Open Access Article
Open Access ArticleFunctional electrospun polymeric materials for bioelectronic devices: a review
Sushmita
Majumder
ab,
Md Mehadi Hassan
Sagor
c and
M Tarik
Arafat
 *c
*c
aDepartment of Chemical Engineering and Materials Science, University of Minnesota, Twin Cities, USA
bDepartment of Materials and Metallurgical Engineering, Bangladesh University of Engineering and Technology (BUET), Dhaka 1200, Bangladesh
cDepartment of Biomedical Engineering, Bangladesh University of Engineering and Technology (BUET), Dhaka 1205, Bangladesh. E-mail: tarikarafat@bme.buet.ac.bd; Tel: +880255167100 ext. 6133
First published on 20th July 2022
Abstract
Bioelectronics has excellent potential to enhance the quality of life. It works by interfacing biomaterials with electronic devices, which are then applied to the monitoring, diagnosis, and treatment of the in vivo and in vitro conditions of the human body. On this account, we have explored numerous works on polymer-based bioelectronics at our disposal to adopt a facile fabrication method – electrospinning. Bioelectronic devices typically encompass biosensors, which detect biological analytes resulting in the detection of biomarkers, and bio-batteries which are able to supply non-intermittent micropower required for operating devices. On the other hand, energy harvesting devices, such as biofuel cells and nanogenerators, have the potential to generate energy. Bio-transistors and bio-actuators are used in flexible, wearable, and portable bioelectronics for human health assessment and effective artificial locomotion. Electrospun polymeric materials have shown advantages such as generating convenient microstructures, improved functionalization, and low manufacturing costs over the last few years. Initially, this article provides an overview of the unique structure and features of electrospun nanofiber materials and their application in bioelectronics. Then, it summarizes the recent progress along with future recommendations to conclude. Thus, this study examines the potential of electrospun polymers in bioelectronics by systematically discussing the fabrication and notable improvement of the devices in medical applications.
1. Introduction
Bioelectronics implies the integration of electronic components with biomaterials intending to uplift the quality of health, environmental state, and security of society. Over the years, extensive research in the field of bioelectronics has led to the development of medical devices, such as glucose monitors for people with diabetes,1,2 cardiac pacemakers, deep-brain stimulators,3,4 and cochlear implants for restoring damaged physiological functions, and profound insight on cell-to-cell and cell-to-environment interactions.5,6 It is interesting to observe how different biodevices’ sizes, shapes, and compositions have evolved to integrate flexibly with human life. For instance, in the past, bioelectronic materials were rigid and had poor compatibility with the human body interface. This hindrance has been overcome by developing flexible biodevices that offer facile and intimate physiological contact with soft organs and tissues.7Since the application of bioelectronic devices is directed toward human health monitoring and involves both in vitro and in vivo analyses, it is of the utmost importance to validate the biocompatible features of the components. Bioelectronic devices have enormously contributed to medical advancement, yet their rigid and bulky structures need downsizing and more biological properties. Furthermore, due to their stiffness, the conventional metallic bioelectronic components fail to match the mechanical similarity to the soft body tissues. Hence, they may render damage to the skin or delicate organs by abrasion.8 Also, bioelectronics should be chemically inert and non-reactive upon contact with the body fluids to avoid delamination or corrosion.9 Usually, the bio-device implanted into the living body is intended to disintegrate after serving its desired purpose. Hence, the biodegradability of the device will impair the body of any second injury during its extraction or removal from the body.10
To fully satisfy the biomedical applications, research has been extended towards developing next-generation functional bioelectronic devices that comply with the abovementioned properties. Polymers, among other materials, have an edge in biocompatibility, flexibility, lightweight, cost-effectiveness, and biodegradability to build bioelectronic components, as shown in Fig. 1. Moreover, the functionality of the polymer structure required for bioelectronic mechanisms can be induced when they get down to micro/nanometers. Electrospinning is a versatile technique to fabricate diverse nanostructures such as nanofibers, hollow or porous configurations, and core–sheath structures. Additionally, electrospun nanostructures may be built into ordered arrays by altering their alignment and stacking order to produce geometries appropriate for interfaces with living organisms.11
This article reviews the electrospinning technique of polymeric materials in fabricating functional bioelectronic devices, such as bio-based sensors, transistors, actuators, capacitors, batteries, and fuel cells. Electrospun polymeric structures have greatly improved the functioning and economic viability of bioelectronic devices. This study will emphasize our developing capacity to integrate functional polymeric structures with electronic materials and enhance our understanding of bioelectronics processing and manufacturing to address various global concerns.
2. Polymers for bioelectronics
Polymers offer a range of diverse properties, being the basis for bioelectronics structure and property design.12 The properties typically investigated for designing bioelectronics include flexibility/stretchability, chemical stability, modulus and stiffness comparable to human tissue and organs, and high conductivity with retaining the elastic properties. The variety and tunability of polymeric materials enable them to be flexible while maintaining enough mechanical strength and electrical conductivity for electrodes, optical transparency for substrates, and biodegradability for sacrificial layers in bioelectronic devices.13,14Commonly used electrode materials include polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT), and polypyrrole (PPy).15–17 Polymers like polydimethylsiloxane, poly(xylylene), and polyimide (PI) act as moisture and dielectric barriers by providing insulation to electronic devices to protect them from degrading upon contact with the body fluids.18–20 Polydimethylsiloxane and polyurethane (PU) are notable materials used in implanted devices to maintain structural integrity and insulate electrical cabling. These polymers offer biocompatibility, chemical and thermal stability, and desirable mechanical and elastomeric properties.21–24
Because of its abundance, biocompatibility, and processability, cellulose is a potential natural polysaccharide that has been widely used in the fabrication of bioelectronics. It possesses admirable properties of hydrophilicity, chirality, biodegradability, and capacity for various chemical modifications. Moreover, cellulose can be chemically modified to its derivative, such as cellulose acetate (CA), which is used as conducting polymers in biosensors and biobatteries.25,26 The chemical functionalization of cellulose and its derivatives is accomplished by breaking the native hydrogen bond network and adding new, minimally processed substituent chemical groups.27 Cellulose-based matrices are ideal for adsorption and immobilization of biological components, giving rise to a suitable polymeric substrate for biosensor applications.28,29
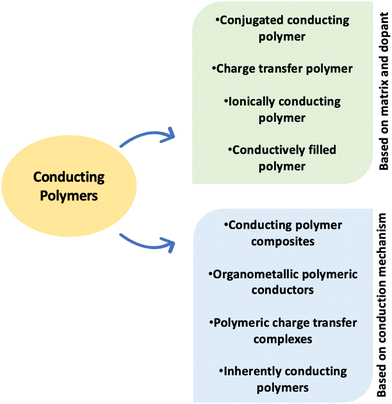
Because of the intricacy of the biological–material interface, there is a strong push to develop sophisticated functional polymeric biomaterials with modified physiochemical and mechanical properties for a variety of bio-applications. Thus, the next level of sophistication for medical devices and implants is achieved by integrating electronic components with the biological medium and analyzing electrical signals. Such devices include cardiac pacemakers, bionic ears, and vagus nerve stimulators.30–32 In recent times, inherently conducting polymers (ICPs), shown in Fig. 2, have been making a mark for bionic devices. ICPs can provide structural, bioactive, and electronic communication, and they are used to generate seamless integration of polymer structures that will take biocommunications to a higher level.
Furthermore, ICPs can be designed to trigger a wide range of biological functions, such as the controlled release of therapeutic drugs and growth factors and the delivery of electrical and mechanical stimuli to the cells and tissues.33–35 Polyacetylene, PANI, PPy, polythiophene, and polyphenylene are the most common ICPs. Many polymeric materials have been tried as working materials in a wide range of bioelectronic devices during the last few decades, with the promise that their properties may be easily adjusted for each application, which will be described in a later part of the review.
3. Electrospinning in bioelectronics
Electrospinning is an electrostatic fiber formation process that utilizes an electrostatic field to produce fibers from polymer dope. The fibers have a thinner diameter and larger surface area, aiding in functionalizing a particular application.36 This process differs from conventional technical methods, such as extrusion and melt blowing, which depend primarily on mechanical forces and geometric boundary conditions. On the other hand, electrospinning is regulated by self-assembly processes produced by electric charges and is controlled by hydrogen bonding, charge transfer interactions, etc.37,38 This approach has been widely used to develop conducting polymer-based nonwoven nanofibrous mats of complex architectures, as illustrated in Fig. 3.39–43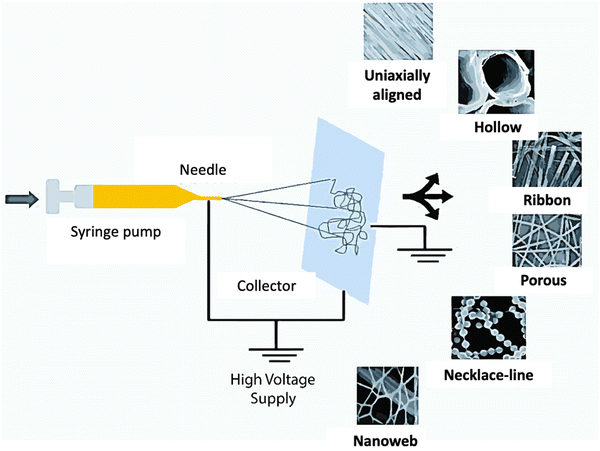 | ||
| Fig. 3 Schematic setup for electrospinning and different morphologies of electrospun polymeric fibers. Adapted from ref. 53 with permission from the Royal Society of Chemistry. | ||
Biocompatible and biodegradable polymers can be electrospun and then coated with ICPs by electrochemical,42 chemical,43,44 or vapor45 phase polymerization processes, or ICP can inherently form the spinning solution and eventually constitute the bulk of the fiber.46,47 These have been reported to be incorporated into electrospun fibrous mats and employed as tissue engineering and drug release conduits. Poly(L-lactide) (PLLA) or poly(lactic-co-glycolic acid) (PLGA) biodegradable polymers have been electrospun with the anti-inflammatory medication dexamethasone onto a gold neural probe, and the fibers have been coated with electrochemically polymerized PEDOT. This enabled the controlled release by using electrostimulation to actuate the nanofibers that caused internal hydrodynamic pressure within the tube and forced the dexamethasone and PLLA degradation products through the cracks in PEDOT casing.42 The adhesion and proliferation of H9c2 rat cardiomyoblast cells were supported by electrospun PANI-gelatin nanofiber mats.47 The key features that make these nanofibrous nonwoven mats suitable for such applications are the 1D confinement characteristic of nanofibers, high surface area and high porosity, and high orientations of structural elements along the fiber direction.48
Flexible bioelectronic systems that mimic natural tissues provide advantages over rigid films and sensors for long-term monitoring without affecting the normal bodily movement and tissue function. Electrospun fibers can be used to fabricate implanted bioelectronic devices.49 For instance, the challenge of achieving good piezoelectric properties with long-term stability in soft materials has been addressed by electrospinning core/shell poly(vinylidene difluoride) (PVDF)/dopamine (DA) nanofibers. This has been accomplished by forming and aligning β-phase PVDF with a solid intermolecular interaction between the –NH2 and –CF2 groups in the polymer. This interaction is responsible for aligning the PVDF chains and promoting β-phase nucleation with a superior and stable piezoelectric performance in implanted devices.50,51 Bioelectronic devices have notable advancements, including flexible/stretchable conductors, transparent electrode development, strain and pressure sensors, and nanogenerators. However, there are a few drawbacks associated with this electrospun bioelectronics, such as the issue of fiber-based devices not lasting long enough because of the unstable electrospinning preparation.52
Recent developments in electrospinning have employed organic polymers with other materials, and the resulting nanofibers exhibit specific electronic, magnetic, and photonic properties. It has been reported that using such electrospun nanofibers in optical and electronic sensors, fuel cells, solar cells, batteries, and energy harvesting and storing devices displays fluorescence, electron conductivities, photovoltaic effects, and photocatalytic effects.37 However, this process has some limitations regarding the fiber deposition rate and uniformity and quality to be enhanced for better performance.
4. Applications of electrospun polymers in bioelectronics
4.1 Biosensors
Biosensors are functional devices consisting of a receptor–transducer system that provides selective quantitative or semi-quantitative analytical information using specific molecular recognition structures.27 Biosensors have largely substituted conventional analytical methodologies for their favorable qualities, including high sensitivity to a particular target, response accuracy, stability, non-toxicity, cost-effectiveness, and mobility. Transducers are the most noteworthy part of the biosensor configuration and are the basis of classification. Biosensors can be classified into optical, electrical, electrochemical, and piezoelectric sensors based on the transducer that translates bio-recognition molecules into measurable entities.54A revolution was brought in the field of biosensors through the development of nanomaterials for the configuration of sensing layers. This enhanced the detection efficiency due to an increased number and activity of surface atoms upon reaching the nanoscale level. Moreover, the nanoscale configuration provides numerous anchoring sites for interacting with the analyte and the sensing molecules. The nanostructure that can tremendously benefit the sensing platform is nanofibers. Nanofibers have become a unique candidate for biosensor applications for their high specific surface area, tunable chemical surfaces, and large pore volume per unit mass.55,56 Electrospinning has always attracted attention over other available techniques for nanofiber synthesis due to its high throughput and capability of producing membranes of various structures, including solid, hollow, porous, multichannel, or whiskers with different conductivities.
Fabrication of polyhydroxybutyrate (PHB) fibers by electrospinning and then dip-coating them with polymethyl methacrylate-co-methacrylic acid, as shown in Fig. 4(a), has been found effective for the detection of dengue virus. This fiber-based sensor system achieved a detection sensitivity of 97% in comparison to the clinical method, with a detection sensitivity of around 76%. This improvement was achieved due to the electrospun fibers with a high surface area providing opportunities for more controlled surface modification and biomolecular interaction for dengue virus detection.57,58 Fiber-based enzyme-linked immunosorbent assay (ELISA) systems were developed using electrospun PLLA and CA substrates to detect the C-reactive protein (CRP) as a cardiac biomarker. The limit of detection (LOD) was measured at 13 pg ml−1, 53 pg ml−1, and 27.32 pg ml−1 for PLLA, CA nanofibers, and cotton microfiber-based platforms, respectively, lower than that of the standard enzyme-linked immunosorbent assays. The results suggested that antibodies tested on the interface of the electrospun PLLA nanofibers became easily immobilized due to the high surface-to-volume ratio and thus showed greater sensitivity to the C-reactive protein (CRP) level.59 In another study, electrospun polystyrene-poly(styrene-co-maleic anhydride) (PS-PSMA) nanofibers immobilized with an aptamer have shown that they could be used to detect proteins. In this aptamers-on-nanofibers-based biosensor, the huge surface area of the nanofibers was the primary reason for a 2500-fold increase in sensitivity and ease of application and manipulation. The paper-based biosensor formed by electrospinning polycaprolactone (PCL) onto a nitrocellulose membrane detects amino acids by forming a hydrophobic coating that enhances the target–gold nanoparticle interaction. The sensitivity of the PCL electrospun-coated test strip was increased nearly tenfold compared to the complete test strip, as illustrated in Fig. 4(b).60 For bacterial and viral pathogen detection, the nitrocellulose nanofibrous membrane was electrospun. The surface was functionalized with an antibody, which detected Escherichia coli O157:H7 and bovine viral diarrhea (BVDV) virus within 8 min. This method provided efficient antibody functionalization and excellent capillary capability.61 The detection scheme is shown in Fig. 4(c). PLA was functionalized with the incorporated biotin to prepare the membrane substrate for the biosensor based on the biotin-streptavidin specific binding to detect synthetic bacterial DNA.62 The conjugated polymer, hydrolyzed poly[2-(3-thienyl) ethanol butoxy carbonyl-methyl urethane] (H-PURET), was electrostatically assembled on the surface of the electrospun CA nanofibrous membrane and acted as the fluorescent probe for the detection of cytochrome c and methyl viologen.63 It was a susceptible optical sensor detecting biological entities (proteins, peptides, and nucleic acids). It has been claimed that conjugated polymer-based sensors have an advantage over fluorescent dye-based sensors in that they display collective features that are extremely sensitive to small perturbations.64 In another work, long rod-shaped M13 viruses were employed to make 1D micro- and nanosized fibers by emulating the spinning mechanism of the silk spider. In micrometer-diameter capillary tubes, liquid crystalline virus suspensions were extruded in glutaraldehyde. 1,1,1,3,3,3-Hexafluoro-2-propanol was used to suspend M13 viruses before they were electrospun into fibers. Polyvinylpyrrolidone (PVP), a highly water-soluble polymer, was combined with M13 viruses to create virus–PVP nanofibers. After resuspending in buffer solution, the virus–PVP electrospun fibers retained their ability to infect bacterial hosts. In biosensor applications, this resulted in beneficial biological activities and a weak catalytic effect.65 Due to the alarming rise of diabetic patients, glucose sensors are drawing considerable research focus. Electrospun polyacrylonitrile nanofibers based on carbon nanotubes (CNTs) have been developed and modified by glucose oxidase for electrochemical glucose sensing. Efficient glucose oxidase immobilization and improved affinity between glucose oxidase and the hydrophilic surface were reported.53 Cholesterol is another biomolecule that requires frequent diagnosis. An amperometric cholesterol biosensor was developed using electrospun PANI nanofibers. The nanofibers were functionalized with more than ten layers of cholesterol oxidase using an electrostatic layer-by-layer adsorption method. It has been reported that the electrostatic interaction can be obtained from such layer-by-layer films assembled by the alternate adsorption of a charged protein and oppositely charged species from solutions.66 Fabrication of the glucose biosensor was later reported by electrospinning a mixture of poly(acrylonitrile-co-acrylic acid) (PANCAA) and multi-walled carbon nanotubes (MWCNTs) to form a nanofibrous membrane that was deposited on a platinum electrode. Their proposed method enhanced the current and improved the reusability of the enzyme electrode (up to 6 times). However, the response time was longer, which needed shortening for diagnostics.67 H2O2 and glucose detection was done using MWCNT and platinum loaded PVDF nanofibers. This non-enzymatic biosensor was highly stable, sensitive, and selective detection of the target analytes.68 Further, electrospun polyurethane (PU) was used as the coating material for implantable glucose biosensors. The PU coating has a submicron fiber diameter and a sub-100-micron thickness that does not hinder the sensing efficiency, and also additional layers can be incorporated to improve biocompatibility.69 Another fascinating material that can be incorporated into the biosensor is graphene quantum dots (GQDs). The construction of a fluorescent and electrochemical biosensor enabled the detection of H2O2 concentration and electrolyte adsorption. The diffusion of the reactants is greatly beneficial because of the high surface area to volume ratio of the 3D electrospun PVA/GQD nanofibrous membrane. Since the addition of H2O2 leads to the fluorescence quelling of GQDs, with an increase in H2O2 concentration, the fluorescence intensity of the nanofibrous membrane declines linearly. Adding glucose oxidase to the created nanofibrous membrane shows high sensitivity and selectivity for glucose detection. Thus, the goal of serving as both a fluorescent and an electrochemical biosensor for determining H2O2 and glucose with high sensitivity is achieved, as depicted in Fig. 4(d).70
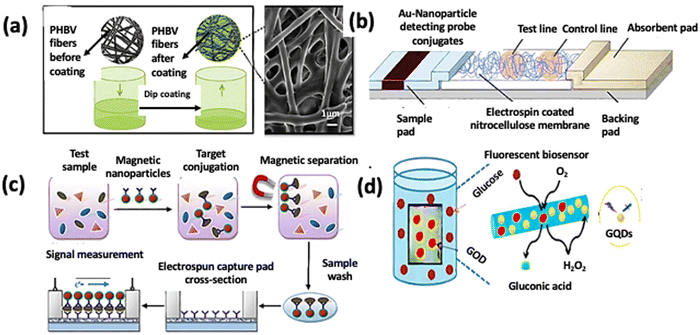 | ||
| Fig. 4 (a) Dip-coating of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers in poly(MMA-co-MAA).57 (b) The PCL electrospun-coated NC membrane is assembled into a conventional lateral flow assay (LFA). Reproduced from ref. 60 with permission from Elsevier. (c) Detection scheme of the lateral flow immunosensor based on the antibody functionalized electrospun capture membrane. Reproduced from ref. 61 with permission from Elsevier. (d) The possible fluorescence detection mechanism of glucose. Reproduced from ref. 70 with permission from the Royal Society of Chemistry. | ||
4.2 Bio-batteries
With the advent of electronic health care devices, batteries in the microampere range and long active life are in demand. Fitting such portable micropower sources to operate machines like cardiac pacemakers is challenging as external recharging or refueling is impossible during prolonged use.71,72In many cases, cellulose or paper-based batteries have already been used for clinical diagnosis. For example, low-cost battery-powered medical devices using cellulose fibers of algal origin and PPy were fabricated as composite structures by laminating multiple stacks of individual layers.73 Despite having a porous and flexible design, these microampere batteries had some limitations, they had minimum cell potential, and the number of available full charge cycles was also less in comparison with Li-ion batteries.
In order to address this, an electrospun polymeric matrix with an atomically thin and malleable separator and electrodes was grown as a monolithic structure.25 The membrane was separately covered with aluminum and silver thin films on the opposite surfaces. After that, it was affixed on sweaty skin in connection with the carbon electrode wires. The bio-battery generated electrical energy from body sweat and reached a power density of 3.38 μW cm−2, a current density of 24.54 μA cm−2, and a voltage of 0.13 V.25 This result was promising as the typical power required for pacemaker operation is 1 μW.
In recent years, bio-batteries have gained the attention of researchers as cellulose-based energy storage devices will have more advantages than the currently used batteries for supplying power to implantable medical devices. Because of the unique structural properties of cellulose, it benefits the development of energy storage devices by improving their electrochemical performance, flexibility, cost competitiveness, and form factor. A group of researchers worked on a fully organic bio-battery that is also activated by biological fluids. The in situ chemical oxidation of PPy and PANI on the CA fibers results in the formation of a polymer matrix, as shown in Fig. 5. Eventually, a polymeric bio-battery was developed by integrating the CA/PPy and CA/PANI composite membranes and separating them with a CA electrospun membrane. Cellulose-based fibers with a high electrical conductivity of 10−2 and 10−1 S cm−1 for CA/PPy and CA/PANI, respectively, and a maximum power density of 1.7 mW g−1 were obtained from the fully developed bio-battery.74
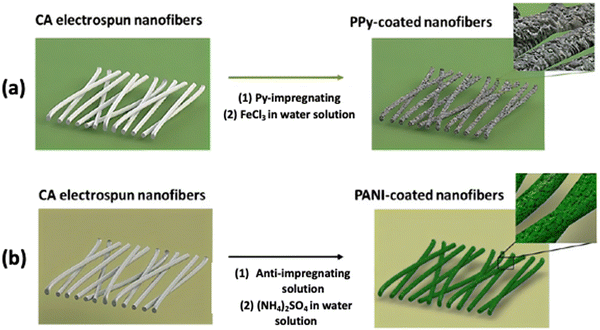 | ||
| Fig. 5 The procedure followed to prepare CA fibers with (a) PPy and (b) PANI by in situ chemical polymerization. Reproduced from ref. 74 with permission from the Royal Society of Chemistry. | ||
4.3 Energy harvesting devices
In addition to bio-batteries, energy harvesting devices, such as biofuel cells (BFCs) and nanogenerators (NGs), are becoming crucial to supply uninterrupted power to healthcare devices. Biofuel cells are bioelectronic devices that rely on the bio-electrocatalytic oxidation of biological fuels, such as lactase or glucose, for energy harvesting applications.75 In contrast to traditional fuel cells that employ expensive metal alloy catalysts and reformed fossil fuels, biofuel cells utilize chemical processes generated by various biological catalysts. Depending on the natural catalyst, biofuel cells can be classified into two groups: enzymatic and microbial fuel cells. Enzymatic fuel cells employ specific isolated enzymes for part of their operation, as shown in Fig. 6(a) and (b), while microbial fuel cells utilize whole organisms containing complete pathways.27 Enzymatic biofuel cells harvesting energy from the metabolites present in biofluids represent a convenient route to eradicate the problem of anatomically compliant power sources. Some applications are shown in Fig. 6(c) and (d).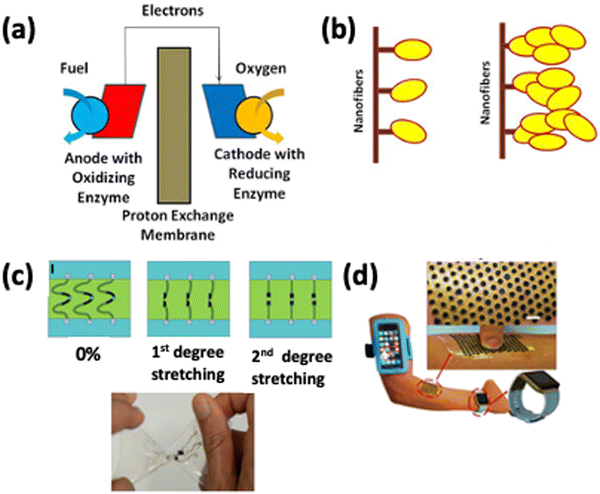 | ||
| Fig. 6 (a) Schematic of enzyme-based biofuel cells, (b) covalently attached enzymes and enzyme aggregates on nanofibers, and (c) a highly stretchable bio-fuel cell. Reprinted with permission from ref. 82. Copyright 2016 American Chemical Society. (d) Stretchable electronic skin-based island-bridge BFC. Reprinted with permission from ref. 83. Copyright 2017 American Chemical Society. | ||
As far back as 1964, glucose oxidase (GOx) was the anodic catalyst for the first enzyme-based biofuel cell.76 After years of advancement and modifications, these biofuel cells still lack the required power density, lifetime, and operational stability. Concurrently, research showed that various nanostructures possess tremendous potential for stabilizing and activating enzymes. Particularly, the enzyme loading capacity will improve significantly if the nanostructures provide a large surface area for enzyme attachments and possibly improve the power density of biofuel cells.77 The covalent attachment of α-chymotrypsin to polystyrene nanofibers of 120 nm diameter was reported. The measured enzyme loading was up to 1.4% (wt/wt), corresponding to a monolayer coverage of the exterior surface of nanofibers of over 27.4%.78 Electrospinning of polyacrylonitrile was used to prepare a 3D nanostructured material. This was done to improve the performance of enzyme electrodes and the power density of biofuel cells. These nanostructures were then functionalized by sputtering a thin film of gold upon them to make them conductive.
The structure enabled a high loading of enzymes and the power density delivered was 1.6 mW cm−2 at 0.75 V, which is five times the power density delivered by the BFC built on flat bioelectrodes.79 Electrospinning was used to create a composite nanofiber by combining a suspension of multi-walled carbon nanotubes (MWCNTs) with a PANI/polyvinyl alcohol (PVA) composite. Biofuel cells operate on the electron transfer principle between the immobilized enzyme and the electrode, which is critical for their success. The schematic of the principle is shown in Fig. 6(a). Electrospun nanostructured electrodes were used to immobilize glucose oxidase and build a bio-anode. Using this nanostructured anode, the maximum current density was 7.5 mA cm−2 at 100 mV s−1vs. Ag/AgCl in 20 mM glucose solution.80 Electrospun PAN fibers coated with gold nanoparticles and MWCNTs were fabricated where the anodic working enzyme glucose oxidase (GOx) was functionalized with poly(N-(3-dimethyl(ferrocenyl)methylammonium bromide) propyl acrylamide) (pFcAc), and this GOx–pFcAc conjugate was immobilized onto the electrospun fiber by physical adsorption.
The adequate wiring of GOX through pFcAc led to a 24-fold increase in current generation efficiency compared to native GOX adsorbed onto the same electrode material.81 The field of soft and flexible electronics has become very popular in the last ten years because they can withstand mechanical changes without affecting the performance. Highly stretchable all-printed CNT-based electrochemical sensors and biofuel cells were developed, capable of withholding strains as high as 500% with no significant effect on their structural integrity and electrochemical performance.82 Such work in constructing highly stretchable CNT-based electrochemical sensors and biofuel cell arrays, as shown in Fig. 6(c), lays the foundation for developing low-cost, highly stretchable, high-performance electronic devices and biofuel cells.82 A deterministically stretchable, high power density wearable electronic skin-based biofuel cell capable of producing energy from human sweat has also been developed, as shown in Fig. 6(d).83
Nanowire-based nanogenerators are novel tools for obtaining biomechanical energy from the human body, for instance, when the lungs inhale and exhale or the heart beats.84 Using piezoelectric nanostructures, nanogenerators convert mechanical energy to electricity. This extraction of mechanical energy from ambient surroundings makes nanogenerators an attractive renewable energy source for various applications. If energy can be generated from our body environment by utilizing the wasted energy invested in walking, speaking, and breathing, these energy sources can potentially meet the energy requirements to operate numerous small devices and personal electronics.85
PVDF is a viable choice of polymer for fabricating nanogenerators as it possesses good piezoelectric and mechanical properties, exhibits chemical stability, and minimizes resistance to outward physical movements at low frequency.86 The piezoelectric properties of PVDF fibers can be improved by spinning with a near-field electrospinning setup with in situ mechanical stretching and electrical poling of aligned nanofibers. In another study, electrospinning of a copolymer of PVDF, specifically poly(vinylidene fluoride–trifluoroethylene) [P(VDF–TrFE)], was carried out, which had an advantage over PVDF in terms of ferroelectricity with the actual Curie transition temperature (Tc) and large electromechanical coupling coefficient.87,88 Their research demonstrated that electrospinning boosted the orientation of the molecular dipoles (CF2) present in [P(VDF–TrFE)], which increased the output current signal when several tens of layers of the electrospun nanofiber web with the same polarization were stacked. Thus, it was expected to obtain a desirable power output for portable electronic devices by simply changing the number of stacked layers. It has been further reported that electrospinning of poly(vinylidene fluoride–hexafluoropropylene) [P(VDF–HFP)] nanofibers loaded with silver nanoparticles can be used to fabricate a polymer-based nanogenerator. A maximum generated voltage of 3 V with a current density of 0.9 μA cm−2 is achieved with that polymer-based nanogenerator.89 It was found that the piezoelectric phase increased due to the functionalization of the nanofibers with silver nanoparticles which enabled the material system to be implanted into wireless sensors and biomedical devices. Later, PEDOT-coated PVDF electrospun nanofibers were developed on neat PVDF nanofibers. The 3D structured nanogenerator thereby provided an enhanced output voltage of 48 V and current of 6 μA upon 8.3 kPa of applied stress amplitude with a superior piezoelectric energy conversion efficiency of 66% compared to that of the single-mat device, which enabled a robust wearable energy harvester that survived fatigue tests over 6 months.90 The work was carried forward and the electrospun PVDF nanofibers were functionalized with MWCNTs to improve the output voltage and power.91 The maximum piezovoltage generated by PVDF nanofiber mats in the presence of 5 wt% MWCNTs is as high as 6 V, while the average capacitor charging power is 81.8 nW, increasing 200% and 44.8%, respectively, compared with bare PVDF nanofiber mats. Zhang et al. first fabricated a wearable all-fiber triboelectric nanogenerator-based insole by simple electrospinning of PVDF, as shown in Fig. 7(a). While walking, this structure was wedged between two conducting fabric electrodes. The maximum output voltage, instantaneous power, and output current of the insole reach 210 V, 2.1 mW, and 45 μA, respectively.92 This fiber-based nanogenerator provided the advantages of efficient energy conversion, user comfort, and low cost. This concept of harvesting energy from human walking has been utilized in smart shoes. Since many piezoelectric materials have limited mechanical durability, their use is limited. To address this issue, a nanocomposite of barium titanate nanoparticles (BT NPs), dispersed in P(VDF-TrFE), was electrospun to produce piezoelectric nanofibers. The functionalization of the polymer matrix with BT NPs leads to fabrication of a robust, flexible, and efficient nanogenerator. Because of its good impact on surface charge density, a porous, spongy structure has been produced to improve the power performance of the nanogenerator. It takes around 72 steps to charge a 4.7 F capacitor. The self-poled nanocomposite nanofibers are largely responsible for the exceptional performance of the NG.93 The triboelectric effect and electrostatic induction work together in a triboelectric nanogenerator to convert the mechanical energy from the outside world into electricity. Electrospun polymer mats (PVDF and poly(3-hydroxybutyrate-co-3-hydroxyvalerate)) with a porous parenchyma-like structure, as shown in Fig. 7(f), were used to create a triboelectric nanogenerator. Cold-compacting post-treatment gave the electrospun mats a porous parenchyma-like structure. Because of its good impact on surface charge density, a porous, spongy structure has been produced to improve the power performance of the nanogenerator. The optimized triboelectric nanogenerator produces a maximum output voltage, short circuit current, and load power of 695 V, 58 μA, and 3.1 W m−2. The performance of the spongy nanogenerator was enhanced by more than three times compared to the NG containing untreated electrospun mats.94 An astonishing discovery in the nanogenerator and diagnostic sensor applications showed that the PLLA nanofibers could show piezoelectricity along the fiber direction, possibly due to the improved crystallinity of the fibers. The electrospun PLLA fibers can produce an 8 pA current and a 20 mV voltage via a simple push–release procedure. Another blood pulse sensor, made of PLLA fibers, was tested and showed an output of roughly 2 pA for the blood pulse.95 In another experiment, it has been found that the enhancement of β-phase crystal formation in PVDF electrospun nanofibers can impact triboelectric nanogenerator applications. The group functionalized the PVDF nanofibers with silver nanowires (AgNWs). Because of the electrostatic interactions between the nanowire surface charges and the PVDF chain dipoles, AgNWs considerably increased the output performance of the triboelectric nanogenerator and aided in creating phase crystals.96 According to research on biodegradable triboelectric nanogenerators, gelatin and electrospun polylactic acid nanofiber membranes can significantly improve the biodegradability of such nanogenerators. They obtained an output voltage of up to 500 V, a short circuit current density of 10.6 mA m−2, and a maximum power density of over 5 W m−2. Thus, the technology harvests energy from heartbeats and other body motions to create a viable green micro-power source for biomedical implants.97 Research on triboelectric nanogenerators recently reported the fabrication of a woven triboelectric nanogenerator utilizing PVDF nanofibers (doped with varying concentrations of MWCNTs) and commercial nylon cloth to harvest energy from human motion. It reaches a high output voltage and current of about 14 V and 0.7 μA, respectively, for the size dimensions of 6 × 6 cm. To capture mechanical energy from human motions, such as tapping, arm movements and walking, a woven triboelectric nanogenerator has been proposed. Since it can operate in contact with several freestanding triboelectric layers, it can effectively capture mechanical energy. As a result, the suggested woven triboelectric nanogenerator shows potential as a power generator for wearable devices and can be extensively used in a variety of other applications.98
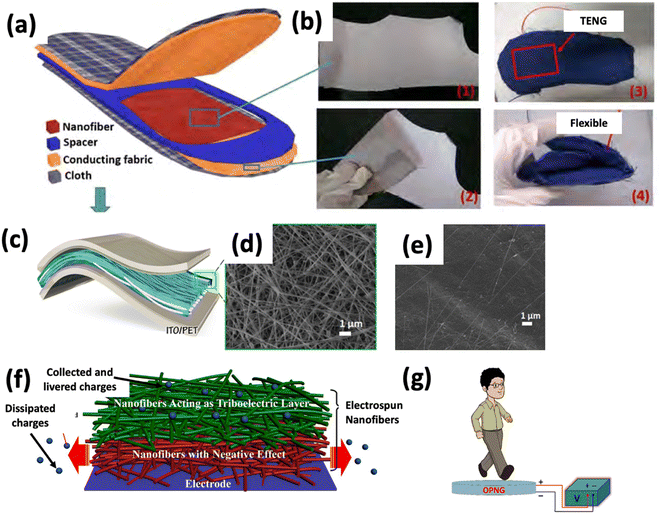 | ||
| Fig. 7 (a) Schematic diagram of the structure of the triboelectric nanogenerator-based insole. (b) Digital photographs of the as-spun PVDF nanofibers on the conducting fabric: (1) front side, (2) backside, and (3) and (4) are images demonstrating flexibility. Reprinted from ref. 92 with permission from Elsevier. (c) Schematic of the nanocomposite NG. Top-view FE-SEM image of electrospun 35 wt% BT-P(VDF-TrFE) nanocomposite nanofibers (d) before and (e) after polydimethylsiloxane coating. Reprinted from ref. 93 with permission from Elsevier. (f) Schematic diagram showing different effects of the electrospun nanofiber mat on the triboelectric nanogenerator. Reprinted from ref. 94 with permission from Elsevier. (g) Sensing performance of the NG based on human walking. Reprinted with permission from ref. 90. Copyright 2018 American Chemical Society. | ||
4.4 Bio-e-skin
E-skins are typically created by overlaying an active nanomaterial on a stretchable surface that adheres to and acts as a sensor for human skin. Bio-e-skin can mimic human skin and facilitate interaction with the skin and organs of the human body. This piezoelectric pressure sensor (PEPS) based bio-e-skin can detect subtle pressure (1 Pa–1 kPa), enabling its application in wearable healthcare systems, artificial intelligence, and prosthetic skin.99–101 In addition, the PEPS possesses high sensitivity and fast response time to realize the full-range of human activities from subtle wrist pulse, intraocular, and intracranial pressure to the movement of synovial joints of the elbows, knees, and fingers.102However, natural piezoelectric materials have yet to receive adequate research attention as e-skins for sensing and evaluating human physiological signals. Cellulose, collagen, and chitin fish gelatin can also be considered due to their ability to exhibit piezoelectric properties due to polar –CONH hydrogen bonding motifs between the polypeptide chains as molecular dipoles. Moreover, it is abundantly present in nature. Gelatin was electrospun to create bio-e-skin for monitoring human physiological signals, and the electrospun gelatin nanofibers demonstrated dynamic pressure sensing capability with outstanding output stability. As a result, the bio-e-skin could precisely detect the glottis opening and closing during swallowing. Therefore, it can be utilized as a breathing monitor and to detect motions of individual fingers, as shown in Fig. 8.102
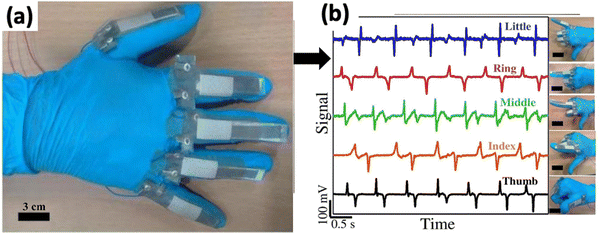 | ||
