A new azodioxy-linked porphyrin-based semiconductive covalent organic framework with I2 doping-enhanced photoconductivity†
Bhaskar
Nath
,
Wen-Hua
Li
,
Jia-Hong
Huang
,
Guan-E.
Wang
,
Zhi-hua
Fu
,
Ming-Shui
Yao
and
Gang
Xu
*
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences (CAS), 155 Yangqiao Road West, Fuzhou, Fujian 350002, PR China. E-mail: gxu@fjirsm.ac.cn
First published on 1st March 2016
Abstract
A room-temperature solution phase reaction was developed to synthesize a covalent organic framework (COF) for the first time. The synthesized azodioxy-linked porphyrin-based COF (POR-COF) possesses a 2D chess board-like structure in the ab-plane and a 1D channel with an open-window size of around 1.9 nm along the c-axis in the modeled crystal structure. The electrical conductivity of POR-COF increases by more than 3 orders of magnitude through I2 doping. The photoconductivity of the I2-doped COF material was also studied firstly. POR-COF shows interesting doping-enhanced photo-current generation.
Organic conductive materials have attracted considerable attention for a long time, not only for their interesting electrical and optoelectronic properties but also for their low cost, low weight and easy process.1 A large number of π-conjugated organic molecules were found to show semiconducting behaviours, where the charge transport originates from the π⋯π interaction between the molecules in the solid state.2 On the other hand, π⋯π interaction is one of the foremost driving forces for assembling π-conjugated molecules to form crystalline states.
Covalent organic frameworks (COFs) are a class of crystalline organic polymers with well-defined porous structures.3 They have received considerable attention from materials chemists as they have appeared as promising materials for catalysis,4 drug delivery,5 gas storage/separation6 and electrical applications.7 For electrical applications, building blocks based on highly electron-rich systems such as pyrene, porphyrin and phthalocyanine have been involved in COF materials due to their intrinsic semiconductive and photoconductive properties. Moreover, the porous structure of COFs makes it very convenient to modulate their electrical properties by doping suitable dopants to form charge transfer complexes.
The key factor for the crystallization of COFs is to find a reaction which can slowly and reversibly form covalent bonds, for example, the formation of boronate esters from boronic acids,7 imines from the condensation of aldehydes and primary amines,8etc. Most COFs reported to date were synthesized under special care, such as the use of harsh experimental conditions (e.g., high temperature and high pressure), inert atmosphere, and heterogeneous reaction mixtures and long reaction time.4–8 The synthesis of COFs under ambient conditions using homogeneous solution phase reaction is still a big challenge. Thus, it is very important to find a rapid and mild synthetic approach for COF materials.
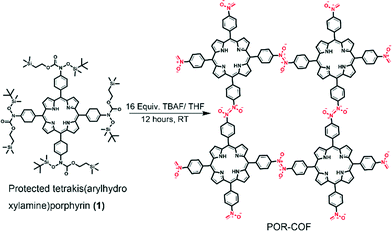
In this work, we report the room-temperature synthesis of a COF using a homogeneous solution phase reaction for the first time to the best of our knowledge. A π-conjugated semiconductive molecule, porphyrin, is connected by azodioxy (–ON![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NO–) linkers to form a two-dimensional COF, namely POR-COF. The structure determination, the gas sorption properties and the electrical properties of POR-COF before and after I2 doping were shown. Notably, it is also observed firstly that the photoconductivity of a COF material can be significantly enhanced by I2 doping.
NO–) linkers to form a two-dimensional COF, namely POR-COF. The structure determination, the gas sorption properties and the electrical properties of POR-COF before and after I2 doping were shown. Notably, it is also observed firstly that the photoconductivity of a COF material can be significantly enhanced by I2 doping.
To synthesize POR-COF, a protected tetrakis(arylhydroxylamine)porphyrin (1) was synthesized as a precursor by following a reported procedure from tetra(p-bromophenyl)porphyrin.9 When this protected tetrakis(arylhydroxylamine)porphyrin (1) was treated with 16 equivalents of 1 M tetrabutylammonium fluoride (TBAF) in THF at room temperature, it formed tetrakis(arylhydroxylamine)porphyrin which is expected to transform into tetrakis(arylnitroso)porphyrin in situ, followed by polymerization to give POR-COF as shown in Scheme 1. Arylhydroxylamines are reactive compounds and have a tendency to be oxidized easily.10 Anderson et al. have reported the oxidation of N-hydroxyamphetamine by aerated toluene to give the corresponding nitroso dimer.10c In this work, the in situ generated tetrakis(arylhydroxylamine)porphyrin has four arylhydroxylamine groups attached to an electron-rich porphyrin unit. The presence of electron-rich groups may further increase the reactivity of the arylhydroxylamine groups. Therefore, they are expected to be oxidized by the aerated solvent to form tetrakis(arylnitroso)porphyrin for further polymerization. The strong absorption peaks at 1735 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 2950–2850 cm−1 (aliphatic C–H stretching) in the FT-IR spectra of 1 disappear in POR-COF, which indirectly demonstrates the complete de-protection of 1 by TBAF (Fig. S2a†). On the other hand, the peak at around 1283 cm−1 confirms the presence of the trans-azodioxy bond (dimeric nitroso group, –ON
O stretching) and 2950–2850 cm−1 (aliphatic C–H stretching) in the FT-IR spectra of 1 disappear in POR-COF, which indirectly demonstrates the complete de-protection of 1 by TBAF (Fig. S2a†). On the other hand, the peak at around 1283 cm−1 confirms the presence of the trans-azodioxy bond (dimeric nitroso group, –ON![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NO–) in POR-COF (Fig. S2b†).11a In general, the N
NO–) in POR-COF (Fig. S2b†).11a In general, the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond length in the trans-azodioxy compound is virtually constant and its Raman-active stretching frequency lies in the range of 1436–1447 cm−1.11b Therefore, the absorption band at 1447 cm−1 in the Raman spectra of POR-COF confirms the presence of the trans-N2O2 dimer (Fig. S3†).11c Arylnitroso compounds are well known to form dimers through both cis- and trans-azodioxy bonds (–ON
N bond length in the trans-azodioxy compound is virtually constant and its Raman-active stretching frequency lies in the range of 1436–1447 cm−1.11b Therefore, the absorption band at 1447 cm−1 in the Raman spectra of POR-COF confirms the presence of the trans-N2O2 dimer (Fig. S3†).11c Arylnitroso compounds are well known to form dimers through both cis- and trans-azodioxy bonds (–ON![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NO–), and most of the organic nitroso compounds exist as dimers in the solid state.12 Interestingly, in the present case, IR and Raman spectra suggest that there is only the trans-azodioxy bond in POR-COF. The 13C cross-polarized magic angle spinning (CP-MAS) solid-state NMR spectrum (Fig. 2a) of POR-COF shows signals at 152.5, 142.0, 132.2 and 118.7 ppm. These chemical shifts are comparable with those of other porphyrin-based COFs.13 Moreover, there is no resonance in the region of 155–170 ppm in the 13C CP-MAS spectrum which suggests the virtual absence of any monomeric nitroso groups and thus excludes the presence of any oligomeric unit. Therefore, from the above experimental evidence, it is reasonable to expect that the tetrakis(arylnitroso)porphyrin building blocks self-polymerize through the formation of azodioxy bonds which may result in the generation of a COF structure.
NO–), and most of the organic nitroso compounds exist as dimers in the solid state.12 Interestingly, in the present case, IR and Raman spectra suggest that there is only the trans-azodioxy bond in POR-COF. The 13C cross-polarized magic angle spinning (CP-MAS) solid-state NMR spectrum (Fig. 2a) of POR-COF shows signals at 152.5, 142.0, 132.2 and 118.7 ppm. These chemical shifts are comparable with those of other porphyrin-based COFs.13 Moreover, there is no resonance in the region of 155–170 ppm in the 13C CP-MAS spectrum which suggests the virtual absence of any monomeric nitroso groups and thus excludes the presence of any oligomeric unit. Therefore, from the above experimental evidence, it is reasonable to expect that the tetrakis(arylnitroso)porphyrin building blocks self-polymerize through the formation of azodioxy bonds which may result in the generation of a COF structure.
POR-COF has excellent thermal stability which remains stable up to 350 °C as observed from the TG experiment (Fig. S5†). Therefore, it can be activated at 150 °C to remove the lattice solvent molecules inside the framework for gas sorption measurement. The N2 adsorption–desorption experiment using POR-COF at 77 K (Fig. 2b) shows a type I isotherm which suggests its micro-porous nature. The BET surface area of POR-COF was found to be 447 m2 g−1 which is similar to the reported values for other 2D COFs.14 The pore size distribution plot of POR-COF obtained using the BJH method (Barrett–Joyner–Halenda method) reveals a pore width of around 1.95 nm (Fig. S6†).
SEM (scanning electron microscopy) and TEM (tunneling electron microscopy) images show that POR-COF has a nanoparticle morphology with a size of around 150–200 nm which is too small to be used for determining the crystal structure (Fig. S7 and S8†). Powder X-ray diffraction (PXRD) measurement indicates that POR-COF is a crystalline material (Fig. 1b). Most of the peaks in its PXRD pattern are too broad to undergo Rietveld refinement. However, according to the describable peaks in the PXRD pattern, gas sorption analysis, IR, Raman as well as NMR spectra, a possible crystal structure model of POR-COF was simulated. The simulated structure has a P4 space group with a = b = 39.0 Å, c = 3.5 Å and α = β = γ = 90° (Fig. 1a). In this modeled structure, one porphyrin unit is connected to four neighboring porphyrins through trans-azodioxy bonds to form a 2D chess board-like structure in the ab-plane. The 2D sheets of POR-COF are not perfectly planar; the connecting aryl rings lie in a different plane from the central porphyrin units. These 2D sheets further pack with each other through π⋯π interactions along the c-axis to produce a 1D channel with an open-window size of around 1.9 nm which agrees very well with the data analysis of N2 sorption measurement.
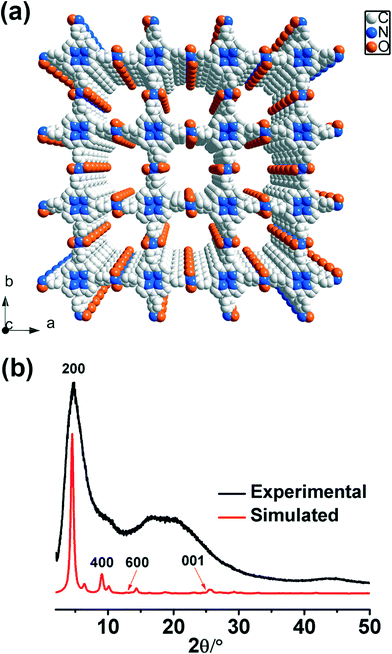 | ||
| Fig. 1 (a) Modeled crystal structure of POR-COF; (b) simulated and experimental PXRD patterns of POR-COF. | ||
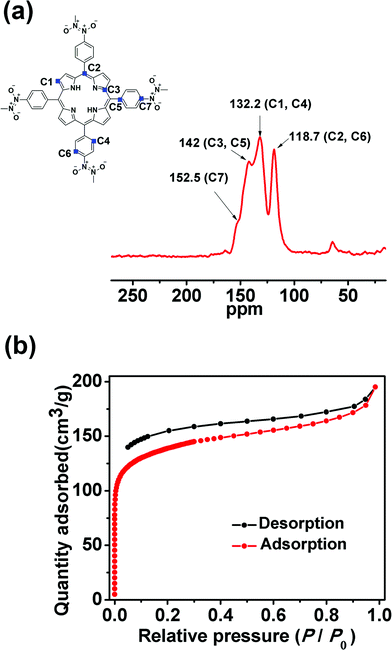 | ||
| Fig. 2 (a) Solid-state cross-polarized magic angle spinning (CP-MAS) 13C-NMR spectrum of POR-COF; (b) nitrogen adsorption–desorption curve of POR-COF. | ||
A two-probe method was employed to investigate the conductive properties of POR-COF. A pressed pallet of POR-COF was prepared by mixing it with 20 wt% polymethyl methacrylate (PMMA), where both faces of the pallet were painted with silver paint and dried in air for a few hours before the measurement. As shown in Fig. 3a and S9,† the I–V curve of POR-COF shows almost a linear profile indicating an ohmic contact between the sample and electrodes. The electrical conductivity of POR-COF is 4.6 × 10−11 S cm−1 at room temperature in air. This value is comparable with the reported conductivity of other 2D COFs.15 The temperature-dependent electrical conductivity of POR-COF was also measured. The conductivity of POR-COF increases almost linearly with increasing temperature (Fig. S10†), which indicates its semiconductive nature. In general, COF materials exhibit lower intrinsic conductivity, and it can be enhanced through doping of appropriate guest molecules.16 Due to its porous structure, POR-COF can be easily doped with I2 by using a simple solution method. The sample was prepared by immersing POR-COF in a solution of n-hexane containing I2 for 30 hours, filtered and washed many times with n-hexane and then dried in an oven at 70 °C overnight. After doping with I2, POR-COF shows its color change from purple to black indicating the formation of a charge transfer complex. The formation of the charge transfer complex is also evident in the electronic spectra as there are significant changes of the absorption in the region of 700–1200 nm when compared with that of pure POR-COF, which is typical for I2-doped 2D COF materials (Fig. S4†).17 The electronic conductivity of I2-doped POR-COF was measured exactly using the same method as that for pure POR-COF, which also shows a linear I–V plot (Fig. 3a). Compared to that of pure POR-COF, the conductivity of I2-doped POR-COF increases by more than 3 orders of magnitude and reaches 1.52 × 10−7 S cm−1. Tetraphenylporphyrin is a p-type semiconductor18 and iodine is an electron acceptor. Doping POR-COF with iodine results in charge transfer from the host to the dopant and creates free holes to migrate through the organic framework under irradiation. Therefore, this improvement in conductivity upon doping with I2 indicates that there is a remarkable amount of holes generated and POR-COF is a p-type semiconductor.
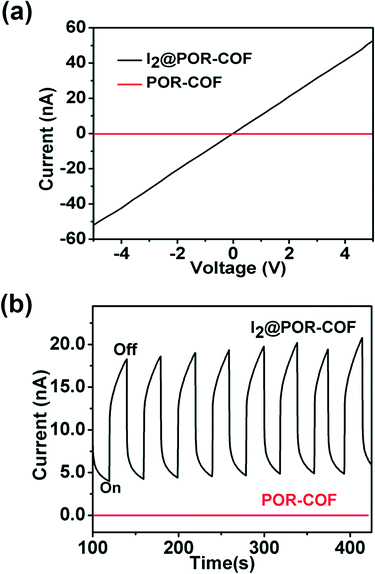 | ||
| Fig. 3 (a) I–V profiles of POR-COF and I2-doped POR-COF; (b) photo-current of POR-COF with the light on and off from a 300 W xenon lamp. | ||
As shown in Fig. 3b, the photoconductivity of both POR-COF and I2-doped POR-COF was studied. The experiments were performed by sandwiching the film samples between two ITO glasses and irradiating with light generated from a xenon lamp. The POR-COF thin film shows a very low current and almost no response to light irradiation. Interestingly, it is observed for the first time that I2 doping can significantly enhance the photoconductive response of a COF material. The current of I2-doped POR-COF sharply rose upon irradiation by light with the on–off ratio greater than one order of magnitude. This photocurrent can be reversibly switched many times with almost no deterioration (Fig. 3b). Similar to other material systems, this I2 doping-induced photoconductivity enhancement may also be attributed to the fact that the doped I2 can make the host material more photosensitive, decrease the energy barrier for charge transport and improve the mobility of holes by trapping electrons.19
Conclusions
In conclusion, a homogeneous solution phase reaction under ambient conditions was developed to synthesize a COF material for the first time. The conductivity of the synthesized POR-COF can increase by more than three orders of magnitude through doping with iodine, indicating the hole-transport nature of this material. Moreover, I2-doped POR-COF shows significantly enhanced photo-current generation when irradiated with light and it can be generated reversibly without any decline in the on–off ratio. This doping-induced enhancement in the photo-electrical properties of POR-COF may open up a new opportunity to tailor the semiconductive properties of COF materials for their future applications.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21550110194, 51402293 and 21401193) and China Post-doctoral Science Foundation (2015M570562). B. N. acknowledges the Chinese Academy of Sciences (CAS) for the PIFI fellowship.Notes and references
- W. Brutting, Physics of Organic Semiconductors, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed.
- (a) L. Briseno, J. Aizenberg, Y. J. Han, R. A. Penkala, H. Moon, A. J. Lovinger, C. Kloc and Z. Bao, J. Am. Chem. Soc., 2005, 127, 12164 CrossRef PubMed; (b) L. Zhang, A. Fonari, Y. Liu, A. L. M. Hoyt, H. Lee, D. Granger, S. Parkin, T. P. Russell, J. E. Anthony, J. L. Bredas, V. Coropceanu and A. L. Briseno, J. Am. Chem. Soc., 2014, 136, 9248 CrossRef CAS PubMed; (c) J. Huang and M. Kertesz, J. Am. Chem. Soc., 2007, 129, 1634 CrossRef CAS PubMed.
- (a) X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010 RSC; (b) S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548 RSC.
- (a) S. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Science, 2015, 349, 1208 CrossRef CAS PubMed; (b) S. Y. Ding, J. Gao, Q. Wang, Y. Zhang, W. G. Song, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816 CrossRef CAS PubMed.
- Q. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu and Y. Yan, J. Am. Chem. Soc., 2015, 137, 8352 CrossRef CAS PubMed.
- J. L. Mendoza-Cortes, W. A. Goddard, III, H. Furukawa and O. M. Yaghi, J. Phys. Chem. Lett., 2012, 3, 2671 CrossRef CAS PubMed.
- (a) Y. Ye, L. Zhang, Q. Peng, G. Wang, Y. Shen, Z. Li, L. Wang, X. Ma, Q. Chen, Z. Zhang and S. Xiang, J. Am. Chem. Soc., 2015, 137, 913 CrossRef CAS PubMed; (b) S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., 2009, 121, 5547 CrossRef; (c) X. Ding, L. Chen, Y. Honsho, X. Feng, O. Saengsawang, J. Guo, A. Saeki, S. Seki, S. Irle, S. Nagase, V. Parasuk and D. Jiang, J. Am. Chem. Soc., 2011, 133, 14510 CrossRef CAS PubMed.
- (a) S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524 CrossRef CAS PubMed; (b) B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328 CrossRef CAS PubMed; (c) C. R. DeBlase, K. E. Silberstein, T. T. Truong, H. D. Abruña and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821 CrossRef CAS PubMed.
- (a) D. Beaudoin and J. D. Wuest, Tetrahedron Lett., 2011, 52, 2221 CrossRef CAS; (b) D. Beaudoin, T. Maris and J. D. Wuest, Nat. Chem., 2013, 5, 830 CrossRef CAS PubMed.
- (a) E. Bamberger and A. Rising, Liebigs Ann. Chem., 1901, 316, 257 CrossRef CAS; (b) S. Horiyama, K. Suwa, M. Yamaki, H. Kataoka, T. Katagi, M. Takayama and T. Takeuchi, Chem. Pharm. Bull., 2002, 5, 996 CrossRef; (c) B. Lindeke and E. Anderson, Acta Pharm. Suec., 1975, 12, 183 CAS.
- (a) W. Luttke, Z. Elektrochem., 1957, 61, 976 CAS; (b) B. G. Gowenlock and K. J. McCullough, J. Chem. Soc., Perkin Trans. 2, 1989, 551 RSC; (c) A. Cruger and N. LeCalve, Spectrochim. Acta, Part A, 1975, 31, 581 CrossRef.
- (a) B. G. Gowenlock and G. B. Richter-Addo, Chem. Soc. Rev., 2005, 34, 797 RSC; (b) I. Halasz, I. Biljan, P. Novak, E. Mestrovic, J. Plavec, G. Mali, V. Smrecki and H. Vancik, J. Mol. Struct., 2009, 918, 19 CrossRef CAS.
- (a) X. Feng, L. Chen, Y. Dong and D. Jiang, Chem. Commun., 2011, 47, 1979 RSC; (b) S. Kandambeth, D. B. Shinde, M. K. Panda, B. Lukose, T. Heine and R. Banerjee, Angew. Chem., 2013, 125, 13290 CrossRef.
- S. Y. Ding, J. Gao, Q. Wang, Y. Zhang, W. G. Song, C. Y. Shu and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816 CrossRef CAS PubMed.
- S. Duhovićand and M. Dinca, Chem. Mater., 2015, 27, 5487 CrossRef.
- S. L. Cai, Y. B. Zhang, A. B. Pun, B. He, J. Yang, F. M. Toma, I. D. Sharp, O. M. Yaghi, J. Fan, S. R. Zheng, W. G. Zhang and Y. Liu, Chem. Sci., 2014, 5, 4693 RSC.
- S. Jin, T. Sakurai, T. Kowalczyk, S. Dalapati, F. Xu, H. Wei, X. Chen, J. Gao, S. Seki, S. Irle and D. Jiang, Chem. – Eur. J., 2014, 20, 14608 CrossRef CAS PubMed.
- (a) M. V. Martinez-Diaz, G. de la Torre and T. Torres, Chem. Commun., 2010, 46, 7090 RSC; (b) A. W. Hains, Z. Liang, M. A. Woodhouse and B. A. Gregg, Chem. Rev., 2010, 110, 6689 CrossRef CAS PubMed; (c) J. D. Zimmerman, V. V. Diev, K. Hanson, R. R. Lunt, E. K. Yu, M. E. Thompson and S. R. Forrest, Adv. Mater., 2010, 22, 2780 CrossRef CAS PubMed.
- R. Bahri, R. K. Bali and B. R. Sood, J. Phys. D: Appl. Phys., 1980, 13, L39 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, IR and Raman spectra, 1H-NMR, TGA, electronic spectra, and conductivity measurements. See DOI: 10.1039/c6ce00168h |
| This journal is © The Royal Society of Chemistry 2016 |