Synergistic enhancement of photocatalytic hydrogen evolution in ZnIn2S4/CuWO4via an S-scheme heterojunction and the photothermal effect†
Dafeng
Zhang
a,
Dong
Zhang
b,
Fuping
Zhao
a,
Yutong
Zhao
a,
Hengshuai
Li
 *b,
Junchang
Liu
*b,
Junchang
Liu
 *a,
Xue-Yang
Ji
*a,
Xue-Yang
Ji
 a,
Xipeng
Pu
a,
Xipeng
Pu
 a and
Huayang
Zhang
a and
Huayang
Zhang
 *c
*c
aSchool of Materials Science and Engineering, Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, Liaocheng University, Liaocheng, 252000, P. R. China. E-mail: liujunchang@lcu.edu.cn
bSchool of Physics Science and Information Technology, Shandong Key Laboratory of Optical Communication Science and Technology, Liaocheng University, Liaocheng, 252000, P. R. China. E-mail: lihengshuai@lcu.edu.cn
cSchool of Chemical Engineering, The University of Adelaide, Adelaide, South Australia 5005, Australia. E-mail: huayang.zhang@adelaide.edu.au
First published on 13th November 2024
Abstract
The construction of integrated photothermal materials and photocatalysts has emerged as a promising approach to enhance photocatalytic hydrogen evolution reaction (HER) activity, yet the underlying synergistic mechanisms remain poorly understood. In this study, we successfully synthesized ZnIn2S4/CuWO4 (ZIS/CWO) S-scheme heterojunctions, combining photothermal and photocatalytic functionalities to improve HER efficiency. The CuWO4 component acted as a photothermal energy source, elevating the system's temperature, enhancing charge transfer, and boosting the energy available for the photogenerated carriers in ZnIn2S4. Additionally, the S-scheme heterojunction effectively suppressed the recombination of photogenerated charge carriers, further improving the photocatalytic performance. Under visible light irradiation for 3 h, the ZIS/CWO-3 heterojunction achieved a hydrogen evolution rate of 6.27 ± 0.04 mmol g−1 h−1, which is 21.7 ± 0.9 times higher than that of pure ZnIn2S4 (0.29 ± 0.01 mmol g−1 h−1). This work presents a robust strategy for designing integrated photothermal–photocatalytic systems with significantly improved HER performance.
1. Introduction
The global energy crisis and environmental degradation caused by the overreliance on fossil fuels have intensified the need for sustainable and clean energy solutions.1–3 Hydrogen (H2), recognized as an ideal clean fuel and energy carrier, has gained considerable attention due to its high energy density and zero carbon emissions, thereby driving a new wave of research in recent years.4,5 Among the various hydrogen production methods, photocatalytic water splitting is particularly attractive for its ability to harness solar energy, an abundant and renewable resource.6,7 However, the practical application of most photocatalysts is hindered by low solar energy conversion efficiency, primarily due to severe recombination of photogenerated charge carriers, sluggish charge migration, and insufficient light absorption.8–10 Thus, improving the solar energy utilization efficiency of photocatalysts is a pressing research challenge.11,12 Among the potential candidates, ZnIn2S4 (ZIS), a ternary metal sulfide, has drawn significant attention owing to its facile synthesis, tunable morphology, and appropriate band gap for photocatalysis.13,14 Nonetheless, pure ZIS suffers from severe photocorrosion and rapid recombination of photogenerated carriers, leading to suboptimal photocatalytic performance.15,16 Consequently, it is imperative to design rational strategies to enhance the photocatalytic hydrogen evolution efficiency of ZIS.In recent years, photothermal catalysis has gained increasing attention in the photocatalytic hydrogen evolution reaction (HER).17,18 This is attributed to the ability of photothermal materials to enhance photocatalytic processes by locally increasing the temperature around the catalyst.19,20 The underlying mechanism is primarily associated with generating high-energy hot carriers from the decay of localized surface plasmon resonance (LSPR) in the material. These carriers release heat into the surrounding environment, raising the local temperature and inducing a thermal effect that promotes catalytic activity.21,22 Therefore, the rational design of integrated systems combining photothermal materials with photocatalysts holds great potential for developing high-performance photocatalysts for H2 production. For example, Wang et al.23 synthesized catalysts with excellent performance using the LSPR effect of Cu2−xS combined with ZIS. The LSPR effect, induced by the collective oscillation of carriers due to Cu deficiencies in the Cu2−xS lattice, converted light energy into thermal energy, enhancing the photocatalytic hydrogen evolution performance of ZIS. Zhang et al.24 constructed an integrated photothermal/photocatalytic system for hydrogen production using Co3O4@ZIS, which demonstrated an enhanced hydrogen evolution rate—3.5 times higher than that of pure ZIS—due to the synergistic effects of the heterojunction and the photothermal effect. Guo et al. further enhanced photothermal-assisted photocatalytic H2 production by coupling ZIS nanosheets with FeS2 hollow spheres.25 The H2 evolution rate of the composite material could reach 5.05 mmol g−1 h−1, which was 47.9 times higher than that of pure ZIS. Recently, U. Bharagav et al.26 introduced CuWO4 (CWO) as a nanomaterial with photothermal properties, although the photothermal effect of CWO was not thoroughly explored in their work. Consequently, integrating CWO with ZIS into a photothermal/photocatalytic system offers a promising strategy to enhance the efficiency of photocatalytic H2 production.
Given that CWO has been scarcely reported in photocatalytic H2 production systems, density functional theory (DFT) calculations were performed to predict the theoretical feasibility of ZIS/CWO heterojunctions. Notably, the Gibbs free energy of hydrogen adsorption (ΔGH*) for the ZIS/CWO system was near zero, suggesting its potential suitability for photocatalytic H2 production.27 Based on these predictions, ZIS/CWO S-scheme heterojunction photocatalysts with a synergistic photothermal effect were synthesized via a simple ultrasonic self-assembly method, followed by calcination, which was then applied in a photocatalytic HER system. A comprehensive analysis, including characterization tests, H2 evolution experiments, and further theoretical calculations, was conducted to investigate the factors contributing to the enhanced photocatalytic activity of ZIS/CWO. Finally, a plausible mechanism for the synergistic photothermal effect of the ZIS/CWO S-scheme heterojunction in the photocatalytic HER was proposed.
2. Experimental
2.1. Synthesis of CWO nanoparticles
CWO nanoparticles were prepared using hydrothermal and calcination methods.26 Specifically, 0.604 g of Cu(NO)3·2H2O and 0.825 g of Na2WO4·2H2O were dissolved in 25 mL of deionized (DI) water, respectively. After complete dissolution, the Na2WO4 solution was slowly dripped into the Cu(NO)3·2H2O solution and stirred at room temperature for 0.5 h. Subsequently, the above solutions were transferred to an 80 mL polytetrafluoroethylene reactor kept in an oven at 180 °C for 24 h. After the reaction was completed, the system was allowed to cool naturally to room temperature. The samples were then collected via centrifugation at 8000 rpm and washed several times with deionized water and ethanol. Subsequently, the obtained samples were calcined in a muffle furnace at a sintering temperature of 500 °C with a heating rate of 2.5 °C min−1 for 2 h. Finally, the powdered samples were collected, successfully yielding CWO nanoparticles.2.2. Synthesis of ZIS/CWO S-scheme heterojunctions
ZIS microspheres were synthesized via a hydrothermal method, as described in our previous work.14 The ZIS/CWO S-scheme heterojunctions were fabricated using a simple ultrasonic self-assembly method followed by calcination. Specifically, a predefined amount of ZIS and CWO powder was dispersed into 30 mL of ethanol. The mixed solution was then sonicated at 75% power (180 W) for 0.5 h. After sonication, the solution was maintained at 60 °C and stirred until the ethanol completely evaporated. The resulting powder was collected and calcined in a muffle furnace at 180 °C with a heating rate of 5 °C min−1 for 2 h. Finally, ZIS/CWO heterojunctions with varying mass ratios (ZIS/CWO-x, where x = 0.5, 1, 3, 5, 7) were obtained.2.3. Characterization
The ESI† includes detailed specifications of the chemicals used in the experiments, characterization methods, photocatalytic test procedures, electrochemical measurement protocols, and the parameters employed for density functional theory (DFT) calculations.3. Results and discussion
3.1. Theoretical prediction
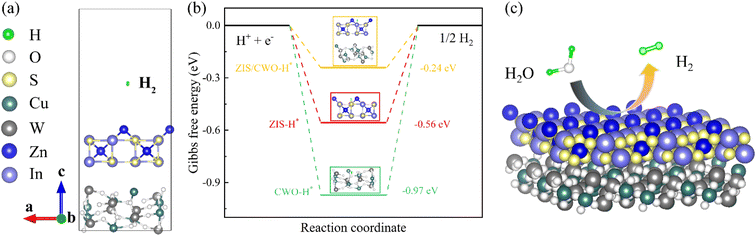
The atomic configuration of the ZIS/CWO S-scheme heterojunction was simulated using DFT calculations (Fig. 1a). Optimization of the theoretical model indicated that H2 could be produced on the ZIS surface when the ZIS/CWO heterojunction was covered with H atoms. Previous studies have suggested that an optimal catalyst should have a Gibbs free energy of hydrogen adsorption (ΔGH*) value close to 0 eV, which reflects a balanced H adsorption/desorption strength during the catalytic process.28,29 Consequently, the photocatalytic HER activities of ZIS, CWO, and ZIS/CWO were assessed by calculating their ΔGH* values. As illustrated in Fig. 1b, pure ZIS and CWO exhibited ΔGH* values of 0.56 eV and −0.97 eV, respectively. In contrast, the ΔGH* value of the ZIS/CWO heterojunction was −0.24 eV, which was closer to the ideal value of 0 eV, indicating enhanced HER activity. This suggests that the ZIS/CWO heterojunction can significantly improve the photocatalytic HER performance of pure ZIS. Additionally, CWO provides excellent electrical conductivity and broad sunlight absorption, while ZIS offers superior catalytic activity for the HER. In conclusion, the design and synthesis of ZIS/CWO S-scheme heterojunction photocatalysts represent a viable strategy for enhancing photocatalytic HER efficiency. A structural model of the ZIS/CWO S-scheme heterojunction was proposed, which is expected to achieve excellent HER performance (Fig. 1c).3.2. Microstructure and element composition
Based on the theoretical predictions, ZIS/CWO S-scheme heterojunctions were successfully synthesized. Initially, pure phases of ZIS and CWO were prepared separately, followed by coupling at high temperature in the presence of ethanol to form the final ZIS/CWO S-scheme heterojunction (Fig. 2a). The morphology and microstructure of the resulting samples were thoroughly examined using Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM). As shown in Fig. 2b, CWO exhibited irregularly shaped nanoparticles with an average particle diameter of approximately 80 nm, as indicated by the particle size distribution (Fig. S1†). ZIS, on the other hand, displayed a microspherical morphology consisting of stacked nanosheets with a radius of about 4 μm (Fig. 2c). In the ZIS/CWO composite (Fig. 2d), the original ZIS morphology was preserved, while CWO nanoparticles were dispersed between the ZIS nanosheets, indicating close contact between the two components without altering their individual morphologies. SEM elemental mapping (Fig. S2†) further confirmed the uniform distribution of W, O, and Cu elements on the surface of the ZIS microspheres. | ||
| Fig. 2 (a) Schematic diagram for the synthesis of ZIS/CWO heterojunctions. SEM images of (b) CWO, (c) ZIS and (d) ZIS/CWO. (e) TEM, (f) HRTEM and (g) SAED pattern of ZIS/CWO. | ||
TEM imaging of the ZIS/CWO composite (Fig. 2e) revealed that the CWO nanoparticles were closely fitted between the ZIS nanosheets, which had an approximate thickness of 50 nm. High-resolution TEM (HRTEM) analysis further characterized the crystal structure of the ZIS/CWO composites, revealing lattice spacings of 3.11 nm and 3.22 nm, corresponding to the (−1 1 1) plane of CWO and the (1 0 2) plane of ZIS, respectively (Fig. 2f). Moreover, Selected Area Electron Diffraction (SAED) patterns displayed three distinct diffraction rings corresponding to the (1 0 2) plane of ZIS and the (1 0 0) and (−1 1 1) planes of CWO (Fig. 2g). These results clearly confirm that the ZIS/CWO composite was self-assembled from ZIS and CWO.
