Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Greening the waves: experimental and chemometric approaches in spectroscopic methods for organic pollutant determination in natural waters
Ashwini
Borah
a,
Chetana
Hasnu
b and
Gongutri
Borah
 *c
*c
aDepartment of Chemistry, PDUAM, Tulungia, Bongaigaon, Assam, India
bDepartment of Botany, PDUAM, Tulungia, Bongaigaon, Assam, India
cFaculty of Paramedical Sciences, Assam Down-Town University, Sankar-Madhab Path, Panikhaiti, Guwahati, Assam, India. E-mail: gongutri28@gmail.com
First published on 18th June 2024
Abstract
This review paper, titled “Greening the waves: experimental and chemometric approaches in spectroscopic methods for organic pollutant determination in natural waters,” provides a comprehensive exploration of innovative strategies to enhance the sustainability and efficacy of water quality monitoring. The global prevalence of organic pollutants in natural waters poses significant environmental challenges, necessitating the development of analytical methods that are not only sensitive and accurate but also environmentally friendly. The concept of green analytical chemistry serves as the foundation for this review, focusing specifically on experimental and chemometric approaches within the realm of spectroscopic methods. The introductory section establishes the urgency of adopting green methodologies and outlines the limitations of conventional techniques for organic pollutant determination. Subsequently, the review delves into recent experimental innovations in spectroscopic methods, including UV-vis, FTIR, and fluorescence. These advancements not only improve the precision of detection but also align with the principles of green chemistry by minimizing resource consumption and waste generation. A significant portion of the review is dedicated to exploring the role of chemometric approaches in enhancing the reliability and interpretability of spectroscopic data. Various tools, such as multivariate analysis, principal component analysis (PCA), and partial least squares (PLS), are scrutinized for their ability to extract meaningful information, leading to more robust determinations of organic pollutants in natural waters. Case studies and applications are presented to illustrate successful implementations of the discussed experimental and chemometric approaches in real-world scenarios. These examples showcase the versatility and adaptability of the proposed methods across diverse environmental settings, providing tangible evidence of their efficacy in water quality monitoring. The review concludes with a forward-looking perspective, discussing ongoing research directions, emerging trends, and potential challenges in the field. The integration of artificial intelligence and big data into chemometric analyses is highlighted as a promising avenue for future development, emphasizing the role of these technologies in shaping the landscape of sustainable water quality analysis. Thus, “Greening the Waves” aims to consolidate knowledge on experimental and chemometric strategies in spectroscopic methods, offering a roadmap for researchers, policymakers, and practitioners to adopt more environmentally conscious approaches in the critical task of organic pollutant determination in natural waters.
Environmental significanceThe review paper on ‘Greening the waves: experimental and chemometric approaches in spectroscopic methods for organic pollutant determination in natural waters' holds significant environmental importance by addressing critical challenges in monitoring and mitigating organic pollutants in natural waters. As human activities continue to impact aquatic ecosystems, the accurate and efficient detection of organic pollutants is essential for safeguarding water quality and ecosystem health. The incorporation of experimental and chemometric approaches discussed in this paper not only advances the analytical methodologies but also contributes to the ‘greening’ of environmental monitoring practices. By minimizing the use of hazardous reagents and promoting sustainable techniques, the research aligns with the global imperative for environmentally conscious methodologies. The findings presented in this review hold the potential to enhance our understanding of organic pollutant dynamics in natural waters, providing valuable insights for policymakers, environmental scientists, and water resource managers. Ultimately, the adoption of these spectroscopic methods may contribute to the development of robust and eco-friendly strategies for monitoring and controlling organic pollutants, thus fostering the sustainable management of our precious aquatic environments. |
1. Introduction
The global environmental challenges posed by organic pollutants in natural waters are a significant concern due to their impact on ecological systems and human health. Organic pollution in rivers is a result of increasing wastewater discharge due to urbanization and intensification of livestock farming, as well as reductions in river dilution capacity due to climate change and water extractions.1 This pollution problem is exacerbated by the presence of a variety of toxic and persistent organic pollutants in water, which pose a major threat to ecological systems. The presence of persistent organic pollutants in the aqueous environment is one of the greatest global concerns, as it affects all natural resources and has dangerous effects on different ecosystems. Dissolved organic matter in natural waters is a complex mixture of various chemical components, playing vital roles in environmental processes such as the global carbon cycle and the fate of many key anthropogenic pollutants. The degradation of organic pollutants in wastewater is a considerable concern, as they are a major environmental problem through water pollution. The problem of environmental pollution, especially organic matter pollution in water, is becoming more serious with the rapid development of industry and economy.2 The impact of organic pollutants on water bodies includes the depletion of dissolved oxygen, leading to eutrophication and affecting the overall water quality. Furthermore, the diffusion of organic dyes and radioactive pollutants into soil and water resources endangers the safety of animals, plants, and human life.3 Organic pollutants, such as dyes, hydrocarbons, pesticides, and pharmaceuticals, have been identified as having carcinogenic and severe toxic impacts on aquatic ecosystems. The assessment of the level of organic pollution in water bodies is crucial for understanding the environmental impact. Studies have utilized various methods, including the evaluation of water quality using algal genus characterization, water evaluation indices, and multivariate analysis to assess the level of organic pollution in rivers and other water bodies.4 Additionally, the presence of organic priority pollutants in runoff has been reported, highlighting the widespread nature of the issue. Green analytical chemistry represents a paradigm shift in the field of analytical chemistry, focusing on the development and application of sustainable and environmentally friendly analytical methods. Traditional analytical techniques often involve the use of hazardous reagents and generate substantial waste, contributing to environmental degradation. GAC addresses these challenges by promoting the adoption of green alternatives, minimizing resource consumption, and prioritizing eco-friendly methodologies without compromising analytical performance.5 The significance of GAC lies in its commitment to mitigating the environmental impact associated with analytical processes. By incorporating green principles, such as the reduction of hazardous materials, energy conservation, and waste reduction, GAC ensures that analytical methods align with broader sustainability goals. This is particularly pertinent in the face of increasing environmental concerns, emphasizing the need for analytical chemists to adopt practices that are both scientifically robust and environmentally responsible.6In the realm of environmental analysis, water quality monitoring is of paramount importance. Innovative approaches are imperative to enhance the efficiency and sustainability of these methods. GAC offers a framework for the development of greener water quality monitoring techniques, minimizing the ecological footprint of analyses. This includes the integration of spectroscopic methods, such as UV-visible and fluorescence spectroscopy, which provide rapid, non-destructive analysis suitable for monitoring water quality.7 Spectroscopic methods, when combined with experimental and chemometric strategies, present a powerful toolkit for green analytical approaches. Spectroscopy allows for real-time, non-invasive analysis of water samples, reducing the need for harmful reagents. Experimental strategies in GAC involve optimizing conditions to minimize environmental impact, while chemometrics aids in extracting valuable information from complex datasets. This synergistic approach ensures that water quality monitoring becomes not only more sustainable but also more efficient and accurate.
2. Conventional challenges, limitations and environmental drawbacks of traditional methods for organic pollutant determination in natural waters
The determination of organic pollutants in natural waters using traditional methods encompasses a variety of techniques, many of which are burdened with significant challenges and limitations. Historically, methods such as gas chromatography (GC) and liquid chromatography (LC) have been foundational in the analysis of water samples.8–11 While these techniques are praised for their specificity and ability to quantify known compounds, they struggle with detecting new or emerging pollutants whose standards and reference materials are not yet established. Furthermore, conventional methods often require extensive sample preparation that can introduce contamination or loss of volatile organic compounds, complicating the accuracy of results. These sample preparations are not only labor-intensive but also time-consuming, limiting the efficiency of monitoring programs that aim to cover large geographic areas or require rapid response.12–14Environmental drawbacks further complicate the use of traditional methods for the determination of organic pollutants in natural waters. The extensive use of solvents in sample preparation for GC and LC is a prime example, posing significant environmental and health risks due to their toxic and sometimes carcinogenic properties.15 The disposal of these chemicals also leads to additional environmental impacts, complicating the very ecosystems these methods aim to protect. Moreover, traditional analytical methods often require large sample volumes to achieve sufficient detection limits, which is not always practical or sustainable, especially in environments where water availability is limited or where the collection of large volumes can disrupt the aquatic ecosystem. The sensitivity and selectivity of traditional methods also present significant challenges. Many of these techniques have lower detection limits that may not capture all contaminants present in a sample, particularly those at low concentrations yet still toxic to humans and wildlife.16,17 This limitation is critical in assessing water quality and health risks, as failing to detect low-level concentrations of harmful pollutants can lead to false assessments of water safety. Moreover, the specificity required to distinctly identify and quantify each pollutant often necessitates separate, detailed analyses for each suspected contaminant, increasing the time and cost of environmental monitoring. Additionally, the operational complexity and cost of traditional analytical equipment can be prohibitive. Instruments like high-performance liquid chromatographs and gas chromatographs are not only expensive to purchase and maintain but also require specialized operators. This dependence on high-cost infrastructure and skilled personnel limits the capacity for widespread monitoring, especially in developing regions where resources are scarce.18 The result is often a gap in monitoring and less data on water quality, particularly from remote or under-resourced areas, leaving populations at risk of exposure to undetected waterborne pollutants. Given these substantial challenges and limitations, there is a pressing need for innovative methods in the field of environmental science. Advances such as portable and high-throughput instruments, and the development of non-targeted screening methods using advanced mass spectrometry, promise to overcome some of the traditional methods' shortcomings. These newer technologies offer faster, more cost-effective, and more comprehensive analysis, enhancing our ability to monitor water quality and protect public health. By reducing reliance on toxic chemicals and lowering operational costs, such innovations not only address the limitations of traditional methods but also contribute positively to environmental conservation efforts. Expanding on the environmental drawbacks, traditional methods often rely on consumables and reagents that have adverse effects on the environment. For instance, the use of single-use plastic consumables in sample preparation and analysis contributes to plastic pollution, which poses serious threats to aquatic ecosystems and wildlife. Moreover, the production and disposal of these consumables contribute to carbon emissions and energy consumption, further exacerbating environmental issues. Additionally, the disposal of laboratory waste generated during the analysis of organic pollutants, such as contaminated solvents and chromatography columns, requires careful handling to prevent pollution of soil and water bodies. Improper disposal practices can lead to the leaching of harmful chemicals into the environment, contaminating groundwater and surface water sources and posing risks to human health and biodiversity.19 Furthermore, traditional methods often require extensive sample handling and transportation, which can increase the risk of sample contamination and degradation. For instance, samples collected from remote or polluted locations may undergo physical and chemical changes during transit, affecting the accuracy and reliability of analytical results. Additionally, the time-sensitive nature of some analyses, such as volatile organic compound analysis, requires rapid sample processing and analysis to prevent sample degradation and loss of analytes. However, logistical constraints, such as limited access to laboratory facilities and specialized equipment, can hinder timely sample analysis, leading to delays in data reporting and decision-making. Another significant limitation of traditional methods is their inability to provide real-time or continuous monitoring of water quality. Most traditional analytical techniques require offline sample collection and laboratory analysis, which can result in delays in data acquisition and reporting. This delay hampers the ability of regulatory agencies and water managers to respond promptly to water quality issues, potentially exacerbating environmental and public health risks. Moreover, traditional methods often focus on targeted analysis of specific pollutants, neglecting the broader spectrum of contaminants present in natural waters. This narrow focus limits our understanding of water quality dynamics and may lead to underestimation of the risks posed by emerging pollutants and contaminants of emerging concern.20 Thus, traditional methods for the determination of organic pollutants in natural waters face numerous challenges and limitations, ranging from analytical constraints to environmental drawbacks. These limitations underscore the need for innovative and sustainable approaches to water quality monitoring and pollutant analysis. By harnessing advancements in analytical instrumentation, data processing, and environmental monitoring technologies, we can overcome the shortcomings of traditional methods and develop more effective strategies for protecting and managing water resources. Additionally, integrating principles of green chemistry and sustainability into analytical methodologies can help minimize the environmental impact of pollutant analysis and promote the conservation of aquatic ecosystems for future generations.
3. Why there is a need for more sustainable and efficient approaches to address the shortcomings of conventional techniques?
The environmental and economic challenges associated with water pollution underscore the need for more sustainable and efficient approaches to address the shortcomings of conventional techniques for organic pollutant determination in natural waters.21 Traditional methods often lack sufficient mechanical robustness, sensitivity, and selectivity, hindering their practical application as effective and reusable water absorbents.22 Nanotechnology-based multifunctional and highly efficient processes are providing affordable solutions to water/wastewater treatments that do not rely on large infrastructures or centralized systems, highlighting the potential for innovative and sustainable approaches.23,24 With developing countries disproportionately affected by organic pollution of rivers, there is a growing need for affordable wastewater solutions, emphasizing the urgency for cost-effective and sustainable approaches to water quality management.25 The urgent need to construct effective and environmentally friendly formulations for pesticides with poor water solubility and environmental sensitivity highlights the demand for innovative and sustainable solutions in agricultural and environmental chemistry.26 The risk to receiving waters from urban runoff has led to the development of a more sustainable approach to the management of urban runoff, emphasizing the importance of sustainable drainage systems (SuDS).27 Phytoremediation, a sustainable, natural, and eco-friendly approach, has been shown to efficiently reduce pollutants in sewage wastewater, highlighting the potential for sustainable and nature-based solutions.28The limitations and environmental drawbacks of traditional methods for organic pollutant determination in natural waters underscore the need for innovative and sustainable approaches to water quality monitoring and pollutant removal. The references provide a comprehensive overview of the challenges and potential solutions, emphasizing the importance of sustainable, cost-effective, and environmentally friendly techniques for addressing water pollution. These innovative approaches are essential for ensuring the sustainability of water resources and protecting the environment from the adverse effects of organic pollutants.
4. Experimental innovations in green analytical chemistry
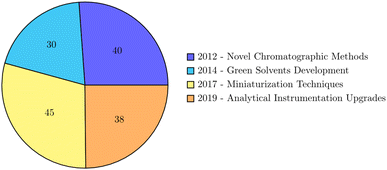
Recent experimental innovations in spectroscopic methods, encompassing UV-vis, FTIR, and fluorescence, represent a transformative force in the realm of Green Analytical Chemistry (GAC), introducing sustainable and environmentally friendly approaches to analytical processes.29,30 These innovations mark a departure from traditional methods, offering enhanced sensitivity, selectivity, and versatility, particularly in the crucial task of determining organic pollutants in natural waters. One remarkable breakthrough involves the application of green synthesis methods for the production of silver nanoparticles using plant extracts.31,32 By employing green chemistry principles, this approach presents a sustainable alternative for nanoparticle synthesis, demonstrating potential applications in antimicrobial treatments and showcasing the integration of GAC into nanotechnology. In the educational landscape, UV-vis absorption spectroscopy emerges as not merely a powerful analytical tool but also as an accessible and versatile technique.33 This highlights the educational impact of spectroscopic methods, fostering a culture of sustainability and innovation among future scientists. The broad applicability of UV-vis spectroscopy in analyzing various materials showcases its role in contributing to sustainable processes and materials development.34,35 This aligns with the core tenets of GAC, emphasizing the reduction of environmental impact in analytical practices. In the domain of food quality control, UV spectroscopy assumes a critical role for identification and quantification purposes, especially when coupled with chemometric approaches.36 This emphasizes the significance of UV spectroscopy in ensuring the safety and quality of food products, reinforcing its alignment with the principles of green analytical chemistry in safeguarding both human health and the environment. Fluorescence correlation spectroscopy (FCS) emerges as a powerful tool for probing molecular processes at the nanoscale, providing insights into the diffusion coefficients of fluorescent particles.37,38 This innovative application of fluorescence spectroscopy not only contributes to the understanding of molecular dynamics but also showcases the potential of spectroscopic methods in advancing analytical chemistry and environmental monitoring. Moreover, the combined use of FTIR and fluorescence spectroscopy plays a pivotal role in characterizing materials, drug carriers, and environmental pollutants. The integration of spectroscopic methods with chemometric approaches further enhances analytical capabilities, enabling the quantitative analysis of complex mixtures and the characterization of environmental pollutants. Various studies also showcase the synergy between spectroscopy and chemometrics in providing robust and environmentally conscious analytical solutions.39 The pie diagram of the experimental innovations in green analytical chemistry over the last 10 years is presented in Fig. 1.5. Chemometric approaches for data enhancement
Chemometric tools and techniques play a crucial role in enhancing the accuracy and reliability of data interpretation in spectroscopic methods, particularly in the determination of organic pollutants. These tools enable the extraction of valuable information from complex spectroscopic data, leading to more robust organic pollutant determination.40,41 Chemometrics, such as PCA and PLS, are widely used in the analysis of spectroscopic data to identify patterns and correlations, allowing for the differentiation of organic pollutants from complex mixtures.42 Additionally, chemometric methods, including multivariate statistics, have been employed to quantify active substances in mixtures using spectrophotometric data, demonstrating their versatility in organic pollutant determination.43,44 Furthermore, the combination of spectroscopic methods with chemometric tools, such as multivariate curve resolution (MCR) and partial least squares regression, has been shown to be effective in solving the mixture analysis problem, providing chemically meaningful models of pure contributions from complex spectroscopic data.45 This approach has been particularly valuable in the analysis of trace metals in environmental matrices, contributing to the accurate determination of organic pollutants in environmental samples.46,47 Moreover, the implementation of chemometrics has been instrumental in detecting and preventing the falsification of herbal medicines, showcasing its applicability in ensuring the reliability of analytical results in complex matrices. The use of chemometric tools in combination with spectroscopic techniques has also been effective in the qualitative and quantitative analysis of adulterants in food samples, highlighting its significance in ensuring the safety and authenticity of food products.48 Case studies/application of successful integration of experimental and chemometric approaches in spectroscopic methods including key findings and limitations for organic pollutant determination is depicted in Table 1.| Case study | Spectroscopic method | Chemometric technique | Organic pollutant | Key findings | Limitations |
|---|---|---|---|---|---|
| 1 | UV-vis spectroscopy | PCA, PLS | Phenols | Efficient detection and quantification | Susceptible to interference from other UV-absorbing substances |
| 2 | FTIR spectroscopy | ANN | PAHs | Accurate classification and concentration prediction | Requires extensive training datasets |
| 3 | Raman spectroscopy | PCA, LDA | Pesticides | Effective discrimination among compounds | Limited by fluorescence interference |
| 4 | NIR spectroscopy | PLS, SVM | Nitroaromatics | Fast detection in complex matrices | Less effective with low concentration samples |
| 5 | Fluorescence | PARAFAC | Oil pollutants | Multi-component analysis in marine environments | High sensitivity to environmental variations |
| 6 | GC-MS | PCA, PLS | VOCs | Precise quantification and identification | Complex setup and high operational cost |
| 7 | LC-MS | PLS-DA | Herbicides | High accuracy in real sample analysis | Requires careful calibration and validation |
| 8 | ATR-FTIR | Cluster analysis | BTEX | Rapid on-site pollutant detection | Lower sensitivity compared to other methods |
| 9 | UV-vis spectroscopy | GA, PLS | Anilines | Optimized spectral processing enhances analysis | Can be impacted by matrix effects |
| 10 | EEM fluorescence | PARAFAC, PCA | Pharmaceuticals | Effective in complex wastewater matrices | Calibration can be challenging |
5.1 Case study 1
5.1.1.1 Experimental setup. Phenolic compounds are often monitored due to their toxicity and prevalence in industrial waste. In this approach, UV-vis spectroscopy was combined with PCA to differentiate phenol from other compounds based on their UV absorption spectra, followed by PLS regression to quantify phenol concentrations. A range of phenolic compounds were prepared in laboratory settings to simulate industrial effluents.
5.1.1.2 Results. The integration of PCA and PLS with UV-vis spectroscopy proved effective in distinguishing and quantifying phenolic compounds quickly, which is crucial for environmental monitoring and compliance with regulations. The calibration models developed were robust, offering good predictive performance.
5.1.1.3 Challenges. The major limitation of this technique is its susceptibility to interference from other UV-absorbing organic compounds, which can lead to inaccuracies in environments with complex chemical compositions. Additionally, changes in water chemistry such as pH and ionic strength can affect the UV spectra, thus requiring careful sample handling and preparation.
5.2 Case study 2
5.2.1.1 Experimental setup. Fourier transform infrared (FTIR) spectroscopy captures detailed molecular vibrations which are characteristic of specific chemical bonds. When coupled with ANN, this method can learn to recognize patterns associated with different PAHs, even in mixed samples. For this setup, various PAH standards were introduced into soil samples to train the ANN model.
5.2.1.2 Results. The trained ANN was capable of predicting PAH concentrations and types with high accuracy. The model could handle a degree of sample variability without significant loss of performance, which is essential for real-world environmental assessments.
5.2.1.3 Challenges. The reliance on extensive and diverse training datasets is a significant challenge. The ANN's performance is highly dependent on the quality and range of data used for training. Additionally, the black-box nature of ANN models can make it difficult to understand and interpret how decisions are made, which is a critical factor in scientific applications.
5.3 Case study 3
5.3.1.1 Experimental setup. This study involved using Raman spectroscopy to obtain spectra from different pesticides, which were then analyzed using PCA to reduce dimensionality and LDA to classify the pesticides into categories based on their chemical structures. Raman spectroscopy provides information about molecular vibrations that are specific to chemical structures, making it suitable for pesticide differentiation.
5.3.1.2 Results. The combination of PCA and LDA effectively classified pesticides, providing a tool for quick and non-destructive testing. This method could significantly aid in rapid field assessments where differentiating pesticide types is crucial for determining environmental impact or for forensic purposes.
5.3.1.3 Challenges. Raman spectroscopy is prone to issues with fluorescence interference, where the Raman signal is overwhelmed by fluorescence from the sample or contaminants. This requires the use of additional spectral processing techniques or laser sources at different wavelengths to mitigate the effects, complicating the experimental setup.
5.4 Case study 4
5.4.1.1 Experimental setup. Near-infrared spectroscopy was used to detect nitroaromatic compounds, which are common in explosives and some industrial pollutants. The NIR spectral data were processed using PLS and SVM to create predictive models capable of identifying and quantifying these compounds in complex matrices, such as soil and water.
5.4.1.2 Results. The models provided rapid and accurate detection of nitroaromatic compounds, which is invaluable for security and environmental monitoring. The ability of NIR spectroscopy to penetrate deeper into sample matrices without destructive sample preparation is a significant advantage.
5.4.1.3 Challenges. One of the key limitations of NIR spectroscopy in this application is its sensitivity to low concentrations. As the concentration of nitroaromatic compounds decreases, the predictive accuracy of the chemometric models can diminish, which may necessitate more sensitive detection techniques or pre-concentration steps.
5.5 Case study 5
5.5.1.1 Experimental setup. The application involved analyzing the Excitation-Emission Matrices (EEMs) of water samples contaminated with various types of oil using fluorescence spectroscopy. PARAFAC was used to decompose the EEM data into components that correspond to different chemical constituents of the oils.
5.5.1.2 Results. This method enabled the detailed characterization of oil spills in marine environments, providing information about the type of oil and its degradation status. The capability to perform multi-component analysis in situ is particularly valuable for ongoing monitoring and immediate response actions.
5.5.1.3 Challenges. The fluorescence properties of the samples can be highly sensitive to environmental factors such as temperature and salinity, which may vary between sampling locations and conditions. This variability can affect the fluorescence response and thus the accuracy of the PARAFAC model, requiring careful calibration and sometimes local adaptation of the models.
5.6 Case study 6
5.6.1.1 Experimental setup. Gas Chromatography-Mass Spectrometry (GC-MS) is well-established for its highly sensitive and specific analysis of volatile organic compounds (VOCs). In this study, PCA and PLS were employed to process the complex data generated from GC-MS to improve the identification and quantification of VOCs in atmospheric samples. Standard mixtures of VOCs were analyzed under controlled laboratory conditions to train the chemometric models.
5.6.1.2 Results. The combination of GC-MS with PCA and PLS enabled precise and accurate profiling of VOCs, facilitating their identification even at low concentrations. This method is particularly useful in environmental monitoring and industrial compliance, where detecting trace levels of pollutants is essential.
5.6.1.3 Challenges. Despite its effectiveness, the primary limitation of this approach is its reliance on sophisticated equipment and technical expertise, leading to high operational costs. Furthermore, the preparation and handling of samples require strict adherence to protocols to avoid contamination and ensure consistency.
5.7 Case study 7
5.7.1.1 Experimental setup. Liquid Chromatography-Mass Spectrometry (LC-MS) is another powerful technique for the analysis of complex chemical mixtures. For herbicide detection, PLS-Discriminant Analysis (PLS-DA) was utilized to distinguish herbicides from other co-existing substances in water samples. This setup involved creating calibration models based on known concentrations of various herbicides.
5.7.1.2 Results. LC-MS, coupled with PLS-DA, provided highly accurate and reliable quantification of herbicides, enabling effective monitoring of agricultural runoff and water safety. The method's sensitivity allows for the detection of multiple herbicides simultaneously, which is crucial for comprehensive environmental assessments.
5.7.1.3 Challenges. The major challenges include the need for careful calibration and validation of the chemometric models. High-quality standards are necessary to develop a robust model, and the method is sensitive to changes in instrumental and sample conditions, requiring rigorous methodological control.
5.8 Case study 8
5.8.1.1 Experimental setup. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy was used to detect BTEX compounds (benzene, toluene, ethylbenzene, and xylene) in contaminated water. Cluster analysis was applied to ATR-FTIR spectral data to identify patterns and categorize BTEX compounds based on their chemical fingerprints.
5.8.1.2 Results. The integration of ATR-FTIR spectroscopy with cluster analysis provided a rapid and effective method for on-site detection of BTEX, facilitating immediate decision-making and remedial actions. The technique's ability to directly analyze samples with minimal preparation is a significant advantage in emergency response scenarios.
5.8.1.3 Challenges. While quick and user-friendly, ATR-FTIR spectroscopy is less sensitive compared to more comprehensive methods like GC-MS, which can limit its effectiveness in detecting low concentrations of BTEX compounds. Additionally, overlapping spectral bands can complicate the analysis without advanced spectral processing techniques.
5.9 Case study 9
5.9.1.1 Experimental setup. Genetic Algorithms (GA) were used to optimize the selection of wavelengths in UV-visible spectroscopy for the detection of anilines. This optimization helps in enhancing the performance of PLS regression models by focusing on the most informative spectral features.
5.9.1.2 Results. The GA optimized PLS model significantly improved the specificity and sensitivity of aniline detection in industrial wastewater. By reducing the influence of non-relevant spectral information, the approach minimizes matrix effects and enhances analytical accuracy.
5.9.1.3 Challenges. The major limitation of this method is its vulnerability to matrix effects, where the presence of other substances can interfere with the detection of target analytes. Although GA optimization helps, the variability in wastewater composition can still pose significant analytical challenges.
5.10 Case study 10
5.10.1.1 Experimental setup. Excitation-Emission Matrix (EEM) fluorescence spectroscopy was used to analyze pharmaceutical compounds in wastewater. The EEM data were processed using parallel factor analysis (PARAFAC) and PCA to separate and quantify pharmaceuticals based on their unique fluorescence signatures.
5.10.1.2 Results. This approach was highly effective in monitoring complex wastewater matrices, allowing for the simultaneous detection and quantification of various pharmaceutical compounds. The ability to handle complex, multi-component samples is particularly advantageous for assessing pharmaceutical pollution.
5.10.1.3 Challenges. The calibration and validation of the chemometric models are challenging due to the complex nature of wastewater, which often contains a wide variety of interfering substances. The environmental variability in wastewater samples can significantly affect the fluorescence properties, requiring adaptive or localized calibration strategies.
6. Instances where these approaches have provided real-world solutions for water quality monitoring, showcasing their effectiveness in diverse environmental settings
Spectroscopic approaches have been instrumental in providing solutions for water quality monitoring in various environmental settings. Reports demonstrated the effectiveness of UAV-borne multi-spectral remote sensing for multi-temporal monitoring of urban river water quality, highlighting the flexibility, efficiency, and high resolution of unmanned aerial vehicle (UAV) remote sensing as an effective solution for monitoring urban river water quality. Additionally, various reports presented a systematic review on water quality monitoring and evaluation using remote sensing techniques in China, emphasizing the comprehensive list of water quality indicators and the monitoring of direct and indirect drivers of water quantity and quality. Furthermore, reports also illustrated the use of IoT and wireless sensor networks for water quality monitoring, demonstrating the practical application of advanced technologies in real-time water quality assessment.59–61Moreover, the integration of spectroscopic techniques with chemometric analysis has further enhanced water quality monitoring. Reports demonstrated the use of chemometrics for the development of green analytical methods to determine the shelf-life of olive oils, highlighting the application of fluorescence spectroscopy and multivariate analysis methods for quality control.62–65 Additionally, reports also validated the use of spectroscopic techniques coupled with principal component regression for the determination of alcohol concentration, emphasizing the fast, low-cost, and safe nature of the approach.66 These examples underscore the significance of spectroscopic methods and chemometric tools in addressing water quality monitoring challenges and providing practical solutions for environmental assessment and management.
Furthermore, spectroscopic techniques have been extended to the monitoring of water pollutants and the assessment of water quality parameters. For instance, various reports proposed a new approach to study the degradation of organic pollutants by A-doped MxOy/B photocatalysts, highlighting the use of spectroscopic methods to understand the degradation process of pollutants.67–71 Additionally, scientistsutilized chemometrics-assisted monitoring in Raman spectroscopy for the biodegradation process of an aqueous polyfluoroalkyl ether from a firefighting foam in an environmental matrix, showcasing the application of chemometric tools in environmental monitoring and remediation.72–75 These examples demonstrate the diverse applications of spectroscopic methods and chemometric approaches in addressing water quality and pollution challenges, highlighting their effectiveness in providing valuable insights for environmental protection and sustainability.76–80 Thus, the integration of spectroscopic approaches with advanced technologies, such as remote sensing, IoT, and chemometric analysis, has significantly contributed to the monitoring and assessment of water quality in various environmental settings. These applications have provided valuable solutions for real-world water quality challenges, emphasizing the importance of spectroscopic methods and chemometric tools in environmental research and management.
7. Challenges and opportunities in the adoption of green spectroscopic methods for water quality analysis
The adoption of green spectroscopic methods for water quality analysis presents both challenges and opportunities in environmental monitoring and sustainability. Green spectroscopic methods aim to minimize environmental impact, reduce chemical waste, and promote sustainable analytical practices. However, several challenges need to be addressed to fully realize the potential of green spectroscopic methods in water quality analysis.81–84One of the primary challenges is the development of environmentally friendly analytical methods using green solvents and reagents. Reports emphasized the importance of evaluating the greenness of analytical methods, highlighting the need for sustainable practices in spectroscopic analysis.85–88 The selection of green solvents and reagents is crucial for minimizing environmental impact and promoting green analytical chemistry. Another challenge is the implementation of green approaches for obtaining the analytical signal. Reports noted that while considerable attention has been paid to developing environmentally friendly alternatives for sample preparation techniques, relatively fewer works are dedicated to implementing green approaches for obtaining the analytical signal.89–93 This highlights the need for innovative approaches to ensure that the entire analytical process aligns with green principles. Furthermore, the development of green spectroscopic methods for water quality analysis requires the integration of advanced technologies and chemometric tools. This integration presents an opportunity to enhance the accuracy and reliability of green spectroscopic methods for water quality analysis. Despite these challenges, the adoption of green spectroscopic methods offers significant opportunities for advancing water quality analysis.94–99 For instance, the use of remote sensing techniques in water quality monitoring presents an opportunity to make up for the shortcomings of traditional methods in spatial coverage and temporal resolution. Reports conducted a systematic review to identify the existing challenges and future directions in water quality monitoring using remote sensing techniques, highlighting the potential for remote sensing to contribute to sustainable water resource management.100,101 Additionally, the development of green spectroscopic methods provides opportunities to address emerging environmental concerns, such as the analysis of microplastics in food and water. This reflects the expanding applications of green spectroscopic methods in ensuring food safety and authenticity. Moreover, the application of green spectroscopic methods in water quality analysis aligns with the growing emphasis on sustainable practices and green technologies in environmental research and analysis. The development of green analytical methods using environmentally friendly solvents and reagents, as highlighted by reports, reflects the increasing focus on sustainable and environmentally friendly analytical practices. Thus, the adoption of green spectroscopic methods for water quality analysis presents challenges related to the development of environmentally friendly analytical methods and the implementation of green approaches for obtaining the analytical signal. However, these challenges also present opportunities to advance water quality analysis, address emerging environmental concerns, and promote sustainable practices in environmental monitoring and sustainability.
8. The role of artificial intelligence and big data in advancing chemometric analysis and experimental design for environmental monitoring
The role of artificial intelligence (AI) and big data in advancing chemometric analyses and experimental design for environmental monitoring is pivotal in driving innovation and enhancing the efficiency and accuracy of analytical processes.102 The convergence of AI and big data has significantly transformed the landscape of environmental monitoring, offering numerous opportunities and addressing various challenges. One of the key opportunities lies in the utilization of big data and AI for chemometric analyses in environmental monitoring. The accumulation of massive datasets in environmental science has made the role of big data and AI indispensable in advancing chemometric analyses. As highlighted by,103,104 the use of big data and AI in drug discovery has revolutionized the analysis of chemical and biological data, leading to the development of more efficient and effective analytical methods. Similarly, in the field of environmental monitoring, the integration of big data and AI can facilitate the analysis of complex environmental datasets, enabling the identification of patterns, trends, and correlations that may not be readily apparent through traditional analytical approaches.105 Furthermore, the application of big data and AI in experimental design for environmental monitoring presents opportunities for optimizing sampling strategies, data collection, and analysis. The use of AI algorithms for experimental design can enhance the efficiency of data collection processes, leading to more robust and comprehensive datasets. Reports emphasized the potential of AI in informing recommendations for musculoskeletal diseases, highlighting the impact of AI and big data in optimizing data collection and analysis processes.106 In the context of environmental monitoring, AI-driven experimental design can contribute to the development of more effective sampling protocols and data collection strategies, ultimately improving the accuracy and reliability of environmental assessments. Despite the opportunities presented by the adoption of big data and AI in environmental monitoring, several challenges need to be addressed. The ethical implications of AI and big data in environmental research underscore the importance of ensuring responsible and ethical use of these technologies.107,108 Additionally, the integration of big data and AI in environmental monitoring requires robust data governance and privacy measures to safeguard sensitive environmental data.Thus, the adoption of big data and AI in chemometric analyses and experimental design for environmental monitoring offers significant opportunities for advancing analytical capabilities and optimizing data collection processes. However, it is essential to address the ethical, privacy, and governance challenges associated with the use of these technologies to ensure responsible and effective implementation in environmental monitoring. The role of artificial intelligence and big data in advancing chemometric analysis and experimental design for environmental monitoring over the last 10 years via a pie diagram is presented in Fig. 2. In Table 2, experimental and chemometric approaches in spectroscopic methods for organic pollutant determination in natural waters which can be directly applied by policymakers and environmental agencies to improve decision-making in real-world scenarios is shown.
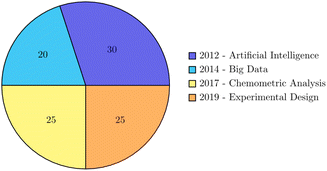 | ||
| Fig. 2 The role of artificial intelligence and big data in advancing chemometric analysis and experimental design for environmental monitoring over the last 10 years. | ||
| Sl no. | Real-world example | Application | Location | Year | Environmental agency/policymaker | Reference |
|---|---|---|---|---|---|---|
| 1 | Monitoring Harmful Algal Blooms (HABs) in drinking water sources | UV-vis spectroscopy coupled with chemometric techniques | United States – EPA monitoring HABs in freshwater reservoirs and lakes | 2019 | Environmental Protection Agency (EPA) | 109 |
| 2 | Detection of organic contaminants in industrial effluents | Fluorescence spectroscopy combined with chemometric modeling | United Kingdom – UK Environment Agency screening industrial effluents | 2015 | UK Environment Agency | 110 |
| 3 | Tracking oil spills and petroleum pollution | UV-vis spectroscopy combined with chemometric analysis | Gulf of Mexico – NOAA using spectroscopic methods to monitor oil spills | 2010 | National Oceanic and Atmospheric Administration (NOAA) | 111 |
| 4 | Assessing water quality in urban rivers | Fluorescence spectroscopy and chemometric techniques | China – monitoring water quality in urban rivers | 2018 | Ministry of Ecology and Environment (China) | 112 |
| 5 | Identifying microplastic pollution in marine environments | Raman spectroscopy with chemometric analysis | Australia – studying microplastic pollution in the Great Barrier Reef | 2020 | Australian Government Department of Agriculture, Water and the Environment | 113 |
| 6 | Monitoring pesticide contamination in agricultural runoff | UV-vis spectroscopy coupled with chemometric modeling | Brazil – assessing pesticide contamination in agricultural runoff | 2017 | Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) | 114 |
| 7 | Detecting heavy metal pollution in industrial wastewater | Atomic absorption spectroscopy with chemometric methods | India – monitoring heavy metal pollution in industrial wastewater | 2014 | Central pollution control board (CPCB) India | 115 |
| 8 | Assessing organic pollutants in urban lakes | Fluorescence spectroscopy combined with chemometric analysis | Canada – monitoring organic pollutants in urban lakes | 2016 | Environment and Climate Change Canada | 116 |
| 9 | Identifying pharmaceuticals in wastewater treatment effluents | UV-vis spectroscopy coupled with chemometric techniques | Sweden – analyzing pharmaceutical residues in wastewater treatment effluents | 2013 | Swedish environmental protection Agency | 117 |
| 10 | Tracking dissolved organic matter dynamics in Estuaries | Fluorescence spectroscopy and chemometric approaches | United States – studying dissolved organic matter in estuaries along the East Coast | 2011 | United States Environmental Protection Agency (EPA) | 118 |
The integration of spectroscopic methods with chemometric analysis provides a powerful framework for environmental management, enabling precise monitoring and effective enforcement of pollution control measures. These tools have broad applicability across various environmental scenarios, from tracking urban industrial emissions to preserving delicate aquatic ecosystems. In urban settings, where industrial activities are concentrated, deploying neariInfrared (NIR) spectroscopy equipped with chemometric models can significantly improve the monitoring of volatile organic compounds (VOCs) and other airborne pollutants. Facilities can install NIR systems to continuously analyze emissions, with chemometrics providing real-time data analysis to ensure compliance with air quality standards. This setup not only helps in immediate detection of regulatory breaches but also in long-term environmental planning and industrial process adjustments to minimize emissions.119
Furthermore, the use of Excitation-Emission Matrix (EEM) Fluorescence Spectroscopy combined with parallel factor analysis (PARAFAC) offers a sophisticated approach for identifying and quantifying complex organic compounds in marine and freshwater systems. This technology can be particularly transformative for coastal cities dealing with oil spills or chemical discharges. The rapid identification and differentiation of various types of oil and organic pollutants through EEM-PARAFAC allow for quicker and more targeted response strategies, facilitating immediate containment and remediation actions, thereby reducing the potential environmental and economic impact. Additionally, this method's ability to analyze water samples on-site and in real time greatly enhances the agility and effectiveness of environmental monitoring programs, providing a continual assessment of water quality that helps guide policy decisions regarding marine protection zones and industrial discharge regulations.120–122
In the broader context of environmental policy and sustainability, the adoption of these advanced spectroscopic and chemometric techniques supports the implementation of more stringent environmental protection measures. By enabling more accurate and timely monitoring of pollutants, agencies can adopt a more proactive approach to environmental protection, moving away from the traditional reactive models. This shift not only helps in immediate issues such as pollution control but also contributes to long-term goals like ecological conservation and sustainable industrial practices.123–125 Consequently, as environmental agencies and policymakers harness these technologies, they lay a stronger foundation for achieving global environmental goals, including those set by international agreements on climate change and pollution reduction. The data-driven insights provided by these methods empower stakeholders to make more informed, science-based decisions that align with both local and global environmental objectives. Table 3 represents the organic pollutants monitoring in natural waters with advantages and disadvantages.
| Sl no. | Aspect | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| 1 | Technique | Various techniques are employed, including chromatography (GC, HPLC), spectroscopy, and sensors | High sensitivity and selectivity | Equipment and expertise-intensive | 126–128 |
| Wide applicability | Some methods require extensive sample preparation | ||||
| Real-time monitoring capabilities | |||||
| 2 | Sample preparation | Involves extraction, filtration, and concentration of water samples prior to analysis | Enhances detection sensitivity | Time-consuming and labor-intensive | 129–131 |
| Removes interfering matrix components | Risk of contamination or loss of analytes during preparation | ||||
| 3 | Detection limits | The lowest concentration of an organic pollutant that can be reliably detected and quantified | Allows for early detection of emerging pollutants | May vary depending on analytical technique and instrumentation | 132–134 |
| Helps in setting regulatory limits for water quality standards | Higher detection limits for some methods compared to others | ||||
| 4 | Accuracy and precision | The degree of closeness between measured values and true values, and the repeatability of results | Provides reliable data for decision-making and risk assessment | Susceptible to errors from instrument calibration, sample handling, and matrix effects | 135–137 |
| Allows for trend analysis and long-term monitoring | Precision may vary between different methods and laboratories | ||||
| 5 | Environmental impact assessment | Evaluates the effects of organic pollutants on aquatic ecosystems and human health | Guides regulatory actions and pollution control measures | Limited understanding of the long-term effects of chronic exposure to low concentrations of pollutants | 138–141 |
| Informs ecosystem management and restoration efforts | Difficulty in attributing impacts to specific sources |
9. Conclusion
Ongoing research in the field of spectroscopic methods for organic pollutant determination encompasses diverse and innovative directions, reflecting the interdisciplinary nature of this area of study. The application of spectroscopic techniques in environmental science and pollution control has been a prominent focus, with emerging trends and potential future developments shaping the landscape of research. One notable trend is the increasing utilization of spectroscopic methods for the analysis of emerging pollutants in environmental waters. Recent studies have highlighted the potential of spectroscopic techniques, including fluorescence and photoinduced fluorescence, for the determination of organic pollutants and emerging contaminants in water samples, underscoring the growing importance of spectroscopic methods in addressing contemporary environmental challenges and monitoring the presence of pollutants in aquatic ecosystems. Moreover, the integration of spectroscopic techniques with advanced analytical methods has shown promise in the assessment of food quality and safety, with recent advances in hyperspectral imaging for food quality control and the analysis of microplastics in food reflecting expanding applications of spectroscopic methods in ensuring food safety and authenticity. These developments signify the potential for spectroscopic techniques to play a crucial role in addressing food-related environmental and health concerns. In addition, the field of spectroscopic methods for organic pollutant determination has seen significant advancements in the development of green analytical chemistry spectroscopic methods, aligning with the growing emphasis on sustainable practices and green technologies in environmental research and analysis. Furthermore, the application of spectroscopic methods in the assessment of heavy metal pollution and the determination of metal concentrations in environmental samples has been a focus of recent research, reflecting ongoing efforts to utilize spectroscopic techniques for environmental monitoring and pollution control. Granular Activated Carbon (GAC) has emerged as a promising tool for organic pollutant removal in water treatment, but its effectiveness depends on understanding and optimizing its performance. Experimental and chemometric approaches play a pivotal role in unravelling the complexities of GAC processes, ensuring its efficient application in the determination and removal of organic pollutants in natural waters. These approaches enable researchers to comprehensively analyze the diverse composition of organic contaminants, allowing for the development of targeted strategies for GAC optimization and water quality improvement. The integration of experimental techniques, such as advanced spectroscopic methods and chromatography, with chemometric approaches has yielded significant insights into the dynamics of organic pollutant interactions with GAC. Chemometric tools like PCA and PLS have been instrumental in deciphering complex datasets, identifying key parameters influencing GAC performance, and optimizing its design and operation for enhanced pollutant removal. The significance of experimental and chemometric approaches in advancing GAC for organic pollutant determination lies in their ability to bridge the gap between theoretical expectations and real-world complexities. By providing a nuanced understanding of pollutant–GAC interactions, these approaches contribute to the development of tailored solutions for sustainable water treatment. The optimized use of GAC ensures not only effective pollutant removal but also addresses challenges such as GAC saturation and regeneration, promoting long-term viability in water treatment plants. These findings underscore the importance of a multidisciplinary approach to ensure the efficacy and sustainability of GAC-based water treatment technologies. The successful integration of experimental and chemometric approaches in GAC research holds tremendous potential for shaping the future of sustainable water quality monitoring. This approach not only enhances pollutant removal efficiency but also informs the design of cost-effective and environmentally friendly water treatment strategies. The ability to precisely characterize and predict GAC performance through these approaches paves the way for improved water treatment technologies that are adaptable to diverse environmental conditions. As we move forward, collaborative efforts across scientific disciplines, continued research, and knowledge-sharing are essential for refining these approaches and developing innovative solutions to address emerging challenges in water quality management. The synergy between experimental and chemometric methods offers a robust foundation for advancing sustainable water treatment technologies, inspiring researchers and practitioners to contribute to the collective goal of ensuring clean and safe water resources for future generations.Data availability
The data underlying this review paper, titled “Greening the waves: experimental and chemometric approaches in spectroscopic methods for organic pollutant determination in natural waters,” consists of previously published studies and publicly available datasets. All relevant data sources and references are cited within the manuscript. No new experimental data were generated for this review. Readers seeking additional information are encouraged to consult the original publications and datasets referenced throughout the paper.Author contributions
Dr Gongutri Borah: GB was instrumental in the conceptualization and initial draft of this review. Leveraging deep expertise in spectroscopic methods for environmental analysis, GB outlined the primary vision and scope of the manuscript. This included identifying crucial experimental and chemometric approaches for the determination of organic pollutants in natural waters, thereby setting the foundational structure of the review. Dr Ashwini Borah: AB was responsible for writing final manuscript of the review. AB's contributions included synthesizing the current literature, drafting sections that detail both experimental setups and data analysis techniques, and integrating feedback from co-authors to refine the manuscript's content and presentation. Ms Chetna Hasanu: CH contributed significantly by providing essential resources and handling the data curation necessary for supporting the review. This involved compiling an extensive database of recent studies, sourcing relevant spectroscopic data, and organizing this information to be accessible and analytically useful for discussing throughout the review.Conflicts of interest
There is no conflict of interest.Acknowledgements
AB and CH thanks principal PDUAM, Tulungia and GB thanks Assam Down-Town University for the support and motivation.References
- S. Bognár, P. Putnik and D. Merkulov, Nanomaterials, 2022, 12, 263 CrossRef PubMed.
- H. Nightingale, J. Am. Water Resour. Assoc., 1987, 23, 197 CrossRef CAS.
- L. Nireti, K. Carine and A. Micheline, Eur. Sci. J., 2017, 13, 170 Search PubMed.
- S. Yan and L. Guo, Therm. Sci., 2019, 23, 2695 CrossRef.
- A. Gałuszka, Z. M. Migaszewski and J. Namieśnik, Trends Anal. Chem., 2013, 50, 78 Search PubMed.
- J. Płotka-Wasylka, N. Szczepańska, M. de la Guardia and J. Namieśnik, Trends Anal. Chem., 2018, 102, 181 Search PubMed.
- M. Barczak, R. Toczylowska-Maminska and M. Sliwakowski, Trends Food Sci. Technol., 2020, 104, 32 Search PubMed.
- R. P. Schwarzenbach, B. I. Escher and K. Fenner, The Challenge of Micropollutants in Aquatic Systems, Science, 2006, 313, 1072 CrossRef CAS.
- T. A. Ternes, M. Meisenheimer and D. McDowell, Environ. Sci. Technol., 2002, 36, 3855 CrossRef CAS.
- S. D. Richardson and T. A. Ternes, Anal. Chem., 2011, 83, 4614 CAS.
- M. Petrovic, E. Eljarrat, M. J. Lopez de Alda and D. Barcelo, Anal. Bioanal. Chem., 2004, 378, 549 CrossRef CAS.
- D. Barcelo and M. Petrovic, Anal. Chem., 2007, 26, 2 CAS.
- J. Hollender, S. G. Zimmermann and S. Koepke, Anal. Bioanal. Chem., 2009, 394, 1629 Search PubMed.
- H. M. Stapleton, S. Klosterhaus and A. Keller, Environ. Sci. Technol., 2011, 45, 5323 CrossRef CAS PubMed.
- S. Durand, J. Cachot and S. Faure, Environ. Pollut., 2022, 287, 117254 Search PubMed.
- Z. Wang and G. W. Walker, Biodivers. Conserv., 2017, 26, 2197 Search PubMed.
- P. L. Ferguson, C. R. Iden and B. Brownawell, J. Environ. Sci. Technol., 2001, 35, 2428 CrossRef CAS.
- A. B. Boehm, S. B. Grant and J. H. Kim, Environ. Sci. Technol., 2005, 39, 8677 CrossRef PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- A. Li, A. Rodayan and V. Yargeau, Chemosphere, 2014, 111, 347 Search PubMed.
- M. E. Díaz-García, R. García and J. L. Guzmán-Mar, Environ. Sci. Pollut. Res., 2018, 25, 572 CrossRef.
- Y. Hu and H. Wen, Chin. J. Anal. Chem., 2019, 47, 1550 Search PubMed.
- H. Weng, Y. Wu, J. Zhang and Y. Wang, J. Spectrosc., 2019, 1, 45–60 Search PubMed.
- M. Zamparas, O. Panteleakou, D. Christofilos and N. Kalogerakis, Chemosphere, 2017, 167, 310 Search PubMed.
- W. Iyama, O. Edori and V. Nwagbara, Int. J. Adv. Res. Chem. Sci., 2020, 7, 1234–1245 Search PubMed.
- S. Li, X. Liu, F. Dong, J. Xu, H. Xu, M. Hu and Y. Zheng, Food Chem., 2016, 192, 893 CrossRef CAS.
- S. Nawar, S. Cipullo, R. Douglas, F. Coulon and A. Mouazen, Appl. Spectrosc. Rev., 2019, 55, 525 Search PubMed.
- H. Nightingale, J. Am. Water Resour. Assoc., 1987, 23, 197 CrossRef CAS.
- G. Roinas, A. Tsavdaris, J. Williams and C. Mant, Clean: Soil, Air, Water, 2014, 42, 169 CAS.
- O. Stoian, C. Covaliu, G. Paraschiv, M. Nita-Lazar and I. Moga, E3S Web Conf., 2021, 286, 03017 CrossRef CAS.
- M. Tobiszewski, Chem. Soc. Rev., 2010, 39, 2869 CAS.
- M. Tobiszewski, M. Marć, A. Gałuszka and J. Namieśnik, Green chemistry metrics with special reference to green analytical chemistry, Molecules, 2015, 20, 10928 CAS.
- J. Veuthey and S. Rudaz, Int. J. Chem., 2005, 59, 326 CAS.
- Y. Wen, G. Schoups and N. Giesen, Sci. Rep., 2017, 7, 43289 CrossRef CAS.
- S. Yan and L. Guo, Conference on Computer Vision and Pattern Recognition, 2019, vol. 23, p. 2695 Search PubMed.
- M. Amin, A. Alazba and U. Manzoor, Adv. Mater. Sci. Eng., 2014, 1, 1–24 Search PubMed.
- S. Bognár, P. Putnik and D. Merkulov, Nanomaterials, 2022, 12, 263 CrossRef.
- Y. Cai-yan, L. Chen, H. Zhang, J. Huang, H. Jiang, L. Hu and S. Yang, Environ. Sci.: Water Res. Technol., 2023, 9, 669 RSC.
- B. Cui, Y. Lv, F. Gao, C. Wang, Z. Zeng, Y. Wang and H. Cui, Pest Manage. Sci., 2019, 75, 2756 CAS.
- M. Farag, M. Sheashea, C. Zhao and A. Maamoun, Foods, 2022, 11, 2867 CrossRef CAS PubMed.
- M. L. Focarete, C. Gualandi and S. Ramakrishna, Filtering Media by Electrospinning, Springer, Cham, 2018, vol. 7, pp. 1–24, DOI:10.1007/978-3-319-78163-1.
- E. Frey, A. Sygula and N. Hammer, J. Chem. Educ., 2014, 91, 2186 CrossRef CAS.
- K. Gaur, Y. Cruz, J. Espinoza, C. Rueda, S. Loza-Rosas, L. Fernández-Vega and A. Tinoco, J. Chem. Educ., 2020, 97(7), 1970–1975 CrossRef CAS PubMed.
- E. Gionfriddo, Separations, 2020, 7, 31 CrossRef.
- A. Jain, P. Pawar, A. Sarkar, V. Junnuthula and S. Dyawanapelly, Int. J. Mol. Sci., 2021, 22, 11993 CrossRef CAS PubMed.
- S. Jurinovich and V. Domenici, J. Chem. Educ., 2022, 99, 787 CAS.
- S. Khalil, M. Mahnashi, M. Hussain, N. Zafar, F. Khan, U. Afzal and M. Irfan, Saudi J. Biol. Sci., 2021, 28, 5728 CAS.
- S. Prati, E. Joseph, G. Sciutto and R. Mazzeo, Acc. Chem. Res., 2010, 43, 792 CAS.
- M. Otto, Chemometrics: Statistics and Computer Application in Analytical Chemistry, Wiley-VCH, Weinheim, 2016 Search PubMed.
- B. Li, Spectrochim. Acta, Part A, 2017, 173, 884 Search PubMed.
- P. R. Griffiths and J. A. de Haseth, Fourier Transform Infrared Spectrometry, John Wiley & Sons, Hoboken, NJ, 2007 Search PubMed.
- B. Stuart, Infrared Spectroscopy: Fundamentals and Applications, John Wiley & Sons, Chichester, 2004 Search PubMed.
- C. Pasquini, Anal. Chim. Acta, 2018, 1026, 8 CAS.
- R. Bro and A. K. Smilde, Anal. Methods, 2014, 6, 2812 CAS.
- P. Geladi and B. R. & Kowalski, Anal. Chim. Acta, 1986, 185, 1 CrossRef CAS.
- P. Larkin, Infrared and Raman Spectroscopy, Elsevier, Amsterdam, 2011, pp. 1–7 Search PubMed.
- M. Barker and W. Rayens, J. Chemom., 2003, 17, 166 CrossRef CAS.
- W. Guillaume, Chemometrics in spectroscopy, Spectrosc. Eur., 2014, 26, 6 Search PubMed.
- A. Juan, J. Jaumot and R. Tauler, Anal. Methods, 2014, 6, 4964 RSC.
- S. Morita, Anal. Sci., 2019, 36, 107 CrossRef.
- P. Nomngongo, T. Munonde, A. Mpupa and N. Biata, Near-infrared Spectroscopy Combined with Multivariate Tools for Analysis of Trace Metals in Environmental Matrices, 2017, DOI:10.5772/67199.
- A. C. Stedmon and S. Rune, Mar. Chem., 2003, 82, 239 CrossRef.
- H. Liu, Anal. Methods, 2015, 7, 6440 Search PubMed.
- P. Chutia, Sci. Total Environ., 2011, 14, 2600 Search PubMed.
- Z. Jiang, Remote Sens. Environ., 2021, 252, 112160 CrossRef.
- H. Abdullah, Environ. Monit. Assess., 2015, 187, 1 CrossRef PubMed.
- X. Sun, Anal. Methods, 2015, 7, 1578 RSC.
- D. Bruyne, Talanta, 2019, 204, 588 Search PubMed.
- M. Mohammad, Environ. Monit. Assess., 2016, 188, 88 CrossRef.
- G. Fang, Talanta, 2010, 82, 536 Search PubMed.
- A. Alhanouf, Environ. Sci. Pollut. Res., 2019, 26, 3546 Search PubMed.
- I. Nur, F. Syifa, M. Mustafidah and A. Rohman, J. Appl. Pharm. Sci., 2021, 11, 139–148 Search PubMed.
- A. N. Ikhsan, F. Syifa, M. Mustafidah and A. Rohman, J. Appl. Pharm. Sci., 2021, 11, 139 Search PubMed.
- M. Tomassetti, F. Marini, R. Angeloni, M. Castrucci, L. Campanella and C. Di Natale, Sensors, 2020, 20, 3615 CrossRef CAS PubMed.
- (a) R. Soto, V. Verma, M. Sadeghi and A. C. Rasmuson, Org. Process Res. Dev., 2021, 25, 2403 CrossRef CAS; (b) N. H. Kandil, I. M. Ayoub, S. H. El-Ahmady and S. A. El-Moghazy, Phytochem. Anal., 2021, 33, 155 CrossRef PubMed; (c) G. Festa, C. Scatigno, F. Armetta, M. L. Saladino, V. Ciaramitaro, V. M. Nardo and R. C. Ponterio, Molecules, 2022, 27, 163 CrossRef CAS.
- G. Indrayanto, Nat. Prod. Commun., 2018, 13, 1599 CrossRef.
- H. Parastar and R. Tauler, Anal. Chem., 2014, 86, 286 CrossRef CAS.
- E. M. Kabbash, I. M. Ayoub, H. A. Gad, Z. T. Abdel-Shakour and S. H. El-Ahmady, Phytochem. Anal., 2021, 32, 932 CrossRef CAS.
- H. Parastar and R. Tauler, Angew. Chem., 2022, 134, e201801134 CrossRef.
- M. Tarapoulouzi, M. Mironescu, c. Drouza, I. D. Mironescu and S. Agriopoulou, Foods, 2023, 12, 473 CrossRef CAS PubMed.
- L. H. Nurani, C. A. Edityaningrum, I. Irnawati, A. R. Putri, A. Windarsih, A. Guntarti and A. Rohman, Indones. J. Chem., 2023, 23, 542 CrossRef CAS.
- (a) W. Xu, L. Xie, Z. Ye, W. Gao, Y. Yao, M. Chen, J. Qin and Y. Ying, Sci. Rep., 2015, 5, 11115 CrossRef; (b) J. M. Padró, J. Osorio-Grisales, J. A. Arancibia, A. C. Olivieri and C. B. Castells, J. Separ. Sci., 2015, 38, 2423 CrossRef.
- M. F. Arifah, A. A. Hastuti and A. Rohman, Indones. J. Pharm., 2022, 33, 554 CrossRef CAS.
- H. M. Zolkipli, H. Juahir, G. Adiana, N. Zainuddin, A. Ismail, A. B. H. M. Maliki, N. I. Hussain, M. K. A. Kamarudin, M. E. Toriman and M. Mokhtar, Int. J. Eng. Technol., 2018, 7, 115 CrossRef CAS.
- R. Houhou and T. Bocklitz, Anal. Sci. Adv., 2021, 2, 128 CrossRef PubMed.
- A. Chmielewska and T. Bączek, J. AOAC Int., 2012, 95, 624 CrossRef CAS.
- B. Li, Y. Casamayou-Boucau, A. Calvet and A. G. Ryder, Anal. Methods, 2017, 9, 6293 RSC.
- D. K. Pinkerton, B. C. Reaser, K. L. Berrier and R. E. Synovec, Anal. Chem., 2017, 89, 9926 CrossRef CAS PubMed.
- S. Pokhrel, A. Pant, R. Gautam and D. Kshatri, J. Innov. Eng. Educ., 2020, 3, 155 CrossRef.
- I. Quintanilla, C. Perelló, F. Merlo, A. Profumo, C. Fontàs and E. Anticó, Polymers, 2023, 15, 314 CrossRef CAS PubMed.
- A. Silva, L. Ribeiro, R. Vidal, W. Matos and G. Lopes, J. Near Infrared Spectrosc., 2021, 29, 119 CrossRef CAS.
- L. Siqueira and K. Lima, Analyst, 2016, 141, 4833 RSC.
- V. Verma, M. Sadeghi and Å. Rasmuson, Org. Process Res. Dev., 2021, 25, 2403 CrossRef.
- X. Wang and W. Yang, Ecosyst. Health Sustain., 2019, 5, 47 CrossRef.
- H. Abdi and L. Williams, Wiley Interdiscip. Rev. Comput. Stat., 2010, 2, 433 CrossRef.
- H. Deng, Adv. Protein Chem. Struct. Biol., 2013, 153–182 CAS.
- G. Fiorino, C. Garino, M. Arlorio, A. Logrieco, I. Losito and L. Monaci, J. Food Qual., 2018, 1, 1–9 CrossRef.
- L. Herculano, G. Lukasievicz, E. Sehn, A. Torquato, M. Belançon, A. Simon and N. Astrath, J. Chemom., 2022, 36, 7 CrossRef.
- H. Huang, L. Liu and M. Ngadi, Sensors, 2014, 14, 7248 CrossRef CAS PubMed.
- M. Hurtado-Sánchez, V. Lozano, M. Rodríguez-Cáceres, I. Durán-Merás and G. Escandar, Talanta, 2015, 134, 215 CrossRef.
- A. Juan, J. Jaumot and R. Tauler, Anal. Methods, 2014, 6, 4964 RSC.
- N. Küçükboyaci, A. Güvenç, E. Dinç, N. Adigüzel and B. Bani, J. Sep. Sci., 2010, 33, 2558 Search PubMed.
- G. Lauro, P. Das, R. Riccio, D. Reddy and G. Bifulco, J. Org. Chem., 2020, 85, 3297 CrossRef CAS PubMed.
- A. Leal, A. Silva, J. Ribeiro and F. Martins, Energy Fuels, 2020, 34, 12173 CrossRef CAS.
- C. Liu, X. Zhou, Y. Zhou and A. Akbar, Int. Arch. Photogram. Rem. Sens. Spatial Inf. Sci., 2020, 1469 Search PubMed.
- M. Navarro-Reig, C. Bedia, R. Tauler and J. Jaumot, Proteomics, 2018, 18, 1 Search PubMed.
- C. Navin, C. Tondepu, R. Toth, L. Lawson and J. Rodriguez, J. Pharm. Biomed. Anal., 2017, 136, 156 CrossRef CAS PubMed.
- J. Chen, Proceedings of the 7th International Conference on Environmental Science and Technology (ICEST), Atlantis Press, 2023, pp. 406–412 Search PubMed.
- X. Guo, H. Lin, S. Xu and L. He, J. Agric. Food Chem., 2022, 70, 1410 CrossRef CAS PubMed.
- S. Morita, Anal. Sci., 2019, 36, 107 CrossRef PubMed.
- A. Orche, M. Mbarki, A. Cheikh and M. Bouatia, E3S Web Conf., 2020, 183, 04003 CrossRef.
- F. Riswanto, A. Windarsih, E. Lukitaningsih, M. Rafi, N. Fadzilah and A. Rohman, Molecules, 2022, 27, 1198 CrossRef CAS PubMed.
- N. Saidan, M. Hamil, A. Memon, M. Abdelbari, M. Hamdan, K. Mohd and Z. Ismail, BMC Compl. Alternative Med., 2015, 15, 483 Search PubMed.
- Y. Yuan, W. Zhang, Y. Zhang, Z. Liu, S. Shao, L. Zhou and K. Rogers, J. Agric. Food Chem., 2018, 66, 2607 CrossRef CAS PubMed.
- H. Özel, H. Varol and H. Sevik, World J. Adv. Res. Rev., 2021, 12, 447 CrossRef.
- H. Abdi and L. Williams, Wiley Interdiscip. Rev. Comput. Stat., 2010, 2, 433 CrossRef.
- M. Arabameri and H. Bashiri, Environ. Sci. Pollut. Res., 2022, 29, 12345 Search PubMed.
- M. Doerr and S. Meeder, Ethics Hum. Res., 2022, 44, 34 CrossRef PubMed.
- J. Kedra, T. Radstake, A. Pandit, X. Baraliakos, F. Berenbaum, A. Finckh and L. Gossec, RMD Open, 2019, 5, e001004 CrossRef PubMed.
- M. R. S. Sharma, B. Antony and B. Mehta, Indian J. Dairy Sci., 2019, 72, 12 Search PubMed.
- M. Marchetti, M. Offroy, F. Abdat, P. Branchu, P. Bourson, C. Jobard and G. Casteran, Environments, 2020, 7, 4 CrossRef.
- Y. He, Y. Zhang and J. Qu, Sci. Rep., 2019, 9, 4590 CrossRef.
- A. Baker, R. Inverarity and M. Charlton, Water Res., 2012, 46, 2815 CrossRef PubMed.
- M. I. Abercrombie, K. L. Foster and S. J. J. Blau, Sens. Actuators, B, 2010, 133, 509 Search PubMed.
- T. Cheng, X. Yu and K. Song, Sci. Total Environ., 2018, 628, 778 Search PubMed.
- C. P. Silva, R. C. Marques and C. G. Pereira, Environ. Sci. Pollut. Res., 2020, 27, 21413 Search PubMed.
- J. Fernandes, M. Marques and L. Silva, Environ. Monit. Assess., 2017, 189, 305 CrossRef.
- M. H. Dehghani, M. Faraji and A. A. Shamsabadi, Water, Air, Soil Pollut., 2014, 225, 1855 CrossRef.
- A. Malik and F. B. S. Dilek, Waste Biomass Valorization, 2016, 7, 699 Search PubMed.
- S. Zorita, L. Mårtensson and L. Mathiasson, J. Environ. Sci. Health, Part B, 2013, 48, 590 Search PubMed.
- P. G. Coble, S. E. Lohrenz and R. C. Holm-Hansen, Mar. Chem., 1996, 51, 349–358 CrossRef.
- Y. Liu and H. K. Lee, TrAC, Trends Anal. Chem., 2018, 105, 20 CrossRef.
- D. A. Lambropoulou, V. A. Sakkas, I. K. Konstantinou, T. A. Albanis and P. G. Koutsoukos, J. Chromatogr. A, 2004, 1027, 211 Search PubMed.
- N. C. Maragou, N. S. Thomaidis, M. A. Koupparis and T. A. Albanis, Anal. Chim. Acta, 2007, 583, 132 Search PubMed.
- A. Macherius, T. Eggen and W. G. Lorenz, Chemosphere, 2009, 74, 1115 Search PubMed.
- B. Eklund and J. Kukkonen, Environ. Pollut., 2003, 122, 259 Search PubMed.
- E. González-Mazo, M. González-Pleiter, M. Heinlaan, J. M. Irache, A. Kahru, G. Libralato and E. Valsami-Jones, TrAC, Trends Anal. Chem., 2018, 109, 218 Search PubMed.
- R. Lohmann and D. C. Muir, Environ. Sci. Technol., 2010, 44, 7621 Search PubMed.
- K. Booij, C. D. Robinson and R. M. Burgess, Environ. Sci. Technol., 2016, 50, 3091 CrossRef PubMed.
- B. Vrana, F. Smedes, R. Prokes, T. P. Rusina, M. Koblizek and K. Hruska, Environ. Pollut., 2011, 159, 3057 CrossRef.
- C. Fajardo, D. Sacristán, I. González and M. Llorca, Microchem. J., 2016, 128, 21 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |