Introduction of taurine (2-aminoethanesulfonic acid) as a green bio-organic catalyst for the promotion of organic reactions under green conditions†
Farhad Shirini*a and
Nader Daneshvarb
aDepartment of Chemistry, College of Science, University of Guilan, 41335, Rasht, Iran. E-mail: shirini@guilan.ac.ir; Fax: +98 131 3233262; Tel: +98 131 3233262
bDepartment of Chemistry, College of Science, University of Guilan, University Campus 2, Iran. E-mail: ndxdaneshvar@gmail.com
First published on 18th November 2016
Abstract
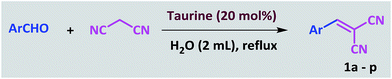
Taurine (2-aminoethanesulfonic acid), a semi-essential amino acid that exists in the human body and numerous other living creatures, is used as a green bio-organic catalyst for the promotion of the Knoevenagel reaction between aldehydes and malononitrile. In the same way, tetraketones can also be produced through a Knoevenagel reaction, followed by Michael addition. 2-Amino-3-cyano-4H-pyran derivatives are simply prepared via a three-component reaction in the presence of taurine as the catalyst. All these reactions are performed in water, a green solvent. The advantages of using of taurine as the catalyst are it is environmentally friendly, low cost, commercially available, easy to separate from the reaction mixture, and has high reusability. Use of this catalyst results in acceptable reaction times, high yields and high purities of the obtained products without utilizing any organic solvents.
Introduction
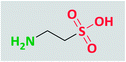
The progress of science has been more and more towards environmentally compatible, or “green” processes, with particular focus on catalysts and other materials in organic chemistry. One aspect of these topics is the application of an alternative reaction medium that is free from the problems associated with the numerous traditional volatile solvents. Using this type of media may also increase the chance of separation and reuse of the catalyst. From the viewpoint of green chemistry, it is better to perform the reactions under solvent-free conditions, but when solvent is necessary, water is the best choice.1 In addition to environmental concerns, chemists prefer to use water as a solvent due to its economic benefits and generally easy separation and work-up conditions. Furthermore, all biological reactions are known to occur in aqueous media. Moreover, numerous organic reactions proceed faster and better in water than in organic solvents.22-Aminoethane sulfonic acid, or taurine (Fig. 1), is an amino acid that is found in high concentration in the tissues of animals. It is one of the constituent members of bile, which can be found in the large intestine and its amount in the average is one-tenth percent of the total weight of the human body.3 Taurine appellation refers to its first isolation from ox bile, named Bos taurus.4 The concentration of taurine in mammalian organs is higher in comparison with the other types of the animals; its concentration in insects and arthropods is less than that in mammals, whereas in plants and bacteria, its concentration is negligible.5 Red algae, although not the brown or green ones, contain high levels of taurine and its N-(1-carboxylated) derivatives, but lichens, mushrooms, mosses, and ferns have very low concentrations of this amino acid.6
In comparison with other homologue amino acids, taurine is structurally different. It is a β-amino acid with a sulfonic acid group instead of a carboxylic acid group. This difference increases its acidity, i.e., in the range of mineral acids (pKa = 1.5), compared to carboxylic acid homologues, and unlike those homologues that are not dissociated at biological pH, taurine is in a zwitterionic state at this pH level, which leads to distinct biological properties.7 According to computational investigations, the neutral conformation of taurine exists in the gas phase, whereas its zwitterionic form exists in water media, which is in agreement with the experimental NMR analysis.8
Taurine has been used for many years as an ingredient in energy drinks and nutrient supplements and has many biological properties such as osmoregulation, immunomodulation and bile salt formation.9
Very recently, silica gel supported taurine was used in the oxidation of sulfides to their corresponding disulfides.10 Moreover, to the best of our knowledge, there are no other reports of the catalytic activity of this β-amino acid for organic transformations.
In recent decades, performance of standard chemical reactions in aqueous media has been often considered, with particular focus on carbon–carbon bond forming transformations.11 Several reactions that have been investigated in this capacity are Diels–Alder, Claisen and Aldol condensations, and radical additions.12
The Knoevenagel reaction, i.e., treatment of an aldehyde with an active methylene group reported by Emil Knoevenagel in 1894, is one of the most important and notable reactions for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formation.13 This reaction is suitable for the preparation of alkenes with electron-withdrawing groups and its products can be used as intermediates for many other types of reactions.14
C bond formation.13 This reaction is suitable for the preparation of alkenes with electron-withdrawing groups and its products can be used as intermediates for many other types of reactions.14
Many conditions have been used to promote the Knoevenagel reaction between aldehydes and malononitrile, including grinding, high pressure, microwaves, and ultrasonics.15 Different types of catalysts have also been utilized; among the most important are cetyltrimethylammonium bromide (CTMAB),16 ReBr(CO)5,17 1,1,3,3-tetramethylguanidium lactate,18 [C4dabco][BF4],19 amine-functionalized polyacrylonitrile fiber,20 Fe3O4 MNPs–guanidine,21 sulfonated carbon/silica composites,22 MP(DNP),23 Na2S/Al2O3,24 silica–L-proline,25 ZnO,26 NixMg1−xFe2O4,27 sodium carbonate,28 hydroxyapatite supported caesium carbonate,29 Tamarindus indica juice,30 L-proline–IL,31 and SiO2–NH4OAc.32
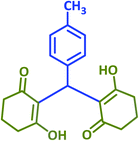
Arylmethylene[bis(3-hydroxy-2-cyclohexene-1-ones)] (tetraketones) firstly were introduced by Merling through the addition of aldehydes with 1,3-diketones via a Knoevenagel reaction followed by a Michael addition.33 These compounds have been extensively used as intermediates for some other important target compounds such as acrilidine diones, thiaxanthenes and xanthene diones.34 Tetraketones have biological activity as antioxidants, lipoxygenases and tyrosinase inhibitors.35 Because of the important properties of these products, several methods have been used to achieve them using various catalysts such as SnCl2/HCl,36 In(OTf)3,37 SmCl3,38 Yb(OTf)3–SiO2 with aniline,39 Fe3O4@SiO2–SO3H,40 PVP-stabilized Ni nanoparticles,41 choline chloride-based deep eutectic,42 nano Fe/NaY zeolite,43 EDDA,44 and Al/MCM-41.45
4H-Pyran and its derivatives have attracted much interests because of their important biological activities, such as anticoagulant, spasmolytic, diuretic, anticancer, antianaphylactin,46 antiallergenic,47 antiproliferative,48 antitumor,49 antibacterial,50 cytotoxic,51 mutagenic52 and sex pheromonal53 activities. These compounds also are present in the structure of some photoactive materials54 and natural products55 and can be used in the synthesis of cosmetics and pigments.56
A large number of catalysts were introduced for the synthesis of 2-amino-3-cyano-4H-pyran derivatives under various conditions; notable among them are hexadecyldimethyl benzyl ammonium bromide,57 1-butyl-3-methyl imidazolium hydroxide,58 2,2,2-trifluoroethanol,59 Ba(OTf)2,60 tetrabutylammonium chloride,61 triazine functionalized ordered mesoporous organosilica,62 tungstic acid functionalized mesoporous SBA-15,63 p-dodecylbenzenesulfonic acid,64 potassium phthalimide,65 red sea sand,66 choline hydroxide,67 IRMOF-3(Zn4O(H2N-TA)3),68 β-cyclodextrins–glycerine,69 tris-hydroxymethylaminomethane,70 squaramide,71 Ce(SO4)2·4H2O,72 poly(vinylpyrrolidonium)hydrogen phosphate,73 L-proline,74 I2,75 and glutamic acid.76
Experimental
All the chemicals for this study were purchased from Merck, Aldrich, and Fluka Chemical Companies and used without further purification. All the products were separated and characterized by their physical properties in comparison with the reported standards. Both the purity determination of the substrates and reaction monitoring were accomplished by thin layer chromatography (TLC) using SIL G/UV 254 silica gel plates. Melting points were determined using a Buchi B-545 apparatus. FT-IR spectra were obtained by a Perkin-Elmer spectrum BX series spectrophotometer (KBr disks). The 1H NMR and 13C NMR spectra were acquired by a Bruker Avance 400 MHz instrument using deuterated solvents.General procedure for the Knoevenagel condensation of aldehydes and malononitrile
In a 25 mL round-bottomed flask, a mixture of aldehyde (1.0 mmol), malononitrile (1.1 mmol) and taurine (0.025 g, 20 mol%) in water (2 mL) was heated at a reflux temperature for the appropriate time. After the conversion, which was monitored by TLC, 10 mL of water was added and stirred for 3 minutes. During this time, the product was precipitated and subsequently separated by filtration. The separated product was washed several times with water. After drying, the pure product was obtained; there was no need for further purification and addition of organic solvent was not necessary. Furthermore, water was evaporated from the filtrate to re-obtain the taurine catalyst.General procedure for the synthesis of tetraketones
In a 25 mL round-bottomed flask, a mixture of aldehyde (1.0 mmol), 1,3-cyclodicarbonyl compound (2.0 mmol) and taurine (0.030 g, 24 mol%) in water (2 mL) was heated at reflux for the appropriate time. After completion of the reaction, which was monitored by TLC, 10 mL of water was added and stirred for 3 minutes. During this time, the product was precipitated and subsequently separated by filtration. The separated product was washed several times with water. After drying, the pure product was obtained; there was no need for further purification and addition of organic solvent was not necessary. Furthermore, water was evaporated from the filtrate to re-obtain the taurine catalyst.General procedure for the synthesis of 2-amino-4H-chromenes
In a 25 mL round-bottomed flask, a mixture of aldehyde (1.0 mmol), 1,3-cyclodicarbonyl compound (1.0 mmol), malononitrile, (1.1 mmol) and taurine (0.030 g, 28 mol%) in water (2 mL) was heated at reflux for the appropriate time. After the reaction was competed, which was monitored by TLC, 10 mL of water was added and stirred for 3 minutes. During this time, the product was precipitated and subsequently separated by filtration. The separated product was washed several times with water. After drying, the pure product was obtained; there was no need for further purification and addition of organic solvent was not necessary. Furthermore, water was evaporated from the filtrate to re-obtain the taurine catalyst.Spectroscopic data of the new compounds
Results and discussion
In recent years, introduction of sulfonic acid based catalysts for the promotion of organic transformations has been an important part of our ongoing research program.77,78 In continuation of these studies, we were interested in investigating the applicability of taurine, a natural, green and commercially available amino acid containing a sulfonic acid group, in the acceleration of organic reactions. The Knoevenagel reaction was selected as the model reaction. In the optimization study, the reaction was initially carried out in the absence of solvent and catalyst and only a little product was obtained. This result was achieved again even when the catalyst was used under solvent-free conditions. Then, the reaction was tested in chloroform, acetonitrile and ethanol with no significant change in the progress of the reaction. In continue the reaction was studied in water as the solvent. The progress of the reaction at room temperature in water was slow, and became even slower as it proceeded; however, the rate was greatly increased at reflux, so the decision was made to pursue these conditions instead. At the end of this study, the optimal amount of catalyst was determined by systematically altering the amount of taurine (Table 1). The Knoevenagel reaction was then performed with various aromatic aldehydes and malononitrile using the optimized amount of the catalyst (20 mol%, 24 mg) in water and under reflux conditions (Scheme 1). As shown in Table 2, various types of aldehydes containing electron-donating and electron-withdrawing groups were successfully converted to the corresponding nitrile products. No distinct substitution effect was observed for this reaction. The isolated products were extremely pure without the need for any costly purification steps, which can be expensive in terms of time, materials and overall yield.