinho and J. P. S. Farinha, Nanoscale, 2021, 13, 17199–17217 RSC.
- V. Hynninen, S. Chandra, S. Das, M. Amini, Y. Dai, S. Lepikko, P. Mohammadi, S. Hietala, R. H. A. Ras, Z. Sun, O. Ikkala and Nonappa, Small, 2021, 17, 2005205 CrossRef CAS PubMed.
- S. M. Saleh, M. K. Almotiri and R. Ali, J. Photochem. Photobiol., A, 2022, 426, 113719 CrossRef CAS.
- C. Kong, Y. Luo, W. Zhang, T. Lin, Z. Na, X. Liu and Z. Xie, RSC Adv., 2022, 12, 12060–12067 RSC.
- J. Shen, Q. Xiao, P. Sun, J. Feng, X. Xin, Y. Yu and W. Qi, ACS Nano, 2021, 15, 4947–4955 CrossRef CAS PubMed.
- L. Li, T. Yang, J. Yang and X. Zhang, Sens. Actuators, B, 2022, 353, 131038 CrossRef CAS.
- H. Chen, Y. Chang, R. Wei and P. Zhang, Anal. Methods, 2022, 14, 1439–1444 RSC.
- S. Li, Q. Ma, C. Wang, K. Yang, Z. Hong, Q. Chen, J. Song, X. Song and H. Yang, Anal. Chem., 2022, 94, 2641–2647 CrossRef CAS PubMed.
- S. Dolui, S. Basu and A. Paul, Mater. Adv., 2022, 3, 3286–3292 RSC.
- Y. Pan, X. Wei, X. Guo, H. Wang, H. Song, C. Pan and N. Xu, Biosens. Bioelectron., 2021, 194, 113611 CrossRef CAS PubMed.
- B. Fu, X. Zheng, H. Li, L. Ding, F. Wang, D.-Y. Guo, W. Yang and Q. Pan, Sens. Actuators, B, 2021, 346, 130502 CrossRef CAS.
- M. Wang, X. Zhou, L. Cheng, M. Wang and X. Su, ACS Appl. Nano Mater., 2021, 4, 9265–9273 CrossRef CAS.
- Z. Zhou, T. Shu, Y. Sun, H. Si, P. Peng, L. Su and X. Zhang, Biosens. Bioelectron., 2021, 192, 113530 CrossRef CAS PubMed.
- Y. Sun, T. Shu, J. Ma, Q. Dai, P. Peng, Z. Zhou, X. Zhou, L. Su and X. Zhang, Anal. Chem., 2022, 94, 3408–3417 CrossRef CAS PubMed.
- Y. Tao, K. Yi, H. Wang, K. Li and M. Li, Sens. Actuators, B, 2022, 361, 131711 CrossRef CAS.
- M. Xie, Y. Wang, L. Liu, X. Wang and H. Jiang, J. Colloid Interface Sci., 2022, 614, 502–510 CrossRef CAS PubMed.
- Y. Cong, X. Wang, S. Zhu, L. Liu and L. Li, ACS Appl. Bio Mater., 2021, 4, 2790–2797 CrossRef CAS PubMed.
- Z. Li, H. Peng, J. Liu, Y. Tian, W. Yang, J. Yao, Z. Shao and X. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 83–90 CrossRef CAS PubMed.
- F. Yu, Z. Cao, S. He, H. Xiang, G. Zhao, L. Yang and H. Liu, Chem. Commun., 2022, 58, 811–814 RSC.
- Y.-F. Kang, B. Zheng, C.-Y. Li, Z.-L. Zhang, H.-W. Tang, Q.-S. Wu and D.-W. Pang, Anal. Chem., 2020, 92, 1292–1300 CrossRef CAS PubMed.
- S. Zhai, W. Hu, C. Fan, W. Feng and Z. Liu, Chem. Commun., 2021, 57, 5542–5545 RSC.
- Y.-T. Yen, Y.-J. Chang, Y.-T. Tseng, C.-Y. Chen, Y.-L. Liu and H.-T. Chang, Sens. Actuators, B, 2022, 353, 131151 CrossRef CAS.
- J. Liang, H. Xiong, W. Wang, W. Wen, X. Zhang and S. Wang, Sens. Actuators, B, 2018, 255, 2170–2178 CrossRef CAS.
- H. Zhu, N. Liu, Z. Wang, Q. Xue, Q. Wang, X. Wang, Y. Liu, Z. Yin and X. Yuan, Nanoscale, 2021, 13, 18996–19003 RSC.
- J. Yang, Y. Peng, S. Li, J. Mu, Z. Huang, J. Ma, Z. Shi and Q. Jia, Coord. Chem. Rev., 2022, 456, 214391 CrossRef CAS.
