Regioselective synthesis of polycyclic sulfones via radical-induced three-component bicyclization cascades†
Xiao-Yan
Qin
,
Long
He
,
Jing
Li
,
Wen-Juan
Hao
*,
Shu-Jiang
Tu
 and
Bo
Jiang
and
Bo
Jiang
 *
*
School of Chemistry & Materials Science, Jiangsu Normal University, Xuzhou, 221116, P. R. China. E-mail: wjhao@jsnu.edu.cn; jiangchem@jsnu.edu.cn
First published on 15th February 2019
Abstract
New radical-triggered three-component bicyclization cascades of 2-alkynyl aryldiazonium tetrafluoroborates with a sulfur dioxide surrogate DABCO·(SO2)2 and internal alkynes such as haloalkynes and ynones have been reported for the first time, leading to 49 examples of polycyclic sulfones with moderate to good yields and high levels of regioselectivity. This transformation initiated by an in situ generated arylsulfonyl radical proceeds efficiently under mild and neutral redox conditions, which provide an easy and metal-free pathway toward the formation of a range of richly decorated indeno[1,2-c]thiochromene 5,5-dioxides.
The development of robust, environmentally friendly, and atom-economical synthetic strategies is a constant driving force in chemical sciences.1 In this regard, bicyclization reaction cascades have proven to be an indispensable and versatile tool for assembling a high degree of complex polycyclic systems and natural products with elaborate cyclic frameworks, owing to their atom-economy, step economy, and high bond-forming and annulation efficiency (two new rings are formed in a single step).2 Specifically, with the resurgence of reliable and controllable radical chemistry, radical-triggered bicyclization reactions have received increased attention in recent years as such transformations could provide efficient preparative protocols for the direct collection of cyclic targets of chemical and biological importance which are difficult to obtain through traditional methods.3 A survey of literature reports reveals that the majority of current bicyclization reactions involve the radical-induced two-component transformations of enynes,4N-cyanamides5 and N-(2-cyanobiphenyl)-acrylamides.6 In sharp contrast, radical-induced multicomponent bicyclization cascades have received very little attention7 as such reactions encounter a great challenge in controlling the behavior of the radicals when trapped in the selection of radical acceptors. Therefore, the design and development of new radical multicomponent bicyclizations is of utmost interest to the organic chemist community.
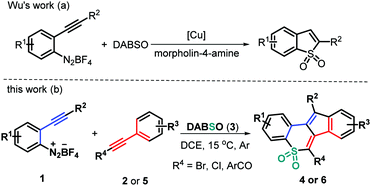
As a valuable class of sulfone-containing molecules, cyclic sulfones are widely prevalent in pharmaceutically important compounds, which have been found to exhibit a broad spectrum of biological activities.8 Moreover, some aryl-annulated sulfones have been widely applied in the field of organic photoconducting materials due to their unique electronical and optical properties.9 In addition, they have also served as versatile intermediates in Diels–Alder reactions10 and Ramberg–Bäcklund rearrangement.11 With these contributions in mind, much effort has been contributed toward identifying efficient methods for assembling structurally important cyclic sulfones, including ring-closing metathesis of acyclic sulfones,12 LDA-induced cyclization of alkynyl sulfones,13 domino cyclization of β-keto sulfones14 or alkynyl(aryl)iodonium salts,15 and the recent SO2 insertion of cyclic diaryliodonium salts16 and others.17 Furthermore, addition of arylsulfonyl radicals, starting from aryldiazonium salts and DABCO–bis(sulfur dioxide) (DABSO), into unsaturated systems has proven to be a reliable and practical SO2 insertion pathway for obtaining structurally diverse sulfones and sulfonamides in a convergent manner.18 For example, Wu and co-workers reported an elegant copper(I)-catalyzed annulation of 2-alkynylaryldiazonium tetrafluoroborates with DABSO in the presence of morpholin-4-amine to afford cyclic sulfonamides through SO2 insertion (Scheme 1a).19 In continuation with our efforts on bicyclization reactions20 and inspired by Wu's work,19 we have considered treating 2-alkynylaryldiazonium tetrafluoroborates 1 with DABSO (3) and haloalkynes 2 to realize the radical three-component bicyclization cascade. Interestingly, this transformation proceeded readily in a highly regioselective manner, resulting in a set of structurally diverse polycyclic sulfones 4 in moderate to good yields (Scheme 1b). Upon using ynones 5 as a radical acceptor to expand the synthetic utility of this protocol, the three-component bicyclization of 1 with 3 and 5 proceeds well through a similar radical addition–cyclization, furnishing good yields of the expected tetracyclic sulfones 6 in a highly regioselective manner (Scheme 1b). Herein, we report these two types of new metal-, catalyst-, and additive-free transformations.
Our initial investigation commenced with the treatment of 2-(phenylethynyl)benzenediazonium tetrafluoroborate (1a) with (bromoethynyl)benzene (2a) and DABSO (3) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at room temperature using 1,2-dichloroethane (DCE) as the reaction medium. The reaction generated indeno[1,2-c]thiochromene 5,5-dioxide 4a in 48% yield (Table S1, entry S1, see ESI†). When the substrate ratio of 1a, 2a and 3 was adjusted to 1.1
1 ratio at room temperature using 1,2-dichloroethane (DCE) as the reaction medium. The reaction generated indeno[1,2-c]thiochromene 5,5-dioxide 4a in 48% yield (Table S1, entry S1, see ESI†). When the substrate ratio of 1a, 2a and 3 was adjusted to 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1, the reaction proceeded more efficiently, providing the desired product 4a in a slightly higher yield (52%, entry S2, ESI†). Further increasing the amount of 2a and 3 led to a relatively lower conversion into product 4a (entries S3 and S4, ESI†). Subsequently, the effect of solvent on this radical transformation was investigated by screening various solvents such as dichloromethane (DCM), acetonitrile (CH3CN), tetrahydrofuran (THF), and 1,4-dioxane. The use of DCM as the reaction medium resulted in a decreased yield of product 4a (45%, entry S5, ESI†), whereas the other three solvents completely suppressed the reaction process (entries S6–S8, ESI†). Elevating the reaction temperature to 30 °C was not beneficial for the transformation, affording 4a in a reduced yield (46%, entry S9, ESI†). In contrast, a decrease of the reaction temperature seems to promote the conversion of 1a into product 4a as a higher yield (60%) was obtained when the reaction temperature was 15 °C (entry S10, ESI†). Further lowering the reaction temperature to 10 °C led to a reduced yield of 52% (entry S11, ESI†). An identical reaction was performed under Ar, delivering product 4a in a slightly higher yield (65%, entry S12, ESI†).
1.1, the reaction proceeded more efficiently, providing the desired product 4a in a slightly higher yield (52%, entry S2, ESI†). Further increasing the amount of 2a and 3 led to a relatively lower conversion into product 4a (entries S3 and S4, ESI†). Subsequently, the effect of solvent on this radical transformation was investigated by screening various solvents such as dichloromethane (DCM), acetonitrile (CH3CN), tetrahydrofuran (THF), and 1,4-dioxane. The use of DCM as the reaction medium resulted in a decreased yield of product 4a (45%, entry S5, ESI†), whereas the other three solvents completely suppressed the reaction process (entries S6–S8, ESI†). Elevating the reaction temperature to 30 °C was not beneficial for the transformation, affording 4a in a reduced yield (46%, entry S9, ESI†). In contrast, a decrease of the reaction temperature seems to promote the conversion of 1a into product 4a as a higher yield (60%) was obtained when the reaction temperature was 15 °C (entry S10, ESI†). Further lowering the reaction temperature to 10 °C led to a reduced yield of 52% (entry S11, ESI†). An identical reaction was performed under Ar, delivering product 4a in a slightly higher yield (65%, entry S12, ESI†).
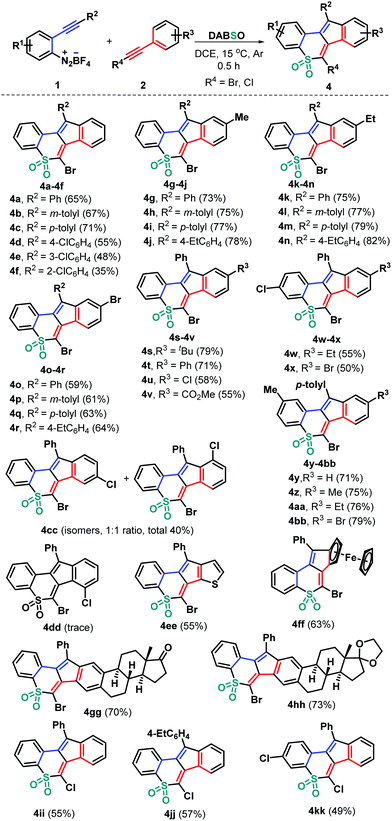
With these acceptable reaction conditions in hand, we then systematically investigated the generality of this metal-free three-component bicyclization cascade toward synthesizing polycyclic sulfones 4 by examining 2-alkynyl aryldiazonium tetrafluoroborate and haloalkyne components (Scheme 2). Different functional groups attached on both 2-arylalkynyl aryldiazonium tetrafluoroborates 1 and bromoalkynes 2 were evaluated by combining with DABSO. Electronically neutral, rich (methyl and ethyl), or poor (chloro) groups at different positions of the arylalkynyl moiety of substrates 1 could show good reactivity, generating the corresponding tetracyclic sulfones 4b–4cc in 35–82% yields. Among them, bromoalkynes bearing both electron-donating (methyl 2b, ethyl 2c, tert-butyl 2d, and phenyl 2e) and electron-withdrawing (chloro 2f, bromo 2g and ester 2h) groups at the para position of the phenyl ring could be successfully engaged in this transformation. The representative chloro and methyl substituents were independently introduced into the internal aryl ring of substrates 1 and were then employed to react with 2 and DABSO under standard conditions. The reactions worked well to deliver the corresponding products 4w–4bb in 50–79% yields. Due to the presence of a chloro group at the meta position of the phenyl ring in the bromoalkyne unit (2i) two inseparable isomers 4cc were obtained in a total yield of 40%. However, bromoalkyne 2j having a 2-chlorophenyl group only provided a trace amount of product 4dd, which failed to be isolated. Moreover, 2-thienyl bromoalkyne 2k proved to be suitable for this transformation, affording tetracyclic sulfone 4ee in 55% yield. Besides, ferrocenyl bromoalkyne 2l would be accommodated, confirming the efficiency of the transformation, as the product 4ff was obtained in 63% yield. The feasible modification of important bioactive molecular frameworks is a basis for the evaluation of a practical protocol. In this context, estrone-derived bromoalkynes 2m and 2n were prepared, which tolerated this radical bicyclization cascade well, giving complex products 4gg and 4hh that comprise both estrone and cyclic sulfone units in 70% and 73% yields, respectively. Notably, chloroalkyne 2o was a good candidate for this radical transformation (4ii–4kk), further highlighting the generality and great potential of the developed synthetic strategy.‡
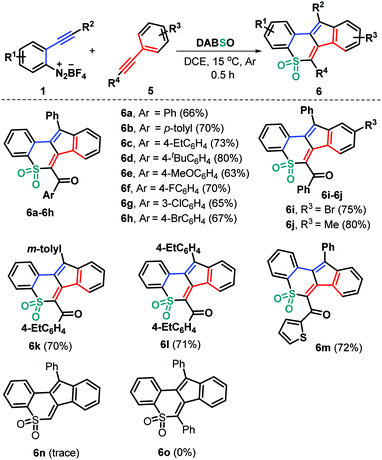
To expand the utility of this methodology, we devoted our next efforts to assessing the feasibility of the current three-component bicyclization cascade toward cyclic sulfone syntheses by exploiting ynones to replace the haloalkyne component (Scheme 3). As anticipated, the reaction of the preformed ynones 5 with substrates 1 and DABSO (3) proceeded readily under the standard reaction conditions, leading to structurally diverse tetracyclic sulfones 6a–6m with generally good yields. Ynones 5 having electron-neutral (H 5a), electron-rich (Me 5b, Et 5c, t-Bu 5d, and MeO 5e) and electron-deficient (F 5f, Cl 5g, and Br 5h) groups attached to the aroyl moiety can participate in the current bicyclization chemistry, thus resulting in the corresponding products 6a–6h in 63–80% yields. Alternatively, the presence of functional groups (R3) including methyl (5i) and bromo (5j) groups residing in the arylalkynyl moiety of the substrates 5 was compatible in this transformation (6i and 6j). Furthermore, the reaction proceeded readily with a variety of functional groups (R2) on the arene ring of substrates 1, giving access to the products 6k and 6l in 70% and 71% yields, respectively. Similarly, the 2-thienyl ynone 5k showed high reactivity, enabling direct radical bicyclization to access the corresponding 2-thienyl substituted product 6m in 72% yield. However, phenylene acetylene 5l and 1,2-diphenylethyne 5m were proved to be ineffective, as the generation of the corresponding products 6n and 6o was completely suppressed under the standard conditions (Scheme 3).
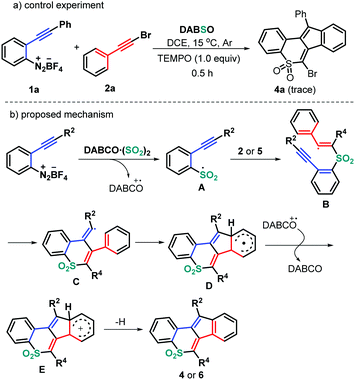
On the basis of the above observations and literature reports, a reasonable mechanism is proposed as shown in Scheme 4. It is well known that the combination of DABSO with aryldiazonium tetrafluoroborates would provide the arylsulfonyl radical A and a tertiary amine (DABCO) radical cation.18 Still, the presence of 2.0 equivalents of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) completely suppressed the formation of the normal product 4a (Scheme 4a), indicating that the reaction process could include a radical mechanism. Therefore, the regioselective radical addition of the in situ generated sulfonyl radical A, from 2-alkynyl aryldiazonium tetrafluoroborates and DABSO, into the triple bond of haloalkynes 2 (or ynones 5) yields the alkenyl radical intermediate B. Intermediate B undergoes a 6-exo-dig cyclization/5-endo-trig cyclization to give the radical intermediate D, followed by a single electron transfer (SET) in the presence of a tertiary amine (DABCO) radical cation to access intermediate E. Finally, the desired products 4 or 6 are produced through a deprotonation process (Scheme 4b).
In conclusion, we have developed two new types of radical three-component bicyclization cascades of 2-alkynyl-aryldiazonium tetrafluoroborate, DABSO and internal alkynes under oxidant- and catalyst-free conditions. This transformation initiated by the in situ generated arylsulfonyl radical proceeds efficiently under mild and neutral redox conditions, regioselectively giving access to indeno[1,2-c]thiochromene 5,5-dioxides with different substituent patterns in moderate to good yields. A tandem radical process with the insertion of sulfur dioxide and 6-exo-dig/5-endo-trig bicyclization is proposed, with the formation of multiple chemical bonds. The transformation is attractive since it provides an easy and metal-free pathway toward the formation of a range of richly decorated polycyclic sulfones. Further investigation on their applications will be carried out in due course.
We are grateful for financial support from the NSFC (No. 21602087 and 21871112), the Outstanding Youth Fund of JSNU (YQ2015003), NSF of Jiangsu Province (BK20160212), and the Qing Lan Project of Jiangsu Education Committee (QL2016006).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) B. M. Trost, Acc. Chem. Res., 2002, 35, 695 CrossRef CAS PubMed; (b) S. Kotha, D. Goyal and A. S. Chavan, J. Org. Chem., 2013, 78, 12288 CrossRef CAS PubMed; (c) K. Banert and O. Plefka, Angew. Chem., Int. Ed., 2011, 50, 6171 CrossRef CAS PubMed; (d) B. M. Trost, F. D. Toste and K. Greenman, J. Am. Chem. Soc., 2003, 125, 4518 CrossRef CAS PubMed; (e) B. Jiang, T. Rajale, W. Wever, S.-J. Tu and G. Li, Chem. – Asian J., 2010, 5, 2318 CrossRef CAS PubMed.
- For selected examples, see: (a) L. Wang, L.-X. Shi, L. Liu, Z.-X. Li, T. Xu, W.-J. Hao, G. Li, S.-J. Tu and B. Jiang, J. Org. Chem., 2017, 82, 3605 CrossRef CAS PubMed; (b) Z.-Z. Chen, S. Liu, G. Xu, S. Wu, J.-N. Miao, B. Jiang, W.-J. Hao, S.-L. Wang, S.-J. Tu and G. Li, Chem. Sci., 2015, 6, 6654 RSC; (c) S. Liu, X.-C. Lan, K. Chen, W.-J. Hao, G. Li, S.-J. Tu and B. Jiang, Org. Lett., 2017, 19, 3831 CrossRef CAS PubMed; (d) Q. Gao, P. Zhou, F. Liu, W.-J. Hao, C. Yao, B. Jiang and S.-J. Tu, Chem. Commun., 2015, 51, 9519 RSC; (e) Y. Tian, L. Tian, X. He, C.-J. Li, X. Jia and J. Li, Org. Lett., 2015, 17, 4874 CrossRef CAS PubMed; (f) S. Jia, S. Su, C. Li, X. Jia and J. Li, Org. Lett., 2014, 16, 5604 CrossRef CAS PubMed; (g) J.-Y. Wang, P. Zhou, G. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Org. Lett., 2017, 19, 6682 CrossRef CAS PubMed.
- For selected reviews, see: (a) M.-H. Huang, W.-J. Hao and B. Jiang, Chem. – Asian J., 2018, 13, 2958 CrossRef CAS PubMed; (b) F. Wang, P. Chen and G. Liu, Acc. Chem. Res., 2018, 51, 2036 CrossRef CAS PubMed; (c) X.-Q. Chu, D. Ge, Z.-L. Shen and T.-P. Loh, ACS Catal., 2018, 8, 258 CrossRef CAS; (d) J. Luo and W.-T. Wei, Adv. Synth. Catal., 2018, 360, 2076 CrossRef CAS; (e) J. Xie, H. Jin and K. S. A. Hashmi, Chem. Soc. Rev., 2017, 46, 5193 RSC; (f) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2016, 55, 58 CrossRef CAS PubMed; (g) X.-H. Ouyang, R.-J. Song and J.-H. Li, Chem. – Asian J., 2018, 13, 2316 CrossRef CAS PubMed.
- For selected examples, see: (a) Z.-J. Shen, H.-N. Shi, W.-J. Hao, S.-J. Tu and B. Jiang, Chem. Commun., 2018, 54, 11542 RSC; (b) Y.-L. Zhu, B. Jiang, W.-J. Hao, J.-K. Qiu, J. Sun, D.-C. Wang, P. Wei, A.-F. Wang, G. Li and S.-J. Tu, Org. Lett., 2015, 17, 6078 CrossRef CAS PubMed; (c) Y. Liu, R.-J. Song, S.-L. Luo and J.-H. Li, Org. Lett., 2018, 20, 212 CrossRef CAS PubMed; (d) M. Hu, R.-J. Song and J.-H. Li, Angew. Chem., Int. Ed., 2015, 54, 608 CAS; (e) B. Liu, C.-Y. Wang, M. Hu, R.-J. Song, F. Chen and J.-H. Li, Chem. Commun., 2017, 53, 1265 RSC; (f) M. Hu, H.-X. Zou, R.-J. Song, J.-N. Xiang and J.-H. Li, Org. Lett., 2016, 18, 6460 CrossRef CAS PubMed.
- (a) J. Zheng, Z.-Y. Deng, Y. Zhang and S.-L. Cui, Adv. Synth. Catal., 2016, 358, 746 CrossRef CAS; (b) J. Zheng, Y. Zhang, D.-H. Wang and S.-L. Cui, Org. Lett., 2016, 18, 1768 CrossRef CAS PubMed; (c) X. Liu, P. Qian, Y. Wang and Y. Pan, Org. Chem. Front., 2017, 4, 2370 RSC.
- (a) X.-X. Li, X.-X. Fang, S.-Y. Zhuang, P. Liu and P.-P. Sun, Org. Lett., 2017, 19, 3580 CrossRef CAS PubMed; (b) Y.-L. Yu, Z.-Q. Cai, W.-W. Yuan, P. Liu and P.-P. Sun, J. Org. Chem., 2017, 82, 8148 CrossRef CAS PubMed; (c) Y.-L. Yu, W.-W. Yuan, H.-S. Huang, Z.-Q. Cai, P. Liu and P.-P. Sun, J. Org. Chem., 2018, 83, 1654 CrossRef CAS PubMed; (d) L.-J. Wu, Y. Yang, R.-J. Song, J.-X. Yu, J.-H. Li and D.-L. He, Chem. Commun., 2018, 54, 1367 RSC.
- For reviews see: (a) G. Qiu, K. Zhou, L. Gao and J. Wu, Org. Chem. Front., 2018, 5, 691 RSC; (b) G. Qiu, L. Lai, J. Cheng and J. Wu, Chem. Commun., 2018, 54, 10405 RSC; (c) G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC; (d) M.-H. Huang, W.-J. Hao, G. Li, S.-J. Tu and B. Jiang, Chem. Commun., 2018, 54, 10791 RSC.
- (a) A. K. Ghosh, W. J. Thompson, P. M. D. Fitzgerald, J. C. Culberson, M. G. Axel, S. P. McKee, J. R. Huff and P. S. Anderson, J. Med. Chem., 1994, 37, 2506 CrossRef CAS PubMed; (b) C. U. Kim, L. R. McGee, S. H. Krawczyk, E. Harwood, Y. Harada, S. Swaminathan, N. Bischofberger, M. S. Chen, J. M. Cherrington, S. F. Xiong, L. Griffin, K. C. Cundy, A. Lee, B. Yu, S. Gulnik and J. W. Erickson, J. Med. Chem., 1996, 39, 3431 CrossRef CAS PubMed; (c) F. Velázquez, M. Sannigrahi, F. Bennett, R. G. Lovey, A. Arasappan, S. Bogen, L. Nair, S. Venkatraman, M. Blackman, S. Hendrata, Y. Huang, R. Huelgas, P. Pinto, K.-C. Cheng, X. Tong, A. T. McPhail and F. G. Njoroge, J. Med. Chem., 2010, 53, 3075 CrossRef PubMed; (d) M. G. Brant and J. E. Wulff, Org. Lett., 2012, 14, 5876 CrossRef CAS PubMed.
- (a) N. S. Simpkins, Sulfones in Organic Synthesis, Pergamon Press, Oxford, 1993 Search PubMed; (b) Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953 CrossRef CAS PubMed; (c) A. Mishra, C. Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS PubMed; (d) L. Duan, J. Qiao, Y. Sun and Y. Qiu, Adv. Mater., 2011, 23, 1137 CrossRef CAS PubMed; (e) M. E. Cinar and T. Ozturk, Chem. Rev., 2015, 115, 3036 CrossRef CAS PubMed.
- For review, see: (a) J. Leonard, A. B. Hague and J. A. Knight, in Organosulfur Chemistry, ed. P. Page, Academic Press, San Diego, 1998, vol. 2, p. 6. For recent examples, see Search PubMed; (b) T. K. Shing and Y. Tang, J. Chem. Soc., 1994, 1, 1625 Search PubMed; (c) J. Leonard, A. B. Hague and M. F. Jones, Tetrahedron Lett., 1997, 38, 3071 CrossRef CAS.
- (a) L. A. Paquette, Org. React., 1977, 25, 1 CAS; (b) L. A. Paquette, Acc. Chem. Res., 1968, 1, 209 CrossRef CAS; (c) D. I. MaGee and E. J. Beck, J. Org. Chem., 2000, 65, 8367 CrossRef CAS PubMed; (d) E. Vedejs and S. P. Singer, J. Org. Chem., 1978, 43, 4884 CrossRef CAS; (e) G. D. McAllister and R. J. K. Taylor, Tetrahedron Lett., 2001, 42, 1197 CrossRef CAS; (f) V. Cere, G. Mantovani, F. Peri, S. Pollicino and A. Rici, Tetrahedron, 2000, 56, 1225 CrossRef CAS.
- Q. Yao, Org. Lett., 2002, 4, 427 CrossRef CAS PubMed.
- M. S. Hossain and A. L. Schwan, Org. Lett., 2011, 13, 5330 CrossRef CAS PubMed.
- Q.-G. Wang, Q.-Q. Zhou, J.-G. Deng and Y.-C. Chen, Org. Lett., 2013, 15, 4786 CrossRef CAS PubMed.
- A. Rodríguez and W. J. Moran, J. Org. Chem., 2016, 81, 2543 CrossRef PubMed.
- M. Wang, S. Chen and X. Jiang, Org. Lett., 2017, 19, 4916 CrossRef CAS PubMed.
- (a) J. P. John and A. V. Novikov, Org. Lett., 2007, 9, 61 CrossRef CAS PubMed; (b) C. Bosset, G. Lefebvre, P. Angibaud, I. Stansfield, L. Meerpoel, D. Berthelot, A. Guérinot and J. Cossy, J. Org. Chem., 2017, 82, 4020 CrossRef CAS PubMed.
- For selected examples, see: (a) H. Wang, S. Sun and J. Cheng, Org. Lett., 2017, 19, 5844 CrossRef CAS PubMed; (b) Z.-J. Shen, Y.-N. Wu, C.-L. He, L. He, W.-J. Hao, A.-F. Wang, S.-J. Tu and B. Jiang, Chem. Commun., 2018, 54, 445 RSC; (c) Y. Wang, L. Deng, J. Zhou, X. Wang, H. Mei, J. Han and Y. Pan, Adv. Synth. Catal., 2018, 360, 1060 CrossRef CAS; (d) K. Sun, Z. Shi, Z. Liu, B. Luan, J. Zhu and Y. Xue, Org. Lett., 2018, 20, 6687 CrossRef CAS PubMed; (e) D. Zheng, J. Yu and J. Wu, Angew. Chem., Int. Ed., 2016, 55, 11925 CrossRef CAS PubMed; (f) K. Zhou, H. Xia and J. Wu, Org. Chem. Front., 2017, 4, 1121 RSC; (g) X. Wang, Y. Li, G. Qiu and J. Wu, Org. Chem. Front., 2018, 5, 2555 RSC; (h) S. Liu, K. Chen, W.-J. Hao, X.-C. Tu, S.-J. Tu and B. Jiang, J. Org. Chem., 2019, 84, 1964 CrossRef CAS PubMed.
- Y. Luo, X. L. Pan, C. Chen, L. Q. Yao and J. Wu, Chem. Commun., 2015, 51, 180 RSC.
- (a) A.-F. Wang, W.-J. Hao, Y.-L. Zhu, G. Li, P. Zhou, S.-J. Tu and B. Jiang, ACS Omega, 2018, 3, 1482 CrossRef CAS; (b) P. Zhou, J.-Y. Wang, T.-S. Zhang, G. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Chem. Commun., 2018, 54, 164 RSC; (c) F. Liu, J.-Y. Wang, P. Zhou, G. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Angew. Chem., Int. Ed., 2017, 56, 15570 CrossRef CAS PubMed; (d) J.-K. Qiu, B. Jiang, Y.-L. Zhu, W.-J. Hao, D.-C. Wang, J. Sun, P. Wei, S.-J. Tu and G. Li, J. Am. Chem. Soc., 2015, 137, 8928 CrossRef CAS PubMed; (e) P. Zhou, W.-J. Hao, J.-P. Zhang, B. Jiang, G. Li and S.-J. Tu, Chem. Commun., 2015, 51, 13012 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1888213 (4g). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc00324j |
| ‡ The crystal of compound 4g belongs to the monoclinic space group P2(1)/n with dimensions of 0.42 × 0.25 × 0.14 mm and a total of 9016 reflections were collected in the 2.63 < θ < 25.02° range by using an ω scan mode and 3240 were independent (Rint = 0.0441), a = 8.4326(7) Å, b = 11.5349(9) Å, c = 18.9409(15) Å, a = g = 90°, β = 91.0070(10)°, V = 1842.1(3) Å3, Mr = 435.32, Z = 4, Dc = 1.570 g cm−3, μ(MoKα) = 2.361 mm−1, F(000) = 880, the final R = 0.0324 and wR = 0.0769. |
| This journal is © The Royal Society of Chemistry 2019 |