To gain further insight into the physical structure of the samples, X-ray Diffraction (XRD) analysis was conducted. As shown in Fig. 3a, the diffraction peaks of ZIS matched the JCPDS card no. 60-2023, with major peaks at 27.7° and 47.2°, corresponding to the (1 0 2) and (1 1 0) lattice planes of ZIS, respectively.30 For CWO (JCPDS no. 88-2069), the primary diffraction peaks were observed at 19.0°, 28.7°, and 30.1°, corresponding to the (1 0 0), (−1 1 1), and (1 −1 1) lattice planes, respectively.26 The diffraction peaks of ZIS in the synthesized ZIS/CWO composites remained relatively unchanged, while those of CWO became more pronounced as its content increased. These results indicated that the synthesis process did not alter the physical phase structures of the original ZIS and CWO.
 | ||
| Fig. 3 (a) XRD patterns of samples. XPS spectra of ZIS, CWO and ZIS/CWO: (b) total survey, (c) S 2p, (d) In 3d, (e) Zn 2p, (f) W 4f, (g) O 1s and (h) Cu 2p. | ||
The surface chemical composition and states of the synthesized samples were further examined by X-ray Photoelectron Spectroscopy (XPS). As shown in Fig. 3b, the ZIS/CWO composite sample contained six expected elements: S, In, Zn, W, O, and Cu. High-resolution XPS spectra for each element were analyzed before and after the composite formation. The S 2p spectrum showed peaks at 161.8 and 163.0 eV, corresponding to S 2p3/2 and S 2p1/2, respectively (Fig. 3c).31,32 The In 3d spectrum displayed peaks at 445.1 and 452.7 eV, representing In 3d5/2 and In 3d3/2, respectively (Fig. 3d).33,34 In the Zn 2p spectrum (Fig. 3e), peaks at 1022.1 and 1045.2 eV were attributed to Zn 2p3/2 and Zn 2p1/2, respectively.35 The binding energies of these three elements in ZIS were positively shifted compared to those in ZIS/CWO.
For CWO, the W 4f spectrum displayed peaks at 35.2, 37.3, and 41.0 eV, corresponding to W 4f7/2, W 4f5/2, and W 5p3/2, indicating the presence of W6+ (Fig. 3f).36 The O 1s spectrum (Fig. 3g) exhibited two peaks at 530.2 eV for the Cu–O bond and 531.2 eV for the W–O bond.26,37 As shown in Fig. 3h, the Cu 2p3/2 spectrum was deconvoluted into two peaks at 932.1 and 932.7 eV, while the Cu 2p1/2 spectrum had peaks at 952.8 and 953.9 eV. Additional satellite peaks were also observed, confirming the presence of Cu2+.38,39 Notably, the binding energies of all elements in CWO were negatively shifted compared to those in ZIS/CWO. This shift in binding energy is consistent with previous studies suggesting that lower binding energy corresponds to higher charge density.40 These findings imply a significant interaction between ZIS and CWO in the heterojunction, with electrons likely flowing from ZIS to CWO. This electron transfer plays a crucial role in enhancing the photocatalytic HER activity.
3.3. Photocatalytic HER activity
The photocatalytic HER activity of the synthesized samples was evaluated under 300 W xenon lamp irradiation using Na2S and Na2SO3 as sacrificial agents. As shown in Fig. 4a, pure CWO exhibited negligible photocatalytic H2 production, likely due to its conduction band (ECB) position being too low to support the reduction reaction. Similarly, pure ZIS showed poor H2 production performance, possibly due to its high carrier recombination rate and severe photocorrosion. Notably, the H2 production activity of the ZIS/CWO composite samples was significantly enhanced upon the formation of the ZIS/CWO S-scheme heterojunction. The H2 evolution rate of the composite samples increased linearly with prolonged irradiation time. As shown in Fig. 4b, after 3 h of visible light irradiation, the ZIS/CWO-3 composite achieved an H2 production rate of 6.27 ± 0.04 mmol g−1 h−1, which was 21.7 ± 0.9 times higher than that of pure ZIS (0.29 ± 0.01 mmol g−1 h−1) and 91.5 ± 13.6 times higher than that of pure CWO (0.07 ± 0.01 mmol g−1 h−1). However, further increasing the CWO content beyond 3% led to a significant decrease in H2 production, possibly due to excess CWO hindering the visible light absorption of ZIS. The apparent quantum efficiency (AQE) of ZIS/CWO-3 under different monochromatic light sources is shown in Fig. 4c, with AQE values of 8.23%, 5.31%, 0.91%, and 0% under irradiation at 420, 450, 500, and 600 nm, respectively. This trend is consistent with the UV-vis diffuse reflectance spectra (DRS) of ZIS/CWO-3. Stability is a critical factor for practical applications, and to evaluate this, five cycling experiments were conducted under the same conditions (Fig. 4d). The results indicated no significant decline in H2 production after five cycles, demonstrating the excellent stability of ZIS/CWO-3. Furthermore, the XRD (Fig. S4†), SEM (Fig. S5†) and XPS (Fig. S6†) results of ZIS/CWO-3 after the cycling tests revealed no significant changes, confirming the structural stability of the material. As shown in Fig. 4e, the performance of ZIS/CWO-3 surpasses that of most previously reported ZIS-based photocatalytic systems and representative photothermal photocatalysts, as detailed in Table S1.†3.4. Factors influencing the enhancement of photocatalytic HER activity
To assess the photothermal effect, infrared thermography was employed to monitor the temperature changes of ZIS, CWO, and ZIS/CWO-3 samples under 300 W Xenon lamp irradiation for 3 minutes at room temperature. Thermal images were captured every 45 seconds. The initial temperature of the sample is depicted in Fig. S7.† Compared to ZIS, CWO demonstrated a significantly higher rate of temperature increase and greater temperature elevation (Fig. 5a and b). The temperature of the ZIS/CWO-3 S-scheme heterojunction reached 68.7 °C after 3 minutes of irradiation, surpassing that of pure ZIS, indicating that the ZIS/CWO-3 heterojunction retained most of the photothermal properties of CWO (Fig. 5c). Given that the actual photocatalytic HER occurs in solution, infrared thermography was also used to observe the temperature changes of the samples during the reaction process. The initial temperatures of different reaction systems are shown in Fig. S8.† As shown in Fig. S9a,† the temperature of the reaction solution without any catalyst did not increase significantly after 3 h of light exposure. However, when ZIS/CWO-3 was introduced, the temperature of the reaction solution increased rapidly, reaching 46.7 °C after 3 h, which was notably higher than the temperature of the ZIS reaction system (36.8 °C) (Fig. S9b and c†). This observation suggests that the strong photothermal effect of ZIS/CWO-3 enhanced the activity of internal particles, accelerated carrier generation, and improved electron conduction across internal electric fields, ultimately leading to a higher rate of the photocatalytic HER. To further elucidate the contributions of the heterojunction and photothermal effects on photocatalytic H2 evolution activity, we compared the impact of the heterojunction on H2 evolution at a fixed temperature and compared the impact of the photothermal effect on H2 evolution by varying the temperature as detailed in the ESI and Fig. S10†. The results confirmed that the enhanced H2 evolution performance of ZIS/CWO-3 was largely due to the formation of heterojunctions, with the photothermal effect further improving this performance. | ||
| Fig. 5 Timeline and photothermal mapping images of (a) ZIS, (b) CWO, and (c) ZIS/CWO-3 under 300 W Xenon lamp irradiation. | ||
As illustrated in Fig. 6a, CWO exhibited excellent absorption across the entire visible light spectrum, whereas ZIS struggled to absorb light with wavelengths longer than 415 nm. Notably, the visible light absorption of ZIS significantly improved when combined with CWO, and the absorption capacity of ZIS/CWO increased progressively with higher CWO content. As shown in Fig. 6b, the band gaps of ZIS and CWO were determined to be 2.42 eV and 2.18 eV, respectively, using the Kubelka–Munk function, consistent with previous studies. Additionally, the N2 adsorption–desorption isotherms of ZIS, CWO, and ZIS/CWO-3 all displayed a type IV hysteresis loop, indicative of mesoporous structures (Fig. 6c).41 The pore size distribution curves (inset of Fig. 6c) further supported the presence of mesopores. As shown in Fig. 6d, ZIS/CWO-3 had the largest specific surface area (72.41 m2 g−1), followed by ZIS (68.79 m2 g−1), while CWO had the smallest surface area (10.52 m2 g−1). These findings suggest that the large specific surface area of ZIS/CWO-3 provides numerous active and adsorption sites, facilitating the photocatalytic reaction. Furthermore, the zeta potential measurement in Fig. 6e indicated that ZIS/CWO-3 exhibited a relatively negative potential, which facilitates the adsorption of H+ ions in solution, thus enhancing the rate of the photocatalytic HER.
The efficiency of carrier separation and transfer is another critical factor influencing the photocatalytic HER activity of the samples. The photoluminescence (PL) spectrum revealed that the peak intensity of ZIS was much higher than that of the composite materials, with ZIS/CWO-3 exhibiting the lowest peak intensity. Furthermore, after loading CWO onto ZIS, a significant red shift was observed in the PL peak. This was since, according to the testing principle of PL, when an electron–hole pair generates a photon through radiative recombination, the electron is at the bottom of the conduction band while the hole is at the top of the valence band. In this case, the photon energy is equal to the band gap width Eg, and the product of the band gap width and the wavelength is constant. Based on the results from DRS testing showing a decreased band gap of the material after recombination, there would be a noticeable redshift in the PL peak.42 This suggests that the charge recombination rate in ZIS/CWO-3 has been significantly reduced. In addition, as shown in Fig. S11,† the time-resolved PL (TRPL) measurements indicated that the average fluorescence lifetime of CWO was 2.43 ns, while those of ZIS and ZIS/CWO-3 were 0.54 ns and 0.47 ns, respectively. This suggested that the loading of CWO could effectively enhance the separation and transfer of photogenerated carriers. As shown in Fig. 6g and h, ZIS/CWO-3 demonstrated a higher photocurrent response and a smaller radius in the fitted electrochemical impedance spectroscopy (EIS) spectra, indicating enhanced carrier mobility in the composite.43
Furthermore, as depicted in Fig. 6i, the H2 production overpotentials required for ZIS and CWO to reach a current density of 10 mA cm−2 were −1638 mV and −1630 mV, respectively. In contrast, the hybrid ZIS/CWO heterojunction required a lower overpotential of −1537 mV, making it easier to facilitate the reduction reaction with protons. These results indicate that the construction of the ZIS/CWO S-scheme heterojunction improved the separation and transfer efficiency of photogenerated carriers, contributing to enhanced photocatalytic HER performance.
3.5. DFT calculations
To further explore the reasons behind the enhanced photocatalytic activity of the synthesized catalysts, density functional theory (DFT) calculations were performed. The band gap widths and electronic state densities of ZIS and CWO were calculated using the Heyd–Scuseria–Ernzerhof (HSE06) method. As illustrated in Fig. 7a and b, both pure ZIS and CWO exhibited direct band gap semiconductor properties. This characteristic contributes to the low photocatalytic HER activity of ZIS and CWO, as the excited electrons can easily return from the ECB to the valence band (EVB). The band gap values calculated using DFT for ZIS and CWO were 2.40 eV and 2.13 eV, respectively, which align well with the values obtained from the UV-vis DRS measurements. Additionally, the Density of States (DOS) plots (Fig. 7a and b, right) showed that the EVB and ECB of ZIS were primarily influenced by p-orbital electrons, while those of CWO were mainly affected by d-orbital electrons. As shown in Fig. S12,† the ZIS/CWO heterojunction did not exhibit a clear band gap feature, likely due to the strong interaction between ZIS and CWO. The DOS of ZIS/CWO was more evenly distributed across the energy range, with no prominent localized spikes, indicating enhanced electronic delocalization in the composite. This result was further supported by the Electron Localization Function (ELF) map (Fig. S13†), where a clear asymmetry in electron localization was observed between the interfaces of ZIS and CWO. Atoms far from the interface exhibited higher electron localization density than those near the interface, suggesting strong interactions between ZIS and CWO. This interaction promotes better charge transfer and enables the ZIS/CWO heterojunction to interact more effectively with H+ ions, leading to superior photocatalytic HER performance. | ||
| Fig. 7 Band structures and DOS of (a) ZIS and (b) CWO. Electrostatic potentials of (c) ZIS and (d) CWO. (e) Simulated charge distributions at the ZIS/CWO interface. | ||
The work functions (Wf) of the ZIS (1 0 2) and CWO (−1 1 1) lattice planes were investigated based on TEM data. As shown in Fig. 7c and d, the Wf of ZIS and CWO were 4.16 eV and 6.65 eV, respectively. According to the definition of the work function, the Fermi energy level (EF) of CWO was significantly lower than that of ZIS.28 This suggests that when ZIS and CWO come into contact to form a heterojunction, free electrons from ZIS would flow to CWO due to the potential difference, resulting in an irreversible electron transfer. Furthermore, by calculating the electron density difference (EDD) at the ZIS/CWO interface, a clear charge exchange behavior was observed, where electrons moved from ZIS to CWO. This finding aligns with the XPS results, confirming the electron transfer between the two materials at the heterojunction interface. This charge redistribution enhances the photocatalytic HER activity by improving charge separation and transfer efficiency.
3.6. Possible photocatalytic HER mechanism
The relative energy band positions of ZIS and CWO were determined using Mott–Schottky (MS) curves. As shown in Fig. 8a, the flat band potentials (Efb) of CWO and ZIS were 0.50 and −0.59 V vs. NHE, respectively, calculated using the equation ENHE = ESCE + 0.244.44 Since the slopes of the MS curves for both ZIS and CWO were positive, this indicates that they are n-type semiconductors. For n-type semiconductors, the ECB is approximately 0.1 V lower than the Efb, resulting in ECB values of 0.40 V and −0.69 V vs. NHE for CWO and ZIS, respectively.45 Based on the equation Eg = EVB − ECB, the EVB values for CWO and ZIS were calculated to be 2.58 and 1.73 V vs. NHE, respectively.46 Given these energy band configurations, two possible interfaces between CWO and ZIS could be formed: either a type-II or S-scheme heterojunction (Fig. 8b). To further investigate the charge transfer mechanism in the ZIS/CWO heterojunction, electron spin resonance (ESR) spectroscopy was used to detect photoexcited ˙OH and ˙O2− species. Under light excitation, both ZIS and ZIS/CWO-3 showed ˙O2− signals, whereas no signal was observed for CWO, likely due to the positive ECB position of CWO. Additionally, as shown in Fig. 8d, both CWO and ZIS/CWO-3 exhibited strong DMPO-˙OH signals, with the signal for ZIS/CWO-3 being stronger. In contrast, none of the samples displayed ˙O2− or ˙OH signals under dark conditions (Fig. S14†), indicating that more photogenerated holes accumulate in the EVB of CWO upon coupling with ZIS. Furthermore, the S-scheme heterojunction charge transfer mechanism of the ZIS/CWO heterojunction was confirmed by in situ XPS measurements. As shown in Fig. S15,† under both dark conditions and 300 W Xenon lamp irradiation, the XPS diffraction peaks for ZIS/CWO-3 did not undergo significant changes. However, there was a noticeable shift in the binding energy values. Compared to the dark conditions, the binding energies of the three elements associated with ZIS in ZIS/CWO-3 shifted to lower values under Xenon lamp irradiation, while the elements associated with CWO shifted to higher binding energies. This suggests that a substantial accumulation of photogenerated electrons occurred on the ZIS side under visible light irradiation, whereas a significant depletion of photogenerated electrons was observed on the CWO side.47,48 This finding is consistent with the ZIS/CWO S-scheme heterojunction charge transfer mechanism.These findings, combined with the DFT calculation results, suggest a tendency for electron transfer from ZIS to CWO when they form a heterojunction. As the potential difference between the two materials reaches dynamic equilibrium, a significant positive charge accumulates on the ZIS side and a corresponding negative charge on the CWO side. This charge separation leads to the formation of an internal electric field at the interface. Consequently, the energy band edge of ZIS bends upward while that of CWO bends downward, following the S-scheme heterojunction pathway (Fig. 8e).49 Compared with the type-II path, the high redox capacity was maintained, and the photogenerated carriers could be better separated.
Based on the experimental results, a rational mechanism for the synergistic photothermal effect of the ZIS/CWO S-scheme heterojunction in promoting photocatalytic water splitting was proposed. As depicted in Fig. 9, under simulated sunlight irradiation, both ZIS and CWO absorb solar energy, generating electron–hole pairs. At the interface, driven by the built-in electric field (IEF), the electrons from the ECB of CWO recombine with the holes in the EVB of ZIS, facilitating efficient charge separation. The remaining photogenerated electrons in the ECB of ZIS were used to reduce H+, generating H2, while the accumulated holes in the EVB of CWO were consumed by SO32− and S2− to produce SO42− and S2O32−.50–52 In addition, the photothermal effect of CWO plays a dual role in enhancing the photocatalytic process. First, it increases the kinetic energy of the photogenerated carriers in ZIS, making them more reactive and accelerating charge separation. Second, the photothermal effect raises the temperature of the reaction system, which promotes the migration and reaction of the reactant molecules. Consequently, combining the S-scheme heterojunction and the photothermal effect synergistically improves the photocatalytic HER activity of the ZIS/CWO composite system.
 | ||
| Fig. 9 Mechanism of photothermal-assistant H2 evolution for the ZIS/CWO S-scheme under visible-light irradiation. | ||
4. Conclusion
In conclusion, ZIS/CWO S-scheme heterojunction photocatalysts were successfully synthesized through an ultrasonic self-assembly process followed by calcination, supported by density functional theory (DFT) calculations. Both the theoretical and experimental results demonstrated that the ZIS/CWO S-scheme heterojunction exhibited outstanding photocatalytic HER activity. The optimized sample, ZIS/CWO-3, achieved a remarkable H2 production rate, 20.8 times higher than that of pristine ZIS (0.30 mmol g−1 h−1). The enhanced photocatalytic performance can be attributed to several factors, including improved light absorption, increased specific surface area, enhanced photogenerated carrier transfer and separation efficiencies, the photothermal effect, and the formation of the S-scheme heterojunction. This study offers valuable insights for the design and development of photothermal–photocatalytic catalysts for efficient H2 production.Data availability
The data that support the findings of this study are available on request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Shandong Provincial Natural Science Foundation (No. ZR2022ME179), Shandong Provincial Key Research and Development Program (Public Welfare Science and Technology Research) (No. 2019GGX103010), Science and Technology Planning Project of Higher School in Shandong Province (No. J18KA243), Liaocheng Key Research and Development Program (policy guidance category) (No. 2022YDSF90), and Liaocheng University High-level Talents & PhD Research Startup Foundation (No. 318051619). H. Zhang acknowledges the support from the Australian Research Council (ARC-Discovery Project DP240102787). We are grateful to Professor Xinghui Liu of the Hubei Institute of Aerospace Chemo Technology for the support·of DFT calculations.References
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, A metal-free polymeric photocatalyst for hydrogen production from water under visible light, Nat. Mater., 2009, 8, 76–80, DOI:10.1038/NMAT2317.
- A. Kudo and Y. Miseki, Heterogeneous photocatalyst materials for water splitting, Chem. Soc. Rev., 2009, 38, 253–278, 10.1039/b800489g.
- H. Q. Zhang, X. J. Zeng, Q. Q. Zhang, Z. L. Zhang, C. L. Jin and R. H. Yu, Dual template-induced construction of three-dimensional porous SiO2/NC/Co-CNTs heterostructure with highly dispersed active sites for efficient oxygen evolution reaction, Tungsten, 2024, 6, 585–595, DOI:10.1007/s42864-023-00253-x.
- Z. Mamiyev and N. O. Balayeva, Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances, Catalysts, 2022, 12, 1316, DOI:10.3390/catal12111316.
- J. Zhang, J. G. Yu, Y. M. Zhang, Q. Li and J. R. Gong, Visible Light Photocatalytic H2-Production Activity of CuS/ZnS Porous Nanosheets Based on Photoinduced Interfacial Charge Transfer, Nano Lett., 2011, 11, 4774–4779, DOI:10.1021/nl202587b.
- G. C. Zuo, Y. T. Wang, W. L. Teo, A. M. Xie, Y. Guo, Y. X. Dai, W. Q. Zhou, D. Jana, Q. M. Xian, W. Dong and Y. L. Zhao, Ultrathin ZnIn2S4 Nanosheets Anchored on Ti3C2TX MXene for Photocatalytic H2 Evolution, Angew. Chem., Int. Ed., 2020, 59, 11287–11292, DOI:10.1002/anie.202002136.
- M. Y. Ma, H. Z. Yu, L. M. Deng, L. Q. Wang, S. Y. Liu, H. Pan, J. W. Ren, M. Y. Maximov, F. Hu and S. J. Peng, Interfacial engineering of heterostructured carbon-supported molybdenum cobalt sulfides for efficient overall water splitting, Tungsten, 2023, 5, 589–597, DOI:10.1007/s42864-023-00212-6.
- Z. Y. Zhang, K. C. Liu, Z. Q. Feng, Y. N. Bao and B. Dong, Hierarchical sheet-on-sheet ZnIn2S4/g-C3N4 heterostructure with highly efficient photocatalytic H2 production based on photoinduced interfacial charge transfer, Sci. Rep., 2016, 6, 19221, DOI:10.1038/srep19221.
- S. Wang, B. C. Zhu, M. J. Liu, L. Y. Zhang, J. G. Yu and M. H. Zhou, Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity, Appl. Catal., B, 2019, 243, 19–26, DOI:10.1016/j.apcatb.2018.10.019.
- W. L. Yu, J. X. Chen, T. T. Shang, L. F. Chen, L. Gu and T. Y. Peng, Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production, Appl. Catal., B, 2017, 219, 693–704, DOI:10.1016/j.apcatb.2017.08.018.
- V. Kumar, S. G. Prasad, M. Kumar, A. Kumar, P. Singh, A. K. Ansu, A. Sharma, T. Alam, A. S. Yadav and D. Dobrota, Nanocomposite Marvels: Unveiling Breakthroughs in Photocatalytic Water Splitting for Enhanced Hydrogen Evolution, ACS Omega, 2024, 9, 6147–6164, DOI:10.1021/acsomega.3c07822.
- T. Li, N. Tsubaki and Z. L. Jin, S-scheme heterojunction in photocatalytic hydrogen production, J. Mater. Sci. Technol., 2024, 169, 82–104, DOI:10.1016/j.jmst.2023.04.049.
- Y. X. Shi, L. L. Li, Z. Xu, F. Guo and W. L. Shi, Construction of full solar-spectrum available S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production, Chem. Eng. J., 2023, 459, 141549, DOI:10.1016/j.cej.2023.141549.
- S. Wang, D. Zhang, D. Zhang, X. Pu, J. Liu, H. Li and P. Cai, A novel hydrangea-like ZnIn2S4/FePO4 S-scheme heterojunction via internal electric field for boosted photocatalytic H2 evolution, J. Alloy. Compd., 2023, 967, 171862, DOI:10.1016/j.jallcom.2023.171862.
- H. T. Fan, Z. Wu, K. C. Liu and W. S. Liu, Fabrication of 3D CuS@ZnIn2S4 hierarchical nanocages with 2D/2D nanosheet subunits p–n heterojunctions for improved photocatalytic hydrogen evolution, Chem. Eng. J., 2022, 433, 134474, DOI:10.1016/j.cej.2021.134474.
- Y. Lu, X. Zou, L. Wang and Y. Geng, Preparation and hydrogen evolution properties of ZnIn2S4/g-C3N4/MoS2 ternary heterojunctions, Liaocheng Univ., 2023, 57–64, DOI:10.19728/j.issn1672-6634.2023040004.
- D. Zhang, M. Zhu, R. Qin, P. Chen, M. Yin, D. Zhang, J. Liu, H. Li, X. Pu and P. Cai, Rational construction of CuFe2O4@C/Cd0.9Zn0.1S S-scheme heterojunction photocatalyst for extraordinary photothermal-assisted photocatalytic H2 evolution, J. Energy Chem., 2024, 92, 240–249, DOI:10.1016/j.jechem.2024.01.050.
- Y. Shi, L. Li, Z. Xu, F. Guo and W. Shi, Construction of full solar-spectrum available S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production, Chem. Eng. J., 2023, 459, 141549, DOI:10.1016/j.cej.2023.141549.
- Y. Li, Y. Wu, Y. Yang, Y. Xiong, Y. Li and Q. Huang, In situ construction of Mn0.2Cd0.8S/NiB composite for highly efficient full spectrum-driven photocatalytic H2 evolution, J. Environ. Chem. Eng., 2023, 11, 109522, DOI:10.1016/j.jece.2023.109522.
- S. Wang, D. Zhang, X. Pu, L. Zhang, D. Zhang and J. Jiang, Photothermal-Enhanced S-Scheme Heterojunction of Hollow Core-Shell FeNi2S4@ZnIn2S4 toward Photocatalytic Hydrogen Evolution, Small, 2024, 20, e2311504, DOI:10.1002/smll.202311504.
- M. M. Gao, T. X. Zhang and G. W. Ho, Advances of photothermal chemistry in photocatalysis, thermocatalysis, and synergetic photothermocatalysis for solar-to-fuel generation, Nano Res., 2022, 15, 9985–10005, DOI:10.1007/s12274-022-4795-3.
- D. Mateo, J. L. Cerrillo, S. Durini and J. Gascon, Fundamentals and applications of photo-thermal catalysis, Chem. Soc. Rev., 2021, 50, 2173–2210, 10.1039/d0cs00357c.
- Y. C. Wang, M. J. Liu, C. X. Wu, J. P. Gao, M. Li, Z. P. Xing, Z. Z. Li and W. Zhou, Hollow Nanoboxes Cu2−xS@ZnIn2S4 Core-Shell S-Scheme Heterojunction with Broad-Spectrum Response and Enhanced Photothermal–Photocatalytic Performance, Small, 2022, 18, 2202544, DOI:10.1002/smll.202202544.
- S. Zhang, G. Zhang, S. Wu, Z. Guan, Q. Li and J. Yang, Fabrication of Co3O4@ZnIn2S4 for photocatalytic hydrogen evolution: insights into the synergistic mechanism of photothermal effect and heterojunction, J. Colloid Interf. Sci., 2023, 650, 1974–1982, DOI:10.1016/j.jcis.2023.07.147.
- K. Chen, Y. Shi, P. Shu, Z. Luo, W. Shi and F. Guo, Construction of core-shell FeS2@ZnIn2S4 hollow hierarchical structure S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production, Chem. Eng. J., 2023, 454, 140053, DOI:10.1016/j.cej.2022.140053.
- U. Bharagav, N. R. Reddy, V. N. Rao, P. Ravi, M. Sathish, M. V. Shankar and M. M. Kumari, CuWO4 as a novel Z-scheme partner to construct TiO2 based stable and efficient heterojunction for photocatalytic hydrogen generation, Int. J. Hydrogen Energ., 2022, 47, 40391–40406, DOI:10.1016/j.ijhydene.2022.07.155.
- J. Jiang, S. Bai, M. Yang, J. Zou, N. Li, J. Peng, H. Wang, K. Xiang, S. Liu and T. Zhai, Strategic design and fabrication of MXenes-Ti3CNCl2@CoS2 core-shell nanostructure for high-efficiency hydrogen evolution, Nano Res., 2022, 15, 5977–5986, DOI:10.1007/s12274-022-4276-8.
- D. Zhang, D. Zhang, D. Fan, J. Liu, H. Li, X. Pu, H. Hu, F. Guo and P. Cai, Decorating Cd0.9Zn0.1S using a magnetic FeCo@ N-doped graphite carbon layer to achieve considerable hydrogen evolution efficiency, ACS Sustain. Chem. Eng., 2024, 12, 8236–8246, DOI:10.1021/acssuschemeng.4c01726.
- X. Yu, J. Zhao, L. Zheng, Y. Tong, M. Zhang, G. Xu, C. Li, J. Ma and G. Shi, Hydrogen Evolution Reaction in Alkaline Media: Alpha- or Beta-Nickel Hydroxide on the Surface of Platinum?, ACS Energy Lett., 2018, 3, 237–244, DOI:10.1021/acsenergylett.7b01103.
- D. Kong, X. Hu, J. Geng, Y. Zhao, D. Fan, Y. Lu, W. Geng, D. Zhang, J. Liu, H. Li and X. Pu, Growing ZnIn2S4 nanosheets on FeWO4 flowers with p-n heterojunction structure for efficient photocatalytic H2 production, Appl. Surf. Sci., 2022, 591, 153256, DOI:10.1016/j.apsusc.2022.153256.
- D. Zhang, D. Zhang, S. Wang, H. Li, J. Liu, X. Pu, P. Chen, R. Qin, H. Hu and P. Cai, Synthesize magnetic ZnFe2O4@C/Cd0.9Zn0.1S catalysts with S-scheme heterojunction to achieve extraordinary hydrogen production efficiency, J. Colloid Interf. Sci., 2024, 657, 672–683, DOI:10.1016/j.jcis.2023.11.159.
- M. H. Fan, C. H. Wang, X. Yu, J. Ding, A. Q. Xiao, Y. Li and W. Y. Huang, MoS2 as a cocatalyst applied in advanced oxidation processes for enhancing degradation of organic pollutants: a review, Tungsten, 2024, 6, 473–487, DOI:10.1007/s42864-023-00252-y.
- S. Wang, D. Zhang, P. Su, X. Yao, J. Liu, X. Pu, H. Li and P. Cai, In situ preparation of mossy tile-like ZnIn2S4/Cu2MoS4 S-scheme heterojunction for efficient photocatalytic H2 evolution under visible light, J. Colloid Interf. Sci., 2023, 650, 825–835, DOI:10.1016/j.jcis.2023.07.052.
- Y. W. Xiao, J. Z. Tian, H. Miao and E. Z. Liu, Facile fabrication of S-scheme MnCo2S4/ZnIn2S4 heterojunction for photocatalytic H2 evolution, J. Alloy. Compd., 2024, 1003, 175636, DOI:10.1016/j.jallcom.2024.175636.
- K. Wu, R. Q. Jiang, Y. L. Zhao, L. Mao, X. Q. Gu, X. Y. Cai and M. S. Zhu, Hierarchical NiCo2S4/ZnIn2S4 heterostructured prisms: High-efficient photocatalysts for hydrogen production under visible-light, J. Colloid Interf. Sci., 2022, 619, 339–347, DOI:10.1016/j.jcis.2022.03.124.
- U. Bharagav, N. R. Reddy, V. N. Koteswararao, P. Ravi, K. Pratap, M. Sathish, K. K. Cheralathan, M. V. Shankar and M. M. Kumari, Heterojunction of CdS Nanocapsules-WO3 Nanosheets Composite as a Stable and Efficient Photocatalyst for Hydrogen Evolution, Energy Fuels, 2020, 34, 14598–14610, DOI:10.1021/acs.energyfuels.0c00597.
