Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Corrin-based chemosensors for the ASSURED detection of endogenous cyanide
Felix
Zelder
*a and
Lucas
Tivana
*b
aInstitute of Chemistry, University of Zurich, Winterthurerstrasse 190, 8057 Zürich, Switzerland. E-mail: felix.zelder@chem.uzh.ch; Web: http://www.felix-zelder.com Fax: +41 44 635 68 03; Tel: +41 44 635 46 24
bFaculty of Engineering, UEM, Av. Mocambique km 1.5, Maputo, Mozambique. E-mail: lucas.tivane@uem.mz; Fax: +25821475311; Tel: +25821443497
First published on 15th October 2014
Abstract
Cassava (Manihot esculenta Crantz) is a staple food for more than 500 million people, especially in Africa and South America. However, its consumption bears risks as it contains cyanogenic glycosides that convert enzymatically to toxic cyanide during cell damage. To avoid serious health problems by unintentional cyanide intake, this dangerous product of decomposition must be removed before consumption. For monitoring such food processing procedures and for controlling the quality and safety of cassava products on the market, a convenient and reliable analytical method for routine applications without laboratory equipment is required. This Perspective summarizes the authors’ work on corrin-based chemosensors for the (‘naked-eye’) detection of endogenous cyanide in cassava samples. Considering selectivity, sensitivity, handling and speed of detection, these systems are superior to currently applied methods. Based on these properties, the development of a test kit for application by rural farmers in remote locations is proposed.
Introduction
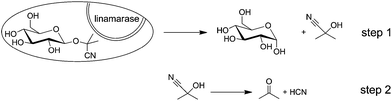
Cassava (Manihot esculenta Crantz) is a staple food in most tropical regions, especially in Africa, the continent with the largest cassava production. It can be cultivated over a wide range of climates and altitudes and on a variety of soils (Fig. 1). Moreover, cassava is tolerant to drought; it is productive in poor soil where other staple crops cannot be grown without intensive inputs.1 For these reasons, cassava represents one of the most important carbohydrate sources for humans and animals in many African regions.However, cassava contains linamarin, a cyanogenic glycoside that releases enzymatically toxic hydrogen cyanide (HCN) after cell damaging (Scheme 1). This self-defence mechanism protects the plant against attacks by certain worms, arthropods and mammals. Due to this natural resistance against animal predation, the majority of farmers in Southern Africa prefer growing bitter varieties with high levels of cyanogens.2,3 Needless to say that these plant constituents and its products of decomposition must be removed before consumption. Combinations of several methods are applied for the elimination of hydrogen cyanide from cassava products including shredding, washing, drying and cooking. Unfortunately, this is not always the case and, as a result, intoxications from poorly processed foodstuffs still occur.4
Cassava related illnesses include tropical ataxic neuropathy, epidemic spastic paraparesis, also known as konzo,5–7 endemic goitre and cretinism.8 These diseases have been reported in the Democratic Republic of Congo, Nigeria and Mozambique.9,10
To avoid such serious health problems, efficient removal of cyanogenic glycosides must be ensured during food processing and needs to be reliably controlled by an efficient analytical method. The cyanogenic potential (CNp) of the crop is defined as the concentration of cyanogenic glycosides and their break down products (cyanohydrins and hydrogen cyanide). The most common analysis of CNp involves three steps (i–iii): (i) extraction of cyanogens from cassava, (ii) hydrolysis of cyanogens to cyanide and (iii) detection of cyanide.11,12 For cyanide detection during CNp analysis, some relatively straightforward and inexpensive methods are nowadays applied by cassava producers and processors. In this context, the most relevant method so far is most likely the semi-quantitative alkaline picrate method.13–15 In this assay, yellow coloured picrate is converted by cyanide into reddish-brown isopurpuric acid.16 Although the picrate method is easy to use, it has certain disadvantages. The reaction is very slow (∼16 hours), the chemical needs special handling and storage, and the response is sometimes imprecise. Also the other commonly applied systems of cyanide detection do not meet the criteria of an ideal diagnostic test for applications in remote settings and situations. Attributes of such tests have been coined ASSURED, standing for affordable, sensitive, selective, user-friendly, rapid, equipment-free and delivered, by the World Health Organisation (WHO).17,18
Having the outstanding affinity of vitamin B12 (“B12”) for cyanide in mind,19 the Zelder group started in 2008 a program for developing B12 derivatives as ASSURED chemosensors for cyanide. These efforts led to the development of aqua, cyano corrinoids (Scheme 2) and are summarized in this Perspective Article.19–25 These metal complexes consist of a central Co(III) ion, an equatorially coordinated tetradendate corrin macrocycle and two axially coordinated ligands, a cyanide and a water molecule.26
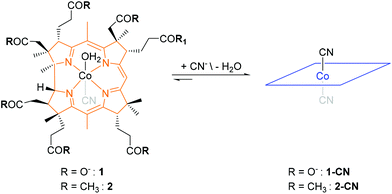 | ||
| Scheme 2 Structural formulas of the corrin-based chemosensors 1, 2 (only one diastereomer is shown). | ||
The complexes convert upon substitution of cobalt coordinated water with cyanide to the corresponding violet-coloured dicyano derivatives (Scheme 2). The absorptions and hence the colour of these metal complexes arise from π to π* transitions of the 14π-electron rich corrin macrocycle and are affected by the nature of the axially coordinated ligands.26
In addition to their high selectivity and sensitivity for cyanide, favourable kinetics makes corrinoids highly attractive for analytical purposes.27,28 Aqua, cyano corrinoids sense cyanide within seconds.27 Indeed, the second order rate constants for the reaction between cyanide and corrinoids (kII ∼ 104 M−1 s−1) resemble more the behaviour of kinetically labile Co(II) compounds than of typical Co(III)-Werner complexes. This behaviour is explained with the strongly donating character of the corrin ring (cis-effect).28 For this reason, ligand substitution reactions in corrinoids are by a factor of up to 103 and 105 faster when compared to reactions with porphyrin-, and tetrammine complexes.29
Detection of endogenous cyanide with corrinoids
In 2009, Männel-Croisé et al. reported on the rapid colorimetric detection of endogenous cyanide for the first time.23,30–32 This application was made possible by exploiting the ideal binding properties of corrinoids for cyanide and the compatibility of the chemosensor with biological matrices. Rapid detection of endogenous biological cyanide was demonstrated directly in colourless biological matrices with chemosensors 1 and 2 (Scheme 2).23Fig. 2 shows a photograph with the pure chemosensor 1 before (a) and after applications to a crude aqueous cassava suspension (b), a crude cassava slurry (d), as well as directly on the surface of a freshly cut cassava slice (c). In all of these samples, the presence of cyanide was indicated by a colour change of the chemosensor from orange to violet. However, when the cassava slurry was repeatedly washed with water, cyanide was successfully removed (e). This behaviour is indicated by the orange colour of the aqua, cyano derivative.
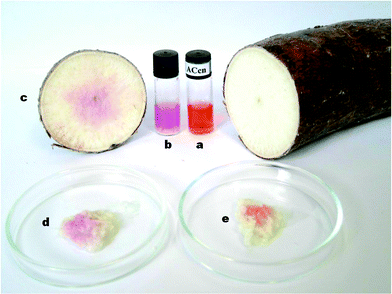 | ||
| Fig. 2 Colour of chemosensor 1 (1 mM) before (a) and after applications to: an aqueous crude cassava extract (b), a freshly cut cassava slice (c), a grinded cassava sample (d) and a thoroughly washed grinded cassava sample (e) (Adapted from ref. 23).23 | ||
On the basis of these examinations, the generality of the method was subsequently underscored by the instantaneous and interference-free detection of endogenous cyanide in various cassava samples such as fresh cassava roots, boiled fresh cassava roots and dried cassava roots.9 The results were in agreement with the quantification of CNp using a combination of isonicotinate and 1,3-dimethylbarbiturate as described by Essers et al.9,33 The superiority of the corrin-based chemosensors compared to this as well as other established systems in terms of handling and speed of detection was strikingly demonstrated by determining the CNp content of fresh cassava samples in less than 5 minutes. For this purpose, only a single drop of fluid squeezed out of the fresh cassava tissue was required. Fresh cassava extracts contain sufficient endogenous linamarase for converting cyanogens to cyanide and no additional steps of sample preparation are therefore required. In contrast to fresh samples, enzymatic degradation of cyanogenic glycosides does not take place in processed cassava roots, such as boiled, dried or roasted roots, most likely due to denaturation of the enzyme. In order to ensure complete conversion of cyanogens to cyanide, the adding of exogenous linamarase is required. It is of advantage that linamarase is easily accessible by extracting the enzyme from the latex of cassava leaves, making the overall procedure of CNp analysis straightforward, fast (∼15 min) and cheap.
In another study, corrin-based chemosensors were also immobilised on hydrophobic white silica material for endogenous cyanide detection in samples of green coloured cassava leaves.20
For this purpose, an experimental filter set-up was developed (Scheme 3). In this system, the coloured plant composites were first removed in a hydrophobic extraction zone and cyanide was then detected with the immobilised chemosensor in a subsequent detection zone.
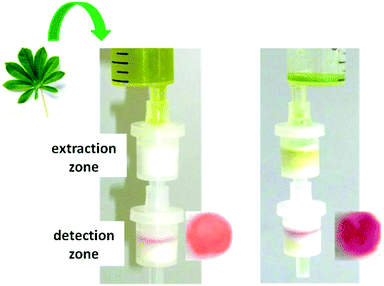 | ||
| Scheme 3 Experimental setup for the detection of cyanide in a raw green-coloured extract of a cassava leaf using extraction and detection zones (adapted from ref. 20).20 | ||
Summary and outlook
Rapid, selective and sensitive detection of biological cyanide makes easy accessible corrin-based chemosensors highly attractive for practical applications. Based on a series of fundamental studies and optimisations, this behaviour was demonstrated for the safe and straightforward detection of endogenous cyanide in cassava, a staple food for more than 500 million people, mostly resident in Africa and South America. Until now, the new method was successfully tested for a large variety of different cassava samples and the results were in agreement with those obtained by an independent analytical method. On the basis of these investigations a new straightforward and quick protocol of routine CNp analysis was developed. Compared to commonly used, rather complex and time-consuming methods, corrin-based chemosensors facilitate handling and sample preparation and enhance greatly the speed of detection. It is beneficial that the presence of endogenous cyanide in cassava products can be detected solely by naked-eye. Therefore, neither the use of laboratory instrumentation nor the interpretation of results by expert-users is principally required. This behaviour is ideal for qualitative and semi-quantitative yes–no analysis by untrained users (i.e. rural farmers, customers) in remote locations (i.e. rural farms and local markets). In these areas the monitoring of food processing procedures or quality controls of cassava products is particularly desired. Unfortunately, until today, ASSURED cyanide tests are not yet on the market. They are, however, expected to improve significantly the quality of life of cassava consuming societies and cassava producers, especially rural farmers. Corrin-based chemosensors exhibit enormous potential, but it remains to be seen whether they will move on from academic research to routine applications in resource limited locations.Acknowledgements
A generous gift of Vitamin B12 from DSM Nutritional Products AG (Basel/Switzerland) is acknowledged. A part of the study was funded by the Swedish International Development Agency (SIDA). F.Z. is a member of the COST action Supramolecular Chemistry in Water (CM 1005).Notes and references
- D. Leihner, in Cassava biology, production and utilization, ed. R. J. Hillocks, J. M. Thresh and A. C. Bellotti, CABI Publishing, Wallingford, UK, 2002, pp. 91–113 Search PubMed.
- A. C. Bellotti and L. Riis, in Proceedings of the international workshop on cassava safety, ed. M. Bokanga, A. J. A. Essers, N. Poulter, H. Rosling and O. Tewe, Acta Horticulturae, New York, 1994, vol. 375, pp. 141–151 Search PubMed.
- L. Chiwona-Karltun, T. Tylleskar, J. Mkumbira, M. Gebre-Medhin and H. Rosling, Int. J. Food Sci. Nutr., 2000, 51, 33–43 CrossRef CAS PubMed.
- F. H. Zelder and C. Männel-Croisé, Chimia, 2009, 63, 58–62 CrossRef CAS.
- H. Rosling, Cassava, cyanides and epidemic spastic paraparesis, a study in Mozambique on dietary cyanide exposure, Upsala University, Upsala, 1986 Search PubMed.
- H. Rosling, Cassava toxicity and food security – A review of health effects of cyanide exposure from cassava and of ways to prevent such effects, Uppsala University, Upsala, 1988 Search PubMed.
- H. Nzwalo and J. Cliff, PLoS Neglected. Trop. Dis, 2011, 5, 1–8 Search PubMed.
- F. Delange, L. O. Ekpechi and H. Rosling, in Proceedings of the international workshop on cassava safety, ed. M. Bokanga, A. J. A. Essers, N. Poulter, H. Rosling and O. Tewe, Acta Horticulturae, New York, 1994, vol. 375, pp. 289–293 Search PubMed.
- L. Tivana, J. Da Cruz Francisco, F. Zelder, B. Bergenstahl and P. Dejmek, Food Chem., 2014, 158, 20–27 CrossRef CAS PubMed.
- D. Nhassico, H. Muquingue, J. Cliff, A. Cumbana and J. H. Bradbury, J. Sci. Food Agric., 2008, 88, 2043–2049 CrossRef CAS.
- M. F. Borges, W. M. G. Fukuda and R. C. Caldas, Rev. Bras. Mandioca, 1993, 12, 75–80 Search PubMed.
- J. H. Bradbury, M. G. Bradbury and S. V. Egan, in Proceedings of the international workshop on cassava safety, ed. M. Bokanga, A. J. A. Essers, N. Poulter, H. Rosling and O. Tewe, Acta Horticulturae, New York, 1994, vol. 375, pp. 87–96 Search PubMed.
- H. J. Williams and T. G. Edwards, J. Sci. Food Agric., 1980, 31, 15–22 CrossRef CAS.
- J. H. Bradbury, Food Chem., 2009, 113, 1329–1333 CrossRef CAS.
- M. G. Bradbury, S. V. Egan and J. H. Bradbury, J. Sci. Food Agric., 1999, 79, 593–601 CrossRef CAS.
- R. G. Smith, J. Am. Chem. Soc., 1929, 51, 1171–1174 CrossRef CAS.
- D. Mabey, R. W. Peeling, A. Ustianowski and M. D. Perkins, Nat. Rev. Microbiol., 2004, 2, 231–240 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2009, 82, 3–10 CrossRef PubMed.
- F. H. Zelder, Inorg. Chem., 2008, 47, 1264–1266 CrossRef CAS PubMed.
- C. Männel-Croisé and F. Zelder, ACS Appl. Mater. Interfaces, 2012, 4, 725–729 Search PubMed.
- C. Männel-Croisé and F. Zelder, Anal. Methods, 2012, 4, 2632–2634 RSC.
- C. Männel-Croisé and F. Zelder, Inorg. Chem., 2009, 48, 1272–1274 CrossRef PubMed.
- C. Männel-Croisé, B. Probst and F. Zelder, Anal. Chem., 2009, 81, 9493–9498 CrossRef PubMed.
- M. K. Freeman and L. G. Bachas, Anal. Chim. Acta, 1990, 241, 119–125 CrossRef CAS.
- S. S. M. Hassan, M. S. A. Hamza and A. E. Kelany, Talanta, 2007, 71, 1088–1095 CrossRef CAS PubMed.
- F. Zelder and R. Alberto, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Elsevier Science, San Diego, 2012, vol. 25, pp. 83–130 Search PubMed.
- B. Aebli, C. Männel-Croisé and F. Zelder, Inorg. Chem., 2014, 53, 2516–2520 CrossRef CAS PubMed.
- S. M. Chemaly, M. Florczak, H. Dirr and H. M. Marques, Inorg. Chem., 2011, 50, 8719–8727 CrossRef CAS PubMed.
- H. M. Marques, J. Chem. Soc., Dalton Trans., 1991, 1437–1442 RSC.
- L. G. Nandi, C. R. Nicoleti, I. C. Bellettini and V. G. Machado, Anal. Chem., 2014, 86, 4653–4656 CrossRef CAS PubMed.
- N. Kumari, S. Jha and S. Bhattacharya, Chem. – Asian J., 2014, 9, 830–837 CrossRef CAS PubMed.
- K. P. Divya, S. Sreejith, B. Balakrishna, P. Jayamurthy, P. Anees and A. Ajayaghosh, Chem. Commun., 2010, 6069–6071 RSC.
- A. A. Essers, M. Bosveld, R. M. Vandergrift and A. G. J. Voragen, J. Sci. Food Agric., 1993, 63, 287–296 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |