Synthesis of chiral graphene structures and their comprehensive applications: a critical review
Animesh
Sinha
a and
Hongyun
So
 *ab
*ab
aDepartment of Mechanical Convergence Engineering, Hanyang University, Seoul 04763, South Korea. E-mail: hyso@hanyang.ac.kr
bInstitute of Nano Science and Technology, Hanyang University, Seoul 04763, South Korea
First published on 23rd July 2024
Abstract
From a molecular viewpoint, chirality is a crucial factor in biological processes. Enantiomers of a molecule have identical chemical and physical properties, but chiral molecules found in species exist in one enantiomer form throughout life, growth, and evolution. Chiral graphene materials have considerable potential for application in various domains because of their unique structural framework, properties, and controlled synthesis, including chiral creation, segregation, and transmission. This review article provides an in-depth analysis of the synthesis of chiral graphene materials reported over the past decade, including chiral nanoribbons, chiral tunneling, chiral dichroism, chiral recognition, and chiral transfer. The second segment focuses on the diverse applications of chiral graphene in biological engineering, electrochemical sensors, and photodetectors. Finally, we discuss research challenges and potential future uses, along with probable outcomes.
1. Introduction
Chiral compounds are defined in chemistry as left/right-handed enantiomers comprising stereoisomers.1 Numerous naturally occurring molecules are chiral, with amino acids (AAs) being prominent examples. Chirality refers solely to the geometry of an object and does not inherently indicate a chiral material's physical or chemical properties.2 The study of chirality has been increasingly popular in recent years and has impacted the biological, genetic, and medical sciences.3–5 Consequently, the increasing prominence of synthesis of numerous types of chiral materials becomes apparent. Nevertheless, there may be difficulties in identifying mirror molecules in chiral compounds because of their weak chiroptical signals.In recent years, active research has been carried out on chiral metamaterials to manipulate the local field and enhance chiroptical signals.6 However, previous studies on chiral metamaterials have mostly concentrated on investigating the techniques and optimal parameters for attaining structure-induced chirality and elucidating its underlying mechanism. Therefore, more research must be performed for improving the properties of materials using different material combinations for practical applications (e.g., functional materials can be used to enhance the applicability of chiral metamaterials).7,8
Similarly, chiral inorganic compounds have attracted considerable attention over the past decade owing to their novel optical properties and feasible applications.9 One of the most distinctive characteristics of chiral inorganic compounds is that their chirality metrics may be adjusted by modifying external factors such as solvents, temperature, and helix handedness. This switchable trait allows for the customization of the optical chiral response, enhancing chiroptical activity.10 Researchers have created and used chiral materials for numerous electronic and biomedical applications for years.11,12 However, most of the reported materials do not have characteristics such as excellent stability, outstanding material interactions, and nontoxic nature, predominantly because most chiral elements are metals or polymers, which are harmful to organisms.
To address these issues, researchers have conducted experiments with different material combinations, and carbon derivatives are among the most effective and widely used materials owing to their numerous unique features, as discussed below. Carbon is Earth's earliest substance and one of its most prevalent elements. People have long endeavored to discover new purposes of carbon, extend its scope of significance, research its characteristics, and provide it with an entirely novel “lifetime value”.13,14 Graphene—an allotrope of carbon—is a promising two-dimensional (2D) material that has attracted attention for various applications. One advantageous property of graphene is that its chemical characteristics can be controlled via an extraneous potential or chemical alteration.15,16 Graphene oxide (GO) is an interesting form of graphene because of its unique chemical structures, which include functional groups with hydrophilic oxygen and small sp2 carbon surrounded by sp3 carbon domains.17 Therefore, integrating this novel substance into designed systems can enhance the functionality of the overall material. In addition, graphene has a problem of self-folding due to π–π stacking, which can be readily controlled with chiral materials.
Moreover, researchers have used chiral metasurfaces (i.e., a programmable feature of graphene) to create functional devices such as polarizers, modulators, and tunable optical sensors.18–21 Furthermore, some researchers discovered techniques for achieving significant development in chiral graphene-based materials using methods such as twisted layering and buckling.22–24 The chirality of graphene is an exciting yet mostly unexplored research area among the many possible avenues of research into functional materials created using graphene. Recent research has indicated that the optical activity of chiral metamaterials made of metal/chalcogenide glass/metal trilayers can be changed in the near-infrared (NIR) region.25 However, achieving ultrasmall chiral features and precise alignment in the optical domain while creating layered chiral metamaterials remains difficult. Significant challenges must be addressed when fabricating chiral graphene biosensors, photodetectors, and chemical sensors. One of the main challenges is finding the right technology to maintain high sensitivity and specificity levels. Specificity refers to a sensor's ability to differentiate between target and non-target biological entities in a sample, which is considered the most crucial characteristic of a sensor.
Fig. 1 summarizes the various forms of chiral graphene and its derivatives, along with their applications. The chiral hybrids are categorized according to their chiral components, and a separate section is dedicated to chiral graphene made from multiple ingredients. This article discusses the methods of chiral fabrication, transfer, and dichroism and their application in catalysts, detectors, and selectors. Additionally, it presents a brief discussion of the chiral species involved in the chirality of graphene materials and their applications in photodetection and biomedicine (e.g., in drug-delivery systems, Alzheimer's disease, Parkinson's disease, etc.). Other relevant topics, such as emerging chiral graphene materials, current challenges, and prospects, are also covered. Altogether, this report provides guidance for researching and developing chiral graphene-based materials and their use in future applications.
2. Chiral graphene synthesis
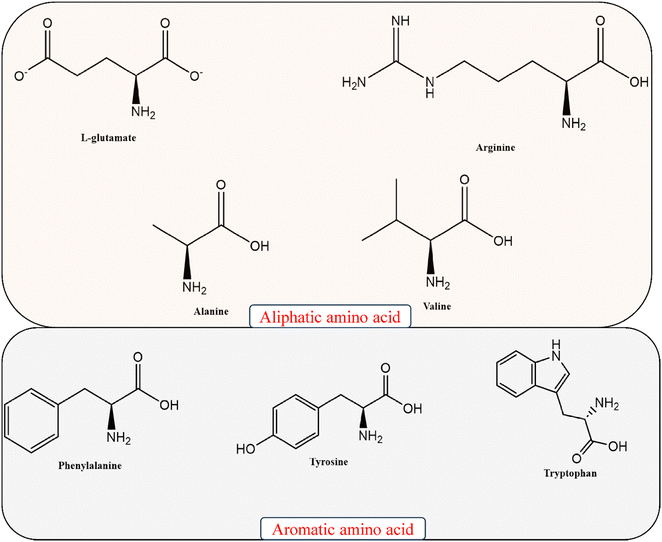
The pharmaceutical sector has grown over the past decade, increasing the interest in chiral synthesis. Although homogeneous symmetric catalysis has advanced significantly in recent years, heterogeneous catalysis has benefits such as easy catalyst separation and recycling, product purification, and the ability to perform chiral chemical processing.26,27 Graphene materials, which have exceptional properties, are promising despite not being uniformly dispersed. Chiral transformation of graphene materials can eliminate their tendency to accumulate and enhance the catalytic interactions.28,29 The following paragraph describes some of the most widely used phenomena in graphene synthesis. Fig. 2 shows the AAs most widely used by researchers according to recent reports.2.1. Synthesis of chiral graphene using aliphatic AAs
Aliphatic AAs are a class of chemical molecules characterized by a pair of amino acids and a group of carboxylic acids. The use of aliphatic AAs for the functionalization of graphene holds promise; however, a precise methodology for synthesizing chiral graphene in this context may differ based on the study methodology and objectives. Creating chiral organic–inorganic blended foundation networks is a complex supramolecular chemical process. These networks are widely used in enantiomeric formation, asymmetric reactions, nonlinear optical activity, and photosynthesis. Interestingly, graphene nanocomposite supramolecular structures, synthesized by adding organic molecules to oxygen-containing groups, have become a popular research area.30Han et al. examined amide formation between the enantiomers (glutamic acid) and the oxygen groups in GO.31 They observed that L,D-glutamic acid (L-Glu) was covalently attached to graphene sheets. The authors noticed that functionalized graphene with redox reactions and chiral recognition was more likely to the heterochiral interaction between graphene GO and the DOPA enantiomer, which not only served as a suitable chiral space as the enantioselective site for targets in electrochemical detection of DOPA enantiomers but also played a role in fast electron-transfer kinetics and additional signal amplification. However, further research on the effect of the temperature on the process and the kinetics of the response to temperature is necessary. Additionally, creating crosslinked polymers is a crucial factor in reaction kinetics. The amount of crosslinkers present can affect the swelling behavior of the system, which allows informed speculations regarding the reasons for a higher level of anti-discrimination.32
The impact on the polymer side chain due to the absorption of amino acids was examined, which affects the overall performance of enantiomeric selection. It is reported that adding too much triphosgene to AA L-glutamic acid with 5-methyl ester can reduce the yield and cause unwanted side effects (e.g., complications in cross-linking) during polymerization.33 Thus, to prevent these side effects. Meng et al. found that L-glutamic acid—a type of chiral selector—had exceptional chiral resolution abilities in combination with GO.34 The Glu-GO membranes exhibited 1–2 orders of magnitude higher flux and far higher selectivity than traditional chiral separation membranes. These materials have an efficient transport mechanism with many free-volume elements. This allows the enantiomer with low, or no binding affinity move quickly through the membrane, allowing high-throughput chiral separation. The authors also showed that modifying the chiral selector in GO-based membranes can lead to effective chiral separation without typical tradeoffs associated with polymer-based membranes.35 This breakthrough introduces new possibilities for enantioselective separation.
Because protein adsorption involves reactions between side chains of AA and nanomaterial substrates, it is crucial to understand the thermodynamics of these interactions to comprehend the chemical process at play.36 Sadhasivam et al. created a new material called chiral MABPP using L-phenylalanine.37 They then synthesized a polyamide (PI)/GO nanocomposite by combining GO with PI. An analysis indicated that GO in a nanocomposite can be evenly distributed and aligned parallel to the film, even at high concentrations. This is because the polyimide matrix and GO nanoparticles (NPs) are strongly bonded, preventing the formation of cavities in the material. Moreover, one notable benefit of obtaining even distribution in nanocomposites is the potential enhancement of electrical, mechanical, or optical characteristics of the material. Dinari et al. examined graphene aggregates in a poly(benzimidazole-amide) (PBIA) matrix, with a focus on their thermal, morphological, intrinsic viscosity, and solubility properties.38 To solve the aggregation problem, the author added GO to PBIA to form a GO/PBIA nanocomposite with an L-leucine moiety in the side chain. This was achieved using a simple and effective ultrasonic wave technique. They found that the carboxyl, hydroxyl, and epoxy functional groups on the outermost layer of GO can form strong bonds with the polymer's amidic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H active groups through covalent and hydrogen bonding. This connection allowed a more even dispersion of nanolayers throughout the polymer substrate.
O and N–H active groups through covalent and hydrogen bonding. This connection allowed a more even dispersion of nanolayers throughout the polymer substrate.
Understanding and managing protein adsorption on nanomaterials can be challenging because of the complex chemical mechanisms involved. This is particularly true in macroscopic systems where multiple protein molecules are present and thermodynamics play a significant role in proteins’ constant adsorption and desorption balance. Hirano et al. created an aromaphilicity index by measuring the energy needed for an AA to bind with graphene.39 This index accurately assesses the affinity of AAs to aromatic carbon surfaces, on the basis of experiments. Aromatic AAs and Arg (arginine) have higher negative binding energies than others. The index is not affected by the chiral angle but increases with curvature. Interactions with aromatic carbon surfaces may foretell the durability and binding location of proteins and polypeptides. The association of an electron across an aromatic heart of AA with the carbon core creates a rigid structure, a twist in the fragrant AA arrangement, and reverse CD absorption depending on the chiral source. The obtained chiral CDs were used in the development and fabrication of a sensitive GO chiral CD glucose sensor by adjusting the GO's catalytic activity.
Chiral graphene synthesis using aliphatic AAs faces several obstacles, including fabrication, purity, and stability. However, the benefits, such as tunability, increased chemical responsiveness, and biological compatibility, outweigh these challenges and significantly expand the spectrum of possibilities compared to non-chiral graphene.
2.2. Synthesis of chiral graphene using aromatic AAs
Chiral aromatic AAs such as tryptophan and phenylalanine, containing rings of aromatic compounds, can be used in graphene synthesis to manipulate and optimize chirality, resulting in chiral graphene with unique properties. Additionally, due to their affordability, availability, and renewability, biomolecules such as amylose and aromatic AAs are preferred over others, such as proteins and DNA. However, organic amylose needs to be implemented as a detector on a graphene-based detection platform.40–42Dragana et al. conducted a study that involved creating a model of a graphene-flake molecule.43 The model had a honeycomb arrangement of carbon atoms, with hydrogen atoms filling in the outermost circle of the graphene flakes. Despite having the same number of connections, amyloids with aromatic AAs interacted more strongly than those without aromatic AAs. The researcher's observation shows that an aromatic ring connected to a graphene flake, which is considered to be a single connection, has stronger interactions than CH–Π and NH–Π interactions. However, the study did not take environmental factors into account. It cannot be concluded that the existence of an aromatic AA in the amyloid sequence is the primary determinant of the interaction energies. The positioning of the side chains to allow stronger interactions between the amyloids and graphene also has a considerable impact.
To explore practical alternatives to enantiomer-specific amino acid identification, Niu et al. developed a new material called rGO-Fc-CD by adding β-CD (cyclodextrin) to GO-Fc (ferrocene) under alkaline conditions.44 This composite can recognize phenylalanine isomers and increase the distance between the layers of GO, enhancing its functionality. The reduction of GO, resulting in rGO-Fc, increases the electrical conductivity. The researchers found that the phenyl group of Phe enantiomers creates a strong hydrogen bond with the –NH2 functional group of L-Phe and secondary hydroxyl bonds on the edge of β-CD. This results in a higher peak current of D-Phe because the insulation of Phe inhibits the electron transfer. Therefore, it is recommended to include L-Phe with β-CD rather than D-Phe. Importantly, adding ferrocene (Fc) molecules to GO has gained significant attention in GO functionalization, which is mainly because it enhances the functionality, dispersibility, and specific surface area of GO. Additionally, Fc's exceptional electronic transmission ability on GO has contributed to the widespread interest in this approach. Similarly, Niu et al. observed that the chiral interactions of SA-CS-NGC (sodium alginate-chitosan-N-doped graphene-CNT) had a stronger attraction to L-Trp (tryptophan) than to D-Trp.45 This is because L-Trp is more easily separated from the electrode's modification layer and reaches the electrode surface, which leads to a stronger electrochemical signal.
When working with nanomaterials, high amplification factors are common. However, ensuring the repeatability and dependability of established methodologies remains a significant scientific challenge. We believe that the advantages of a traditional approach (e.g., chemical synthesis) can be measured by the outstanding productivity, effortless and articulate synthesis, substantial economic benefits, soft reaction conditions, and increasing popularity of nanocomposites such as CuO/ZnO@N-GQDs46 and black phosphorus/graphene47 in chiral applications.
Recently, directed assembly techniques have made significant progress in the synthesis of chiral materials with high stability, good solubility characteristics, and strategically placed functional groups. These materials come in unprecedentedly large sizes and possess the necessary chirality. Extending their supramolecular functionalities in fields such as molecular recognition, host–guest interactions, chiral recognition, and catalysis is expected to be a major focus of future research. This research is crucial for developing nanoscale devices and molecular machinery for displays.
As the use of nanotechnology has grown, opportunities for creating fabricated chiral materials such as nanostructures and their assemblies have emerged—particularly those related to the biochemical, healthcare, and pharmaceutical fields. However, in pharmacological research, the toxicology of chemical substances remains a concern.48–50
Combining these benefits showed how comfortably graphene can be utilized in the biomedical research by overcoming challenges such as scalability, immune responsivity, and long-term effects. As shown in Fig. 3D, adding either D- or L-Phe caused yellow-green fluorescence. This implies that cells treated with TPTA-assembled GQDs (Fig. 3A) with highest occupied molecular orbital (HOMO) (Fig. 3B) and lowest unoccupied molecular orbital (LUMO) (Fig. 3C) produce more yellow-green light than cells treated with L-Phe, suggesting that they can be used to detect D-Phe inside cells using chiral science.51 Thus, the cytotoxicity of the obtained TPTA-assembled GQDs was found to be truncated, and their intracellular imaging potential was relatively high. The authors suggested that the procedure has positive environmental impacts. However, the cell viability observed in the study raises concerns about the reliability of the end product. Additionally, the authors’ argument for minimal toxicity relies solely on color identification, which is not the most precise approach for detecting cytotoxicity.
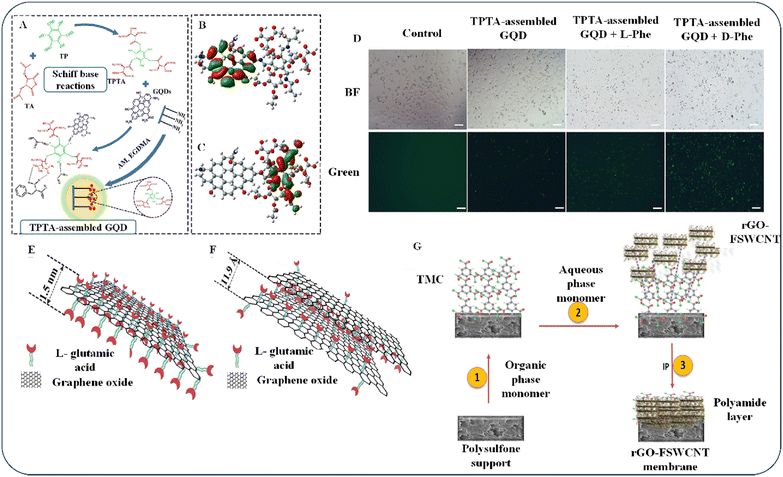 | ||
| Fig. 3 (A) D-Phe's interaction with chiral recognition elements (TPTA/GQDs). (B) HOMO and (C) LUMO diagrams of TPTA/GQDs. Degenerate orbitals (red) and unoccupied orbitals (green). (D) HeLa cells stained with TPTA/GQDs as observed by confocal fluorescence microscopy in the absence and presence of L-Phe or D-Phe. Reproduced with permission from ref. 51 Copyright 2022 Springer Nature. (E) A sandwiched framework of an individual layer of Glu-GO. (F) Glu-GO nanosheets in membranes. Reproduced with permission from ref. 35 Copyright 2017 Elsevier). (G) Mechanism of rGO-FSWCNT membranes’ IP interaction. Reproduced with permission from ref. 52 Copyright 2022 Elsevier. | ||
Chemical synthesis is not the only method for chiral synthesis; enantiomeric identification and separation is also a commonly utilized technique for the synthesis process. Meng et al. (2017) focused on achieving high flux and specificity in membrane separation and found that GO was a suitable candidate.35 They noted that functional groups composed of oxygen on the borders and basal planes of GO sheet materials offer a wide variety of ways to alter the physical and chemical properties of GO and function as spacers to make graphene planes more reactive. The authors created a sandwich structure for Glu-GO using a glutamic acid/GO membrane. They achieved this by covalently attaching glutamic acid to the surface of GO via amide formation and, through epoxide polymerization, inducing an interaction between Glu and GO (as shown in Fig. 3E). The modified Glu “space” did not fill the intersheet gallery area, because GO is not completely oxidized, while epoxide is the only oxygen-containing group (Fig. 3F). Their findings indicated that the chemical treatment of GO is beneficial for creating a larger space that allows larger molecules to permeate. This implies that chiral selector-modified GO-based membranes are a potential easy-to-use alternative to traditional membrane separation. By overcoming the limitations of a tradeoff relationship in polymer-based separation membranes, these membranes introduce a new path for enantioselective separation.
In 2022, Gogoi et al. researched carbonaceous NPs used in nanoporous membranes. They found an imbalance between their permeability and selective transport, which posed a dilemma.52 To address this, they explored using GO as a material that could strike a middle ground. The researchers used the interfacial polymerization (IP) technique to create a hybrid of rGO-functionalized single-walled carbon nanotubes (FSWCNTs) and trimesoylchloride on a polysulfone substrate (as shown in Fig. 3G). They developed an rGO-FSWCNT membrane by adding aqueous treatment solution along with polyamide to the organic phase and letting the solutions drain off the surface of the membrane. They also found that adding AAs increased the chiral membrane's size and charge selectivity. Overall, it may be possible to increase enantioselectivity when treating racemic chemicals with membranes by adding FSWCNTs to the GO sheets. Nevertheless, it is essential to discuss the shortcomings of membrane separation technology, such as chiral selection53–55 and membrane stability,56 which are crucial variables in chirality applications, even though membrane separation technology is energy-efficient and economically viable and represents one of the most intriguing techniques for industrial manufacturing.
Bai et al. recently developed a novel technique for enantiomer separation.57 They developed a thin-film nanocomposite membrane using a GO-based ethylenediamine-β-cyclodextrin (EDA-β-CD) membrane, which was synthesized through IP. To form EDA-β-CD TFNMs, they crosslinked the cellulose acetate (CA) substrate membrane with EDA-β-CD using cross-facial polymerization. The material being used was submerged in the solution of EDA-β-CD and TEA, and a 2.0-mg mL−1 benzenetricarbonyl trichloride solution was poured onto the treated substrate. The TFN membranes were then air-dried for 1 min and cured in a vented oven at 60 °C for 10 min to strengthen the targeted surface (Fig. 4A–C). The authors also performed docking simulations based on molecules to examine the chiral recognition mechanism of polymer-based membranes used for enantioseparation (Fig. 4D–G). They utilized hydrogen bonds to link receptors and ligands and to determine the binding free energy of the system. However, their explanation can be more coherent and elaborate on how bond formation is directly or indirectly related to the thermodynamic stability of membranes. It is recommended to consider additional factors such as the results of feasibility tests, the reaction mechanisms, the design and selection of materials, and the material properties before drawing conclusions regarding the strength of the membranes. The authors cited the literature without a meaningful connection to their work, making their docking result and explanation less significant.
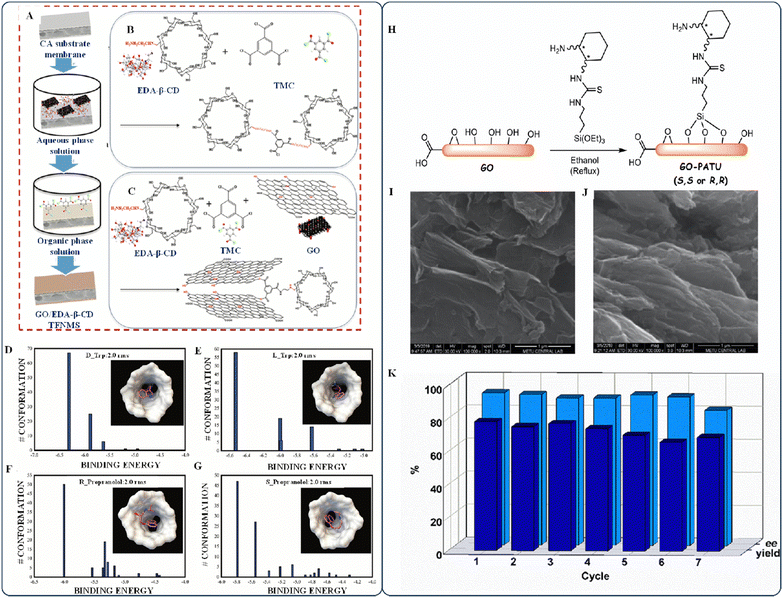 | ||
| Fig. 4 (A) EDA-β-CD TFNMs and GO/EDA-β-CD TFNMs. (B) Chemical modification of EDA-β-CD TFNMs. (C) GO/EDA-β-CD TFNMs. (D) Docking results and the optimized geometry of EDA-β-CD with D-Trp, (E) L-Trp, (F) R-Prop, and (G) S-Prop. Reproduced with permission from ref. 57, Copyright 2022 Elsevier. (H) Preparation of primary amine–thiourea-modified graphene-based C-catalysts. Scanning electron microscopy (SEM) images of (I) (S,S) GO-PATU and (J) (R R) GO-PATU. (K) Reusability experiments of (S,S) GO-PATU in the asymmetric Michael addition of isobutyraldehyde to trans-β-nitrostyrene. Reproduced with permission from ref. 58, Copyright 2022 Elsevier. | ||
Remarkably, Badr et al. proposed a different separation method that uses asymmetric carbon catalysts that are flexible, recyclable, and recoverable.58 Combining the silicon functional group in the saline with the active group containing oxygen in the GO creates a graphene-based carbon catalyst (as shown in Fig. 4H). The SEM images in Fig. 4I and J show the wrinkled nanostructure of the newly developed carbon catalyst. The authors aimed for reusability; however, their reusability experiment showed that the enantioselectivity (ee) decreased after the sixth use, while the yield steadily declined after the third run (Fig. 4K). The yield can be reduced if the chiral centers on the top layer are freed or if structural changes to the catalyst reduce the number of active sites. It remains unclear whether there is a connection between the reactions and the viability of reuse testing. The reusability test yielded somewhat random data, suggesting that a more precise test is necessary for drawing conclusions.