- J. Yu, X. Yao, P. Su, S. Wang, D. Zhang, B. Ge and X. Pu, Construction of Cu3Mo2O9/Mn0.3Cd0.7S S-Scheme Heterojunction for Photocatalytic Hydrogen Production via Water Splitting, Liaocheng Univ., 2024, 52–61, DOI:10.19728/j.issn1672-6634.2023090011.
- S. Kannan, K. Mohanraj and G. Sivakumar, Preparation of Bifunctional CuWO4-Based Heterostructure Nanocomposites for Noble-Metal-Free Photocatalysts, ChemistrySelect, 2017, 2, 4484–4498, DOI:10.1002/slct.201700877.
- O. Y. Khyzhun, T. Strunskus, S. Cramm and Y. M. Solonin, Electronic structure of CuWO4: XPS, XES and NEXAFS studies, J. Alloy. Compd., 2005, 389, 14–20, DOI:10.1016/j.jallcom.2004.08.013.
- Y. Yang, W. Ren, Y. Y. Liu, C. Cai, X. Z. Zheng, S. G. Meng and L. W. Zhang, Construction of shell-core Co2P/Cd0.9Zn0.1S photocatalyst by electrostatic attraction for enhancing H2 evolution, J. Colloid Interf. Sci., 2023, 649, 547–558, DOI:10.1016/j.jcis.2023.06.132.
- D. Kong, H. Fan, D. Yin, D. Zhang, X. Pu, S. Yao and C. Su, AgFeO2 Nanoparticle/ZnIn2S4 Microsphere p–n Heterojunctions with Hierarchical Nanostructures for Efficient Visible-Light-Driven H2 Evolution, ACS Sustain. Chem. Eng., 2021, 9, 2673–2683, DOI:10.1021/acssuschemeng.0c07638.
- L. Wu, F. Su, T. Liu, G. Liu, Y. Li, T. Ma, Y. Wang, C. Zhang, Y. Yang and S. Yu, Phosphorus-doped single-crystalline quaternary sulfide nanobelts enable efficient visible-light photocatalytic hydrogen evolution, J. Am. Chem. Soc., 2022, 144, 20620–20629, DOI:10.1021/jacs.2c07313.
- F. Cao, X. Zhang, X. Niu, X. Lin, T. Wu, S. Zhong, H. Lin, L. Zhao and S. Bai, Upgrading single S-scheme heterojunction to multi-S-Scheme ones for better synergy of photocatalytic CO2 reduction and H2O oxidation: The third component location matters, ACS Catal., 2024, 14, 12529–12540, DOI:10.1021/acscatal.4c03286.
- P. Su, D. Zhang, X. Yao, T. Liang, N. Yang, D. Zhang, X. Pu, J. Liu, P. Cai and Z. Li, Enhanced piezo-photocatalytic performance in ZnIn2S4/BiFeO3 heterojunction stimulated by solar and mechanical energy for efficient hydrogen evolution, J. Colloid Interf. Sci., 2024, 662, 276–288, DOI:10.1016/j.jcis.2024.02.058.
- Z. W. Shao, X. Meng, H. Lai, D. F. Zhang, X. P. Pu, C. H. Su, H. Li, X. Z. Ren and Y. L. Geng, Coralline-like Ni2P decorated novel tetrapod-bundle Cd0.9Zn0.1S ZB/WZ homojunctions for highly efficient visible-light photocatalytic hydrogen evolution, Chinese J. Catal., 2021, 42, 439–449, DOI:10.1016/S1872-2067(20)63597-5.
- P. Su, D. Kong, H. Zhao, S. Li, D. Zhang, X. Pu, C. Su and P. Cai, SnFe2O4/ZnIn2S4/PVDF piezophotocatalyst with improved photocatalytic hydrogen production by synergetic effects of heterojunction and piezoelectricity, J. Adv. Ceram., 2023, 12, 1685–1700, DOI:10.26599/JAC.2023.9220758.
- Y. Zhang, F. Y. Cao, S. Y. Zhao, J. K. Zhang, S. X. Zhong, H. Mao, L. H. Zhao and S. Bai, Efficient charge and proton balance enabled by a 2D/2D S-scheme heterojunction with a nanochamber design for better synergy of photocatalytic CO2 methanation and benzylamine oxidation, Adv. Funct. Mater., 2024, 2413830, DOI:10.1002/adfm.202413830.
- J. S. Li, X. Zhang, X. Q. Xiong, C. L. Wu, Y. X. Jin and K. L. Lv, Breaking Type-I heterojunction limitations: Harnessing an Ohmic-like/S-scheme cascade charge transfer mechanism for enhanced photocatalytic H2 evolution, Sep. Purif. Technol., 2025, 354, 129444, DOI:10.1016/j.seppur.2024.129444.
- L. Y. Zhang, J. J. Zhang, H. G. Yu and J. G. Yu, Emerging S-Scheme Photocatalyst, Adv. Mater., 2022, 34, 2107668, DOI:10.1002/adma.202107668.
- H. B. Huang, Z. B. Fang, K. Yu, J. Lü and R. Cao, Visible-light-driven photocatalytic H2 evolution over CdZnS nanocrystal solid solutions: interplay of twin structures, sulfur vacancies and sacrificial agents, J. Mater. Chem. A, 2020, 8, 3882–3891, 10.1039/c9ta13836f.
- Z. B. Jin, T. T. Wei, L. X. Li, F. Y. Li, R. Tao and L. Xu, Loading Co3N nanoparticles as efficient cocatalysts over Zn0.5Cd0.5S for enhanced H2 evolution under visible light, Dalton Trans., 2019, 48, 2676–2682, 10.1039/c8dt05087b.
- Y. X. Tang, D. F. Zhang, X. X. Qiu, L. Zeng, B. X. Huang, H. Li, X. P. Pu and Y. L. Geng, Fabrication of a NiCo2O4/Zn0.1Cd0.9S p–n heterojunction photocatalyst with improved separation of charge carriers for highly efficient visible light photocatalytic H2 evolution, J. Alloy. Compd., 2019, 809, 151855, DOI:10.1016/j.jallcom.2019.151855.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06654e |
| This journal is © The Royal Society of Chemistry 2024 |