| Entry | Catalyst (mol%) | Solvent | Temp. | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1.0 mmol), malononitrile (1.1 mmol), solvent (2 mL) and required amount of catalyst.b The yields are related to the isolated products.c The reaction was not completed. | |||||
| 1 | — | — | 90 °C | 90 | Tracec |
| 2 | 24 | — | 90 °C | 90 | Tracec |
| 3 | 24 | CHCl3 | Reflux | 90 | Tracec |
| 4 | 12 | EtOH | Reflux | 90 | Tracec |
| 5 | 24 | EtOH | Reflux | 90 | Tracec |
| 6 | 24 | CH3CN | Reflux | 90 | Tracec |
| 7 | 12 | H2O | RT | 90 | Tracec |
| 8 | 24 | H2O | RT | 90 | 87 |
| 9 | 16 | H2O | Reflux | 13 | 91 |
| 10 | 20 | H2O | Reflux | 7 | 98 |
| 11 | 24 | H2O | Reflux | 6 | 95 |
| 12 | 24 | H2O/EtOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Reflux | 20 | 86 |
| Entry | Aldehyde | Product | Symbol | Time (min) | Yielda (%) | Mp (°C) | |
|---|---|---|---|---|---|---|---|
| (Obs.) | (Lit.) | ||||||
| a The yields are related to the isolated products. | |||||||
| 1 |  |
 |
1a | 14 | 86 | 82–83 | 82–84 (ref. 16) |
| 2 | 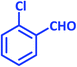 |
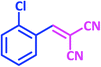 |
1b | 11 | 94 | 92–93 | 93–94 (ref. 27) |
| 3 | 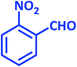 |
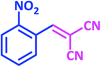 |
1c | 9 | 96 | 138–140 | 139–140 (ref. 21) |
| 4 | 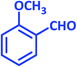 |
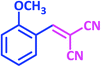 |
1d | 18 | 90 | 81–82 | 80 (ref. 24) |
| 5 | 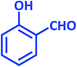 |
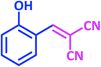 |
1e | 15 | 87 | 99–101 | 98–99 (ref. 29) |
| 6 | 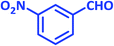 |
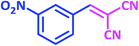 |
1f | 10 | 93 | 103–105 | 103–105 (ref. 27) |
| 7 | 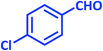 |
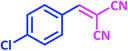 |
1g | 7 | 98 | 161–163 | 161–162 (ref. 27) |
| 8 | 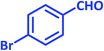 |
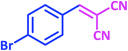 |
1h | 9 | 96 | 156–158 | 159–160 (ref. 21) |
| 9 | 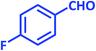 |
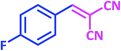 |
1i | 12 | 92 | 122–124 | 122–124 (ref. 28) |
| 10 | 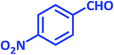 |
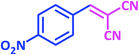 |
1j | 15 | 94 | 161–163 | 157–160 (ref. 22) |
| 11 | 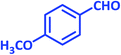 |
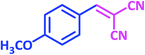 |
1k | 20 | 89 | 115–117 | 112–114 (ref. 22) |
| 12 | 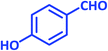 |
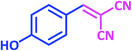 |
1l | 14 | 91 | 186–188 | 185–187 (ref. 27) |
| 13 | 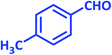 |
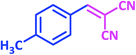 |
1m | 10 | 93 | 136–137 | 137–138 (ref. 24) |
| 14 | 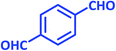 |
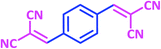 |
1n | 8 | 94 | 295–297 | 298–300 (ref. 30) |
| 15 | 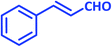 |
 |
1o | 10 | 86 | 125–127 | 124–126 (ref. 22) |
| 16 | 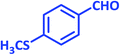 |
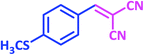 |
1p | 6 | 97 | 155–157 | New product |
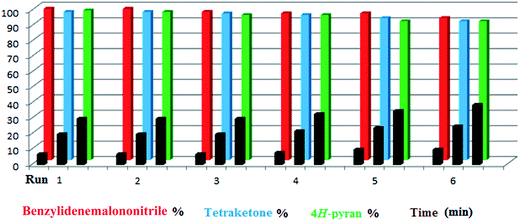
Since taurine is soluble in water, it was easily separated from the products by simple filtration. The filtered solution could be reused in the same reaction without significant loss of catalytic activity. This procedure was repeated six times for the model reaction; each time, the cycle was completed with no significant change in reaction time or yield. During the recycling studies, the product was separated and its melting point was verified after every run to ensure that the purity remained excellent (Fig. 2). It should be mentioned that since the purity of most of the derivatives was too high, we preferred not to use any organic solvent at all; thus, the melting points in this work are related to the non-recrystallized products.
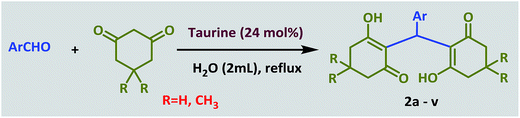
Next, the efficiency of taurine for promoting the synthesis of tetraketone derivatives was investigated via the Knoevenagel reaction, followed by Michael addition of an aldehyde with two equivalents of dimedone or 1,3-cyclohexanedione (Scheme 2).
It should be mentioned that taurine is only suitable to catalyze formation of open chain xanthenes in water and any effort to achieve closed chain xanthenes as single products using this catalyst was not successful.
Only a small amount of product was formed in the absence of a catalyst and solvent at the first step of the optimization of the conditions. When the catalyst was used under solvent-free conditions, a mixture of the products was achieved at different temperatures. As shown in Table 3, the reaction was tested in different solvents and conditions to determine the optimum conditions (entry 10). Furthermore, various aldehydes were used to prepare diverse tetraketone structures. The aldehydes selected for this purpose produced generally high yields of the desired products with acceptable reaction times and do not require further purification (Table 4). Just like the previous reaction, reusability of the catalyst was studied by selecting a typical reaction and using the catalyst-containing filtrate solution in a new reaction mixture. The process showed good reusability over 6 cycles (Fig. 2).
| Entry | Catalyst (mol%) | Solvent | Temp. | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1.0 mmol), dimedone (2.0 mmol), solvent (2 mL) and required amount of the catalyst.b The yields are related to the isolated products.c The reaction was not completed. | |||||
| 1 | — | — | 90 °C | 120 | Tracec |
| 2 | 24 | — | 60–90 °C | 120 | Mixturec |
| 3 | 24 | CHCl3 | Reflux | 120 | Tracec |
| 4 | 12 | EtOH | Reflux | 120 | Tracec |
| 5 | 24 | EtOH | Reflux | 120 | 30c |
| 6 | 24 | CH3CN | Reflux | 120 | Tracec |
| 7 | 12 | H2O | RT | 120 | 20c |
| 8 | 24 | H2O | RT | 120 | 35c |
| 9 | 16 | H2O | Reflux | 33 | 91 |
| 10 | 24 | H2O | Reflux | 20 | 96 |
| 11 | 28 | H2O | Reflux | 17 | 92 |
| 12 | 20 | H2O/EtOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Reflux | 30 | 83 |
| Entry | Aldehyde | Product | Symbol | Time (min) | Yielda (%) | Mp (°C) | |
|---|---|---|---|---|---|---|---|
| (Obs.) | (Lit.) | ||||||
| a The yields are related to the isolated products. | |||||||
| 1 | 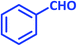 |
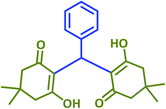 |
2a | 10 | 95 | 181–183 | 185 (ref. 42) |
| 2 | 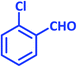 |
 |
2b | 15 | 93 | 194–196 | 199–200 (ref. 43) |
| 3 |  |
 |
2c | 10 | 92 | 242–243 | 244–246 (ref. 38) |
| 4 |  |
 |
2d | 25 | 87 | 181–183 | 184–186 (ref. 43) |
| 5 |  |
 |
2e | 20 | 91 | 209–211 | 208–209 (ref. 40) |
| 6 |  |
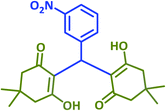 |
2f | 25 | 94 | 195–197 | 197–199 (ref. 40) |
| 7 | 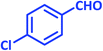 |
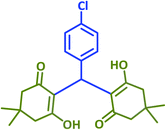 |
2g | 20 | 97 | 139–141 | 138–141 (ref. 42) |
| 8 | 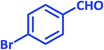 |
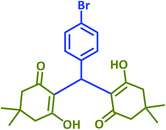 |
2h | 15 | 96 | 159–161 | 154–156 (ref. 43) |
| 9 | 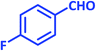 |
 |
2i | 15 | 90 | 183–185 | 184–186 (ref. 43) |
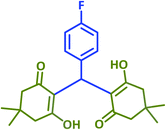
| 10 |  |
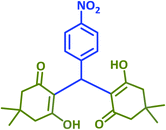 |
2j | 45 | 91 | 185–187 | 188–190 (ref. 43) |
| 11 | 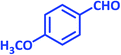 |
 |
2k | 15 | 90 | 142–144 | 146–148 (ref. 38) |
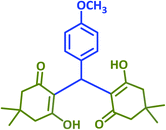
| 12 | 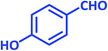 |
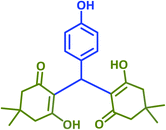 |
2l | 20 | 95 | 182–183 | 180 (ref. 40) |
| 13 | 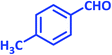 |
 |
2m | 25 | 85 | 133–135 | 132 (ref. 41) |
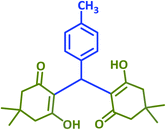
| 14 | 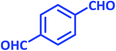 |
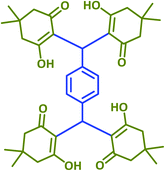 |
2n | 25 | 85 | 220–221 | 225 (ref. 42) |
| 15 | 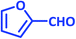 |
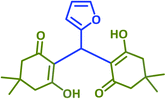 |
2o | 15 | 92 | 151–153 | 146 (ref. 41) |
| 16 | 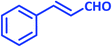 |
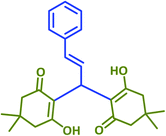 |
2p | 30 | 92 | 215–218 | 213–215 (ref. 38) |
| 17 | 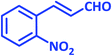 |
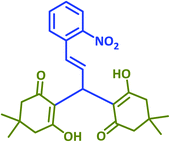 |
2q | 45 | 93 | 174–176 | New product |
| 18 | 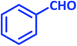 |
 |
2r | 15 | 89 | 219–220 | 214–216 (ref. 43) |
| 19 | 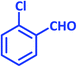 |
 |
2s | 20 | 93 | 203–205 | 199–200 (ref. 42) |
| 20 | 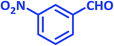 |
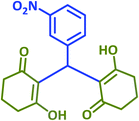 |
2t | 30 | 96 | 207–209 | 209–211 (ref. 36) |
| 21 | 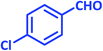 |
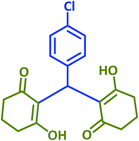 |
2u | 25 | 91 | 199–200 | 201–203 (ref. 43) |
| 22 | 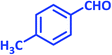 |
 |
2v | 30 | 90 | 189–191 | 190–191 (ref. 39) |
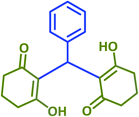
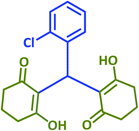
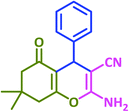
Ultimately, a one-pot, three-component reaction system was designed containing an aldehyde, either dimedone or 1,3-cyclohexanedione, and malonitrile, to test the ability of taurine to catalyze the formation of 2-amino-3-cyano-4H-pyran derivatives.
Optimization of the conditions was performed using a typical reaction of 4-chlorobenzaldehyde, dimedome and malononitrile under a variety of conditions and in the presence of different amounts of taurine as the catalyst. As shown in Table 5, the best results were obtained in refluxing water using 35 mg (28 mol%) catalyst (entry 10).
| Entry | Catalyst (mol%) | Solvent | Temp. | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1.0 mmol), malononitrile (1.1 mmol) and dimedone (1.0 mmol), solvent (2 mL) and required amount of the catalyst.b The yields are related to the isolated products.c The reaction was not completed. | |||||
| 1 | — | — | 90 °C | 120 | Tracec |
| 2 | 24 | — | 60–90 °C | 120 | Tracec |
| 3 | 24 | CHCl3 | Reflux | 120 | Tracec |
| 4 | 12 | EtOH | Reflux | 120 | Tracec |
| 5 | 24 | EtOH | Reflux | 120 | Tracec |
| 6 | 24 | CH3CN | Reflux | 120 | Tracec |
| 7 | 12 | H2O | RT | 115 | 90 |
| 8 | 24 | H2O | RT | 90 | 91 |
| 9 | 16 | H2O | Reflux | 65 | 90 |
| 10 | 28 | H2O | Reflux | 30 | 97 |
| 11 | 32 | H2O | Reflux | 26 | 94 |
| 12 | 28 | H2O/EtOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Reflux | 45 | 88 |
After optimization of the reaction conditions (Scheme 3), different types of aldehydes containing electron-donating and electron-withdrawing groups were subjected to the same reaction. The results showed that all types of aldehydes performed well to give the corresponding products in good to excellent yields.
1,3-Cyclohexanedione was also used in some reactions in place of dimedone and the desired products were obtained (Table 6, entries 20–26). The purity of the isolated products was again such that there was no need for further purification or even recrystallization of the products.
| Entry | Aldehyde | Product | Symbol | Time (min) | Yielda (%) | Mp (°C) | |
|---|---|---|---|---|---|---|---|
| (Obs.) | (Lit.) | ||||||
| a The yields are related to the isolated products. | |||||||
| 1 | 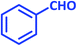 |
 |
3a | 65 | 82 | 231–233 | 232–234 (ref. 73) |
| 2 | 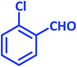 |
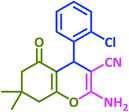 |
3b | 25 | 92 | 209–211 | 211–213 (ref. 73) |
| 3 | 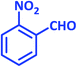 |
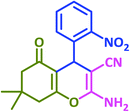 |
3c | 15 | 94 | 222–225 | 228 (ref. 69) |
| 4 | 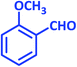 |
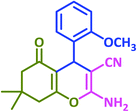 |
3d | 70 | 81 | 204–206 | 196–199 (ref. 73) |
| 5 | 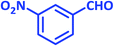 |
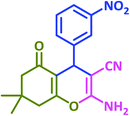 |
3e | 45 | 98 | 211–213 | 211–213 (ref. 72) |
| 6 | 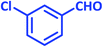 |
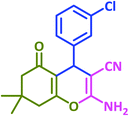 |
3f | 30 | 96 | 224–226 | 228–230 (ref. 72) |
| 7 |  |
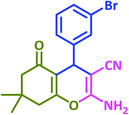 |
3g | 25 | 93 | 287–289 | 289–291 (ref. 72) |
| 8 |  |
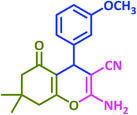 |
3h | 35 | 92 | 190–192 | 188–190 (ref. 72) |
| 9 | 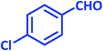 |
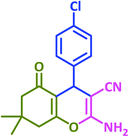 |
3i | 30 | 97 | 208–210 | 208–210 (ref. 73) |
| 10 | 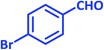 |
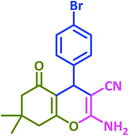 |
3j | 30 | 95 | 203–205 | 201–203 (ref. 73) |
| 11 | 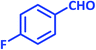 |
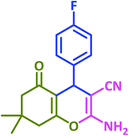 |
3k | 25 | 97 | 210–212 | 214–215 (ref. 73) |
| 12 | 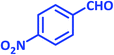 |
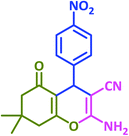 |
3l | 30 | 96 | 180–182 | 181–183 (ref. 73) |
| 13 |  |
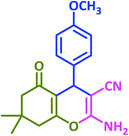 |
3m | 65 | 91 | 193–195 | 196–198 (ref. 70) |
| 14 |  |
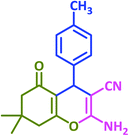 |
3n | 45 | 87 | 217–219 | 217–219 (ref. 70) |
| 15 |  |
 |
3o | 50 | 94 | 220–222 | 222–224 (ref. 70) |
| 16 |  |
 |
3p | 35 | 93 | 266–269 | 270–275 (ref. 77) |
| 17 | 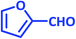 |
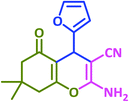 |
3q | 30 | 90 | 223–226 | 223–225 (ref. 73) |
| 18 | 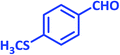 |
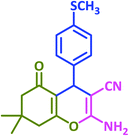 |
3r | 60 | 89 | 211–212 | 208–209 (ref. 58) |
| 19 | 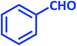 |
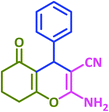 |
3s | 50 | 93 | 212–214 | 212–213 (ref. 79) |
| 20 | 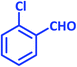 |
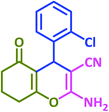 |
3t | 20 | 91 | 209–210 | 210–212 (ref. 57) |
| 21 |  |
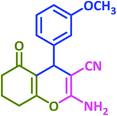 |
3u | 50 | 94 | 202–204 | 202–204 (ref. 57) |
| 22 | 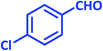 |
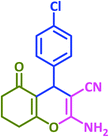 |
3v | 40 | 93 | 221–223 | 224–226 (ref. 57) |
| 23 | 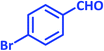 |
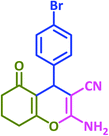 |
3w | 35 | 98 | 237–240 | 234–235 (ref. 63) |
| 24 | 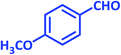 |
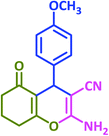 |
3x | 45 | 85 | 202–204 | 206–208 (ref. 63) |
| 25 | 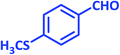 |
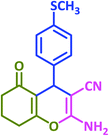 |
3y | 50 | 92 | 217–219 | New product |
As we have mentioned previously, the reusability of the catalyst was investigated in the model reactions for all three transformations studied (products were 1g, 2g and 3i). In each case, six consecutive runs showed excellent reusability (Fig. 2).
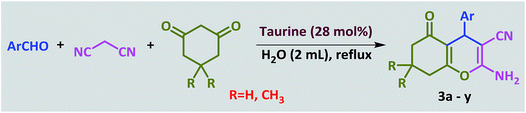
Plausible mechanisms for the abovementioned reactions as catalyzed by taurine are shown in Scheme 4. Taurine acts as a bifunctional donor–acceptor reagent in which the aldehyde carbonyl site is activated by taurine and then attacked by the negatively activated methylene group in malononitrile. Elimination of water in the Knoevenagel reaction results in arylidene malononitriles that can be separated by filtration (compounds 1a–1p). Moreover, it is believed that the taurine-activated aldehyde can be attacked by a taurine-enolized β-dicarbonyl. Then, after losing water and forming an arylidene dicarbonyl in the Knoevenagel reaction, this species can react with another enolized β-dicarbonyl, leading to a tetraketone (products 2a–2v). For the three component system, the Knoevenagel product is again formed, followed by Michael addition with a β-dicarbonyl. Lastly, enolization occurs, followed by amine–enamine tautomerization in the presence of taurine to produce the 4H-pyran derivative (products 3a–3y).
The results of this study were compared with some existing literature reports in order to better illustrate the utility of taurine in accelerating the reactions under study (Table 7). In each case, the taurine-catalyzed reaction had an advantage wherein it utilized a green catalyst and a nontoxic solvent, featured easy separation of product and catalyst, offered catalyst reusability, and resulted in lower reaction times and higher yields.
| Product | Catalyst | Amount | Conditions | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| a Current work. | |||||
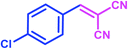 |
SiO2–L-proline25 | 10 mol% (0.100 g) | CH3CN/80 °C | 540 | 95 |
| L-Proline–IL31 | 30 mol% | 80 °C | 1440 | 96 | |
| MNPs–guanidine21 | 0.39 mol% (0.005 g) | H2O–PEG/RT | 150 | 96 | |
| SiO2–NH4OAc32 | 0.200 g | CH2Cl2/reflux | 450 | 90 | |
| HAP–Cs2CO3 (ref. 29) | 0.200 g | H2O/80 °C | 180 | 86 | |
| CTMAB16 | 0.50 mmol | H2O/RT | 90 | 94 | |
| ZnO26 | 0.500 g | H2O/RT | 90 | 86 | |
| Na2S/Al2O3 (ref. 24) | 20 mol% on 0.500 g | CH2Cl2/reflux | 30 | 90 | |
| Taurinea | 20 mol% (0.025 g) | H2O/reflux | 7 | 98 | |
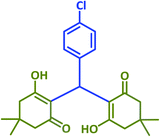 |
SmCl3 (ref. 38) | 20 mol% | 120 °C | 20 | 95 |
| EDDA44 | 30 mol% | THF/reflux | 240 | 97 | |
| Fe3O4@SiO2–SO3H40 | 0.010 g | H2O/RT | 80 | 83 | |
| Al/MCM-41 (ref. 45) | 0.100 g | EtOH/reflux | 120 | 88 | |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea42 urea42 |
1 mL | 80 °C | 120 | 86 | |
| Fe/NaY43 | 0.025 g | EtOH/reflux | 70 | 98 | |
| Taurinea | 24 mol% (0.030 g) | H2O/reflux | 20 | 97 | |
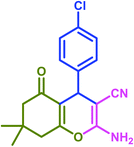 |
β-Cyclodextrin69 | 0.227 g | Aq glyserin/40 °C | 30 | 90 |
| IRMOF–Zn complex68 | 4 mol% | 60 °C | 300 | 90 | |
| [Ch][OH]67 | 10 mol% | H2O/80 °C | 60 | 86 | |
| Red sea sand66 | 0.500 g | EtOH/reflux | 280 | 85 | |
| Glutamic acid74 | 20 mol% | EtOH/reflux | 40 | 91 | |
| DBSA64 | 20 mol% | H2O/reflux | 240–420 | 69 | |
| TFE62 | 2 mL | Reflux | 300 | 95 | |
| I2 (ref. 75) | 10 mol% | DMSO/120 °C | 210 | 88 | |
| L-Proline76 | 10 mol% | EtOH/reflux | 120 | 72 | |
| HDMBAB57 | 2.4 mol% | H2O/80–90 °C | 450 | 90 | |
| TBAC61 | 10 mol% | H2O/reflux | 120 | 98 | |
| Taurinea | 28 mol% (0.035 g) | H2O/reflux | 30 | 97 | |
Conclusions
The general advantages of taurine as a catalyst in the studied reactions are as follows: it is non-toxic for humans and other living organisms, it offers ease of separation of products and catalyst, it does not require use of organic solvents, it features excellent catalyst recyclability and reusability, it is highly stable and easy to store, and finally, it is commercially available at a low price. All of these points are in full compliance with the requirements of green chemistry.Acknowledgements
We are thankful to the University of Guilan Research Council for partial support of this work.References
- (a) B. C. Ranu and K. Chattopadhyay, Eco-Friendly Synthesis of Fine Chemicals, ed. R. Ballini, Royal Society of Chemistry, Cambridge, UK, 2009, ch. 5 Search PubMed; (b) R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- C. Ameta and K. L. Ameta, Water: A Benign Solvent for the Synthesis of Various Organic Moieties, Springer, India, 2014, pp. 231–252 Search PubMed.
- R. J. Huxtable, Physiol. Rev., 1992, 72, 101 CAS.
- H. Demarcay, Ann. Pharm., 1838, 27, 270 CrossRef.
- (a) L. E. Ericson and B. Carlson, Ark. Kemi, 1954, 6, 511 Search PubMed; (b) G. T. Reynoso and B. A. d. Gamboa, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1982, 73, 95 CrossRef CAS.
- (a) H. Kataoka and N. Ohnishi, Agric. Biol. Chem., 1986, 50, 1887 CAS; (b) M. Kuriyama, Nature, 196l, 192, 962 Search PubMed.
- (a) R. J. Huxtable, Physicochemical properties of taurine: introduction, in Taurine in Nutrition and Neurology, ed. R. J. Huxtable and H. Pasantes-Morales, Plenum, New York, 1982, pp. 1–4 Search PubMed; (b) R. J. Huxtable and A. Leslie, Trends Pharmacol. Sci., 1986, 7, 481 CrossRef CAS.
- l. K. Song and Y. K. Kang, Comput. Theor. Chem., 2013, 1025, 8 CrossRef.
- (a) G. P. Salz and D. A. Davis, Aquaculture, 2015, 437, 215 CrossRef; (b) J. M. Menzie, C. Pan, H. Prentice and J. Y. Wu, Amino Acids, 2014, 46, 31 CrossRef CAS PubMed; (c) G. B. Schuller-Levis and E. Park, Neurochem. Res., 2004, 29, 117 CrossRef CAS PubMed; (d) A. F. Hofmann, J. Biochem., 1963, 89, 57 CrossRef CAS.
- H. M. Shen, H. B. Ji, H. X. Shi, Y. B. She, W. J. Zhou, H. K. Wu, W. B. Yu and N. Ai, Tetrahedron Lett., 2015, 56, 4494 CrossRef CAS.
- C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS.
- (a) D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS; (b) R. Breslow, U. Maitra and D. Rideout, Tetrahedron Lett., 1983, 24, 1901 CrossRef CAS; (c) P. A. Griero, K. Yoshida and P. Garner, J. Org. Chem., 1983, 48, 3137 CrossRef.
- L. F. Tietze and U. Beifuss, Comprehensive Organic Synthesis, Oxford, UK, 1991, ch. 1, pp. 341–394 Search PubMed.
- F. Freeman, Chem. Rev., 1969, 69, 591 CrossRef CAS PubMed.
- (a) A. McCluskey, P. J. Robinson, T. Hill, J. L. Scott and J. K. Edwards, Tetrahedron Lett., 2002, 43, 3117 CrossRef CAS; (b) G. Jenner, Tetrahedron Lett., 2001, 42, 243 CrossRef CAS; (c) S. Balalaie and N. Nemati, Synth. Commun., 2000, 30, 869 CrossRef CAS; (d) J. S. Yadav, B. V. S. Reddy, A. K. Basak, B. Visali, A. V. Narsaiah and K. Nagaiah, Eur. J. Org. Chem., 2004, 111, 546 CrossRef; (e) J. M. Nulty, J. A. Steere and S. Wolf, Tetrahedron Lett., 1998, 39, 8013 CrossRef.
- S. Wang, Z. Ren, W. Cao and W. Tong, Synth. Commun., 2001, 31, 673 CrossRef CAS.
- W. X. Zuo, R. Hua and X. Qiu, Synth. Commun., 2004, 34, 3219 CrossRef CAS.
- J. Zhang, T. Jiang, B. Han and A. Zhu, Synth. Commun., 2006, 36, 3305 CrossRef CAS.
- D. Z. Xu, Y. Liu, S. Shi and Y. Wang, Green Chem., 2010, 12, 514 RSC.
- G. Li, J. Xiao and W. Zhang, Green Chem., 2012, 14, 2234 RSC.
- A. Rostami, B. Atashkar and H. Gholami, Catal. Commun., 2013, 37, 69 CrossRef CAS.
- P. Gupta, M. Kour, S. Paul and J. H. Clark, RSC Adv., 2014, 4, 7461 RSC.
- S. K. Panja, N. Dwivedi and S. Saha, RSC Adv., 2015, 5, 65526 RSC.
- M. Heravi, K. Bakhtiari, S. Taheri and H. A. Oskooi, J. Chin. Chem. Soc., 2007, 54, 1557 CrossRef CAS.
- R. Raid and M. Gupta, Monatsh. Chem., 2015, 146, 645 CrossRef.
- M. Basude, P. Sunkara and V. S. Puppala, J. Chem. Pharm. Res., 2013, 9, 46 Search PubMed.
- S. L. Khillare, A. O. Dhokte, M. K. Lande and B. R. Arbad, Int. J. Chem. Phys. Sci., 2014, 5, 96 Search PubMed.
- A. Pasha, M. Manjula, P. Krishnappa and V. Jayashankara, Indian J. Clin. Biochem., 2010, 49, 1428 Search PubMed.
- M. Gupta, R. Gupta and M. Anand, Beilstein J. Org. Chem., 2009, 5, 68 Search PubMed.
- R. Pal, Int. J. Anal. Chem., 2014, 2, 27 Search PubMed.
- C. Zhuo, D. Xian, W. Jian-wei and X. Hui, ISRN Org. Chem., 2011, 2011, 676789 Search PubMed.
- R. Gupta, M. Gupta, S. Paul and R. Gupta, Bull. Korean Chem. Soc., 2009, 30, 2419 CrossRef CAS.
- G. Merling, Ann. Chem., 1894, 278, 20 CrossRef.
- Q. H. To, Y. R. Lee and S. H. Kim, Bull. Korean Chem. Soc., 2012, 33, 1170 CrossRef CAS.
- (a) P. Shanmugasundram, K. J. Prabahar and V. T. Ramakrishnan, J. Heterocycl. Chem., 1993, 30, 1003 CrossRef; (b) P. Shanmugasundram, P. Murugan, V. T. Ramakrishnan, N. Srividya and P. Ramamurthy, J. Heterocycl. Chem., 1996, 7, 17 Search PubMed; (c) T. Josephrajan and V. T. Ramakrishnan, Can. J. Chem., 2007, 85, 572 CrossRef CAS; (d) P. Murugan, P. Shanmugasundram, V. T. Ramakrishnan, B. Venkatachalapathy, N. Srividya, P. Ramamurthy, K. Gunasekaran and D. Velmurugan, J. Chem. Soc., Perkin Trans. 2, 1998, 999 RSC; (e) K. J. Parabahar, K. Rajagopalan and V. T. Ramakrishnan, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1989, 28, 952 Search PubMed; (f) T. Josephrajan, V. T. Ramakrishnan, G. Kathiravan and J. Muthumary, ARKIVOC, 2005, xi, 124 Search PubMed.
- K. M. Khan, G. M. Maharvi, M. T. H. Khan, A. J. Shaikh, Sh. Perveen, S. Begum and M. I. Choudhary, Bioorg. Med. Chem., 2006, 14, 344 CrossRef CAS PubMed.
- D. H. Jung, Y. R. Lee, S. H. Kim and W. S. Lyoo, Bull. Korean Chem. Soc., 2009, 30, 1989 CrossRef CAS.
- A. Ilangovan, S. Malayappasamy, S. Muralidharan and S. Maruthamuthu, Chem. Cent. J., 2011, 5, 81 CrossRef CAS PubMed.
- V. Rao, M. K. Muthyala and A. Kumar, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2011, 50, 1128 Search PubMed.
- F. Nemati, M. M. Heravi and R. Saeedi-Rad, Chin. J. Catal., 2012, 33, 1825 CrossRef CAS.
- J. Khurana and K. Vij, J. Chem. Sci., 2012, 124, 907 CrossRef CAS.
- N. Azizi, S. Dezfooli and M. M. Hashemi, C. R. Chim., 2013, 16, 997 CrossRef CAS.
- M. Tajbakhsh, M. Heidary and R. Hossseinzadeh, Res. Chem. Intermed., 2015, 42, 1425 CrossRef.
- D. H. Jung, Y. R. Lee, S. H. Kim and W. S. Lyoo, Bull. Korean Chem. Soc., 2009, 30, 1989 CrossRef CAS.
- M. Rastroshan, S. Sayyahi, V. Zare-Shahabadi and R. Badri, J. Iranian Chem. Res., 2012, 5, 265 Search PubMed.
- (a) L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517 CrossRef CAS; (b) J. Bloxham, C. P. Dell and C. W. Smith, Heterocycles, 1994, 38, 399 CrossRef CAS; (c) G. A. M. Nawwar, F. M. Abdelrazek and R. H. Swcllam, Arch. Pharm., 1991, 342, 875 CrossRef; (d) J. Zamocka, E. Misikova and J. Durinda, Pharmazie, 1991, 46, 610 CAS.
- K. Gorlitzer, A. Dehre and E. Engler, Arch. Pharm., 1983, 316, 264 CrossRef CAS PubMed.
- C. P. Dell and C. W. Smith, European Patent Applications EP 537 949 21, Apr 1993Chem. Abstr. 119, 139102d, 1993.
- S. J. Mohr, M. A. Chirigos, F. S. Fuhrman and J. W. Pryor, Cancer Res., 1975, 35, 3750 CAS.
- R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2007, 17, 6459 CrossRef CAS PubMed.
- A. Zonouzi, R. Mirzazadeh, M. Safavi, S. K. Ardestani, S. Emami and A. Foroumadi, Iran. J. Pharm. Res., 2013, 12, 679 CAS.
- A. H. Bedair, H. A. Emam, N. A. El-Hady, K. A. R Ahmed and A. M. El-Agrody, Farmaco, 2001, 56, 965 CrossRef CAS PubMed.
- G. Bianchi and A. Tava, Agric. Biol. Chem., 1987, 51, 2001 CAS.
- D. Armetso, W. M. Horspool, N. Martin, A. Ramos and C. Seaone, J. Org. Chem., 1989, 54, 3069 CrossRef.
- S. Hatakeyama, N. Ochi, H. Numata and S. Takano, J. Chem. Soc., Chem. Commun., 1988, 17, 1202 RSC.
- G. P. Ellis, Chromenes, chromanones, and chromones, in The Chemistry of Heterocyclic Compounds, ed. A. Weissberger and E. C. Taylor, JohnWiley, New York, NY, USA, 1977, ch. 2, pp. 11–139 Search PubMed.
- T. S. Jin, A. Q. Wang, F. Shi, L. S. Han, L. B. Liu and T. S. Li, ARKIVOC, 2006, xiv, 78 Search PubMed.
- B. C. Ranu, S. Banerjee and S. Roy, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2008, 47, 1108 Search PubMed.
- S. Khaksar, A. Rouhollahpour and S. Mohammadzadeh Talesh, J. Fluorine Chem., 2012, 141, 11 CrossRef CAS.
- A. Kumar and M. S. Rao, Green Chem. Lett. Rev., 2012, 5, 283 CrossRef CAS.
- H. Mehrabi and N. Kamali, J. Iran. Chem. Soc., 2012, 9, 599 CrossRef CAS.
- J. Mondal, A. Modak, M. Nandi, H. Uyama and A. Bhaumik, RSC. Adv., 2012, 2, 11306 RSC.
- S. K. Kundu, J. Mondal and A. Bhaumik, Dalton Trans., 2013, 42, 10515 RSC.
- E. Sheikhhosseini, D. Ghazanfari and V. Nezamabadi, Iran. J. Catal., 2013, 3, 197 Search PubMed.
- M. G. Dekamin and M. Eslami, Green Chem., 2014, 16, 4914 RSC.
- N. M. A. El-Rahman and R. M. Borik, World Appl. Sci. J., 2014, 31, 1 Search PubMed.
- H. Hu, F. Qiu, A. Yin, J. Yang and H. Meng, Int. J. Mol. Sci., 2014, 15, 6897 CrossRef CAS PubMed.
- S. Rostamnia and A. Morsali, Inorg. Chim. Acta, 2014, 411, 113 CrossRef CAS.
- S. R Kamat, A. H. Mane, S. M Arde and R. S. Salunkhe, Int. J. Pharm., Chem. Biol. Sci., 2014, 4, 1012 Search PubMed.
- K. S. Pandit, P. V. Chavan, U. V. Desai, M. A. Kulkarni and P. Wadgaonkar, New J. Chem., 2015, 36, 4452 RSC.
- W. Sun, P. Zhang, J. Fan, S. Chen and Z. Zhang, Synth. Commun., 2010, 40, 578 Search PubMed.
- M. Eslami and E. Mosaddegh, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 3134 CrossRef.
- F. Shirini, O. Goli-Jolodar, M. Akbari and M. Seddighi, Res. Chem. Intermed., 2015, 42, 4733 CrossRef.
- F. K. Behbahani and F. Alipour, Guyana J. Sci., 2015, 28, 387 Search PubMed.
- R. S. Bhosale, C. V. Magar, K. S. Solanke, S. B. Mane, S. S. Choudhary and R. P. Pawar, Synth. Commun., 2007, 37, 4353 CrossRef CAS.
- F. Hatamjafari, J. Chem. Health Risks, 2016, 6, 133 Search PubMed.
- (a) F. Shirini, M. Mamaghani, T. Mostashari-Rad and M. Abedini, Bull. Korean Chem. Soc., 2010, 31, 2399 CrossRef CAS; (b) F. Shirini, M. A. Zolfigol and M. Abedini, J. Iran. Chem. Soc., 2010, 7, 603 CrossRef CAS; (c) F. Shirini, M. Mamaghani and S. V. Atghia, Catal. Commun., 2011, 12, 1088 CrossRef CAS; (d) F. Shirini and N. G. Khaligh, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 2156 CrossRef CAS; (e) F. Shirini, M. Mamaghani and S. V. Atghia, Appl. Clay Sci., 2012, 58, 67 CrossRef CAS; (f) M. Seddighi, F. Shirini and M. Mamaghani, RSC Adv., 2013, 3, 24046 RSC.
- M. Abedini, F. Shirini and A. Shahriari, Curr. Org. Chem., 2015, 19, 2011 CrossRef CAS.
- Y. Gao and D. Du, Tetrahedron: Asymmetry, 2012, 23, 1343 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR, 1H NMR & 13C NMR of new products. See DOI: 10.1039/c6ra15432h |
| This journal is © The Royal Society of Chemistry 2016 |