Regarding the aforementioned synthesis processes, several aspects can be enhanced. Such improvements include reducing the length of the synthesis process, improving the yield's dependability, considering human and environmental factors, and ensuring that the developed device and prepared materials are safe and reliable. Table 1 highlights the most widely used combination of materials for synthesis, which involves two different types of AAs (aliphatic and aromatic). The table also indicates the usefulness of their material combinations. The pharmaceutical industry recognizes the value of producing AAs for chiral synthesis and enantiomer separation. However, researchers can use organic and synthetic catalysts to create chiral molecules, which can be used to detect harmful diseases. In the past decade, advancements in biological chemistry, protein chemical terminology, and molecular engineering have provided researchers unrestricted access to various proteins and cultured microbes for chemical synthesis. As the use of AAs increases in chiral science, using catalysts to produce chiral molecules appears to be a promising approach. Researchers are also exploring simple and facile techniques such as miniemulsion polymerization59 and electrocatalytic reduction60 for synthesizing polymeric chiral NPs using AAs.
| Type | Materials used | Functions | Types of bonds | Application | Advantages | Ref. |
|---|---|---|---|---|---|---|
| Aliphatic AAs | Cobalt(II) L-glutamate (H2O)·H2O | Chiral material | Hydrogen bonds | Chiral synthesis | Chiral supramolecules may develop when bilayers interact, which leads to a network of ample chiral avenues connecting glutamate molecules, interlinking the components into an intricate but highly symmetric 3D array | 30 |
| Poly-(γ-benzyl-L-glutamate) | Alignment medium to crosslink with polymer and can also be used for strain-induced alignment. | Residual dipolar coupling | Chiral synthesis | Produces crosslinked poly-(-benzyl-L-glutamate) gels for organic solvents that allow precise residual dipolar coupling measurements and is an aligning medium based on chiral polypeptides, which can distinguish between enantiomers and has the freedom to scale up or down that crosslinked polymer gels provide | 32 | |
| Ply(γ-methyl-L-glutamate) | Membrane used for chiral separation | Covalent bonds | Chiral separation | Utilization of three different alcohols for ester swap membrane formation and enantioselective permeation behavior | 33 | |
| (1) L-Glutamic acid graphene hybrid (LGO)/D-glutamic acid graphene hybrid (DGO) | (1) Chiral properties and excellent electrochemical activity | Covalent bonds | Chiral synthesis | Electrochemical detection may benefit from the rapid electron-transfer kinetics and high signal strength of the graphene nanocomposite's chiral structure | 31 | |
| (2) 3,4-Dihydroxyphenylalanine (DOPA) enantiomers | (2) Chiral enantiomer for chiral recognition | |||||
| (1) Glutamic acid-GO nanosheets | (1) Membrane for chiral separation | Covalent bonds | Chiral separation | Regarding chiral separations, Glu-GO membranes achieve a steady, intriguing throughput orders of magnitude higher than those of typical polymer-based and inorganic membranes | 35 | |
| (2) Chiral separation material | (2) D,L-Phenylalanine | |||||
| (1) L-Glutamic acid/GO/poly (L-glutamic acid sodium) | (1) Use for chiral separation | Covalent bonds | Chiral separation | Compared with traditional membrane materials, Glu-GO/PLGA membranes have superior chiral separation capabilities, with increased selectivity coming at the cost of a negligible reduction in flux | 34 | |
| (2) D,L-Phenylalanine | (2) Chiral separation material | |||||
| Pristine graphene/graphene oxide/amino acids | Amino acids interact with graphene derivatives, and the interaction depends on the graphene surface oxygen density | Hydrogen bonds | Biomolecular system design and optimization for biological detection and therapeutic delivery. | Hydrophobic interactions of aliphatic side chains are crucial to understanding how aliphatic molecules interact with the graphene sheet | 36 | |
| Poly(benzimidazole-amide), chiral diacid, aromatic diamine, tetra-n-butylammonium bromide | Used as chiral synthesis materials | Covalent and hydrogen bonds | Synthesis of chiral poly(benzimidazole-amide) | Chiral green and economical process for the synthesis of GO/PBIA nanocomposites, which have excellent thermal stability | 38 | |
| Graphene/BOC-L-alanine | Used as a catalyst for direct asymmetric aldol reactions | Covalent bonds | Synthesis of chiral heterogeneous catalysts | As excellent chiral organo-catalysts, amino acids covalently adsorbed on graphene catalyze an immediate asymmetric aldol process with high selectivity | 61 | |
| (1) Graphene/CNT, aliphatic amino acids | (1) Amino-acid interaction with graphene/CNT | Covalent bonds | Identifying the aromaphilicity index of 20 proteinogenic amino acids | Aromaphilicity index is calculated from the free energy of an amino acid binding to graphene. This indicator can reveal the stability and location of protein and polypeptide binding on heterocyclic carbon surfaces, which control the creation of protein adsorption layers | 39 | |
| (2) Arginine | (2) Form bonds with DNA | |||||
| (1) Phenylalanine/GO | (1) Used in chiral synthesis | Covalent bonds | Synthesis of chiral material | Strong interfacial bonding, wide dispersion, and strong flame-resistant capabilities are believed to cause significant improvements in thermal processing, conductivity, and structural performance | 37 | |
| (2) 3,3′,4,4′-Benzophenone tetracarboxylic dianhydride | (2) Chiral material | |||||
| (1) Tyrosine | (1) As a chiral source | Covalent bonds | Electrochemical biosensor for glucose detection | Using aliphatic amino acids, chiral sources can impart the handedness of carbon spots via surface modification. Thermal polymerization of citric acid and chiral AA yielded chiral CDs, and changing the reaction temperature altered the handedness of the CDs | 40 | |
| (2) Citric acid/(L-/D-Tyr) | (2) To form chiral carbon dots | |||||
| (3) GO-chiral carbon dots enzyme | (3) For glucose detection | |||||
| Aromatic AAs | (1) Black phosphorus/graphene nanocomposite | (1) Used as an active Raman substrate in the chiral discrimination of amino acids | Covalent bonds | Discrimination of enantiomers | The authors presented a technique that uses the complementary photo-electronic properties of a pair of diodes to amplify the Raman signal asymmetrically. Using black phosphorus/graphene, it may be possible to perform chiral screening of amino acids and discriminate other substances, including isomeric versions of proteins | 47 |
| (2) Amino acids: phenylalanine; 3,4-dihydroxyphenylalanine; tryptophan; leucine; and isoleucine | 2. For chiral discrimination | |||||
| (1) Amyloid β-sheet segments/graphene flakes | (1) Sample combination for DFT calculations | Hydrogen bonds | Energy assessments of interconnected structures using amyloid-sheet/graphene flake models | According to the authors, the amyloid β-sheets exhibited significant interactions with graphene in the computational modeling of amyloid/graphene aggregates. They found that the chemical reactions between graphene and amyloids are stronger than those between amyloid β-sheets | 43 | |
| (2) β-Amyloid peptide | (2) Peptide that causes Alzheimer's disease | |||||
| (1) Anthracene-labeled amylose/rGO (reduced graphene oxide)/amino acid | 1. Chiral sensing system | Hydrogen bonds | Chiral sensor to recognize a tryptophan enantiomer | The authors claim that their colorimetric and electrochemical sensors have a detection sensitivity for L-tryptophan that is >100 times higher than those of previously reported sensors | 41 | |
| (2) Tryptophan | (2) Enantiomer to be recognized | |||||
| (1) Thionine–graphene, dsDNA (double stranded DNA) | (1) Chiral platform for tryptophan enantiomer sensing | Covalent bonds | Chiral biosensor for tryptophan | As a nano-bionic interface, thionine/graphene/dsDNA provides a large amount of electron transfer to the chiral substrate for detecting enantiomers in the presence of Cu(II) | 42 | |
| (2) Tryptophan | (2) Harmful protein | |||||
| (1) Ferrocene/GO | (1) Accelerate intermolecular charge transfer | Hydrogen bonds | Chiral recognition | Graphene intercalates, exfoliates, and accelerates intermolecular charge transport when ferrocene is noncovalently stacked on GO | 44 | |
| (2) L-Phe and D-Phe | (2) Used in aqueous solution for electrochemical sensing | |||||
| (3) Phenylalanine | (3) For chiral recognition | |||||
| (1) N-doped graphene-CNT | (1) Substrate material | Hydrogen bonds | Chiral sensor for Trp (Tryptophan) enantiomers | Because the two distinct diastereoisomeric enantiomer–selector combinations formed have different degrees of steric hindrance, L-Trp is more easily dissociated from the electrode alteration layer and transported nearer to the electrode surface, leading to a stronger electrochemical signal | 45 | |
| (2) N-(3-dimethyl aminopropyl)-N-ethyl carbodiimide hydrochloride, N-hydroxy succinimide | (2) Use in the preparation of chiral selector | |||||
| (3) L-tryptophan, D-tryptophan | (3) Chiral recognition | |||||
| (4) Sodium alginate, chitosan | (4) Chiral selector | |||||
| (1) Permethylated-β-cyclodextrin | (1) Recognition function | Covalent bonds | Amino-acid detection and chiral recognition | Real-time, electrical identification of amino acids with varying structures and chirality using a single molecule. The system works in either an acidic or alkaline environment, making it a versatile resource for identifying crucial chemicals in ecological or biological systems and gaining deeper knowledge of biological processes | 62 | |
| (2) single-layer graphene | (2) Electrode substrate | |||||
| (3) Graphene-molecule-graphene single-molecule junctions | (3) Chiral detection | |||||
| (1) Nitrogen-doped GQD | (1) Organocatalyst | Hydrogen bonds | To synthesize chiral 2-amino-4H-chromenes using multicomponent reactions | L-proline/N-GQDs/CuO/ZnO composite nanotechnology is an innovative recoverable heterogeneous catalyst; it is now possible to synthesize chromenes and their derivatives in a single step | 46 | |
| (2) L-proline | (2) Chiral molecule | |||||
| (3) CuO/ZnO/N-GQDs/L-proline | (3) Nanocatalyst | |||||
| (1) 1,3,5 triformylphloroglucinol-functionalized chiral (+)-diacetyl-L-tartaric anhydride/GQD | (1) Chiral recognition element | Hydrogen bonds | D-Phenylalanine detection | Detection of D-phenylalanine in HeLa cells using TPTA-GQDs and chiroptical imaging with a smartphone indicated low toxicity and high specificity | 51 | |
| (2) D-Phenylalanine (D-Phe) | (2) Biomarker for diseases | |||||
3. Different forms of chiral graphene
3.1. Chiral nanoribbons
Owing to its distinctive physical features, graphene has excellent electronic properties including a high charge-carrier mobility. In 1996, Fujita et al. reported graphene ribbons as a theoretical framework to study the effects of graphene's boundaries and nanoscale dimensions.63 By creating nanometer-wide strips of graphene, which are also known as graphene nanoribbons (GNRs), it is possible to manipulate graphene's electrical characteristics. GNRs can have a wide variety of electrical structural patterns, which are achieved by varying the dimensions and crystallographic orientations of the edges. Zigzag GNRs have non-bonding edge states that exhibit a different type of magnetic ordering from the electronic gaps in the bands of GNRs that cut across the armchair lattice direction.64–67 These properties vary significantly depending on the nanoribbon width. Despite their potential, the widespread use of GNRs in electrical applications faces several materials science hurdles. These include the need for homogenous dielectric conditions, encapsulation, and low contact resistances at metal–GNR interfaces. Numerous factors influence the application of nanoribbons to electronic devices. The implications of GNRs for the electronic applications of chiral graphene are presented below.• In GNR electron transport, heating effects dominate ambient temperature effects that can surmount the transport barrier under high-bias conduction.68
• Whether a nanoribbon can be employed in electronic devices is determined by its bandgap—an essential property of all electronic materials.69
• Doping is considered a crucial factor as the bandgap of GNRs can influence the electronic properties of the system.70
Oteyza et al. studied chiral GNRs on Au(111), Ag(111), and Cu(111).71 They discovered that using monomers to grow selective and atomically precise GNRs on the Au(111), Ag(111), and Cu(111) substrates substantially increases the length of GNRs and reduces the required temperature for the process. In addition, Merino-Dez et al. extensively examined the electrical and structural properties of chiral GNRs on Au(111) to better understand their behavior in future electronic devices. They explored the construction of heterostructures with complementary GNRs and achieved GNRs with a bandgap of 0.67 ± 0.06 eV, an effective mass of ∼0.35 m0, and energy-level alignment.72 However, researchers continue to face challenges related to stability and potential defects such as vacancies,73 lines,74 and lattice defects,75 on nanoribbons when exposed to other metallic materials.
Scanning tunneling microscopy (STM) is a useful method for exploring how band engineering connects chiral edges and defect structures and detecting issues in a system. It also aids in comprehending the impact of spin interactions. Berdonces-Layunta et al. discovered how oxygen exposure affects the electronic structure of nanoribbons.76Fig. 5A and B show an open-shell resonant system with two Clar sextets in each unit cell and two radicals on the C atoms in the middle of the zigzag segments. This structure affects the stability and reactivity of aromatic hydrocarbons. When oxygen hits one side, it forms ketones and successive Clar sextets, while the nature of the radical on the other side changes, making it more responsive and easier to interact with an outside agent. Although initially counterintuitive after O2 exposure, the number of defects (type II) is doubled with hydrogenated sp3 carbon atoms. In the nc-AFM image (Fig. 5C and D, center), the two flaws are more visible. Hydrogenated sp3 C atoms can be easily dehydrogenated through tip-induced manipulation; however, changing ketone-linked C atoms under controlled conditions is impossible. Owing to oxidation on one side of the ribbon, C atoms are doubly hydrogenated, stabilizing the facing radicals previously produced on neighboring stripes. However, the STM image (Fig. 5C and D, right) shows no noticeable shift in the nc-AFM contrast. The controlled low oxygen pressures may be a lower limit for ribbon harshness compared with a conventional processing and transfer operation during GNR-based device fabrication. Although the stripes are generally closed shells in nature, the investigated zigzag edge segments exhibit only a moderate degree of stability (Fig. 5E). There are still many questions about the oxidizing organisms and resulting products that must be distinguished, and it is unclear whether unidentified atmospheric contaminants during the annealing process or naturally occurring oxidation at room temperature are the causes. While a case-by-case investigation may be necessary, it is plausible that many additional graphene nanostructures that exhibit zigzag edges have low strength, requiring a rethinking of strategies for their application in practical devices.
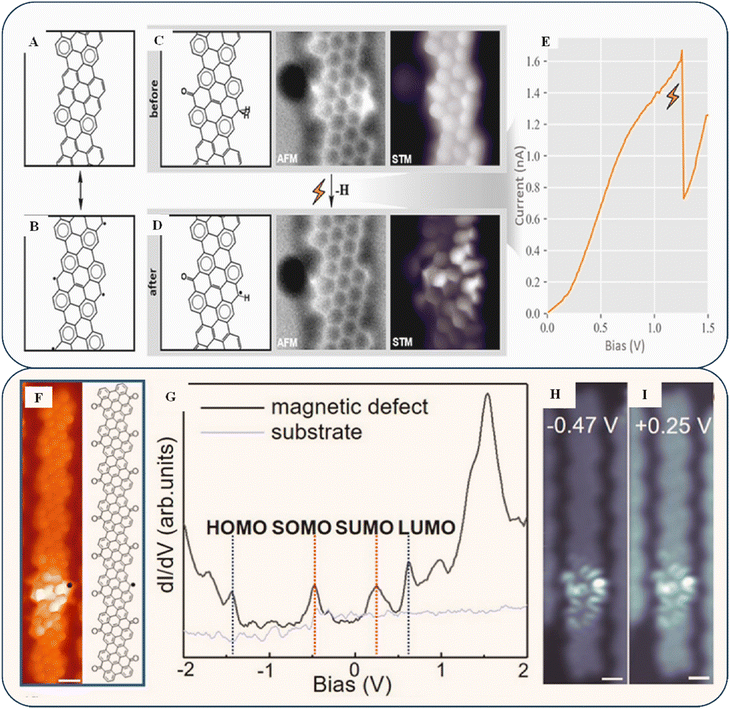 | ||
| Fig. 5 (A) Closed-shell GNRs, i.e., one of the resonant elements. (B) Their open-shell counterparts have more Clar sextets. (C) nc-AFM and BR-STM images show the chemical structure of a damaged unit cell including a ketone and a doubly hydrogenated C atom. (D) Identical framework after tip-induced dehydrogenation. (E) Discontinuity in the double-hydrogenated C atom's bias ramp due to its dehydrogenation process. Reproduced with permission from ref. 76 Copyright 2021 American Chemical Society. (F) BR-STM image of a K-chGNR with a single defect. (G) dI/dV spectra at the identical position (black line) and the bare Au (111) substrate (grey line). (H) Constant-height dI/dV maps recorded at V = −0.47 V, Vrms = 20 mV. (I) Constant-height dI/dV maps recorded at V = 0.25 V, Vrms = 20 mV. Reproduced with permission under a Creative Commons CC-BY license from ref. 77 Copyright 2022 American Chemical Society. | ||
Other methods involve forming metallic edge bands, which can transform the ribbons into topological structures.78 The production of chiral GNRs is a new way to make graphene ribbons with metallic edge bands, which can then be converted into topological states in graphene derivatives (1D and 2D nanoribbons) that are suitable for a wide range of potential future applications. Recently, Wang et al. reported two types of chiral GNRs: one with ketone functionalization at its edges and another without host radical pairs. These two types have significantly different shapes and interact magnetically.77 In Fig. 5F, a CO-functionalized probe exhibiting a single defect, and its chemical structure are shown. When a low bias is used in BR-STM, the unit cell without a ketone exhibits better contrast, indicating the presence of low-energy states. Fig. 5H and I display the differential conductance band (dI/dV) at the location indicated in Fig. 5G. Because of the smaller bandgap, radical states become more hybridized with the molecular orbitals at the border. Thus, they disperse more extensively into neighboring unit cells, leading to a smaller Coulomb gap. When the two radicals combine, they can either form a closed-shell neutral state with a double-occupied HOMO or remain in an open-shell neutral shape with the accompanying SOMOs/SUMOs (singly occupied/unoccupied molecular orbitals). This depends on whether the energy of the hybridization is lower than the energy of the Coulomb repulsion among the electrons in each shell. As discussed previously, GNR topologies can be precisely modified using several methods; nevertheless, they have not been used in practice for protecting GNRs. Previous studies have indicated that the width of GNRs affects their electrical characteristics. Despite this, it remains challenging to create heterostructures with chirality-controllable GNRs.
3.2. Chiral tunneling
Chiral tunneling is one of the primary phenomena determining graphene's unique electronic characteristics. Klein/chiral tunneling preserves the intense mobility of charge carriers despite unavoidable homogeneities given potential applications.79,80 The chirality of charge transport in a graphene sheet guarantees faultless tunneling through the material for electrons that strike a boundary in the expected direction.81 To realize the optimal conditions for chiral tunneling, the electrostatic field must be tuned so that the redistribution of the electron charge concentration does not affect how Dirac electrons move.82In 2013, He et al. proposed preventing field-effect transistors produced from graphene single-layer structures from exhibiting conductivity characteristics when turned off. The ideal chiral tunneling in a single graphene layer precludes the creation of conventional semiconductor devices.83 Therefore, it is apparent to seek a solution where chiral tunneling influences the properties of graphene to develop an application that is both effective and efficient. Accordingly, researchers have identified bilayer graphene (BLG) as a candidate for chiral tunneling.84–86 When a pair of carbon atoms, or graphene layers, are stacked, a gap can be created and regulated with relative ease by using an electric field from the outside. In contrast to the linear energy range of single-layer graphene (SLG), the elliptic energy spectrum of BLG structures consists of four distinct energy levels, providing a better tunneling ability.87 In 2014, Gorbachev et al. reported that a graphene particle was discovered on a hexagonal boron nitrate substrate, resulting in an imbalance of atomic sites.88 The identical phenomenon was observed in a geometry akin to BLG. A non-local impedance was identified, and its ability to withstand elevated temperatures is desirable for potential quantum-computing applications requiring non-dissipative currents.
Varlet et al. demonstrated that certain “magic angles” (as shown in Fig. 6A) exist at which the transmission rate increases abruptly to 1 for small incidence angles,89 which results in a lower resultant conductivity than SLG. However, electrostatic shields in BLG are effective at keeping carriers inside (as shown in Fig. 6B and C). Iurov et al. and Jois et al. reported the possibility of creating electronic devices with valuable features that depend significantly on the tunneling conductivity, the effect of the electric current on electron doping, and subsequent adjustment by irradiation.89,90 When three or more graphene layers are twisted, they form long-wavelength moiré patterns (as shown in Fig. 6D and E). Moiré pattern systems have attracted attention for experimentally observing various abnormal and highly correlated phases, and they provide a valuable testing ground for these phenomena.91
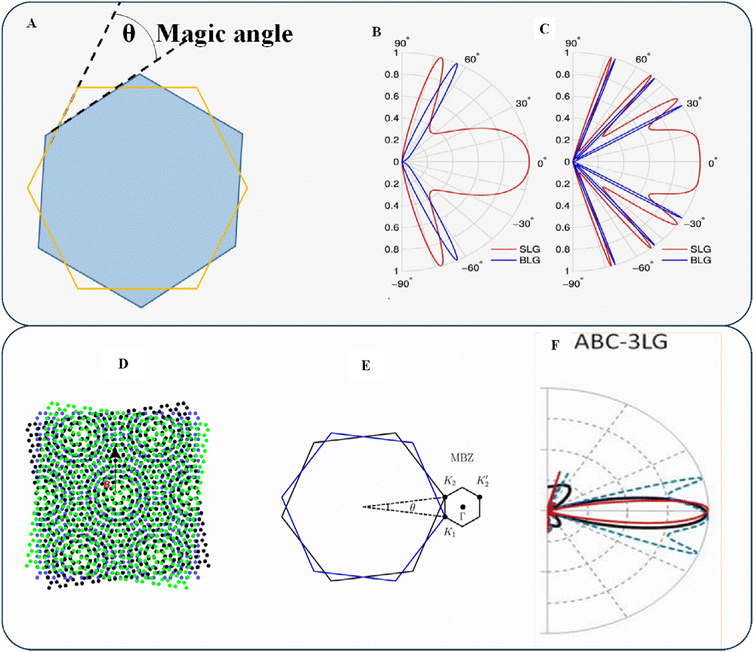 | ||
| Fig. 6 (A) Twisted layer indicating the magic angle. (B) Calculated transmission probability T as a function of the incidence angle for SLG (red) and BLG (blue) with potential barrier thicknesses. (C) Density values represent the SLG and BLG incident energies and barrier heights. Reproduced with permission from ref. 89 Copyright 2015 Wiley-VCH. (D) Moiré pattern of twisted trilayer graphene. (E) Moiré Brillouin zone (MBZ). Reproduced with permission from ref. 91 Copyright 2023 Elsevier. (F) Transmission (T) for ABC-3LG as a function of the electron incidence angle θ. Reproduced with permission from ref. 92 Copyright 2012 American Institute of Physics. | ||
Kumar et al. demonstrated that ABC-3LG (as shown in Fig. 6F) has a pair of restricted angles (θ = ±30°) and that most transmission occurs at angles of |θ| < 30°, making trilayer graphene an effective electron collimator.92 This helps prevent excessive tissue exposure outside the region of interest, minimizing irradiation loss and undue exposure risk. While stacking numerous graphene layers is the easiest way to alter the band structure of graphene, its chiral tunneling transport property is one of its most intriguing and unexpected features. Despite its potential use in reducing energy consumption, this phenomenon has a significant drawback: it makes electrons difficult to contain. Field-effect transistors (FETs) and other switching devices are fundamental to modern electronics, but their implementation has yet to be improved by graphene. However, new techniques have allowed researchers to control the electrical characteristics of layered materials without altering their atomic structures. The simplest method is to bend consecutive layers around each other and change the conductivity of the system.
3.3. Chiral dichroism
Circular dichroism (CD) is a spectroscopic method for measuring the variance in absorbance between left- and right-circularly polarized radiation.93 It is beneficial for analyzing the structures of materials, including secondary systems, and identifying molecule interactions.94 Researchers must create flat, thin crystal structures to incorporate circular polarizers into nanodevices. However, these systems show low wavelength CD spectra because atomic thin films are inherently achiral, making structural aspects predominantly difficult.95–97 Owing to surface plasmonic resonance, CD signals from chiral metal nanostructures are typically more robust than those from chiral biomolecules. These highly chiral metal nanostructures are widely used in biosensing.98,99Recently, Deng et al. proposed a method of triggering the CD effect in an achiral V-shaped metallic nanostructure using a graphene grating.100 They found that the induced CD effect has certain limitations in applications. Specifically, it can only be utilized in a particular type of nanostructure, owing to the difficulty of adjusting the structure to fit a chiral nanostructure. While symmetry breaking can achieve the CD effect, it modifies the optical properties of the original system. Additionally, chiral nanostructures are challenging to fabricate and are the only source for all CDs. Thus, limiting the ocular mode by changing the refractive index using an electric field to customize CD effects quickly and efficiently is recommended. To analyze the transmission and CD spectra of a mid-infrared achiral nanostructure, the researchers employed COMSOL Multiphysics. Fig. 7B depicts a unit cell containing a V-shaped metal nanostructure (G-MV), which is an elemental component. The visual appearance of the G-MV system in typical artificial CD chiral nanostructures can be changed by altering the design, adjusting parameters, or altering the Fermi energy. However, in this scenario, merely altering the Fermi energy of graphene changes the visual aspect of the G-MV system.
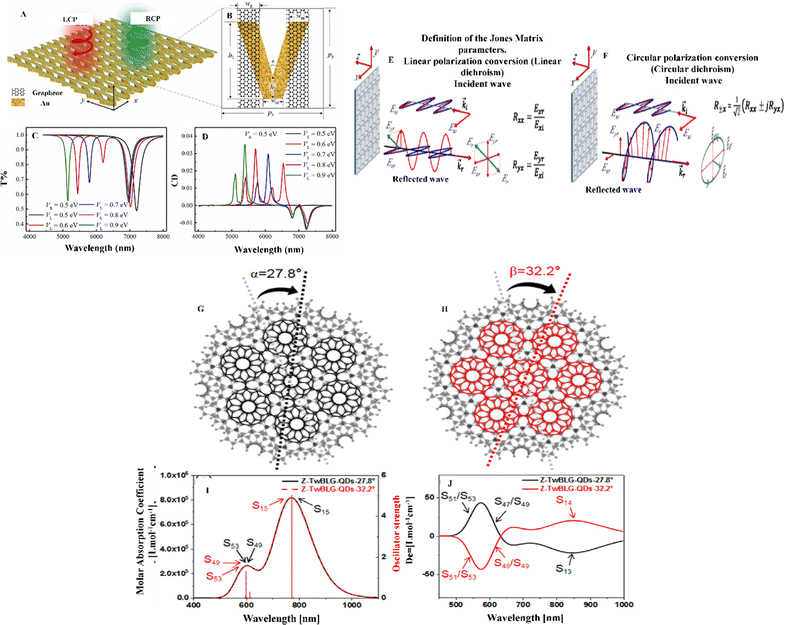 | ||
| Fig. 7 (A) Periodic array of graphene gratings with V-shaped metal nanostructures (G-MV). (B) Unit cell of one of the G-MVs and its parameters. Its open-shell counterpart exhibits a larger quantity of Clar sextets. (C) Transmission spectrum of a single graphene grating with different VL values and a constant VR value. (D) CD spectrum of a G-MV system with different VL values and a constant VR value. Bias gradient on the twofold hydrogenated C atom illustrating the discontinuity resulting from the dehydrogenation process. Reproduced with permission from ref. 100 Copyright 2022 Elsevier. (E) Jones matrix coefficients of reflection with linear polarization, defined for an x-polarized incident wave. (F) Two circularly polarized waves revolving in opposing directions combine to form the reflected wave. Reproduced with permission from ref. 101 Copyright 2020 American Physical Society. (G) Molecular structures of Z-TwBLG-QDs-27.8° and (H) Z-TwBLG-QDs-32.2°. (I) Graphical representation of the UV-vis spectra of Z-TwBLG-QDs-27.8° and Z-TwBLG-QDs-32.2°. (j) Electronic circular dichroism (ECD) spectra of Z-TwBLG-QDs-27.8° and Z-TwBLG-QDs-32.2°. Reproduced with permission under a Creative Commons CC-BY license from ref. 102 Copyright 2022 MDPI. | ||
Fig. 7A shows how the transmission of a graphene grating changes for left circularly polarized (LCP) and right circularly polarized (RCP) incident light as VL (Fermi energy) changes while VR remains constant. When the negative interactions between the left and right graphene gratings (GL and GR) become stronger, the Fermi energy gap between the gratings narrows, causing a slight redshift in the second dip generated by the electric dipoles on GR (Fig. 7C). When VL is equal to VR, the electric dipole length is proportional to the wavelength, making the electron resonance's redshift (black line) on GL and GR simple. As the two transmission dips are adjusted by changing VL, the CD patterns (Mode-GMV1 and Mode-GMV2) also exhibit a redshift as VL decreases, as shown in Fig. 7D. However, the CD modes (Mode-GMV3 and Mode-GMV4) resulting from the second transmission dip are unavoidable due to interactions between the graphene grating and small metal structures. The authors developed a versatile method for generating and manipulating CD effects for polarization detectors and biosensing applications. The proposed approach is intended for use in biosensor applications; however, as the metal structure reacts with graphene, cytotoxicity must be considered along with the design structure.
In 2017, Kong et al. discovered that multiple graphene nanodisks assembled into chiral graphene can provide powerful CD in the mid-infrared range. These graphene-based chiral structures’ CD properties can be precisely altered using electrical or finely tuned chemical doping.103 Graphene plasmons are sensitive to chemicals that adhere to graphene, making chiral devices ideal for environmental monitoring and surveillance.
The configuration of nanostructures, the direction of incoming light, and the chirality of the surrounding medium can all help to explain the relationship between asymmetries and the CD effect.104 Understanding the CD mechanism and improving the CD are now possible owing to this research. However, improving the CD in pure-plane multilayer nanostructures is a new challenge.
When a linearly polarizing electric field is incident on a graphene metascreen, the reflection spectrum transitions from linear polarization to circular polarization, as discovered by Amen et al. in 2020 using a Jones matrix analysis.101 The two techniques for converting an x-directed incidence are displayed in Fig. 7E and F. It is reasonable to suppose that the electric-field components along the x- and y-axis, i.e., Exi and Eyi, respectively, are incident and that those along the z-axis, i.e., Exr and Eyr, are reflected in the general case. The following equation describes the Jones matrix:
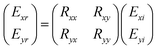 | (1) |
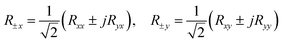 | (2) |
The following equation expresses the transformation of linearly polarizing waves penetrating elliptically polarized reflected waves in the Jones calculus:
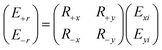 | (3) |
Here, the subscript ±x denotes the right- and left-handed elements of the reflected waves. From eqn (2) and (3), it is clear that a completely circularly polarized reflection wave can be obtained only if the x and y components exhibit equivalent readings. It is essential to understand how the transformation of polarization phases depends on the range of reflections spanning from purely linear dichroic behavior to circularly dichroic behavior, which depends on the tunable response to the frequency of the chiral metascreen derived from graphene.
Furthermore, to create chiral structures using graphene, researchers alter the structural parameters and reconfigure frameworks to adjust the CD impacts of systems. According to Wang et al., the CD impulses can be dynamically modified by altering the Fermi energies of various graphene bilayer split ring (BSR) components.105 Dynamic calibration of the CD pattern indicates that the BSR chirality changes when the CD signal flips from affirmative to adverse or vice versa. Chiral structural formation presents numerous challenges, such as the difficulty of visually observing chiral structures in organic substances and the rotating motion of the polarization plane hindering the development of chiral metamaterials. However, chirality can be accurately determined by evaluating the CD. Owing to the restricted range of the conductivity of most metals, the CD is present only in a confined electromagnetic frequency range and is difficult to control. Therefore, an adaptable regulator of CD is needed, and precise control of CD is expected to be achieved by designing a metasurface with a unique structure and an appropriate material. Many studies have been performed on the tunable CD structure over the past decade, and most focused on the design of tunable metasurfaces,106–111 tunable chemical potential,112etc.
Likewise, Dai et al. presented a method for adjusting the spacing between two graphene nanosheets by manipulating the van der Waals forces between the layers.102 The center of the graphene nanosheet was used as the origin, and a specific “magic angle” was used to rotate the top of the graphene nanosheet. Fig. 7G and H show periodic complementarity when the contorted orientations are 27.8° and 32.2°. The ultraviolet-visible (UV-vis) spectroscopy analysis indicates that Z-TwBLG-QDs-27.8°/32.2° coincide, as shown in Fig. 7I. The primary absorption peak of Z-TwBLG-QDs-32.2° is at approximately 772.4 nm and is caused by a pair of high states of excitation (S15 and S16).
By contrast, the secondary absorption peak is caused by a couple of excited states (S49 and S53). At a twisted angle of 27.8°, the S47/S49 area of the ECD spectrum (Fig. 7J) exhibits high rotational activity, whereas at 32.2°, the S48/S49 region does so. The molar absorption coefficients of Z-TwBLG-QDs-27.8°/32.2° exhibit an axisymmetric relationship owing to the symmetry of the rotatory resilience, suggesting that the molecular system has symmetric CD. The main objective was to evaluate the tangible mechanism of molecular chirality in superlattices. Additionally, the researchers investigated the antisymmetric chirality of zigzag-edge TwBLG-QDs through the statistical topology of the electron density and periodic complementary twist angles. By varying the barrier height and energy, they observed a change in the chirality of twisted BLG from complete tunnel reflection to partial reflection. However, more information will be necessary to understand the method to alter the twisting angle, which affects the chirality of BLG, particularly in the case of CD.
Patwari et al. introduced a non-bonding interaction between GO and microscopic molecules that can be studied using vibrational CD.113 Researchers have numerous opportunities to study CD despite its limitations, such as proteins embedded in membranes, which can significantly scatter the CD spectra during analysis. It is physically unfeasible for a molecule to react differently to the spatial aspects of light waves according to chirality, such as whether the wave shape is right- or left-handed. Nonetheless, CD is useful for identifying the secondary structures of proteins and can aid in estimating protein conformation in biomedical applications.
4. Applications of chiral graphene materials
The use of chiral materials has become a fascinating research area. Advancements in nanoscale chirality have been made by incorporating molecular chirality into nanoscale building blocks, giving rise to valuable applications. Recent developments in this field include the creation of chiral electromotive detectors, photodetectors, and sensors for biological purposes. Medicinal systems for drug delivery have also been developed, and directions for future research have been proposed.4.1. Chiral graphene separation
Chiral membrane separation can be achieved by utilizing the inherent susceptibility of the membrane to enantiomers.114 Enantiomers are structural isomers with the same chemical and physical properties. However, in the creation and development of chiral compounds, they often exist as a single enantiomer to maintain purity, as different enantiomers can have distinct physiological effects on living beings.115–117 The agricultural and nutritional supplement industries increasingly demand pure compounds with specific enantiomeric compositions.10 Homochiral materials are molecular building blocks of identical enantiomers and are crucial for chiral separation procedures. They possess chiral receptors that selectively interact with specific enantiomers, allowing for their recognition and separation. In recent years, significant research has been conducted on homochirality using chiral polymeric materials (CPMs) and chiral metal–organic frameworks (CMOFs) for chiral recognition and separation. These materials have numerous advantages, such as large specific surface areas, distinct pore dimensions, the ability to adjust homochiral pores/cavities, convenient functionalization, and diverse structural characteristics.118–120 Therefore, it is essential to develop an interest in designing and fabricating chiral separation materials such as chiral polymer materials (CPMs) and chiral metalorganic frameworks (CMOFs) to achieve successful chiral separation. However, these materials may be challenging to regulate because of external variables, and their use can lead to contamination.121,122 The exceptional mechanical capabilities, large specific surface area, and unique layered nanostructure of graphene materials differentiate them. They can transport substantial volumes of extraneous substances owing to their low mass-transfer barrier and accessible exposed surfaces. Graphene has various potential applications as a separation, adsorption, and support material.123–128 The science of enantiomer separation can significantly benefit from the use of chiral graphene materials. Therefore, a comprehensive and well-organized review of the studies that have been conducted on chiral graphene compounds for enantiomer separation is needed.129Enantiomeric compounds have presented significant scientific and technical challenges—particularly in the case of pharmaceuticals with identical chemical structures but specific biological functions. Separating and resolving two enantiomers efficiently and cost-effectively is a significant challenge. Incorporating a chiral component into graphene materials might potentially result in the emergence of high-performance chiral separation membranes, as graphene films have a more moderate effect owing to their structure and stacking characteristics.
In 2021, Li et al. created GO-based membranes to separate racemates using β-CD, which had a substantially higher enantioselective affinity to the L-enantiomer than to the D-enantiomer, leading to a slowed transport mechanism and extraordinary enantioselectivities, with the enantiomeric excess value being close to 100% in the β-cyclodextrin graphene oxide membrane (β-CD-GOM).133 Because of the significant differences in how β-cyclodextrin (β-CD) interacts with two opposite enantiomers, combined with the gaps between adjacent GOs, β-CD can make tight and appropriate contact with enantiomers, assisting chiral recognition communication.
Despite the wealth of information on membrane separation, many questions need to be answered. For example, it is not easy to control the synthesis of CPMs. Meanwhile, materials based on metalorganic frameworks (MOFs) are effective at separating molecules, but they are not acid-resistant and can be structurally unstable in acidic environments. Thus, scientists are exploring new materials to create membranes that can selectively transport enantiomer molecules and achieve effective chiral separation.
Liu et al. embedded a protein onto the GO substrate.134 They speculated that protein compounds can serve a comparable function in chiral separation membranes with their distinct forms (primary, secondary, and tertiary). By implanting bovine serum albumin (BSA)—a chiral selector—onto a GO sheet with multiple hydrophobic bonding sites (as shown in Fig. 8A), they assessed the enantioseparation efficacy of a membrane for D,L-Phe (as shown in Fig. 8B). The GO-BSA membrane was ready for use after brief vacuum filter cleaning, and the results indicated that it could separate the D and L-Phe enantiomers with an accuracy of ∼96%. Molecular docking results indicated that L-Phe and D-Phe attached to BSA in slightly different ways and with different strengths. Comparing BSA and L-Phe (Fig. 8C) with D-Phe (Fig. 8D) revealed that the binding side and nature of intermolecular forces differed. The interaction of the chiral pathways created by BSA with functional groups on a membrane is believed to be the primary way chiral substances are separated. Molecular docking simulations support this idea. Functional groups within proteins interact through electrostatic, hydrophobic, and hydrogen-bonding interactions. An appropriate chiral molecule is needed to create a chiral pathway between membranes for the specific identification and efficient separation of chiral molecules. The size of the tracks in the membrane can be regulated to control the percentage of chiral particles associated with inactive transit and make selected active transport work better through sieving and intermolecular forces. The author claims that BSA's chiral channel structure on a membrane and its interaction with racemates are critical factors in the stereoselectivity required to separate chiral substances. Additionally, one of the most common functional-group interactions is chemical interaction, which is significantly affected by the protein microstructure. However, a few points need to be discussed thoroughly.
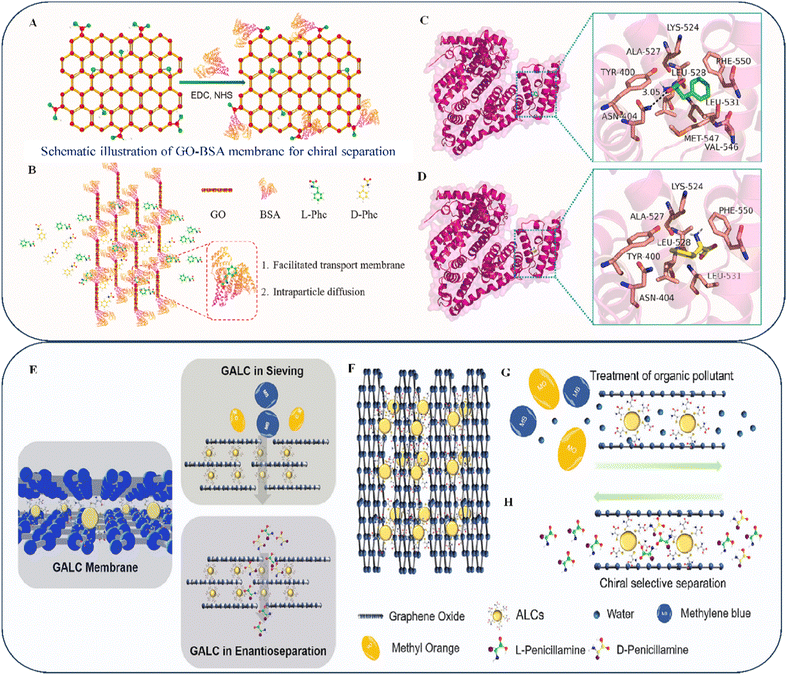 | ||
| Fig. 8 (A) GO-BSA composite reaction. (B) Using GO-BSA membranes, we can selectively isolate Phe enantiomers. The solute travels in a left-to-right path of penetration. Molecular docking (MD) results for BSA (pink) with (C) L-Phe (green) and (D) D-Phe (yellow). Reproduced with permission from ref. 134 Copyright 2023 Elsevier. (E) GALC membranes used for filtration and concentration measurement. (F) Intelligent control of interlayer spacing via pH-responsive amphoteric NP intercalation. (G) Techniques for the separation of organic dyes using GALC membranes. (H) Effect of enantioselective permeation on GALC membranes. Reproduced with permission from ref. 135 Copyright 2023 Elsevier. | ||
• Appropriate proteins must be selected to function as chiral selectors in chiral membrane separation.
• The intermolecular forces required for the separation process and the size of the channel needed for transporting molecules must be determined.
• Chiral membrane separation needs an efficient transport system to separate a membrane; thus, a transport mechanism must be selected.
In addition, Liu et al. found that by adjusting the pH level (as shown in Fig. 8F), the electrostatic forces associated with negatively charged GO nanosheets could be modulated, creating stable and adjustable channels in a membrane (as shown in Fig. 8E).135 The GALC membrane efficiently rejects molecular dyes (Fig. 8G) with variable absorption rates and different charges. Additionally, the membrane has chiral separation properties owing to the unique chiral detection function performed by L-Cys on Au nanoparticles as shown in Fig. 8H. The results indicated that L-Pen always experienced more significant changes than D-Pen, regardless of the membrane thickness, because of the increase in hydrodynamic susceptibility associated with a thicker membrane. This suggests that L- and D-Pen connect to L-Cys to varying degrees, resulting in the preferential movement of L-compounds across the GALC membrane compared with D-enantiomers. The nanochannels exhibiting chiral selection features increased with the film thickness, improving the enantioseparation effectiveness of the membrane. As the feed contact increased, the enantiomer flow increased, exhibiting ideal penetration behavior. However, as the volume of the substance increased, the separation factor decreased, because more molecules participated in the indirect diffusion process as the volume of the material increased.
According to the authors, the pH level affects the separation efficiency by influencing the attraction generated by chiral selection and racemic molecules. Altering the pH level can affect the Π–Π interaction of graphene. In future studies, the size variation of molecules and electrostatic effects, which may affect the critical factor of separation, should be considered. The use of 2D membrane substances can be a straightforward and successful method for achieving chiral separation—particularly in ecological management and pharmaceuticals.
In 2020, Meng et al. discovered that the enantioseparation of GO-based membranes was sensitive to the strength of the interactions between chiral selectors and probes.138 They investigated whether weaker contacts could boost enantioselectivity and synthesized GO sheets modified with L-Phe.
Membrane separation primarily concentrates on two key aspects of chiral separation. First, several chiral molecules can easily functionalize graphene and GO materials. Second, graphene has a high throughput due to its structure and active sites. However, in the case of adsorption separation, the presence of oxygen functionalities indicates the possibility of modification of GO using doping or covalent immobilization, which creates new opportunities for designing advanced adsorbents with an excellent capacity for adsorption and selectivity for heavy metals.
Fig. 9B–F from the paper of Liu et al. show that the GO-Cys membrane contained elements such as C, O, S, and N. This confirmed that cysteine was successfully added to the GO nanosheets.139 Energy-dispersive X-ray spectroscopy (EDS) mapping indicated that N and S atoms were well distributed in the system, confirming that cysteine modified the GO surface. However, any difference found in the absorption could have been due to the residual sulfates that were washed with sulfuric acid. The SEM mapping (Fig. 9A) supports these findings.
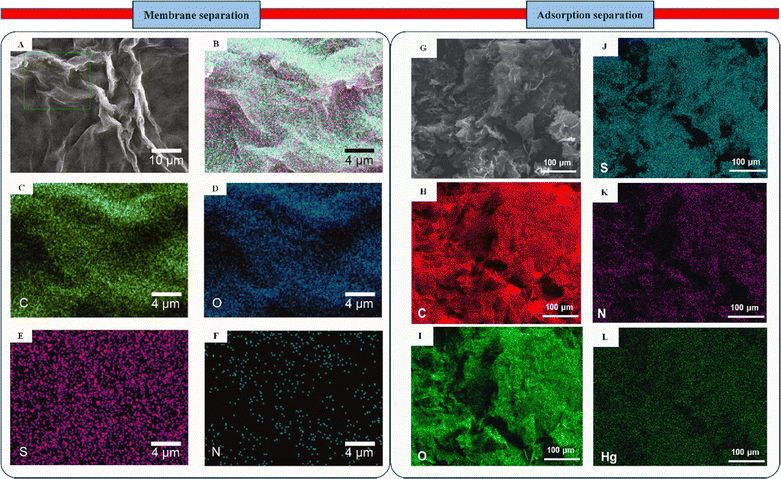 | ||
| Fig. 9 (A) SEM image and (B)–(F) EDS map of the GO-Cys membrane, indicating the presence of C, O, S, and N. Methods for using GALC membranes to isolate organic dyes. Reproduced with permission from ref. 139 Copyright 2021 American Chemical Society. (G) SEM image of Hg(II)-adsorbed Cyst-prGO; (J)–(L) matching the EDS map of Cyst-prGO comprising C, O, S, N, and Hg. Reproduced with permission from ref. 140 Copyright 2018 American Chemical Society. | ||
In comparison to chiral membrane separation, it is crucial to investigate the adsorption isotherm of Cyst-prGO for mercury (Hg(II)) removal, as emphasized by Yap et al.140 This will clarify how the adsorbent and adsorbate function together in practice. The elemental mapping of Cyst-prGO in Fig. 9J–L confirms the presence of C, O, S, and N due to the effective incorporation of functional groups with the GO sheet. Moreover, the even allotment of Hg (Fig. 7G) indicates interactions with elements such as N, O, or S on the adsorbent's surface. The divalent Hg ion has unoccupied orbitals, allowing it to form a metal–chelate complex when it interacts with ligands that provide their lone electron pairs to the ion. The heteroatomic electronegativity on the adsorbent surface can help examine the process of Hg(II) adsorption onto Cyst-prGO. O has stronger attraction to single electrons than N or S, as the electronegativity for electron donors increases in the system. Therefore, because S forms a stronger connection with Hg than N or O, it has a stronger propensity to exchange the lone pair of electrons.
The author conducted experiments with various toxic elements in water, including Pb, Cu, and Cd. In contrast to Hg, other toxic elements did not exhibit effective adsorption separation results. Less effectivity indicates the importance of why and how material selection must be considered critically, as factors like environmental friendliness, including the efficiency of the chiral separation, can be achieved by altering the design considerations or switching to a different combination of materials. However, there is still no evidence of finding a thumb rule for selecting materials as it depends on the requirements.
In 2014, Ahmed et al. researched the enantioselective separation of 12 groups of pharmacological racemates.143 Their findings indicated that a chiral stationary phase with high enantioselectivity could be produced by encapsulating a low concentration of SWCNTs inside a monolithic polymer backbone. If a higher concentration of SWCNTs is used, their chiral discriminating ability can be lost, making it difficult to reproduce results. It remains unclear whether the quantity of CNTs used in the separation process can influence the effectiveness of the technique. Additionally, inexpensive and ecofriendly materials must be identified for chiral separation. Graphene has recently attracted considerable attention because of its remarkable chemical versatility and ability to adsorb hydrophobic substances and carbon-derived ring structures, which are due to its sizeable delocalized π-electron system.
Li et al. developed a technique (Fig. 10A) for removing polar non-steroidal anti-inflammatory drugs (NSAIDs) from various water-based matrices by ultra-precision liquid chromatography with a diode array detector (HPLC-DAD).144 They used an innovative type of magnetized polyethyleneimine tweaked reduced graphene oxide (Fe3O4@PEI-RGO) as an adsorbent for the first time. The adsorption kinetics and mechanisms of four different adsorbents (Fe3O4@PEI, Fe3O4-rGO, Fe3O4@PEI-GO, and Fe3O4@PEI-rGO) (Fig. 10B) and aromatic acids were analyzed. Fe3O4@PEI-rGO exhibited the highest adsorption capacity for polar NSAIDs owing to its interaction-triggered Fe3O4-rGO. Additionally, the effectiveness of the proposed Fe3O4@PEI-rGO magnetic solid-phase extraction (MSPE) technique was evaluated by measuring NSAID concentrations in various water sources (municipal water, river water, and groundwater). Fig. 10C presents chromatograms of unadulterated and adulterated water samples. The MSPE method increased the extraction efficiency as the strong absorption ability of the Fe3O4@PEI-rGO composite contributed to the broad linearity spectrum, minimal identification limit, and excellent recovery of the proposed method. The study indicated that π–π interactions of graphene provide a wide range of options for future separation techniques; however, further investigation is needed for identifying foreign materials to combine with graphene to use as a chromatographic separator. Additionally, the study highlighted the importance of enantiomeric variants in identification and the broad range of chiral selectors for analytes with considerable structural diversity.
