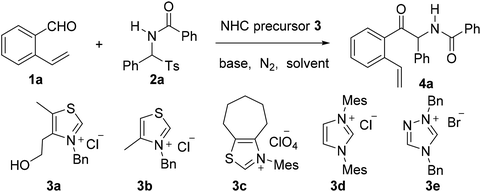
Construction of novel bridged aromatic ring-fused oxazocine frameworks via an N-heterocyclic carbene-catalyzed azabenzoin reaction and radical-initiated cascade cyclization†
Shuang-Shuo
Niu
and
Ying
Cheng
 *
*
College of Chemistry, Beijing Normal University, Beijing 100875, China. E-mail: ycheng2@bnu.edu.cn
First published on 2nd June 2021
Abstract
A novel and efficient method for the construction of 1,5-methanobenzo[f][1,3]oxazocin-6-one compounds from o-vinyl benzaldehydes and N-acylarylimines has been developed. The synthesis proceeded through the sequential NHC-catalyzed azabenzoin reaction and radical-initiated regioselective intramolecular cascade cyclizations. This protocol features mild conditions, good functional group tolerance and high yields of products. Novel 6,10-methanopyrido[3,2-f][1,3]oxazocin-5-ones could also be synthesized from 2-vinylnicotinaldehyde and N-acylarylimines based on this method. Capitalizing on the operational simplicity and use of efficient C–C and C–X bond-forming reactions, this protocol combining NHC-catalyzed and radical-initiated reactions enables the assembly of bridged aromatic ring-fused oxazocine derivatives with versatile functional and structural diversities.
Introduction
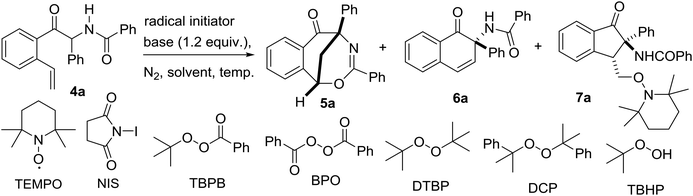
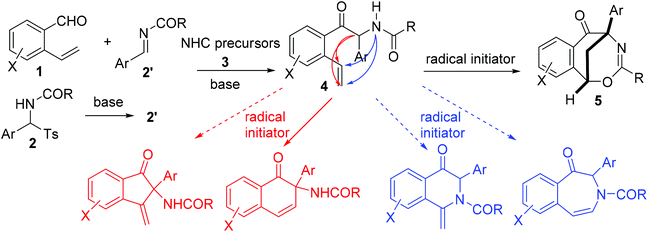
Over the last few decades, radical-initiated cyclization reactions1 have been shown to be powerful methods for the construction of structurally diverse complex carbocyclic and heterocyclic compounds.2 They have often been used as key steps in the total synthesis of biologically active natural products and pharmaceuticals.3,4 For example, the TBPB-initiated cascade cyclization between 3-arylethynyl-[1,1′-biphenyl]-2-carbonitriles and sulfinic acids produced tetracyclic cyclopenta[gh]phenanthridines,2a while the SmI2-triggered radical–radical cyclization cascades of barbiturates produced tricyclic 2a1-hydroxy-hexahydro-4,5a-diazaacenaphthylene-3,5-diones containing up to five contiguous stereocenters.2b On the other hand, nickel-catalyzed intramolecular radical tandem cyclization of alkyl bromide-tethered alkylidenecyclopropanes furnished benzo[b]naphtho[1,2-d]azepines.2c Remarkably, the radical reactions proceed smoothly under relatively mild reaction conditions with a high level of regio- and stereocontrol,5 and particularly a high degree of functional group tolerance. Furthermore, the radical cascade cyclizations are able to efficiently deliver unprecedented polycyclic molecular architectures from simple starting materials in a single synthetic operation.1,2 As a consequence, the development of novel radical-initiated cascade cyclization reactions is of great importance and highly desirable.We have been interested for many years in the exploration of new multicatalytic systems enabling the divergent synthesis of complex molecules from simple and inexpensive chemicals, mainly combining N-heterocyclic carbenes with Lewis acids, Brønsted bases and transition metal catalysts.6 Since N-heterocyclic carbenes are well-known organic catalysts for the azabenzoin reaction between aldehydes and imines to produce various α-aminoketones,7 we envisioned that 2-amido-2-aryl-1-(o-alkenylphenyl)ethanone 4 derived from the NHC-catalyzed reaction of o-alkenyl benzaldehydes with N-acylarylimines would undergo different intramolecular radical cyclizations in the presence of radical initiators to form fused five-, six- or seven-membered carbocyclic or heterocyclic compounds (Scheme 1). To our surprise, under the sequential NHC- and radical-mediated reaction conditions, the reaction of o-vinyl benzaldehyde 1a with N-(phenyl(tosyl)methyl)benzamide 2a, which is the precursor of N-benzoylphenylmethanimine, afforded unexpectedly a novel bridged eight-membered heterocycle 5a (Scheme 1). Reported herein is a novel and highly efficient method for the construction of complex 1,5-methanobenzo[f][1,3]oxazocin-6-one compounds.
 | ||
| Scheme 1 Proposed NHC- and radical-mediated cascade reactions between o-vinyl benzaldehydes and N-acylarylimines. | ||
Results and discussion
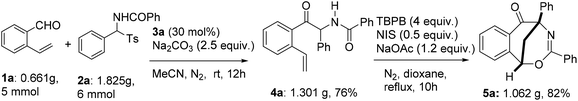
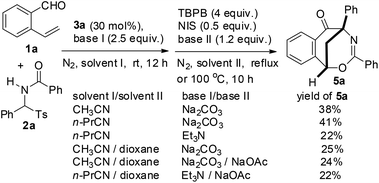
Aiming to develop a cascade reaction, we commenced our study with the examination of the NHC-catalyzed reaction of o-vinyl benzaldehyde 1a with N-benzoylphenylimine 2a′ and the radical-mediated reaction of the resulting adduct 4a, separately. N-Benzoylphenylimine 2a′ was generated in situ from the reaction of N-(phenyl(tosyl)methyl)benzamide 2a with a base. A series of heterocyclic azolium salts including thiazolium salts 3a–3c, imidazolium salt 3d and triazolium salt 3e were employed as NHC precursors. In acetonitrile and at ambient temperature, thiazoliums 3a–3c (20 mol%) upon reaction with Et3N (1.5 equiv.) were able to promote the azabenzoin reaction of 1a with 2a to give 2-benzamido-2-phenyl-1-(2-vinylphenyl)ethanone 4a in 13–35% yields (Table 1, entries 1–3). The highest yield of 4a was obtained from the reaction catalyzed by thiazolium salt 3a. In contrast, imidazolium 3d and triazolium 3e were virtually ineffective towards the azabenzoin reaction of 1a with 2a. Increasing the loading of thiazolium salt 3a to 30 mol% slightly improved the yield of 4a to 39%, while decreasing the loading of catalyst 3a to 10 mol% reduced the yield to 10% (Table 1, entries 6 and 7). With the use of thiazolium salt 3a as an optimized NHC precatalyst, we then found that the action of the base employed was crucial to this reaction. For example, when the loading of Et3N was increased to 2.5 equiv., the yield of 4a greatly improved to 71% (Table 1, entry 8). The replacement of Et3N with Na2CO3 further increased the yield of 4a to 80%. However, DABCO led to the formation of the product in a slightly lower yield (63%), while the use of NaOAc and Cs2CO3 dramatically diminished the yield of product 4a (Table 1, entries 9–12). After the screening of NHC catalysts and bases, the solvent effect was studied. It was found that the reactions of 1a with 2a catalyzed by thiazolium salt 3a and Na2CO3 provided the product in lower yields (14–61%) in dichloromethane, chloroform, DMF and THF, and the reaction was totally retarded in 1,4-dioxane (Table 1, entries 13–17). Finally, the influence of temperature on the reaction was examined. While the reaction at 40 °C gave a similar result to that at ambient temperature, either a higher temperature such as in refluxing CH3CN or a lower temperature (0 °C) drastically decreased the chemical yield of product 4a (entries 18–20). Thus, the catalytic reaction using thiazolium salt 3a (30 mol%) as a precatalyst and Na2CO3 (2.5 equiv.) as a base in acetonitrile at room temperature appears to be optimal conditions for the azabenzoin reaction of o-vinyl benzaldehyde 1a and N-(phenyl(tosyl)methyl)benzamide 2a.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | NHC catalyst 3 (mol%) | Base (equiv.) | Solvent | Temp. (°C) | Yield of 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Under nitrogen protection, a mixture of reactants 1a (0.5 mmol) and 2a (0.5 mmol), catalyst 3 and a base in a dry solvent (5 mL) was stirred for 12 h at the temperature indicated in the table. b Isolated yield. | |||||
| 1 | 3a (20) | Et3N (1.5) | MeCN | r.t. | 35 |
| 2 | 3b (20) | Et3N (1.5) | MeCN | r.t. | 31 |
| 3 | 3c (20) | Et3N (1.5) | MeCN | r.t. | 13 |
| 4 | 3d (20) | Et3N (1.5) | MeCN | r.t. | — |
| 5 | 3e (20) | Et3N (1.5) | MeCN | r.t. | — |
| 6 | 3a (30) | Et3N (1.5) | MeCN | r.t. | 39 |
| 7 | 3a (10) | Et3N (1.5) | MeCN | r.t. | 9 |
| 8 | 3a (30) | Et3N (2.5) | MeCN | r.t. | 71 |
| 9 | 3a (30) | DABCO (2.5) | MeCN | r.t. | 63 |
| 10 | 3a (30) | Na 2 CO 3 (2.5) | MeCN | r.t. | 80 |
| 11 | 3a (30) | NaOAc (2.5) | MeCN | r.t. | 15 |
| 12 | 3a (30) | Cs2CO3 (2.5) | MeCN | r.t. | 29 |
| 13 | 3a (30) | Na2CO3 (2.5) | DCM | r.t. | 61 |
| 14 | 3a (30) | Na2CO3 (2.5) | CHCl3 | r.t. | 45 |
| 15 | 3a (30) | Na2CO3 (2.5) | DMF | r.t. | 51 |
| 16 | 3a (30) | Na2CO3 (2.5) | THF | r.t. | 14 |
| 17 | 3a (30) | Na2CO3 (2.5) | Dioxane | r.t. | — |
| 18 | 3a (30) | Na2CO3 (2.5) | MeCN | 40 | 75 |
| 19 | 3a (30) | Na2CO3 (2.5) | MeCN | Reflux | 43 |
| 20 | 3a (30) | Na2CO3 (2.5) | MeCN | 0 | 7 |
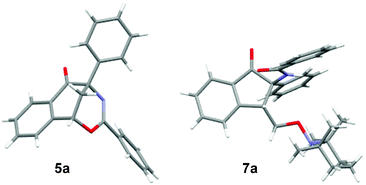
After the optimization of the reaction conditions for the formation of 2-benzamido-2-phenyl-1-(2-vinylphenyl)ethanone 4a from o-vinyl benzaldehyde 1a and N-(phenyl(tosyl)methyl)benzamide 2a, we studied the radical-mediated reaction of 4a. Initially, 2,2,6,6-tetramethylpiperidinooxy (TEMPO) was employed as a radical initiator. In refluxing dioxane, the reaction of 4a mediated by 2, 3 and 4 equiv. of TEMPO, respectively, produced an interesting bridged eight-membered heterocyclic compound, namely 3,5-diphenyl-1,5-methanobenzo[f][1,3]oxazocin-6-one 5a, in 38–60% yields, along with the formation of two minor products, 2-benzamido-2-phenylnaphthalen-1-one 6a (12–22%) and polysubstituted 2,3-dihydroinden-1-one derivative 7a (19–22%) (Table 2, entries 1–3) (see the X-ray structures of 5a and 7a in Fig. 1). No other products proposed in Scheme 1 were isolated. To improve the selectivity in the formation of 1,5-methanobenzo[f][1,3]oxazocin-6-one 5a and naphthalen-1-one 6a, other radical initiators were investigated. It was found that in the presence of N-iodosuccinimide (NIS, 0.5 equiv.) and Na2CO3 (1.2 equiv.), the reaction of 4a produced bridged heterocycle 5a in 31% yield without the formation of naphthalen-1-one 6a. Increasing the loading of NIS was not beneficial because only 15% yield of 5a was isolated from the reaction using 2 equiv. of NIS as the radical precursor (Table 2, entries 4 and 5). When a series of peroxide oxidants including t-butyl hydroperoxide (TBHP), di-t-butylperoxide (DTBP), dicumyl peroxide (DCP), t-butyl peroxybenzoate (TBPB) and dibenzoyl peroxide (BPO) were applied as radical initiators, the reactions of 4a yielded very complex mixtures, in which product 5a or 6a was not observed (Table 2, entries 6–10). Intriguingly, however, when a peroxide including TBHP, DTBP, DCP, BPO or TBPB (4 equiv.) was used in combination with NIS (0.5 equiv.) and Na2CO3 (1.2 equiv.), the reactions of 4a gave 5a in 24–86% yields, with no naphthalen-1-one 6a being detected (Table 2, entries 11–15). The best yield of product 5a (86%) was achieved from the reaction promoted by a combination of TBPB (4 equiv.), NIS (0.5 equiv.) and Na2CO3 (1.2 equiv.). It should be noted that in the absence of Na2CO3, the reaction of 4a with TBPB and NIS did not proceed to form 5a. To further improve the production of bridged heterocyclic product 5a, the base and iodide additives were screened while using TBPB as the radical initiator. We discovered that MnI2, FeI2 and NaI were also effective iodide sources, giving good yields of product 5a (81–84%) (Table 2, entries 17–19). Pleasingly, the use of Et3N and NaOAc instead of Na2CO3 improved the yield of 5a to 91% and 92%, respectively (Table 2, entries 20 and 21). A lower yield of 5a (70%) was obtained when DABCO was used as a base. Finally, the reactions of 4a promoted by a combination of TBPB (4 equiv.), NIS (0.5 equiv.) and NaOAc (1.2 equiv.) were examined in different solvents at 80–100 °C. Except for the reaction in refluxing 1,4-dioxane, other reactions in refluxing acetonitrile, hot butyronitrile, DMF and 1,1,2-trichloroethane (100 °C oil bath temperature) produced product 5a in lower yields (24–79%) (Table 2, entries 23–26).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Oxidant (equiv.) | Additive (equiv.) | Base | Solvent | Temp. (°C) | Yieldb (%) | ||
|---|---|---|---|---|---|---|---|---|
| 5a | 6a | 7a | ||||||
| a Reaction conditions: Under nitrogen protection, a mixture of the reactant 4a (0.5 mmol), radical initiator, additive and base was stirred in a refluxing solvent (5 mL) or at 100 °C for 10 h. b Isolated yield. c Oil bath temperature. | ||||||||
| 1 | TEMPO (2) | — | — | Dioxane | Reflux | 38 | 22 | 22 |
| 2 | TEMPO (3) | — | — | Dioxane | Reflux | 53 | 18 | 21 |
| 3 | TEMPO (4) | — | — | Dioxane | Reflux | 60 | 12 | 19 |
| 4 | NIS (0.5) | Na2CO3 | Dioxane | Reflux | 31 | — | — | |
| 5 | NIS (2) | Na2CO3 | Dioxane | Reflux | 15 | — | — | |
| 6 | TBHP (4) | — | Na2CO3 | Dioxane | Reflux | — | — | — |
| 7 | DTBP (4) | — | Na2CO3 | Dioxane | Reflux | — | — | — |
| 8 | DCP (4) | — | Na2CO3 | Dioxane | Reflux | — | — | — |
| 9 | TBPB (4) | — | Na2CO3 | Dioxane | Reflux | — | — | — |
| 10 | BPO (4) | — | Na2CO3 | Dioxane | Reflux | — | — | — |
| 11 | TBHP (4) | NIS (0.5) | Na2CO3 | Dioxane | Reflux | 38 | — | — |
| 12 | DTBP (4) | NIS (0.5) | Na2CO3 | Dioxane | Reflux | 24 | — | — |
| 13 | DCP (4) | NIS (0.5) | Na2CO3 | Dioxane | Reflux | 65 | — | — |
| 14 | BPO (4) | NIS (0.5) | Na2CO3 | Dioxane | Reflux | 37 | — | — |
| 15 | TBPB (4) | NIS (0.5) | Na2CO3 | Dioxane | Reflux | 86 | — | — |
| 16 | TBPB (4) | NIS (0.5) | — | Dioxane | Reflux | — | — | — |
| 17 | TBPB (4) | MnI2 (0.5) | Na2CO3 | Dioxane | Reflux | 82 | — | — |
| 18 | TBPB (4) | FeI2 (0.5) | Na2CO3 | Dioxane | Reflux | 84 | — | — |
| 19 | TBPB (4) | NaI (0.5) | Na2CO3 | Dioxane | Reflux | 81 | — | — |
| 20 | TBPB (4) | NIS (0.5) | NaOAc | Dioxane | Reflux | 92 | — | — |
| 21 | TBPB (4) | NIS (0.5) | Et3N | Dioxane | Reflux | 91 | — | — |
| 22 | TBPB (4) | NIS (0.5) | DABCO | Dioxane | Reflux | 70 | — | — |
| 23 | TBPB (4) | NIS (0.5) | NaOAc | MeCN | Reflux | 51 | — | — |
| 24 | TBPB (4) | NIS (0.5) | NaOAc | n-PrCN | 100c | 79 | — | — |
| 25 | TBPB (4) | NIS (0.5) | NaOAc | DMF | 100c | 75 | — | — |
| 26 | TBPB (4) | NIS (0.5) | NaOAc | Cl2CHCH2Cl | 100c | 24 | — | — |
After identifying the optimized conditions for the NHC-catalyzed reaction of o-vinyl benzaldehyde 1a with N-(phenyl(tosyl)methyl)benzamide 2a and for the radical-mediated reaction of 2-benzamido-2-phenyl-1-(2-vinylphenyl)ethanone 4a, we tried the one-pot NHC- and radical-mediated sequential reaction of o-vinyl benzaldehyde 1a and N-(phenyl(tosyl)methyl)benzamide 2a. The sequential reactions in a one-pot fashion were first performed using the same base and solvent. We have shown that the optimized solvents for the azabenzoin reaction and radical reaction were acetonitrile and 1,4-dioxane, respectively, and the NHC-catalyzed reaction did not take place in 1,4-dioxane. On the other hand, Na2CO3 and Et3N appeared as the most suitable bases for the NHC catalysis, while in the radical reaction, NaOAc, Et3N and Na2CO3 acted as beneficial additives, leading to the formation of product 5a in good to excellent yields (see Tables 1 and 2). Taking consideration of the optimized solvents, bases, and temperature and their compatibility in the two reactions, we performed the one-pot sequential reaction of 1a with 2a using Na2CO3 or Et3N as the sole base in acetonitrile or butyronitrile. From these reactions, 1,5-methanobenzo[f][1,3]oxazocin-6-one 5a was isolated in 22–41% yields (Scheme 2). Great effort was made to improve the efficiency of the one-pot reaction. For instance, the one-pot sequential reaction was then conducted in acetonitrile or butyronitrile with the use of Na2CO3 or Et3N in the first step, followed by the addition of 1,4-dioxane or 1,4-dioxane and NaOAc, which were the optimal solvent and base in the radical cyclization reaction of 4a. Disappointingly, however, only 22–25% yields of product 5a were isolated when different solvents and bases were used in two steps. The main reason for the low yields of 5a from the one-pot operation was most probably the incompatibility of the conditions for the two individual steps.
 | ||
| Scheme 2 One-pot NHC- and radical-mediated sequential reaction of o-vinyl benzaldehyde 1a and N-(phenyl(tosyl)methyl)benzamide 2a. | ||
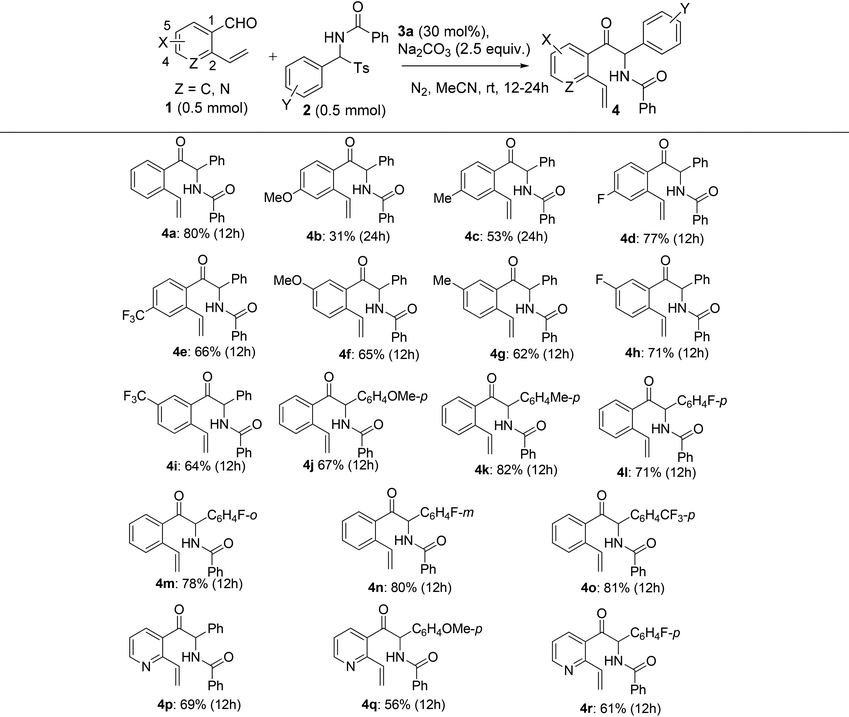
Since the one-pot NHC- and radical-mediated sequential reaction of o-vinyl benzaldehyde 1a and N-(phenyl(tosyl)methyl)benzamide 2a did not provide high synthetic efficiency, and the bridged benzooxazocinone compounds 5 appeared to be important in terms of their unique structure and versatility in organic synthesis, we turned our attention to the synthesis of 5 by means of a stepwise method. Logically, we first examined the scope of the NHC-catalyzed azabenzoin reaction. Under the optimized conditions for the NHC-catalyzed reaction, we investigated the reaction of o-vinyl aromatic aldehydes 1 with N-(aryl(tosyl)methyl)benzamides 2 which bear different substituents. As summarized in Table 3, the reaction displayed good tolerance toward both electron-donating and electron-withdrawing groups attached to both substrates, although the electronic nature and substitution pattern of the substituents influenced the reactivity of the reaction and chemical yields of 2-aryl-2-benzamido-1-(2-vinylaryl)-1-ethanone products 4. For example, the introduction of an electron-withdrawing group to the para-position of the aldehyde group of substrates 1 had marginal influence on the reaction, as the reactions of 4-fluoro- and 4-trifluoromethyl-2-vinylbenzaldehydes 1d and 1e with 2a produced products 4d and 4e in 77% and 66% yields, respectively. However, an electron-donating group at the para-position of aldehyde decreased the reactivity of substrates 1. This was evident for the reaction of 4-methoxy- and 4-methyl-2-vinylbenzaldehydes 1b and 1c, which produced 4b and 4c in lower yields (31% and 53%, respectively) even though the reaction time was prolonged from 12 h to 24 h. When the substituent was moved from the para-position to the meta-position of benzaldehydes 1, no matter whether it was an electron-donating or electron-withdrawing group (5-OMe, 5-Me, 5-F and 5-CF3), the reactions of 1f–1i with 2a proceeded equally well to give products 4f–4i in 62%–71% yields. Being different from the substituent effect observed for o-vinyl benzaldehydes 1, the electronic nature and substitution pattern of the groups attached to N-benzoylarylimines exerted much weaker influence on the reaction. All N-(aryl(tosyl)methyl)benzamides 2 substituted with a p-OMe, p-Me, p-F, o-F, m-F or p-CF3 group reacted smoothly with o-vinyl benzaldehyde 1a to afford products 4j–4o in 67%–82% yields. Finally, a heteroaromatic substrate, 2-vinylnicotinaldehyde 1k, was also found to react analogously with 2a, 2b and 2d, producing 2-aryl-2-benzamido-1-(2-vinyl-3-pyridinyl)-1-ethanones 4p–4r in 56–69% yields (Table 3).
| a Reaction conditions: Under a nitrogen atmosphere and at room temperature, a mixture of N-(aryl(tosyl)methyl)benzamides 2 (0.5 mmol), thiazolium salt 3a (0.15 mmol), Na2CO3 (1.25 mmol) and o-vinyl aromatic aldehydes 1 (0.5 mmol) in dry acetonitrile (5 mL) was stirred for 12–24 h at room temperature. b Isolated yield. |
|---|
|
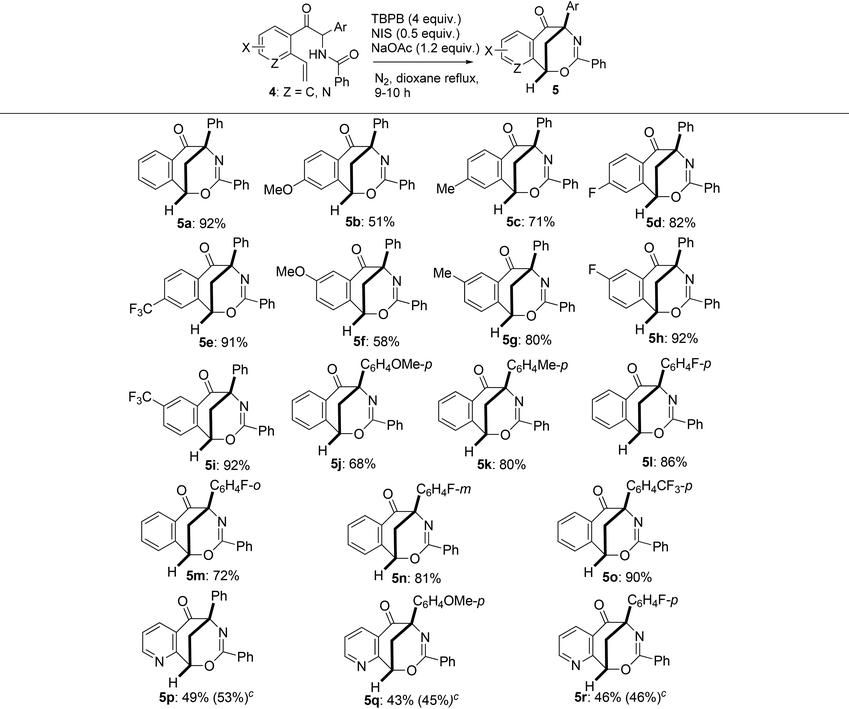
With various 2-aryl-2-benzamido-1-(2-vinylaryl)-1-ethanones (also namely N-(1-aryl-1-(2-vinylaroyl)methyl)benzamides) 4 in hand, we then studied the generality of the radical-mediated intramolecular cyclization of 4 to the synthesis of bridged heterocyclic molecules. The reaction was found to tolerate a wide range of the substrates which contain different electron-donating and electron-withdrawing groups. As presented in Table 4, only reactants 4b, 4f and 4j, which bear a methoxy group on the 1- or 2-phenyl ring, gave moderate yields of 1,5-methanobenzo[f][1,3]oxazocin-6-ones 5b, 5f and 5j (51–68% yields); all other substrates 4 containing a methyl, fluorine or trifluoromethyl group at different positions of each aryl moiety produced the corresponding products 5 in good to excellent yields (71–92%). Under the identical reaction conditions, 2-aryl-2-benzamido-1-(2-vinyl-3-pyridinyl)-1-ethanones 4p–4r produced 6,10-methanopyrido[3,2-f][1,3]oxazocin-5-ones 5p–5r in lower yields (43–49%). The low chemical yields of 5p–5r were due to the formation of many inseparable by-products which were observed by TLC. Varying the reaction conditions, including bases, reaction temperature, and the loads of oxidants, bases and NIS, did not significantly improve the yields of bridged pyrido[3,2-f][1,3]oxazocine derivatives. For example, products 5p–5r were obtained in 45–53% yields from the reaction of 4p–4r initiated by TBPB (2 equiv.), NIS (0.5 equiv.) and Et3N (2 equiv.) in refluxing 1,4-dioxane (see Table 4, the yields in parentheses).
| a Reaction conditions: Under a nitrogen atmosphere and at room temperature, 2-aryl-2-benzamido-1-(o-vinylaryl)-1-ethanones 4 (0.5 mmol), NaOAc (0.6 mmol), NIS (0.25 mmol), TBPB (2 mmol) and dry 1,4-dioxane (5 mL) were added to a dry Schlenk tube. The reaction mixture was stirred in refluxing 1,4-dioxane for 9–10 h under the protection of nitrogen. b Isolated yield. c The yields in parentheses were obtained from the reaction using TBPB (2 equiv.), NIS (0.5 equiv.) and Et3N (2 equiv.). |
|---|
|
It was worth addressing that the NHC- and radical-mediated two-step sequential reactions were scalable. The reaction of o-vinyl benzaldehyde 1a with N-(phenyl(tosyl)methyl)benzamide 2a was carried out readily on a gram scale under the optimized conditions, with the desired intermediate 4a and product 5a being synthesized in 76% and 82% yields, respectively (Scheme 3).
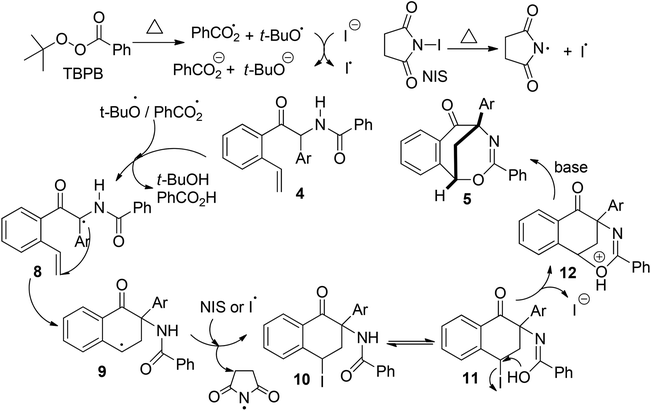
In this NHC- and radical-mediated reaction sequence, the formation of 2-aryl-2-benzamido-1-(2-vinylaryl)-1-ethanones 4 from o-vinyl aromatic aldehydes 1 and N-(aryl(tosyl)methyl)benzamides 2 proceeded via an azabenzoin reaction.7 On the other hand, the formation of 1,5-methanobenzo[f][1,3]oxazocin-6-ones 5 from 2-aryl-2-benzamido-1-(2-vinylaryl)-1-ethanones 4 in the presence of radical initiators TBPB and NIS is intriguing. To account for the unexpected formation of bridged heterocyclic compounds 5, a radical-initiated cascade cyclization mechanism was proposed. As depicted in Scheme 4, the homolytic cleavage of peroxides TBPB and NIS under pyrolysis produces tert-butoxy and benzoate radicals and the iodine radical, respectively. These radicals would abstract the most vulnerable α-hydrogen atom from compounds 4 to generate radical intermediates 8. An intramolecular radical addition to the vinyl group of 8 leads to the formation of 3,4-dihydronaphthalen-1-one radicals 9. The radicals 8 are added predominately to the terminal carbon rather than the internal carbon of the vinyl group because the former reaction forms a more stable benzyl radical than the methyl radical in the latter one. Subsequently, the reaction of 3,4-dihydronaphthalen-1-one radicals 9 with NIS or the iodine radical provides 2-aryl-4-iodo-3,4-dihydronaphthalen-1-one-2-benzamides 10, which tautomerize with 2-aryl-4-iodo-3,4-tetrahydronaphthalen-1-one-2-benzimidic acid 11. Finally, a base-promoted intramolecular nucleophilic substitution reaction between benzyliodide and hydroxyl groups of intermediates 11 leads to the formation of 1,5-methanobenzo[f][1,3]oxazocin-6-one products 5.
 | ||
| Scheme 4 Proposed mechanism for the radical-initiated intramolecular cyclization of 2-aryl-2-benzamido-1-(2-vinylaryl)-1-ethanones 4. | ||
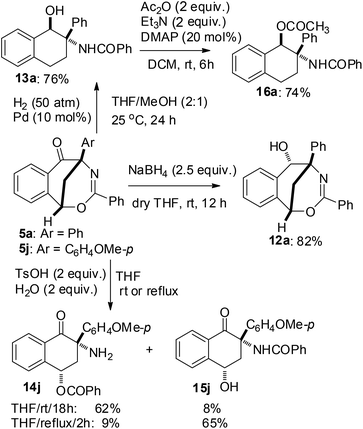
The synthetic value of this sequential reaction protocol was further demonstrated by investigating the transformation of 1,5-methanobenzo[f][1,3]oxazocin-6-ones 5 (Scheme 5). For example, in dry THF and at ambient temperature, the selective reduction of the carbonyl group of compound 5a was achieved with NaBH4 (2.5 equiv.) to furnish 1,5-methanobenzo[f][1,3]oxazocin-6-ol 12a in 82% yield. On the other hand, in a mixture of THF and MeOH (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 2
MeOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
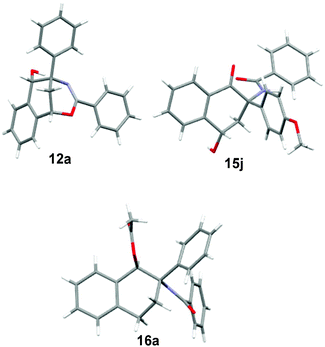
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 25 °C, the Pd–C catalyzed hydrogenation reaction of 5a under 50 atm H2 gas produced N-(1-hydroxy-2-phenyl-2-tetrahydronaphthalenyl)benzamide 13a in 76% yield. Furthermore, the acid hydrolysis of 5j led to the formation of two highly functional 1-tetralones 14j and 15j. The selective synthesis of 3-amino-3-(p-methoxyphenyl)-4-oxo-1-tetrahydronaphthalenyl benzoate 14j (62% yield) and N-(4-hydroxy-2-(p-methoxyphenyl)-1-oxo-2-tetrahydronaphthalenyl)benzamide 15j (65% yield) was achieved by the treatment of compound 5j with TsOH (2 equiv.) and H2O (2 equiv.) in THF at room temperature and in refluxing THF, respectively. The structures of products 12a, 13a, 14j and 15j were elucidated on the basis of spectroscopic data. Both the reduction reactions of 5 by NaBH4 and H2 gas formed a new chiral carbon which was substituted by a hydroxy group, while the acid hydrolysis of 5 did not form a new chiral center. The polysubstituted tetrahydronaphthalen-1-ol 13a did not give satisfactory single crystals for X-ray analysis. Therefore, compound 13a was converted into 1-tetrahydronaphthalenyl acetate 16a by acylation with acetic anhydride in the presence of triethylamine and DMAP. Finally, the structures and relative configurations of compounds 12a, 15j and 16a were determined by single crystal X-ray diffraction analysis (see the X-ray structures of 12a, 15j and 16a in Fig. 2).
1) at 25 °C, the Pd–C catalyzed hydrogenation reaction of 5a under 50 atm H2 gas produced N-(1-hydroxy-2-phenyl-2-tetrahydronaphthalenyl)benzamide 13a in 76% yield. Furthermore, the acid hydrolysis of 5j led to the formation of two highly functional 1-tetralones 14j and 15j. The selective synthesis of 3-amino-3-(p-methoxyphenyl)-4-oxo-1-tetrahydronaphthalenyl benzoate 14j (62% yield) and N-(4-hydroxy-2-(p-methoxyphenyl)-1-oxo-2-tetrahydronaphthalenyl)benzamide 15j (65% yield) was achieved by the treatment of compound 5j with TsOH (2 equiv.) and H2O (2 equiv.) in THF at room temperature and in refluxing THF, respectively. The structures of products 12a, 13a, 14j and 15j were elucidated on the basis of spectroscopic data. Both the reduction reactions of 5 by NaBH4 and H2 gas formed a new chiral carbon which was substituted by a hydroxy group, while the acid hydrolysis of 5 did not form a new chiral center. The polysubstituted tetrahydronaphthalen-1-ol 13a did not give satisfactory single crystals for X-ray analysis. Therefore, compound 13a was converted into 1-tetrahydronaphthalenyl acetate 16a by acylation with acetic anhydride in the presence of triethylamine and DMAP. Finally, the structures and relative configurations of compounds 12a, 15j and 16a were determined by single crystal X-ray diffraction analysis (see the X-ray structures of 12a, 15j and 16a in Fig. 2).
Both 1,5-methanobenzo[f][1,3]oxazocin-6-ones and 6,10-methanopyrido[3,2-f][1,3]oxazocin-5-ones are new bridged heterocyclic compounds that no studies have been reported before. In contrast, a large number of 1-tetralone derivatives have been documented in the literature. 1-Tetralones not only are useful building blocks in organic synthesis,8 but also represent important scaffolds in a wide range of natural products and biologically active molecules of medicinal and agrochemical interest.9 This work provides a new method for the synthesis of highly functionalized 1-tetralone derivatives which would be further modified easily on the structures.
Conclusions
We have provided a two-step reaction method for the efficient synthesis of novel bridged heterocyclic compounds, namely 1,5-methanobenzo[f][1,3]oxazocin-6-ones, from simple and inexpensive starting materials. The method involved NHC-catalyzed azabenzoin condensation between o-vinyl aromatic aldehydes and N-benzoylarylimines and the radical-mediated cascade cyclizations of 2-aryl-2-benzamido-1-(2-vinylaryl)-1-ethanones. The intramolecular regioselective addition of the 2-C radical of 2-aryl-2-benzamido-1-(2-vinylaryl)-1-ethanones to the terminal carbon rather than the internal carbon of the vinyl group forms 3,4-dihydronaphthalen-1-one rather than 2,3-dihydroinden-1-one intermediates. This method could also be used in the assembly of the novel 6,10-methanopyrido[3,2-f][1,3]oxazocin-5-one framework from 2-vinylnicotinaldehyde and N-acylarylimines. The transformations of the resulting bridged heterocyclic compounds demonstrated the applications of this protocol not only in bridged aromatic ring-fused 1,3-oxazocines, but also in highly functional tetralone derivatives.Author contributions
Shuang-Shuo Niu performed the experiments. Ying Cheng conceived and supervised the work, analysed the data and wrote this manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21772015).Notes and references
- Some reviews on the radical cyclization reactions: (a) L. Yet, Free Radical in the Synthesis of Medium-Sized Ring, Tetrahedron, 1999, 55, 9349–9403 Search PubMed; (b) M.-H. Huang, W.-J. Hao and B. Jiang, Recent Advances in Radical-Enabled Bicyclization and Annulation/1,n-Bifunctionalization Reactions, Chem. – Asian J., 2018, 13, 2958–2977 Search PubMed; (c) J. Xuan and A. Studer, Radical cascade cyclization of 1,n-enynes and diynes for the synthesis of carbocycles and heterocycles, Chem. Soc. Rev., 2017, 46, 4329–4346 Search PubMed; (d) A. J. McCarroll and J. C. Walton, Programming organic molecules: design and management of organic syntheses through free-radical cascade processes, Angew. Chem., Int. Ed., 2001, 40, 2224–2248 Search PubMed.
- Examples for the construction of complex cyclic compounds via radical intramolecular cyclization reactions: (a) N. Zhou, M. Wu, M. Zhang, X. Zhou and W. Zhou, TBPB-initiated cascade cyclization of 3-arylethynyl-[1,1′-biphenyl]-2-carbonitriles with sulfinic acids: access to sulfone-containing cyclopenta[gh]phenanthridines, Org. Biomol. Chem., 2020, 18, 1733–1737 Search PubMed; (b) H.-M. Huang and D. J. Procter, Radical–Radical Cyclization Cascades of Barbiturates Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls, J. Am. Chem. Soc., 2016, 138, 7770–7775 Search PubMed; (c) B. Jiang, J.-X. Liu, Y. Wei and M. Shi, Nickel-Catalyzed Synthesis of Benzo[b]naphtho[1,2-d]azepine via Intramolecular Radical Tandem Cyclization of Alkyl Bromide-Tethered Alkylidenecyclopropanes, Org. Lett., 2018, 20, 6229–6233 Search PubMed; (d) P. S. Mahajan and S. B. Mhaske, Silver-Mediated Oxidative Decarboxylative Intramolecular Asymmetric Radical Cyclization (Csp3–Csp2) via Memory of Chirality: Access to Circumdatin Alkaloids, Org. Lett., 2018, 20, 2092–2095 Search PubMed.
- Reviews on the synthesis of natural products via radical reactions: (a) U. Koert, Radical Reactions as Key Steps in Natural Product Synthesis, Angew. Chem., Int. Ed. Engl., 1996, 35, 405–407 Search PubMed; (b) K. Hung, X. Hu and T. J. Maimone, Total synthesis of complex terpenoids employing radical cascade processes, Nat. Prod. Rep., 2018, 35, 174–202 Search PubMed.
- Examples for the synthesis of natural products via radical intramolecular cyclization reactions: (a) X. Wang, F. Liu, J. Yun, Z. Feng, J. Jiang, Y. Yang and P. Zhang, Iron-Catalyzed Synthesis of the Hexahydrocyclopenta[c]furan Core and Concise Total Synthesis of Polyflavanostilbene B, Angew. Chem., Int. Ed., 2018, 57, 10127–10131 Search PubMed; (b) Z. Yuan, X. Hu, H. Zhang, L. Liu, P. Chen, M. He, X. Xie, X. Wang and X. She, Total synthesis of conosilane A via a site-selective C–H functionalization strategy, Chem. Commun., 2018, 54, 912–915 Search PubMed; (c) W. Liu and B. Wang, Synthesis of (±)-Merrilactone A by a Desymmetrization Strategy, Chem. – Eur. J., 2018, 24, 16511–16515 Search PubMed.
- Reviews on the stereocontrol of radical reactions: (a) G. Bar and A. F. Parsons, Stereoselective radical reactions, Chem. Soc. Rev., 2003, 32, 251–263 Search PubMed; (b) T. V. RajanBabu, Stereochemistry of intramolecular free-radical cyclization reactions, Acc. Chem. Res., 1991, 24, 139–145 Search PubMed; (c) H. Miyabe and Y. Takemoto, Enantioselective radical cyclizations: a new approach to stereocontrol of cascade reactions, Chem. – Eur. J., 2007, 13, 7280–7286 Search PubMed.
- (a) Y.-J. Liu, Y.-L. Ding, S.-S. Niu, J.-T. Ma and Y. Cheng, N-Heterocyclic, Carbene/Palladium Cascade Catalysis: Construction of 2,2-Disubstitiuted Benzofuranones from the Reaction of 3-(2-Formylphenoxy)propenoates with Allylic Esters, J. Org. Chem., 2018, 83, 1913–1923 Search PubMed; (b) Z.-Y. Wang, Y.-L. Ding, S.-N. Li and Y. Cheng, N-Heterocyclic Carbene/Lewis Acid Dual Catalysis for the Divergent Construction of Enantiopure Bridged Lactones and Fused Indenes, J. Org. Chem., 2016, 81, 11871–11881 Search PubMed; (c) Z.-Y. Wang, Y.-L. Ding, G. Wang and Y. Cheng, Chiral N-Heterocyclic Carbene/Lewis Acid Cooperative Catalysis in the Reaction of 2-Aroylvinylcinnamaldehydes: A Switch of Reaction Pathway by Lewis Acid Activation, Chem. Commun., 2016, 52, 788–791 Search PubMed; (d) H.-Y. Dang, Z.-T. Wang and Y. Cheng, Changing Reaction Pathways of the Dimerization of 2-Formylcinnamates by N-Heterocyclic Carbene/Lewis Acid Cooperative Catalysis: An Unusual Cleavage of Carbon-Carbon Bond, Org. Lett., 2014, 16, 5520–5523 Search PubMed; (e) J.-T. Ma and Y. Cheng, Construction of Enantiopure Imine Bridged Benzo[c]azepinones by a Silver (I) and Chiral N-Heterocyclic Carbene Multicatalytic Reaction Sequence of N′-(2-Alkynylbenzylidene)hydrazides and Cyclopropanecarbaldehydes, Org. Chem. Front., 2020, 7, 3459–3467 Search PubMed; (f) Y.-f. Tong, J.-h. Mao, S. Wu, Y. Zhao and Y. Cheng, Changing the Reaction Pathway by NHC/Brønsted Base Cooperative Catalysis: Highly Stereoselective Synthesis of Multifunctional Benzo[a]fluoren-11-ones from the Dimerization of 2-(Aroylvinyl)arylaldehydes, J. Org. Chem., 2014, 79, 2075–2081 Search PubMed; (g) Y. Zhao, Z.-T. Wang and Y. Cheng, N-Heterocyclic, Carbene/Brønsted Base Cascade Catalysis: Base-Controlled Selective Synthesis of Multifunctional Benzofuran-3-ones or Flavone Derivatives from the Reaction of 3-(2-Formylphenoxy)propenoates with Imines, Adv. Synth. Catal., 2014, 356, 2580–2590 Search PubMed.
- Examples of the NHC-catalyzed reactions between aldehydes and imines: (a) J. A. Murry, D. E. Frantz, A. Soheili, R. Tillyer, E. J. J. Grabowski and P. J. Reider, Synthesis of α-Amido Ketones via Organic Catalysis: Thiazolium-Catalyzed Cross-Coupling of Aldehydes with Acylimines, J. Am. Chem. Soc., 2001, 123, 9696–9697 Search PubMed; (b) G.-Q. Li, L.-X. Dai and S.-L. You, Thiazolium-derived N-Heterocyclic Carbene-catalyzed Cross-coupling of Aldehydes with Unactivated Imines, Chem. Commun., 2007, 852–854 Search PubMed; (c) S. M. Mennen, J. D. Gipson, Y. R. Kim and S. J. Miller, Thiazolylalanine-Derived Catalysts for Enantioselective Intermolecular Aldehyde-Imine Cross-Couplings, J. Am. Chem. Soc., 2005, 127, 1654–1655 Search PubMed.
- Some examples for the synthesis of bioactive compounds using 1-tetralone derivatives as reactants: (a) R.-Y. Yang, D. Kizer, H. Wu, E. Volckova, X.-S. Miao, S. M. Ali, M. Tandon, R. E. Savage, T. C. K. Chan and M. A. Ashwell, Synthetic methods for the preparation of ARQ 501 (β-Lapachone) human blood metabolites, Bioorg. Med. Chem., 2008, 16, 5635–5643 Search PubMed; (b) M. A. E. Pinto-Bazurco Mendieta, M. Negri, C. Jagusch, U. Müller-Vieira, T. Lauterbach and R. W. Hartmann, Synthesis, Biological Evaluation, and Molecular Modeling of Abiraterone Analogues: Novel CYP17 Inhibitors for the Treatment of Prostate Cancer, J. Med. Chem., 2008, 51, 5009–5018 Search PubMed; (c) R. Ortega, H. Hübner, P. Gmeiner and C. F. Masaguer, Aromatic ring functionalization of benzolactam derivatives: New potent dopamine D3 receptor ligands, Bioorg. Med. Chem. Lett., 2011, 21, 2670–2674 Search PubMed; (d) M. Odagi, K. Furukori, K. Takayama, K. Noguchi and K. Nagasawa, Total Synthesis of Rishirilide B by Organocatalytic Oxidative Kinetic Resolution: Revision of Absolute Configuration of (+)-Rishirilide B, Angew. Chem., Int. Ed., 2017, 56, 6609–6612 Search PubMed.
- Some examples for natural products and biologically active compounds containing a 1-tetralone scaffold: (a) J. G. Allen and S. J. Danishefsky, The Total Synthesis of (±)-Rishirilide B, J. Am. Chem. Soc., 2001, 123, 351–352 Search PubMed; (b) S. Yao, C.-P. Tang, C.-Q. Ke and Y. Ye, Abietane Diterpenoids from the Bark of Cryptomeria fortunei, J. Nat. Prod., 2008, 71, 1242–1246 Search PubMed; (c) K. P. Devkota, D. Covell, T. Ransom, J. B. McMahon and J. A. Beutler, Inhibition of Human Colon Carcinoma Cells by Sesquiterpenoids and Tetralones of Zygogynum calothyrsum, J. Nat. Prod., 2013, 76, 710–714 Search PubMed; (d) J. M. Nyangulu, K. M. Nelson, P. A. Rose, Y. Gai, M. Loewen, B. Lougheed, J. W. Quail, A. J. Cutler and S. R. Abrams, Synthesis and biological activity of tetralone abscisic acid analogues, Org. Biomol. Chem., 2006, 4, 1400–1412 Search PubMed; (e) J. Leng, H.-L. Qin, K. Zhu, I. Jantan, M. A. Hussain, M. Sher, M. W. Amjad, M. Naeem-ul-Hassan, W. Ahmad and S. N. A. Bukhari, Evaluation of multifunctional synthetic tetralone derivatives for treatment of Alzheimer's disease, Chem. Biol. Drug Des., 2016, 88, 889–898 Search PubMed; (f) L. J. Legoabe, M. M. Van der Walt and G. Terre'Blanche, Evaluation of 2-benzylidene-1-tetralone derivatives as antagonists of A1 and A2 adenosine receptors, Chem. Biol. Drug Des., 2018, 91, 234–244 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectral data and copies of NMR spectra. CCDC 2072676 (5a), 2072678 (7a), 2072679 (12a), 2072680 (15j) and 2072681 (16a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1qo00483b |
| This journal is © the Partner Organisations 2021 |