| Fig. 8 (a) Monitoring swallowing motions using relative changes in the output voltage signal. (b) Data glove with five attached bio-e-skins demonstrating relative changes in the output signal while bending and stretching individual glove finger motions. Reprinted from ref. 102 with permission from Elsevier. | ||
Electrospun PLLA was used as bio-e-skin to detect tactile stimuli. Furthermore, PLA is chiral due to the asymmetric carbon in the lactic acid monomer. If the L-lactide is polymerized, the PLA polymer is an L-type PLA or poly(L-lactic acid) (PLLA) (PDLA). Shear piezoelectricity occurs when these polymers are drawn or stretched. PLA films are very transparent and lack intrinsic polarization, making them non-pyroelectric. PLLA is an optically active polymer that is biocompatible and biodegradable. Therefore, it is a suitable material for in vivo applications due to its bio-piezoelectricity.103 Furthermore, the synthesized e-skin demonstrated excellent sensitivity and stability, with a detection limit as low as 18 Pa. Thus, it appeared as a promising bioelectronic device for monitoring health systems, voice recognition, and real-time wrist pulse detection.104
Piezoelectric sensors that can be utilized as electronic skin have been developed. Electrospinning was used to create a nanofiber mat with a delicate nanoscale geometry, allowing for effective polarization and consequently improved piezoelectric properties. A poly(vinylidenefluoride-co-trifluoroethylene) (PVDF-TrFE) nanofiber mat was placed between two elastomer sheets with sputtered electrodes as a piezoelectric active layer. The sensory system was sensitive to μ-scale mechanical stimuli and capable of detecting deformations with a resolution of 1 μm. As a result of the high flexibility and robustness of the components, the performance of the device could be measured repeatedly without losing accuracy. According to researchers, skin displacements of less than 10 μm caused by a heartbeat were found to operate with this skin-attachable tiny sensor.105
Self-powered electronic skin (bio-e-skin) is an innovation in the modern realm of health monitoring and prosthetics. A self-powered e-skin fabricated from electrospun PVDF yarns of nanofibers coated with PEDOT, shown in Fig. 9(a), showed that the multi-level hierarchical structure of the e-skin yielded a high sensitivity of 18.376 kPa−1 at ∼100 Pa, a broad pressure range of 0.002–10 kPa, and a quick response time of 15 ms, as well as a high durability of 7500 cycles. They also proposed that these nonwoven structures of e-skin could be utilized in making electronic textiles.106
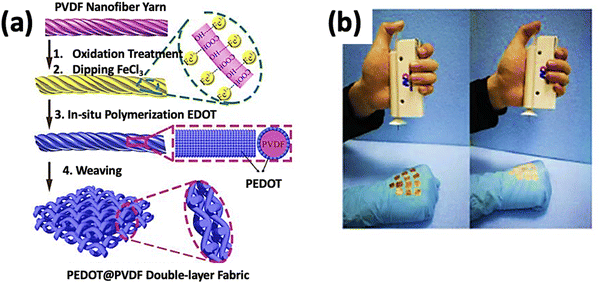 | ||
| Fig. 9 (a) Preparation of PVDF electrospun yarns of nanofibers coated with PEDOT. Reprinted from ref. 106 with permission from Springer Nature. (b) The process of fabricating e-skin by using a handheld electrospinning device. Reprinted with permission from ref. 108. Copyright 2018 American Chemical Society. | ||
Silk fibroin (SF) is a promising candidate for an e-skin substrate to develop bio-e-skin. Since silk fibroin is not electrically conductive, the application of this material in the e-skin substrate is quite limited. However, SF can be made into a highly conductive structure through a simple heat treatment procedure without affecting its desirable properties for e-skin. The group used carbonized electrospinning to make silk nanofiber membranes economically on a commercial scale. The pressure sensor was fabricated by integrating this membrane with planar polydimethylsiloxane films. This bio-e-skin revealed a sensitivity of 34.47 kPa−1 for a wide pressure range, an ultralow detection limit of 0.8 Pa, and a swift response time of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.107
000 cycles.107
A crucial problem in artificial intelligence and robotics is the difficulty in integrating low-level sensorimotor skills in robots compared to high-level reasoning. Polyvinylidene fluoride (PVDF) nanofibers were electrospun to create a sensor based on a single-electrode piezoelectric nanogenerator, as shown in Fig. 9(b). However, this group's piezoelectric nanogenerator has the edge over e-skins that use single-triboelectric nanogenerators to detect dynamic movements but not temperature changes.108
A self-powered and self-cleaning e-skin based on electrospun PVDF/TiO2 nanofibers was developed to detect body motion and degrade organic pollutants. The photocatalytic activity of TiO2 and the piezoelectric effect of PVDF are combined in a single physical/chemical process, resulting in effective organic pollutant degradation on the e-skin. The photocatalytic activity of TiO2 and the piezoelectric effect of PVDF are coupled in a single physical/chemical process that renders efficient degradation of organic pollutants on the e-skin. The developed e-skin caused rapid degradation of methylene blue in an aqueous solution under ultrasonic vibration and UV irradiation.109 As a further step, multi-walled carbon nanotubes (MWCNTs) were incorporated into the PVDF solution and electrospun into nanofibers to improve the sensing performance of piezoelectric nanogenerators as e-skin. The sensitivity was shown to improve with the incorporation of MWCNTs which created induction charges on the PVDF matrix surface and initiated β-phase nucleation. It has been possible due to the feasibility of the electrospinning technique allowing nucleation of the electro-active β-phase and alignment of the molecular dipoles simultaneously.110
4.5 Bio-transistors
Bio-transistors are often known as organic transistors and referred to as Bio-FETs. They refer to a transistor with a bio-sensitive layer that can detect nucleic acids and proteins. A Bio-FET system comprises a semiconducting field-effect transistor acting as a transducer separated from the biological recognition element by an insulator layer. The application of a Bio-FET as an electrical device is expanding day by day due to its low cost, flexibility, and biocompatibility. This polymer-based device is manufactured and studied extensively as a fundamental building component in logic devices and display switches.111 Frequently, in a FET system, the sensing elements are immobilized on the semiconductor path that is connected to the source (S) and drain (D) electrodes to capture the targets (usually via high specificity and binding affinity). A bias potential is applied and modulated to a third electrode named the gate.Similarly, a typical Bio-FET has distinctive parts, namely, source, gate, drain, and two significant compartments: one that converts the biochemical signal into an electronic signal, and another that displays a biological output corresponding to the previous call.112 These devices are sensors to detect biochemical signals from the chemical gradient. It is worth mentioning that the gate of a Bio-FET plays an essential role in this aspect. In comparison with other processes, electrospinning stands out because of its capability to fabricate nanofibers with a controlled morphology in a continuous process at a large scale. Furthermore, polymer-based electrospun nanofibers can contribute to forming an enhanced semiconductor path. The solid stretching force and the shape of the electrospinning process are better able to handle the crystallinity or orientation of the polymer along its long axis. Additionally, electrospun polymers have a powerful ability to create thin films at the nanoscale.113–115
A 1D polymer-based field-effect transistor can be fabricated from electrospun poly(3-hexylthiophene) nanofibers of 100–500 nm size, and chloroform solution was used on electrodes for electrospinning. The structure of the transistor is depicted in Fig. 10(a). The FET displayed a hole field-effect movement of 0.03 cm2 V−1 s−1. In the saturation region, on the other hand, while operating in the accumulation mode, a current on/off ratio of 103 was found. The group also suggested that the impact of polymer chain alignment can be investigated more carefully by fabricating a polymer-based electrospun nanofiber FET in an inert environment and expected an ushering new era of chemical and biological sensors due to the enhancement of the Bio-FET.116
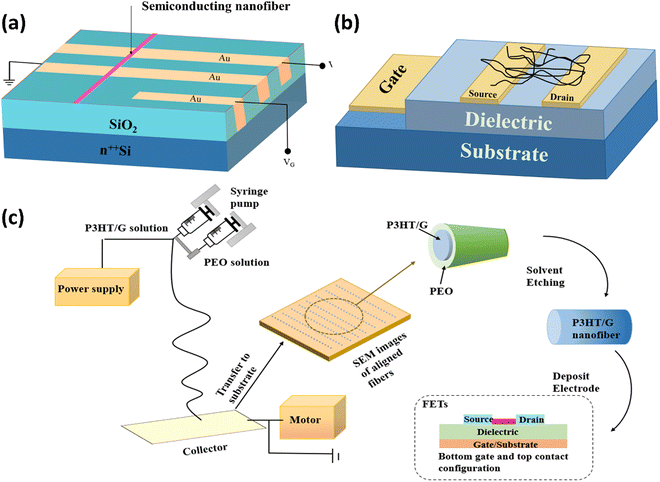 | ||
| Fig. 10 (a) Structure of the electrospun nanofiber-based FET. Reprinted from ref. 116 with the permission of AIP Publishing. (b) Schematic of the FET. Reprinted with permission from ref. 119. Copyright 2005 American Chemical Society. (c) Procedure for the fabrication of P3HT/graphene composite nanofibers and device configuration of the FET. Reproduced from ref. 117 with permission from the Royal Society of Chemistry. | ||
Further, carrier mobility could increase due to the geometrical confinement from electrospun nanofibers. During electrospinning, the strong stretching force reduces grain boundaries, and the π–π molecular packing is enhanced with a highly ordered orientation. The graphene flake was distributed uniformly in the electrospun nanofibers made from poly(3-hexylthiophene) (P3HT) based nanofibers, as illustrated in Fig. 10(b), which functioned as a conducting bridge between poly(3-hexylthiophene) domains in the composite. With a maximum hole mobility of 1.82 cm2 V−1 s−1, their Bio-FET demonstrated exceptional environmental stability for such transfer properties and a moderately high ION/IOFF of 5.88 × 104. Thus, they figured out that carrier mobility can be enhanced if the geometrical confinement of the nanofibers could control the graphene aggregation.117Fig. 10(c) illustrates the P3HT/graphene composite nanofiber fabrication process and FET device configuration. Another study showed that PANI/polyethylene oxide could be used to increase the active semiconducting layer of FETs. They reported that saturation currents were shallow in the electrospun polymer-based FET in the electrospun devices. Polyethylene typically functions as an insulator. As a result, it was suggested that eliminating polyethylene oxide from the nanofibers might decline the non-conducting barriers to the charge transport between PANI chains. Electrospinning was therefore recommended by Pinto and his group as a straightforward way of producing 1D polymer-based FETs.118 Binary blends of poly[2-methoxy-5-(2-ethylhexoxy)1,4-phenylenevinylene] and poly(3-hexylthiophene) [MEH-PPV/PHT], as well as poly[2-methoxy-5-(2-ethylhexoxy)1,4-phenylenevinylene] and poly(9,9-dioctylfluorene) [MEH-PPV/PFO], were used to compare their tunability, stability, composition dependency, and optical and electronic properties aiming to analyze their feasibility in field-effect transistors. Since PHT was present in the MEH-PPV/PHT blend, significant energy transfer was noticed. In contrast, no efficient energy transfer was found for the MEH-PPV/PFO blend. The hole mobility dropped from 1 × 10−4 cm2 (V s)−1 in 20 wt% MEH-PPV blend nanofibers to 5 × 10−6 cm2 (V s)−1 at 70 wt%. Almost the same hole mobility can be gained by compensating for the reduced channel area of transistors as the active hole movement fluctuates from 5 × 10−5 to 1 × 10−3 cm2 (V s)−1. Because of this, it is believed that electrospun nanofibers made from conjugated polymer blends might effectively contribute to field-effect transistors, which could be crucial in developing futuristic electronic nanostructures.119
4.6 Bio-actuators