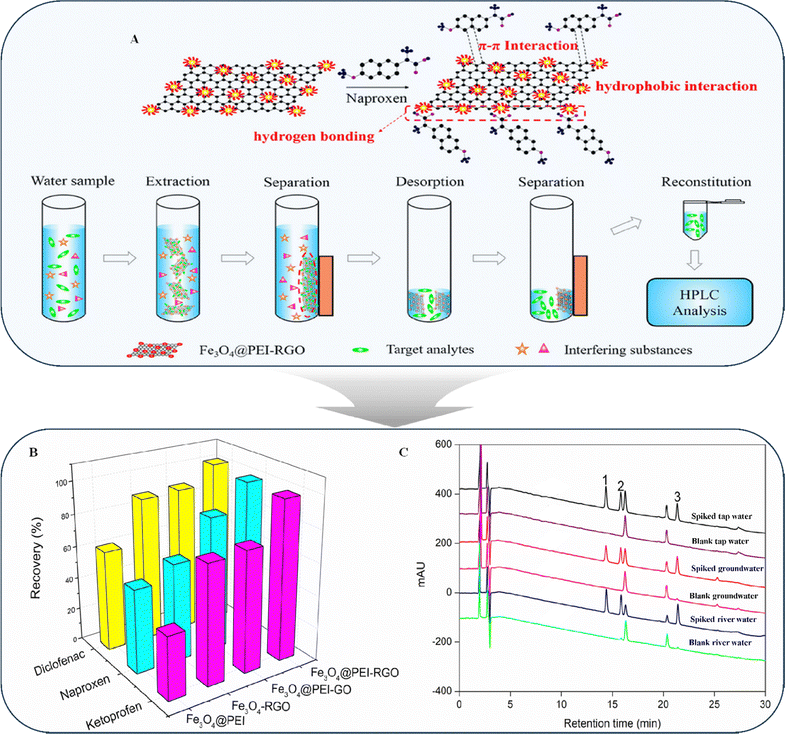 | ||
| Fig. 10 (A) Fabrication of the Fe3O4@PEI-RGO composite. (B) Extraction efficacy of Fe3O4@PEI-RGO and its effect on alternative adsorbents. (C) Chromatograms of spiked water (tap water, groundwater, and river water) and blanked water (tap water, groundwater, and river water) extracted by Fe3O4@PEI-RGO-based MSPE. Peak 1: ketoprofen; peak 2: naproxen; and peak 3: diclofenac. Reproduced with permission from ref. 144, Copyright 2021 American Chemical Society. | ||
Determining the optimal stationary phase and chromatographic conditions for separating analytes involves trial-and-error testing. This procedure is expensive and time-consuming, although theoretically inexpensive.145,146 Achieving optimal chromatography conditions requires careful selection of the system layout to ensure a high rate of substance recovery. Additional scientific evidence is required to fully comprehend the intricate characteristics of achiral phenomena, alternative figures-of-merit in chromatography, and the probable interactions among putative achiral sample constituents. Moreover, it is essential to determine how various parameters, such as the quantity of the chiral selector, percentage of GO, pH of the buffer, and applied voltage, affect the separation process.
4.2. Chiral recognition
The phenomenon of molecular chirality is essential in understanding crucial issues such as the origin of biological homochirality.147 Accurate identification is of considerable interest, as identifying biomolecular chirality is critical to drug development and transportation owing to its role in recognizing molecules and their functions.148–150 The trajectory of carrier motion and molecule chirality influence the electron spin orientation during transport across chiral compounds. Additionally, the orientation and chirality of chiral molecules affect electron transport. This creates a spin-dependent mechanism where the molecules’ chirality and carriers determine the most desirable spin orientations. Thus, the chirality-induced spin selectivity (CISS) effect can be used to constantly monitor chirality deviations during chemical reactions. This enhances our understanding of chiral symmetry breaking and helps explain its physical origin.151–154Yang et al. developed a method to vaporize Ni and Ag electrodes on graphene field-effect transistors.155 This allows combining single-molecule junction (Fig. 11A) setups to detect chirality and improve reactions. The researchers used a magnetized ferromagnetic electrode to test the system and monitored fluctuations in the chirality of the molecular moiety in real time. They observed reaction modifications at low and high temperatures (60–120 K) that benefited from the resulting high-voltage fields of electricity on the exteriors, indicating a reduced transition state as a result of stereoselectivity and the phenomenon of chiral asymmetry (Fig. 11B). In Fig. 11C–J, the central figures on the left side show the magnification of the sample, whereas the right side of the prominent figures shows the sensitivity to the chiral inversion histogram at different current levels. Because of steric hindrance, it is almost impossible to change the direction between the R and S arrangements of the studied outcome, which is called chiral inversion. Achiral maleimide is necessary as an intermediary for R–S conversion (Fig. 11K). However, it is unclear whether the current fluctuations impact the results of the chirality identification method. Comparison of the thermodynamic properties at the ensemble level, which is unproductive, is commonly used to differentiate molecules. In addition, the development and fabrication of structure-specific detection machinery are necessary to achieve a higher precision in identifying various compounds. Furthermore, molecular devices must covalently anchor the molecule's asymmetric reaction center to relate spin orientations to the molecular framework156 and achieve asymmetric catalysis157,158 using a spin current.
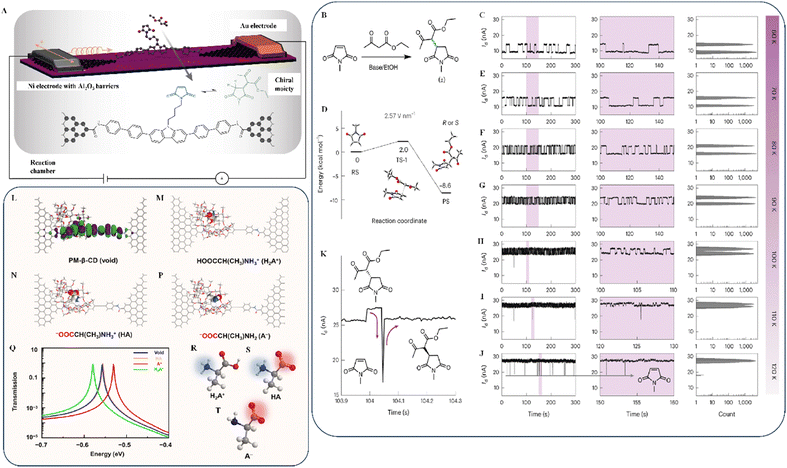 | ||
| Fig. 11 (A) Schematic of the single-molecule spin valve system used to determine Michael addition chirality. (B) Schematic of the molecular junction measurement of the chiral Michael addition process. (C) Michael addition potential energy surface at 2.57 V nm. Monitoring of the current at different temperatures: (D) 60 K; (E) 70 K; (F) 80 K; (G) 90 K; (H) 100 K; (I) 110 K; (J) and 120 K. (K) Typical electrical signal time sequence at 110 K, with high temporal precision. Between the R and S arrangements, maleimide is always present. Reproduced with permission from ref. 155 Copyright 2023 Springer Nature. (L) Schematic of the estimated frontier molecular orbitals of the molecular machine. Typical molecular shapes formed by the union of oppositely charged AAs: (M) cation; (N) zwitterion; and (P) anion. (Q) Similar spectrum of transmission under no bias voltage. For the cases where an anion, a zwitterion, and a cation are present in the cavity, the transmission curves are red, yellow, and green, respectively. The blue curve corresponds to an empty cavity. (R)–(T) Cation, zwitterion, and anion depictions of AAs. Reproduced with permission under a Creative Commons CC-BY license from ref. 62, Copyright 2021 AAAS. | ||
Additionally, studies on the enantiomer recognition of AAs, the constituents of proteins, and the essential parts of pharmaceuticals with different physiological functions may provide valuable information regarding how chiral molecules are accepted and what roles they play in biological systems.159,160 The analysis performed by Liu et al. revealed how the present flip-flops are related to host–guest dynamics.62 They used density functional theory (DFT) at a low voltage and discovered that the largest packed chemical orbital is the most critical determinant of conductivity (p-HOMO). At low voltage, their DFT analysis indicated that the perturbed highest occupied molecular orbital (p-HOMO) is essential for conductivity. Transmission spectra based on the Landauer formula161 support this. Fig. 11L–P and 11R–T show several typical configurations during PM-β-CD and AA association and dissociation, respectively. They determined the tendency of electrical conductance, where the anion shows the highest and the cation shows the lowest electrical conductance. To achieve the various phases of conductance, it was discovered that the transmission spectra of these arrangements varied significantly close to the Fermi level of the electrodes. As shown in Fig. 11Q, the molecular conductance was higher when the p-HOMO of PM-β-CD was closer to the electrode’ Fermi level. The opposite was observed in the case of cation formation in the hole; reducing the conductance caused the p-HOMO of PM-β-CD to move away from the electrode Fermi level. Aside from its ability to detect AAs and distinguish chirality in acidic and alkaline environments, this system is useful for identifying various biological substances in natural or artificial environments. It provides a fundamental understanding of the molecular processes involved in life. Comparing the thermodynamic characteristics of ensembles is a common way to differentiate molecules. Therefore, it is essential to develop a universal molecular methodology to increase the accuracy of detecting target molecules in other contexts.
4.3. Chiral transfer
A material possesses chirality because of molecular asymmetry in a noncovalent assembly. There are several methods for producing chirality, e.g., creating multiple helices, twisting a cyclic ligand, or placing binding ligands in distinct positions around an ion,162 which helps to develop rigid materials with unique structures and shapes, such as those with chiroptical properties.163,164 Chiral substances with biological value are crucial in our daily lives, making it essential to understand chirality for developing new production methods. Chirality can be induced using the classic covalent technique or by shifting the enantiomerically pure models, such as DNA, to proactive achiral compounds.165,166 Noncovalent interactions, such as hydrogen bonds, coordinating conversations, electrostatic charges, and van der Waals forces, may promote the transfer of chirality from achiral molecules, which is referred to as triggered supramolecular chirality.167Basu et al. observed chirality transfer from the graphene surface to alkoxyphenylbenzoate (APB) and 4-cyano-4′-pentyl biphenyl (CB)—two achiral liquid crystals.168 They achieved this by sonicating pure graphene in ethanol (1 mg mL−1) for 10 h, adding liquid crystal APB/CB, and replicating the mixture. Moreover, Maoro et al. established one of the most straightforward strategies for transferring the chirality of GO.169 In their experiment, they drop-cast a water-based suspension of GO under dynamically started CW and CCW swirls to efficiently transfer chirality in GO by causing successive layering, as observed in the mirror image of the CD spectra (Fig. 12A). In subsequent studies on chirality transfer, Đorđević et al. and Fernández-Garca et al. investigated the use of chirality transfer in the generation of supramolecular complexes with carbon derivatives. They discovered that carbon derivatives (e.g., fullerene and GQD) functioned as highly effective recyclable catalysts in the transfer of hydrogen reactions during supramolecular structure assembly and that they could initiate chirality transfer in carbon derivatives through chirality transfer in carbon derivatives.170,171
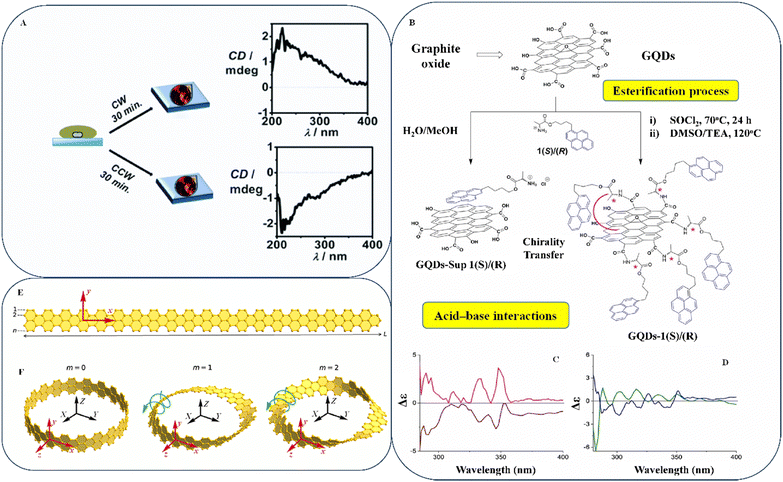 | ||
| Fig. 12 (A) Deposition through droplet casting during mixing. A small magnet is used to continuously swirl the GO solution as it is poured over a glass substrate. Reproduced with permission from ref. 169, Copyright 2016 Royal Society of Chemistry. (B) Amidation interaction with compound 1(R) or 1(S) leads to the formation of chiral GQDs-1(R) and GQDs-1(S). (C) CD spectra of supramolecular constructed GQD-sup 1(R) (brown) and GQD-sup 1(S) (magenta) in DMSO. (D) Blue (GQD-1(R)) and green (GQD-1(S)) CD spectra of aggregates in DMSO. Reproduced with permission from ref. 172 Copyright 2022 Wiley-VCH. (E) Nanoribbon of planar graphene with length L and a width of n atoms. (F) Twisting the nanoribbon m times around its x-axis and joining the resulting ends produces a circular graphene nanostrip. Reproduced with permission under a Creative Commons CC-BY license from ref. 173 Copyright 2023 American Chemical Society. | ||
Similarly, Vázquez-Nakagawa et al. fabricated complex chiral GQD supramolecular frameworks.172 To do this, they changed the structure of GQDs using chiral derivatives of pyrene to obtain a chiral nanostructured material. Fig. 12B shows the GQDs-1(R)/1(S) molecules synthesized through two steps: esterification and acid–base interactions. The researchers utilized GQDs with SOCl2 for 24 h to change the carboxylic acid group into acid chlorides. Subsequently, the solution was reacted in situ with 1(R) or 1(S) in DMSO-TEA at 120 °C for 48 h. Finally, the pyrene counterparts 1(S) and 1(R) were used to fabricate supramolecularly functionalized GQDs-1(R) and GQDs-1(S) from covalent bonds between GQDs and pyrene. Significant chirality transfer was confirmed using the CD spectra, where a positive cotton effect was observed in the absorption wavelength range of 320–350 nm for GQDs-sup 1(S), and an adverse effect was observed in the identical absorption range for GQDs-sup 1(R) (Fig. 12C).
Similarly, the CD assays for GQDs-1(S) and GQDs-1(R) in dimethyl sulfoxide (DMSO) demonstrated the opposite cotton impact alongside a comparable zero-cross point in the identical absorption band (Fig. 12D). The existence of hydrogen bonds makes it easier to construct a chiral structure utilizing chiral transfer in GO. Nonetheless, the external driving force to make GO a feasible candidate for chiral transfer remains unclear. Furthermore, although several research articles on the fabrication of a supramolecular structure have been published, some comments concerning the design statement and material selection of a supramolecular form need to be clarified. Multiple authors independently considered “homochirality,” which indicates that all the molecules of an element have the same enantiomer.174–177 It can change through chiral transfers from patched chiral dendritic clusters using CD and a 2D rotating frame with a specific twist angle.178 Wang et al. recently published evidence for an accurate chirality transport mechanism for GNRs in the creation of targeted GNRs in the topmost atomic layer of insulating h-BN, furnishing the necessary building blocks of a thin ultra-large-scale incorporated circuitry to realize a topological electronic state that can be used for quantum computing.179 Identifying the spiral and rotational motion of GNRs along their axis is essential for understanding the effects of graphene nanostrips’ topologically challenging shape on their optical properties and the robustness of their irregular edge terrain. Fig. 12E presents the results of a detailed investigation of the visual behavior of graphene nanostrips performed by Razzhivina et al.173 They noticed that the complex topology results from the cyclic boundaries that differentiate the energy bands in the system.
The twisted graphene nanostrips (TGNs) are only visible for transformations oriented across the entire nanostrip (Fig. 12F). By contrast, absorption occurs through transitions polarized both along and across the axis of the nanoribbon. To better understand the chiral optical responses of graphene nanostrips created with different precursor molecules, it is necessary to evaluate their complex topological and physical characteristics with greater precision. This will introduce new avenues for studying the effects of complex topology on various materials. Modifying the chemical bonding reactions between chiral organic compounds and inorganic frameworks is necessary for achieving high spin-polarization responsiveness in chiral molecules, which triggers lattice distortions. This will help uncover the origins of spin-polarization-based phenomena. However, it is challenging to confirm the effects of chemical connection interactions on spin-related features of chiral structures using previously described methodologies.
4.4. Chiral graphene photodetector applications
Over the past few years, researchers have extensively studied various 2D compounds to evaluate their potential for use in photodetector applications. Photodetectors play a crucial role in modern transmission and detection devices. For example, compounds such as h-BN (hexagonal boron nitride, TMDs (transition metal dichalcogenides), and BP (black phosphorus), which have different bandgaps, have been utilized for photodetectors that detect visible, ultraviolet (UV), and NIR light.180,181 By contrast, graphene lacks a bandgap, making it effective for detecting wideband light. This is particularly useful in hyperspectral scanning and monitoring systems.Interestingly, the recognition of graphene's unique and beneficial properties has led to the exploration of other 2D crystals and hybrid structures. Moreover, it has been observed that graphene/semiconductor heterojunction light detectors, which operate based on the photogate phenomenon, demonstrate remarkably high amplification. Additionally, the localized characteristics of semiconductor components on photogenerated carriers contribute to comprehending stereoselectivity and chiral symmetry-breaking mechanisms.182–184 Advanced IR detectors have been developed using narrow-bandgap group III/V semiconductors. Despite noteworthy progress in materials and manufacturing, the lack of broadband absorption and a fast response continues to hinder system performance. However, emerging nanomaterials with unique physical and optical properties present promising opportunities to advance infrared photodetector technology.185–189 Further, molecules with varying structures and properties can exhibit dichroism, which increases proportionally to the chirality parameter.190 Thus, many research groups are exploring the potential of these compounds for chiral sensing instruments, biochemical and metabolite sensors, and photodetectors.191
Graphene and CNTs are outstanding ultrasensitive substances for photodetectors because of their unique optical features, such as broadband and adaptable light absorption.192,193 Despite previous studies on metal/graphene structures indicating promising photoreactivity, the poor transmittance of an individual sheet of carbon atoms severely constrained the ability to be used in photodetector applications.194 Liu et al. reported that photodetectors based on a quasi-2D SWNT-graphene blended film outperformed graphene and SWNTs with regard to not only photoconductive gain (∼105) but also the response time (100 μs) and ultra-broadband sensitivity (covering the communications band at 1.5 μm).195 Despite the higher photodetection capacity achieved using the hybrid model of the chiral structure, a few points need to be thoroughly discussed. The authors asserted that the hybrid model is superior to the individual model, citing the lower absorption intensities and rapid amalgamation of excited electrons of exfoliated graphene. However, other models, such as doped graphene, rGO, and other graphene derivatives, are yet to be investigated. Mustaqeem et al. further investigated the CMOF/graphene paradigm, in which the porous shape of CMOFs traps light to increase light harvesting and the production of electron–hole pairs. Because of the substantial carrier mobility of graphene, the generated charges can migrate to the graphene layer, significantly increasing the photocurrent.196 Although the authors reported that their method is effective, their results are insufficient for drawing conclusion regarding its lifetime and cost-effectiveness.
Peng et al. discussed a graphene photodetector capable of directly detecting photon spin singularity motion states associated with LCP and RCP incident light.197 However, they failed to provide sufficient evidence for the relationship between the electrode shape and photon detection. The chirality and resonant wavelength of the photodetector are determined by the chirality and geometry of the electrodes. However, combining electrode shapes in a single device to detect combinatorial variables such as wavelengths and the orbit's angular momentum has yet to be considered. By contrast, Li et al. presented the idea of polarimetry. They reported that chiral plasmonic metasurfaces facilitate polarimetry and allow state of polarization (SoP) light analysis at a resolution of several microns.198 While it is possible to partition the chip into individual light components, this option is complex and requires extra space. Instead, incorporating metasurface-integrated polarimetry within the chip reduces manufacturing and component costs while allowing complex spatial analysis and changing of light beams. Full-Stokes polarized imaging and on-chip polarimetry will change the upcoming generation of hybrid integrated nano-electronic-photonic circuits.
Spin light, i.e., circularly polarized light (CPL), is used in various fields, including pharmaceutical screening, optical science, and biological sensing, owing to the sensitivity of newly developed photodetectors to the spin state of light. Wei et al. reported that the efficiencies and sensitivities of integrated light detectors primarily depend on their shapes.199 They demonstrated that their approach could transform the light emitted from the near field into a voltage and transmit electrical signals to an external circuit using a small area of graphene. The Poincaré sphere (as shown in Fig. 13A) is helpful to explain the concept of an ideal detector specific to CPL. However, developing high-performance and inflexible CPL photodetectors has been challenging. Fig. 13B shows a SEM image of a prototype of the CPL-specific photodetector. This device has two ports, a circular few-layer graphene device channel, and a T-shaped arrangement of circularly arranged nanoantennae.
 | ||
| Fig. 13 (A) Poincaré sphere CPL photodetector, whose photoresponse Vph is independent of all Stokes parameters other than the fourth Stokes parameter of the incoming light. (B) Ring-shaped device phony-color SEM image. Color scheme: red for graphene, yellow for plasmonic nanostructures and electrodes, and grey for the substrate. (C) Sensitivity of the device to the four Stokes parameters was determined by rotating a QWP and monitoring its electrical output. (D) SEM images of fabricated graphene sheets and ribbon devices. (E) Ribbon system can direct photocarriers more effectively and have a higher internal resistance after the removal of the extra graphene, reducing the energy loss and increasing the electrical output. (F) Reported sheet and ribbon device polarization-dependent photovoltages. (G) Detailed optical ellipticity measurements at a low incident power of 1.8 μW. (H) Photovoltages were measured during the off-and-on periods of LCP and RCP lighting. Orange color denotes a light condition with an illumination power of 260 μW. (I) Measured I–V curves with drain–source distortion. (J) Measured gate-tunable photoresponse. Reproduced with permission from ref. 199, Copyright 2022 Springer Nature. | ||
Fig. 13C shows the procedures used in the experiment to investigate the responsivities of the photographic detectors to a quartet of Stokes indices. The incident ray was modified using a quarter-wave plate (QWP) in the experiment.
Close examination of the photoresponse of the graphene ribbon has indicated that a small piece of graphene is necessary to transform the visible near field into voltages and send electrical impulses to the external loop. The photoresponse can be improved by altering the shape of the graphene layer.
Fig. 13D shows two L-shaped cascaded appliances utilizing graphene ribbons to achieve near-field chirality. Fig. 13E shows a simulated illustration of the photoresponse effect related to CPL. Although the device size was identical to that shown in Fig. 13F, the photoresponse of the ribbon device was higher than that of the sheet device. The instrument could measure elasticity as low as 0.03° Hz−1/2 at a power of 1.8 W, as shown in Fig. 13G. Fig. 13H shows the LCP and RCP photovoltages during 500-Hz on/off cycles. The current–voltage (I-V) dependence in Fig. 13I is linear, revealing LCP and RCP illumination, with the I-V lines shifting upward and downward to demonstrate their inverse photoresponses. When a gate voltage is applied across it, the four-column system achieves a peak responsiveness of 392 V W−1 of single-column nanostructures, as shown in Fig. 13J. Non-uniform rotation of nanostructures creates different geometric phases that can determine the polarization states, e.g., in geometric metasurfaces, which allow CPL responses to accumulate while linearly polarized light disappears. Closer inspection reveals that only a small patch of graphene is needed to transform the optical near field into current and transmit electrical impulses to an exterior network. Consequently, the transformation of the graphene layer can be exploited to enhance the light responsiveness. Although CPL photodetector research has made substantial progress in recent years, several critical difficulties need to be resolved before these technologies can be utilized in real-world applications. Some crucial issues are presented below.
• CPL detectors’ mobility needs to be improved, and the photoresponsivity of polarized light detectors is substantially lower than that of polarized light sensors.
• The CPL detector's performance must be adequate to differentiate LCP/RCP by frequency modulation or appropriate material selection.
• One of the essential goals of this field of study should be the creation of novel chiral materials with high nobilities and significant CD values.
• Chirality, quantum geometrical effects, and unusual surface states with atypical degrees of freedom will provide many intriguing optical phenomena.
CPL photodetectors constructed from chiral materials will become more relevant in optical detection research as more chiral substances with superior electrical characteristics and higher CD values are developed and advanced device technologies are implemented.
4.5. Chiral graphene biomedical applications
Chirality, which exists at all levels (molecular to supramolecular) in nature, can be altered by circular polarization and affects the chemical, biological, and structural properties of materials.200,201 Numerous experiments have proven that the naturally occurring properties of materials, such as cell and growth adhesion, protein absorption, and antimicrobial activity, are dependent on chirality.202–204 Advances in molecular imaging allow us to observe biological processes within living cells—even at the subcellular level—in real time. Chirality can be considered a bioinspired system for science and technology, providing high optical activity and transparency, facilitating the creation of biosensors,205 achieving a negative refractive index206 in certain parts of the electromagnetic radiation spectrum,207 and producing chiral inorganic nanomaterials.208 Compared with non-chiral nanomaterials, bioinspired chiral nanomaterials have high selectivity and precision, making them ideal for biomedical and biochemical applications, such as sensing and catalysis.209–211Because of its physical compliance and fluorescence-quenching ability, graphene and its analogs have attracted considerable attention for biological applications. These materials have unique properties that make them appropriate for fluorescent biosensors with intracellular imaging ability.212,213 A recent study indicated that enantio-analysis of biomarkers with chiral moieties can produce accurate and quick results in identifying early-stage gastric cancer cells.214 This chiral nanomaterial is physiologically acceptable and can be used for designing new chiral therapeutic systems and other biological applications. Researchers have combined graphene with other components to achieve the desirable physical compatibility, controlled release, and enhanced drug-loading capacity.215–222 One such technology involves using magnetic nanocomposites comprising β-CD-conjugated GO, which respond well to an external magnetic field and can hold the stationary phase in place in microchannels, allowing the rapid screening of chiral compounds in large quantities.223
Xie et al. discovered that a biomimetic system utilizing a β-CD-based PI membrane has the potential for drug identification and evaluation.224 Additionally, β-CD-based GO composites are effective for separating and enantio-separating organic substances in pollution-related treatments,225 adsorbing AAs,226 producing therapeutic intermediates,227 and detecting cancer cells.228 Chiral drugs can interact enantioselectively with chiral carriers and the human body because of the unique chiral environment inside. Therefore, analyzing the delivery characteristics of chiral drugs is crucial as the use of chiral medicines continues to increase significantly.
In 2022, Wang et al. reported the early detection of Alzheimer's disease using polymer/GO sensors supported by machine learning (Fig. 14A).238 The authors recommend establishing an optimal structure incorporating polymers and GO to create a robust platform for the easy, precise, and high-throughput detection of a wide range of biological analytes. However, the protein selection could be more arbitrary and prohibitively expensive. While few studies have been carried out on using graphene and its derivatives for diagnosing Alzheimer's disease, recent studies have indicated that graphene quantum dots (GQDs) can also help diagnose Alzheimer's disease.239–242
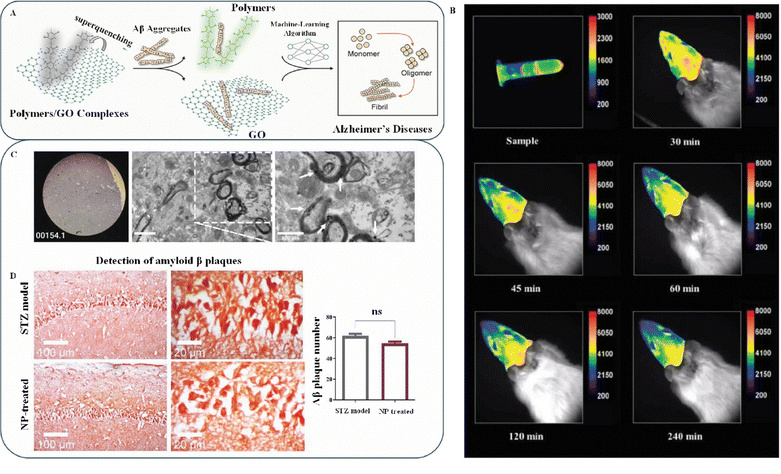 | ||
| Fig. 14 (A) Polymer/GO composite structure for detecting Alzheimer's disease. Reproduced with permission from ref. 238 Copyright 2022 American Chemical Society. (B) Time-lapse fluorescence illustrations acquired in vivo show the accumulation of CS/GQD NPs in the brain after intravenous (IN) administration. (C) TEM revealed the presence of CS/GQD NPs within the myelinated axons of hippocampal neurons. (D) Illustration of amyloid plaques stained with Congo red in STZ-induced models and NP-treated groups. Reproduced with permission from ref. 243, Copyright 2023 Wiley-VCH. | ||
In 2023, Mohebichamkhorami et al. conducted a study on a potential treatment for Alzheimer's disease.243 They combined chitosan and graphene quantum NPs that could cross the blood–brain barrier (BBB). The researchers employed real-time in vivo bioimaging (Fig. 14B) to observe the effects of intranasal administration of chitosan/GQD NPs in rats. After 30 min, the rat's brain showed two bright green fluorescence patches, which began to spread after 45 min. The NPs were evenly dispersed throughout the brain 2 h later, and the fluorescence faded after 4 h. Histological studies revealed an accumulation of NPs in the brain parenchyma and myelinated neurons. NPs formed aggregates in myelinated axons and were implanted in unmyelinated ones, while extracellular NPs were rare (Fig. 14C). However, no significant difference in the development of amyloid plaques was observed between the NP-treated group and the streptozotocin-induced model group (Fig. 14D, top). The authors attributed this to the small percentage of GQDs used as conductors inside the NP structure. NPs are ultrasmall and can quickly move across olfactory neurons, allowing them to enter neural cells, where they have a minimal impact on Aβ plaques in the intercellular space (Fig. 14D, bottom). The study suggests that NPs are excellent for in vivo imaging of the cerebral cortex by intranasal administration and may provide an intranasal bioimaging strategy. With further modifications, NPs can become a viable medicament for neurodegenerative diseases. Identifying the best possible nanomaterial combination to optimize the target efficiency and rescale production is essential, which plays a vital role in drug delivery and early diagnosis of diseases.
In 2014, Xu et al. discovered that L-DOPA is far more effective than D-DOPA for treating Parkinson's disease.249 They also developed a novel enzyme with enantioselectivity and an NIR photothermal effect that detects H2O2 (hydrogen peroxide) inside cells using “–COOH”-modified GO in a chiral metallo-supramolecular system. Additionally, they successfully separated the enantiomers of L-DOPA—a chiral drug used to treat Parkinson's disease.
In a recent study, Goswami et al. discovered that reduced GQDs (rGQDs) experience substantial fluorescence activation of L-DOPA in water-based formulations and synthetic urine with an effective recognition range.250Fig. 15A shows the microwave-assisted pyrolysis process for composing rGQDs. The researchers found that after interacting with L-DOPA, the rGQDs/L-DOPA particles became large and clumped together (Fig. 15C); however, before the interaction, the particle sizes were small and dispersed (Fig. 15B). Fluorescence emission measurements indicated that the clumping caused the emission. The well-known “salt-screening effect” experiment indicated that the electrostatic bonds among rGQDs and L-DOPA drive such aggregation. The intensity of the mixed solution did not increase despite increasing amounts of L-DOPA (Fig. 15D). A highly energized salt in the water-soluble solution prevents the other ingredients from making electrostatic contact.
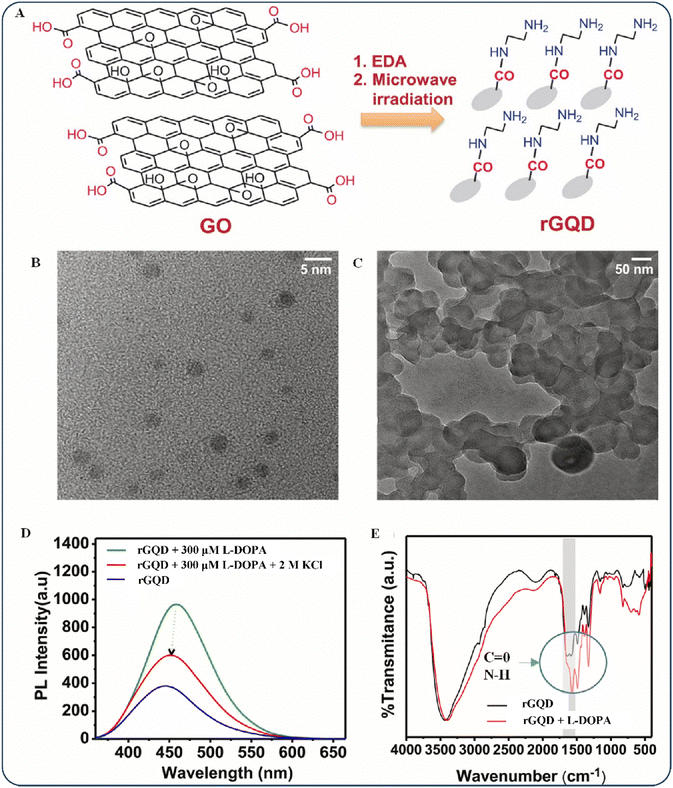 | ||
Fig. 15 (A) Synthesis of rGQDs from graphite. TEM images of (B) rGQDs (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm) and (C) rGQDs + L-DOPA (50 nm) and (C) rGQDs + L-DOPA (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm). (D) rGQD fluorescence spectrum for a combination of L-DOPA and 2 M KCl. (E) FT-IR spectra of rGQDs and rGQDs + L-DOPA. Congo red staining micrograph. Reproduced with permission from ref. 250 Copyright 2022 Elsevier. nm). (D) rGQD fluorescence spectrum for a combination of L-DOPA and 2 M KCl. (E) FT-IR spectra of rGQDs and rGQDs + L-DOPA. Congo red staining micrograph. Reproduced with permission from ref. 250 Copyright 2022 Elsevier. | ||
As shown in Fig. 15E, in a Fourier transform infrared (FT-IR) experiment, the rGQDs formed hydrogen bonds with the analyte through its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H groups. The experiment indicated the potential of rGQDs for Parkinson's disease diagnosis through the detection of L-DOPA. While some disagree with the justification behind the choice of rGQDs, the underlying mechanisms depend on hydrogen bonds that can be generated with most carbon derivatives. The fact that the author was not confined to a purely materialistic design and testing is a significant strength of the study. The electrical properties of the biosensor they developed remain constant throughout a wide range of bending degrees and do not change following an upsurge in relative humidity. This may facilitate the development of wearable L-DOPA sensors. Interactions between biomolecules and biostructures with inherent chirality and synthetic chiral materials are almost limitless. The development of productive chiral production and the optimization of chiral configurations remain central to chiral manufacturing. Generating materials with significant photonic activity across solid and liquid phases will be a future challenge requiring an enormous amount of chiral manufacturing. Various chirality-based biosensors have been created, but they must be clinically optimized before being implemented into reagents and devices. Future fine-scale disease and environment monitoring will significantly benefit from the increased sensitivity and reliability of chirality-based biosensors in various approaches.
O and N–H groups. The experiment indicated the potential of rGQDs for Parkinson's disease diagnosis through the detection of L-DOPA. While some disagree with the justification behind the choice of rGQDs, the underlying mechanisms depend on hydrogen bonds that can be generated with most carbon derivatives. The fact that the author was not confined to a purely materialistic design and testing is a significant strength of the study. The electrical properties of the biosensor they developed remain constant throughout a wide range of bending degrees and do not change following an upsurge in relative humidity. This may facilitate the development of wearable L-DOPA sensors. Interactions between biomolecules and biostructures with inherent chirality and synthetic chiral materials are almost limitless. The development of productive chiral production and the optimization of chiral configurations remain central to chiral manufacturing. Generating materials with significant photonic activity across solid and liquid phases will be a future challenge requiring an enormous amount of chiral manufacturing. Various chirality-based biosensors have been created, but they must be clinically optimized before being implemented into reagents and devices. Future fine-scale disease and environment monitoring will significantly benefit from the increased sensitivity and reliability of chirality-based biosensors in various approaches.
In 2020, Zabol et al. conducted a study using molecular dynamics (MD) simulations to identify the binding of cytarabine to functionalize GO (Fig. 16A).258 Their research indicated a strong interaction between the cytarabine molecule and the functionalized GO (fGO) nanosheet. This suggests that dopamine-functionalized GO may be an effective carrier for transporting cytarabine—an anticancer medication—to tumors. Furthermore, Wang et al. investigated a theoretical framework for loading chiral molecules of drugs by assembling a D-phenylalanine derivative gelator alongside GO. This approach is expected to pioneer chiral drug delivery.259 Mirza-Aghayan et al. proposed a facile and efficient method for creating innovative multi-functionalized rGO by reacting GO with dopamine, HAuCl4/NaBH4, and doxorubicin (Dox) in that order under moderate conditions.260 SEM images of rGO/DA (Fig. 16B, left), rGO/DA/Au NP composites (Fig. 16B, center), and rGO/DA/Au NPs/Dox (Fig. 16B, right) indicated that the Au NPs were evenly distributed and did not clump together. A photothermal study indicated that the surface temperature of rGO/DA/Au NPs/Dox increased rapidly within 5 min of exposure to a visible lamp (Fig. 16C). This suggests the promising future of the material as a photothermal compound in biological systems.
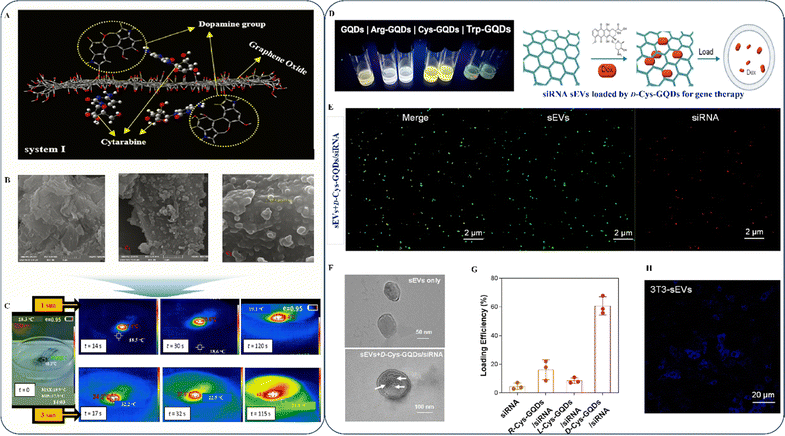 | ||
| Fig. 16 (A) fGO/CYT system's MD snapshots at different levels of drug loading across the fGO surface. Reproduced with permission from ref. 258 Copyright 2020 Elsevier. SEM images of (B) rGO/DA, rGO/DA/Au NPs, and rGO/DA/Au NPs/Dox hybrid nanomaterials. (C) Time-lapse thermal images of an rGO/DA/Au NPs/Dox composite substance. Reproduced with permission from ref. 260 Copyright 2022 Elsevier. (D) Illustrations of chiral GQDs with various ligands. (E) Confocal micrograph of siRNA (red)-loaded sEVs (green) facilitating D-Cys-GQDs. (F) TEM images showing D-Cys-GQDs aiding siRNA penetration. (G) Efficacy of dynamic loading of chiral Cys-GQDs/siRNA into sEVs. (H) 48 h of confocal visualization of DU145 cells subjected to 3T3 sEVs. Reproduced with permission from ref. 261 Copyright 2023 American Chemical Society. | ||
Regarding drug-delivery materials that distinguish between stereoisomers, it is essential to prioritize biological compatibility to avoid neurotoxicity. Although GQDs have a fixed chiral organization, their conformational distribution may be irregular, leading to minor chiral events that can affect cell lifespan.262 Nonetheless, GQDs are stable in high electrolyte concentrations and have strong luminescence, making them a dependable nanocarrier for targeted drug delivery. They have a single-layer structure, and their high crystallinity enhances the fluorescence; additionally, doping with different heteroatoms can broaden the emission spectrum.263,264 Small extracellular vesicles (sEVs) are known to transport bioactive elements such as proteins and nucleic acids from source cells to cell recipients, promoting intercellular communication.265–267 In recent years, sEVs have attracted interest as drug-delivery systems owing to their physiological and practical features, such as their minimal antigenicity, simultaneous transport of multiple drugs, advanced cargo loading techniques, biomarker-driven diagnostics, prolonged circulation time, non-toxicity, deep tissue penetration, potentiated intended effects, and ability to cross the BBB.268
Recently, Zhang et al. found that chiral GQDs can enter sEVs through a lipid membrane and load sEVs passively with some tuning.261 For investigating the role of ligands in determining permeation rates and the effectiveness of d-Cys-GQDs in permeating sEVs of chiral GQDs into sEVs, two different ligands—tryptophan and arginine—were used with GQDs (Fig. 16D). Dox-loaded sEVs can enhance medicinal benefits; nevertheless, incorporating Dox or other frequently used drugs into sEVs is challenging, limiting their application as drug-delivery agents. Using confocal fluorescence microscopy to measure the colocalization information of the marked siRNA (red) and sEV (green) allowed the researchers to determine that chiral-Cys-GQDs/siRNA was the optimal loading condition for identifying the oncogene Pygo2 in prostate cancer as a target for treatment (Fig. 16E). Furthermore, TEM revealed that d-Cys-GQDs/siRNA colocalized with sEVs, making black dots appear close to individual sEVs instead of the naked ones (Fig. 16F). In this circumstance, the authors referred to the proportion of functional sEVs that can encapsulate medicines due to their drug-loading effectiveness. The findings indicated that chiral GQDs could effectively load siRNAs into the sEVs and that the sEVs maintained their structural integrity during the loading operation (Fig. 16G). To demonstrate the efficiency of d-Cys-GQDs-loaded (3T3) sEVs in eliminating cancer cells and inducing knockdown of the target gene, researchers examined fluorescence signals in the cytoplasm of DU145 cells cultured with the sEVs for 48 h (Fig. 16H). The results indicated that chiral GQDs improve drug incorporation, representing a practical, generic, and feasible strategy for drug loading that may facilitate large-scale fabrication of therapeutic sEVs for clinical trials.
Many reports have indicated that the luminescence properties of GQDs decrease over time.269–271 However, GQDs are promising for managing drug delivery, allowing medication to be targeted and its effectiveness to be monitored. This is particularly important when there are concerns about unwanted side effects or ineffective dosages of drugs. As more efficient synthesis processes emerge, significant progress is expected in studies on chiral interactions in GQDs and cells and the development of drug-delivery agents.
4.6. Chiral graphene sensor applications
Luo et al. (2021) developed an innovative electrochemical sensor for detecting tyrosine (Tyr) enantiomers.280 Tyr was selected because it is an essential AA, and L-Tyr deficiency causes symptoms such as anxiety, depressive disorders, irritability, and pain. By contrast, excess L-Tyr in the body causes problems with DNA replication. The researchers used rGO in combination with a chiral selector known as chiral hetero-multifaceted [3,2,1] (CHMF) to enhance and transmit electrochemical signals, as shown in Fig. 17A. The enantiorecognition ability was assessed through differential pulse voltammetry (DPV), where a glassy carbon electrode (GCE) failed to distinguish between Tyr enantiomers (Fig. 17B). However, modified electrodes such as rGO/GCE, CHMF/GCE, and rGO-CHMF-GCE samples exhibited different results. This is because rGO has a higher conductivity and electrocatalytic efficiency than bare electrodes, resulting in a higher determination sensitivity (as shown in Fig. 17C). The current of the rGO/GCE electrode increased by 3-fold (to 17 A) compared with that of the bare GCE. In addition, the host–guest interactions with the CHMF enriched the substrates on the surface of the adapted electrode (as shown in Fig. 17D). Changing the GCE with CHMF and rGO (rGO-CHMF/GCE) showed a selective response to l/D-Tyr enantiomers by increasing the currents in the voltammograms, which led to a higher current ratio and a wider potential window for detection (Fig. 17E). Overall, the findings suggest that the detection of enantiomers in electrochemical systems can be significantly improved using rGO and CHMF. Although CHMF is a potential candidate for the Tyr sensing application, there was no evidence of chiral formation in their study. Additionally, they did not consider the toxicity of CHMF, which must be considered in future research.
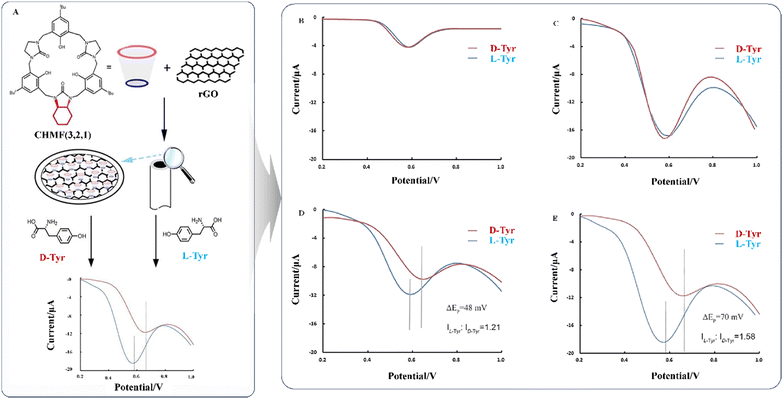 | ||
| Fig. 17 (A) Schematic of the electrochemical sensor for enantiorecognition. DPV of Tyr enantiomers on (B) a bare GCE, (C) rGO/GCE, (D) CHMF/GCE, and (E) rGO-CHMF/GCE. Reproduced with permission from ref. 280 Copyright 2021 Elsevier. | ||
In 2022, Sun et al. proposed a method for recognizing chiral molecules using an organic ligand electrochemical sensor.281 The significance of β-CD in the human body lies in its ability to function as a cholesterol-binding agent. To enhance the electroconductivity of a crosslinked MOF (CLMOF), researchers modified the GCE with GO to create a GO-CLMOF/GCE electrochemical sensor. The sensor underwent successful testing with a urine sample.
However, further research is required on the hydrophobic and hydrophilic nature of β-CD and its behavior in structural formation related to the chemical arrangement to make efficient chiral sensors. While researchers claim that MOFs are valuable tools for developing electrochemical sensors, CMOFs, in combination with β-CD, show remarkable potential in stereochemistry applications. During electrochemical determination, substances being tested to undergo oxidation or reduction, which can lead to the accumulation of deactivation products on the electrode surface. This can reduce the accuracy of analyte detection.13,282 The evaluation procedure may alter the spatial architecture and chiral recognition points necessary for enantiomer identification. Additionally, breakdown or occupancy by reaction products makes precise determination difficult. Therefore, developing renewable sensors is essential for successful chiral electrochemical analysis in real-world scenarios. Enantioselective detection using chiral electrochemical sensors has gained popularity. However, these sensors have a significant drawback: their structures cannot be reused. This is because the chiral biorecognition components tend to be deactivated during measurements, making it necessary to reconstruct the sensing platform after each determination, which is an expensive and labor-intensive procedure that may require several hours or days. Hence, it is necessary to develop a sensing platform that can be used effectively in real-world scenarios for enantioselective analysis.
| Sensor | Electrode/substrate materials | Enantiomers | pH | Detection/target sample | Linearity range | Limit of detection | Ref. |
|---|---|---|---|---|---|---|---|
| Electrochemical sensor | GO-CLMOF/GCE | Mandelic acid | pH = 6 | Human urine | 0.5–30 mM | D-MA-0.15 mM | 281 |
| L-MA-0.09 mM | |||||||
| GO-(S, S)-CIL-GCE | L/D-Tryptophan, (R)-/(S)-mandelic acid, (R)-/(S)-malic acid, L/D-tyrosine | pH = 5.5–7 | 0.5 mM CuCl2 and a 0.1 M PBS solution | 2.0 mg mL−1 | — | 279 | |
| L-NGQDs/GCE, D-NGQDs/GCE | L/D-Cysteine | pH = 3 | Tartaric acid | 0.5 mM | — | 13 | |
| CHMF [3,2,1]-functionalized rGO | Tyrosine | pH = 7 | Human serum | 0.1–10 μM | L-Tyr-78 nM | 280 | |
| D-Tyr-83 nM | |||||||
| 3D-NGMWCNT@ (S, S)-CIL/GCE | Tryptophan | pH = 6 | Human urine | 0.01–5 mM | L-Trp-0.024 μM | 278 | |
| Human serum | D-Trp-0.055 μM | ||||||
| urchin-CMOF/rGO | Tryptophan | pH = 6.5 | Human cerebrospinal fluid | 40, 80, 100 μM | — | 286 | |
| GCE/rGO-TsPro-Cs | Naproxen | pH = 7 | Biological fluids | 20–500 μM | R-Nap-0.4 μM | 287 | |
| S-Nap-0.9 μM | |||||||
| rGO-PDA-L-Lys/GCE | Tryptophan | pH = 7 | Human urine | 1–6 mM | D-Trp-0.2 μM | 288 | |
| Human serum | L-Trp-2.3 μM | ||||||
| TBO@rGO/GCE | Naproxen | pH = 6 | Bovine serum albumin | 5.0 × 10−4–5.0 × 10−3 mol L−1 | 3.3 × 10−7 mol L−1 | 289 | |
| N-rGO/β-CD-Cu-CMC/GCE | Tryptophan | pH = 7 | Human urine | 0.01–5 mM | L-Trp-0.063 μM | 290 | |
| Human serum | D-Trp-0.0035 μM | ||||||
| SA-CS-NGC/GCE | Tryptophan | pH = 7 | Human urine | 0.1–0.9 mM | — | 45 | |
| Human serum | |||||||
| 3D-G/HP-β-CD/GCE | Tryptophan | pH = 7 | Amino acids | 0.5–175 μM | L-Trp-9.6 nM | 291 | |
| D-Trp-38 nM | |||||||
| 3D-rGO/Pd @ Au/CM-β-CD | Tyrosine | pH = 7 | Glucose (Glu), ascorbic acid (AA), dopamine (DA), 5-hydroxytryptamine, serine | 0.8–130 μM | L-Trp-52 nM | 292 | |
| D-Trp-96 nM | |||||||
| RGO/PhenCu/GCE | Tryptophan | pH = 5.3–7.0 | Biological sample | 3–6 mM | — | 293 | |
| NH2-GQDs/β-CD/GCE | Tryptophan | pH = 7 | Physiological substances (glycine, alanine, proline, tyrosine, methionine, serine, glutamate, arginine, phenylalanine, threonine, lysine, histidine, glutamine, valine, lactose, glucose, fructose, and maltose) | 1.0–30.0 μM | L-Trp- 0.65 μM | 294 | |
| D-Trp-0.12 μM | |||||||
| GR-MIP/GCE | Levodopa | pH 1.3 | Human blood | 0.4–100 μM | 0.012 μM | 295 | |
| RGO-Au/L-Glu/GCE | Tryptophan | pH = 6 | Amino acids (lysine, mandelic acid, and phenylalanine) | 1–5 mM | L-Trp- 0.28 mM | 296 | |
| RGO-Au/d-Glu/GCE | D-Trp- 0.86 mM | ||||||
| Biosensor | GO-Au NRs | D-Amino acid | pH = 7 | Valine | 5 × 10−4−30 mM | 1.09 × 10−9 mM | 297 |
| γ-CD-GO | D-Phenylalanine | pH = 10.5 | Zebrafish | 0.1 μg mL−1 | F-L-Phe-52 × 10−6 M | 298 | |
| F-D-Phe-2.3 × 10−6 M | |||||||
| D-His-ZIF-8 @ CoFe-PDA/GCE | Tryptophan | — | Human urine | 1–6 mM | L-Trp-0.066 mM | 299 | |
| Human serum | D-Trp-0.15 mM | ||||||
| Terahertz | Amino acid | pH = 7.4 | Arginine, methionine, phenylalanine | 0.01 g mL−1 | L-Phe- 60.5 nmol | 300 | |
| L-Met- 67 nmol | |||||||
| PMB/PILs/rGO/Au NPs/AN | — | pH = 7.4 | SARS-CoV-2 protein | 0.1–1000 ng mL−1 | 38 pg mL−1 | 301 | |
| Chiral-induced spin selectivity Au/Ni | Double-stranded-DNA | pH = 7.2 | Dengue virus | — | 0.12 pM | 302 | |
| Graphene field-effect transistor | DNA | pH = 7.4 | Serum and cell supernatants | 10 pg mL−1–800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ng mL−1 ng mL−1 |
3.24 pg mL−1 | 303 | |
| Micro-tapered long-period fibre grating/GO | — | pH = 4.5 | Pepsin | 1–1000 ng mL−1 | 25.79 ng mL−1 | 304 | |
| L/D-CNT/PPy/Pt NPs@β-CD | Amino acids | pH = 7.4 | L-tyrosine tablets | 3–30 μM | 0.107 nM | 305 |
Detecting specific proteins through DNA hybridization in biosensing can be difficult because of steric hindrances caused by proteins. However, in 2022, Chen et al. developed a portable sensor device that can detect vascular endothelial growth factor (VEGF) using unique materials, including graphene.303 VEGF is a significant factor that regulates essential biological processes such as tumor growth, vascular penetration, and tissue proliferation. The researchers used a graphene field-effect transistor (GFET) with a captured DNA strand anchored on the channel intersection to identify a second DNA strand and assess its protein content (Fig. 18A). This allowed the sensor to amplify the detection signal through DNA hybridization. To make the design easier to understand, the researchers added a DNA strand (T1) with a different aptamer sequence to the graphene medium interface and then released the aptamer to create a double-stranded DNA/aptamer complex (P1-T2) (Fig. 18B). However, selecting the appropriate aptamer for proteins is a significant challenge, and the detection of proteins depends heavily on the forms of aptamers used in the examination process. Therefore, additional study is required to identify the appropriate aptamer/protein pairings, which may improve future results.
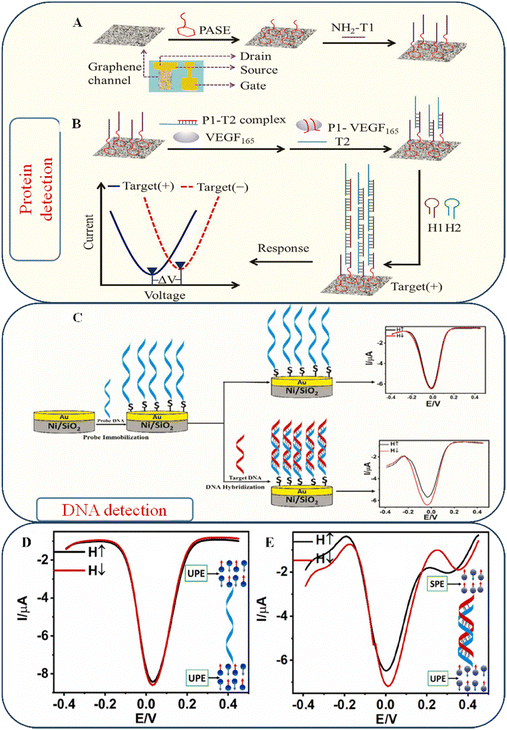 | ||
| Fig. 18 (A) Overarching structure of the biosensor, including a working graphene channel. (B) Detection of VEGF165 using a GFET-based sensing approach. Reproduced with permission from ref. 303 Copyright 2022 Elsevier. (C) Diagrammatic presentation of the spin-based electrochemical hybridization for DNA detection. (D) DPV curves showing the I–V relationship of SAMs for (C) probe-DNA. (E) Hybridization probe DNA based on target DNA sequences that are a perfect match. Reproduced with permission from ref. 302, Copyright 2023 Elsevier. | ||
Labeling DNA without changing its underlying structure is a complex method that requires a sophisticated approach.306 Recently, Bangruwa et al. developed a method for detecting the dengue virus using a DNA hybridized sensor that utilizes spin recognition.302 The first step of the two-step process involves chemically processing and immobilizing single-stranded DNA (ssDNA) molecules on an Au/Ni device. In the second step, modified probe DNA is used together with the target DNA in an electrochemical process that employs chiral-induced spin selectivity. Using spin-based DPV, the authors detected a specific part of the dengue virus that is not targeted by drugs. The spin polarization remained constant when the I–V curves of probe-DNA monolayers were measured in different magnetic-field directions (as shown in Fig. 18C). However, DPV measurements (Fig. 18D and E) confirmed the presence of the dengue virus. While the DNA detection results are valid, there is a considerable human error. The spin filtration of DNA is highly dependent on its molecular structure, and even minor changes in conformation can significantly impact spin polarization values because DNA is double-stranded. Therefore, it is essential to carefully examine external disturbances, such as current errors, as they can influence the hybridization process.
Similar to proteins, AAs are essential for various biological processes, including physiological control and human metabolism. It is crucial to identify susceptible and immediate chiral AAs for drug development, food analysis, and clinical disease diagnosis, as discussed in Section 2. The synthesis and chiral structure of AAs are significant in this regard. In 2022, Zhou et al. discovered that charging Au NPs modifies the dielectric constant of the interface.297 This allows an enzyme to attach to the Au nanorods (NRs) using gold-binding peptides (GBPs) that merge with them, enhancing the enzyme's positioning for capturing its target and facilitating the transmission of electrons from the enzyme to the Au NRs. An improved artificial receptor is necessary for detecting chiral AAs in living organisms. This can be achieved through a Terahertz biosensor298 and a zebrafish model sensor,300 which have excellent evaluation and solid anti-interference capabilities. Enantioselective labeling and chiro-selective graphene-based probes can provide ideal nanoplatforms for tracking chiral recognition processes in vivo. However, the sensitivity of the metasurface biosensor relies upon the number of analyte receptors it has, which can affect its ability to detect chiral AA enantiomers in living beings.
4.7. Impacts of chiral graphene in a neuromorphic artificial intelligence (AI) system
The relevance of memristors in artificial neuronal synapses has grown significantly due to the fast expansion of neuromorphic computing. Memristors using 2D materials in neuromorphic computing need help scaling up their performance to satisfy the rigorous demands of real-world applications. However, the potential of graphene in simulating brain-controlled senses (i.e., tactile sensors, taste sensors, gas sensors, etc.) is a fascinating area of research.307–310 Graphene physical sensors detect external environments and generate output signals for task recognition, whereas ML techniques use data to establish the relationship between known environments and output signals. Several authors have shown that chirality and other parameters may directly affect the mechanical properties of graphene structures and may indirectly complicate the use of chiral graphene in neuromorphic devices controlled by AI and machine learning.311,312 Zhang et al. found that the relationship with chirality becomes more pronounced whenever the tension intensifies in the graphene sheets.312 Luckily, the incorporation of foreign materials (i.e., O, N, etc.) in the graphene structure has the potential to alter the electron orbitals and thus affect the crystal structure. In a recent publication, Maity and their team investigated N2-incorporated graphene using DFT calculations. They devised a spin-controlled spectral analyzer based on graphene that could make a unique synthetic image pattern. They also used deep learning techniques inspired by neuromorphic vision to find chiral combinations or isomer blenders.313 Furthermore, chiral graphene sensors may use ML to perform statistical analysis, categorization, and diagnosis while considering accuracy limitations. In this era of ever-smaller electronic devices, scientists in the field of molecular electronics are concentrating on ways to employ self-organizing molecular building blocks in their research. Nevertheless, an essential concern in molecular electronics is how the junction acts—as a tunnel barrier or a pathway for electron or hole conduction through the molecule. Thus, the CISS effect as a new alternative becomes more noticeable when regulating spin transfer via molecules.314,315 Intriguingly, Naaman and their team explained that CNTs and graphene can increase spin–orbital coupling (SOC) by overlapping p-orbitals in neighboring atoms. In contrast, SOC in chiral molecules results in changes in the energy of the spin states of the moving electron.316 Also, Schranghamer et al. created graphene memristive synaptic devices that could significantly affect the progress in making energy-efficient devices for neuromorphic computing.317Even though there is little real-world evidence showing how chiral graphene directly affects the properties of neuromorphic devices, researchers today have yet to generate much qualitative data and empirical analysis. Nevertheless, the use of chiral graphene has the potential to provide a favorable perspective and possibilities for the advancement of affordable and energy-efficient neuromorphic devices for future generations.
5. Challenges and prospects
Despite various challenges in chiral synthesis, a chiral graphene structure in the chiral world has significant implications for the future of biology, pharmacy, and chemical engineering. We discussed chiral graphene synthesis and its application in various fields in previous sections. In each section, we extensively covered the advantages and limitations of chiral graphene. We intended to provide ideas to the researchers to encourage the development of new functional materials integrating the advantages of graphene and chiral materials. However, despite some advancements in chiral graphene synthesis, achieving precise control over the synthesis of chiral graphene structures remains a complex process. Significant challenges, such as developing efficient methods for synthesizing complex chiral graphene structures, still need to be addressed. We also recommend researchers to explore new synthesis processes such as asymmetric synthesis of chiral graphene, which involves the use of chiral catalysts to selectively produce one enantiomer of chiral graphene. We recommend an article published by Zhao and their team to explore more synthesis processes to gain new insights into chiral graphene structures.318 However, the unique properties of chiral graphene structures give rise to several issues that require further exploration. Future studies should focus on the following points:• While programmable chiral metamaterials lack the responsiveness required for information or data transmission systems, they are promising for detecting batches or constant flow reactive strategies in biological and pharmaceutical applications. Chiroptic substances will be investigated for various applications because they have unique potential for polarization conversion or switching outside the chemical domain. When proactive chiroptical metasurface materials convert polarization with almost no loss, this will open interesting new possibilities for polarizing division doubling in analog optoelectronic computing. Chirality, classical geometrical implications, and exotic states of surfaces with atypically rich environments provide opportunities for discovering novel optical phenomena.
• New methods of detecting the chiral configuration of a molecule in the specimen are crucial in the pharmaceutical sector for advancing the treatment of cardiac diseases, diabetes, and hypertension. Innovative techniques for determining chirality, such as nuclear magnetic resonance spectroscopy and Raman optical activity, have been developed, but more precise approaches are needed to detect interactions between biomolecules and chiral structures and explain enantioselective surface processes.
• The use of nanomaterials in graphene chiral separation techniques has recently attracted considerable attention. Chiral detachment is a challenging and expensive process, and isolating intermediate and impurities of an individual racemic is essential. Chiral separation of bioactive chemicals across membranes is impacted by the pore size and shape of chiral graphene structures, affecting chiral restoration during computational chemistry. Additionally, further study is needed to determine how mechanical whirling creates chiral separations.
• On-surface reactions are an exciting area of study for the transfer of chirality, and a large amount of further research is needed. The structural chirality of precursors and their transfer from helical chirality to linear chirality through single-molecule reactions are significant aspects of chiral transfer that require more studies. Although chiral organic reconfigurable structures can be synthesized and combined with other molecules to form complex systems, further research is needed to create and develop technologies that can be employed directly in the transfer process.
• Because of its widespread use in imaging and biosensor applications, CD has attracted considerable attention in the field of optoelectronic devices. The frequency and amplitude of the CD spectrum shift whenever the Fermi energy and graphene nanostructures are modified. Rotational and longitudinal dichroism are typical techniques for controlling polarized light; however, a chiral metascreen based on graphene's adjustable frequency response is advantageous. Further research is needed to identify more active metasurfaces to comprehend the fundamental reaction mechanism, enantioselective components, racemization, enantiomeric separation, drug-transport mechanism, and quality assurance of protein-based drugs.
• Most of the references mentioned earlier pertain to the domains of sensors and biomedicine. In the biomedical area, it is not a prerequisite for graphene or its derivatives to be conductive. Consequently, most researchers do not prioritize the investigation of chiral graphene conductivity. Nevertheless, the increasing need for adaptable electronics and conductive ink319–323 presents a lucrative opportunity for researchers to establish themselves as innovators in this domain and discover novel uses for chiral graphene. In recent years, researchers in printing technology have extensively investigated graphene-based materials.324,325 These materials have been used in many applications, such as graphene-based conductive inks and wearable and printed sensors.326,327 In addition, several critical technical parameters and issues must be addressed to develop conductive inks and printing systems that effectively use carbon derivatives' exceptional capabilities in printed electronics.
In general, chiral graphene materials show the potential to emerge as a significant material in the realm of advanced science, facilitating the mass manufacture of enantiomeric substances. Continuing research endeavors are anticipated to provide novel chiral graphene materials, potentially propelling the exploration of basic chirality in natural systems. The biggest challenge that persists practically is synthesizing chiral graphene materials on an immense scale while maintaining excellent stability and affordable prices. Furthermore, the existing state of research is limited to the study of AAs and the determination of a select number of chiral pharmaceuticals and small to medium scale scientific studies. Hence, more investigations should be conducted on the real-world application of such nanomaterials to expedite the advancement of chiral graphene materials and establish a prominent position in the scientific domain.
6. Conclusion
Our review provides a comprehensive analysis of the synthesis and applications of chiral graphene materials; however, it was impossible to cover every aspect of this fascinating subject. Chirality plays a unique role in living organisms and offers promising opportunities for researchers. We thoroughly examined recent research on the synthesis of chiral graphene, including its drawbacks and potential solutions. While computer-aided design (e.g., molecular modeling, design of chiral catalysts, and chiral descriptors) is the focus of most studies to design chiral structures to make superimposable mirror images, further research is needed to understand chiral phenomenon's underlying chemical and physical mechanisms. In addition, various design tools and methods provide rational arguments for differentiating chiral analytes, a compelling synthesis of chiral structures, and broad applicability in sensing, pharmaceuticals, and photodetection.Ethical approval
This article does not include any studies with human participants or animals performed by any of the author.Consent for publication
All authors have given their consent for publication.Data availability
Data will be made available on request.Conflicts of interest
The authors declare no competing interest.Acknowledgements
Hongyun So is grateful for the financial support from the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT of Republic of Korea (No. RS-2024-00359316 and RS-2023-00260527).References
- P. Cintas, Angew. Chem., Int. Ed., 2007, 46, 4016–4024 CrossRef CAS PubMed.
- J. D. Marth, Nat. Cell Biol., 2008, 10, 1015 CrossRef CAS PubMed.
- S. A. Wolf, D. D. Awschalom, R. A. Buhrman, J. M. Daughton, S. von Molnár, M. L. Roukes, A. Y. Chtchelkanova and D. M. Treger, Science, 2001, 294, 1488–1495 CrossRef CAS PubMed.
- S. Wang, R. Li, C. Zhang, Y. Li, B. Li and Y. Yang, Mater. Lett., 2013, 106, 71–74 CrossRef CAS.
- H. F. Krug and P. Wick, Angew. Chem., Int. Ed., 2011, 50, 1260–1278 CrossRef CAS PubMed.
- Y. Guan, Z. Wang, B. Ai, C. Chen, W. Zhang, Y. Wang and G. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 50192–50202 CrossRef CAS PubMed.
- P. Jiao, H. Hasni, N. Lajnef and A. H. Alavi, Mater. Des., 2020, 187, 108214 CrossRef CAS.
- P. Jiao, T. Chen and Y. Xie, Compos. Struct., 2021, 256, 113053 CrossRef CAS.
- L. Xiao, T. An, L. Wang, X. Xu and H. Sun, Nano Today, 2020, 30, 100824 CrossRef CAS.
- Y. Duan and S. Che, Adv. Mater., 2023, 35, 202205088 Search PubMed.
- L. Song, S. Wang, N. A. Kotov and Y. Xia, Anal. Chem., 2012, 84, 7330–7335 CrossRef CAS PubMed.
- W. Yan, L. Xu, W. Ma, L. Liu, L. Wang, H. Kuang and C. Xu, Small, 2014, 10, 4293–4297 CrossRef CAS PubMed.
- H. Pei, F. Chen, W. Guo, Q. Jia, R. Guo, N. Liu and Z. Mo, J. Electrochem. Soc., 2021, 168, 067515 CrossRef CAS.
- S. Luo, Y. Wang, X. Tong and Z. Wang, Nanoscale Res. Lett., 2015, 10, 199 CrossRef PubMed.
- L. Lin, X. Xu, J. Yin, J. Sun, Z. Tan, A. L. Koh, H. Wang, H. Peng, Y. Chen and Z. Liu, Nano Lett., 2016, 16, 4094–4101 CrossRef CAS PubMed.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- J. T. Kim and C.-G. Choi, Opt. Express, 2012, 20, 3556 CrossRef CAS PubMed.
- A. Farmani, M. Miri and M. H. Sheikhi, IEEE Photonics Technol. Lett., 2018, 30, 153–156 CAS.
- C. Cen, H. Lin, J. Huang, C. Liang, X. Chen, Y. Tang, Z. Yi, X. Ye, J. Liu, Y. Yi and S. Xiao, Sensors, 2018, 18, 4489 CrossRef PubMed.
- L. Zhang, M. Farhat and K. N. Salama, Sensors, 2020, 20, 2347 CrossRef CAS PubMed.
- P. J. Evans, J. Ouyang, L. Favereau, J. Crassous, I. Fernández, J. Perles and N. Martín, Angew. Chem., Int. Ed., 2018, 57, 6774–6779 CrossRef CAS PubMed.
- C. M. Cruz, I. R. Márquez, I. F. A. Mariz, V. Blanco, C. Sánchez-Sánchez, J. M. Sobrado, J. A. Martín-Gago, J. M. Cuerva, E. Maçôas and A. G. Campaña, Chem. Sci., 2018, 9, 3917–3924 RSC.
- K. Kato, Y. Segawa, L. T. Scott and K. Itami, Angew. Chem., 2018, 130, 1351–1355 CrossRef.
- T. Cao, L. Zhang, R. E. Simpson, C. Wei and M. J. Cryan, Opt. Express, 2013, 21, 27841 CrossRef PubMed.
- Y. Zhao, M. A. Belkin and A. Alù, Nat. Commun., 2012, 3, 870 CrossRef CAS PubMed.
- Y. Zhou, S. Wu, H. Zhou, H. Huang, J. Zhao, Y. Deng, H. Wang, Y. Yang, J. Yang and L. Luo, Environ. Int., 2018, 121, 523–537 CrossRef CAS PubMed.
- J. Ceramella, D. Iacopetta, A. Franchini, M. De Luca, C. Saturnino, I. Andreu, M. S. Sinicropi and A. Catalano, Appl. Sci., 2022, 12, 10909 CrossRef CAS.
- V. K. Vashistha, Chirality, 2022, 34, 833–847 CrossRef CAS PubMed.
- G. Qing, S. Zhao, Y. Xiong, Z. Lv, F. Jiang, Y. Liu, H. Chen, M. Zhang and T. Sun, J. Am. Chem. Soc., 2014, 136, 10736–10742 CrossRef CAS PubMed.
- Y. Zhang, M. K. Saha and I. Bernal, CrystEngComm, 2003, 5, 34–37 RSC.
- Q. Han, Q. Xia, D. Guo, C. Li and Y. Fu, Anal. Methods, 2015, 7, 5387–5390 RSC.
- T. Montag and C. M. Thiele, Chem. – Eur. J., 2013, 19, 2271–2274 CrossRef CAS PubMed.
- C. Thoelen, M. De bruyn, E. Theunissen, Y. Kondo, I. F. J. Vankelecom, P. Grobet, M. Yoshikawa and P. A. Jacobs, J. Membr. Sci., 2001, 186, 153–163 CrossRef CAS.
- C. Meng, Q. Chen, H. Tan, Y. Sheng and H. Liu, J. Membr. Sci., 2018, 555, 398–406 CrossRef CAS.
- C. Meng, Y. Sheng, Q. Chen, H. Tan and H. Liu, J. Membr. Sci., 2017, 526, 25–31 CrossRef CAS.
- F. Rahmani, S. Nouranian, M. Mahdavi and A. Al-Ostaz, J. Nanopart. Res., 2016, 18, 320 CrossRef.
- B. Sadhasivam, M. F. Rigana, B. Rukmanikrishnan and S. Muthusamy, Polym. Bull., 2018, 75, 829–849 CrossRef CAS.
- M. Dinari, E. Salehi and A. Abdolmaleki, Ultrason. Sonochem., 2018, 41, 59–66 CrossRef CAS PubMed.
- A. Hirano and T. Kameda, ACS Appl. Nano Mater., 2021, 4, 2486–2495 CrossRef CAS.
- M. Zhang, Y. Ma, H. Wang, B. Wang, Y. Zhou, Y. Liu, M. Shao, H. Huang, F. Lu and Z. Kang, ACS Appl. Mater. Interfaces, 2021, 13, 5877–5886 CrossRef CAS PubMed.
- W. Wei, K. Qu, J. Ren and X. Qu, Chem. Sci., 2011, 2, 2050 RSC.
- L. Guo, Q. Zhang, Y. Huang, Q. Han, Y. Wang and Y. Fu, Bioelectrochemistry, 2013, 94, 87–93 CrossRef CAS PubMed.
- D. M. Božinovski, P. V. Petrović, M. R. Belić and S. D. Zarić, Chem. Phys. Chem., 2018, 19, 1226–1233 CrossRef PubMed.
- X. Niu, Z. Mo, X. Yang, C. Shuai, N. Liu and R. Guo, Bioelectrochemistry, 2019, 128, 74–82 CrossRef CAS PubMed.
- X. Niu, X. Yang, Z. Mo, J. Wang, Z. Pan, Z. Liu, C. Shuai, G. Liu, N. Liu and R. Guo, Bioelectrochemistry, 2020, 131, 107396 CrossRef CAS PubMed.
- P. Babaei and J. Safaei-Ghomi, Mater. Chem. Phys., 2021, 267, 124668 CrossRef CAS.
- V. Ranc and Z. Chaloupková, Anal. Chim. Acta, 2020, 1129, 69–75 CrossRef CAS PubMed.
- S. W. Smith, Toxicol. Sci, 2009, 110, 4–30 CrossRef CAS PubMed.
- W. Ma, L. Xu, A. F. de Moura, X. Wu, H. Kuang, C. Xu and N. A. Kotov, Chem. Rev., 2017, 117, 8041–8093 CrossRef CAS PubMed.
- A. Döring, E. Ushakova and A. L. Rogach, Light: Sci. Appl., 2022, 11, 75 CrossRef PubMed.
- Y. Zhao, Y. Zhang, H. Liu and B. Sun, Anal. Bioanal. Chem., 2022, 414, 4885–4896 CrossRef CAS PubMed.
- M. Gogoi, R. Goswami, A. Borah and S. Hazarika, Sep. Purif. Technol., 2022, 283, 120201 CrossRef CAS.
- X. Shang, C. H. Park, G. Y. Jung, S. K. Kwak and J. H. Oh, ACS Appl. Mater. Interfaces, 2018, 10, 36194–36201 CrossRef CAS PubMed.
- S. Mallakpour, Amino Acids, 2011, 40, 487–492 CrossRef CAS PubMed.
- S. Mallakpour, P. Asadi and M. R. Sabzalian, Amino Acids, 2011, 41, 1215–1222 CrossRef CAS PubMed.
- Y.-P. Xue, C.-H. Cao and Y.-G. Zheng, Chem. Soc. Rev., 2018, 47, 1516–1561 RSC.
- X. Bai, J. Ke, X. Qiu, H. Liu, Y. Ji and J. Chen, Sep. Purif. Technol., 2022, 290, 120833 CrossRef CAS.
- S. M. Badr, M. Azlouk, E. Zor, H. Bingol and M. Durmaz, Mol. Catal., 2022, 526, 112383 CrossRef CAS.
- L. C. Preiss, L. Werber, V. Fischer, S. Hanif, K. Landfester, Y. Mastai and R. Muñoz-Espí, Adv. Mater., 2015, 27, 2728–2732 CrossRef CAS PubMed.
- Y. Fang, X. Liu, Z. Liu, L. Han, J. Ai, G. Zhao, O. Terasaki, C. Cui, J. Yang, C. Liu, Z. Zhou, L. Chen and S. Che, Chemistry, 2023, 9, 460–471 CrossRef CAS.
- M. Sadiq, R. Aman, K. Saeed, M. S. Ahmad and M. A. Zia, Mod. Res. Catal., 2015, 04, 43–49 CrossRef CAS.
- Z. Liu, X. Li, H. Masai, X. Huang, S. Tsuda, J. Terao, J. Yang and X. Guo, Sci. Adv., 2021, 7, eabe4365 CrossRef CAS PubMed.
- M. Fujita, K. Wakabayashi, K. Nakada and K. Kusakabe, J. Phys. Soc. Jpn., 1996, 65, 1920–1923 CrossRef CAS.
- K. Nakada, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 17954–17961 CrossRef CAS PubMed.
- M. Ezawa, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 045432 CrossRef.
- Y.-W. Son, M. L. Cohen and S. G. Louie, Phys. Rev. Lett., 2006, 97, 216803 CrossRef PubMed.
- O. V. Yazyev, Rep. Prog. Phys., 2010, 73, 056501 CrossRef.
- M. Y. Han and P. Kim, Nano Convergence, 2014, 1, 1 CrossRef PubMed.
- Z. Geng, B. Hähnlein, R. Granzner, M. Auge, A. A. Lebedev, V. Y. Davydov, M. Kittler, J. Pezoldt and F. Schwierz, Ann. Phys., 2017, 529, 1700033 CrossRef.
- Z. Chen, A. Narita and K. Müllen, Adv. Mater., 2020, 32, 2001893 CrossRef CAS PubMed.
- D. G. de Oteyza, A. García-Lekue, M. Vilas-Varela, N. Merino-Díez, E. Carbonell-Sanromà, M. Corso, G. Vasseur, C. Rogero, E. Guitián, J. I. Pascual, J. E. Ortega, Y. Wakayama and D. Peña, ACS Nano, 2016, 10, 9000–9008 CrossRef CAS PubMed.
- N. Merino-Díez, J. Li, A. Garcia-Lekue, G. Vasseur, M. Vilas-Varela, E. Carbonell-Sanromà, M. Corso, J. E. Ortega, D. Peña, J. I. Pascual and D. G. de Oteyza, J. Phys. Chem. Lett., 2018, 9, 25–30 CrossRef PubMed.
- N. Gorjizadeh, A. A. Farajian and Y. Kawazoe, Nanotechnology, 2009, 20, 015201 CrossRef PubMed.
- N. Lu, H. Guo, W. Hu, X. Wu and X. C. Zeng, J. Mater. Chem. C, 2017, 5, 3121–3129 RSC.
- K. Rallis, P. Dimitrakis, I. G. Karafyllidis, A. Rubio and G. C. Sirakoulis, IEEE Trans. Nanotechnol., 2021, 20, 151–160 CAS.
- A. Berdonces-Layunta, J. Lawrence, S. Edalatmanesh, J. Castro-Esteban, T. Wang, M. S. G. Mohammed, L. Colazzo, D. Peña, P. Jelínek and D. G. de Oteyza, ACS Nano, 2021, 15, 5610–5617 CrossRef CAS PubMed.
- T. Wang, S. Sanz, J. Castro-Esteban, J. Lawrence, A. Berdonces-Layunta, M. S. G. Mohammed, M. Vilas-Varela, M. Corso, D. Peña, T. Frederiksen and D. G. de Oteyza, Nano Lett., 2022, 22, 164–171 CrossRef CAS PubMed.
- J. Li, S. Sanz, N. Merino-Díez, M. Vilas-Varela, A. Garcia-Lekue, M. Corso, D. G. de Oteyza, T. Frederiksen, D. Peña and J. I. Pascual, Nat. Commun., 2021, 12, 5538 CrossRef CAS PubMed.
- M. I. Katsnelson, K. S. Novoselov and A. K. Geim, Nat. Phys., 2006, 2, 620–625 Search PubMed.
- T. Tudorovskiy, K. J. A. Reijnders and M. I. Katsnelson, Phys. Scr., 2012, T146, 014010 CrossRef.
- C. Bai and X. Zhang, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 075430 CrossRef.
- A. V. Parafilo, I. V. Krive, E. N. Bogachek, U. Landman, R. I. Shekhter and M. Jonson, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 045427 CrossRef.
- W.-Y. He, Z.-D. Chu and L. He, Phys. Rev. Lett., 2013, 111, 066803 CrossRef PubMed.
- C.-S. Park, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 115423 CrossRef.
- J.-B. Qiao and L. He, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 075410 CrossRef CAS.
- V. Kleptsyn, A. Okunev, I. Schurov, D. Zubov and M. I. Katsnelson, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 165407 CrossRef.
- M. Barbier, P. Vasilopoulos, F. M. Peeters and J. M. Pereira, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 155402 CrossRef.
- R. V. Gorbachev, J. C. W. Song, G. L. Yu, A. V. Kretinin, F. Withers, Y. Cao, A. Mishchenko, I. V. Grigorieva, K. S. Novoselov, L. S. Levitov and A. K. Geim, Science, 2014, 346, 448–451 CrossRef CAS PubMed.
- A. Varlet, M.-H. Liu, D. Bischoff, P. Simonet, T. Taniguchi, K. Watanabe, K. Richter, T. Ihn and K. Ensslin, Phys. Status Solidi RRL, 2016, 10, 46–57 CrossRef CAS.
- A. Iurov, L. Zhemchuzhna, G. Gumbs, D. Huang and P. Fekete, Phys. Rev. B, 2022, 105, 115309 CrossRef CAS.
- N. Benlakhouy, A. Jellal and H. Bahlouli, Ann. Phys., 2023, 449, 169202 CAS.
- S. Bala Kumar and J. Guo, Appl. Phys. Lett., 2012, 100, 163102 CrossRef.
- N. Purdie, J. Am. Chem. Soc., 1996, 118, 12871 CrossRef CAS.
- J. Cao, H.-J. Yin and R. Song, Front Mater. Sci., 2013, 7, 83–90 CrossRef.
- M. Decker, M. W. Klein, M. Wegener and S. Linden, Opt. Lett., 2007, 32, 856 CrossRef CAS PubMed.
- M. Kuwata-Gonokami, N. Saito, Y. Ino, M. Kauranen, K. Jefimovs, T. Vallius, J. Turunen and Y. Svirko, Phys. Rev. Lett., 2005, 95, 227401 CrossRef PubMed.
- E. Suárez Morell, L. Chico and L. Brey, 2D Mater., 2017, 4, 035015 CrossRef.
- W. Ma, H. Kuang, L. Xu, L. Ding, C. Xu, L. Wang and N. A. Kotov, Nat. Commun., 2013, 4, 2689 CrossRef PubMed.
- H. T. Maune, S. Han, R. D. Barish, M. Bockrath, W. A. G. III, P. W. K. Rothemund and E. Winfree, Nat. Nanotechnol., 2010, 5, 61–66 CrossRef CAS PubMed.
- J. Deng, W. F. Lim, J. Chen and H. J. Quah, Optik, 2022, 251, 168351 CrossRef CAS.
- M. Amin, O. Siddiqui and M. Farhat, Phys. Rev. Appl., 2020, 13, 024046 CrossRef CAS.
- G. Dai, X. Chen, Y. Jin and J. Wang, Molecules, 2022, 27, 6525 CrossRef CAS PubMed.
- X.-T. Kong, R. Zhao, Z. Wang and A. O. Govorov, Nano Lett., 2017, 17, 5099–5105 CrossRef CAS PubMed.
- Y. Wang, J. Dong, Z. Wang, S. Zhou, Q. Wang, Q. Han, W. Gao, K. Ren and J. Qi, Opt. Express, 2019, 27, 33869 CrossRef CAS PubMed.
- T. Wang, Y. Wang, L. Luo, L. Wang and Z. Zhang, Plasmonics, 2017, 12, 829–833 CrossRef CAS.
- L. Ju, B. Geng, J. Horng, C. Girit, M. Martin, Z. Hao, H. A. Bechtel, X. Liang, A. Zettl, Y. R. Shen and F. Wang, Nat. Nanotechnol., 2011, 6, 630–634 CrossRef CAS PubMed.
- H. Yan, X. Li, B. Chandra, G. Tulevski, Y. Wu, M. Freitag, W. Zhu, P. Avouris and F. Xia, Nat. Nanotechnol., 2012, 7, 330–334 CrossRef CAS PubMed.
- V. W. Brar, M. S. Jang, M. Sherrott, J. J. Lopez and H. A. Atwater, Nano Lett., 2013, 13, 2541–2547 CrossRef CAS PubMed.
- F. Li, T. Tang, Y. Mao, L. Luo, J. Li, J. Xiao, K. Liu, J. Shen, C. Li and J. Yao, Ann. Phys., 2020, 532, 2000065 CrossRef CAS.
- Y. Cui, X. Wang, H. Jiang and Y. Jiang, J. Phys. D: Appl. Phys., 2022, 55, 135102 CrossRef CAS.
- Y. Zhang, H. Liu, R. Xu, Z. Qin, C. Teng, S. Deng, M. Chen, Y. Cheng, H. Deng, H. Yang, S. Qu and L. Yuan, Opt. Express, 2021, 29, 21020 CrossRef CAS PubMed.
- H. Zhou, S. Su, H. Ma, Z. Zhao, Z. Lin, W. Qiu, P. Qiu, B. Huang and Q. Kan, Opt. Express, 2020, 28, 31954 CrossRef CAS PubMed.
- M. Omor Faruk Patwary, M. Mahbubur Rahman, M. Khalid Bin Islam, M. Ackas Ali, M. A. Halim and F. Ahmed, Comput. Theor. Chem., 2022, 1207, 113503 CrossRef CAS.
- L. A. Nguyen, H. He and C. Pham-Huy, Int. J. Biomed. Sci., 2006, 2, 85–100 CrossRef CAS PubMed.
- Q. Cheng, H. Pei, Q. Ma, R. Guo, N. Liu and Z. Mo, Chem. Eng. J., 2023, 452, 139499 CrossRef CAS.
- P. O. Carvalho, Q. B. Cass, S. A. Calafatti, F. J. Contesini and R. Bizaco, Braz. J. Chem. Eng., 2006, 23, 291–300 CrossRef CAS.
- V. L. E. Lima, Quim. Nova, 1997, 20, 657–663 CrossRef CAS.
- F. Wang, D. Pizzi, Y. Lu, K. He, K. J. Thurecht, M. R. Hill, P. J. Marriott, M. M. Banaszak Holl, K. Kempe and H. Wang, Angew. Chem., Int. Ed., 2023, 135, e202212139 CrossRef.
- Y. Lu, H. Zhang, Y. Zhu, P. J. Marriott and H. Wang, Adv. Funct. Mater., 2021, 31, 2101335 CrossRef CAS.
- J. Shen and Y. Okamoto, Chem. Rev., 2016, 116, 1094–1138 CrossRef CAS PubMed.
- M. Gogoi, R. Goswami, A. Borah, H. Sarmah, P. Rajguru and S. Hazarika, Sep. Purif. Technol., 2021, 279, 119704 CrossRef CAS.
- Y. Lu, H. Zhang, J. Y. Chan, R. Ou, H. Zhu, M. Forsyth, E. M. Marijanovic, C. M. Doherty, P. J. Marriott, M. M. B. Holl and H. Wang, Angew. Chem., Int. Ed., 2019, 58, 16928–16935 CrossRef CAS PubMed.
- F. Perreault, A. Fonseca de Faria and M. Elimelech, Chem. Soc. Rev., 2015, 44, 5861–5896 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- R. D. Martínez-Orozco, H. C. Rosu, S.-W. Lee and V. Rodríguez-González, J. Hazard. Mater., 2013, 263, 52–60 CrossRef PubMed.
- B. Qiu, M. Xing and J. Zhang, Chem. Soc. Rev., 2018, 47, 2165–2216 RSC.
- D. Akinwande and D. Kireev, Nature, 2019, 576, 220–221 CrossRef CAS PubMed.
- R. You, Y. Liu, Y. Hao, D. Han, Y. Zhang and Z. You, Adv. Mater., 2020, 32, 1901981 CrossRef CAS PubMed.
- K. B. Jirage and C. R. Martin, Trends Biotechnol., 1999, 17, 197–200 CrossRef CAS PubMed.
- H. Gong, S. Zhang, N. Liu, J. Zhang, Q. Chen and H. Liu, Sep. Purif. Technol., 2022, 288, 120642 CrossRef CAS.
- H. Tan, T. Liu, X. Zhang, Q. Shan, J. Chen, Z. Li, H. Ihara and H. Qiu, Anal. Chem., 2020, 92, 13630–13633 CrossRef CAS PubMed.
- Y. Zhu, X. Li, Z. Bai, Y. Zeng, H. Jiang, X. Bai and R. Li, New J. Chem., 2023, 47, 11852–11858 RSC.
- X. Li, Q. Chen, X. Tong, S. Zhang and H. Liu, J. Membr. Sci., 2021, 634, 119350 CrossRef CAS.
- J. Liu, T. Chu, M. Cheng, Y. Su, G. Zou and S. Hou, J. Membr. Sci., 2023, 668, 121198 CrossRef CAS.
- J. Liu, G. Zou and S. Hou, Chem. Eng. J., 2023, 467, 143366 CrossRef CAS.
- D. D. Medina and Y. Mastai, Isr. J. Chem., 2018, 58, 1330–1337 CrossRef CAS.
- Q. Tang, N. Li, Q. Lu, X. Wang and Y. Zhu, Polymers, 2019, 11, 1737 CrossRef CAS PubMed.
- C. Meng, S. Zhang, Q. Chen, X. Li and H. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 10893–10901 CrossRef CAS PubMed.
- J. Liu, W. Yuan, C. Li, M. Cheng, Y. Su, L. Xu, T. Chu and S. Hou, ACS Appl. Mater. Interfaces, 2021, 13, 49215–49223 CrossRef CAS PubMed.
- P. L. Yap, S. Kabiri, D. N. H. Tran and D. Losic, ACS Appl. Mater. Interfaces, 2019, 11, 6350–6362 CrossRef CAS PubMed.
- W. H. Brooks, W. C. Guida and K. G. Daniel, Curr. Top. Med. Chem., 2011, 11, 760–770 CrossRef CAS PubMed.
- S. D. Chambers, F. Svec and J. M. J. Fréchet, J. Chromatogr. A, 2011, 1218, 2546–2552 CrossRef CAS PubMed.
- M. Ahmed, M. M. A. Yajadda, Z. J. Han, D. Su, G. Wang, K. (Ken) Ostrikov and A. Ghanem, J. Chromatogr. A, 2014, 1360, 100–109 CrossRef CAS PubMed.
- N. Li, J. Chen and Y.-P. Shi, Talanta, 2019, 191, 526–534 CrossRef CAS PubMed.
- P. Sajonz, W. Schafer, X. Gong, S. Shultz, T. Rosner and C. J. Welch, J. Chromatogr. A, 2007, 1145, 149–154 CrossRef CAS PubMed.
- R. Sancho and C. Minguillón, Chem. Soc. Rev., 2009, 38, 797 RSC.
- L. D. Barron, Strategies of Life Detection, Springer US, Boston, MA, 2008, pp. 187–201 Search PubMed.
- J. Yeom, U. S. Santos, M. Chekini, M. Cha, A. F. de Moura and N. A. Kotov, Science, 2018, 359, 309–314 CrossRef CAS PubMed.
- K. Banerjee-Ghosh, O. Ben Dor, F. Tassinari, E. Capua, S. Yochelis, A. Capua, S.-H. Yang, S. S. P. Parkin, S. Sarkar, L. Kronik, L. T. Baczewski, R. Naaman and Y. Paltiel, Science, 1979, 2018(360), 1331–1334 Search PubMed.
- R. R. E. Steendam, J. M. M. Verkade, T. J. B. van Benthem, H. Meekes, W. J. P. van Enckevort, J. Raap, F. P. J. T. Rutjes and E. Vlieg, Nat. Commun., 2014, 5, 5543 CrossRef CAS PubMed.
- H. Al-Bustami, G. Koplovitz, D. Primc, S. Yochelis, E. Capua, D. Porath, R. Naaman and Y. Paltiel, Small, 2018, 14, 1801249 CrossRef PubMed.
- T. Kurihara, M. Kojima, T. Yoshino and S. Matsunaga, Asian J. Org. Chem., 2020, 9, 368–371 CrossRef CAS.
- T. Nanda, S. K. Banjare, W.-Y. Kong, W. Guo, P. Biswal, L. Gupta, A. Linda, B. V. Pati, S. R. Mohanty, D. J. Tantillo and P. C. Ravikumar, ACS Catal., 2022, 12, 11651–11659 CrossRef CAS.
- V. Ondruš, L. Fišera and V. Bradac, ARKIVOC, 2001, v, 60–67 Search PubMed.
- C. Yang, Y. Li, S. Zhou, Y. Guo, C. Jia, Z. Liu, K. N. Houk, Y. Dubi and X. Guo, Nat. Chem., 2023, 15, 972–979 CrossRef CAS PubMed.
- R. Naaman, Y. Paltiel and D. H. Waldeck, Acc. Chem. Res., 2020, 53, 2659–2667 CrossRef CAS PubMed.
- T.-H. Lo, Y.-C. Weng, C.-F. Pai and J. G. Lin, AIP Adv., 2020, 10, 025120 CrossRef CAS.
- Y. Li, N. Zhang and K. Wang, Sci. China Inf. Sci., 2022, 65, 122404 CrossRef.
- C. Han, X. Hou, H. Zhang, W. Guo, H. Li and L. Jiang, J. Am. Chem. Soc., 2011, 133, 7644–7647 CrossRef CAS PubMed.
- H. Ouldali, K. Sarthak, T. Ensslen, F. Piguet, P. Manivet, J. Pelta, J. C. Behrends, A. Aksimentiev and A. Oukhaled, Nat. Biotechnol., 2020, 38, 176–181 CrossRef CAS PubMed.
- S. S. Pershoguba, T. Veness and L. I. Glazman, Phys. Rev. Lett., 2019, 123, 067001 CrossRef CAS PubMed.
- J. Crassous, Chem. Soc. Rev., 2009, 38, 830 RSC.
- T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- M. Raynal, P. Ballester, A. Vidal-Ferran and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2014, 43, 1660–1733 RSC.
- R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872–3890 CrossRef CAS PubMed.
- J. Labuta, J. P. Hill, S. Ishihara, L. Hanyková and K. Ariga, Acc. Chem. Res., 2015, 48, 521–529 CrossRef CAS PubMed.
- S. Huang, H. Yu and Q. Li, Adv. Sci., 2021, 8, 2002132 CrossRef PubMed.
- R. Basu, D. Kinnamon and A. Garvey, J. Appl. Phys., 2015, 118, 114302 CrossRef.
- A. Di Mauro, R. Randazzo, S. F. Spanò, G. Compagnini, M. Gaeta, L. D’Urso, R. Paolesse, G. Pomarico, C. Di Natale, V. Villari, N. Micali, M. E. Fragalà, A. D’Urso and R. Purrello, Chem. Commun., 2016, 52, 13094–13096 RSC.
- L. Đorđević, F. Arcudi, A. D’Urso, M. Cacioppo, N. Micali, T. Bürgi, R. Purrello and M. Prato, Nat. Commun., 2018, 9, 3442 CrossRef PubMed.
- J. M. Fernández-García, P. J. Evans, S. Filippone, M. A. Herranz and N. Martín, Acc. Chem. Res., 2019, 52, 1565–1574 CrossRef PubMed.
- M. Vázquez-Nakagawa, L. Rodríguez-Pérez, N. Martín and M. A. Herranz, Angew. Chem., Int. Ed., 2022, 134, e202211365 CrossRef.
- M. E. Razzhivina, I. D. Rukhlenko and N. V. Tepliakov, J. Phys. Chem. Lett., 2023, 14, 4426–4432 CrossRef CAS PubMed.
- Q. Sallembien, L. Bouteiller, J. Crassous and M. Raynal, Chem. Soc. Rev., 2022, 51, 3436–3476 RSC.
- D. G. Blackmond, Chem. Rev., 2020, 120, 4831–4847 CrossRef CAS PubMed.
- G. Laurent, D. Lacoste and P. Gaspard, Proc. Natl. Acad. Sci, 2021, 118, e2012741118 CrossRef CAS PubMed.
- S. F. Ozturk, Z. Liu, J. D. Sutherland and D. D. Sasselov, Sci. Adv., 2023, 9, eadg8274 CrossRef CAS PubMed.
- B. Shen, Y. Kim and M. Lee, Adv. Mater., 2020, 32, 1905669 CrossRef CAS PubMed.
- H. S. Wang, L. Chen, K. Elibol, L. He, H. Wang, C. Chen, C. Jiang, C. Li, T. Wu, C. X. Cong, T. J. Pennycook, G. Argentero, D. Zhang, K. Watanabe, T. Taniguchi, W. Wei, Q. Yuan, J. C. Meyer and X. Xie, Nat. Mater., 2021, 20, 202–207 CrossRef CAS PubMed.
- H. Jiang, J. Wei, F. Sun, C. Nie, J. Fu, H. Shi, J. Sun, X. Wei and C.-W. Qiu, ACS Nano, 2022, 16, 4458–4466 CrossRef CAS PubMed.
- H. Fang and W. Hu, Adv. Sci., 2017, 4, 1700323 CrossRef PubMed.
- G. Konstantatos, M. Badioli, L. Gaudreau, J. Osmond, M. Bernechea, F. P. G. de Arquer, F. Gatti and F. H. L. Koppens, Nat. Nanotechnol., 2012, 7, 363–368 CrossRef CAS PubMed.
- Y. Lee, J. Kwon, E. Hwang, C.-H. Ra, W. J. Yoo, J.-H. Ahn, J. H. Park and J. H. Cho, Adv. Mater., 2015, 27, 41–46 CrossRef CAS PubMed.
- H. Jiang, C. Nie, J. Fu, L. Tang, J. Shen, F. Sun, J. Sun, M. Zhu, S. Feng, Y. Liu, H. Shi and X. Wei, Nanophotonics, 2020, 9, 3663–3672 CrossRef CAS.
- C. R. Dean, A. F. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim, K. L. Shepard and J. Hone, Nat. Nanotechnol., 2010, 5, 722–726 CrossRef CAS PubMed.
- M. Liu, X. Yin, E. Ulin-Avila, B. Geng, T. Zentgraf, L. Ju, F. Wang and X. Zhang, Nature, 2011, 474, 64–67 CrossRef CAS PubMed.
- H. Yuan, X. Liu, F. Afshinmanesh, W. Li, G. Xu, J. Sun, B. Lian, A. G. Curto, G. Ye, Y. Hikita, Z. Shen, S.-C. Zhang, X. Chen, M. Brongersma, H. Y. Hwang and Y. Cui, Nat. Nanotechnol., 2015, 10, 707–713 CrossRef CAS PubMed.
- N. Liu, H. Tian, G. Schwartz, J. B.-H. Tok, T.-L. Ren and Z. Bao, Nano Lett., 2014, 14, 3702–3708 CrossRef CAS PubMed.
- A. Rogalski, Infrared Phys. Technol., 2011, 54, 136–154 CrossRef.
- T. Wu, J. Kessler and P. Bouř, Phys. Chem. Chem. Phys., 2016, 18, 23803–23811 RSC.
- M. Hentschel, M. Schäferling, X. Duan, H. Giessen and N. Liu, Sci. Adv., 2017, 3, e1602735 CrossRef PubMed.
- P. Avouris, M. Freitag and V. Perebeinos, Nat. Photonics, 2008, 2, 341–350 CrossRef CAS.
- F. H. L. Koppens, T. Mueller, P. Avouris, A. C. Ferrari, M. S. Vitiello and M. Polini, Nat. Nanotechnol., 2014, 9, 780–793 CrossRef CAS PubMed.
- T. Mueller, F. Xia and P. Avouris, Nat. Photonics, 2010, 4, 297–301 CrossRef CAS.
- Y. Liu, F. Wang, X. Wang, X. Wang, E. Flahaut, X. Liu, Y. Li, X. Wang, Y. Xu, Y. Shi and R. Zhang, Nat. Commun., 2015, 6, 8589 CrossRef CAS PubMed.
- M. Mustaqeem, S. Kamal, N. Ahmad, P.-T. Chou, K.-H. Lin, Y.-C. Huang, G.-Y. Guo, C. R. Paul Inbaraj, W.-K. Li, H.-C. Yao, K.-L. Lu and Y.-F. Chen, Mater. Today NANO, 2023, 21, 100303 CrossRef CAS.
- J. Peng, B. P. Cumming and M. Gu, Opt. Lett., 2019, 44, 2998 CrossRef CAS PubMed.
- L. Li, J. Wang, L. Kang, W. Liu, L. Yu, B. Zheng, M. L. Brongersma, D. H. Werner, S. Lan, Y. Shi, Y. Xu and X. Wang, ACS Nano, 2020, 14, 16634–16642 CrossRef CAS PubMed.
- J. Wei, Y. Chen, Y. Li, W. Li, J. Xie, C. Lee, K. S. Novoselov and C.-W. Qiu, Nat. Photonics, 2023, 17, 171–178 CAS.
- P. Vukusic, Science, 1979, 2009(325), 398–399 Search PubMed.
- V. Sharma, M. Crne, J. O. Park and M. Srinivasarao, Science, 1979, 2009(325), 449–451 Search PubMed.
- D. W. Green, J.-M. Lee, E.-J. Kim, D.-J. Lee and H.-S. Jung, Adv. Mater. Interfaces, 2016, 3, 1500411 CrossRef.
- X. Dou, N. Mehwish, C. Zhao, J. Liu, C. Xing and C. Feng, Acc. Chem. Res., 2020, 53, 852–862 CrossRef CAS PubMed.
- L. Luo, G. Li, D. Luan, Q. Yuan, Y. Wei and X. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 19371–19377 CrossRef CAS PubMed.
- W. Chen, A. Bian, A. Agarwal, L. Liu, H. Shen, L. Wang, C. Xu and N. A. Kotov, Nano Lett., 2009, 9, 2153–2159 CrossRef CAS PubMed.
- J. B. Pendry, Science, 2004, 306, 1353–1355 CrossRef CAS PubMed.
- B. Wang, J. Zhou, T. Koschny, M. Kafesaki and C. M. Soukoulis, J. Opt. A: Pure Appl. Opt., 2009, 11, 114003 CrossRef.
- Y. Liu and X. Zhang, Chem. Soc. Rev., 2011, 40, 2494 RSC.
- J. Fan and N. A. Kotov, Adv. Mater., 2020, 32, 1906738 CrossRef CAS PubMed.
- J. Lv, X. Gao, B. Han, Y. Zhu, K. Hou and Z. Tang, Nat. Rev. Chem., 2022, 6, 125–145 CrossRef PubMed.
- H.-Y. Ahn, S. Yoo, N. H. Cho, R. M. Kim, H. Kim, J.-H. Huh, S. Lee and K. T. Nam, Acc. Chem. Res., 2019, 52, 2768–2783 CrossRef CAS PubMed.
- G. Reina, J. M. González-Domínguez, A. Criado, E. Vázquez, A. Bianco and M. Prato, Chem. Soc. Rev., 2017, 46, 4400–4416 RSC.
- P. T. Yin, S. Shah, M. Chhowalla and K.-B. Lee, Chem. Rev., 2015, 115, 2483–2531 CrossRef CAS PubMed.
- R.-I. Stefan-van Staden, R.-M. Ilie-Mihai, L. Magerusan, M. Coros and S. Pruneanu, Anal. Bioanal. Chem., 2020, 412, 3199–3207 CrossRef CAS PubMed.
- J. Liu, L. Cui and D. Losic, Acta Biomater., 2013, 9, 9243–9257 CrossRef CAS PubMed.
- R. G. Bai and G. A. Husseini, Biomimetic Nanoengineered Materials for Advanced Drug Delivery, Elsevier, 2019, pp. 149–168 Search PubMed.
- C. L. Weaver, J. M. LaRosa, X. Luo and X. T. Cui, ACS Nano, 2014, 8, 1834–1843 CrossRef CAS PubMed.
- A. M. L. Oliveira, M. Machado, G. A. Silva, D. B. Bitoque, J. Tavares Ferreira, L. A. Pinto and Q. Ferreira, Nanomaterials, 2022, 12, 1149 CrossRef CAS PubMed.
- R. Lakshmanan and N. Maulik, Can. J. Physiol. Pharmacol., 2018, 96, 869–878 CrossRef CAS PubMed.
- G. Cellot, L. Jacquemin, G. Reina, A. Franceschi Biagioni, M. Fontanini, O. Chaloin, Y. Nishina, A. Bianco and L. Ballerini, ACS Appl. Nano Mater., 2022, 5, 17640–17651 CrossRef CAS PubMed.
- C. McCallion, J. Burthem, K. Rees-Unwin, A. Golovanov and A. Pluen, Eur. J. Pharm. Biopharm., 2016, 104, 235–250 CrossRef CAS PubMed.
- S. M. Hosseini, S. Mazinani, M. Abdouss, H. Kalhor, K. Kalantari, I. S. Amiri and Z. Ramezani, Polym. Bull., 2022, 79, 541–554 CrossRef CAS.
- R.-P. Liang, C.-M. Liu, X.-Y. Meng, J.-W. Wang and J.-D. Qiu, J. Chromatogr. A, 2012, 1266, 95–102 CrossRef CAS PubMed.
- G. Xie, W. Tian, L. Wen, K. Xiao, Z. Zhang, Q. Liu, G. Hou, P. Li, Y. Tian and L. Jiang, Chem. Commun., 2015, 51, 3135–3138 RSC.
- S. Mahpishanian and H. Sereshti, J. Chromatogr. A, 2017, 1485, 32–43 CrossRef CAS PubMed.
- X. Xu, Y. Zhang, B. Wang, L. Luo, Z. Xu and X. Tian, RSC Adv., 2018, 8, 32538–32544 RSC.
- F. Qie, J. Guo, B. Tu, X. Zhao, Y. Zhang and Y. Yan, Chem. – Asian J, 2018, 13, 2812–2817 CrossRef CAS PubMed.
- Y. Zhou, W. Jiang, H. Wu, F. Liu, H. Yin, N. Lu and S. Ai, Microchim. Acta, 2019, 186, 488 CrossRef PubMed.
- A. Snoun, T. Bouchrika and O. Jemai, 2022, pp. 534–547.
- J. A. Soria Lopez, H. M. González and G. C. Léger, 2019, pp. 231–255.
- C. R. Jack, D. S. Knopman, W. J. Jagust, R. C. Petersen, M. W. Weiner, P. S. Aisen, L. M. Shaw, P. Vemuri, H. J. Wiste, S. D. Weigand, T. G. Lesnick, V. S. Pankratz, M. C. Donohue and J. Q. Trojanowski, Lancet Neurol., 2013, 12, 207–216 CrossRef CAS PubMed.
- Y. Fan, S. Ou-yang, D. Zhou, J. Wei and L. Liao, Chirality, 2022, 34, 760–781 CrossRef CAS PubMed.
- T. T. Nguyen, T. T. D. Nguyen, T. K. O. Nguyen, T. K. Vo and V. G. Vo, Biomed. Pharmacother., 2021, 139, 111623 CrossRef CAS PubMed.
- H. Zhang, T. Fan, W. Chen, Y. Li and B. Wang, Bioact Mater., 2020, 5, 1071–1086 Search PubMed.
- R. Zacharia, H. Ulbricht and T. Hertel, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 155406 CrossRef.
- I. Ahmad, A. Mozhi, L. Yang, Q. Han, X. Liang, C. Li, R. Yang and C. Wang, Colloids Surf., B, 2017, 159, 540–545 CrossRef CAS PubMed.
- K. Hou, J. Zhao, H. Wang, B. Li, K. Li, X. Shi, K. Wan, J. Ai, J. Lv, D. Wang, Q. Huang, H. Wang, Q. Cao, S. Liu and Z. Tang, Nat. Commun., 2020, 11, 4790 CrossRef CAS PubMed.
- H. Wang, M. Chen, Y. Sun, L. Xu, F. Li and J. Han, Anal. Chem., 2022, 94, 2757–2763 CrossRef CAS PubMed.
- M. Walton-Raaby, R. Woods and S. Kalyaanamoorthy, Int. J. Mol. Sci., 2023, 24, 9476 CrossRef CAS PubMed.
- H. Tang, Y. Li, A. Kakinen, N. Andrikopoulos, Y. Sun, E. Kwak, T. P. Davis, F. Ding and P. C. Ke, Phys. Chem. Chem. Phys., 2022, 24, 86–97 RSC.
- A. Mars, M. Hamami, L. Bechnak, D. Patra and N. Raouafi, Anal. Chim. Acta, 2018, 1036, 141–146 CrossRef CAS PubMed.
- K. Tak, R. Sharma, V. Dave, S. Jain and S. Sharma, ACS Chem. Neurosci., 2020, 11, 3741–3748 CrossRef CAS PubMed.
- F. Mohebichamkhorami, M. Faizi, M. Mahmoudifard, A. Hajikarim-Hamedani, S. S. Mohseni, A. Heidari, Y. Ghane, M. Khoramjouy, M. Khayati, R. Ghasemi, H. Zali, S. Hosseinzadeh and E. Mostafavi, Small, 2023, 19, 2207626 CrossRef CAS PubMed.
- A. Wood-Kaczmar, S. Gandhi and N. W. Wood, Trends Mol. Med., 2006, 12, 521–528 CrossRef CAS PubMed.
- P. Jenner, Acta Neurol. Scand., Suppl., 1991, 136, 6–15 CrossRef CAS PubMed.
- C. Hao, A. Qu, L. Xu, M. Sun, H. Zhang, C. Xu and H. Kuang, J. Am. Chem. Soc., 2019, 141, 1091–1099 CrossRef CAS PubMed.
- N.-N. Zhang, H.-R. Sun, S. Liu, Y.-C. Xing, J. Lu, F. Peng, C.-L. Han, Z. Wei, T. Sun, B. Yang and K. Liu, CCS Chem., 2022, 4, 660–670 CrossRef CAS.
- S. Fahn, Mov. Disord, 2008, 23, S497–S508 CrossRef PubMed.
- C. Xu, C. Zhao, M. Li, L. Wu, J. Ren and X. Qu, Small, 2014, 10, 1841–1847 CrossRef CAS PubMed.
- K. J. Goswami, B. Gogoi and N. Sen Sarma, Sens. Actuators, B, 2022, 350, 130892 CrossRef CAS.
- Y. W. H. Jeon, R. Zhu and G. Kim, Front. Chem., 2023, 11, 1207579 CrossRef PubMed.
- H. Park, A. Otte and K. Park, J. Controlled Release, 2022, 342, 53–65 CrossRef CAS PubMed.
- N. Borzooee Moghadam, M. Avatefi, M. Karimi and M. Mahmoudifard, J. Mater. Chem. B, 2023, 11, 2568–2613 RSC.
- M. S. Islam, F. Renner, S. Azizighannad and S. Mitra, Colloids Surf., B, 2020, 189, 110827 CrossRef CAS PubMed.
- A. Jafari, K. K. Chenab, H. Malektaj, F. Farshchi, S. Ghorbani, A. Ghasemiamineh, M. Khoshakhlagh, B. Ashtari and M.-R. Zamani-Meymian, FlatChem, 2022, 34, 100381 CrossRef CAS.
- S. K. Singh, M. K. Singh, P. P. Kulkarni, V. K. Sonkar, J. J. A. Grácio and D. Dash, ACS Nano, 2012, 6, 2731–2740 CrossRef CAS PubMed.
- Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876–10877 CrossRef CAS PubMed.
- M. Zaboli, H. Raissi, N. R. Moghaddam and F. Farzad, J. Mol. Liq., 2020, 301, 112458 CrossRef CAS.
- X. Wang, B. Wu, Y. Zhang and C. Feng, Polym. Chem., 2022, 13, 1685–1694 RSC.
- M. Mirza-Aghayan, M. Heidarian, M. Mohammadi and R. Boukherroub, Appl. Surf. Sci., 2022, 583, 152568 CrossRef CAS.
- Y. Zhang, G. Kim, Y. Zhu, C. Wang, R. Zhu, X. Lu, H.-C. Chang and Y. Wang, ACS Nano, 2023, 17, 10191–10205 CrossRef CAS PubMed.
- A. Brotchie, Nat. Rev. Mater., 2016, 1, 16006 CrossRef.
- T. K. Henna and K. Pramod, Mater. Sci. Eng., C, 2020, 110, 110651 CrossRef CAS PubMed.
- S. Javadian, K. Najafi, S. M. Sadrpoor, F. Ektefa, N. Dalir and M. Nikkhah, J. Mol. Liq., 2021, 331, 115746 CrossRef CAS.
- Y. Guo, H. Wang, L. Huang, L. Ou, J. Zhu, S. Liu and X. Xu, MedComm, 2021, 2, 17–26 CrossRef CAS PubMed.
- A. Kottorou, F.-I. Dimitrakopoulos, G. Diamantopoulou, F. Kalofonou, M. Stavropoulos, K. Thomopoulos, T. Makatsoris, A. Koutras and H. Kalofonos, Cancers, 2023, 15, 1685 CrossRef CAS PubMed.
- E. Gómez-de-Mariscal, M. Maška, A. Kotrbová, V. Pospíchalová, P. Matula and A. Muñoz-Barrutia, Sci. Rep., 2019, 9, 13211 CrossRef PubMed.
- Y. Takakura, A. Matsumoto and Y. Takahashi, Biol. Pharm. Bull., 2020, 43, 576–583 CrossRef CAS PubMed.
- S. Saeidi, B. Rezaei, N. Irannejad and A. A. Ensafi, J. Power Sources, 2020, 476, 228647 CrossRef CAS.
- P.-C. Wu, J.-Y. Wang, W.-L. Wang, C.-Y. Chang, C.-H. Huang, K.-L. Yang, J.-C. Chang, C.-L. L. Hsu, S.-Y. Chen, T.-M. Chou and W.-S. Kuo, Nanoscale, 2018, 10, 109–117 RSC.
- Z. Qian, J. Ma, X. Shan, L. Shao, J. Zhou, J. Chen and H. Feng, RSC Adv., 2013, 3, 14571 RSC.
- M. Trojanowicz, Electrochem. Commun., 2014, 38, 47–52 CrossRef CAS.
- F. Burg, S. Breitenlechner, C. Jandl and T. Bach, Chem. Sci., 2020, 11, 2121–2129 RSC.
- X. Niu, X. Yang, H. Li, J. Liu, Z. Liu and K. Wang, Microchim. Acta, 2020, 187, 676 CrossRef CAS PubMed.
- Q. Zhang, L. Guo, Y. Huang, Y. Wang, Q. Han and Y. Fu, Anal. Methods, 2013, 5, 4397 RSC.
- H. Imanzadeh, Y. Sefid-Sefidehkhan, H. Afshary, A. Afruz and M. Amiri, J. Pharm. Biomed. Anal., 2023, 230, 115390 CrossRef CAS PubMed.
- H. Hou, S. Tang, M. Liu, F. Zhang, A. Liang, L. Sun, L. Geng, B. Xie, Y. Yi and A. Luo, New J. Chem., 2023, 47, 8558–8565 RSC.
- N. Liu, J. Liu, X. Niu, J. Wang, R. Guo and Z. Mo, Microchim. Acta, 2021, 188, 163 CrossRef CAS PubMed.
- D. Wu, W. Tan, Y. Yu, B. Yang, H. Li and Y. Kong, Anal. Chim. Acta, 2018, 1033, 58–64 CrossRef CAS PubMed.
- H. Luo, H. Li, Q. Ge, H. Cong, Z. Tao and M. Liu, Microchem. J., 2021, 164, 105949 CrossRef CAS.
- X. Sun, Z. Fu, M. Zhang, H. Fu, C. Lin, J. Kuang, H. Zhang and P. Hu, Microchem. J., 2022, 183, 108074 CrossRef CAS.
- T. Gong, S. Zhu, S. Huang, P. Gu, Y. Xiong, J. Zhang and X. Jiang, Anal. Chim. Acta, 2022, 1191, 339276 CrossRef CAS PubMed.
- M. Machado, A. M. L. Oliveira, G. A. Silva, D. B. Bitoque, J. Tavares Ferreira, L. A. Pinto and Q. Ferreira, Nanomaterials, 2022, 12, 1624 CrossRef CAS PubMed.
- S. Shahriari, M. Sastry, S. Panjikar and R. S. Raman, Nanotechnol., Sci. Appl., 2021, 14, 197–220 CrossRef CAS PubMed.
- R. Zhao, X. Liu, J. Li and Y. Zhang, Phys. Chem. Chem. Phys., 2022, 24, 28325–28332 RSC.
- J. Guo, X. Wei, H. Lian, L. Li, X. Sun and B. Liu, ACS Appl. Nano Mater., 2020, 3, 3675–3683 CrossRef CAS.
- L. Zagitova, Y. Yarkaeva, V. Zagitov, M. Nazyrov, S. Gainanova and V. Maistrenko, J. Electroanal. Chem., 2022, 922, 116744 CrossRef CAS.
- X. Yang, X. Niu, Z. Mo, R. Guo, N. Liu, P. Zhao and Z. Liu, Electrochim. Acta, 2019, 319, 705–715 CrossRef CAS.
- L. Guo, Y. Huang, Q. Zhang, C. Chen, D. Guo, Y. Chen and Y. Fu, J. Electrochem. Soc., 2014, 161, B70–B74 CrossRef CAS.
- X. Niu, X. Yang, Z. Mo, N. Liu, R. Guo, Z. Pan and Z. Liu, Microchim. Acta, 2019, 186, 557 CrossRef PubMed.
- W. Liang, Y. Rong, L. Fan, W. Dong, Q. Dong, C. Yang, Z. Zhong, C. Dong, S. Shuang and W.-Y. Wong, J. Mater. Chem. C, 2018, 6, 12822–12829 RSC.
- Q. Niu, P. Jin, Y. Huang, L. Fan, C. Zhang, C. Yang, C. Dong, W. Liang and S. Shuang, Analyst, 2022, 147, 880–888 RSC.
- H. Gou, J. He, Z. Mo, X. Wei, R. Hu and Y. Wang, RSC Adv., 2015, 5, 60638–60645 RSC.
- Q. Xiao, S. Lu, C. Huang, W. Su, S. Zhou, J. Sheng and S. Huang, J. Iran. Chem. Soc., 2017, 14, 1957–1970 CrossRef CAS.
- L. Lin, H.-T. Lian, X.-Y. Sun, Y.-M. Yu and B. Liu, Anal. Methods, 2015, 7, 1387–1394 RSC.
- H. Pei, J. Wang, X. Jin, X. Zhang, W. Liu, R. Guo, N. Liu and Z. Mo, J. Electroanal. Chem., 2022, 913, 116283 CrossRef CAS.
- Z. Zhou, Z. Yang, L. Xia and H. Zhang, Biosens. Bioelectron., 2022, 198, 113836 CrossRef CAS PubMed.
- Y. He, Z. Ye, F. Zhu, T. Qiu, X. Dai, Y. Xie, S. Zou, Q. Dong, W. Zhang, J. Ma and X. Mao, Molecules, 2023, 28, 3700 CrossRef CAS PubMed.
- X. Niu, R. Zhao, S. Yan, H. Li, J. Yang, K. Cao, X. Liu and K. Wang, Microchim. Acta, 2023, 190, 230 CrossRef CAS PubMed.
- W. Shi, F. Fan, L. Ma, T.-R. Zhang, J.-Y. Liu, J.-R. Cheng, X. Wang and S. Chang, Opt. Laser Technol., 2023, 162, 109274 CrossRef CAS.
- X. Yang, Z.-Z. Yin, G. Zheng, M. Zhou, H. Zhang, J. Li, W. Cai and Y. Kong, Bioelectrochemistry, 2023, 151, 108375 CrossRef CAS PubMed.
- N. Bangruwa, P. k Bhartiya and D. Mishra, Sens. Actuators, B, 2023, 382, 133447 CrossRef CAS.
- L. Chen, G. Li, A. Yang, J. Wu, F. Yan and H. Ju, Sens. Actuators, B, 2022, 351, 130964 CrossRef CAS.
- X. Kang, R. Wang, M. Jiang, E. Li, Y. Li, T. Wang and Z. Ren, Sens. Actuators, Rep., 2023, 5, 100139 CrossRef.
- G. Ning, H. Wang, M. Fu, J. Liu, Y. Sun, H. Lu, X. Fan, Y. Zhang and H. Wang, Electroanalysis, 2022, 34, 316–325 CrossRef CAS.
- H. Imran, P. N. Manikandan and V. Dharuman, J. Electroanal. Chem., 2019, 835, 10–21 CrossRef CAS.
- S. Ghosh, A. Pannone, D. Sen, A. Wali, H. Ravichandran and S. Das, Nat. Commun., 2023, 14, 6021 CrossRef CAS PubMed.
- T. Hayasaka, A. Lin, V. C. Copa, L. P. Lopez, R. A. Loberternos, L. I. M. Ballesteros, Y. Kubota, Y. Liu, A. A. Salvador and L. Lin, Microsyst. Nanoeng., 2020, 6, 50 CrossRef CAS PubMed.
- Y. Lee, J. Kim, B. Jang, S. Kim, B. K. Sharma, J.-H. Kim and J.-H. Ahn, Nano Energy, 2019, 62, 259–267 CrossRef CAS.
- S. Huang, A. Croy, A. L. Bierling, V. Khavrus, L. A. Panes-Ruiz, A. Dianat, B. Ibarlucea and G. Cuniberti, Appl. Phys. Rev., 2023, 10, 021406 CAS.
- M. Huang, Z. Li and H. Zhu, Adv. Intell. Syst., 2022, 4, 2200077 CrossRef.
- Z. Zhang, Y. Hong, B. Hou, Z. Zhang, M. Negahban and J. Zhang, Carbon, 2019, 148, 115–123 CrossRef CAS.
- A. Maity, Y. Hershkovitz-Pollak, R. Gupta, W. Wu and H. Haick, Adv. Mater., 2023, 35, 2209125 CrossRef CAS PubMed.
- N. Sukenik, H. Alpern, E. Katzir, S. Yochelis, O. Millo and Y. Paltiel, Adv. Mater. Technol., 2018, 3, 1700300 CrossRef.
- B. Göhler, V. Hamelbeck, T. Z. Markus, M. Kettner, G. F. Hanne, Z. Vager, R. Naaman and H. Zacharias, Science, 2011, 331, 894–897 CrossRef PubMed.
- R. Naaman, Y. Paltiel and D. H. Waldeck, Nat. Rev. Chem., 2019, 3, 250–260 CrossRef CAS.
- T. F. Schranghamer, A. Oberoi and S. Das, Nat. Commun., 2020, 11, 5474 CrossRef CAS PubMed.
- B. Zhao, S. Yang, J. Deng and K. Pan, Adv. Sci., 2021, 8, 2003681 CrossRef CAS PubMed.
- F. Torrisi, T. Hasan, W. Wu, Z. Sun, A. Lombardo, T. S. Kulmala, G.-W. Hsieh, S. Jung, F. Bonaccorso, P. J. Paul, D. Chu and A. C. Ferrari, ACS Nano, 2012, 6, 2992–3006 CrossRef CAS PubMed.
- C. Backes, A. Bianco, C. Casiraghi, F. Galembeck, R. K. Gupta, M. C. Hersam, A. R. Kamali, M. Kolíbal, V. Kolosov, V. Kumar, W. H. Lee, N. Martsinovich, M. Melchionna, K. Müllen, A. Oyarzun, V. Palermo, M. Prato, P. Samori, S. Sampath, A. Silvestri, D. Sirbu, R. Sui, A. Turchanin, C. Wetzl, I. A. Wright, Z. Xia and X. Zhuang, Faraday Discuss., 2021, 227, 141–162 RSC.
- J. Du, G. Fu, X. Xu, A. M. Elshahawy and C. Guan, Small, 2023, 19, 2207833 CrossRef CAS PubMed.
- P. Wang, B. Barnes, Z. Huang, Z. Wang, M. Zheng and Y. Wang, Adv. Mater., 2021, 33, 2005890 CrossRef CAS PubMed.
- J. Mohanraj, D. Durgalakshmi and A. Rakkesh, J. Electrochem. Soc., 2020, 167, 067523 CrossRef CAS.
- S. Lu and A. D. Franklin, Nanoscale, 2020, 12, 23371–23390 RSC.
- A. Grillo, Z. Peng, A. Pelella, A. Di Bartolomeo and C. Casiraghi, ACS Nano, 2023, 17, 1533–1540 CrossRef CAS PubMed.
- Z. Peng, A. Grillo, A. Pelella, X. Liu, M. Boyes, X. Xiao, M. Zhao, J. Wang, Z. Hu, A. Di Bartolomeo and C. Casiraghi, Mater. Horiz., 2024, 11, 1344–1353 RSC.
- A. Maity, Y. Milyutin, V. D. Maidantchik, Y. H. Pollak, Y. Broza, R. Omar, Y. Zheng, W. Saliba, T. Huynh and H. Haick, Adv. Sci., 2022, 9, 2203639 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |