Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A self-reporting AIE probe with a built-in singlet oxygen sensor for targeted photodynamic ablation of cancer cells†
Youyong
Yuan
a,
Chong-Jing
Zhang
a,
Shidang
Xu
a and
Bin
Liu
*ab
aDepartment of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, 117585, Singapore
bInstitute of Materials Research and Engineering, Agency for Science, Technology and Research (A*STAR), 3 Research Link, 117602, Singapore. E-mail: cheliub@nus.edu.sg
First published on 23rd November 2015
Abstract
The real-time monitoring of reactive oxygen species (ROS, particularly singlet oxygen) generation during photodynamic therapy is a great challenge due to the extremely short half-life and small radius of action. To tackle this issue, we herein report a bioprobe composed of a red emissive photosensitizer (PS) with aggregation-induced emission (AIE) characteristics and a fluorogenic green emissive rhodol dye conjugated via a singlet oxygen cleavable aminoacrylate (AA) linker. The probe emits red fluorescence in water, and the red emissive PS can be used for probe self-tracking. Upon image-guided light irradiation, the generated singlet oxygen cleaves the AA linker to yield green fluorescence turn-on of rhodol, which offers real-time and in situ monitoring of singlet oxygen generation during photodynamic ablation of cancer cells, providing a strategy for the early evaluation of the therapeutic effect.
Introduction
Targeted drug delivery has been extensively studied for cancer therapy.1 However, precisely answering when, where, and how the therapeutic agents are delivered and what their functions are remain challenging. Recently, theranostic delivery systems that combine therapy and diagnostic imaging have attracted great attention in biomedical research.2 They offer the opportunity to evaluate therapeutic regimes and provide useful information for dose adjustment and prognosis of individual patients to achieve the ultimate goal of personalized medicine.3 The systems require real-time monitoring of cancer therapy, particularly if the process can be detected in a non-invasive manner. Recently, a fluorophore or contrast agent conjugated with a chemotherapy drug via a tumor-specific responsive linker has been developed for the real-time and in situ reporting of drug activation.4 The design principle mainly relies on the change of fluorescence intensity or magnetic resonance imaging (MRI) signal concomitantly occurring with the drug activation.Compared to chemotherapy drugs, photosensitizer (PS) drugs have recently received increased attention because photodynamic therapy (PDT) does not acquire drug resistance, and it shows limited side effects as its toxicity is light-controllable.5 PDT is based on the concept that PSs generate cytotoxic reactive oxygen species (ROS), particularly singlet oxygen, upon light irradiation to induce cell death. Although some fluorescent probes have been developed for singlet oxygen detection based on fluorescence changes,6 the separate administration of the PS and the probe does not guarantee them to have the same location in cells.6b As singlet oxygen has very short lifetime (<40 ns) and a small radius of action (<20 nm),7 it remains a challenge to monitor singlet oxygen generation during PDT in real-time and in situ at the subcellular level. The design of a probe that can simultaneously image and ablate cancer cells with real-time monitoring of the singlet oxygen generation during PDT is technically challenging and it is practically not available yet.
Traditional PSs, such as porphyrin, with large planar structures show aggregation-caused quenching (ACQ) with weak fluorescence and reduced singlet oxygen generation in solid state or in aggregates due to π–π stacking.8 Recently, fluorogens with aggregation-induced emission characteristics (AIEgens) have received extensive attention for biosensors and bioimaging.9 Distinctively, AIEgens emit intensively in aggregated state due to the restriction of intramolecular motions.9a More recently, AIEgen PSs with high signal-to-background ratio for fluorescence imaging and efficient singlet oxygen generation in aggregates have been developed for image-guided PDT.10
As a proof of concept, in this contribution, by taking advantages of AIE PSs, we designed a probe for imaging, ablating cancer cells and real-time monitoring of singlet oxygen generation during PDT. The probe is composed of an AIE PS and a fluorogenic rhodol dye conjugated through a singlet oxygen cleavable aminoacrylate (AA) linker.11 The probe is red emissive in aqueous media, which can be utilized for probe self-tracking. Upon image-guided light irradiation, the green fluorescence of rhodol intensifies greatly as the generated singlet oxygen can cleave the AA linker to release the highly emissive fluorophore 6-hydroxy-3H-xanthen-3-one,12 which can be used for the real-time and in situ monitoring of singlet oxygen generation during PDT.
Results and discussion
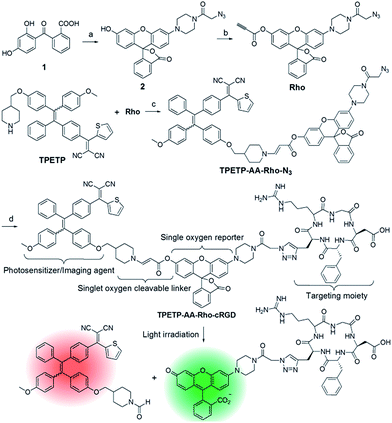
The synthetic route to the probe is shown in Scheme 1. Compound 2 was obtained by the reaction of compound 1 with 2-azidoacetic acid and then it was further esterified with propiolic acid. The AIE PS (TPETP) was synthesized according to Scheme S1.† It reacted with compound 3 through an yne-amine click reaction to yield TPETP-AA-Rho-N3. The probe was obtained after the click reaction between TPETP-AA-Rho-N3 and alkyne-functionalized cRGD, and was denoted as TPETP-AA-Rho-cRGD. The detailed synthetic procedure and characterization data of the probe are shown in Fig. S1–S8.†The absorption spectrum of TPETP is shown in Fig. S9A,† which shows a shoulder peak at 430 nm and an emission maximum at 650 nm. The quantum yield (Φ) of TPETP is determined to be 0.13 ± 0.01 using 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (DCM) as the standard (Φ = 0.43). The AIE property of TPETP was confirmed by studying its PL spectra in DMSO/water mixtures with different water fractions (Fig. 1A). The formation of TPETP aggregates in aqueous solution was also confirmed (Fig. S9†). The probe TPETP-AA-Rho-cRGD forms aggregates in DMSO and phosphate buffered saline (PBS) mixture (v/v = 1/199) with sizes of 140 ± 13 nm (Fig. 1B). The absorption and emission spectra of the probe are shown in Fig. 1C. The probe shows an absorption peak at 430 nm with a very weak shoulder at 510 nm, indicating that Rho exists in the ring closed form.12 Upon excitation at 430 nm, the emission spectrum of the probe is similar to that of TPETP and the green fluorescence of Rho is weak. The singlet oxygen generation of the probe upon visible light (λ = 400–700 nm) irradiation was studied using 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) as an indicator. As shown in Fig. 1D and S10,† only in the presence of TPETP was a decrease in the ABDA absorption observed, an indication of singlet oxygen generation. The singlet oxygen quantum yield (Φ) of TPETP in DMSO/PBS (v/v = 1/199) was determined to be 0.68 using Rose Bengal (RB) as the standard photosensitizer (ΦRB = 0.75 in water), which is much higher than the clinically used PSs such as Photofrin® (Φ = 0.28) or Laserphyrin® (Φ = 0.48).7 The absorbance decrease of ABDA was inhibited in the presence of the singlet oxygen scavenger ascorbic acid (Asc).
The photoluminescence (PL) change of the probe upon visible light irradiation was also monitored over different durations of light irradiation. As shown in Fig. 1E, the fluorescence signal of Rho shows a quick and steady increase upon light irradiation, and the fluorescence is 19-fold brighter than the original Rho emission from the probe after light irradiation for 7 minutes. In contrast, no significant fluorescence increase is observed when Asc is present. It should be noted that the absorption and emission of the compounds Rho and TPETP do not change upon light irradiation under the same conditions. It was also found that the probe fluorescence is pH independent, but after light illumination the fluorescence of the green emissive product is pH dependent (Fig. S11†), which agrees with the fluorescent properties of 6-hydroxy-3H-xanthen-3-one.12 The selectivity of the probe towards different ROS including singlet oxygen (1O2), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), superoxide (O2˙−), and hydroxyl radicals (˙OH) in N2-purged PBS was also studied. As shown in Fig. S11C,† the fluorescence of Rho shows significant enhancement only in the presence of singlet oxygen, which confirms that the AA linker can be selectively cleaved by singlet oxygen.11b Reverse-phase HPLC and mass analyses further confirmed the anticipated linker cleavage after light irradiation (Fig. S12†). To assess whether the probe can monitor the singlet oxygen generation in situ, we evaluated the correlation between the absorbance decrease of ABDA and the fluorescence increase of Rho. The results shown in Fig. 1F clearly demonstrate that the fluorescence increase of Rho is linearly (R2 = 0.98) correlated with singlet oxygen generation, which indicates that the fluorescence changes of Rho can be used for real-time monitoring of singlet oxygen generation during PDT.
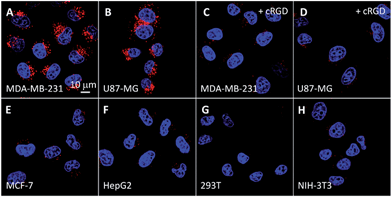
Receptor mediated cancer therapy is attractive as it can facilitate the cellular internalization through receptor mediated endocytosis. To demonstrate the feasibility of cancer targeting, the probe was incubated with αvβ3 integrin overexpressed MDA-MB-231 and U87-MG cancer cells and low αvβ3 integrin expressed MCF-7 and HepG2 cancer cells as well as NIH/3T3 and 293T normal cells. The red fluorescence in U87-MG and MDA-MB-231 cells increases with incubation time (Fig. S13 and S14†) and the intensities are much higher than the other cell lines (Fig. 2). However, the fluorescence signal is greatly inhibited when the cells are pretreated with cRGD, confirming that the probe is internalized by cancer cells via receptor-mediated endocytosis. To identify the cellular location of the probe after αvβ3 integrin mediated endocytosis, colocalization experiments were performed by co-staining the probe labelled cells with a fluorescent endo-/lysosome tracker or mitochondria tracker. The co-localized results shown in Fig. S13† demonstrate that the probe is entrapped in endo-/lysosomes, but not mitochondria, after the cellular internalization, which agrees with the nanoaggregate nature of the probe.
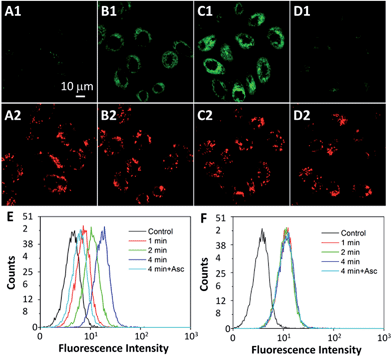
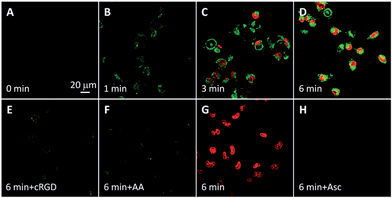
The intracellular singlet oxygen generation induced Rho fluorescence change was studied by confocal microscopy. As shown in Fig. 3, the fluorescence signal is weak without light irradiation, but the fluorescence of Rho is increased along with the light irradiation while that of TPETP remains constant. After 4 min of light irradiation, the green fluorescence is very strong. In contrast, the fluorescence of Rho remains weak after light irradiation when the singlet oxygen scavenger Asc is presented. The fluorescence changes of Rho and TPETP upon light irradiation were also confirmed by flow cytometric analysis (Fig. 3E and F). Similar phenomena were also observed in U87-MG cells (Fig. S15†). The real-time and in situ monitoring of the Rho fluorescence change provides a convenient and reliable strategy for self-feedback of singlet oxygen generation during PDT.
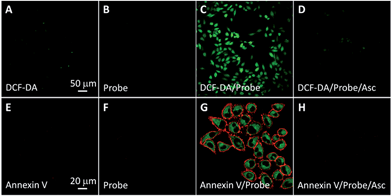
The singlet oxygen generation of TPETP in cells upon light irradiation was studied using 2′,7′-dichlorofluorescin diacetate (DCF-DA) as a cell-permeable indicator. DCF-DA is non-fluorescent but can be oxidized by ROS to yield highly fluorescent 2′,7′-dichlorofluorescein (DCF). As shown in Fig. 4A–D, strong green fluorescence can only be observed when the cells were treated with TPETP followed by light irradiation. When the singlet oxygen scavenger Asc is added, the DCF fluorescence is negligible which further confirms the singlet oxygen generation. The singlet oxygen induced cell apoptosis was studied by Cy5-tagged annexin V as an indicator. Cy5-annexin V can distinguish apoptotic cells from viable ones as annexin V can bind to phosphatidylserine expressed on apoptotic cellular membrane. As shown in Fig. 4E–H and S16,† the red fluorescence of Cy5 is only observed on cell surfaces when the cells were treated with the probe followed by light irradiation. These results demonstrate that the probe can generate singlet oxygen in cells and induce cell apoptosis after exposure to light.
The probe-induced cell necrosis upon light irradiation was subsequently monitored by propidium iodide (PI) as an indicator, which only stains the nuclei of necrotic cells. The probe incubated MDA-MB-231 and U87-MG cells were irradiated with light for different time with TPETP as positive control. As shown in Fig. 5 and S17,† before light irradiation, both green fluorescence of Rho and red fluorescence of PI are negligible. Along with the light irradiation, the green fluorescence is increased and the red fluorescence from the nuclei is observed in some cells, indicating the generated singlet oxygen can turn on the Rho fluorescence and induce cell necrosis. The fluorescence signals of Rho and PI are weak when the cells were pretreated with cRGD or in the presence of Asc. To confirm that the cell necrosis is induced by the singlet oxygen generation of TPETP with light irradiation, the MDA-MB-231 cells were also treated with free TPETP and strong PI fluorescence can be observed while the fluorescence signal is negligible in the presence of Asc. These results confirm that the fluorescence change of Rho can be used to report singlet oxygen generation and predict the therapeutic effect in real-time.
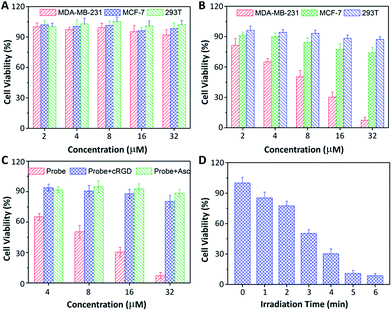
Low dark toxicity but high toxicity upon light irradiation is highly essential for phototherapy to minimize side effects and enhance the therapeutic outcome. The cytotoxicity of the probe-incubated cells in the dark or with light irradiation was evaluated by MTT assay. As shown in Fig. 6, the probe shows no apparent toxicity to MDA-MB-231, MCF-7 and 293T cells in dark conditions. Upon light irradiation, the probe only shows significant cytotoxicity to MDA-MB-231 cells while the toxicity to MCF-7 and 293T cells is minimal. The half-maximal inhibitory concentration (IC50) of the probe to MDA-MB-231 cells in the dark and upon light irradiation are 219.1 and 8.3 μM, respectively. The phototoxicity index,13 which is the ratio between the IC50 values in the dark and upon light irradiation, was calculated to be 26.4, indicating that the probe is a good phototherapeutic agent. When the MDA-MB-231 cells were pretreated with free cRGD or Asc, the toxicity of the probe was greatly diminished. In addition, the cytotoxicity of the probe to MDA-MB-231 cells is dependent on the irradiation time. Similar results were also observed for U87-MG, HepG2 and NIH/3T3 cells (Fig. S18†). It should be noted that Rho is almost non-toxic to all the tested cells regardless of light irradiation (Fig. S19†).
Conclusions
In conclusion, we have developed a novel probe for the theranostic photodynamic ablation of cancer cells. The probe contains an AIE PS which can efficiently generate singlet oxygen upon light irradiation and the intrinsic red fluorescence can be used for image-guided PDT. The cell experiments confirmed that the probe can be selectively taken up by αvβ3 integrin overexpressed cancer cells and localized in endo-lysosomes, which can be tracked by the red fluorescence of TPETP. Upon image-guided light irradiation, the singlet oxygen generation in cells is able to induce cell death, which can be monitored by the fluorescence change of Rho. Therefore, our probe design provides a new strategy for potential theranostic PDT with real-time monitoring of singlet oxygen generation through non-invasive fluorescence signal changes, which offers a new avenue for early evaluation of the PDT therapeutic effect. Further development of the AIE PSs with long wavelength absorption will allow us to conduct in vivo PDT in the near future.Acknowledgements
We thank the SMART (R279-000-378-592), the Ministry of Education (R279-000-391-112), Singapore NRF Investigatorship (R279-000-444-281) and the Institute of Materials Research and Engineering of Singapore (IMRE/14-8P1110) for financial support.Notes and references
- R. Langer, Nature, 1998, 392, 5–10 CAS.
- (a) J. Xie, S. Lee and X. Y. Chen, Adv. Drug Delivery Rev., 2010, 62, 1064–1079 CrossRef CAS PubMed; (b) R. Kumar, W. S. Shin, K. Sunwoo, W. Kim, S. Koo, S. Bhuniy and J. S. Kim, Chem. Soc. Rev., 2015, 44, 6670–6683 RSC.
- R. L. Schilsky, Nat. Rev. Drug Discovery, 2010, 9, 363–366 CrossRef CAS PubMed.
- (a) M. H. Lee, Z. Yang, C. W. Lim, Y. H. Lee, S. Dongbang, C. Kang and J. S. Kim, Chem. Rev., 2013, 113, 5071–5109 CrossRef CAS PubMed; (b) S. Maiti, N. Park, J. H. Han, H. M. Jeon, J. H. Lee, S. Bhuniya, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2013, 135, 4567–4572 CrossRef CAS PubMed; (c) J. N. Liu, J. W. Bu, W. B. Bu, S. J. Zhang, L. M. Pan, W. P. Fan, F. Chen, L. P. Zhou, W. J. Peng, K. L. Zhao, J. L. Du and J. L. Shi, Angew. Chem., Int. Ed., 2014, 53, 4551–4555 CrossRef CAS PubMed; (d) T. T. Zou, J. Liu, C. T. Lum, C. Ma, R. C. Chan, C. N. Lok, W. M. Kwok and C. M. Che, Angew. Chem., Int. Ed., 2014, 53, 10119–10123 CrossRef CAS PubMed; (e) Y. Z. Min, J. M. Li, F. Liu, E. K. L. Yeow and B. G. Xing, Angew. Chem., Int. Ed., 2014, 53, 1012–1016 CrossRef CAS PubMed; (f) X. L. Hu, G. H. Liu, Y. Li, X. R. Wang and S. Y. Liu, J. Am. Chem. Soc., 2015, 137, 362–368 CrossRef CAS PubMed; (g) Y. Y. Yuan, R. T. K. Kwok, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2014, 136, 2546–2554 CrossRef CAS PubMed; (h) J. Tian, L. Ding, H. Ju, Y. Yang, X. Li, Z. Shen, Z. Zhu, J. Yu and C. Yang, Angew. Chem., Int. Ed., 2014, 53, 9544–9549 CrossRef CAS PubMed; (i) X. M. Wu, X. R. Sun, Z. Q. Guo, J. B. Tang, Y. Q. Shen, T. D. James, H. Tian and W. H. Zhu, J. Am. Chem. Soc., 2014, 136, 3579–3588 CrossRef CAS PubMed.
- L. Cheng, C. Wang, L. Z. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- (a) K. Pu, A. J. Shuhendler and J. Rao, Angew. Chem., Int. Ed., 2013, 52, 10325–10329 CrossRef CAS PubMed; (b) S. Kim, T. Tachikawa, M. Fujitsuka and T. Majima, J. Am. Chem. Soc., 2014, 136, 11707–11715 CrossRef CAS PubMed.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181–3209 RSC.
- J. B. Birks. Photophysics of Aromatic Molecules; Wiley: London, 1970 Search PubMed.
- (a) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed; (b) R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC; (c) X. Zhang, X. Zhang, L. Tao, Z. Chi, J. Xu and Y. Wei, J. Mater. Chem. B, 2014, 2, 4398–4414 RSC; (d) J. Liang, B. Tang and B. Liu, Chem. Soc. Rev., 2015, 44, 2798–2811 RSC; (e) K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC; (f) X. R. Wang, J. M. Hu, G. Y. Zhang and S. Y. Liu, J. Am. Chem. Soc., 2014, 136, 9890–9893 CrossRef CAS PubMed; (g) Q. L. Hu, M. Gao, G. X. Feng and B. Liu, Angew. Chem., Int. Ed., 2014, 53, 14225–14229 CrossRef CAS PubMed; (h) A. D. Shao, Y. S. Xie, S. J. Zhu, Z. Q. Guo, S. Q. Zhu, J. Guo, P. Shi, T. D. James, H. Tian and W. H. Zhu, Angew. Chem., Int. Ed., 2015, 54, 7275–7280 CrossRef CAS PubMed; (i) X. D. Xue, Y. Y. Zhao, L. R. Dai, X. Zhang, X. H. Hao, C. Q. Zhang, S. D. Huo, J. Liu, C. Liu, A. Kumar, W. Q. Chen, G. Z. Zou and X. J. Liang, Adv. Mater., 2014, 26, 712–717 CrossRef CAS PubMed; (j) A. Shao, Z. Guo, S. Zhu, S. Zhu, P. Shi, H. Tian and W. Zhu, Chem. Sci., 2014, 5, 1383–1389 RSC; (k) Z. L. Xie, C. J. Chen, S. D. Xu, J. Li, Y. Zhang, S. W. Liu, J. R. Xu and Z. G. Chi, Angew. Chem., Int. Ed., 2015, 54, 7181–7184 CrossRef CAS PubMed.
- (a) F. Hu, Y. Y. Huang, G. X. Zhang, R. Zhao, H. Yang and D. Q. Zhang, Anal. Chem., 2014, 86, 7987–7995 CrossRef CAS PubMed; (b) Y. Yuan, C. J. Zhang, M. Gao, R. Zhang, B. Z. Tang and B. Liu, Angew. Chem., Int. Ed., 2015, 54, 1780–1786 CrossRef CAS PubMed; (c) S. Xu, Y. Yuan, X. Cai, C. Zhang, F. Hu, J. Liang, G. Zhang, D. Zhang and B. Liu, Chem. Sci., 2015, 6, 5824–5830 RSC; (d) Y. Yuan, G. Feng, W. Qin, B. Z. Tang and B. Liu, Chem. Commun., 2014, 50, 8757–8760 RSC; (e) E. G. Zhao, H. Q. Deng, S. J. Chen, Y. N. Hong, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2014, 50, 14451–14454 RSC.
- (a) S. H. Lee, M. K. Gupta, J. B. Bang, H. Bae and H. J. Sung, Adv. Healthcare Mater., 2013, 2, 908–915 CrossRef CAS PubMed; (b) M. Y. Jiang and D. Dolphin, J. Am. Chem. Soc., 2008, 130, 4236–4237 CrossRef CAS PubMed.
- (a) Y. Urano, Anal. Sci., 2008, 24, 51–53 CrossRef CAS PubMed; (b) B. C. Dickinson and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 9638–9639 CrossRef CAS PubMed; (c) J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- H. Huang, B. Yu, P. Zhang, J. Huang, Y. Chen, G. Gasser, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2015, 54, 14049–14052 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of the intermediates; molecular orbital data. See DOI: 10.1039/c5sc03583j |
| This journal is © The Royal Society of Chemistry 2016 |