The growing relationship between soft actuation devices and developing biomedical applications is spurring the development of new biocompatible and sensitive materials to behave as bio-actuators in a human-friendly way. Bio-actuators can play a significant role in bridging the gap between soft actuating materials and novel biomedical applications because bio-actuators have so many structural and functional development possibilities in bio-mimetic muscles, bone prostheses, affinity membranes, and drug delivery carriers. They have already been a top priority for many people. They are mainly used in the innovative adaptive biostructures related to multi-network concepts and integrity among living organisms.Cellulose-based polymeric solutions are mostly preferred for renewable natural resources, sustainability, and environmental pollution elimination for bio-actuators. As the pure cellulose actuator has limited bending ability, low actuation voltage, and low power consumption, many researchers would like to dope the cellulose polymer with metal particles, carbon nanotubes (CNTs), carbon nanofibers (CNFs), fullerenes (C60), and graphene to improve the functionality of the actuator. In the aspect of biocompatibility, it has been found that fibroblast cell attachment with the membrane of electrospun cellulose acetate and PANI/CA (polyaniline-cellulose acetate) membranes at various weight percentages of 0.1 wt% and 0.5 wt% demonstrated biocompatibility by the proper attachment and viability of the fibroblast cells.120 The SEM and confocal images of living cells regarding cell attachments on three electrospun membranes are shown in Fig. 11(a). These images show comparable nanofibrous structures in membrane surfaces with varying PANI contents. The PANI conducting polymer does not produce much difference in electrospun membranes, but it builds well-defined nano-porous structures. Electrospun nanofibers shrink as the PANI conducting polymer loading increases to 0.5 wt%, resulting in well-defined nano-porous networking architectures. Both ion migration and piezoelectricity in cellulose actuators are critical parameters for high-performance actuation, and the high porosity of electrospun membranes can facilitate ion migration. The 0.5 wt% PANI/CA membrane does have a high degree of porosity, which is advantageous for actuator applications. In another recent experiment by Ling Wang et al. on the electrospun scaffold for cardiac tissue engineering, PLA (poly-L-lactic acid)/PANI conductive nanofibrous scaffolds were used as a remarkable potential biocompatible material in enhancing cardiomyocyte maturation, spontaneous beating, and forming cardiomyocyte-based 3D bio-actuators.121 The biological viability of another cellulose acetate-based hybrid (fullerenol reinforced) membrane was tested through bacterial culture where Escherichia coli K-12 were developed in Luria–Bertani (LB) liquid medium at 37 °C and injected on the surface of MacConkey agar plates; the hybrid membranes were placed on the surface of each of the inoculated plates. Fullerenol reinforced cellulose acetate membranes were found to be very compatible for allowing the growth of cells, with additional advantages of its novel crystalline structure (i.e., increase in crystallization, the piezoelectric effect of cellulose acetate, electrostrictive behavior, porosity, thermal stability, and hydrophilic functionalization).122 At even minute concentrations of fullerene C60 and carbon nanotubes, electrospun CA nanofibers acquire great tensile strength for the interactions between the hydroxyl moieties of fullerene and cellulose.123 The effects of the aligned cellulose film in improving the overall performance of electro-active paper (EA-Pap) + actuators have also been analyzed.124 The experiment included electrospinning and the wet-drawn stretching method. Because of the aligned cellulose molecular chains, the produced in-plane stresses were found to behave proportionately in response to the applied electric film, which reveals an increase in the piezoelectricity of EA-Pap, which is very important for the affinity of the membranes. Fan Wang and co-workers improved the PVDF–graphene (polyvinylidene fluoride and graphene) actuator, acquiring more significant bending deformation under low input voltage.125 Electrochemical doping of the PEDOT:PSS electrode layers and the increased ionic conductivity of the membrane were involved.
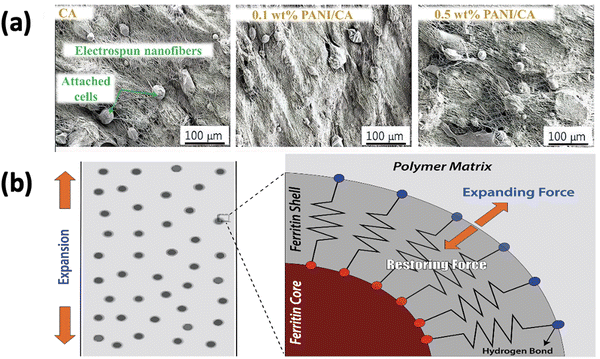 | ||
| Fig. 11 (a) SEM images of three electrospun membranes. Reprinted from ref. 120 with permission from Elsevier. (b) The elastic spring model: a ferritin shell that produces elastic restoring forces when a hybrid nanofibrous hydrogel is expanded. Reprinted from ref. 126 with permission from John Wiley and Sons. | ||
The concept of nanocomposite hydrogels is newly introduced in biomimetic engineering to promote the mechanical properties of bio-actuators. Ferritin-based nanofibrous hydrogels form an elastic spring model generating elastic restoring forces during expansion and contraction. The functional groups of ferritin establish a synergy between ferritin shells and the PVA (polyvinyl alcohol) matrix (–COOH and –NH2) and PVA (–OH), which enables frequent movements of any biostructure like artificial muscles without severe creeping, mimicking the natural one (i.e., skeletal muscles generate about 0.35 MPa stress and contract at 50% per second).126 The elastic spring model is shown in Fig. 11(b). However, hydrogel performance during pH switching is improved if pH-sensitive chitosan is added.
Electrospun bio-actuators are used in drug delivery, one of the most promising biomedical applications, especially for sustained drug release, due to their biocompatibility and sensitive actuation capability, and can reliably function as bio-use-oriented actuators in a human-friendly manner. The polymer choice, drug–polymer compatibility, drug physicochemical properties, formulation properties, and electrospinning technique are all factors that influence the drug delivery method.127 Electrospun fibers have the potential to provide controlled drug release, heavy drug loading (up to 60%), excellent drug entrapment (up to 100%), and cut down expenses for these processes. Besides synthetic polymers like polyurethane (PU), polycarbonate, and nylon-6, natural polymers such as silk, collagen, gelatin, alginate, and chitosan are often used. The drug-releasing policies are designed considering targeted areas, drug receptors, and duration of action through controlling the rate of diffusion, the solubility of drug and polymer, the thickness of polymeric fibers, the degradation rate of the biodegradable polymeric capsule, and the geometry of non-degradable, semi-crystalline and glassy polymers. With a proper PCL/PLGA fiber composition ratio, Carson et al. could tailor the release of tenofovir (10–40 wt%) in 24 hours or >30 days. The experiment also illustrated that azidothymidine, maraviroc, raltegravir, and tenofovir disoproxil fumarate disperse rapidly through a similar PCL/PLGA fiber formulation, tenofovir. However, a deeper understanding of the interaction between polymeric properties and clinical purposes leads to an advanced world of biomimetics.128
5. Future perspectives
Electrospun nanostructures have a place in the production of bioelectronic devices because they offer more functionality and are good for bio-applications because they are biocompatible, biodegradable, and have high porosity. However, many vital challenges need to be addressed for electrospun bioelectronics to overcome its infancy stage and become prominent in the large-scale production of commercial bioelectronic devices. First, the biosensors based on electrospun polymeric substrates focus on detecting many biomarkers (common metabolites and electrolytes). They often encounter malfunctioning or disintegration upon prolonged contact with body fluids. Therefore, the materials should be devised and functionalized to achieve more efficient electrochemical selectivity, sensitivity, stability, and mechanical integrity so that the in situ monitoring becomes reliable, repeatable, and comfortable. The mechanical robustness is still a bottleneck that hampers today's polymer-based bioelectronics. It is crucial to maintain mechanical stability and flexibility simultaneously as well. The devices require being flexible due to the curvilinear surface of the body but at the same time structurally robust for their efficient use and repeatability.Poor mechanical characteristics, such as elongation at breaking and tensile strength, limit electrospun nanofibers in many applications. Improved spinning parameters, new polymer ingredients, and post-treatment techniques are typical factors to boost the mechanical strength of nanofibers. It is possible to obtain fibers with desirable mechanical characteristics by developing electrospinning setups and fabricating different fibrous assemblies more organized at high production rates. In addition, fiber alignment of electrospun nanofibers can improve the tensile and dielectric properties and enhance the overall mechanical characteristics, making them suitable for many structural and multifunctional applications. Each method has some merits and demerits to consider. However, combining two balanced techniques could enhance the mechanical properties with fewer disadvantages compared to a single one.129–131
A significant drawback of the electrospinning process is its low production speed. However, productivity can be improved drastically by avoiding the possibility of clogging the spinneret. Moreover, needle-less electrospinning and multi-needle electrospinning for single structure nanofibers on a commercial level have been developed to address this problem. A one-stepped pyramid-shaped copper spinneret was previously used to commercially produce a vast volume of core–sheath fibers. Another suitable approach for the large-scale production of core/sheath fibers could be air-blowing-assisted electrospinning. A high flow rate is maintained through the nozzle with high airflow.132 Airflow evaporates the extra solvent from the nozzle due to the high flow rate, ultimately optimizing the electrospinning process and making it a promising technique for large-scale production.133 A few nozzle-type electrospinning techniques are used for a high production rate in the pharmaceutical field. Free surface electrospinning, melt electrospinning, and alternating current electrospinning processes have also been utilized to increase the production of electrospun nanofibers to industrial levels.134
Additionally, nanoparticles being added into the electrospinning polymer for functionality enhancement may represent a risk of toxicity and non-biocompatibility. Thus, a complete evaluation of these functional nanomaterials in the devices needs to be ensured to prevent undesired side effects in the body. It should be mentioned that contemporary electrospun polymer-based bioelectronic devices are not widely used in industrial production. They are still in the development stage and are created on a lab scale. Suppose the features mentioned above are upgraded in the fabrication methods. In that case, more and more electrospun bioelectronic devices will be rolled out of the research labs and used in practical applications with a remarkably higher contribution to human life and the environment.
6. Conclusion
Current advances in electrospinning and polymer electrospinning and functionalization have enhanced medical devices with higher biocompatibility, reliability, and accuracy in human health monitoring. Considering the flexibility and curvilinear surface of the human body, polymers such as CA, PLLA, polydimethylsiloxane, PANI functionalized with nanoparticles, or MWCNTs stand out as the most feasible choice of material for use in biodevices. Polymers and electrospinning processes are frequently used in bioelectronic devices to build real-time monitoring devices such as biosensors capable of detecting cancer biomarkers, viruses, and target biomolecules. Biosensors frequently use bio-transistors, which preferably use electrospun polymeric materials owing to their advantage in generating controlled morphology. Moreover, power-generating devices, such as bio-batteries, BFCs, and nanogenerators, rapidly increase with increased charge density by generating electrical energy from body fluids. It has alleviated the problem of requiring a constant power supply to the body-mounted biodevices for health monitoring. The spectrum of electrospinning has broadened from conventional polymers to synthetic ones since its basic setup, and technological realizations have been recognized. In addition, 1D nonwoven polymer structures allowed more remarkable modification and improvement in perceiving the electronic, mechanical, and optical properties. Thus, electrospinning can help advance bioelectronic gadgets that address our ever-changing health problems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study acknowledges the Basic Research Grant (2021) of Bangladesh University of Engineering and Technology (BUET).References
- J. Wang, Electrochemical glucose, Biosensors, 2008, 814–825 CAS.
- Y. Fu, Recent advances in electrochemical glucose biosensors: A review, RSC Adv., 2015, 3, 4473–4491, 10.1039/C2RA22351A.
- A. L. Benabid, Deep brain stimulation for Parkinson's disease, Curr. Opin. Neurobiol., 2003, 13, 696–706, DOI:10.1016/j.conb.2003.11.001.
- M. L. Kringelbach, N. Jenkinson, S. L. F. Owen and T. Z. Aziz, Translational principles of deep brain stimulation, Nat. Rev. Neurosci., 2007, 623–635, DOI:10.1038/nrn2196.
- J. Rivnay and G. G. Malliaras, The rise of organic bioelectronics, Chem. Mater., 2014, 26(1), 679–685, DOI:10.1021/cm4022003.
- A. Zhang and C. M. Lieber, Nano-bioelectronics, Chem Rev., 2016, 116(1), 215–257, DOI:10.1021/acs.chemrev.5b00608.
- M. Mehrali, S. Bagherifard, M. Akbari, A. Thakur, B. Mirani, M. Mehrali, M. Hasany, G. Orive, P. Das, J. Emneus, T. L. Andresen and A. Dolatshahi-Pirouz, Blending electronics with the human body: A pathway toward a cybernetic future, Adv. Sci., 2018, 5(10), 1700931, DOI:10.1002/advs.201700931.
- J. K. Nguyen, D. J. Park, J. L. Skousen, A. E. Hess-Dunning, D. J. Tyler, S. J. Rowan, C. Weder and J. R. Capadona, Mechanically-compliant intracortical implants reduce the neuroinflammatory response, J. Neural Eng., 2014, 11(5), 056014, DOI:10.1088/1741-2560/11/5/056014.
- R. Chen, A. Canales and P. Anikeeva, Neural recording and modulation technologies, Nat. Rev. Mater., 2017, 2, 1–16, DOI:10.1038/natrevmats.2016.93.
- S. Kang, R. K. J. Murphy, S. Hwang, S. M. Lee, D. V. Harburg, N. A. Krueger, J. Shin, P. Gamble, H. Cheng, S. Yu, Z. Liu, J. G. Mccall, M. Stephen, H. Ying, J. Kim, G. Park, R. C. Webb, C. H. Lee, S. Chung, D. S. Wie, A. D. Gujar, B. Vemulapalli, A. H. Kim, K. Lee, J. Cheng, Y. Huang, S. H. Lee, P. V. Braun, W. Z. Ray and J. A. Rogers, Bioresorbable silicon electronic sensors for the brain, Nature, 2016, 530(7588), 71–76, DOI:10.1038/nature16492.
- J. Xue, J. Xie, W. Liu and Y. Xia, Electrospun nanofibers: New concepts, materials, and applications, Acc. Chem. Res., 2017, 50, 1976–1987, DOI:10.1021/acs.accounts.7b00218.
- X. Wu and H. Peng, Polymer-based flexible bioelectronics, Sci. Bull., 2019, 64, 634–640, DOI:10.1016/j.scib.2019.04.011.
- G. Lanzani, Materials for bioelectronics: Organic electronics meets biology, Nat. Mater., 2014, 1–2, DOI:10.1038/nmat4021.
- E. Cuttaz, J. Goding, C. Vallejo-Giraldo, U. Aregueta-Robles, N. Lovell, D. Ghezzi and R. A. Green, Conductive elastomer composites for fully polymeric, flexible bioelectronics, Biomater. Sci., 2019, 7, 1372–1385, 10.1039/c8bm01235k.
- J. R. Capadona, O. Van Den Berg, L. A. Capadona, M. Schroeter, S. J. Rowan, D. J. Tyler and C. Weder, A versatile approach for the processing of polymer nanocomposites with self-assembled nanofibre templates, Nat Nanotechnol, 2007, 765–769, DOI:10.1038/nnano.2007.379.
- J. Y. Oh, S. Kim, H. Baik and U. Jeong, Conducting polymer dough for deformable electronics, Adv. Mater., 2016, 4455–4461, DOI:10.1002/adma.201502947.
- M. Zhao, M. Bj, L. Cao, H. Wang and J. Pelto, Polypyrrole coating on poly(lactide/glycolide) – β -tricalcium phosphate screws enhances new bone formation in rabbits, Biomed Mater., 2015, 10(6), 065016, DOI:10.1088/1748-6041/10/6/065016.
- S. Park, S. W. Heo, W. Lee, D. Inoue, Z. Jiang, K. Yu, H. Jinno, D. Hashizume, M. Sekino, T. Yokota, K. Fukuda, K. Tajima and T. Someya, Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics, Nature, 2018, 561(7724), 516–521, DOI:10.1038/s41586-018-0536-x.
- Z. Xiang, S. C. Yen, N. Xue, T. Sun, W. M. Tsang, S. Zhang, L. De Liao, N. V. Thakor and C. Lee, Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle, J. Micromech. Microeng., 2014, 24(6), 065015, DOI:10.1088/0960-1317/24/6/065015.
- S. C. B. Mannsfeld, B. C. Tee, R. M. Stoltenberg, C. V. H. Chen, S. Barman, B. V. O. Muir, A. N. Sokolov, C. Reese and Z. Bao, Microstructured rubber dielectric layers, Nat. Mater., 2010, 9, 859–864, DOI:10.1038/nmat2834.
- A. Mata and A. J. Fleischman, Characterization of polydimethylsiloxane (PDMS) Properties for biomedical micro/nanosystems, Biomed. Microdevices., 2005, 2, 281–293 CrossRef PubMed.
- T. Stöver and T. Lenarz, Biomaterials in cochlear implants, GMS Curr. Top. Otorhinolaryngol. Head Neck Surg., 2009, 8, 1–22, DOI:10.3205/cto000062.
- V. Barbaro, C. Bosi, S. Caiazza, P. Chistolini, D. Ialongo and P. Rosa, Implant effects on polyurethane and silicone cardiac pacing leads in humans: Insulation measurements and 8EM observations, Biomaterials, 1985, 6, 28–32, DOI:10.1016/0142-9612(85)90034-1.
- K. Ye, R. Felimban, K. Traianedes, S. E. Moulton, G. G. Wallace, J. Chung, A. Quigley, P. F. M. Choong and D. E. Myers, Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed Chitosan Scaffold, PLoS One, 2014, 9(6), e99410, DOI:10.1371/journal.pone.0099410.
- A. C. Baptista, J. I. Martins, E. Fortunato, R. Martins, J. P. Borges and I. Ferreira, Thin and flexible bio-batteries made of electrospun cellulose-based membranes, Biosens. Bioelectron., 2011, 26, 2742–2745, DOI:10.1016/j.bios.2010.09.055.
- X. Li, Q. Feng, K. Lu, J. Huang, Y. Zhang, Y. Hou, H. Qiao, D. Li and Q. Wei, Encapsulating enzyme into metal–organic framework during in situ growth on cellulose acetate nanofibers as self-powered glucose biosensor, Biosens. Bioelectron., 2021, 171, 112690, DOI:10.1016/j.bios.2020.112690.
- A. Baptista, I. Ferreira and J. Borges, Cellulose-based bioelectronic devices, Adv Mater., 2021, 33(28), e2000619 CrossRef PubMed.
- M. W. Frey, Electrospinning cellulose and cellulose derivatives, Polym. Rev., 2008, 48, 378–391, DOI:10.1080/15583720802022281.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Cellulose: Fascinating biopolymer and sustainable raw material, Angew. Chem., Int. Ed., 2005, 44, 3358–3393, DOI:10.1002/anie.200460587.
- W. C. Ross, E. R. Lee, P. Fessenden and K. R. Holmes, 38f/j, IEEE Trans. Biomed. Eng., 1990, 37, 1118–1120 CrossRef PubMed.
- A. Norlin, J. Pan and C. Leygraf, Investigation of Electrochemical Behavior of Stimulation/Sensing Materials for Pacemaker Electrode Applications: I. Pt, Ti, and TiN Coated Electrodes, J. Electrochem. Soc., 2005, 152(2), J7–J15, DOI:10.1149/1.1842092.
- G. M. Clark and R. J. Hallworth, A multiple-electrode array for a cochlear implant, J. Laryngol. Otol., 1976, 90, 623–627, DOI:10.1017/S0022215100082529.
- R. T. Richardson, B. Thompson, S. Moulton, C. Newbold, M. Ghee, A. Cameron, G. Wallace, R. Kapsa, G. Clark and S. O. Leary, The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons, Biomaterials, 2007, 28, 513–523, DOI:10.1016/j.biomaterials.2006.09.008.
- R. O. L. Anger, Stimulation of neurite outgrowth using an electrically, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 8948–8953 CrossRef PubMed.
- A. Gelmi, M. J. Higgins and G. G. Wallace, Biomaterials physical surface and electromechanical properties of doped polypyrrole biomaterials, Biomaterials, 2010, 31, 1974–1983, DOI:10.1016/j.biomaterials.2009.11.040.
- N. Bhardwaj and S. C. Kundu, Electrospinning: A fascinating fiber fabrication technique, Biotechnol. Adv., 2010, 28, 325–347, DOI:10.1016/j.biotechadv.2010.01.004.
- S. Agarwal, A. Greiner and J. H. Wendorff, Functional materials by electrospinning of polymers, Prog. Polym. Sci., 2013, 38, 963–991, DOI:10.1016/j.progpolymsci.2013.02.001.
- Z.-M. Huang, Y.-Z. Zhang, M. Kotaki and S. Ramakrishna, A review on polymer nanofibers by electrospinning and their applications in nanocomposites, Compos. Sci. Technol., 2003, 63, 2223–2253, DOI:10.1016/S0266-3538(03)00178-7.
- D. Li and Y. Xia, Electrospinning of nanofibers: Reinventing the wheel?, Adv. Mater., 2004, 16, 1151–1170, DOI:10.1002/adma.200400719.
- D. H. Reneker, A. L. Yarin, E. Zussman and H. Xu, Electrospinning of nanofibers from polymer solutions and melts, Adv. Appl. Mech., 2007, 41, 15–21, DOI:10.1016/S0065-2156(07)41002-X.
- A. Greiner and J. H. Wendorff, Electrospinning: A fascinating method for the preparation of ultrathin fibers angewandte, Angew. Chem., Int. Ed., 2007, 5670–5703, DOI:10.1002/anie.200604646.
- M. R. Abidian, D. H. Kim and D. C. Martin, Conducting-polymer nanotubes for controlled drug release, Adv. Mater., 2006, 18, 405–409, DOI:10.1002/adma.200501726.
- J. Y. Lee, C. A. Bashur, A. S. Goldstein and C. E. Schmidt, Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications, Biomaterials, 2009, 30, 4325–4335, DOI:10.1016/j.biomaterials.2009.04.042.
- T. Sudwilai, J. J. Ng, C. Boonkrai, N. Israsena, S. Chuangchote and P. Supaphol, Polypyrrole-coated electrospun poly(lactic acid) fibrous scaffold: Effects of coating on electrical conductivity and neural cell growth, J. Biomater. Sci., Polym. Ed., 2014, 25, 1240–1252, DOI:10.1080/09205063.2014.926578.
- X. Liu, J. Chen, K. J. Gilmore, M. J. Higgins, Y. Liu and G. G. Wallace, Guidance of neurite outgrowth on aligned electrospun polypyrrole/poly(styrene-β-isobutylene-β-styrene) fiber platforms, J. Biomed. Mater. Res., Part A, 2010, 94, 1004–1011, DOI:10.1002/jbm.a.32675.
- I. S. Chronakis, S. Grapenson and A. Jakob, Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties, Polymer, 2006, 47, 1597–1603, DOI:10.1016/j.polymer.2006.01.032.
- M. Li, Y. Guo, Y. Wei, A. G. MacDiarmid and P. I. Lelkes, Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications, Biomaterials, 2006, 27, 2705–2715, DOI:10.1016/j.biomaterials.2005.11.037.
- F. Dotti, A. Varesano, A. Montarsolo, A. Aluigi, C. Tonin and G. Mazzuchetti, Electrospun porous mats for high efficiency filtration, J. Ind. Text., 2007, 37, 151–162, DOI:10.1177/1528083707078133.
- X. Wan, Y. Zhao, Z. Li and L. Li, Emerging polymeric electrospun fibers: From structural diversity to application in flexible bioelectronics and tissue engineering, Exploration, 2022, 2, 20210029, DOI:10.1002/exp.20210029.
- T. Li, M. Qu, C. Carlos, L. Gu, F. Jin, T. Yuan, X. Wu, J. Xiao, T. Wang, W. Dong, X. Wang and Z. Q. Feng, High-performance poly(vinylidene difluoride)/dopamine core/shell piezoelectric nanofiber and its application for biomedical sensors, Adv. Mater., 2021, 33(3), 2006093, DOI:10.1002/adma.202006093.
- M. M. Alam, S. Lee, M. Kim, K. S. Han, V. A. Cao and J. Nah, Ultra-flexible nanofiber-based multifunctional motion sensor, Nano Energy, 2020, 72, 104672, DOI:10.1016/j.nanoen.2020.104672.
- Y. Wang, T. Yokota and T. Someya, Electrospun nanofiber-based soft electronics, NPG Asia Mater., 2021, 13(1), 1–22, DOI:10.1038/s41427-020-00267-8.
- V. A. Online, J. Ding and G. Wei, Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications, RSC Adv., 2014, 4(94), 52598–52610, 10.1039/C4RA07848A.
- L. M. Lechuga, L. G. Carrascosa and M. Moreno, Nanomechanical biosensors: A new sensing tool, TrAC, Trends Anal. Chem., 2006, 25, 196–206, DOI:10.1016/j.trac.2005.09.006.
- Z. Rezaei and M. Mahmoudifard, Pivotal role of electrospun nanofibers in microfluidic diagnostic systems – a review, J. Mater. Chem. B, 2019, 7(30), 4602–4619, 10.1039/c9tb00682f.
- S. Sadir, M. P. Prabhakaran, D. H. B. Wicaksono and S. Ramakrishna, Fiber based enzyme-linked immunosorbent assay for C-reactive protein, Sens. Actuators, B, 2014, 205, 50–60, DOI:10.1016/j.snb.2014.08.051.
- S. Hosseini, P. Azari, M. M. Aeinehvand, H. A. Rothan, I. Djordjevic, S. O. Martinez-Chapa and M. J. Madou, Intrant ELISA: A novel approach to fabrication of electrospun fiber mat-assisted biosensor platforms and their integration within standard analytical well plates, Appl. Sci., 2016, 6(11), 336, DOI:10.3390/app6110336.
- S. Hosseini, P. Azari, M. Jiménez-Moreno, A. Rodriguez-Garcia, B. Pingguan-Murphy, M. Madou and S. Martínez-Chapa, Polymethacrylate coated electrospun PHB Fibers as a functionalized platform for bio-diagnostics: Confirmation analysis on the presence of immobilized IgG antibodies against dengue virus, Sensors, 2017, 17, 2292, DOI:10.3390/s17102292.
- S. Sadir, M. P. Prabhakaran and D. H. B. Wicaksono, Chemical Fiber based enzyme-linked immunosorbent assay for C-reactive protein, Sens. Actuators, B, 2014, 205, 50–60, DOI:10.1016/j.snb.2014.08.051.
- C. H. T. Yew, P. Azari, J. R. Choi, F. Li and B. Pingguan-Murphy, Electrospin-coating of nitrocellulose membrane enhances sensitivity in nucleic acid-based lateral flow assay, Anal. Chim. Acta, 2018, 1009, 81–88, DOI:10.1016/j.aca.2018.01.016.
- Y. Luo, S. Nartker, H. Miller, D. Hochhalter, M. Wiederoder, S. Wiederoder, E. Setterington, L. T. Drzal and E. C. Alocilja, Biosensors and Bioelectronics Surface functionalization of electrospun nanofibers for detecting E. coli O157: H7 and BVDV cells in a direct-charge transfer biosensor, Biosens. Bioelectron., 2010, 26, 1612–1617, DOI:10.1016/j.bios.2010.08.028.
- D. Li, M. W. Frey and A. J. Baeumner, Electrospun polylactic acid nanofiber membranes as substrates for biosensor assemblies, J. Membr. Sci., 2006, 279, 354–363, DOI:10.1016/j.memsci.2005.12.036.
- X. Wang, Y. Kim, C. Drew, B. Ku, J. Kumar and L. A. Samuelson, Electrostatic assembly of conjugated polymer thin layers on electrospun nanofibrous membranes for biosensors, Nano Lett., 2004, 8–11 Search PubMed.
- D. T. Mcquade, A. E. Pullen and T. M. Swager, Conjugated polymer-based chemical sensors, Chem Rev., 2000, 100(7), 2537–2574 CrossRef CAS PubMed.
- S. W. Lee and A. M. Belcher, Virus-based fabrication of micro- and nanofibers using electrospinning, Nano Lett., 2004, 4, 387–390, DOI:10.1021/nl034911t.
- Y. J. Shin and J. Kameoka, Amperometric cholesterol biosensor using layer-by-layer adsorption technique onto electrospun polyaniline nanofibers, J. Ind. Eng. Chem., 2012, 18, 193–197, DOI:10.1016/j.jiec.2011.11.009.
- W. Zhen-Gang, W. You, X. Hui, L. Guang and X. Zhi-Kang, Carbon nanotube-filled nanofibrous membranes electrospun from poly(acrylonitrile-co-acrylic acid) for glucose biosensor, J. Phys. Chem. C, 2009, 113, 2955–2960, DOI:10.1021/jp807047s.
- P. Zhang, X. Zhao, X. Zhang, Y. Lai, X. Wang, J. Li, G. Wei and Z. Su, Electrospun doping of carbon nanotubes and platinum nanoparticles into the β-phase polyvinylidene difluoride nanofibrous membrane for biosensor and catalysis applications, ACS Appl. Mater. Interfaces, 2014, 6, 7563–7571, DOI:10.1021/am500908v.
- N. Wang, K. Burugapalli, W. Song, J. Halls, F. Moussy and A. Ray, Biomaterials Electrospun fi bro-porous polyurethane coatings for implantable glucose biosensors, Biomaterials, 2013, 34, 888–901, DOI:10.1016/j.biomaterials.2012.10.049.
- P. Zhang, X. Zhao, Y. Ji, Z. Ouyang, X. Wen, J. Li, Z. Su and G. Wei, Electrospinning graphene quantum dots into a nanofibrous membrane for dual-purpose fluorescent and electrochemical biosensors, J. Mater. Chem. B, 2015, 3(12), 2487–2496, 10.1039/C4TB02092H.
- P. X. Gao, J. Song, J. Liu and Z. L. Wang, Nanowire piezoelectric nanogenerators on plastic substrates as flexible power sources for nanodevices, Adv. Mater., 2007, 19, 67–72, DOI:10.1002/adma.200601162.
- D. Linden and T. B. Reddy, Handbook of batteries, Fuel Energy Abstr., 1995, 4(36), 265, DOI:10.5860/choice.33-2144.
- G. Nyström, A. Razaq, M. Strømme, L. Nyholm and A. Mihranyan, Ultrafast all-polymer paper-based batteries, Nano Lett., 2009, 9, 3635–3639, DOI:10.1021/nl901852h.
- A. C. Baptista, I. Ropio, B. Romba, J. P. Nobre, C. Henriques, J. C. Silva, J. I. Martins, J. P. Borges and I. Ferreira, Cellulose-based electrospun fibers functionalized with polypyrrole and polyaniline for fully organic batteries, J. Mater. Chem. A, 2018, 6(1), 256–265, 10.1039/C7TA06457H.
- J. Kim, I. Jeerapan, J. R. Sempionatto, A. Barfidokht, R. K. Mishra, A. S. Campbell, L. J. Hubble and J. Wang, Wearable bioelectronics: Enzyme-based body-worn electronic devices, Acc. Chem. Res., 2018, 51, 2820–2828, DOI:10.1021/acs.accounts.8b00451.
- A. T. Yahiro, S. M. Lee and D. O. Kimble, Bioelectrochemistry. I. Enzyme utilizing bio-fuel cell studies, Biochim. Biophys. Acta, Spec. Sect. Biophys. Subj., 1964, 88, 375–383, DOI:10.1016/0926-6577(64)90192-5.
- J. Kim, H. Jia and P. Wang, Challenges in biocatalysis for enzyme-based biofuel cells, Biotechnol. Adv., 2006, 24, 296–308, DOI:10.1016/j.biotechadv.2005.11.006.
- H. Jia, G. Zhu, B. Vugrinovich, W. Kataphinan, D. H. Reneker and P. Wang, Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts, Biotechnol. Prog., 2002, 18, 1027–1032, DOI:10.1021/bp020042m.
- D. Selloum, A. A. Chaaya, M. Bechelany, V. Rouessac, P. Miele and S. Tingry, A highly efficient gold/electrospun PAN fiber material for improved laccase biocathodes for biofuel cell applications, J. Mater. Chem. A., 2014, 2, 2794–2800, 10.1039/c3ta14531j.
- A. M. Asiri, Electrospun polyaniline/polyvinyl alcohol/multi-walled carbon nanotubes nanofibers as promising bioanode material for biofuel cells, J. Electroanal. Chem., 2017, 789, 181–187, DOI:10.1016/j.jelechem.2017.02.025.
- A. S. Campbell, H. Murata, S. Carmali, K. Matyjaszewski, M. F. Islam and A. J. Russell, Polymer-based protein engineering grown ferrocene-containing redox polymers improve current generation in an enzymatic biofuel cell, Biosens. Bioelectron., 2016, 86, 446–453, DOI:10.1016/j.bios.2016.06.078.
- A. J. Bandodkar, I. Jeerapan, J. M. You, R. Nuñez-Flores and J. Wang, Highly stretchable fully-printed CNT-based electrochemical sensors and biofuel cells: Combining intrinsic and design-induced stretchability, Nano Lett., 2016, 16, 721–727, DOI:10.1021/acs.nanolett.5b04549.
- A. J. Bandodkar, J. M. You, N. H. Kim, Y. Gu, R. Kumar, A. M. V. Mohan, J. Kurniawan, S. Imani, T. Nakagawa, B. Parish, M. Parthasarathy, P. P. Mercier, S. Xu and J. Wang, Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat, Energy Environ. Sci., 2017, 10, 1581–1589, 10.1039/c7ee00865a.
- R. Yang, Y. Qin, C. Li, G. Zhu and Z. L. Wang, Converting biomechanical energy into electricity by a muscle-movement-driven nanogenerator, Nano Lett., 2009, 9, 1201–1205, DOI:10.1021/nl803904b.
- Z. L. Wang, Towards self-powered nanosystems: From nanogenerators to nanopiezotronics, Adv. Funct. Mater., 2008, 18, 3553–3567, DOI:10.1002/adfm.200800541.
- H. Kawai, The Piezoelectricity of poly(vinylidene fluoride), Jpn. J. Appl. Phys., 1969, 8, 975–976, DOI:10.1143/jjap.8.975.
- D. Mandal, S. Yoon and K. J. Kim, Origin of piezoelectricity in an electrospun poly(vinylidene fluoride-trifluoroethylene) nanofiber web-based nanogenerator and nano-pressure sensor, Macromol. Rapid Commun., 2011, 32, 831–837, DOI:10.1002/marc.201100040.
- L. F. Brown, Design considerations for piezoelectric polymer ultrasound transducers, IEEE Int. Symp. Appl. Ferroelectr., 2000, 1, 265–270, DOI:10.1109/isaf.2000.941553.
- D. Mandal, K. Henkel and D. Schmeißer, Improved performance of a polymer nanogenerator based on silver nanoparticles doped electrospun P(VDF-HFP) nanofibers, Phys. Chem. Chem. Phys., 2014, 16, 10403–10407, 10.1039/c3cp55238a.
- K. Maity and D. Mandal, Applications of polymer, composite, and coating materials all organic high performance piezoelectric nanogenerator with multilayer assembled electrospun nanofibers mats for self-powered multifunctional sensor all organic high performance piezoelectric N, ACS Appl Mater Interfaces, 2018, 10(21), 18257–18269, DOI:10.1021/acsami.8b01862.
- H. Yu, T. Huang, M. Lu, M. Mao, Q. Zhang and H. Wang, Enhanced power output of an electrospun PVDF/MWCNTs-based nanogenerator by tuning its conductivity, Nanotechnology, 2013, 24(40), 405401, DOI:10.1088/0957-4484/24/40/405401.
- T. Huang, C. Wang, H. Yu and H. Wang, Human walking-driven wearable all-fiber triboelectric nanogenerator containing electrospun polyvinylidene fluoride piezoelectric nano fibers, Nano Energy, 2015, 226–235, DOI:10.1016/j.nanoen.2015.01.038.
- S. Siddiqui, D. Kim, E. Roh, L. T. Duy, T. Q. Trung, M. T. Nguyen and N. Lee, A durable and stable piezoelectric nanogenerator with nanocomposite nanofibers embedded in an elastomer under high loading for a self-powered sensor system, Nano Energy, 2017, 30, 434–442, DOI:10.1016/j.nanoen.2016.10.034.
- B. Yu, H. Yu, H. Wang, Q. Zhang and M. Zhu, Nano energy high-power triboelectric nanogenerator prepared from electrospun mats with spongy parenchyma-like structure, Nano Energy, 2017, 34, 69–75, DOI:10.1016/j.nanoen.2017.02.010.
- G. Zhao, B. Huang, J. Zhang, A. Wang, K. Ren and Z. L. Wang, Electrospun poly(L-lactic acid) nanofibers for nanogenerator and diagnostic sensor applications, Macromol. Mater. Eng., 2012, 201600476, DOI:10.1002/mame.201600476.
- S. Cheon, H. Kang, H. Kim, Y. Son, J. Y. Lee, H. Shin, S. Kim and J. H. Cho, High-performance triboelectric nanogenerators based on electrospun polyvinylidene fluoride–silver nanowire composite nanofibers, Adv. Funct. Mater., 2018, 1703778, DOI:10.1002/adfm.201703778.
- R. Pan, W. Xuan, J. Chen, S. Dong, H. Jin, X. Wang, H. Li and J. Luo, Fully biodegradable triboelectric nanogenerators based on electrospun polylactic acid and nanostructured gelatin films, Nano Energy, 2018, 45, 193–202, DOI:10.1016/j.nanoen.2017.12.048.
- M. O. Shaikh, Y. Huang, C. Wang and C. Chuang, Wearable woven triboelectric nanogenerator utilizing electrospun PVDF nanofibers for mechanical energy harvesting, Micromachines, 2019, 10(7), 438 CrossRef PubMed.
- I. J. Gomez, B. Arnaiz, M. Cacioppo, F. Arcudi and M. Prato, Nitrogen-doped carbon nanodots for bioimaging and delivery of paclitaxel, J. Mater. Chem. B, 2018, 6(35), 5540–5548, 10.1039/C8TB01796D.
- G. Schwartz, B. C. K. Tee, J. Mei, A. L. Appleton, D. H. Kim, H. Wang and Z. Bao, Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring, Nat. Commun., 2013, 4(1), 1–8, DOI:10.1038/ncomms2832.
- S. Gong, W. Schwalb, Y. Wang, Y. Chen, Y. Tang, J. Si, B. Shirinzadeh and W. Cheng, A wearable and highly sensitive pressure sensor with ultrathin gold nanowires, Nat. Commun., 2014, 5, 1–8, DOI:10.1038/ncomms4132.
- S. K. Ghosh, P. Adhikary, S. Jana, A. Biswas, V. Sencadas, S. D. Gupta, B. Tudu and D. Mandal, Electrospun gelatin nanofiber based self-powered bio-e-skin for health care monitoring, Nano Energy, 2017, 36, 166–175, DOI:10.1016/j.nanoen.2017.04.028.
- M. Ando, H. Kawamura, H. Kitada, Y. Sekimoto, T. Inoue and Y. Tajitsu, Pressure-sensitive touch panel based on piezoelectric poly(L-lactic acid) film, Jpn. J. Appl. Phys., 2013, 52(9S1), 09KD17, DOI:10.7567/JJAP.52.09KD17.
- A. Sultana, S. K. Ghosh, V. Sencadas, T. Zheng, M. J. Higgins, T. R. Middya and D. Mandal, Human skin interactive self-powered wearable piezoelectric bio-e-skin by electrospun poly-L-lactic acid nanofibers for non-invasive physiological signal monitoring, J. Mater. Chem. B, 2017, 5, 7352–7359, 10.1039/c7tb01439b.
- S. H. Park, H. B. Lee, S. M. Yeon, J. Park and N. K. Lee, Flexible and stretchable piezoelectric sensor with thickness-tunable configuration of electrospun nanofiber mat and elastomeric substrates, ACS Appl. Mater. Interfaces, 2016, 8, 24773–24781, DOI:10.1021/acsami.6b07833.
- Y. Zhou, J. He, H. Wang, K. Qi, N. Nan, X. You, W. Shao, L. Wang, B. Ding and S. Cui, Highly sensitive, self-powered and wearable electronic skin based on pressure-sensitive nanofiber woven fabric sensor, Sci. Rep., 2017, 1–9, DOI:10.1038/s41598-017-13281-8.
- Q. Wang, M. Jian, C. Wang and Y. Zhang, Carbonized silk nanofiber membrane for transparent and sensitive electronic skin, Adv. Funct. Mater., 2017, 27(9), 1605657, DOI:10.1002/adfm.201605657.
- X. Wang, W. Z. Song, M. H. You, J. Zhang, M. Yu, Z. Fan, S. Ramakrishna and Y. Z. Long, Bionic single-electrode electronic skin unit based on piezoelectric nanogenerator, ACS Nano, 2018, 12(8), 8588–8596, DOI:10.1021/acsnano.8b04244.
- C. Dong, Y. Fu, W. Zang, H. He, L. Xing and X. Xue, Self-powering/self-cleaning electronic-skin basing on PVDF/TiO2 nanofibers for actively detecting body motion and degrading organic pollutants, Appl. Surf. Sci., 2017, 416, 424–431, DOI:10.1016/j.apsusc.2017.04.188.
- B. Mahanty, K. Maity, S. Sarkar and D. Mandal, Science direct human skin interactive self-powered piezoelectric e-skin based on PVDF/MWCNT electrospun nanofibers for non-invasive health care monitoring, Mater. Today: Proc., 2020, 21, 1964–1968, DOI:10.1016/j.matpr.2020.01.282.
- R. González and N. J. Pinto, Electrospun poly(3-hexylthiophene-2,5-diyl) fiber field effect transistor, Synth. Met., 2005, 151, 275–278, DOI:10.1016/j.synthmet.2005.05.007.
- C. A. Vu and W. Y. Chen, Field-effect transistor biosensors for biomedical applications: Recent advances and future prospects, Sensors, 2019, 19, 4214, DOI:10.3390/s19194214.
- N. J. Pinto, R. Gonzalez, A. T. Johnson and A. G. MacDiarmid, Electrospun hybrid organic/inorganic semiconductor Schottky nanodiode, Appl. Phys. Lett., 2006, 89, 3–6, DOI:10.1063/1.2227758.
- F. Garnier, Scope and limits of organic-based thin-film transistors, Philos. Trans. R. Soc., A, 1997, 355, 815–827, DOI:10.1098/rsta.1997.0046.
- H. C. Chang, C. L. Liu and W. C. Chen, Flexible nonvolatile transistor memory devices based on one-dimensional electrospun P3HT:Au hybrid nanofibers, Adv. Funct. Mater., 2013, 23, 4960–4968, DOI:10.1002/adfm.201300283.
- H. Liu, C. H. Reccius and H. G. Craighead, Single electrospun regioregular poly(3-hexylthiophene) nanofiber field-effect transistor, Appl. Phys. Lett., 2005, 87, 1–3, DOI:10.1063/1.2149980.
- C. J. Lin, C. L. Liu and W. C. Chen, Poly(3-hexylthiophene)-graphene composite-based aligned nanofibers for high-performance field effect transistors, J. Mater. Chem. C, 2015, 3, 4290–4296, 10.1039/c5tc00399g.
- N. J. Pinto, A. T. Johnson, A. G. MacDiarmid, C. H. Mueller, N. Theofylaktos, D. C. Robinson and F. A. Miranda, Electrospun polyaniline/polyethylene oxide nanofiber field-effect transistor, Appl. Phys. Lett., 2003, 83, 4244–4246, DOI:10.1063/1.1627484.
- A. Babel, D. Li, Y. Xia and S. A. Jenekhe, Electrospun nanofibers of blends of conjugated polymers: Morphology, optical properties, and field-effect transistors, Macromolecules, 2005, 38, 4705–4711, DOI:10.1021/ma047529r.
- C. H. Hong, S. J. Ki, J. H. Jeon, H. lian Che, I. K. Park, C. D. Kee and I. K. Oh, Electro-active bio-composite actuators based on cellulose acetate nanofibers with specially chopped polyaniline nanoparticles through electrospinning, Compos. Sci. Technol., 2013, 87, 135–141, DOI:10.1016/j.compscitech.2013.08.006.
- L. Wang, Y. Wu, T. Hu, B. Guo and P. X. Ma, Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators, Acta Biomater., 2017, 59, 68–81, DOI:10.1016/j.actbio.2017.06.036.
- J. Li, S. Vadahanambi, C. D. Kee and I. K. Oh, Electrospun fullerenol-cellulose biocompatible actuators, Biomacromolecules, 2011, 12, 2048–2054, DOI:10.1021/bm2004252.
- J. Li, C. D. Kee, S. Vadahanambi and I. K. Oh, A novel biocompatible actuator based on electrospun cellulose acetate, Adv. Mater. Res., 2011, 214, 359–363, DOI:10.4028/www.scientific.net/AMR.214.359.
- G. Y. Yun, H. S. Kim, J. Kim, K. Kim and C. Yang, Effect of aligned cellulose film to the performance of electro-active paper actuator, Sens. Actuators, A, 2008, 141, 530–535, DOI:10.1016/j.sna.2007.10.014.
- F. Wang, S. Y. Ko, J. O. Park, S. H. Park and C. D. Kee, Electro-active polymer actuator based on PVDF and graphene through electrospinning, Adv. Mater. Res., 2015, 1105, 311–314, DOI:10.4028/www.scientific.net/amr.1105.311.
- M. K. Shin, G. M. Spinks, S. R. Shin, S. I. Kim and S. J. Kim, Nanocomposite hydrogel with high toughness for bioactuators, Adv. Mater., 2009, 21, 1712–1715, DOI:10.1002/adma.200802205.
- S. F. Chou, D. Carson and K. A. Woodrow, Current strategies for sustaining drug release from electrospun nanofibers, J. Control. Release., 2015, 220, 584–591, DOI:10.1016/j.jconrel.2015.09.008.
- D. Carson, Y. Jiang and K. A. Woodrow, Tunable release of multiclass anti-HIV drugs that are water-soluble and loaded at high drug content in polyester blended electrospun fibers, Pharm. Res., 2016, 33, 125–136, DOI:10.1007/s11095-015-1769-0.
- Y. Han, Y. Xu, S. Zhang, T. Li, S. Ramakrishna and Y. Liu, Progress of improving mechanical strength of electrospun nanofibrous membranes, Macromol. Mater. Eng., 2020, 305, 1–13, DOI:10.1002/mame.202000230.
- Y. Wang and L. Chen, Cellulose nanowhiskers and fiber alignment greatly improve mechanical properties of electrospun prolamin protein fibers, ACS Appl. Mater. Interfaces, 2014, 6, 1709–1718, DOI:10.1021/am404624z.
- T. U. Rashid, R. E. Gorga and W. E. Krause, Mechanical properties of electrospun fibers—a critical review, Adv. Eng. Mater., 2021, 23(9), 2100153, DOI:10.1002/adem.202100153.
- G. Jiang and X. Qin, An improved free surface electrospinning for high throughput manufacturing of core–shell nanofibers, Mater. Lett., 2014, 128, 259–262, DOI:10.1016/j.matlet.2014.04.074.
- G. Duan and A. Greiner, Air-blowing-assisted coaxial electrospinning toward high productivity of core/sheath and hollow fibers, Macromol. Mater. Eng., 2019, 304, 2–6, DOI:10.1002/mame.201800669.
- P. Vass, E. Szabó, A. Domokos, E. Hirsch, D. Galata, B. Farkas, B. Démuth, S. K. Andersen, T. Vigh, G. Verreck, G. Marosi and Z. K. Nagy, Scale-up of electrospinning technology: Applications in the pharmaceutical industry, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, 1–24, DOI:10.1002/wnan.1611.
| This journal is © The Royal Society of Chemistry 2022 |