- I. M. Khan, S. Niazi, L. Yue, Y. Zhang, I. Pasha, M. K. I. Khan, W. Akhtar, A. Mohsin, M. F. J. Chughati and Z. Wang, Talanta, 2022, 241, 123228 CrossRef CAS PubMed.
- S. Qian, Z. Wang, Z. Zuo, X. Wang, Q. Wang and X. Yuan, Coord. Chem. Rev., 2022, 451, 214268 CrossRef CAS.
- H. Shen, C. Xu, F. Sun, M. Zhao, Q. Wu, J. Zhang, S. Li, J. Zhang, J. W. Y. Lam and B. Z. Tang, ChemMedChem, 2022, 17, e202100578 CrossRef CAS PubMed.
- M.-M. Zhang, X.-Y. Dong, Y.-J. Wang, S.-Q. Zang and T. C. W. Mak, Coord. Chem. Rev., 2022, 453, 214315 CrossRef CAS.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Aggregate, 2021, 2, 114–132 CrossRef.
- S. Bothra and S. K. Sahoo, Sensing and Biosensing with Optically Active Nanomaterials, ed. S. K. Sahoo, Elsevier, 1st edn, 2022, pp. 207–242 Search PubMed.
- L. Zhao, Y. Zhou, G. Niu, F. Gao, Z. Sun, H. Li and Y. Jiang, Part. Part. Syst. Charact., 2022, 2100231 CrossRef CAS.
- S. Zhan, J. Jiang, Z. Zeng, Y. Wang and H. Cui, Coord. Chem. Rev., 2022, 455, 214381 CrossRef CAS.
- M. T. Nguyen, L. Deng and T. Yonezawa, Soft Matter, 2022, 18, 19–47 RSC.
- D. Ungor, Á. Juhász, N. Varga and E. Csapó, Adv. Colloid Interface Sci., 2022, 301, 102616 CrossRef CAS PubMed.
- A. Gonzàlez-Rosell, C. Cerretani, P. Mastracco, T. Vosch and S. M. Copp, Nanoscale Adv., 2021, 3, 1230–1260 RSC.
- I. Zare, D. M. Chevrier, A. Cifuentes-Rius, N. Moradi, Y. Xianyu, S. Ghosh, L. Trapiella-Alfonso, Y. Tian, A. Shourangiz-Haghighi, S. Mukherjee, K. Fan and M. R. Hamblin, Mater. Today, 2021 DOI:10.1016/j.mattod.2020.10.027.
- M. Sharifi, S. H. Hosseinali, R. H. Alizadeh, A. Hasan, F. Attar, A. Salihi, M. S. Shekha, K. M. Amen, F. M. Aziz, A. A. Saboury, K. Akhtari, A. Taghizadeh, N. Hooshmand, M. A. El-Sayed and M. Falahati, Talanta, 2020, 212, 120782 CrossRef CAS PubMed.
- L. Wang, M. H. Kafshgari and M. Meunier, Adv. Funct. Mater., 2020, 30, 2005400 CrossRef CAS.
- N. Sarfraz and I. Khan, Chem. – Asian J., 2021, 16, 720–742 CrossRef CAS PubMed.
- H. Cui, Z.-S. Shao, Z. Song, Y.-B. Wang and H.-S. Wang, J. Mater. Chem. C, 2020, 8, 14312–14333 RSC.
- T.-Q. Yang, B. Peng, B.-Q. Shan, Y.-X. Zong, J.-G. Jiang, P. Wu and K. Zhang, Nanomaterials, 2020, 10, 261 CrossRef CAS PubMed.
- M. Z. Iqbal, I. Ali, W. S. Khan, X. Kong and E. Dempsey, Mater. Des., 2021, 205, 109694 CrossRef.
- A. Mooradian, Phys. Rev. Lett., 1969, 22, 185–187 CrossRef CAS.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Aggregate, 2021, 2, 114–132 CrossRef.
- S. Link, A. Beeby, S. FitzGerald, M. A. El-Sayed, T. G. Schaaff and R. L. Whetten, J. Phys. Chem. B, 2002, 106, 3410–3415 CrossRef CAS.
- E. S. Shibu, M. A. H. Muhammed, T. Tsukuda and T. Pradeep, J. Phys. Chem. C, 2008, 112, 12168–12176 CrossRef CAS.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS PubMed.
- N. Goswami, Q. Yao, Z. Luo, J. Li, T. Chen and J. Xie, J. Phys. Chem. Lett., 2016, 7, 962–975 CrossRef CAS PubMed.
- R. Antoine and V. Bonačić-Koutecký, Liganded silver and gold quantum clusters. Towards a new class of nonlinear optical nanomaterials, Springer Nature, Cham, Switzerland, 2018, pp. 11–12 Search PubMed.
- G. Wang, T. Huang, R. W. Murray, L. Menard and R. G. Nuzzo, J. Am. Chem. Soc., 2005, 127, 812–813 CrossRef CAS PubMed.
- G. Wang, R. Guo, G. Kalyuzhny, J.-P. Choi and R. W. Murray, J. Phys. Chem. B, 2006, 110, 20282–20289 CrossRef CAS PubMed.
- P. Londoño-Larrea, J. P. Vanegas, D. Cuaran-Acosta, E. Zaballos-García and J. Pérez-Prieto, Chem. – Eur. J., 2017, 23, 8137–8141 CrossRef PubMed.
- K. L. Dimuthu, M. Weerawardene and C. M. Aikens, J. Am. Chem. Soc., 2016, 138, 11202–11210 CrossRef PubMed.
- M. Zhou and Y. Song, J. Phys. Chem. Lett., 2021, 12, 1514–1519 CrossRef CAS PubMed.
- D. Chen and J. Li, Nanoscale Horiz., 2020, 5, 1355–1367 RSC.
- L. Zhu, Y. Zeng, M. Teubner, B. Grimm-Lebsanft, A. R. Ziefuß, C. Rehbock, M. A. Rübhausen, S. Barcikowski, W. J. Parak and I. Chakraborty, ACS Appl. Nano Mater., 2021, 4, 3197–3203 CrossRef CAS.
- S. Kundu, B. Ghosh, S. Nandi, M. Ghosh, A. Pyne, J. Chatterjee and N. Sarkar, ACS Appl. Bio Mater., 2020, 3, 4282–4293 CrossRef CAS PubMed.
- J.-U. Kim, S.-H. Cha, K. Shin, J. Y. Jho and J.-C. Lee, J. Am. Chem. Soc., 2005, 127, 9962–9963 CrossRef CAS PubMed.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed.
- N. Goswami, F. Lin, Y. Liu, D. T. Leong and J. Xie, Chem. Mater., 2016, 28, 4009–4016 CrossRef CAS.
- H. Chang, N. S. Karan, K. Shin, M. S. Bootharaju, S. Nah, S. I. Chae, W. Baek, S. Lee, J. Kim, Y. J. Son, T. Kang, G. Ko, S.-H. Kwon and T. Hyeon, J. Am. Chem. Soc., 2021, 143, 326–334 CrossRef CAS PubMed.
- A. J. Moro, J. Avó, M. Malfois, F. Zaccaria, C. F. Guerra, F. J. Caparrós, L. Rodríguez and J. C. Lima, Dalton Trans., 2020, 49, 171–178 RSC.
- W. Sun, L. Luo, Y. Feng, Y. Cai, Y. Zhuang, R.-J. Xie, X. Chen and H. Chen, Angew. Chem., Int. Ed., 2020, 59, 9914–9921 CrossRef CAS PubMed.
- Y. Hua, Y. Wang, X. Kang, F. Xu, Z. Han, C. Zhang, Z.-Y. Wang, J.-Q. Liu, X. Zhao, X. Chen and S.-Q. Zang, J. Nanobiotechnol., 2021, 19, 1–14 CrossRef PubMed.
- H. Zou, J. Zhang, C. Wu, B. He, Y. Hu, H. H. Y. Sung, R. T. K. Kwok, J. W. Y. Lam, L. Zheng and B. Z. Tang, ACS Nano, 2021, 15, 9176–9185 CrossRef CAS PubMed.
- T. Pan, T. Zhou, Y. Tu and J. Yan, Talanta, 2021, 227, 122197 CrossRef CAS PubMed.
- Y. Zi, D. Xu, C. Li, F. Qu and X.-E. Zhao, Sens. Actuators, B, 2021, 345, 130243 CrossRef CAS.
- Y. Li, S. Teng, M. Wang, B. Duan and Z. Huang, Sens. Actuators, B, 2021, 330, 129290 CrossRef CAS.
- J. M. Carnerero, A. Jimenez-Ruiz, P. M. Castillo and R. Prado-Gotor, ChemPhysChem, 2017, 18, 17–33 CrossRef CAS PubMed.
- D. Bera and N. Goswami, J. Phys. Chem. Lett., 2021, 12, 9033–9046 CrossRef CAS PubMed.
- C. Zhou, C. Sun, M. Yu, Y. Qin, J. Wang, M. Kim and J. Zheng, J. Phys. Chem. C, 2010, 114, 7727–7732 CrossRef CAS PubMed.
- L. Li, Z. Li, H. Zhang, S. Zhang, I. Majeed and B. Tan, Nanoscale, 2013, 5, 1986–1992 RSC.
- S. Palmal, S. Basiruddin, A. R. Maity, S. C. Ray and N. R. Jana, Chem. – Eur. J., 2013, 19, 943–949 CrossRef CAS PubMed.
- X. Wen, P. Yu, Y.-R. Toh, A.-C. Hsu, Y.-C. Lee and J. Tang, J. Phys. Chem. C, 2012, 116, 19032–19038 CrossRef CAS.
- J. K. Sahu, S. A. Lone and K. K. Sadhu, Langmuir, 2022, 38, 5865–5873 CrossRef CAS PubMed.
- D. Bain, S. Maity and A. Patra, Chem. Commun., 2020, 56, 9292–9295 RSC.
- S. Chakraborty, D. Bain, S. Maity, S. Kolay and A. Patra, J. Phys. Chem. C, 2022, 126, 2896–2904 CrossRef CAS.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- K. Nobusada and T. Iwasa, J. Phys. Chem. C, 2007, 111, 14279–14282 CrossRef CAS.
- M. Y. Sfeir, H. Qian, K. Nobusada and R. Jin, J. Phys. Chem. C, 2011, 115, 6200–6207 CrossRef CAS.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A. Emwas, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- X.-Y. Xie, P. Xiao, X. Cao, W.-H. Fang, G. Cui and M. Dolg, Angew. Chem., Int. Ed., 2018, 57, 9965–9969 CrossRef CAS PubMed.
- R. Galassi, M. M. Ghimire, B. M. Otten, S. Riccia, R. N. M. Jr, R. M. Almotawa, D. Alhmoud, J. F. Ivy, A.-M. M. Rawashdeh, V. N. Nesterov, E. W. Reinheimer, L. M. Daniels, A. Burinia and M. A. Omary, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E5042–E5051 CrossRef CAS PubMed.
- D. Wang, R. Cai, S. Sharma, J. Jirak, S. K. Thummanapelli, N. G. Akhmedov, H. Zhang, X. Liu, J. L. Petersen and X. Shi, J. Am. Chem. Soc., 2012, 134, 9012–9019 CrossRef CAS PubMed.
- J. S. Mohanty, P. L. Xavier, K. Chaudhari, M. S. Bootharaju, N. Goswami, S. K. Pal and T. Pradeep, Nanoscale, 2012, 4, 4255–4262 RSC.
- N. Zhang, Y. Si, Z. Sun, L. Chen, R. Li, Y. Qiao and H. Wang, Anal. Chem., 2014, 86, 11714–11721 CrossRef CAS PubMed.
- B. Zhenga, J. Zhenga, T. Yua, A. Sanga, J. Dua, Y. Guoa, D. Xiaoa and M. M. F. Choi, Sens. Actuators, B, 2015, 221, 386–392 CrossRef.
- Q. Yuan, X. Kang, D. Hu, C. Qin, S. Wang and M. Zhu, Dalton Trans., 2019, 48, 13190–13196 RSC.
- S. Basu, M. P. Bakulic, H. Fakhouri, I. Russier-Antoine, C. Moulin, P.-F. Brevet, V. Bonac-Koutecky and R. Antoine, J. Phys. Chem. C, 2020, 124, 19368–19374 CrossRef CAS.
- X. Hu, Y. Zhang, T. Ding, J. Liu and H. Zhao, Front. Bioeng. Biotechnol., 2020, 8, 990 CrossRef PubMed.
- Y. Li, T. Zhai, J. Chen, J. Shi, L. Wang, J. Shen and X. Liu, Chem. – Eur. J., 2022, 28, e202103736 CAS.
- H. Tada, Dalton Trans., 2022, 51, 3383–3393 RSC.
- I. Zare, M. T. Yaraki, G. Speranza, A. H. Najafabadi, A. Shourangiz-Haghighi, A. B. Nik, B. B. Manshian, C. Saraiva, S. J. Soenen, M. J. Kogan, J. W. Lee, N. V. Apollo, L. Bernardino, E. Araya, D. Mayer, G. Mao and M. R. Hamblin, Chem. Soc. Rev., 2022, 51, 2601–2680 RSC.
- R. Petrucci, M. Bortolami, P. D. Matteo and A. Curulli, Nanomaterials, 2022, 12, 959 CrossRef CAS PubMed.
- P. Yuan, R. Ma, N. Gao, M. Garai and Q.-H. Xu, Nanoscale, 2015, 7, 10233–10239 RSC.
- R. Kazan, B. Zhang and T. Bürgi, Dalton Trans., 2017, 46, 7708–7713 RSC.
- L. Bazán-Díaz, R. Mendoza-Cruz, J. J. Velázquez-Salazar, G. Plascencia-Villa, F. M. Ascencio-Aguirre, H. J. Ojeda-Galván, R. Herrera-Becerra, G. Guisbiers and M. José-Yacamán, Langmuir, 2018, 34, 9394–9401 CrossRef PubMed.
- Z. Chen, W. Ding, Y. Gu, S. Gao, D. Yun, C. Wang, W. Li and F. Sun, Langmuir, 2020, 36, 13928–13936 CrossRef CAS PubMed.
- S. Li, W. Tian and Y. Liu, Nanoscale, 2021, 13, 16847–16859 RSC.
- Y. Li, M. Zhou and R. Jin, Adv. Mater., 2021, 33, 2006591 CrossRef CAS PubMed.
- A. Cantelli, G. Guidetti, J. Manzi, V. Caponetti and M. Montalti, Eur. J. Inorg. Chem., 2017, 5068–5084 CrossRef CAS.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- X. Y. Wong, D. Quesada-González, S. Manickam, S. Y. New, K. Muthoosamy and A. Merkoçi, Sci. Rep., 2021, 11, 2375 CrossRef CAS PubMed.
- Q. Li, R. Zhou, Y. Sun, D. Xiao, M. Liu, D. Zhao, S. Peng, Y. Chen and Y. Lin, ACS Appl. Mater. Interfaces, 2021, 13, 11708–11720 CrossRef CAS PubMed.
- V. Jain, S. Bhagat and S. Singh, Sens. Actuators, B, 2021, 327, 128886 CrossRef CAS.
- L. Zhang, M. Zhang and Y. Wu, J. Mol. Struct., 2014, 1069, 245–250 CrossRef CAS.
- A.-M. Hada, A.-M. Craciun, M. Focsan, R. Borlan, O. Soritau, M. Todea and S. Astilean, Talanta, 2021, 225, 121960 CrossRef CAS PubMed.
- D. Ungor, A. Barbasz, A. Czyzowska, E. Csapó and M. Ocwieja, Colloids Surf., B, 2021, 200, 111593 CrossRef CAS PubMed.
- J. Li, C. Xiao, W. Wei, R. Xiao, H. Yao and H. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 36632–36643 CrossRef CAS PubMed.
- Y. Niu, T. Ding, J. Liu, G. Zhang, L. Tong, X. Cheng, Y. Yang, Z. Chen and B. Tang, Talanta, 2021, 223, 121745 CrossRef CAS PubMed.
- S. Chakraborty, A. Nandy, S. Ghosh, N. K. Das, S. Parveen, S. Datta and S. Mukherjee, Analyst, 2021, 146, 1455–1463 RSC.
- L. Yan, Y. Cai, B. Zheng, H. Yuan, Y. Guo, D. Xiao and M. M. F. Choi, J. Mater. Chem., 2012, 22, 1000–1005 RSC.
- Y. Tao, K. Yi, H. Hu, D. Shao and M. Li, J. Mater. Chem. B, 2021, 9, 94–100 RSC.
- Y. Zhang, H. Jiang and X. Wang, Anal. Chim. Acta, 2015, 870, 1–7 CrossRef CAS PubMed.
- P. S. Devi, S. Banerjee, S. R. Chowdhury and G. S. Kumar, RSC Adv., 2012, 2, 11578–11585 RSC.
- Y. Yu, Z. Luo, Y. Yu, J. Y. Lee and J. Xie, ACS Nano, 2012, 6, 7920–7927 CrossRef CAS PubMed.
- E. R. Gran, F. Bertorelle, H. Fakhouri, R. Antoine, M. P. Bakulić, Ž. S. Maršić, V. Bonačić-Koutecký, M. Blain, J. Antel and D. Maysinger, Nanoscale, 2021, 13, 3173–3183 RSC.
- L. Yang, B. Zhang, L. Fu, K. Fu and G. Zou, Angew. Chem., Int. Ed., 2019, 58, 6901–6905 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X.-D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- T. Pan, T. Zhou, Y. Tu and J. Yan, Talanta, 2021, 227, 122197 CrossRef CAS PubMed.
- F. Bertorelle, I. Russier-Antoine, N. Calin, C. Comby-Zerbino, A. Bensalah-Ledoux, S. Guy, P. Dugourd, P.-F. Brevet, Ž. Sanader, M. Krstić, V. Bonačić-Koutecký and R. Antoine, J. Phys. Chem. Lett., 2017, 8, 1979–1985 CrossRef CAS PubMed.
- I. Russier-Antoine, F. Bertorelle, M. Vojkovic, D. Rayane, E. Salmon, C. Jonin, P. Dugourd, R. Antoine and P.-F. Brevet, Nanoscale, 2014, 6, 13572–13578 RSC.
- F. Bertorelle, C. Moulin, A. Soleilhac, C. Comby-Zerbino, P. Dugourd, I. Russier-Antoine, P.-F. Brevet and R. Antoine, ChemPhysChem, 2018, 19, 165–168 CrossRef CAS PubMed.
- P. Huang, S. Li, N. Gao and F. Wu, Analyst, 2015, 140, 7313–7321 RSC.
- V. Venkatesh, A. Shukla, S. Sivakumar and S. Verma, ACS Appl. Mater. Interfaces, 2014, 6, 2185–2191 CrossRef CAS PubMed.
- C.-C. Huang, Z. Yang, K.-H. Lee and H.-T. Chang, Angew. Chem., Int. Ed., 2007, 46, 6824–6828 CrossRef CAS PubMed.
- J. Sun, J. Zhang and Y. Jin, J. Mater. Chem. C, 2013, 1, 138–143 RSC.
- F. Aldeek, M. A. H. Muhammed, G. Palui, N. Zhan and H. Mattoussi, ACS Nano, 2013, 7, 2509–2521 CrossRef CAS PubMed.
- Y. Li, S. Yi, Z. Lei and Y. Xiao, RSC Adv., 2021, 11, 14678–14685 RSC.
- Z. Lei, J.-J. Li, Z.-A. Nan, Z.-G. Jiang and Q.-M. Wang, Angew. Chem., Int. Ed., 2021, 60, 14415–14419 CrossRef CAS PubMed.
- J. Wang, Z.-Y. Wang, S.-J. Li, S.-Q. Zang and T. C. W. Mak, Angew. Chem., Int. Ed., 2021, 60, 5959–5964 CrossRef CAS PubMed.
- X.-K. Wan, W. W. Xu, S.-F. Yuan, Y. Gao, X.-C. Zeng and Q.-M. Wang, Angew. Chem., Int. Ed., 2015, 54, 9683–9686 CrossRef CAS PubMed.
- X.-S. Han, X. Luan, H.-F. Su, J.-J. Li, S.-F. Yuan, Z. Lei, Y. Pei and Q.-M. Wang, Angew. Chem., Int. Ed., 2020, 59, 2309–2312 CrossRef CAS PubMed.
- K. Pyo, N. H. Ly, S. Y. Yoon, Y. Shen, S. Y. Choi, S. Y. Lee, S.-W. Joo and D. Lee, Adv. Healthcare Mater., 2017, 6, 1700203 CrossRef PubMed.
- L. Kacenauskaite, N. Bisballe, R. Mucci, M. Santella, T. Pullerits, J. Chen, T. Vosch and B. W. Laursen, J. Am. Chem. Soc., 2021, 143, 1377–1385 CrossRef CAS PubMed.
- D. J. Lewis, T. M. Day, J. V. MacPherson and Z. Pikramenou, Chem. Commun., 2006, 1433–1435 RSC.
- C.-Z. Li, K. B. Male, S. Hrapovic and J. H. T. Luong, Chem. Commun., 2005, 3924–3926 RSC.
- H. He, C. Xie and J. Ren, Anal. Chem., 2008, 80, 5951–5957 CrossRef CAS PubMed.
- G. F. Walsh and L. D. Negro, Nano Lett., 2013, 13, 786–792 CrossRef CAS PubMed.
- M. Saini, Y. Masirkar, R. Varshney, P. Roy and K. K. Sadhu, Chem. Commun., 2017, 53, 6199–6202 RSC.
- M. Saini, A. Verma, K. Tomar, P. K. Bharadwaj and K. K. Sadhu, Chem. Commun., 2018, 54, 12836–12839 RSC.
- K. Bharti, S. A. Lone, A. Singh, S. Nathani, P. Roy and K. K. Sadhu, Front. Chem., 2021, 9, 639090 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- X. Yuan, X. Dou, K. Zheng and J. Xie, Part. Part. Syst. Charact., 2015, 32, 613–629 CrossRef.
- M. Linden, A. J. Bunningen, L. Amidani, M. Bransen, H. Elnaggar, P. Glatzel, A. Meijerink and F. M. F. Groot, ACS Nano, 2018, 12, 12751–12760 CrossRef PubMed.
- A. Sannigrahi, S. Chowdhury, I. Nandi, D. Sanyal, S. Chall and K. Chattopadhyay, Nanoscale Adv., 2019, 1, 3660–3669 RSC.
- X. Qu, Y. Li, L. Li, Y. Wang, J. Liang and J. Liang, J. Nanomater., 2015, 2015, 1–23 Search PubMed.
- S. L. Fereja, P. Li, J. Guo, Z. Fang, Z. Zhang, Z. Zhuang, X. Zhang, K. Liu and W. Chen, Talanta, 2021, 233, 122469 CrossRef CAS PubMed.
- Q. Li, L. Li, L. Chen, C. Wang, C. Li, K. Li and Y. Lin, J. Nanosci. Nanotechnol., 2020, 20, 692–700 CrossRef CAS PubMed.
- R. Dai, W. Deng, P. Hu, C. You, L. Yang, X. Jiang, X. Xiong and K. Huang, Microchem. J., 2018, 139, 1–8 CrossRef CAS.
- B. Zheng, J. Zheng, T. Yu, A. Sang, J. Du, Y. Guo, D. Xiao and M. M. F. Choi, Sens. Actuators, B, 2015, 221, 386–392 CrossRef CAS.
- H. Huang, H. Li, J.-J. Feng and A.-J. Wang, Sens. Actuators, B, 2016, 223, 550–556 CrossRef CAS.
- D. Hikosou, S. Saita, S. Miyata, H. Miyaji, T. Furuike, H. Tamura and H. Kawasaki, J. Phys. Chem. C, 2018, 122, 12494–12501 CrossRef CAS.
- K. Brach, M. Waszkielewicz, J. Olesiak-Banska, M. Samoc and K. Matczyszyn, Langmuir, 2017, 33, 8993–8999 CrossRef CAS PubMed.
- T. Ye and X. An, New J. Chem., 2019, 43, 569–572 RSC.
- M. Liu, N. Li, Y. He, Y. Ge and G. Song, Microchim. Acta, 2018, 185, 147 CrossRef PubMed.
- Z. Yin, Z. Wang, X. Dai, N. Liu, S. Wang, G. Li, F. Du and X. Yuan, ACS Sustainable Chem. Eng., 2020, 8, 15336–15343 CrossRef CAS.
- R. Kobayashi, Y. Nonoguchi, A. Sasaki and H. Yao, J. Phys. Chem. C, 2014, 118, 15506–15515 CrossRef CAS.
- T. Udayabhaskararao, Y. Sun, N. Goswami, S. K. Pal, K. Balasubramanian and T. Pradeep, Angew. Chem., Int. Ed., 2012, 51, 2155–2159 CrossRef CAS PubMed.
- J. Sun, H. Wuab and Y. Jin, Nanoscale, 2014, 6, 5449–5457 RSC.
- Y. Yang, Y. Sun, S. Liao, Z. Wu and R. Yu, Anal. Methods, 2016, 8, 7237–7241 RSC.
- S. Ristig, D. Kozlova, W. Meyer-Zaika and M. Epple, J. Mater. Chem. B, 2014, 2, 7887–7895 RSC.
- R. D. Corpuz, Y. Ishida, M. T. Nguyen and T. Yonezawa, Langmuir, 2017, 33, 9144–9150 CrossRef CAS PubMed.
- F. Yu, P. Luo, Y. Chen, H. Jiang and X. Wang, Anal. Methods, 2021, 13, 2575–2585 RSC.
- Q. Liu, X. Yan, Q. Lai and X. Su, Sens. Actuators, B, 2019, 282, 45–51 CrossRef CAS.
- W.-Y. Chen, G.-Y. Lan and H.-T. Chang, Anal. Chem., 2011, 83, 9450–9455 CrossRef CAS PubMed.
- S. Liu and S. Pang, Microchim. Acta, 2018, 185, 426 CrossRef PubMed.
- T. Li, H. Yi, Y. Liu, Z. Wang, S. Liu, N. He, H. Liu and Y. Deng, J. Biomed. Nanotechnol., 2018, 14, 150–160 CrossRef CAS PubMed.
- Y. Zhang, H. Jiang, W. Ge, Q. Li and X. Wang, Langmuir, 2014, 30, 10910–10917 CrossRef CAS PubMed.
- J. Liu, X.-X. Yuan, H.-W. Li and Y. Wu, J. Mater. Chem. C, 2017, 5, 9979–9985 RSC.
- Z. Suo, X. Hou, J. Chen, X. Liu, Y. Liu, F. Xing, Y. Chen and L. Feng, J. Phys. Chem. C, 2020, 124, 21094–21102 CrossRef CAS.
- S. Pang and S. Liu, Anal. Methods, 2017, 9, 6713–6718 RSC.
- F. Meng, F. Gan and G. Ye, Microchim. Acta, 2019, 186, 371 CrossRef PubMed.
- L. Fu, X. Gao, S. Dong, H.-Y. Hsu and G. Zou, Anal. Chem., 2021, 93, 4909–4915 CrossRef CAS PubMed.
- L. Tian, Y. Li, T. Ren, Y. Tong, B. Yang and Y. Li, Talanta, 2017, 170, 530–539 CrossRef CAS PubMed.
- S. Pramanik, A. Saha and P. Sujatha Devi, RSC Adv., 2015, 5, 33946–33954 RSC.
- W. Wu, T. Zhou and S. Zhou, Chem. Mater., 2009, 21, 2851–2861 CrossRef CAS.
- R. Contreras-Caceres, P. Alonso-Cristobal, D. Mendez-Gonzalez, M. Laurenti, A. Maldonado-Valdivia, F. Garcia-Blanco, E. L. Cabarcos, A. Fernandez-Barbero, J. Lopez-Romero and J. Rubio-Retama, Langmuir, 2014, 30, 15560–15567 CrossRef CAS PubMed.
- L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. D. Gobbo, R. G. AbdulHalim, M. Eddaoudi, D. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970–11975 CrossRef CAS PubMed.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922–926 CrossRef CAS PubMed.
- C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578–11581 CrossRef CAS PubMed.
- M. Linden, A. Barendregt, A. J. Bunningen, P. T. K. Chin, D. Thies-Weesie, F. M. F. Groot and A. Meijerink, Nanoscale, 2016, 8, 19901–19909 RSC.
- C. M. Andolina, A. C. Dewar, A. M. Smith, L. E. Marbella, M. J. Hartmann and J. E. Millstone, J. Am. Chem. Soc., 2013, 135, 5266–5269 CrossRef CAS PubMed.
- P.-C. Chen, J.-Y. Ma, L.-Y. Chen, G.-L. Lin, C.-C. Shih, T.-Y. Lin and H.-T. Chang, Nanoscale, 2014, 6, 3503–3507 RSC.
- S. Sculfort and P. Braunstein, Chem. Soc. Rev., 2011, 40, 2741–2760 RSC.
- X. Kang, S. Wang, Y. Song, S. Jin, G. Sun, H. Yu and M. Zhu, Angew. Chem., Int. Ed., 2016, 55, 3611–3614 CrossRef CAS PubMed.
- S. Wang, Y. Song, S. Jin, X. Liu, J. Zhang, Y. Pei, X. Meng, M. Chen, P. Li and M. Zhu, J. Am. Chem. Soc., 2015, 137, 4018–4021 CrossRef CAS PubMed.
- F. Nie, L. Ga, J. Ai and Y. Wang, RSC Adv., 2018, 8, 13708–13713 RSC.
- E. Mahmoudi, A. Hajian, M. Rezaei, A. Afkhami, A. Amine and H. Bagheri, Microchem. J., 2019, 145, 242–251 CrossRef CAS.
- C.-X. Zhang, Y.-C. Gao, H.-W. Li and Y. Wu, ACS Appl. Nano Mater., 2020, 3, 9318–9328 CrossRef CAS.
- Y.-C. Gao, C. Wang, C.-X. Zhang, H.-W. Li and Y. Wu, Microchim. Acta, 2021, 188, 50 CrossRef CAS PubMed.
- V. G. Deepagan, M. N. Leiske, N. L. Fletcher, D. Rudd, T. Tieu, N. Kirkwood, K. J. Thurecht, K. Kempe, N. H. Voelcker and A. Cifuentes-Rius, Nano Lett., 2021, 21, 476–484 CrossRef CAS PubMed.
- X.-Y. Wang, J. Zhang, J. Yin, S. H. Liu and B. Z. Tang, Mater. Chem. Front., 2021, 5, 368–374 RSC.
- Q. Li, C. J. Zeman, G. C. Schatz and X. W. Gu, ACS Nano, 2021, 15, 16095–16105 CrossRef CAS PubMed.
- R. Liu, L. Bao, S. Zhang, Z. Wu, J. Zhou, C. Liu and R. Yu, J. Mater. Chem. B, 2020, 8, 11001–11009 RSC.
- M. A. Abbas, P. V. Kamat and J. H. Bang, ACS Energy Lett., 2018, 3, 840–854 CrossRef CAS.
- K. Bharti and K. K. Sadhu, Results Chem., 2022, 4, 100288 CrossRef CAS.
- Y. Zhang, N. Feng, S. Zhou and X. Xin, Nanoscale, 2021, 13, 4140–4150 RSC.
Footnote |
| † Both the authors contributed equally in this review. |
| This journal is © The Royal Society of Chemistry 2022 |