Abortive reaction leads to selective adsorbate rotation
Yi-Fang
Lai
a,
Lydie
Leung
a,
Matthew J.
Timm
 b,
Gilbert C.
Walker
b,
Gilbert C.
Walker
 a and
John C.
Polanyi
a and
John C.
Polanyi
 *a
*a
aLash Miller Chemical Laboratories, Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario M5S 3H6, Canada. E-mail: john.polanyi@utoronto.ca
bDepartment of Physical Chemistry, University of Graz, Heinrichstrasse 28, 8010 Graz, Austria
First published on 25th January 2024
Abstract
Electron-induced dissociation of a fluorocarbon adsorbate CF3 (ad) at 4.6 K is shown by Scanning Tunnelling Microscopy (STM) to form directed energetic F-atom ‘projectiles’ on Cu(110). The outcome of a collision between these directed projectiles and stationary co-adsorbed allyl ‘target’ molecules was found through STM to give rotational excitation of the target allyl, clockwise or anti-clockwise, depending on the chosen collision geometry. Molecular dynamics computation linked the collisional excitation of the allyl target to an ‘abortive chemical reaction’, in which the approach of the F-projectile stretched an H–C bond lifting the allyl above the surface, facilitating isomerization from ‘Across’ to ‘Along’ a Cu row.
Introduction
Bimolecular chemical reactions have been shown to occur if reagents collide with sufficient energy and a favorable orientation.1 Gas-phase studies of molecular collisions have provided insights into the reaction dynamics, despite averaging over the reagent impact parameter (the miss-distance) and averaging over the angle of approach.2,3 Alignment of the reagents at a surface provides a means to restrict the impact parameter. Such surface-aligned reactions can occur between a recoiling reagent produced by photolysis or electron-induced dissociation, and a stationary adsorbed target molecule.4–14 Recent studies of this type employing Scanning Tunnelling Microscopy (STM) have shown that a molecular projectile, CF2, can be aimed at a stationary target at different impact parameters.15,16 The ability to choose the impact parameter allows control over the collision outcome. At small impact parameters such collisions can result in a reactive outcome.15–17 In the case of an atomic projectile, an F-atom, the one-dimensional collision along its path with a CF3 target resulted in a substitution reaction where the incoming atom ‘knocked-on’ an F-atom in the target.18 A directed to-and-fro knock-on reaction involving an F-atom projectile and a row of stationary targets has been shown to resemble the motion in a Newton’s cradle.19Here we report an STM study of collisions of an energetic F-atom projectile with a stationary allyl target on Cu(110), at impact parameters in the region of 1–2 Å. Almost half of these collisions led to rotation of the allyl from ‘Across’ to ‘Along’ a Cu row. Molecular dynamics calculations gave evidence of an abortive H-atom abstraction reaction as the cause of the observed efficient rotational isomerization of the allyl.
Methods
Experimental
All experiments were performed in a low-temperature UHV-STM (Omicron) with a base pressure of <3.0 × 10−11 mbar. The Cu(110) sample was cleaned by repeated cycles of Argon sputtering (0.6 keV, 7 μA) and annealing at 800 K. The surface cleanliness, less than 1% contaminants, was determined by STM. The constant-current mode was used to record the STM images at 4.6 K. The bias is given with respect to the sample. Both iodotrifluoromethane (CF3I; SynQuest, 99%) and allyl bromide (C3H6Br; Aldrich, 99%) were deposited via a capillary tube pointing to the Cu sample. Chemisorbed CF3 were obtained from the thermal dissociation of CF3I on Cu(110) at 79.5 K, as previously reported.17–19 The target molecule, allyl (C3H5), was then generated by dosing allyl bromide on the Cu sample, which dissociated upon adsorption at 11 K.The F-atom projectile was produced from the electron-induced dissociation of CF3. The STM tip was placed over the CF3 radical with the feedback loop off. The current was recorded as a function of time at a constant bias of +1.4 V. A subsequent STM image showed the position of the products after the collision between the F-atom projectile and the allyl Across. The positions of the products following collision were obtained using the WSxM software.20
Theory
Density Functional Theory (DFT) calculations, as implemented in the Vienna Ab initio Simulation Package (VASP),21,22 were performed on the SciNet supercomputer Niagara cluster.23,24 The structure relaxations used the projected augmented method25,26 and the generalized-gradient approximation with the Perdew–Burke–Ernzerhof functional.27 A semi-empirical dispersion correction (DFT-D3) generated by Grimme was added to account for van der Waals interactions.28 The energy cutoff for the plane-wave basis was set to 400 eV. The Cu(110) surface was modelled by a (4 × 10) slab composed of 200 Cu-atoms in five layers, separated by a 17 Å vacuum layer. All atoms except for the bottom two layers were fully relaxed until the residual force on each atom was less than 0.01 eV Å−1. The Tersoff–Hamann approximation29 was employed to produce the STM-image simulations, which were then visualized with the HIVE-STM program.30An Impulsive Two-State (I2S) model, described in detail elsewhere,12,13,31–33 was used to model the formation of the F-atom projectile. In the I2S model, the system is promoted from equilibrium on the ground state to a repulsive upper state by transferring an electron from the F-atom core to its valence shell, so as to reproduce the effect of added charge. Molecular dynamics (MD) were calculated for the excited system for a time, t*. Once the system was returned to the ground state, with the velocities and atomic positions obtained from the excited state, the dynamics were followed until a final state was reached. For the abortive reaction, the maximum allyl rotation was calculated with t* = 7.5 fs. For the abstraction reaction, the t* was set at 8.0 fs which was the minimum time needed to obtain the reaction products, HF and allyl radical. The MD calculations were performed using a time-step of 0.5 fs, under microcanonical conditions.
