Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective sensing of saccharides using simple boronic acids and their aggregates†
Xin Wu
a,
Zhao Li
a,
Xuan-Xuan Chen
a,
John S. Fossey
b,
Tony D. James
c and
Yun-Bao Jiang
*a
aDepartment of Chemistry, College of Chemistry and Chemical Engineering, MOE Key Laboratory of Analytical Sciences, and Collaborative Innovation Center of Chemistry for Energy Materials (iChEM), Xiamen University, Xiamen 361005, China. E-mail: ybjiang@xmu.edu.cn
bSchool of Chemistry, University of Birmingham, Birmingham, West Midlands B15 2TT, UK
cDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK
First published on 16th July 2013
Abstract
The reversible boronic acid–diol interaction empowers boronic acid receptors' saccharide binding capacities, rendering them a class of lectin mimetic, termed “boronlectins”. Boronic acids follow lectin functions not just in being able to bind saccharides, but in multivalent saccharide binding that enhances both affinity and selectivity. For almost a decade, efforts have been made to achieve and improve selectivity for given saccharide targets, most notably glucose, by using properly positioned boronic acids, offering multivalent interactions. Incorporation of several boronic acid groups into a covalent framework or non-covalent assembly of boronic acid are two general methods used to create such smart sensors, of which the latter resembles lectin oligomerisation that affords multivalent saccharide-binding architectures. In this review, we discuss supramolecular selective sensing of saccharides by using simple boronic acids in their aggregate forms, after a brief survey of the general aspects of boronic acid-based saccharide sensing.
 Zhao Li, Xuan-Xuan Chen, Xin Wu and Yun-Bao Jiang | Xin Wu (third from left) received his BS degree in chemistry from Xiamen University in 2011. He has been a PhD graduate under Professor Yun-Bao Jiang since 2011 and will soon join Professor P. A. Gale at the University of Southampton to continue his PhD work. He is currently working on boronic acids in self-assembly for sensing of saccharides, hydroxy acids and anions. |
Zhao Li (left) is an associate professor at Xiamen University. She completed her first degree in Chemical Engineering in 1989 at Jiangnan University. After obtaining her MS degree from Xiamen University in 1997 she joined the University as an assistant professor. She was awarded a PhD in 2008 for her work with Professor Yun-Bao Jiang and spent one year as a postdoctoral fellow with Dr Hua-qiang Zeng at the National University of Singapore before taking up her current position in 2009. Her research is centred on foldamer-based sensing. |
Xuan-Xuan Chen (second from left) is currently a last-year undergraduate at Xiamen University. She will soon receive her BS degree and continue to pursue her PhD study in the fall of 2013. She has been a member in the group of Professor Yun-Bao Jiang since 2011. She has been since working on supramolecular chirality from metal coordination polymers and dye aggregates. |
 John S. Fossey | John S. Fossey is a lecturer and Royal Society Industry Fellowship holder at the University of Birmingham. He completed his first degree in chemistry in 2000 at Cardiff University of Wales. In 2004 he was awarded a PhD from Queen Mary University London. He then worked as a JSPS postdoctoral research fellow with Professor Shū Kobayashi at the University of Tokyo. He then took up a temporary position at the University of Bath before moving to the University of Birmingham to take up his current position in 2008. |
 Tony D. James | Tony D. James is a Professor at the University of Bath. He completed his first degree in Chemistry in 1986 at the University of East Anglia. In 1991 he was awarded a PhD from the University of Victoria in Canada. He then worked as a Postdoctoral Research Fellow at the Chemirecognics Project in Kurume with Seiji Shinkai. In 1995 he returned to the UK as a Royal Society Research Fellow at the School of Chemistry at the University of Birmingham, moving to the Department of Chemistry at the University of Bath in September 2000. |
Yun-Bao Jiang (right) is currently a professor of chemistry at Xiamen University. He obtained his PhD in 1990 with Prof. Guo-Zhen Chen in Xiamen University and did his postdoc research with Prof. K. A. Zachariasse at Max-Planck-Institute for Biophysical Chemistry, Germany (AvH fellow) and with Prof. Chi-Ming Che at University of Hong Kong. He has been an invited professor at ENS Cachan (2008) and a visiting professor at National University of Singapore (2008). Research in his group focuses on photophysics of electron/proton transfer and supramolecular sensing. |
1. Introduction
The effectiveness of boronic acids as receptors in chemosensors for a range of biologically important species is perhaps nowhere more evident than in the myriad of publications related to this area. The target species to which boronic acids are most often applied are saccharides. These neutral polyhydroxyl guests have high solvation enthalpies in aqueous solutions that are difficult for synthetic chemosensors to overcome as efficiently as naturally occurring lectins that operate solely by non-covalent interactions.There is yet another challenge, the binding selectivity, since many saccharides show no structural difference except configurations of certain stereocentres, making them hard to be distinguished between. These challenges are reflected by the relatively slow progress in the development of completely non-covalent interaction-based synthetic glucose sensors for real world applications.1 In this context, sensors utilising biological recognition elements, including those based on enzymes or sugar binding proteins (lectins), have been developed and are widely used. Synthetic chemosensors, however, are more desired in terms of the stability, low cost, and oxygen independence. Boronic acid-based synthetic chemosensors therefore represent promising alternatives for saccharide sensing.
The reversible covalent interaction of boronic acids with cis-1,2- or 1,3-diols to form five- or six-membered cyclic esters, respectively, has proved sufficiently strong, enabling binding of saccharides at mM or sub-mM levels, rendering boronic-acid based saccharide sensing in biologically relevant scenarios possible. The strength of boronic acid binding to saccharides is determined by the orientation and relative position of hydroxyl groups, thus boronic acids can differentiate structurally similar saccharide molecules. It is now known that monoboronic acids exhibit inherent fructose selectivity among monosaccharides.2 This is because of the high abundance in solution of the form of fructose that contains a syn-periplanar pair of hydroxyl groups available for boronic acid binding, i.e. the β-D-fructofuranose form whose relative percentage of total fructose is 25% in D2O at 31 °C.3 In contrast, with glucose, the available α-D-glucofuranose form makes up only 0.14% of the total composition in D2O at 27 °C (Scheme 1).4 In line with these facts the binding constant of phenylboronic acid with fructose is 4370 M−1 while that with glucose is 110 M−1.2 The generally observed binding affinity of phenylboronic acids with monosaccharides follows the order of fructose > galactose > mannose > glucose.2 In general both the saccharide affinity and selectivity of simple monoboronic acid based sensors are poor. This can be substantially improved by using properly positioned multiboronic acids. In this regard it is of interest to note that the use of multiboronic acids parallels the approach nature takes to enhance saccharide affinity by lectins that contain multiple binding sites, in which two cases the multivalency effect seem to operate.
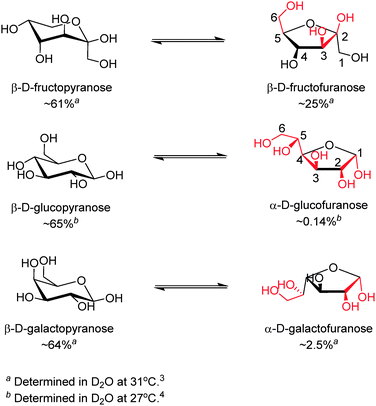 | ||
| Scheme 1 Equilibria between the dominant form (left) and the form that contains a syn-periplanar anomeric hydroxyl pair (right) of D-fructose (top), D-glucose (middle) and D-galactose (bottom).3–5 Potential boronic acid binding sites are highlighted in red. Positions for hydroxyl groups are numbered in β-D-fructofuranose and α-D-glucofuranose. | ||
Multivalency6,7 is actually a key working principle of molecular recognition in biological systems, which makes cumulative use of individually weak interactions. Briefly, binding between two multivalent (i.e. having more than one binding site) entities involving n (n > 1) binding events occurs with an affinity higher than the sum of n individual monovalent interactions. This is similar to the chelate effect in coordination chemistry where a tridentate ligand can have a higher binding affinity for a metal than three related monodentate ligands. Note that the interaction between a multivalent receptor and several monovalent substrates is not multivalency, although multivalent receptors do bind monovalent substrates with a higher binding constant than monovalent receptors simply because of a higher total concentration of binding sites. Of particular significance among the multivalency effects found in nature is that observed in the reversible lectin–saccharide interaction, which has been termed the “cluster-glycoside effect”.8,9 The interaction between a lectin binding site and a monosaccharide molecule, as a combination of non-covalent interactions such as hydrogen bonds, hydrophobic interactions and van der Waals interactions, with binding constants typically ranging from 103–104 M−1, is relatively weak and less specific compared to those of other biological recognition events. The binding constant of a lectin subunit with an oligosaccharide is, however, substantially higher, up to 106 M−1 as a result of spatial extension of the binding sites, which is the operating principle of small lectins and is referred to as subsite multivalency. Further affinity enhancement for oligosaccharide recognition is achieved through clustering of binding sites via (i) non-covalent aggregation of lecton polypeptides into oligomeric structures (e.g. the hepatic asialoglycoprotein receptor10) or (ii) repeating binding sites within a single lectin polypeptide (e.g. the macrophage mannose receptor11). Multivalent architectures containing several binding sites created via both (i) and (ii) allow multivalent interactions with oligosaccharides with high specificity and dramatically increased binding constants up to ca. 109 M−1. This is central to the cluster glycoside effect and has made the lectin–saccharide interactions operate in a variety of biological processes,12 such as control of glycoprotein traffic, cell adhesion, immune response, bacterial invasion, and tumor metastasis.
While the term “multivalency” is not commonly used for a diboronic acid receptor, probably because of the low valence of glucose compared to oligo- and polysaccharides, the successful development of glucose selective boronic acid sensors, where diboronic acids can often bind glucose substantially more strongly than fructose is a manifestation of multivalency. Although a single boronic acid–diol interaction, by definition, is already divalent when considering the formation of each B–O–C bond as monovalent (note: in some cases with boronic acid–fructose binding, the boronic acid–diol motif can be further stabilised by an additional B–O–C linkage, resulting in a tridentate boronate ester13), we here define binding of one boronic acid moiety involving the formation of two or three B–O–C bonds as a valence, for the sake of convenience in the following discussions. Under this definition, β-D-fructofuranose, which is the fructose isomer that participates in boronic acid binding, can be seen as a monovalent ligand, whereas the α-glucofuranose form of glucose can be seen as divalent and can potentially bind two boronic acid moieties via its two binding sites at the 1,2- and 3,5,6-positions (Scheme 1). Although fructose was also shown by Norrild and Eggert to be bound by two boronic acids in its β-pyranose form,13 that was observed at high boronic acid concentration (in a NMR spectroscopic study) and in the presence of excess fructose, which is unrealistic for sensing in most biologically relevant settings. Actually almost all sensing studies using boronic acid at 10−4–10−6 M have identified fructose as a monovalent ligand with boronic acids binding to its β-furanose form. Using this difference in the number of valences for glucose and fructose, two typical monosaccharides, remarkable progress has been made over the past decade in developing boronic acid-based sensors selective for glucose, by following a general concept that suitably arranged multiple boronic acid groups favor multivalent saccharides.
Sensors selective for other saccharides and glycosylated species have also been reported employing the same strategy, but less than those for glucose. The majority of such efforts are driven by the demand for practical sensors selective for glucose which is of great biological and clinical importance. The other reason is that the shorter distance and favorable orientation of the two potential binding sites in glucose make rational sensor design feasible. Mechanisms for creating supramolecular boronic acid sensors selective for saccharides, by covalently incorporating multiple boronic acid groups into one structural framework or non-covalently assembling simple boronic acid into aggregates are summarised in Fig. 1.
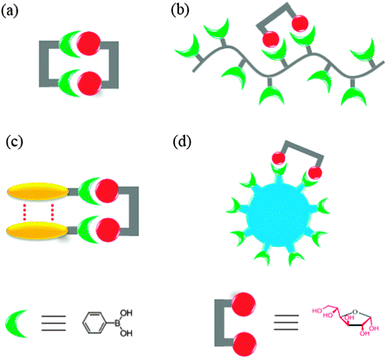 | ||
Fig. 1 Cartoon representation of four strategies for selective saccharide sensing via multivalent boronic acid–saccharide interactions, exemplified by glucose sensing. (a) Synthetic diboronic acids that form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic boronate esters with glucose, (b) boronic acid-containing polymers that bind glucose with two of the pendant boronic acid moieties, (c) aggregation of simple boronic acids via non-covalent interactions to allow multivalent glucose binding, and (d) boronic acid-conjugated nanomaterials as multivalent scaffolds. Note that the aggregates shown in (c) can be aggregates or saccharide binding altered aggregates of boronic acid, or saccharide binding induced aggregates of boronic acid. 1 cyclic boronate esters with glucose, (b) boronic acid-containing polymers that bind glucose with two of the pendant boronic acid moieties, (c) aggregation of simple boronic acids via non-covalent interactions to allow multivalent glucose binding, and (d) boronic acid-conjugated nanomaterials as multivalent scaffolds. Note that the aggregates shown in (c) can be aggregates or saccharide binding altered aggregates of boronic acid, or saccharide binding induced aggregates of boronic acid. | ||
Glucose selective boronic acid-based sensors are typically diboronic acids that are able to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic boronate esters with glucose (Fig. 1a). The multivalent diboronic acid–glucose interaction leads to a higher affinity for glucose than for fructose, in contrast to the higher affinity for fructose observed with monoboronic acids. The first breakthrough in this area was by Shinkai and co-workers in 199414 and was quickly followed by the publication of other more structurally diverse diboronic acids, each having their own advantages and disadvantages. Diboronic acid-based sensors have also been developed for other saccharides and glycosylated species for which multivalent binding is possible. However, many of the reported diboronic acid sensors exhibited several disadvantages, including substantial synthetic demand and poor water solubility.
1 cyclic boronate esters with glucose (Fig. 1a). The multivalent diboronic acid–glucose interaction leads to a higher affinity for glucose than for fructose, in contrast to the higher affinity for fructose observed with monoboronic acids. The first breakthrough in this area was by Shinkai and co-workers in 199414 and was quickly followed by the publication of other more structurally diverse diboronic acids, each having their own advantages and disadvantages. Diboronic acid-based sensors have also been developed for other saccharides and glycosylated species for which multivalent binding is possible. However, many of the reported diboronic acid sensors exhibited several disadvantages, including substantial synthetic demand and poor water solubility.
Alternatives to these diboronic acids, are the boronic acid-containing polymers (Fig. 1b), in which multiple boronic acid moieties are covalently attached to the polymer backbone and thereby allow more than one pendant boronic acid moieties to interact with a saccharide molecule. In fact, both approaches resemble the cluster glycoside effect by a single lectin polypeptide containing many binding sites.11 It is of interest, from a biomimetic point of view, to question whether synthetic “boronlectins” can follow another approach; that taken by naturally occurring lectins, i.e. the non-covalent aggregation,10 to achieve higher affinity and selectivity for a given saccharide species. Indeed, it has been recently demonstrated that the covalent framework of multiboronic acids can be replaced by the non-covalent self-assembly of simple boronic acids (Fig. 1c), in which boronic acid molecules can come together and result in glucose selectivity, comparable to the best synthetic multiboronic acids reported. Selective saccharide sensing has also been realised by the use of nanomaterials (Fig. 1d) to which multiple boronic acids are covalently attached.
In this review, saccharide selective sensors based on supramolecular boronic acids are presented and discussed, which are categorised into the aforementioned four sensing mechanisms (Fig. 1), of which those using the self-assembly of boronic acids are of primary importance. We begin with a brief discussion of the basic principles of molecular boronic acid based saccharide sensing and key issues in sensor design. For detailed discussions on molecular boronic acid–diol interaction for saccharide sensing, several excellent reviews,15–18 book chapters19,20 and a monograph5 are recommended.
Before moving onto the next sections, we need to clarify what is meant by the concept of selectivity. While the term “selective” is used in almost all sensing publications, we should remember the two types of selectivity, namely the binding selectivity and the response selectivity.21 Traditionally, selectivity is pursued by choosing an appropriate receptor that binds thermodynamically more favorably to the target species of interest than to potential interfering species. The selectivity achieved can be termed “binding selectivity” or “thermodynamic selectivity”. While there are sensors that exhibit “selectivity” in terms of signal transduction, where the affinity of a given target to the sensor might not be the highest among species that can interact with the sensor, yet upon host–guest interaction the given target creates an exclusive response, showing response selectivity. In assessment of the sensor performance, the two kinds of “selectivity” should not be confused. If a sensor exhibits only response selectivity without binding selectivity, coexistence of the interfering substance will lead to a negative deviation due to a competitive binding of the interfering species for the sensor. The concurrence of excellent binding selectivity and response selectivity is without doubt desired, but difficult to achieve, especially for boronic acid-based sensors for glucose. Conformational changes or self-assembly induced by glucose are means to elicit glucose-selective responses where monovalent fructose is unable to induce the same change. This, however, precludes the use of structurally rigid diboronic acids that might be favorable for selective binding for glucose over fructose. Therefore, in order to evaluate the practical applicability of a saccharide-selective sensor, especially those designed for glucose, competitive experiments are needed to check the extent to which the coexistence of other saccharides, especially fructose, interfere with the glucose sensing. To demonstrate binding selectivity, many publications report binding constants deduced from spectroscopic titrations, which may not correctly predict the outcome when interferants coexist, since kinetic factors may operate. A sensor that shows a modest selectivity (e.g. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in either binding or response might have rather limited practical applicability, yet these sensors deserve attention. The methodologies used to deliver this selectivity could be instructive in the development of “better” sensors. Therefore, some modestly selective sensors are included in this review.
1) in either binding or response might have rather limited practical applicability, yet these sensors deserve attention. The methodologies used to deliver this selectivity could be instructive in the development of “better” sensors. Therefore, some modestly selective sensors are included in this review.
2. Key issues for boronic acid-based saccharide sensor design
The boronic acid–diol interaction is highly pH-dependent. It is therefore necessary to consider the thermodynamic cycle shown in Scheme 2, when designing boronic acid-based saccharide sensors. Boronic acids are known to become more acidic upon diol binding, with pKa′ of the boronate ester lower by 2–4 units than the pKa of the boronic acid. Note that exceptions exist with extremely weakly binding diols when pKa′ was found to be slightly higher than pKa.22 Formation constants of anionic, tetrahedral boronate esters (Ktet) are in general higher than that of the neutral, trigonal boronate esters (Ktrig) by 2–4 orders of magnitude, since KaKtet = Ka′Ktrig. It is deduced from the larger value of Ktet compared to Ktrig that higher pH favours diol binding when most of the boronic acid exists in the anionic form. Experimentally, however, the optimal binding pH is not necessarily higher than the pKa of the boronic acid, which is attributed to the ionisation of diol moieties.23 Furthermore, the optimal binding pH is not necessarily the optimal pH for saccharide sensing since signal modulation should be taken into account. Generally, sensors are designed to exhibit differing signal outputs of the boronic acid moiety in neutral and negatively charged forms, so that measurable changes can be observed in buffered solutions in which the originally neutral boronic acid becomes anionic upon saccharide binding. In that case the optimal pH for sensing lies between pKa′ and pKa. Higher pH can be used for sensors whose signal transduction does not rely on the acidity change of the boron centre, for example sensors that show fluorescence enhancement upon binding saccharides, that leads to fluorophore rigidisation. It is also of significance to note from Scheme 2 that upon binding to saccharides a negatively charged boronate ester forms at high pH, thus a positive charge, such as that from ammonium or pyridinium cation, may be incorporated into the structure of the sensor to facilitate the boronic acid–saccharide interaction.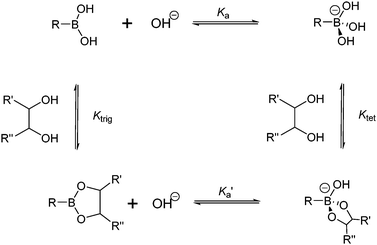 | ||
| Scheme 2 Thermodynamic cycle of boronic acid ionisation and interaction with cis-1,2-diols; Ka and Ka′ represent ionisation constants of boronic acids and boronate esters, respectively, Ktrig and Ktet represent formation constants of trigonal and tetrahedral boronate esters formed from boronic acid and boronate anion, respectively. | ||
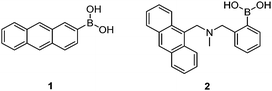
Early investigations into boronic acid–saccharide interactions date back to 1959 when Lorand and Edwards determined the saccharide binding constants of boric acid and phenylboronic acid using a pH-depression method.2 The first boronic acid-based saccharide sensor, however, came over 30 years later with the seminal work of Yoon and Czarnik in 1992.24 The earliest sensor 1 showed a fluorescence quenching response to saccharides, which is usually the case when the boron centre is directly attached to the fluorophore. Sensor 2 developed by James et al.25 was the first boronic acid-based saccharide sensor that displayed a fluorescence “Off–On” response, due to inhibition of the photo-induced electron transfer (PET) upon saccharide binding.
In addition to selectivity and sensitivity, other criteria are important for the biological applications of these boronic acid based sensors, for example, in vivo continuous glucose monitoring. For effective continuous measurement the systems must be reversible, have rapid response time, be stable, non-toxic, and function at physiological pH. Most of the synthetic boronic acid-based sensors perform well in terms of reversibility and response time, as dictated by the nature of the boronic acid–diol interaction. Several other aspects have also been improved, for example, for the sensor to function at physiological pH, the acidity of the boron centre has been increased via introduction of electron-withdrawing substituents to the boronic acid moiety26 or by using the B–N interaction.25,27,28
3. Strategies for saccharide selectivity via construction of multiboronic acid scaffolds
3.1 Synthetic diboronic acids that form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 cyclic boronate esters with glucose or other saccharides
1 cyclic boronate esters with glucose or other saccharides
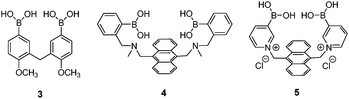
The possibility for multivalent saccharide binding by a diboronic acid sensor was first explored by Tsukagoshi and Shinkai in 1991.29 Achiral diboronic acid 3 displayed an induced CD signal in the presence of D-glucose, D-maltose and D-cellobiose but remained CD-silent in the presence of monovalent D-fructose. It was proposed that the CD signal arises from cyclic boronate esters formed, which was confirmed by subsequent NMR spectroscopic studies.30 Although the binding constant of 3 with glucose was as high as 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1, 3 is not a good optical sensor due to lack of a chromophore that absorbs in the visible region.
000 M−1, 3 is not a good optical sensor due to lack of a chromophore that absorbs in the visible region.
The first glucose-selective boronic acid-based fluorescence sensor 4 was designed and prepared by Shinkai et al. in 1994,14 following their previous success in developing the PET sensor 2. Compound 4 showed a high glucose binding constant of 3981 M−1, compared to that with fructose (316 M−1) and galactose (158 M−1). The 4–glucose complex was initially assigned to the 1,2:4,6-α-glucopyranose diboronate complex (Fig. 2a) and was later shown by Norrild et al.31 to be the 1,2:3,5,6-α-glucofuranose diboronate complex (Fig. 2b) formed in aqueous solutions. The initially-deduced product was found to form only under completely anhydrous conditions. It was proposed in later studies that the C–H⋯π interactions between the hydrocarbon skeleton of glucose and the extended aromatic surface of the anthracene core in 4 might also contribute to the high affinity to glucose.32 Similar to 2, 4 displays fluorescence enhancement responses to saccharides, and the high glucose affinity allows glucose sensing at sub-mM levels, a concentration range over which other saccharides show much weaker fluorescence enhancement. Competitive experiments showed that fructose and galactose at 0.1 mM exerted negligible interference for glucose sensing, which was in good agreement with the largest binding constants of 4 with glucose, among the monosaccharides. Fructose and galactose at mM level, however interfere as they lead to substantial fluorescence enhancement. Takeuchi and co-workers incorporated 4 into a polymer matrix to create fluorescent hydrogel microbeads33 and hydrogel fibers34 for in vivo studies. The microbeads or fibres were implanted into the ear skin of mice and the fluorescence signal was detected via transdermal transmission, enabling successful continuous monitoring of blood glucose concentrations. While the microbeads dispersed from the implantation site and are therefore not suitable for long-term use, implanted fibres display better applicability and can continuously monitor glucose concentrations for up to 140 days and are easily removed non-surgically.
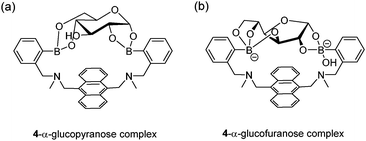 | ||
| Fig. 2 Structures of initially-deduced 4–α-glucopyranose complex formed in MeOD (a) and the latter assigned 4–α-glucofuranose complex (b) formed under aqueous conditions. | ||
Norrild and co-workers reported a pyridinium derivative of 4, 5.35 The positively charged pyridinium cation improves the water solubility and increases the acidity of the boron centre, thus allowing glucose sensing in completely aqueous solution at physiological pH, with a high binding constant of 2512 M−1. NMR spectroscopic studies indicate that 5 binds to α-glucofuranose at its 1,2,3,5-hydroxyl groups. 5 shows fluorescence enhancement in the presence of either glucose or galactose, with selectivity for glucose at sub-mM levels. A drawback of 5 is that its fluorescence response toward glucose is significantly reduced in the presence of galactose or fructose.
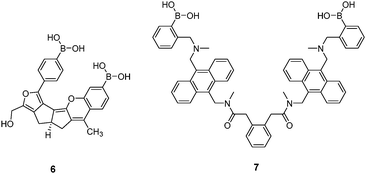
Diboronic acid 636 is a sophisticated example designed by Drueckhammer and co-workers. It binds to α-glucopyranose form instead of the α-glucofuranose (binding with 3, 4 and 5) form of glucose (Scheme 1). Computer-guided design followed by an eight-step synthesis afforded 6 that contains a pair of almost parallel-oriented boronic acid moieties attached to a highly rigid covalent framework. Compound 6 exhibits a much higher affinity for glucose, 400-fold greater than those of other saccharides, with a glucose binding constant of 4.0 × 104 M−1. The 400-fold binding selectivity for glucose over fructose and galactose is the highest reported to date. Compound 6 represents an “extreme” example in which a highly rigid diboronic acid scaffold matches perfectly the structure of glucose, thanks to an elaborate design and formidable synthetic efforts.
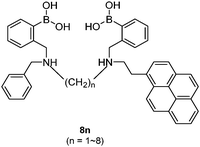
Diboronic acid 737 also shows remarkable glucose selectivity with a glucose binding constant of 1472 M−1, which is 43-fold higher than that of fructose and 49-fold higher than galactose. Another advantage of 7 is that glucose induces a fluorescence enhancement by up to 7 times while the fluorescence enhancement is less than double for fructose and galactose. In the search for a glucose-selective diboronic acid, James et al.38 have developed a modular approach and prepared a series of compounds 8n with varying spacer length from n = 1 to n = 8. Sensor 86 was identified as the best glucose sensor in the series, with binding constants 960 M−1 for glucose, 760 M−1 for fructose, 660 M−1 for galactose and 70 M−1 for mannose. While glucose sensing is carried out in mixed solvents in the case of 5, 6, 7 and 8n, Singaram et al.39 reported a structurally simple and water-soluble cationic diboronic acid 9 with glucose selectivity 1.8-fold higher than fructose and 11 times greater than galactose in aqueous solution. A fluorescent sensing ensemble was created by mixing cationic 9 and an anionic fluorescence reporter dye 10. This ensemble shows enhanced fluorescence in the presence of saccharides since saccharide binding induces an ionisation of the boronic acid moieties of 10 leading to new negative charges and thus the dissociation of the assembly of 9 and 10 originally held together by electrostatic attraction (Scheme 3).
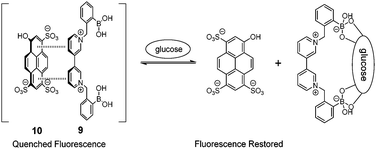 | ||
| Scheme 3 Glucose-binding induced dissociation of the electrostatic assembly of 9 and 10. | ||
While rigid diboronic acids are desired for high binding selectivity for glucose, flexible diboronic acid sensors may adapt to different conformations with regard to whether a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic or a 1
1 cyclic or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 acyclic complex is formed, generating different spectroscopic outputs from these two species that means a “response” selectivity. An elegant example is the fluorescent molecular tweezer 11.40 The mechanism for selective saccharide sensing by 11, is shown in Fig. 3. In the absence of a saccharide, stacking between the two pyrene fluorophores of 11 leads to a long-wavelength excimer emission. While formation of a 1
2 acyclic complex is formed, generating different spectroscopic outputs from these two species that means a “response” selectivity. An elegant example is the fluorescent molecular tweezer 11.40 The mechanism for selective saccharide sensing by 11, is shown in Fig. 3. In the absence of a saccharide, stacking between the two pyrene fluorophores of 11 leads to a long-wavelength excimer emission. While formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 boronate with fructose does not interrupt the stacking and the excimer emission remains intact, upon binding to glucose, galactose or mannose, however, a 1
2 boronate with fructose does not interrupt the stacking and the excimer emission remains intact, upon binding to glucose, galactose or mannose, however, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 boronate is formed in which the conformational change brought about by the binding forces the pyrene moieties to split apart, weakening pyrene excimer emission. The binding constants follow the order of fructose > glucose ≫ galactose > mannose. Although fructose has a higher affinity with 11 than glucose, it does not show an excimer quenching response.
1 boronate is formed in which the conformational change brought about by the binding forces the pyrene moieties to split apart, weakening pyrene excimer emission. The binding constants follow the order of fructose > glucose ≫ galactose > mannose. Although fructose has a higher affinity with 11 than glucose, it does not show an excimer quenching response.
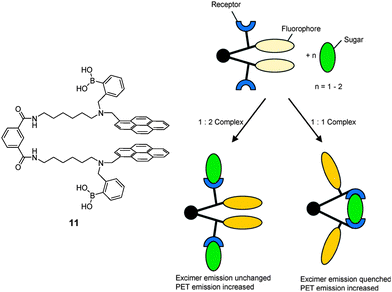 | ||
| Fig. 3 Cartoon scheme of binding of tweezer 11 with monovalent saccharide (left) and divalent saccharide (right). Reproduced from ref. 40 with permission from The Royal Society of Chemistry. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic 3–disaccharide boronate and the knowledge that the stilbene fluorophore becomes more fluorescent when the rotation of the ethylene double bond in the lowest excited state is blocked. Compound 12 indeed shows a 3-fold fluorescence enhancement in the presence of the disaccharide melibiose that can interact with 12 via its 4,6-cis- and 1′,2′-cis-diols. Other disaccharides and monosaccharides induce less or no fluorescence enhancement, because of lower or no tendency of forming 1
1 cyclic 3–disaccharide boronate and the knowledge that the stilbene fluorophore becomes more fluorescent when the rotation of the ethylene double bond in the lowest excited state is blocked. Compound 12 indeed shows a 3-fold fluorescence enhancement in the presence of the disaccharide melibiose that can interact with 12 via its 4,6-cis- and 1′,2′-cis-diols. Other disaccharides and monosaccharides induce less or no fluorescence enhancement, because of lower or no tendency of forming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic boronate due to unfavorable diol orientation, shorter inter-diol distance or the presence of only one set of cis-diols.
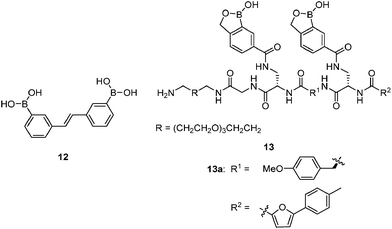
1 cyclic boronate due to unfavorable diol orientation, shorter inter-diol distance or the presence of only one set of cis-diols.By screening a large library of structurally diverse bis(boroxole) receptors, Hall and co-workers successfully identified a receptor that selectively targets tumor marker Thomsen–Friedenreich antigen disaccharide Gal-β-1,3-GalNAc (Fig. 4).42 The combinatorial strategy43 is well suited for identifying receptors for structurally complicated substrates such as oligosaccharides, for which rational design of the receptor structure appears unfeasible. The benzoboroxole moiety44 was chosen as the binding site for hexopyranoside, instead of the boronic acid moiety that is unable to target pyranoside. The bis(boroxole) library 13 consisted of 400 receptors that differ in spacer R1 (out of 20 amino acid residues) and the capping group R2 (from 20 carboxylic acids). Additional interactions such as hydrogen bonding, hydrophobic packing and CH–π interactions were made possible by choosing R1 and R2 that contain suitable functional groups for these interactions, in attempt to better discriminate structurally related saccharides. The identified receptor 13a is indeed highly specific for Gal-β-1,3-GalNAc, with a binding constant of 1.1 × 103 M−1 which is in the same order of magnitude as most of the diboronic acid–glucose interactions and matches lectin–saccharide interactions.
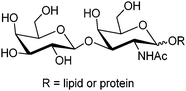 | ||
| Fig. 4 Structure of Thomsen–Friedenreich antigen disaccharide Gal-β-1,3-GalNAc. | ||
While most of the reported diboronic acid sensors show glucose selectivity among monosaccharides, there are a few examples of galactose-selective boronic acid sensors. Among the modular series 8n (n = 1–8), increase in the spacer length, from 86 to 87 and 88, leads to the inversion of selectivity from glucose to galactose. This was explained in that galactose has a longer inter-diol distance and opposed diol orientations of α-galactopyranose in its twist boat conformation thereby requiring a larger binding pocket than that for glucose.5
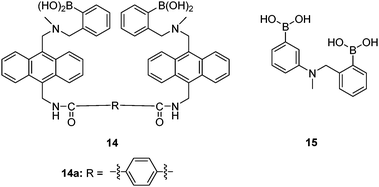
Sialyl Lewis X (sLeX, Fig. 5) is a tetrasaccharide vital to cell–cell recognition. It exists in large quantities at tumor cell surface and its essential role in human fertilisation has just been revealed.45 Selective binding of sLeX or LeX was achieved by Wang et al.46 in 2002, using a combinatorial approach from library 14 with different spacer (R). The identified diboronic acid binder 14a for sLeX was later shown to specifically label sLeX on hepatocellular carcinoma cells in vivo.47
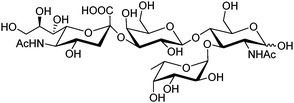 | ||
| Fig. 5 Structure of sialyl Lewis X. | ||
Free sialic acid (e.g. Neu5Ac, Fig. 6), an important tumor marker, contains a glycerol side chain and an anomeric α-hydroxyl acid moiety both able to bind to the boronic acid group. Diboronic acid 1548 was designed by Houston and co-workers for sialic acid sensing. Demonstrated in this work is an interesting approach to improve selectivity when multivalent binding and monovalent binding are efficiently discriminated. Binding of either one of the two boronic acid moieties results in divergent responses, fluorescence “On” when only the ortho-substituted boronic acid was bound while “Off” when only the meta-substituted boronic acid moiety was bound. Therefore when saccharide binding occurs in a weak, monovalent manner, both “On” and “Off” species exist, resulting in little overall change in fluorescence. Binding of both boronic acid moieties of 15 with multivalent sialic acid significantly quenches the fluorescence (“Off” state), which does not occur for monovalent binding unless other saccharide interferants exist at high concentration when 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 15–saccharide complexes form.
2 15–saccharide complexes form.
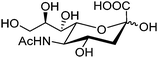 | ||
| Fig. 6 Structure of sialic acid Neu5Ac. | ||
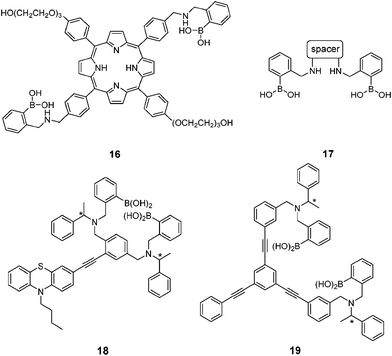
Ginsenosides (e.g. Rb1, Fig. 7) are bioactive species found in ginseng. The group of natural products contains a steroid core to which two saccharide substitutions are attached that allow multivalent boronic acid binding. Porphyrin-cored diboronic acid 149 designed by Anslyn et al. serves as a fluorescent sensor for ginsenosides and shows ginsenoside-dependant binding constants and extents of fluorescence quenching. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic boronate formed from 16 and ginsenosides was supported by fitting of binding isotherm and by ESI-MS studies. A library of diboronic acids 1750 with various spacers were later used in combination with three catechol-containing indicator dyes to construct multicomponent sensing arrays for discrimination and classification of ginsenosides and ginsengs. The three-component sensing ensembles, each consisting of a diboronic acid receptor and a pair of indicators, respond differently toward ginsenosides. These ensembles were then employed as 96-well plate sensing arrays that afforded fingerprints for both ginsenosides and ginsengs. Chiral fluorescent diboronic acids 18 and 19 were also applied to ginsenosides sensing.51
1 cyclic boronate formed from 16 and ginsenosides was supported by fitting of binding isotherm and by ESI-MS studies. A library of diboronic acids 1750 with various spacers were later used in combination with three catechol-containing indicator dyes to construct multicomponent sensing arrays for discrimination and classification of ginsenosides and ginsengs. The three-component sensing ensembles, each consisting of a diboronic acid receptor and a pair of indicators, respond differently toward ginsenosides. These ensembles were then employed as 96-well plate sensing arrays that afforded fingerprints for both ginsenosides and ginsengs. Chiral fluorescent diboronic acids 18 and 19 were also applied to ginsenosides sensing.51
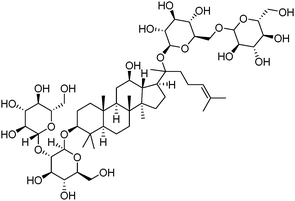 | ||
| Fig. 7 Structure of ginsenoside Rb1. | ||
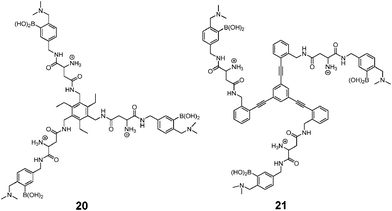
As a widely-used anticoagulant, heparin is a polysaccharide that consists mainly of repeating disaccharide units of iduronic acid and glucosamine residues (Fig. 8). Triboronic acids 2052 and 2153 were designed by Anslyn and co-workers for heparin sensing. Both 20 and 21 bind heparin selectively, with binding constants of 3.8 × 104 M−1 and 1.4 × 108 M−1, respectively, with 21 being more selective. The much higher heparin affinity of 21 was attributed to a larger cavity that allows binding of an extended oligosaccharide surface. In the case of 20, heparin was detected through its displacement of pyrocatechol violet dye that leads to a colour change, differing from 21 that contains a fluorophore that undergoes fluorescence quenching upon heparin binding. As a result of the high binding affinity of 21, it is able to determine heparin in serum at clinical relevant levels.
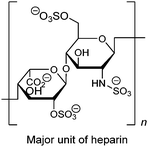 | ||
| Fig. 8 Structure of major unit of heparin. | ||
3.2 Boronic acid-containing polymeric sensors selective for multivalent saccharides
To allow multivalent binding between the sensor and a multivalent saccharide target, boronic acid-containing polymers represent a simpler option than elaborately designed diboronic acids. Multivalent binding between a polymeric boronic acid and saccharides that contain more than one set of cis-diols is possible, in which case the flexible polymer backbone can adjust its conformation upon saccharide binding such that the boronic acid moieties may fit to the positions and orientations of the diols. This implies however a less pre-organised multivalent binding scaffold and hence a lower binding selectivity than rigid diboronic acids designed specifically for a certain target. Indeed in the case of glucose sensing, attempts were made for selective glucose binding using boronic-acid containing polymers, yet with less satisfying results.54 Impressive results were obtained only from those systems in which formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–boron complexes creates two adjacent negative charges or causes crosslinking within the polymer network.
2 glucose–boron complexes creates two adjacent negative charges or causes crosslinking within the polymer network.
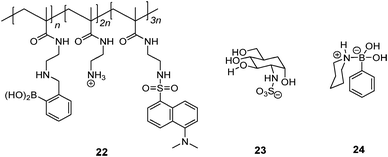
Schrader et al.55 reported a polymeric heparin sensor that represents an excellent example of multivalent boronic acid–diol interactions. The cationic boronic acid containing polymeric sensor 22 binds heparin with a huge binding constant of 3 × 107 M−1, allowing heparin to be detected at a 30 nM level. The individual monovalent binding was studied by using a heparin fragment 23 and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phenylboronic acid–piperidine complex 24 to represent a single binding unit of 22. The monovalent binding constant is <70 M−1, which is 5–6 orders of magnitude lower than the multivalent binding constant of 22 with heparin, nicely demonstrating the dramatic effect of multivalency.
1 phenylboronic acid–piperidine complex 24 to represent a single binding unit of 22. The monovalent binding constant is <70 M−1, which is 5–6 orders of magnitude lower than the multivalent binding constant of 22 with heparin, nicely demonstrating the dramatic effect of multivalency.
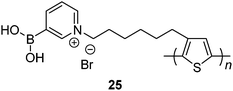
Water-soluble zwitterionic polythiophene-based sensor 25 reported by Liu et al.56 was expected to afford multivalent boronic acid–saccharide interactions. Indeed, disaccharide lactose shows the highest affinity among the tested saccharides, binding constants of 25 with mannose, fructose, glucose, galactose and lactose being 3.33 × 104 M−1, 1.13 × 105 M−1, 1.23 × 105 M−1, 1.69 × 105 M−1 and 4.60 × 105 M−1, respectively. It is of note that the binding constants of glucose and galactose that contain two sets of cis-diols are also higher than that of monovalent fructose. Multivalent binding of 25 with lactose, galactose and glucose is therefore confirmed.
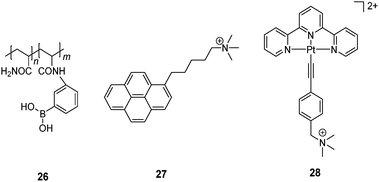
Yam et al.57 developed a glucose-selective sensing ensemble that consists of a water-soluble boronic acid-containing polymer 26 and a cationic fluorescence probe 27. Compound 26 exists in its neutral form at pH 9.0 in the absence of saccharides while 27 is molecularly dissolved and therefore emits only its monomer fluorescence of the pyrene fluorophore at 375 nm. Upon glucose binding to the boronic acid group at pH 9.0, the boronate of 26 is then a polyanion. Cationic 27 is thus induced to aggregate along the polymeric chain through electrostatic interaction, as manifested by the excimer emission at 492 nm from 27. Although other saccharides can also induce the transformation of 26 into a polyanion, glucose leads to the strongest excimer emission of 27 among the tested saccharides, arabinose, galactose, mannose, lactose and sucrose. The glucose-selective response is likely to result from the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–boron complexes which brings two pendant boronic acid moieties into close proximity and generates two adjacent negative charges which favors the aggregation of 27. Other saccharides have lower tendencies of such 1
2 glucose–boron complexes which brings two pendant boronic acid moieties into close proximity and generates two adjacent negative charges which favors the aggregation of 27. Other saccharides have lower tendencies of such 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding and the resultant boronate anions along the polymer chain are far apart because of the electrostatic repulsion. A similar glucose sensing ensemble was later developed that consists of 26 and a cationic platinum complex 28.58 Again, glucose binding leads to electrostatic assembly of the then anionic 26–boronate and 28, facilitating aggregation of 28 via metallophilic and/or π–π interactions as confirmed by the 3MMLCT emission of 28 observed at 800 nm. This ensemble was successfully applied to monitoring α-glucosidase activity.
2 binding and the resultant boronate anions along the polymer chain are far apart because of the electrostatic repulsion. A similar glucose sensing ensemble was later developed that consists of 26 and a cationic platinum complex 28.58 Again, glucose binding leads to electrostatic assembly of the then anionic 26–boronate and 28, facilitating aggregation of 28 via metallophilic and/or π–π interactions as confirmed by the 3MMLCT emission of 28 observed at 800 nm. This ensemble was successfully applied to monitoring α-glucosidase activity.
Solid-phase polymeric boronic acid sensors were also reported. Glucose selective hydrogels of boronic acid-containing polymers exhibit optical or mechanical change due to glucose-induced hydrogel shrinkage when glucose crosslinks two boronic acid moieties within the polymer network.59 Asher and co-workers developed a series of colorimetric glucose-sensing photonic crystals (PCCA). Embedded within hydrogels of boronic acid-containing polymers are crystalline colloidal arrays (CCA) whose diffraction in the visible spectral region shifts to the red when the hydrogel swells and to the blue when it shrinks. Their earliest investigation of saccharide sensing used a boronic acid–polyacrylamide (BA-AA) PCCA60 that shows diffraction red-shift toward all the tested saccharides due to boronic acid ionisation that lead to a Donnan potential and hence hydrogel swelling. The BA-AA PCCA was however fructose selective. The second generation of PCCA incorporated Na+ chelating polyethylene glycol (PEG) units.59 The resultant BA-PEG-AA sensor shows selective diffraction blue-shift with glucose because of the crosslinking induced shrinkage, whereas a red-shift was observed in the presence of fructose for a similar reason observed with BA-AA sensor. Na+ was indispensable for glucose-induced diffraction blue-shift, which was ascribed to ion-paring that stabilises the dianion glucose complex (Fig. 9). Further optimisations were carried out to improve the response time61 and the concentration range of glucose sensing.62 Other glucose-sensing hydrogel materials have been reported following similar strategies. Instead of photonic crystals, silver-based holographic gratings63,64 and an optical fibre65 were used as indicators for the glucose-induced hydrogel shrinkage. Some of these systems have been successfully applied to assays of glucose in tear fluid61 and blood.65 In addition to those optical sensors, a synthetic chemomechanical polymer66 was reported that undergoes a reversible glucose-selective contraction.
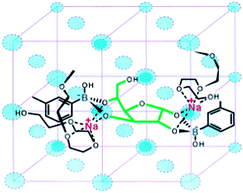 | ||
| Fig. 9 Glucose binding to two boronic acid moieties of BA-PEG-AA. The binding motif is stabilised by PEG-Na+ complex. Reproduced with permission from Anal. Chem., 2003, 75, 2316–2323. Copyright 2003 American Chemical Society. | ||
3.3 Self-assembly of simple boronic acids for selective glucose sensing
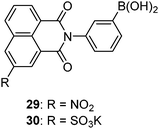
Compared to saccharide selective sensors in which two or more boronic acids have to be positioned in a well-controlled manner, monoboronic acid-based sensors offer advantages such as facile syntheses, structural diversity, and ease in improving water solubility. Monoboronic acids that exist discretely in water would follow their intrinsic binding selectivity among monosaccharides and show the highest affinity for fructose.2 Nevertheless, Heagy's group was able to achieve glucose selectivity using monoboronic acid sensors 2967 and 30;68 here the selectivity refers to the optical responses that glucose produces higher optical response than fructose and galactose, whereas the binding constants with fructose were found higher than those with glucose. Stoichiometry in the glucose–29 complex was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from the Job plot, which precludes the possibility that it is the formation of a 2
1 from the Job plot, which precludes the possibility that it is the formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 glucose–sensor complex that leads to the glucose selectivity. This seemingly “abnormal” response that is not dictated by the binding affinity was attributed to a conformational restriction of the fluorophore upon saccharide binding that influences the excited-state photophysics of the formed boronate ester.
1 glucose–sensor complex that leads to the glucose selectivity. This seemingly “abnormal” response that is not dictated by the binding affinity was attributed to a conformational restriction of the fluorophore upon saccharide binding that influences the excited-state photophysics of the formed boronate ester.Monoboronic acids functioning in that way, however, remain rare. 29 and 30 are actually the only two examples reported to date. It has indeed been envisioned that aggregation of monoboronic acid molecules via non-covalent interactions would lead to supramolecular assemblies that then contain two or more boronic acid moieties. If well positioned in the assembly, they would enable saccharide interaction in a multivalent manner. Among reported sensing systems based on this strategy, diboronic acids were also employed as building blocks which, unlike those presented in Section 3.1, are structurally simple and unable to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cyclic boronate esters with saccharides. Therefore, instead of “monoboronic acids”, we refer to all boronic acids discussed in this section as “simple boronic acids.” The assemblies of these boronic acids bear close resemblance to oligomeric aggregates of lectin polypeptides that afford cluster glycoside effects in multivalent binding with oligosaccharide.9 Currently, success of this strategy has been limited to glucose-selective sensors, yet in principle it could be applied to the design and development of sensors for other saccharide targets, especially those bearing a high number of valences, i.e. oligo- and polysaccharides. Further classifications can be made for these self-assembled glucose sensing systems, based on (i) whether the assembly of boronic acids is induced by glucose or already exists in the absence of glucose and (ii) whether a multivalent template is required to direct the boronic acid molecules for assembly. Glucose selective sensors classified based on (ii) are presented below.
1 cyclic boronate esters with saccharides. Therefore, instead of “monoboronic acids”, we refer to all boronic acids discussed in this section as “simple boronic acids.” The assemblies of these boronic acids bear close resemblance to oligomeric aggregates of lectin polypeptides that afford cluster glycoside effects in multivalent binding with oligosaccharide.9 Currently, success of this strategy has been limited to glucose-selective sensors, yet in principle it could be applied to the design and development of sensors for other saccharide targets, especially those bearing a high number of valences, i.e. oligo- and polysaccharides. Further classifications can be made for these self-assembled glucose sensing systems, based on (i) whether the assembly of boronic acids is induced by glucose or already exists in the absence of glucose and (ii) whether a multivalent template is required to direct the boronic acid molecules for assembly. Glucose selective sensors classified based on (ii) are presented below.
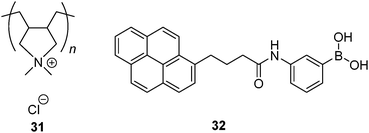
The concept of non-covalently assembling simple boronic acids for the purpose of glucose selectivity was first realised by Kanekiyo and Tao in 2005.69 Polycation 31 was used as a multivalent template, on which multiple copies of 32 in its anionic form assemble via electrostatic interaction, resulting in a multivalent scaffold that contains multiple boronic acids. Excimer emission of 32 bound to 31 is weak in the absence of glucose and it is dramatically enhanced upon glucose binding. This was attributed to the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–32 boronate that brings two pyrene fluorophores into close proximity necessary for excimer formation. The 1
2 glucose–32 boronate that brings two pyrene fluorophores into close proximity necessary for excimer formation. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding motif was supported by the fact that excimer emission drops at higher glucose concentration. Other saccharides, fructose, galactose and ribose, induce little enhancement in excimer emission. In the absence of 31 no excimer emission was observed even when glucose was present, which confirms the role of the multivalent template 31 in promotion of the 1
2 binding motif was supported by the fact that excimer emission drops at higher glucose concentration. Other saccharides, fructose, galactose and ribose, induce little enhancement in excimer emission. In the absence of 31 no excimer emission was observed even when glucose was present, which confirms the role of the multivalent template 31 in promotion of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–32 binding.
2 glucose–32 binding.
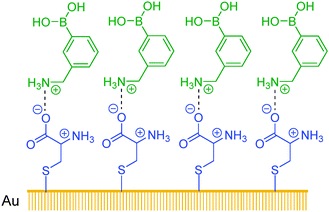
Sun et al.70 have developed assemblies of cysteine ligands on a gold surface as a multivalent template. 3-Aminophenylboronic acid in its protonated form assembled on top of the cysteine layer, via its electrostatic interaction with the negatively charged carboxylate group of the cysteine ligand. The self-assembled bilayer (SAB, Scheme 4) thus formed contains on its surface multiple boronic acid groups which are good for glucose binding. The SAB displays highly sensitive fluorescence quenching response following the order of glucose > galactose > fructose > mannose, with quenching constants at the order of 108 M−1 that are by six orders of magnitude higher than those observed in the solution phase. 3-Aminophenylboronic acid as a monoboronic acid shows in bulk solutions a binding selectivity for fructose at a quenching constant of 102 M−1. The SAB method is extremely sensitive for glucose, capable of sensing glucose at sub-nM level, with a substantially improved selectivity for glucose over fructose.
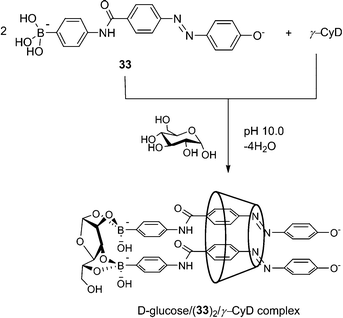 | ||
Scheme 4 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex of 33 with γ-CD in the presence of D-glucose. Adapted from ref. 71 with permission from The Royal Society of Chemistry. 1 inclusion complex of 33 with γ-CD in the presence of D-glucose. Adapted from ref. 71 with permission from The Royal Society of Chemistry. | ||
The Hayashita group has succeeded in creating glucose-selective sensors using γ-cyclodextrin (γ-CyD) inclusion complexes of boronic acid.71 Here γ-CyD can be regarded as a “divalent” template in that it can potentially include two boronic acids within its large and hydrophobic cavity. In the absence of glucose, only the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 33/γ-CyD complex is formed, whereas glucose binding leads to the formation of 2
1 33/γ-CyD complex is formed, whereas glucose binding leads to the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 33/γ-CyD complex (Scheme 5) as indicated by CD spectra and the changes in absorption spectra. With fructose, however, no such 2
1 33/γ-CyD complex (Scheme 5) as indicated by CD spectra and the changes in absorption spectra. With fructose, however, no such 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex was observed and only very minor changes in the absorption spectra were noted. The “dimer” of boronic acid 33 in this case is induced by glucose, since without glucose 33 exists in its monomeric form within the γ-CyD cavity. It is worth noting that for this three-component assembly, without a third component, neither the 1
1 inclusion complex was observed and only very minor changes in the absorption spectra were noted. The “dimer” of boronic acid 33 in this case is induced by glucose, since without glucose 33 exists in its monomeric form within the γ-CyD cavity. It is worth noting that for this three-component assembly, without a third component, neither the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–33, nor the 2
2 glucose–33, nor the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 33/γ-CyD complex forms to an observable extent in solution, since their individual binding constants are too low to be determinable by isothermal titrations. In the presence of a third component at a fixed concentration, e.g. glucose at 30 mM or γ-CyD at 3.0 mM, the binding constants of the remaining two components are (2.7 ± 0.4) × 107 M−2 for 2
1 33/γ-CyD complex forms to an observable extent in solution, since their individual binding constants are too low to be determinable by isothermal titrations. In the presence of a third component at a fixed concentration, e.g. glucose at 30 mM or γ-CyD at 3.0 mM, the binding constants of the remaining two components are (2.7 ± 0.4) × 107 M−2 for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 33/γ-CyD complex and (3.0 ± 0.5) × 107 M−2 for 1
1 33/γ-CyD complex and (3.0 ± 0.5) × 107 M−2 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–33 complex. The dramatic increase in the two-component affinities in the presence of a third component is again a result of the multivalency effect in the binding enhancement.
2 glucose–33 complex. The dramatic increase in the two-component affinities in the presence of a third component is again a result of the multivalency effect in the binding enhancement.
 | ||
Scheme 5 Interaction of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 35-γ-CyD complex with D-glucose and D-fructose. 2 35-γ-CyD complex with D-glucose and D-fructose. | ||
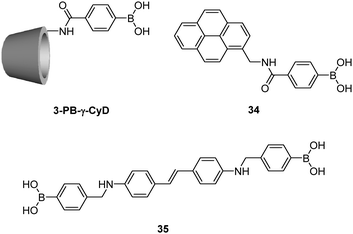
Using γ-CyD, Hayashita et al.72 later developed another supramolecular sensor selective for glucose (Fig. 10). In this example, γ-CyD was modified with a phenylboronic acid (PB) moiety at the secondary hydroxyl rim to yield 3-PB-γ-CyD, when the monoboronic acid guest 34 is included within this modified cyclodextrin cavity, a multivalent scaffold results. In the absence of saccharide, the 34/3-PB-γ-CyD inclusion complex exists in equilibrium with free 34 at an inclusion constant of (2.4 ± 0.4) × 103 M−1. A significant increase of the apparent inclusion constant to (6.5 ± 0.9) × 103 M−1 was observed in the presence of 30 mM glucose. At the same concentration of fructose, however, a slightly decreased value of (2.1 ± 0.3) × 103 M−1 was found. Thus the 34/3-PB-γ-CyD inclusion complex shows a positively cooperative binding with glucose whereas a slightly negatively cooperative binding with fructose. The former observation was attributed to the multivalent interaction between 34/3-PB-γ-CyD complex and glucose that results in a three-component ensemble, while the observation with fructose is probably due to steric hindrance between two fructose molecules that are bound to the 34/3-PB-γ-CyD complex. The 34/3-PB-γ-CyD ensemble undergoes the largest fluorescence enhancement upon binding to glucose among the tested monosaccharides, since glucose binding further strengthens the 34/3-PB-γ-CyD inclusion complex. Competitive experiments with monosaccharides coexisting at concentrations similar to those in blood serum gave satisfactory results, with an average error of ca. 4%.
 | ||
| Fig. 10 Boronic acid-containing SAB reported by Sun and co-workers.72 | ||
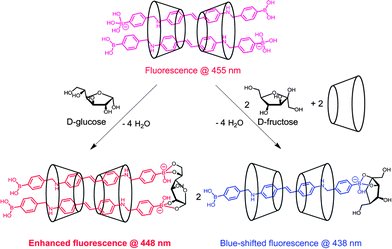
Jiang and co-workers73 reported another γ-CyD-based supramolecular ensemble sensor selective for glucose, relying on the ability of γ-CyD to include two stilbeneboronic acid guests to which the two valences of γ-CyD are transferred. The long and hydrophobic characters of the boronic acid guest 35 are likely the reasons for forming the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 35-γ-CyD inclusion complex. The higher thermodynamic stability established by the 2
2 35-γ-CyD inclusion complex. The higher thermodynamic stability established by the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding mode allows the multivalent scaffold to form in the absence of glucose. Actually, the formation of the 2
2 binding mode allows the multivalent scaffold to form in the absence of glucose. Actually, the formation of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 inclusion complex is close to saturation at 1 mM γ-CyD, since later addition of glucose induces only ca. 10% increase of the CD signal observed from 2
2 inclusion complex is close to saturation at 1 mM γ-CyD, since later addition of glucose induces only ca. 10% increase of the CD signal observed from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 35-γ-CyD (Fig. 3a in ref. 73) whose value was shown to be proportional to the concentration of the 2
2 35-γ-CyD (Fig. 3a in ref. 73) whose value was shown to be proportional to the concentration of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex. A one-fold fluorescence enhancement was observed upon addition of glucose, ascribed to the rigidification of the fluorophore upon glucose binding to the two boronic acids within the γ-CyD cavity, forming a 1
2 complex. A one-fold fluorescence enhancement was observed upon addition of glucose, ascribed to the rigidification of the fluorophore upon glucose binding to the two boronic acids within the γ-CyD cavity, forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–35/γ-CyD supramolecular complex (Scheme 5, left). While only a minor drop in the inclusion complex stability was observed upon fructose binding to the 34/3-PB-γ-CyD complex, complete disassembly of the 2
2 glucose–35/γ-CyD supramolecular complex (Scheme 5, left). While only a minor drop in the inclusion complex stability was observed upon fructose binding to the 34/3-PB-γ-CyD complex, complete disassembly of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 35/γ-CyD by fructose binding was found (Scheme 5, right). The latter was supported by the disappearance of the bisignate CD signal, together with a blue shift of the fluorescence emission, reflecting the thermodynamic instability of the 2
2 35/γ-CyD by fructose binding was found (Scheme 5, right). The latter was supported by the disappearance of the bisignate CD signal, together with a blue shift of the fluorescence emission, reflecting the thermodynamic instability of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 fructose-35/γ-CyD ensemble possibly due to the effect of severe steric hindrance between two adjacent fructose molecules. The 2
2 fructose-35/γ-CyD ensemble possibly due to the effect of severe steric hindrance between two adjacent fructose molecules. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 35/γ-CyD complex hence serves as a multivalent scaffold that both enhances glucose affinity and to some extent inhibits the binding of fructose, which explains the observed higher apparent binding constant with glucose (1048 ± 64 M−1) than with fructose (789 ± 47 M−1). The fluorescence enhancement response was exclusive to glucose. Competitive experiments with saccharide interferent at 0.1 mM and tests in artificial urine samples showed relative deviations below 30% for glucose sensing at sub-mM levels.
2 35/γ-CyD complex hence serves as a multivalent scaffold that both enhances glucose affinity and to some extent inhibits the binding of fructose, which explains the observed higher apparent binding constant with glucose (1048 ± 64 M−1) than with fructose (789 ± 47 M−1). The fluorescence enhancement response was exclusive to glucose. Competitive experiments with saccharide interferent at 0.1 mM and tests in artificial urine samples showed relative deviations below 30% for glucose sensing at sub-mM levels.
In addition to the benefit of sensitivity improvement, aggregation of dye also affords a means of tuning selectivity, as is the case with glucose sensing. Intuitively, aggregation of boronic acid-containing dyes could yield a supramolecular multiboronic acid architecture that shows high glucose affinity as a result of multivalency. An alternative approach is to use aggregation induced by glucose, which provides the possibility of employing fluorescence or absorption changes accompanying the monomer–aggregate transition as highly sensitive responses toward glucose. It is reasonable to predict the achievability of selective induction of aggregation or aggregate structures by glucose, since glucose, among monosaccharides, has the highest tendency to form diboronate esters. Implied in this strategy is the requirement of one glucose molecule to interact with two boronic acid molecules instead of two boronic acid moieties within one molecule or bound by a template. This would not be likely when the boronic acid used as a sensor is present at 10−4–10−5 M because of the low glucose affinity of the monoboronic acid, unless additional interactions are involved. The boronic acid moiety has thus been attached to an aromatic core that could afford hydrophobic and/or π–π interactions to facilitate the boronic acid “dimer”–glucose interaction motif.
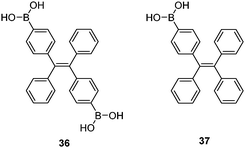
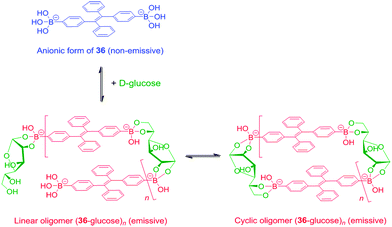
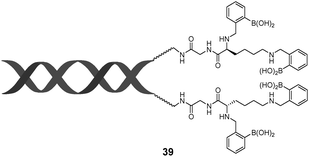
Tang et al. reported a tetraphenylethene (TPE)-cored diboronic acid 3675 that represents a specific “light-up” sensor for glucose. In the absence of saccharides in alkaline conditions, 36 in its dianionic form is weakly emissive. Fluorescence enhancement up to ∼5.4 fold was observed upon addition of glucose, while that induced by fructose, galactose and mannose is much lower. It was proposed that it is the oligomers formed between bifunctional 36 and divalent glucose (Scheme 6) that restricts the intramolecular rotations (RIR) of the phenyl rotors that leads to the fluorescence enhancement. Other monosaccharides are unable to induce such oligomerisation of 36, resulting in only minor fluorescence enhancements. The oligomerisation mechanism is supported by the observation of an emission drop at high glucose concentration and the negligible fluorescence change produced by glucose with the monoboronic acid counterpart 37. Thermodynamically it is not possible that only the interactions of the two boronic acid moieties in 36 with the two binding sites of glucose could lead to appreciable oligomerisation of 36 at a low concentration of 50 μM.76 We thus infer that other interactions such as hydrophobic and π–π interactions between TPE moieties within the oligomer may operate. No binding constants were reported, yet competitive experiments showed that the fluorescence enhancement produced by 4 mM glucose decreased by 60% when 0.1 mM of fructose co-exists, which reveals the much higher affinity of 36 towards fructose than glucose. The relatively low glucose affinity might result from the absence of multivalent interaction since each glucose molecule binds each 36 monovalently, i.e. via a single linkage.
While multivalency is probably absent in glucose sensing by 36, another aggregation-based glucose sensor 3877 we recently developed clearly manifests the multivalency effect, with higher apparent affinity to glucose than to fructose. Sensor 38 contains a hydrophobic pyrene fluorophore that is linked via a cationic pyridinium to the boronic acid moiety. The latter becomes hydrophilic when bound to saccharides in alkaline solutions. The amphiphilicity of the 38–saccharide complex establishes the basis for its supramolecular aggregation in aqueous solution. In alkaline (pH 10) aqueous solution 38 at 0.1 mM exists in small aggregates that exhibit only pyrene monomer fluorescence at ca. 390 nm. Glucose binding to 38 leads to the development of pyrene excimer emission at 510 nm together with a minor increase in monomer emission. In the case of fructose binding, however, only a modest enhancement of the monomer fluorescence was observed. This suggests that the aggregation of 38 is promoted by glucose but not fructose binding (Fig. 11). Indeed, the hydrodynamic diameter of 38 aggregates increases dramatically from ca. 500 to 2000 nm when glucose is added. In agreement with the emission variation, the diameter of aggregates reduces to ca. 200 nm in the presence of fructose. Although 38 already aggregates in the absence of glucose, no excimer emission was observed. The fact that excimer emission was observed upon glucose binding thus indicates that glucose binding leads to not only a higher extent of aggregation but also a well-ordered aggregate structure suitable for excimer emission. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–38 boronate was assumed responsible for the formation of larger aggregates and the strong excimer emission. An apparent binding constant of 38 with glucose of 1.9 × 106 M−2 (with an apparent 1
2 glucose–38 boronate was assumed responsible for the formation of larger aggregates and the strong excimer emission. An apparent binding constant of 38 with glucose of 1.9 × 106 M−2 (with an apparent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry) was found whose square root (1378 M−1) is higher than the binding constant with fructose 353 M−1 (1
2 stoichiometry) was found whose square root (1378 M−1) is higher than the binding constant with fructose 353 M−1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry). The higher glucose affinity is a result of multivalency-enhanced binding, although the 1
1 stoichiometry). The higher glucose affinity is a result of multivalency-enhanced binding, although the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–38 binding motif is not multivalent itself. Multivalency occurs not in the binding between glucose and the boronic acids but in the supramolecular aggregation of the 1
2 glucose–38 binding motif is not multivalent itself. Multivalency occurs not in the binding between glucose and the boronic acids but in the supramolecular aggregation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–38 boronate. The 1
2 glucose–38 boronate. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–38 boronate now contains two pyrene moieties. Self-association of the 1
2 glucose–38 boronate now contains two pyrene moieties. Self-association of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–38 boronate is driven by a divalent aromatic π–π stacking between two pyrene moieties from neighbouring complexes, which gives rise to a much higher tendency for the assembly of the 1
2 glucose–38 boronate is driven by a divalent aromatic π–π stacking between two pyrene moieties from neighbouring complexes, which gives rise to a much higher tendency for the assembly of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–38 boronate than the unbound 38 and the 1
2 glucose–38 boronate than the unbound 38 and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 38-fructose boronate. Aggregation of the 1
1 38-fructose boronate. Aggregation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glucose–38 boronate facilitates the glucose binding that results in a higher apparent glucose affinity, which is crucial for the reduction of interference from fructose, cf. 38 when compared with 36.
2 glucose–38 boronate facilitates the glucose binding that results in a higher apparent glucose affinity, which is crucial for the reduction of interference from fructose, cf. 38 when compared with 36.
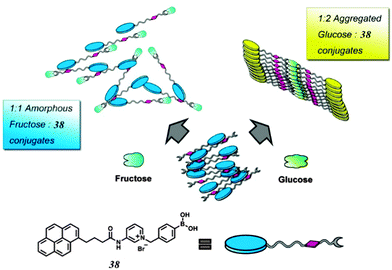 | ||
Fig. 11 Cartoon representation of different aggregation behavior of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 38–fructose and 2 1 38–fructose and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 38–glucose complexes. Adapted with permission from J. Am. Chem. Soc., 2013, 135, 1700–1703. Copyright 2013 American Chemical Society. 1 38–glucose complexes. Adapted with permission from J. Am. Chem. Soc., 2013, 135, 1700–1703. Copyright 2013 American Chemical Society. | ||
Instead of employing aggregates of dye molecules, Anslyn et al.'s boronic acid-functionalised DNA 3978 may also represent a non-covalent assembly of boronic acids capable of achieving multivalency. Two single-strand (ss) oligonucleotides with complementary base sequences were functionalised with diboronic acid functionalities. Upon DNA hybridisation, the tetraboronic acid scaffold 39 forms. No appreciable saccharide binding by the diboronic acid duplex was seen while significant binding of galactose, maltose and trehalose by the tetraboronic acid DNA duplex was observed. Surprisingly, essentially no binding was observed with fructose, the strongest monovalent saccharide binder for boronic acids.
3.4 Boronic acid-conjugated nanomaterials for selective saccharide sensing
Quantum dots and nanochannels have been established as platforms for covalently assembling boronic acids. Zhou and co-workers prepared phenylboronic acid-functionalised CdTe/ZnTe/ZnS quantum dots (PBA-QDs) for glucose sensing (Fig. 12).79 With increasing glucose concentration, photoluminescence of PBA-QDs undergoes quenching and red shift (585 to 609 nm). Dynamic light scattering data and TEM images indicate that these are caused by glucose induced aggregation of PBA-QDs. Lactate that contains one cis-diol induces significantly less change in the hydrodynamic diameters and photoluminescence. The observed glucose-mediated assembly of PBA-QDs was attributed to the interparticle bridging by glucose binding to two boronic acid moieties, respectively, with two PBA-QDs. Although glucose does not bind two boronic acid moieties from the same single PBA-QD and the boronic acid–glucose interaction is thus not multivalent, it appears that the crosslinking of boronic acid moieties by glucose is possible because of the inherent tendency of the nanoparticles to undergo aggregation. The PBA-QDs are able to enter mouse tumor cells thus enabling intracellular glucose assays in vivo. | ||
| Fig. 12 Glucose-mediated assembly of PBA-modified CdTe/ZnTe/ZnS QDs. Reproduced with permission from ref. 79. Copyright 2010 John Wiley and Sons. | ||
In addition to glucose sensing, boronic acid-conjugated nanoparticles have been employed for sensing of the polysaccharide dextran which has more valences than divalent glucose. Zhang and co-workers prepared silver particles coated by thiolate ligands that contain ca. 1% thiolate boronic acid for dextran detection.80 In this case, particle aggregation occurred only with high-valence dextran and not with glucose. The boronic acid-capped silver particle shows an 11-fold binding selectivity for dextran over glucose, which is significantly higher than the 1.5-fold selectivity of the uncapped boronic acid.
A glucose-responsive biomimetic single nanochannel (Fig. 13) was recently reported by Li et al.81 The single conical-shaped nanochannel containing multiple boronic acid moieties on the inner wall was fabricated by chemical etching of a polyethylene terephthalate (PET) membrane followed by coupling of 3-aminophenylboronic acid to the carboxylate groups on the inner wall. The boronic acid-functionalised PET nanochannel displayed a significant decrease in the ionic transmembrane current when exposed to glucose, whereas the change was negligible in the presence of mannose, xylose, galactose, ascorbic acid, glycine and urea. It was proposed that glucose binds two adjacent boronic acid moieties on the inner wall, which leads to steric hindrance and thus a decrease in the transmembrane current. The process is fully reversible by switching between pH 4.49 (under which condition glucose does not bind to boronic acid moieties on the inner-wall) and pH 7.38, and the switching can be cycled many times.
 | ||
| Fig. 13 Glucose responsive biomimetic nanochannel. Reproduced from ref. 81 with permission from The Royal Society of Chemistry. | ||
4. Conclusions and perspectives
We have discussed boronic acid based supramolecular sensors selective for given saccharides under the framework of multivalent interactions. It has been clearly demonstrated that artificial “boronlectins” compare well with natural lectins in binding saccharides in a selective and multivalent manner, through clustering multiple binding sites either within the same covalent framework, or by non-covalent interactions via hierarchical assembly. Remarkably, selectivity inversion from fructose to glucose has been achieved by boronic acids with surprisingly simple structures in their aggregated state that affords multivalent binding which is unlikely from simple boronic acids. Notably some of these multivalent boronic acid sensors have shown biomedical applications.82,83From a practical point of view, selectivity has always been a key issue for boronic acid-based saccharide sensors. As for glucose sensing, while rigid diboronic acids that respond via saccharide binding-induced increase in the boron acidity suffer from interference from fructose at high concentrations, boronic acids, both molecular and supramolecular, that respond selectively to glucose are generally not so rigid and therefore more susceptible to competitive binding of the coexisting fructose. This is why binding selectivity and response selectivity are difficult to be converged. Although many sensors can satisfy the selectivity requirement for the sensing of blood glucose (normal concentration at 4–7 mM) at fructose concentration lower than 0.1 mM, glucose sensors free from the interference of fructose at higher concentration may be required, for example for patients with fructosuria. Sensor 6, developed by Drueckhammer et al. appears the most satisfying in terms of selectivity in both sensing and response, yet the inherent synthetic challenges may prevent its practical application. On the other hand, self-assembled multivalent sensors require much less synthetic effort whilst affording facile discrimination between multivalent and monovalent binding events. The synthetic ease should also allow more space in sensor structural optimisations for a variety of purposes. Further improvement of the binding selectivity remains the primary issue to be addressed, since no reported self-assembled sensor is able to rival Drueckhammer's diboronic acid in glucose selectivity. Thermodynamically, enhanced selectivity could be realised by increasing the stability of the multivalent aggregates or by introducing ortho-substitution84 into the boronic acid moiety. Those boronic acid sensors that already exhibit modest to good selectivity for glucose might be taken to create more selective sensors via derivation into assemble forms. Another possible approach is to tune the kinetics of sensor binding events, i.e. the exploration of sensors that not only bind to glucose strongly but also more rapidly than they do with fructose.
Compared with synthetic multiboronic acids that have been well developed, self-assembled simple boronic acid-based sensors are still in their infancy, whose potentials remain largely unexplored. The biomedical applications of boronic acid aggregates could go far beyond sensing, e.g. if a boronic acid selectively aggregates in the presence of a tumor marker and shows subsequent cytotoxicity85 due to aggregation, promising anti-cancer drugs could be developed.
In summary, boronic acids that afford multivalent interactions with saccharides, created by assembling boronic acid moieties covalently within a molecular or polymeric framework or non-covalently in their aggregated forms, have been shown to achieve improved selectivity for given saccharides, in particular glucose, and are functionally similar to lectins. Further efforts are still required regarding the selectivity for a broad scope of saccharides and biocompatibility of the boronic acid sensors, which we believe can be explored by integrating synthetic and supramolecular chemistry with material sciences. Given the rich knowledge available for the construction of self-assembled materials, we are very optimistic that the future development of simple boronic acid based saccharide selective sensors will benefit from multivalency derived from aggregation.
Acknowledgements
This work was supported by the Ministry of Science and Technology of China via grant 2011CB910403 and by the National Science Foundation of China through grants 91127019, 21275121 and J1210014. The international collaboration has been facilitated by the network of CASE (Catalysis and Sensing for our Environment) and supported by the NSFC-RS joint grant awarded to Yun-Bao Jiang and Tony D. James. John Fossey and Tony James thank the EPSRC (DT/F00267X/1) for support. We are grateful to our co-workers and students for their creative work, part of which is presented here.Notes and references
- C. Ke, H. Destecroix, M. P. Crump and A. P. Davis, Nat. Chem., 2012, 4, 718–723 CrossRef CAS.
- J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769–774 CrossRef CAS.
- S. J. Angyal, Adv. Carbohydr. Chem. Biochem., 1984, 42, 15–68 CrossRef CAS.
- S. J. Angyal, Adv. Carbohydr. Chem. Biochem., 1991, 49, 19–35 CrossRef.
- T. D. James, M. D. Phillips and S. Shinkai, Boronic Acids in Saccharide Recognition, The Royal Society of Chemistry, Cambridge, UK, 2006 Search PubMed.
- C. Fasting, C. A. Schalley, M. Weber, O. Seitz, S. Hecht, B. Koksch, J. Dernedde, C. Graf, E.-W. Knapp and R. Haag, Angew. Chem., Int. Ed., 2012, 51, 10472–10498 CrossRef CAS.
- J. D. Badjić, A. Nelson, S. J. Cantrill, W. B. Turnbull and J. F. Stoddart, Acc. Chem. Res., 2005, 38, 723–732 CrossRef.
- Y. C. Lee and R. T. Lee, Acc. Chem. Res., 1995, 28, 321–327 CrossRef CAS.
- J. M. Rini, Annu. Rev. Biophys. Biomol. Struct., 1995, 24, 551–577 CrossRef CAS.
- H. F. Lodish, Trends Biochem. Sci., 1991, 16, 374–377 CrossRef CAS.
- M. E. Taylor, K. Bezouska and K. Drickamer, J. Biol. Chem., 1992, 267, 1719–1726 CAS.
- H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637–674 CrossRef CAS.
- J. C. Norrild and H. Eggert, J. Chem. Soc., Perkin Trans. 2, 1996, 2583–2588 RSC.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed. Engl., 1994, 33, 2207–2209 CrossRef.
- S. D. Bull, M. G. Davidson, J. M. H. van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y.-B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS.
- J. S. Fossey, F. D'Hooge, J. M. H. van den Elsen, M. P. Pereira Morais, S. I. Pascu, S. D. Bull, F. Marken, A. T. A. Jenkins, Y.-B. Jiang and T. D. James, Chem. Rec., 2012, 12, 464–478 CrossRef CAS.
- Z. Guo, I. Shin and J. Yoon, Chem. Commun., 2012, 48, 5956–5967 RSC.
- R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2012, 48, 1106–1123 Search PubMed.
- J. S. Fossey and T. D. James, in Supramolecular Chemistry: From Molecules to Nanomaterials, ed. P. A. Gale and J. W. Steed, John Wiley & Sons Ltd., 2012, vol. 3, pp. 1346–1379 Search PubMed.
- J. S. Fossey and T. D. James, in Reviews in Fluorescence, ed. C. D. Geddes, Springer Ltd., 2009, pp. 103–118 Search PubMed.
- A. Bencini and V. Lippolis, Coord. Chem. Rev., 2012, 256, 149–169 CrossRef CAS.
- G. Springsteen and B. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205–11209 CrossRef CAS.
- J. Yoon and A. W. Czarnik, J. Am. Chem. Soc., 1992, 114, 5874–5875 CrossRef CAS.
- T. D. James, K. R. A. S. Sandanayake and S. Shinkai, J. Chem. Soc., Chem. Commun., 1994, 477–478 RSC.
- A.-J. Tong, A. Yamauchi, T. Hayashita, Z.-Y. Zhang, B. D. Smith and N. Teramae, Anal. Chem., 2001, 73, 1530–1536 CrossRef CAS.
- J. D. Larkin, J. S. Fossey, T. D. James, B. R. Brooks and C. W. Bock, J. Phys. Chem. A, 2010, 114, 12531–12539 CrossRef CAS.
- B. E. Collins, P. Metola and E. V. Anslyn, Supramol. Chem., 2013, 25, 79–86 CrossRef CAS.
- K. Tsukagoshi and S. Shinkai, J. Org. Chem., 1991, 56, 4089–4091 CrossRef CAS.
- Y. Shiomi, M. Saisho, K. Tsukagoshi and S. Shinkai, J. Chem. Soc., Perkin Trans. 1, 1993, 2111–2117 RSC.
- M. Bielecki, H. Eggert and J. C. Norrild, J. Chem. Soc., Perkin Trans. 2, 1999, 449–456 RSC.
- J. D. Larkin, K. A. Frimat, T. M. Fyles, S. E. Flower and T. D. James, New J. Chem., 2010, 34, 2922–2931 RSC.
- H. Shibata, Y. J. Heo, T. Okitsu, Y. Matsunaga, T. Kawanishi and S. Takeuchi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 17894–17898 CrossRef CAS.
- Y. J. Heo, H. Shibata, T. Okitsu, T. Kawanishi and S. Takeuchi, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 13399–13403 CrossRef CAS.
- H. Eggert, J. Frederiksen, C. Morin and J. C. Norrild, J. Org. Chem., 1999, 64, 3846–3852 CrossRef CAS.
- W. Yang, H. He and D. G. Drueckhammer, Angew. Chem., Int. Ed., 2001, 40, 1714–1718 CrossRef CAS.
- V. V. Karnati, X. Gao, S. Gao, W. Yang, W. Ni, S. Sankar and B. Wang, Bioorg. Med. Chem. Lett., 2002, 12, 3373–3377 CrossRef CAS.
- S. Arimori, M. L. Bell, C. S. Oh, K. A. Frimat and T. D. James, J. Chem. Soc., Perkin Trans. 1, 2002, 803–808 RSC.
- S. Gamsey, A. Miller, M. M. Olmstead, C. M. Beavers, L. C. Hirayama, S. Pradhan, R. A. Wessling and B. Singaram, J. Am. Chem. Soc., 2007, 129, 1278–1286 CrossRef CAS.
- M. D. Phillips, T. M. Fyles, N. P. Barwell and T. D. James, Chem. Commun., 2009, 6557–6559 RSC.
- K. R. A. S. Sandanayake, K. Nakashima and S. Shinkai, J. Chem. Soc., Chem. Commun., 1994, 1621–1622 RSC.
- A. Pal, M. Bérubé and D. G. Hall, Angew. Chem., Int. Ed., 2010, 49, 1492–1495 CrossRef CAS.
- D. Stones, S. Manku, X. Lu and D. G. Hall, Chem.–Eur. J., 2004, 10, 92–100 CrossRef CAS.
- M. Bérubé, M. Dowlut and D. G. Hall, J. Org. Chem., 2008, 73, 6471–6479 CrossRef.
- P.-C. Pang, P. C. N. Chiu, C.-L. Lee, L.-Y. Chang, M. Panico, H. R. Morris, S. M. Haslam, K.-H. Khoo, G. F. Clark, W. S. B. Yeung and A. Dell, Science, 2011, 333, 1761–1764 CrossRef CAS.
- W. Yang, S. Gao, X. Gao, V. V. R. Karnati, W. Ni, B. Wang, W. B. Hooks, J. Carson and B. Weston, Bioorg. Med. Chem. Lett., 2002, 12, 2175–2177 CrossRef CAS.
- W. Yang, H. Fan, X. Gao, S. Gao, V. V. R. Karnati, W. Ni, W. B. Hooks, J. Carson, B. Weston and B. Wang, Chem. Biol., 2004, 11, 439–448 CrossRef CAS.
- S. M. Levonis, M. J. Kiefel and T. A. Houston, Chem. Commun., 2009, 2278–2280 RSC.
- A. E. Hargrove, R. N. Reyes, I. Riddington, E. V. Anslyn and J. L. Sessler, Org. Lett., 2010, 12, 4804–4807 CrossRef CAS.
- X. Zhang, L. You, E. V. Anslyn and X. Qian, Chem.–Eur. J., 2012, 18, 1102–1110 CrossRef CAS.
- Y. Wu, H. Guo, X. Zhang, T. D. James and J. Zhao, Chem.–Eur. J., 2011, 17, 7632–7644 CrossRef CAS.
- Z. Zhong and E. V. Anslyn, J. Am. Chem. Soc., 2002, 124, 9014–9015 CrossRef CAS.
- A. T. Wright, Z. Zhong and E. V. Anslyn, Angew. Chem., Int. Ed., 2005, 44, 5679–5682 CrossRef CAS.
- Y. Egawa, R. Gotoh, T. Seki and J.-i. Anzai, Mater. Sci. Eng., C, 2009, 29, 115–118 CrossRef CAS.
- W. Sun, H. Bandmann and T. Schrader, Chem.–Eur. J., 2007, 13, 7701–7707 CrossRef CAS.
- C. Xue, F. Cai and H. Liu, Chem.–Eur. J., 2008, 14, 1648–1653 CrossRef CAS.
- C. Yu and V. W.-W. Yam, Chem. Commun., 2009, 1347–1349 RSC.
- C. Y.-S. Chung, K. H.-Y. Chan and V. W.-W. Yam, Chem. Commun., 2011, 47, 2000–2002 RSC.
- V. L. Alexeev, A. C. Sharma, A. V. Goponenko, S. Das, I. K. Lednev, C. S. Wilcox, D. N. Finegold and S. A. Asher, Anal. Chem., 2003, 75, 2316–2323 CrossRef CAS.
- S. A. Asher, V. L. Alexeev, A. V. Goponenko, A. C. Sharma, I. K. Lednev, C. S. Wilcox and D. N. Finegold, J. Am. Chem. Soc., 2003, 125, 3322–3329 CrossRef CAS.
- M. Ben-Moshe, V. L. Alexeev and S. A. Asher, Anal. Chem., 2006, 78, 5149–5157 CrossRef CAS.
- M. M. Ward Muscatello, L. E. Stunja and S. A. Asher, Anal. Chem., 2009, 81, 4978–4986 CrossRef CAS.
- A. M. Horgan, A. J. Marshall, S. J. Kew, K. E. S. Dean, C. D. Creasey and S. Kabilan, Biosens. Bioelectron., 2006, 21, 1838–1845 CrossRef CAS.
- K. E. S. Dean, A. M. Horgan, A. J. Marshall, S. Kabilan and J. Pritchard, Chem. Commun., 2006, 3507–3509 RSC.
- S. Tierney, B. M. H. Falch, D. R. Hjelme and B. T. Stokke, Anal. Chem., 2009, 81, 3630–3636 CrossRef CAS.
- G. K. Samoei, W. Wang, J. O. Escobedo, X. Xu, H.-J. Schneider, R. L. Cook and R. M. Strongin, Angew. Chem., Int. Ed., 2006, 45, 5319–5322 CrossRef CAS.
- H. Cao, D. I. Diaz, N. DiCesare, J. R. Lakowicz and M. D. Heagy, Org. Lett., 2002, 4, 1503–1505 CrossRef CAS.
- Z. Cao, P. Nandhikonda and M. D. Heagy, J. Org. Chem., 2009, 74, 3544–3546 CrossRef CAS.
- Y. Kanekiyo and H. Tao, Chem. Lett., 2005, 34, 196–197 CrossRef CAS.
- X.-Y. Sun, B. Liu and Y.-B. Jiang, Anal. Chim. Acta, 2004, 515, 285–290 CrossRef CAS.
- C. Shimpuku, R. Ozawa, A. Sasaki, F. Sato, T. Hashimoto, A. Yamauchi, I. Suzuki and T. Hayashita, Chem. Commun., 2009, 1709–1711 RSC.
- M. Kumai, S. Kozuka, M. Samizo, T. Hashimoto, I. Suzuki and T. Hayashita, Anal. Sci., 2012, 28, 121 CrossRef CAS.
- X. Wu, L.-R. Lin, Y.-J. Huang, Z. Li and Y.-B. Jiang, Chem. Commun., 2012, 48, 4362–4364 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- Y. Liu, C. Deng, L. Tang, A. Qin, R. Hu, J. Z. Sun and B. Z. Tang, J. Am. Chem. Soc., 2011, 133, 660–663 CrossRef CAS.
- T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687–5754 CrossRef CAS.
- Y.-J. Huang, W.-J. Ouyang, X. Wu, Z. Li, J. S. Fossey, T. D. James and Y.-B. Jiang, J. Am. Chem. Soc., 2013, 135, 1700–1703 CrossRef CAS.
- A. E. Hargrove, A. D. Ellington, E. V. Anslyn and J. L. Sessler, Bioconjugate Chem., 2011, 22, 388–396 CrossRef CAS.
- W. Wu, T. Zhou, A. Berliner, P. Banerjee and S. Zhou, Angew. Chem., Int. Ed., 2010, 49, 6554–6558 CrossRef CAS.
- J. Zhang, C. D. Geddes and J. R. Lakowicz, Anal. Biochem., 2004, 332, 253–260 CrossRef CAS.
- Z. Sun, C. Han, L. Wen, D. Tian, H. Li and L. Jiang, Chem. Commun., 2012, 48, 3282–3284 RSC.
- C. F. Dai, A. Sagwal, Y. F. Cheng, H. J. Peng, W. X. Chen and B. H. Wang, Pure Appl. Chem., 2012, 84, 2479–2498 CrossRef CAS.
- J. N. Cambre and B. S. Sumerlin, Polymer, 2011, 52, 4631–4643 CrossRef CAS.
- J. S. Hansen, J. B. Christensen, T. I. Solling, P. Jakobsen and T. Hoeg-Jensen, Tetrahedron, 2011, 67, 1334–1340 CrossRef CAS.
- Y. Kuang and B. Xu, Angew. Chem., Int. Ed., 2013, 52, 6944–6948 CrossRef CAS.
Footnote |
| † Dedicated to Professor Seiji Shinkai to celebrate his 70th birthday. |
| This journal is © The Royal Society of Chemistry 2013 |