Results and discussion
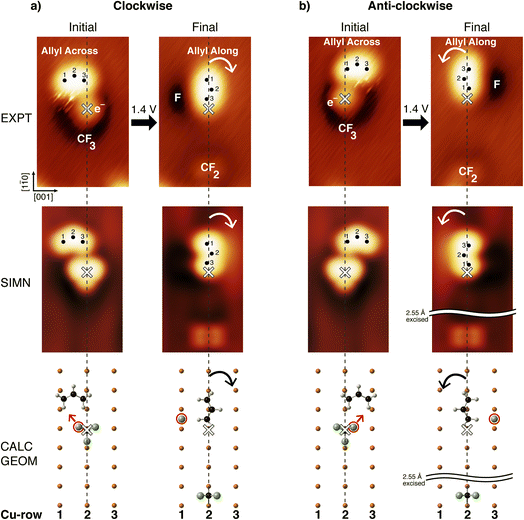
On the Cu(110) surface the allyl was observed to have two adsorption geometries formed in equal numbers, with their long axes lying Across or Along the Cu rows. This observed ratio for the two geometries is consistent with that previously reported.34 The computed geometry for the allyl Across configuration places the curved allyl over a four-fold hollow site, with the H-atoms of the terminal carbon atoms of allyl adjacent to Cu atoms. In the case of the allyl Along configuration, the curved allyl is centered over a Cu atom along a Cu row. Computational results gave an adsorption energy of 0.91 eV for the Across configuration, slightly lower than the 1.15 eV for the Along configuration, making the switch from Across to Along exoergic.In Fig. 1a, the initial state shows an STM image recorded at 4.6 K of CF3 and allyl Across, adsorbed as nearest neighbours. The allyl is located between Cu-rows 1 and 2, and the CF3 is found adsorbed on Cu-row 2 (vertical dashed line). The CF3 was found preferentially on the inside of the curved allyl Across, as shown in the calculated geometry. The CF3 is located on the right-hand side of the allyl centre-of-mass, near the allyl C atom denoted as 3. Localized ‘streaks’ observed between the curved side of the allyl and the CF3 are indicative of long-range interactions between the F-atom and the neighbouring allyl. The final state shown in Fig. 1a, was obtained by injecting 1.4 eV electrons from the STM tip into the CF3 radical, at the location of the white cross. This led to electron-induced dissociation of a C–F bond, generating an F-atom projectile and a CF2 at the surface as indicated in the figure.15–19
The final state comprised an allyl Along and a CF2 adsorbed on the same Cu-row 2, and an F-atom located on the adjacent Cu-row 1. The final position of the F-atom projectile indicated that the broken C–F bond was the one nearest to the allyl Across in the initial state, since the products of the electron-induced dissociation recoil along the bond direction.35 The collision between the F-atom and the allyl Across occurred at a negative impact parameter, with the recoiling F travelling towards C-atom 1 of allyl Across (to the left of the allyl centre-of-mass – see calculated geometries in Fig. 1a). This collision resulted in a clockwise rotation of allyl from Across to Along. The calculated geometries in the simulations are in good agreement with observation, confirming the given assignment of the reagents and products.
The initial state in Fig. 1b consists of an allyl Across located between Cu-rows 2 and 3, and a neighbouring CF3 found on Cu-row 2 (vertical dashed line). In this case the collision between the F-atom and the allyl Across occurred at a positive impact parameter, with the F-atom travelling towards C-atom 3 of allyl Across, resulting in an anti-clockwise rotation of the allyl. The final state STM image shows the allyl Along and the CF2 radical adsorbed on the same Cu-row 2, and the F-atom on Cu-row 3 adjacent to the allyl. The initial and final states in Fig. 1b are essentially mirror-images of those in Fig. 1a.
In addition to rotation, translation of the allyl Along was observed by one or two unit-cells in the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10] direction, attributable in either case to attractive interactions between the allyl and the CF2 radical. The allyl rotation was observed for ∼41% of collisions between the F-atom and the allyl. The balance of the cases gave no rotation; Ntotal = 114 cases.
10] direction, attributable in either case to attractive interactions between the allyl and the CF2 radical. The allyl rotation was observed for ∼41% of collisions between the F-atom and the allyl. The balance of the cases gave no rotation; Ntotal = 114 cases.
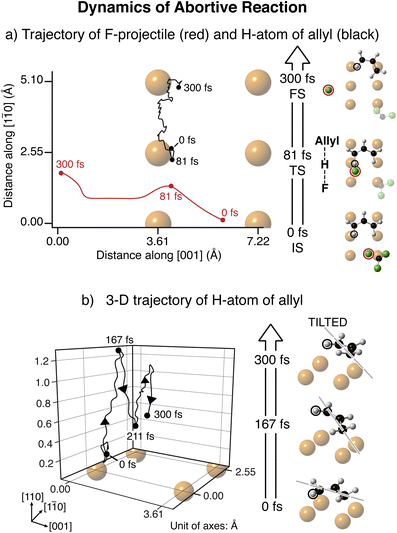
Molecular dynamics calculations were performed for the rotational energy-transfer between the F-atom projectile and the allyl Across (for which the initial state was shown in Fig. 1a). The impulsive two-state model employed to describe the electronic excitation of the CF3 resulted in a recoil of atomic F. The trajectories of the F-atom projectile and the terminal H-atom of allyl, are shown in Fig. 2a, with visualizations of the initial, transition and final states. The F-atom recoiled from CF3 with 0.82 eV, gained after the system had resided in the excited state for t* = 7.5 fs, which is the maximum t* for an abortive (failed) reaction.
On the neutral ground state, the F-atom projectile approached the allyl Across at an impact parameter of −1.3 Å and an angle of 65° from the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction. As the F-atom travelled towards the allyl, at 81 fs the C–H bond extended by 0.26 Å (∼23%) due to the F-atom attracting the H-atom. Subsequently, the C–H bond returned to its original length, with energy released. The released energy was transferred to internal states of the allyl causing the H-atom to accelerate along the [1
0] direction. As the F-atom travelled towards the allyl, at 81 fs the C–H bond extended by 0.26 Å (∼23%) due to the F-atom attracting the H-atom. Subsequently, the C–H bond returned to its original length, with energy released. The released energy was transferred to internal states of the allyl causing the H-atom to accelerate along the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction. At 300 fs, the allyl target was computed to rotate 36.5°, about 1/3 of the experimentally observed rotation.
0] direction. At 300 fs, the allyl target was computed to rotate 36.5°, about 1/3 of the experimentally observed rotation.
Fig. 2b shows dynamical details of the 3D trajectory for the same H-atom of allyl Across shown in Fig. 2a. The C–H bond-stretch induced by the F-atom projectile at 81 fs resulted in lifting of the allyl above the surface, freeing it to rotate and reach its maximum height at 167 fs. Simultaneously the allyl rotated in a clockwise direction, with the carbon atom furthest away from the collision acting as pivot. The allyl then travelled towards the Cu surface and bounced a second time at 211 fs.
Fig. 3 shows the same dynamics as Fig. 2, diagrammatically at successive times. Initially at t = 0 fs, the electron-induced dissociation of CF3 was obtained by adding an electronic-charge to the F-atom of CF3 adjacent to the allyl Across, as indicated by the ‘explosion’ in the C–F bond. At t = 81 fs, the I2S model shows the F-atom recoiling along the direction of the breaking C–F bond in CF3. This F-atom projectile approached and interacted with the H, inside the curve of the allyl Across to form a transition state F–H–C. At 94 fs, the C–H was vibrationally excited, as evidenced by the C–H bond-stretch. Rotational momentum was gained as the allyl bounced from the surface; by 300 fs the allyl had rotated 36.5° clockwise.
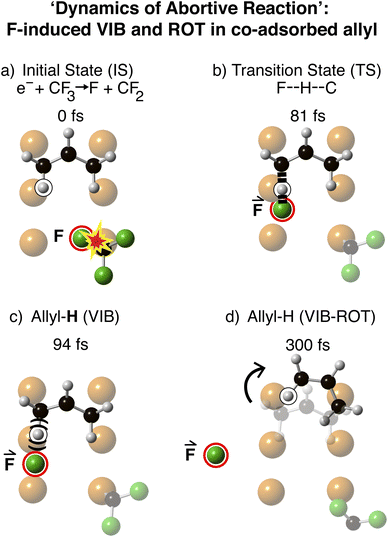 | ||
| Fig. 3 Molecular dynamics for clockwise rotation in the collision between an F-projectile and allyl across target, at a negative impact parameter b = − 1.3 Å. (a) The initial state (t = 0 fs) corresponds to that in Fig. 1a. The explosion marks the C–F bond-excitation to anti-bond. The F-projectile is highlighted in red. (b) Formation of the transition state indicated by black dashed lines, linking F–H and H–allyl. (c) Vibrationally excited H–allyl resulting from the collision between F-projectile and H–allyl. (d) Computed clockwise-rotated position of the allyl at 300 fs. | ||
The final state for anti-clockwise rotation observed in Fig. 1b was the result of a collision with the F-projectile at a positive impact parameter of +1.3 Å. The molecular dynamics were a mirror image of Fig. 3, with the F-atom colliding at the right-end carbon of the allyl. This resulted in a maximum rotation of ∼36.5° anti-clockwise.
The observed rotational excitation of the allyl is a consequence of the C–H bond stretch. This C–H bond stretch occurs en route to an abstraction reaction in which the F-atom projectile would abstract the terminal H-atom of allyl. This abstraction reaction was not observed, since it is calculated to be endoergic by ∼1 eV. Fig. 4 shows the computed abstraction of the terminal H-atom of allyl for an F-atom projectile with an enhanced kinetic energy of 0.88 eV. The enhanced F-atom kinetic energy enabled abstraction of the H-atom to form HF. The abortive reaction (Fig. 2 and 3) excited the C–H bond stretch, leading, in multiple encounters between the allyl and the surface, to rotation of the allyl from Across to Along.
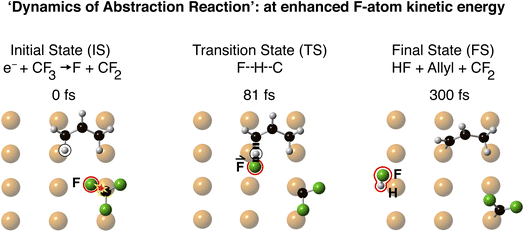 | ||
| Fig. 4 Computed molecular dynamics at an enhanced kinetic energy between the F-projectile and its allyl Across target, at b = −1.3 Å, leading to abstraction reaction. The initial state (t = 0 fs) corresponds to the initial state in Fig. 1a. The explosion in the anti-bond due to electronic excitation, marks the C–F bond being broken. The F-projectile is highlighted in red in the IS, TS and FS. The transition state F–H–C is indicated by dashed lines joining F–H and H–C. The HF product is highlighted at 300 fs, showing the reaction products, HF + allyl, at enhanced kinetic energy. | ||
Conclusions
We report an STM study of inelastic scattering in collisions F + allyl on Cu(110), at kinetic energies below and above the barrier to H-atom abstraction. Molecular dynamics showed collision-induced rotation of the allyl target from Across the Cu rows to Along, as observed. The dynamics link this rotational isomerization to C–H bond extension in the allyl, along the path to HF formation in a process we term ‘abortive’ reaction. Here, therefore, we show the importance of the mixing of degrees of freedom in abortive reaction. The dynamics link the extension of the C–H bond, which lifts the allyl product, with efficient ro-vibrational excitation. At translation energies >0.85 eV for the F-projectile, the potential-energy surface predicts an abstraction reaction to give HF.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was funded in part by the American Chemical Society Petroleum Research Fund (PRF# 65800-ND5) and the Natural Sciences and Engineering Research Council of Canada (RGPIN-2022-04790). Computations were performed on the Niagara cluster at SciNet HPC Consortium. SciNet is funded by Innovation, Science and Economic Development Canada (ISED), the Government of Ontario, and the University of Toronto.References
- R. D. Levine, Molecular Reaction Dynamics, Cambridge University Press, 2005 Search PubMed.
- D. R. Herschbach, Angew. Chem., Int. Ed., 1987, 26, 1221–1243 CrossRef.
- Y. T. Lee, Angew. Chem., Int. Ed., 1987, 26, 939–951 CrossRef.
- E. B. D. Bourdon, J. P. Cowin, I. Harrison, J. C. Polanyi, J. Segner, C. D. Stanners and P. A. Young, J. Phys. Chem., 1984, 88, 6100–6103 CrossRef CAS.
- E. B. D. Bourdon, P. Das, I. Harrison, J. C. Polanyi, J. Segner, C. D. Stanners, R. J. Williams and P. A. Young, Faraday Discuss. Chem. Soc., 1986, 82, 343–358 RSC.
- J. C. Polanyi and A. H. Zewail, Acc. Chem. Res., 1995, 28, 119–132 CrossRef CAS.
- C. E. Tripa and J. T. Yates Jr, Nature, 1999, 398, 591–593 CrossRef CAS.
- L. J. Lauhon and W. Ho, Faraday Discuss., 2000, 117, 249–255 RSC.
- S.-W. Hla, L. Bartels, G. Meyer and K.-H. Rieder, Phys. Rev. Lett., 2000, 85, 2777–2780 CrossRef CAS PubMed.
- P. Maksymovych, D. C. Sorescu, K. D. Jordan and J. T. Yates Jr, Science, 2008, 322, 1664–1667 CrossRef CAS PubMed.
- T. Kumagai, A. Shiotari, H. Okuyama, S. Hatta, T. Aruga, I. Hamada, T. Frederiksen and H. Ueba, Nat. Mater., 2012, 11, 167–172 CrossRef CAS PubMed.
- Z. Ning and J. C. Polanyi, J. Chem. Phys., 2012, 137, 091706 CrossRef PubMed.
- Z. Ning and J. C. Polanyi, Angew. Chem., Int. Ed., 2013, 52, 320–324 CrossRef CAS PubMed.
- M. E. Vaida and T. M. Bernhardt, in Ultrafast Phenomena in Molecular Sciences, ed. R. de Nalda and L. Bañares, Springer International Publishing, Switzerland, 2014, pp. 231–261 Search PubMed.
- K. Anggara, L. Leung, M. J. Timm, Z. Hu and J. C. Polanyi, Sci. Adv., 2018, 4, eaau2821 CrossRef CAS PubMed.
- K. Anggara, L. Leung, M. J. Timm, Z. Hu and J. C. Polanyi, Faraday Discuss., 2019, 214, 89–103 RSC.
- M. J. Timm, L. Leung and J. C. Polanyi, J. Am. Chem. Soc., 2021, 143, 12644–12649 CrossRef CAS PubMed.
- M. J. Timm, L. Leung, K. Anggara and J. C. Polanyi, Commun. Chem., 2021, 4, 14 CrossRef CAS PubMed.
- L. Leung, M. J. Timm and J. C. Polanyi, Chem. Commun., 2021, 57, 12647–12650 RSC.
- I. Horcas, R. Fernández, J. M. Gómez-Rodríguez, J. Colchero, J. Gómez-Herrero and A. M. Baro, Rev. Sci. Instrum., 2007, 78, 013705 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- C. Loken, D. Gruner, L. Groer, R. Peltier, N. Bunn, M. Craig, T. Henriques, J. Dempsey, C.-H. Yu, J. Chen, L. J. Dursi, J. Chong, S. Northrup, J. Pinto, N. Knecht and R. van Zon, J. Phys.: Conf. Ser., 2010, 256, 012026 CrossRef.
- M. Ponce, R. van Zon, S. Northrup, D. Gruner, J. Chen, F. Ertinaz, A. Fedoseev, L. Groer, F. Mao, B. C. Mundim, M. Nolta, J. Pinto, M. Saldarriage, V. Slavnic, E. Spence, C.-H. Yu and W. R. Peltier, in Practice and Experience in Advanced Research Computing (PEARC’19), ACM, New York, NY, USA, 2019, vol. 34, pp. 1–8 Search PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Anthony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- J. Tersoff and D. R. Hamann, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 31, 805–813 CrossRef CAS PubMed.
- D. E. P. Vanpoucke and G. Brocks, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 241308 CrossRef.
- L. Leung, T. Lim, Z. Ning and J. C. Polanyi, J. Am. Chem. Soc., 2012, 134, 9320–9326 CrossRef CAS PubMed.
- A. Eisenstein, L. Leung, T. Lim, Z. Ning and J. C. Polanyi, Faraday Discuss., 2012, 157, 337–353 RSC.
- Z. Ning and J. C. Polanyi, Z. Phys. Chem., 2013, 227, 1501–1510 CAS.
- O. MacLean, K. Huang, L. Leung and J. C. Polanyi, J. Am. Chem. Soc., 2017, 139, 17368–17375 CrossRef CAS PubMed.
- L. Leung, T. Lim, Z. Ning, J. C. Polanyi, W. Ji and C.-G. Wang, J. Phys. Chem. C, 2015, 119, 26038–26045 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |